Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - PDS Biotechnology Corp | s002055x1_8k.htm |
Exhibit 99.1

Corporate Presentation February 2018 ®

Disclaimer This presentation and any statements of representatives of Edge related thereto that are not historical in nature (including but not limited to upcoming milestones) contain, or may contain, among other things, certain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements may include, without limitation, statements with respect to Edge's plans, objectives, projections, expectations and intentions and other statements identified by words such as "projects," "may," "will," "could," "would," "should," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions. These statements are based upon the current beliefs and expectations of Edge's management and are subject to significant risks and uncertainties. Actual results may differ significantly from those set forth in the forward-looking statements. These forward-looking statements involve certain risks and uncertainties that are subject to change based on various risk factors (many of which are beyond Edge's control) as described under the heading "Risk Factors" in Edge's filings with the United States Securities and Exchange Commission. 01

Business development opportunities consistent with strategic focus Success of lead asset EG-1962 in aneurysmal subarachnoid hemorrhage (aSAH) via EVD Market expansion opportunities for EG-1962 Other routes of administration of EG-1962 for aSAH (Cisternal, Lumbar) Other indications in SAH (e.g. Non-aneurysmal, acute DCI, traumatic SAH) Other indications outside of SAH Proprietary PRECISA™ technology: Platform to develop future products Strong balance sheet Strategy To Maximize Long-Term Value 02 Ongoing Phase 3 study supported by positive Phase 2 data

Current Pipeline Product Candidate Formulation Development INDEnabling Phase 1 Aneurysmal Subarachnoid Hemorrhage Intraventricular (EVD) Delivery Intracisternal Administration Lumbar Administration Chronic Subdural Hematoma EG-1962 EG-1962 EG-1962 EG-1964 Phase 2 Phase 3 Product Development Pipeline 03 Atrial Fibrillation EG-1965

Recent and Expected Key Milestones 04 Received pediatric waiver from EMA for EG-1962 Initiated study of EG-1962 cisternal delivery Secured $18M from RDO EG-1962 cisternal PK data update Entered into commercial supply agreement for EG-1962 Initiated EG-1962 lumbar PK animal study NEWTON 2 futility analysis in 4Q’17 EG-1962 health economic data NEWTON 2 full top-line data in late 2018 Submit IND for second PRECISA product in 2018 P P P P P 1H’17 2H’17 1H’18 2H’18 NEWTON 2 interim analysis in early 2018 P P P

SAH (bleeding into subarachnoid space in the brain) is life-threatening with limited treatment options Aneurysmal SAH (aSAH), most severe form of SAH, is caused by a ruptured brain aneurysm Delayed cerebral ischemia (DCI) occurs days after SAH / aSAH; causes brain tissue death and is associated with poor patient outcomes Aneurysmal Subarachnoid Hemorrhage (aSAH) 05 Patient demographics 85-90% of patients make it alive to the hospital 83% of poorer grade patients (WFNS grades 2-4 with GOSE <6) die or suffer permanent brain damage within 90 days * Admitted Inpatients using 2013 National Inpatient Sample data. 6 SAH Patients Annually EG-1962Market ExpansionOpportunity~50% EG-1962 InitialPatientOpportunity~50% SAH U.S. Population~35,000 patients aSAH U.S. Population*~20,000 patients ~600,000 Worldwide ~100,000 North America,EU, Japan Avg. age of aSAH patient = 52

Only 17% of poorer grade patients (WFNS grades 2-4 with GOSE >6) return to favorable or normal status - sub-optimal nimodipine in the brain Current Management of aSAH Nimodipine (L-type Ca channel antagonist) is standard of care (SoC) to prevent DCI and improve outcomes, but prognosis is poor and complications are serious 06 Aneurysm RuptureExternal Ventricular Drain (EVD) Universal administration of oral nimodipine (Class 1, Level A) Surgical / Endovascular Aneurysm Repair ICU Management(Watch & Wait) Stabilize Secure Prevent Treatment limiting side effects; systemic hypotension in up to 50% Short half life (45-minutes) requires heavy nursing burden;2 pills every 4 hours x 21 days – – – Reduce delayed ischemia / improve patient outcomes Standard of care in U.S., Europe, Asia (excl. Japan) Treats multiple deleterious effects of calcium influx after aSAH + + + Discharge to:In/Out Patient, Rehab, Skilled Nursing Home, Physical/Occupational Therapy Recovery

EG-1962 Differentiation and Existing Data ®

EG-1962 provides sustained high drug exposure directly at the site of brain injury Ongoing Phase 3 study for aSAH (EVD) Positive Phase 2 (EVD) and preclinical results Potential for significant health economic impact EG-1962: Potential to Significantly Improve Outcomes, Safety and Convenience 07 Delivers 100 to 1,000 times the concentration of nimodipine directly to the brain with sustained delivery over at least 21 days vs. current standard of care oral nimodipine Has the potential to transform the management of aSAH and improve patient outcomes Unable to get similar EG-1962 type concentrations of nimodipine into the brain with oral nimodipine without causing dose-limiting, potentially serious hypotension Multiple Routes of Administration(EVD, Cisternal, Lumbar) EG-1962

NEWTON 2 Phase 3 Study Replicates Major Elements of Phase 2 Protocol 08 Comparing safety and efficacy of EG-1962 versus oral nimodipine 10 ~75 centers (North America, EU, Australasia)1 to 1 randomization; double-blind/double-dummyStratified by WFNS, Region Primary Endpoints Secondary & Health Economic Endpoints Study Sample Size(n = 374) Neurological outcome measured at 90 days after aSAH using GOSE Safety profile of EG-1962 compared to oral nimodipineCognitive assessment using the Montreal Cognitive Assessment (MoCA)ICU and hospital LoS; discharge dispositionUse of rescue therapy Full study results at 374 patientsFutility analysis at 150 patientsInterim analysis at 210 patients Study Design

NEWTON 2 Interim Analysis Possible Scenarios No formal futility analysis. However, DMC may recommend amending or terminating the study for safety concerns or based on a benefit:risk assessment that does not justify additional subject enrollment. 09 DMC may recommend stopping enrollment and waiting for efficacy data from additional patients enrolled after 210 up to the interim analysis, if the study nearly missed the threshold for stopping at interim. DMC may recommend proceeding to full enrollment (n=374) as planned. DMC may recommend stopping the study for an efficacy win, based on showing a ~20% absolute difference in favorable outcomes versus control arm.

Maximum feasible dose identified (primary endpoint) 800 mg Positive safety and tolerability observed (secondary endpoint) NEWTON Study – Key Highlights 10 Phase 1/2, international, multi-center, randomized, controlled, open-label study All patients tolerated administration 73 patients completed (6 cohorts): 55 EG-1962, 18 oral nimodipine PK characterized up to 800 mg dose (secondary endpoint) Exploratory endpoints all favorable towards EG-1962

Glasgow Outcome Scale (GOSE) Unfavorable Outcome Favorable Outcome 1 2 6 3 4 5 7 8 Dead Vegetative State Lower Severe Disability Upper Severe Disability Lower Moderate Disability Upper Moderate Disability Lower Good Recovery Upper Good Recovery EG-1962 Cohorts 1-5(n=46) 59% Oral nimodipine(n=18) 28% >2x Historical Data*(n=151) 17% 28% 6% ~5x <1% EG-1962 Cohorts 1-5(n=46) Historical Data*(n=151) Oral nimodipine(n=18) NEWTON Study Suggested EG-1962 Potential to Improve Patient Outcomes Favorable Outcome (GOSE 6-8) GOSE 8 11 * Hänggi D, Etminan N, Macdonald RL, Steiger, HJ. NEWTON - Nimodipine microparticles to Enhance recovery While reducing TOxicity after subarachNoid hemorrhage. Neurocritical Care. Published Online: 13 February 2015.

EG-1962 Oral nimodipine 12 (n=13/45) (n=11/18) 29% 61% VasospasmReduced by 52% (n=6/45) (n=6/18) 13% 33% DCIReduced by 61% (n=11/45) (n=10/18) 24% 56% Rescue Therapy Reduced by 57% Median 13.5 (n=45) (n=18) 10 12 14 16 18 17 ICU Length of Stay (LoS) Reduced by 3.5 days (20%) (n=45) (n=18) Median 22.5 20 22 24 26 28 25 Hospital LoS Reduced by 2.5 days (10%) Additional Data from NEWTON Study Provides Further Rationale for Premium Pricing

Market Access andCommercial Strategy ®

Average charge per aSAH patient is >$300,000 and >$400,000 with EVD, excluding physician fees* Physician related fees ICU and hospital stay Rescue therapy to treat delayed cerebral ischemia and vasospasm Mechanical ventilation Securing aneurysm (clip / coil) Primary drivers for charges+: aSAH Among Most Expensive Diseases Treated in U.S. Hospitals * Company analysis of NIPS claims data+ Company analysis of Premier database 13

14 Reimbursement Overview for Hospital Care and aSAH Medicare(DRG) Fixed Rate(DRG/Per Diem) % Charges Fixed Rate(Capitation) Additional Payment Mechanisms:Stop-loss | Carve-out Note: In certain situations, contracts may preclude pharmacy charges from qualifying a case for payment under the stop-loss clause Commercial Payer Government Payer Additional Payment Mechanisms:Outlier | New Technology Add-on (NTAP) Mechanisms in place to support hospital payments for sickest patients and new innovations aSAH high volume hospitals are large teaching or academic centers, which typically have greater leverage in negotiating stop-loss coverage and carve-outs
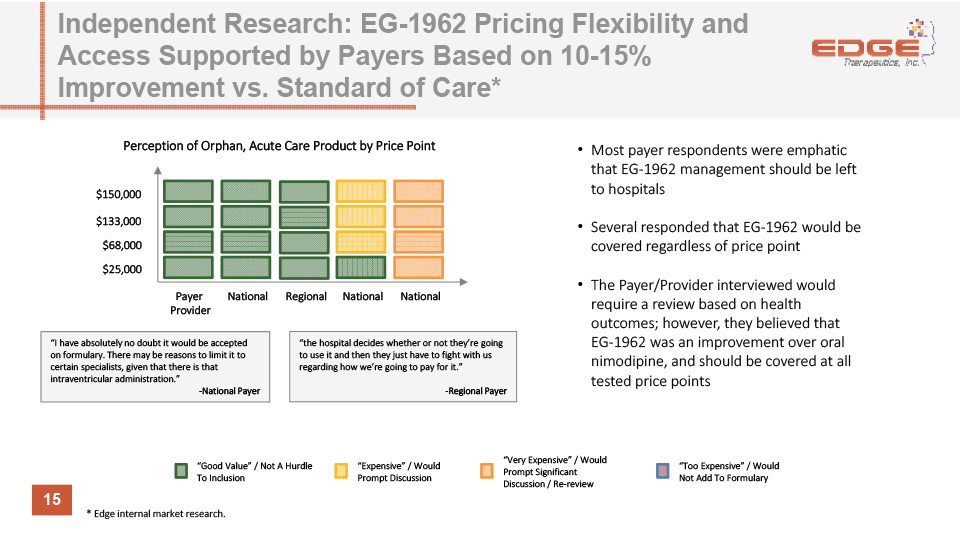
15 Independent Research: EG-1962 Pricing Flexibility and Access Supported by Payers Based on 10-15% Improvement vs. Standard of Care* “Good Value” / Not A Hurdle To Inclusion “Very Expensive” / Would Prompt Significant Discussion / Re-review “Expensive” / Would Prompt Discussion “Too Expensive” / Would Not Add To Formulary $150,000 $133,000 $68,000 $25,000 Payer Provider Most payer respondents were emphatic that EG-1962 management should be left to hospitalsSeveral responded that EG-1962 would be covered regardless of price pointThe Payer/Provider interviewed would require a review based on health outcomes; however, they believed that EG-1962 was an improvement over oral nimodipine, and should be covered at all tested price points National National National Regional “I have absolutely no doubt it would be accepted on formulary. There may be reasons to limit it to certain specialists, given that there is that intraventricular administration.”-National Payer “the hospital decides whether or not they’re going to use it and then they just have to fight with us regarding how we’re going to pay for it.”-Regional Payer Perception of Orphan, Acute Care Product by Price Point * Edge internal market research.
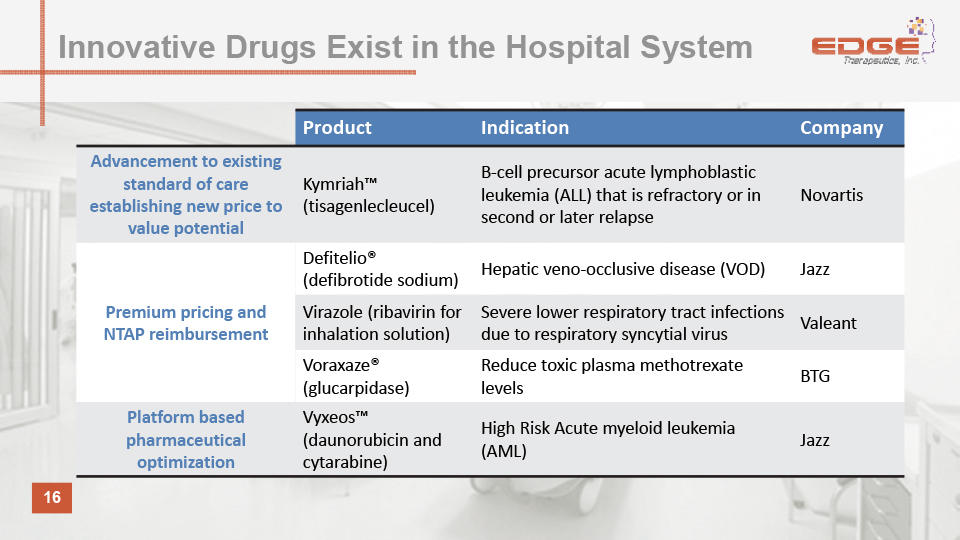
16 Product Indication Company Advancement to existing standard of care establishing new price to value potential Kymriah™ (tisagenlecleucel) B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse Novartis Premium pricing and NTAP reimbursement Defitelio® (defibrotide sodium) Hepatic veno-occlusive disease (VOD) Jazz Virazole (ribavirin for inhalation solution) Severe lower respiratory tract infections due to respiratory syncytial virus Valeant Voraxaze® (glucarpidase) Reduce toxic plasma methotrexate levels BTG Platform based pharmaceutical optimization Vyxeos™ (daunorubicin and cytarabine) High Risk Acute myeloid leukemia (AML) Jazz Innovative Drugs Exist in the Hospital System

Commercial Strategy Strong relationships with key hospital decision makers North America:Small, targeted sales force of ~35 representatives Europe:Similar-sized sales force to NA ~300 hospitals accountfor 90% of all aSAH patients Drivers for rapid adoption (U.S. launch): EG-1962 administration does not materially change current physician behavior / treatment protocol Pharmacoeconomic benefit: preventing DCI saves hospitals and managed care organizations substantial costs, plus outpatient rehab / nursing home costs 17

Four-Layered Approach to Maintain Barrier-to-Entry 4 issued U.S. patents (including composition of matter to 2033), 32 issued foreign patents and more than 50 U.S. and foreign pending patent applications Intellectual Property Manufacturing know-how & trade secrets Precisa development platform Ability to prove bioequivalence Potentially difficult for competitors to prove bioequivalence (i.e., human trial required) 18 Potentially eligible for 7 years of U.S. marketing exclusivity Orphan Drug Designation

EG-1962 Summary Pharmacokinetic rationale Improved outcomes vs nimodipine without off-target side effects in Phase 2 study; supports premium pricing rationale De-risked path to market High Barriers to Entry 19 Pivotal phase 3 design replicates key elements of phase 2 protocol 505(b)(2) regulatory pathway allows for reduced development burden Orphan designation – potentially eligible for 7 years of U.S. marketing exclusivity Composition of matter patent in U.S. to 2033

Questions?

EG-1962 achieved steady state sustained release of nimodipine 100x-1,000x higher nimodipine concentrations in CSF vs. oral nimodipine Pharmacokinetic Data Supports Clinical Benefit of EG-1962 20

Steady-state plasma concentration measured in EG-1962-treated patients was below 30 ng/ml, the level observed to cause systemic hypotension with oral nimodipine (n=0/54) 0.0% Hypotension 16.7% (n=3/18) EG-1962 Was Not Observed To Increase Risk of Hypotension EG-1962 Oral Nimodipine 21

Unfavorable Outcome Favorable Outcome 1 2 6 3 4 5 7 8 Dead Vegetative State Lower Severe Disability Upper Severe Disability Lower Moderate Disability Upper Moderate Disability Lower Good Recovery Upper Good Recovery EG-1962: Favorable Outcomes in NEWTON Study by Cohort 22 56% (n=5) 78% (n=7) 67% (n=6) 40% (n=4) 56% (n=5) Cohort 1 (100 mg; n=9) Oral nimodipine(n=18) Cohort 2(200 mg; n=9) Cohort 3(400 mg;n=9) Cohort 4(600 mg; n=10) Cohort 5(800 mg; n=9) EG-1962 Active Control (n=5) 28% HistoricalData 17% Favorable Outcome

EG-1962: Clinical Benefit Observed Across Disease Severities 23 Overall: Over 2x improvement in favorable outcome rate WFNS 2 = Over 2x improvement in favorable outcome rate WFNS 4 = Nearly 2x improvement in favorable outcome rate
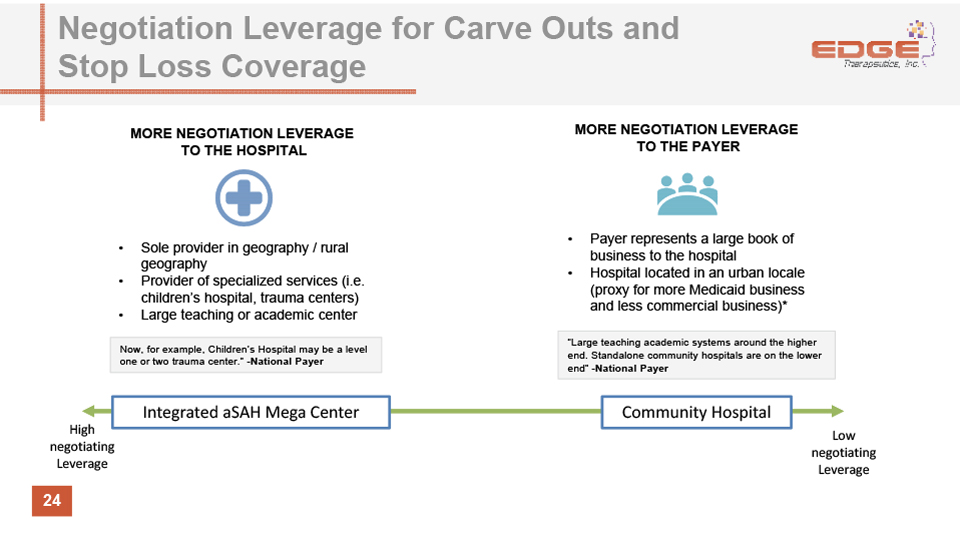
Negotiation Leverage for Carve Outs and Stop Loss Coverage 24 MORE NEGOTIATION LEVERAGE TO THE HOSPITAL MORE NEGOTIATION LEVERAGE TO THE PAYER Sole provider in geography / rural geographyProvider of specialized services (i.e. children’s hospital, trauma centers)Large teaching or academic center Payer represents a large book of business to the hospitalHospital located in an urban locale (proxy for more Medicaid business and less commercial business)* “Large teaching academic systems around the higher end. Standalone community hospitals are on the lower end” -National Payer Now, for example, Children’s Hospital may be a level one or two trauma center.” -National Payer High negotiating Leverage Low negotiating Leverage Integrated aSAH Mega Center Community Hospital

Precisa™ Development Platform Proprietary, programmable, biodegradable polymer-based development platform Controlled & Sustained Release Optimize product Program release profileSpecific blend of polymers Initial release profileSustained release profile Define product profileIdentify therapeuticEngineer polymerPhysical / chemical properties Identify unmetclinical condition Targeted Delivery 25

February 2018 (Nasdaq: EDGE) ®
