Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Alkermes plc. | alks-20180214ex991ac074b.htm |
| 8-K - 8-K - Alkermes plc. | alks-20180214x8k.htm |
Exhibit 99.2
|
Alkermes® Patient inspired Fourth Quarter and Year-End 2017 Financial Results February 14, 2018 |
|
Forward-Looking Statements and Non-GAAP Financial Information Certain statements set forth in this presentation constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: future financial and operating performance, business plans or prospects; the likelihood of continued revenue growth from the company’s commercial products, including the growth of VIVITROL and ARISTADA; the therapeutic and commercial value of the company’s marketed and development products and patient access to such products; expectations concerning the timing and results of clinical development activities, including the timing of the phase 3 clinical trial enrollment completion and data readout for ALKS 3831, the timing of data from the EVOLVE-MS-2 head-to-head gastrointestinal study and the potential $50 million option payment by Biogen, the submission of the NDA for BIIB098, the acceptance of the NDA for ALKS 5461 by the FDA, and the timing of FDA review of the NDA for ALKS 5461; and expectations concerning the timing and results of commercial activities, including the expected launch of ALKS 5461. The company cautions that forward-looking statements are inherently uncertain. Although the company believes that such statements are based on reasonable assumptions within the bounds of its knowledge of its business and operations, the forward-looking statements are neither promises nor guarantees and they are necessarily subject to a high degree of uncertainty and risk. Actual performance and results may differ materially from those expressed or implied in the forward-looking statements due to various risks and uncertainties. These risks and uncertainties include, among others: the unfavorable outcome of litigation, including so-called “Paragraph IV” litigation and other patent litigation, related to any of our products, which may lead to competition from generic drug manufacturers; data from clinical trials may be interpreted by the FDA in different ways than we interpret it; the FDA may not agree with our regulatory approval strategies or components of our filings, such as clinical trial designs, conduct and methodologies; clinical development activities may not be completed on time or at all; the results of our clinical development activities may not be positive, or predictive of real-world results or of results in subsequent clinical trials; regulatory submissions may not occur or be submitted in a timely manner; the company and its licensees may not be able to continue to successfully commercialize their products; there may be a reduction in payment rate or reimbursement for the company’s products or an increase in the company’s financial obligations to governmental payers; the U.S. Food and Drug Administration or regulatory authorities outside the U.S. may make adverse decisions regarding the company’s products; the company’s products may prove difficult to manufacture, be precluded from commercialization by the proprietary rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; and those risks and uncertainties described under the heading “Risk Factors” in the company’s most recent Annual Report on Form 10-K and in subsequent filings made by the company with the U.S. Securities and Exchange Commission (“SEC”), which are available on the SEC’s website at www.sec.gov. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the company disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation. Non-GAAP Financial Measures: This presentation includes information about certain financial measures that are not prepared in accordance with generally accepted accounting principles in the U.S. (GAAP), including non-GAAP net income/(loss) and non-GAAP net income/(loss) per share. These non-GAAP measures are not based on any standardized methodology prescribed by GAAP and are not necessarily comparable to similar measures presented by other companies. Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP financial measures can be found in the Alkermes plc Current Report on Form 8-K filed with the SEC on Feb. 14, 2018. Note Regarding Trademarks: The company is the owner of various U.S. federal trademark registrations (®) and other trademarks (TM), including ARISTADA®, VIVITROL® and NanoCrystal®. Any other trademarks referred to in this presentation are the property of their respective owners. Appearances of such other trademarks herein should not be construed as any indicator that their respective owners will not assert their rights thereto. Alkermes® © 2018 Alkermes All rights reserved. |
|
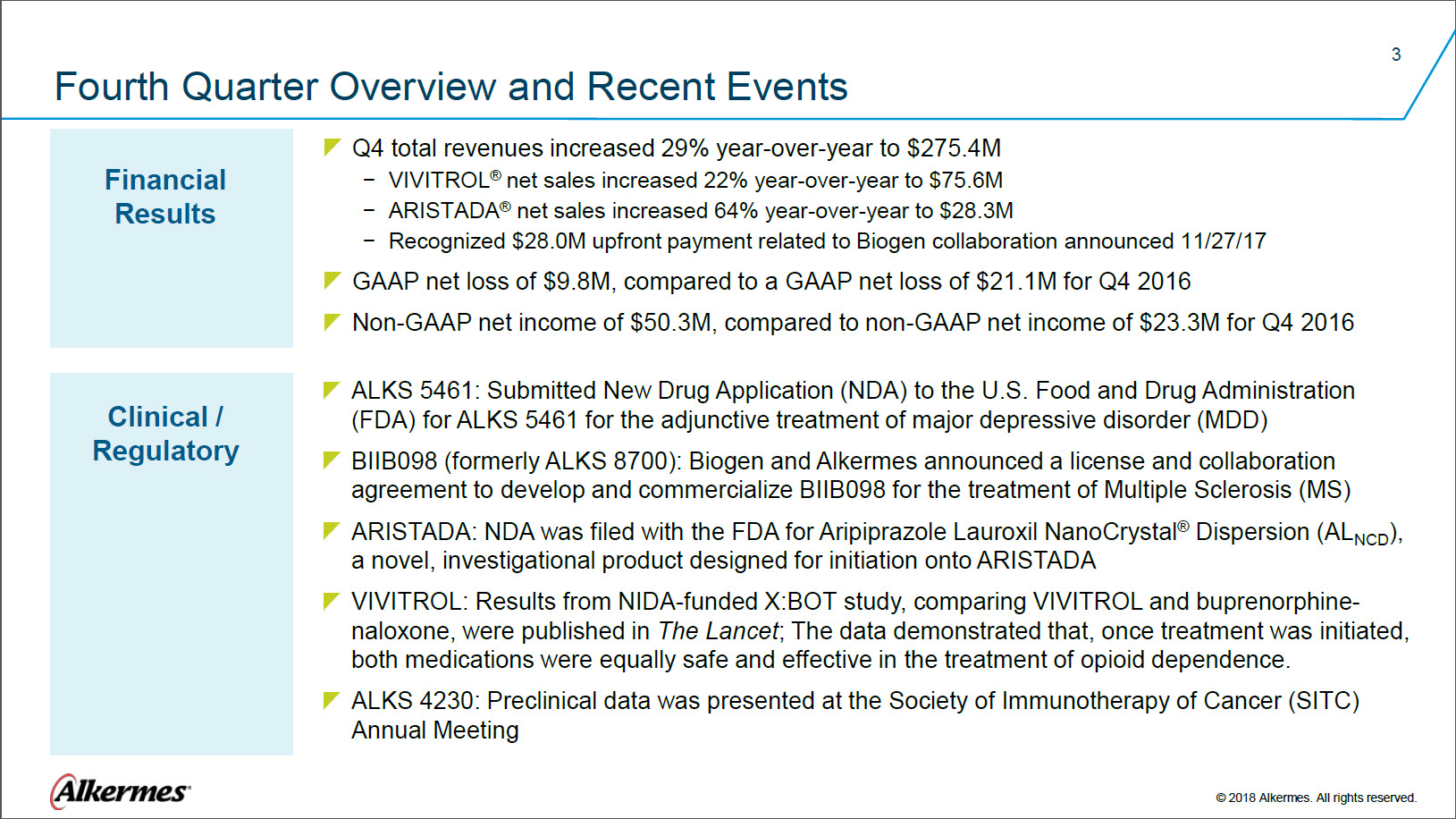
Fourth Quarter Overview and Recent Events Financial Results Q4 total revenues increased 29% year-over-year to $275.4M VIVITROL® net sales increased 22% year-over-year to $75.6M ARISTADA® net sales increased 64% year-over-year to $28.3M Recognized $28.0M upfront payment related to Biogen collaboration announced 11/27/17 GAAP net loss of $9.8M, compared to a GAAP net loss of $21.1M for Q4 2016 Non-GAAP net income of $50.3M, compared to non-GAAP net income of $23.3M for Q4 2016 Clinical / Regulatory
ALKS 5461: Submitted New Drug Application (NDA) to the U.S. Food and Drug Administration BIIB098 (formerly ALKS 8700): Biogen and Alkermes announced a license and collaboration agreement to develop and commercialize BIIB098 for the treatment of Multiple Sclerosis (MS)
ARISTADA: NDA was filed with the FDA for Aripiprazole Lauroxil NanoCrystal® Dispersion (ALNCD), VIVITROL: Results from NIDA-funded X:BOT study, comparing VIVITROL and buprenorphine-naloxone, were published in The Lancet; The data demonstrated that, once treatment was initiated, both medications were equally safe and effective in the treatment of opioid dependence. ALKS 4230: Preclinical data was presented at the Society of Immunotherapy of Cancer (SITC) Annual Meeting Alkermes® © 2018 Alkermes. All rights reserved. |
|
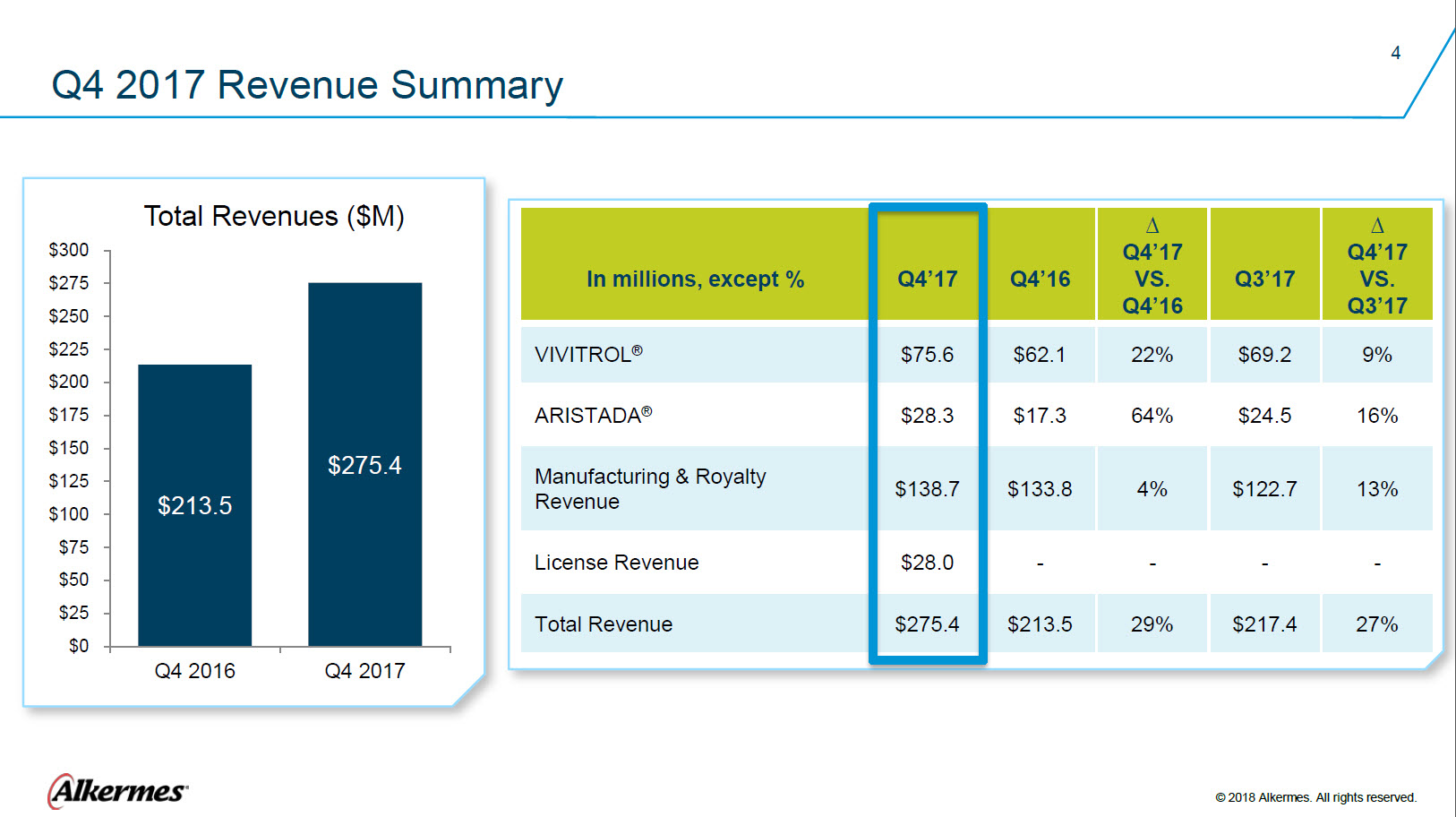
Q4 2017 Revenue Summary Total Revenues ($M) $300 $275 $250 $225 $200 $175 $150 $125 $100 $75 $50 $25 $0 Q4 2016 $213.5 Q4 2017 $275.4 In millions, except % Q4’17 Q4’16 ∆ Q4’17 VS. Q4’16 Q3’17 ∆ Q4’17 VS. Q3’17 VIVITROL ® ARISTADA ® Manufacturing & Royalty Revenue License Revenue Total Revenue $75.6 $62.1 22% $69.2 9% $28.3 $17.3 64% $24.5 16% $138.7 $133.8 4% $122.7 13% $28.0 - - - - $275.4 $213.5 29% $217.4 27% Alkermes® © 2018 Alkermes. All rights reserved. |
|
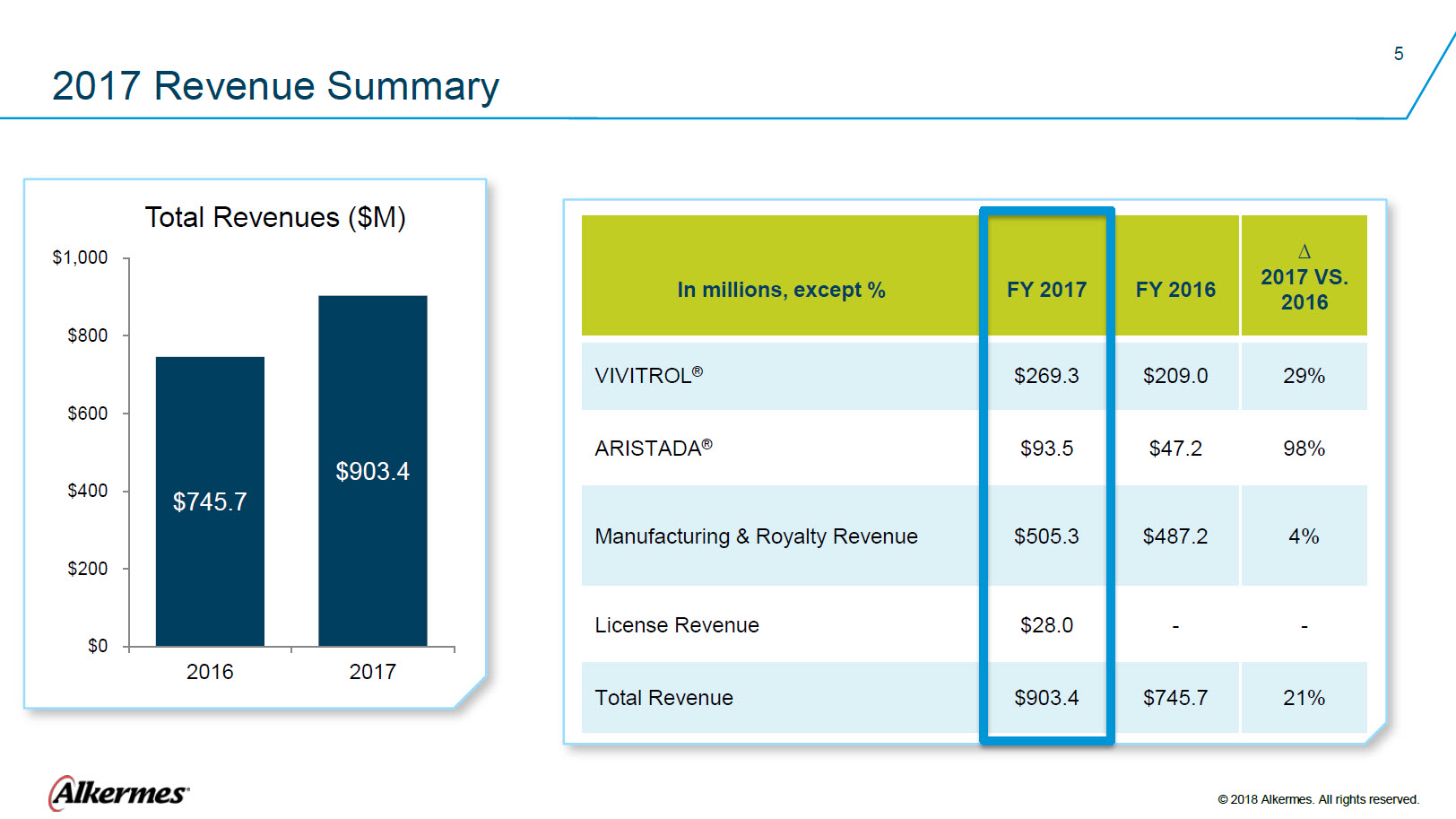
2017 Revenue Summary Total Revenues ($M) $1,000 $800 $600 $400 $200 $0 $745.7 2016 $903.4 2017 In millions, except % VIVITROL ® ARISTADA® Manufacturing & Royalty Revenue License Revenue Total Revenue FY2017 $269.3 $93.5 $505.3 $28.0 $903.4 FY 2016
$47.2 $487.2 0 $745.7 ∆2017 VS. 2016 29% 98% 4% 0 21% Alkermes ® © 2018 Alkermes. All rights reserved |
|
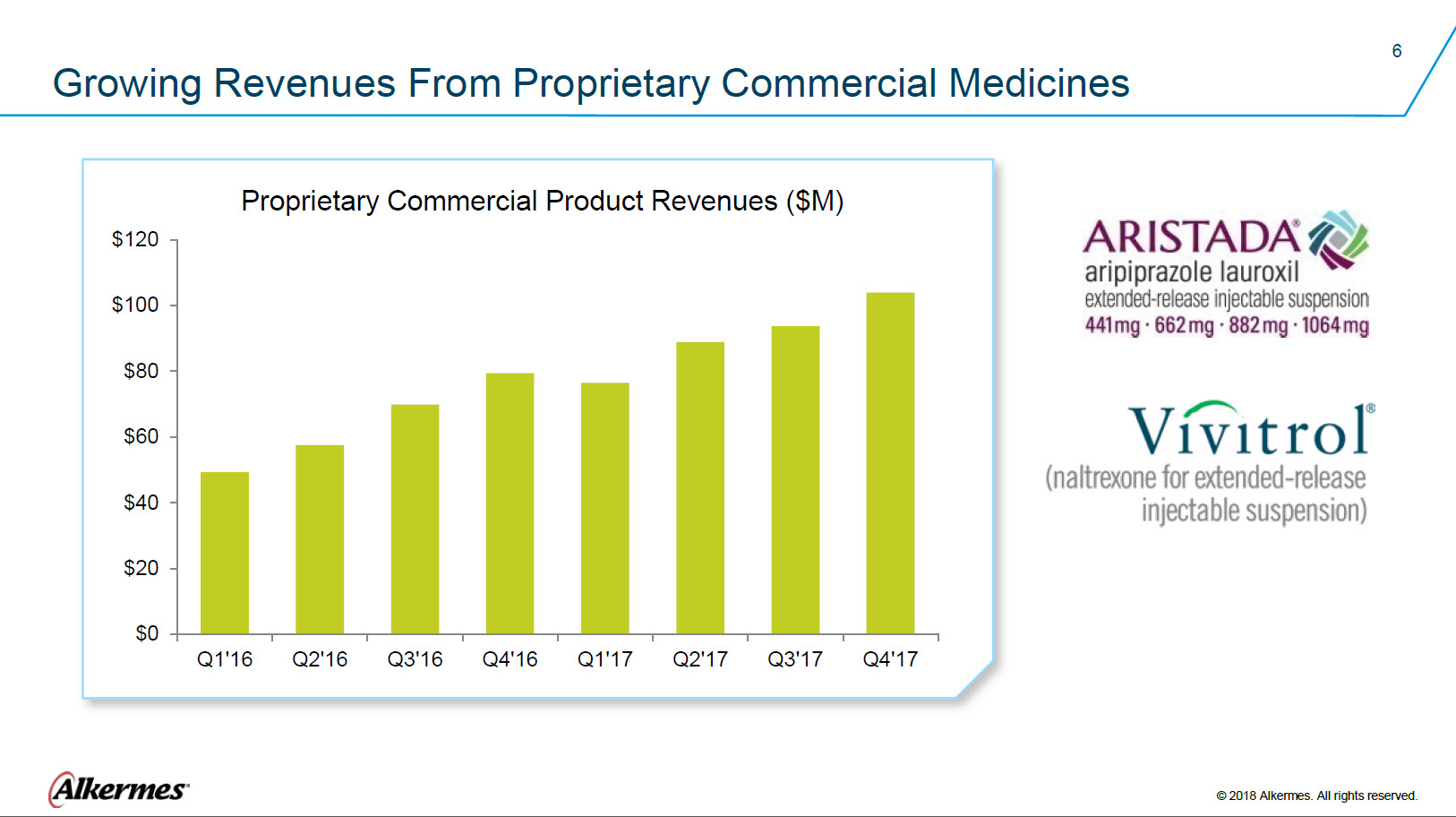
Growing Revenues From Proprietary Commercial Medicines Proprietary Commercial Product Revenues ($M) $120, $100 $80 $60 $40 $20 $0 Q1’16 Q2’16 Q3’16 Q4’16 Q1’17 Q2’17 Q3’17 Q4’17 ARISTADA® aripiprazole lauroxil extended-release injectable suspension 441 mg 662 mg 882 mg 1064 mg VIVITROL® (naltrexone for extended-release injectable suspension) Alkermes® © 2018 Alkermes. All rights reserved. |
|
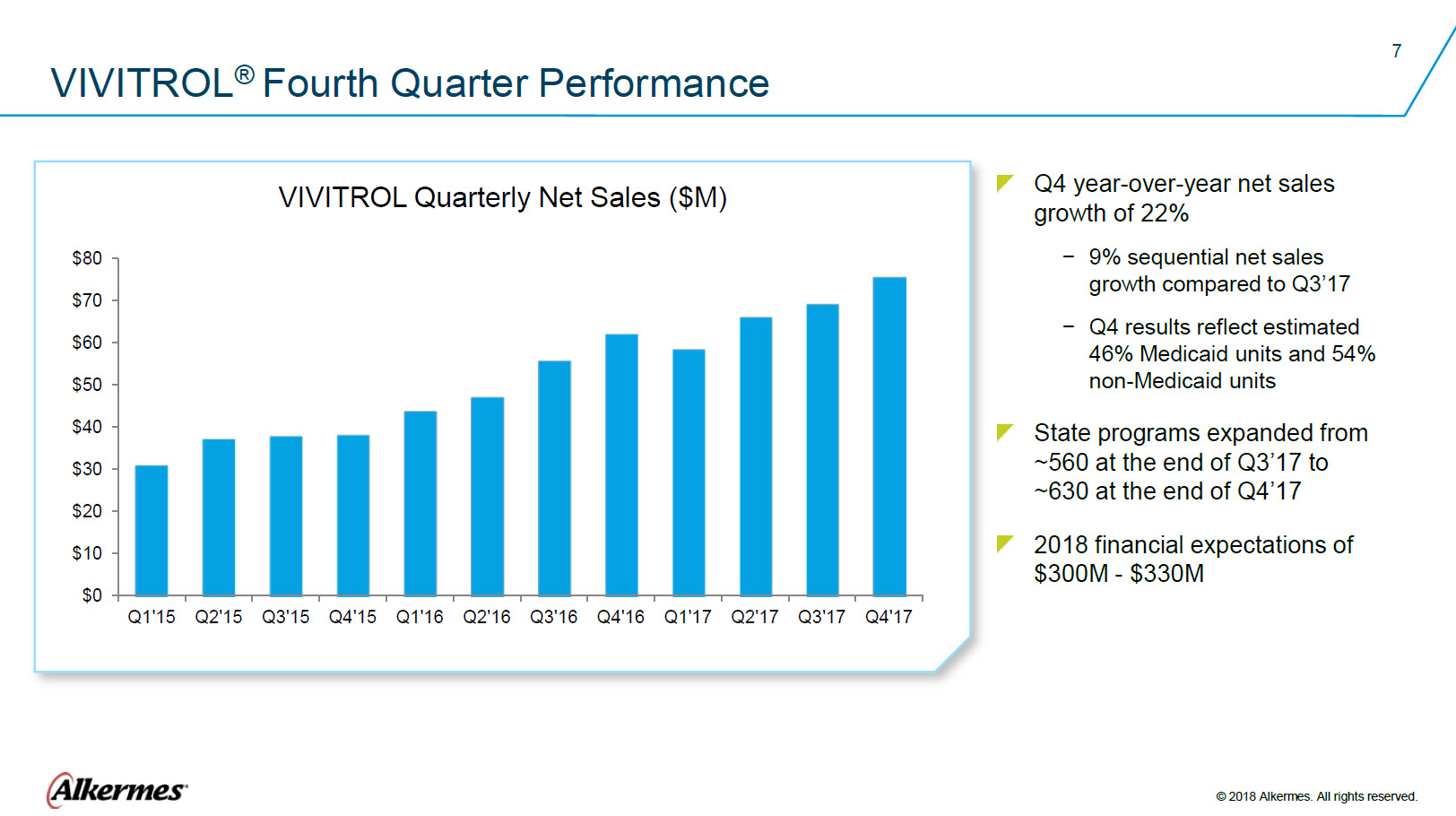
VIVITROL® Fourth Quarter Performance VIVITROL Quarterly Net Sales ($M) $80 $70 $60 $50 $40 $30 $20 $10 $0 Q1’15 Q2’15 Q3’15 Q4’15 Q1’16 Q2’16 Q3’16 Q4’16 Q1’17 Q2’17 Q3’17 Q4’17
Q4 year-over-year net sales
9% sequential net sales
Q4 results reflect estimated
State programs expanded from ~560 at the end of Q3’17 to
2018 financial expectations of Alkermes ® ©2018 Alkermes. All rights reserved. |
|
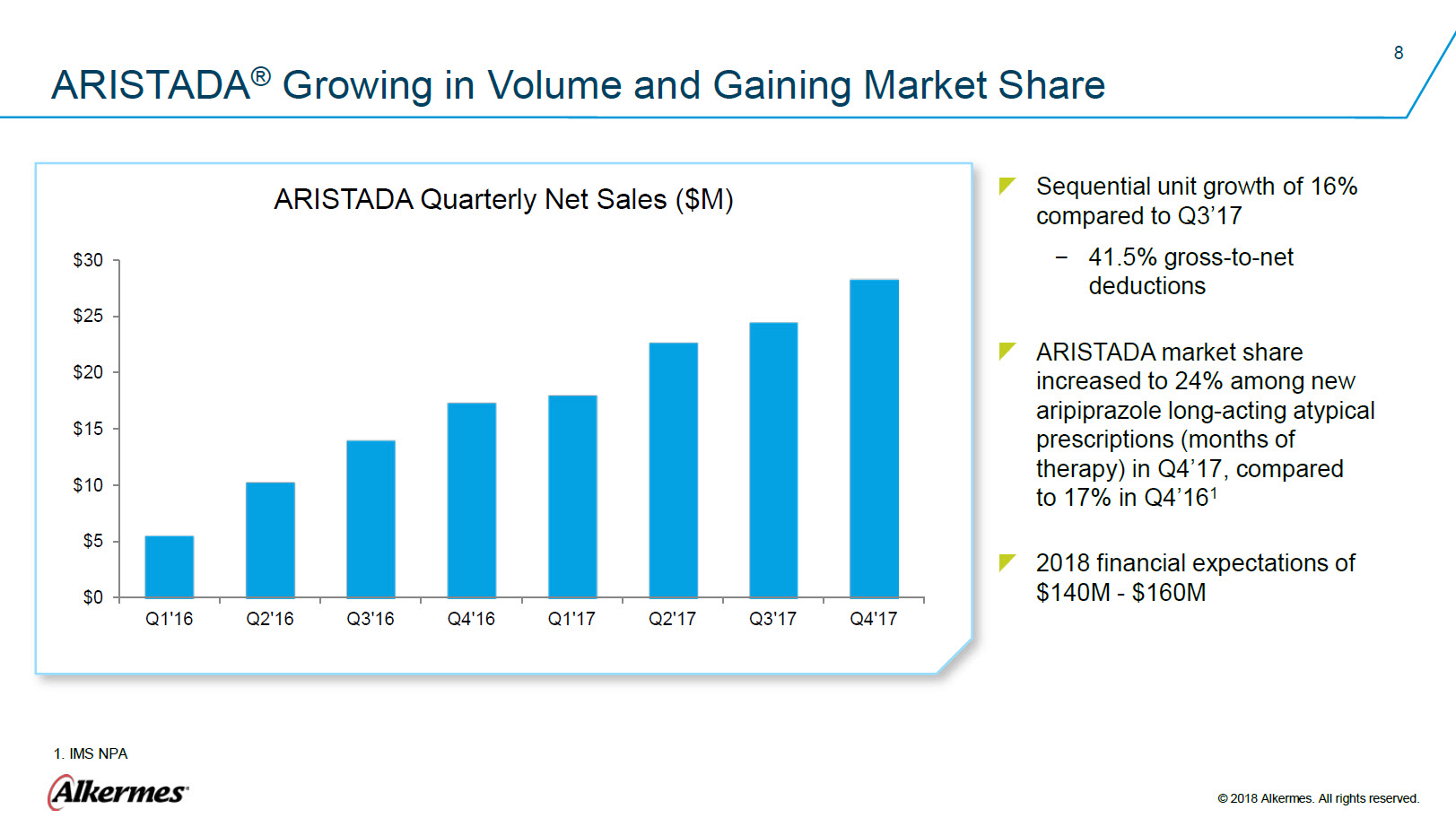
ARISTADA® Growing in Volume and Gaining Market Share ARISTADA Quarterly Net Sales ($M) $30 $25 $20 $15 $10 $5 $0 Q1’16 Q2’16 Q3’16 Q4’16 Q1’17 Q2’17 Q3’17 Q4’17 Sequential unit growth of 15% compared to Q3’17 41.5% gross-to-net deductions ARISTADA market share increased to 24% among new aripiprazole long-acting atypical prescriptions (months of therapy) in Q4’17, compared to 17% in Q4’161 2018 Financial expectations of $140M - $160M 1. IMS NPA Alkermes® © 2018 Alkermes. All rights reserved. |
|
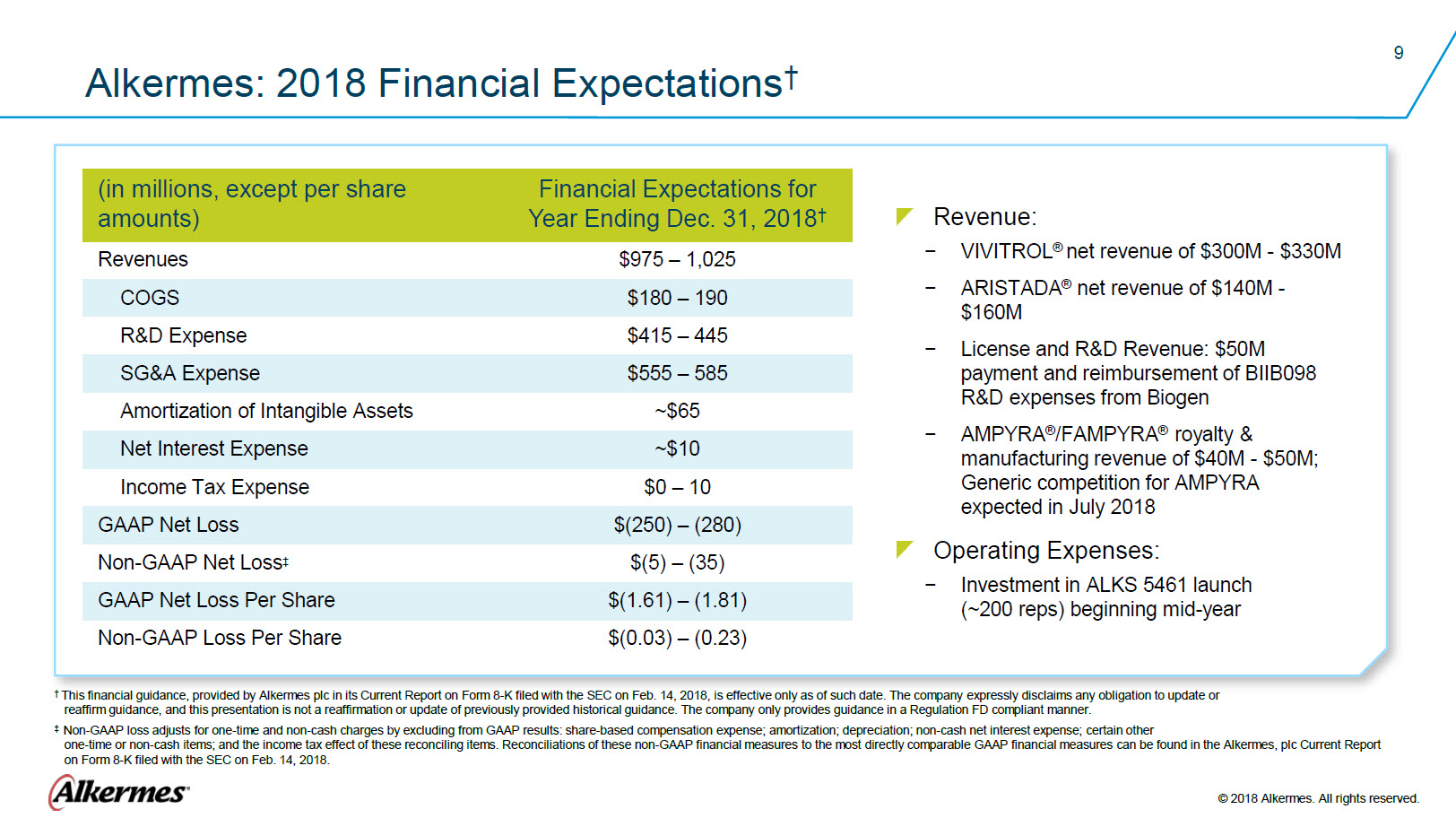
Alkermes: 2018 Financial Expectations † (in millions, except per share amounts) Financial Expectations for Year Ending Dec. 31, 2018†Revenues $978 – 1,025 COGS $180 – 190 R&D Expense $415 -–445 SG&A Expense $555 - 585 Amortization of intangibles ~$65 Net Interest Expense ~$10 Income tax expense $0 – 10 GAAP Net Loss $(250) – (280) Non-GAAP Net Loss‡ $(5) – (35) GAAP Net Loss Per Share $(1.61) – (1.81) Non-GAAP Loss Per Share $(0.03) – (0.23) Revenue: VIVITROL® net revenue of $300M - $330M ARISTADA® net revenue of $140M - $160M License and R&D Revenue: $50M payment and reimbursement of BIIB098 R&D expenses from Biogen AMPYRA®/FAMPYRA® royalty & manufacturing revenue of $40M - $50M; Generic competition for AMPYRA expected in July 2018 Operating Expenses: Investment in ALKS 5461 launch (~200 reps) beginning mid-year.
†This financial guidance, provided by Alkermes plc in its Current Report on Form 8-K filed with the SEC on Feb. 14, 2018, is effective only as of such date. The company expressly disclaims any obligation to update or
‡ Non-GAAP loss adjusts for one-time and non-cash charges by excluding from GAAP results: share-based compensation expense; amortization; depreciation; non-cash net interest expense; certain other Alkermes® © 2018 Alkermes. All rights reserved. |
|
VIVITROL®: Confluence of Data, Policy and Funding Being Integrated Into Response to Opioid Crisis Data underscore utility of VIVITROL for opioid dependence Results from NIDA’s X:BOT study, comparing extended-release naltrexone (VIVITROL) and buprenorphine-naloxone, were published in The Lancet Data from the study demonstrated that, once treatment was initiated, both medicines were equally safe and effective in the treatment of opioid dependence Policymakers activating to address opioid epidemic at national level Focus on implementation of Comprehensive Addiction and Recovery Act FDA taking steps to promote more widespread use of medication-assisted treatment (MAT) State and federal dollars are being allocated; funding slow to reach treatment system Federal budget passed last week included $6B to address the opioid epidemic and mental health programs Last year’s 21st Century Cures Act provided $1B; Have not yet seen that funding flow from the states into changing the treatment system Alkermes® © 2018 Alkermes. All rights reserved. |
|
ARISTADA®: Focused on Patient-Centered Treatment Options NDA submitted to FDA for Aripiprazole Lauroxil NanoCrystal® Dispersion (ALNCD) for initiation onto ARISTADA PDUFA date of June 30, 2018 New initiation regimen designed to replace need for concomitant three weeks of oral aripiprazole Provides an extended-release aripiprazole lauroxil formulation having a smaller particle size than ARISTADA, enabling faster dissolution and leading to more rapid achievement of therapeutic levels of aripiprazole New phase 3b study utilizing ALNCD plus two-month ARISTADA compared to current market leader INVEGA SUSTENNA® initiated in November 2017 Two-month ARISTADA dose gaining traction 9% of total ARISTADA prescriptions in Q4 Alkermes® © 2018 Alkermes. All rights reserved. |
|
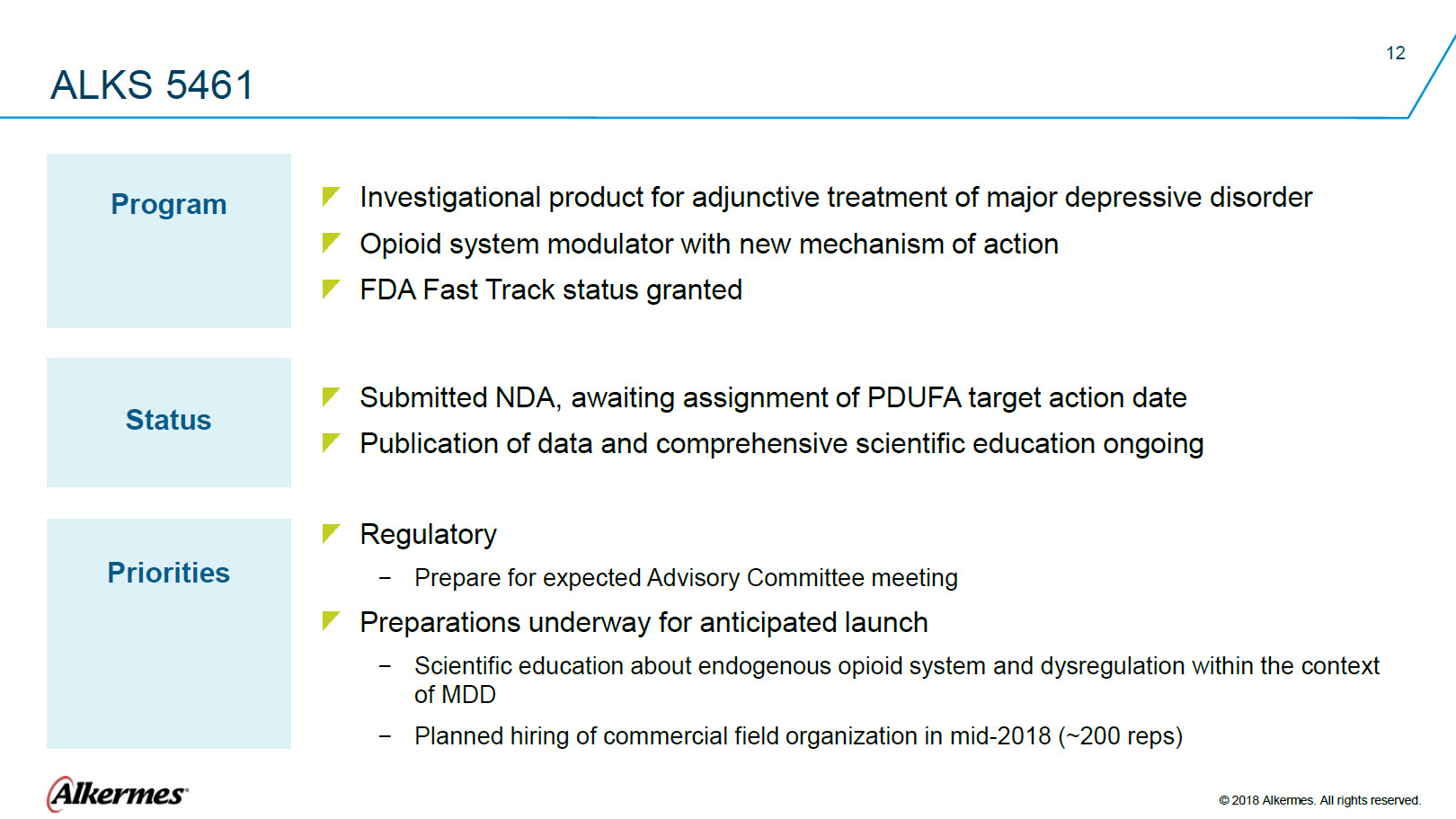
ALKS 5461 Program Investigational product for adjunctive treatment of major depressive disorder Opioid system modulator with new mechanism of action FDA Fast Track status granted Status Submitted NDA, awaiting assignment of PDUFA target action date Publication of data and comprehensive scientific education ongoing Priorities Regulatory Prepare for expected Advisory Committee meeting Preparations underway for anticipated launch Scientific education about endogenous opioid system and dysregulation within the context of MDD Planned hiring of commercial field organization in mid-2018 (~200 reps) |
|
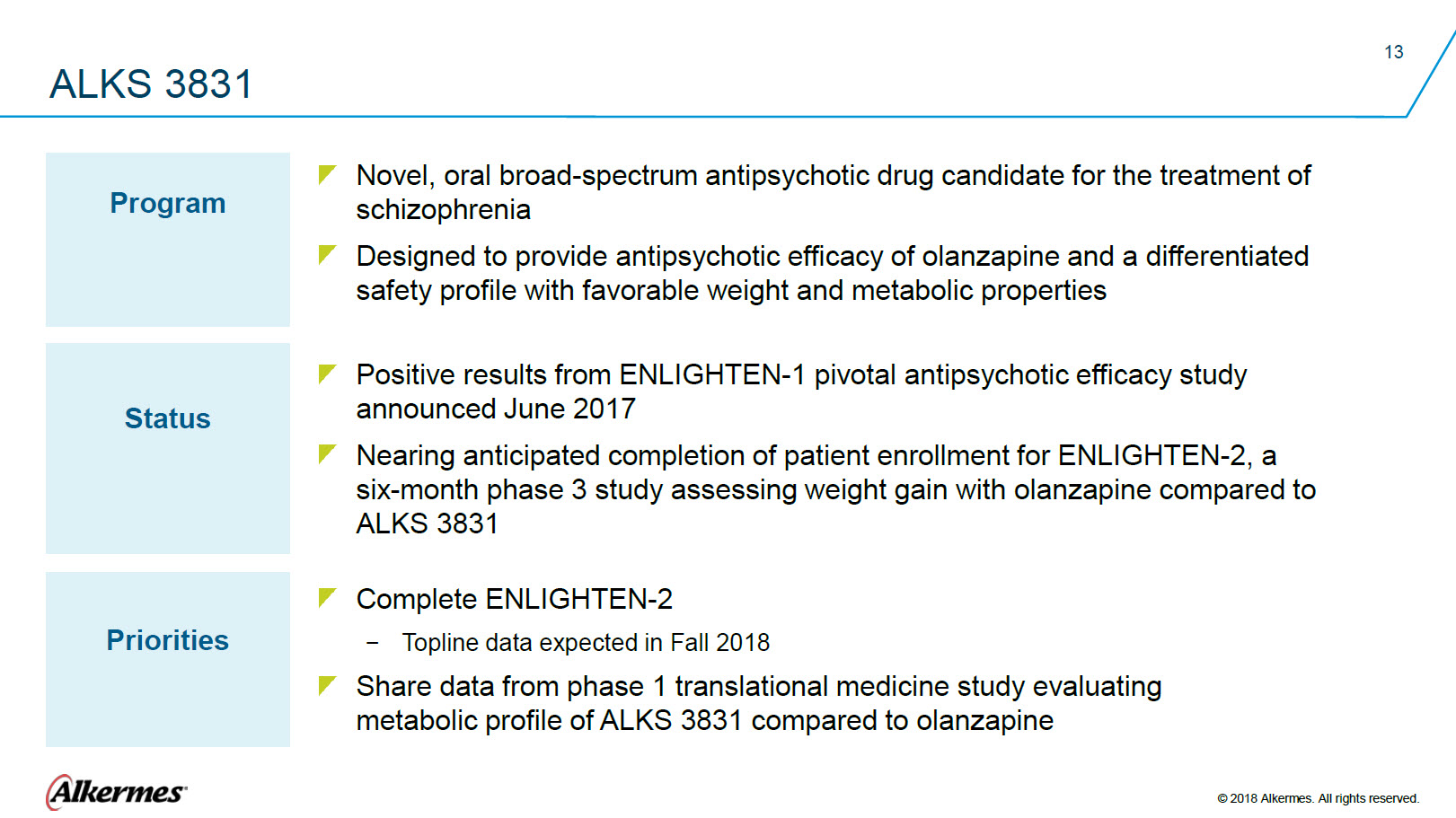
ALKS 3831 Program Novel, oral broad-spectrum antipsychotic drug candidate for the treatment of schizophrenia Designed to provide antipsychotic efficacy of olanzapine and a differentiated safety profile with favorable weight and metabolic properties Status
Positive results from ENLIGHTEN-1 pivotal antipsychotic efficacy study
Nearing anticipated completion of patient enrollment for ENLIGHTEN-2, a Priorities Complete ENLIGHTEN-2 Topline data expected in Fall 2018 Share data from phase 1 translational medicine study evaluating metabolic profile of ALKS 3831 compared to olanzapine Alkermes® © 2018 Alkermes. All rights reserved. |
|
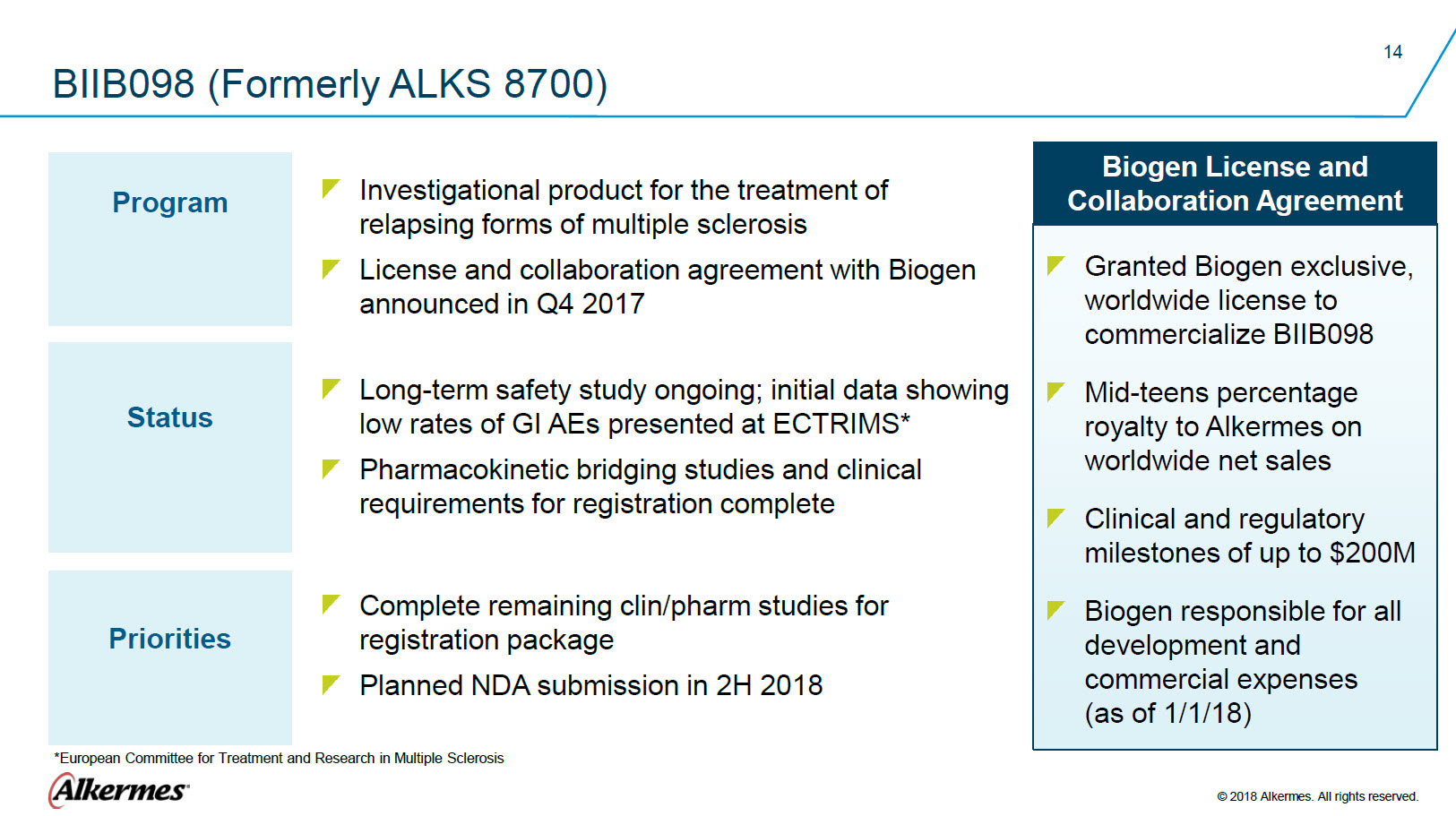
BIIB098 (Formerly ALKS 8700) Program Investigational product for the treatment of relapsing forms of multiple sclerosis License and collaboration agreement with Biogen announced in Q4 2017 Status Long-term safety study ongoing; initial data showing low rates of GI AEs presented at ECTRIMS*
Pharmacokinetic bridging studies and clinical Priorities Complete remaining clin/pharm studies for registration package Planned NDA submission in 2H 2018 Biogen License and Collaboration Agreement Granted Biogen exclusive, worldwide license to commercialize BIIB098 Mid-teens percentage royalty to Alkermes on worldwide net sales Clinical and regulatory milestones of up to $200M
Biogen responsible for all development and commercial expenses * European Committee for Treatment and Research in Multiple Sclerosis Alkermes® © 2018 Alkermes. All rights reserved. |
|
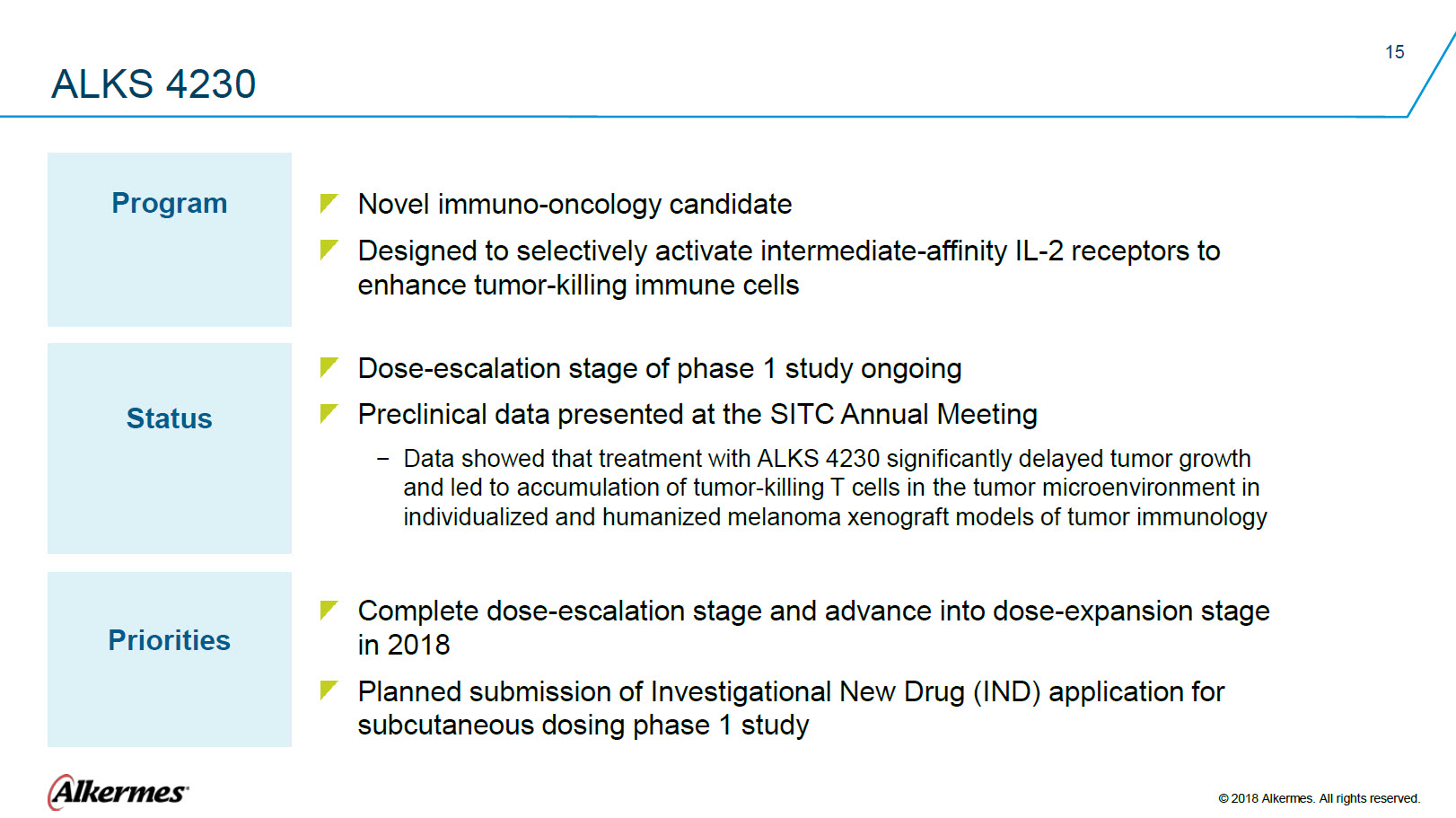
ALKS 4230 Program Novel immuno-oncology candidate Designed to selectively activate intermediate-affinity IL-2 receptors to enhance tumor-killing immune cells Status Dose-escalation stage of phase 1 study ongoing Preclinical data presented at the SITC Annual Meeting
Data showed that treatment with ALKS 4230 significantly delayed tumor growth Priorities Complete dose-escalation stage and advance into dose-expansion stage in 2018 Planned submission of Investigational New Drug (IND) application for subcutaneous dosing phase 1 study Alkermes® © 2018 Alkermes. All rights reserved. |
|
www.alkermes.com © 2018 Alkermes. All rights reserved |