Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - PIERIS PHARMACEUTICALS, INC. | d519809dex993.htm |
| EX-99.1 - EX-99.1 - PIERIS PHARMACEUTICALS, INC. | d519809dex991.htm |
| 8-K - 8-K - PIERIS PHARMACEUTICALS, INC. | d519809d8k.htm |
Exhibit 99.2
Company Overview
We are a clinical stage biotechnology company that discovers and develops Anticalin-based drugs to target validated disease pathways in a unique and transformative way. Our pipeline includes immuno-oncology multi-specifics tailored for the tumor microenvironment, an inhaled Anticalin protein to treat uncontrolled asthma and a half-life-optimized Anticalin protein to treat anemia. Our proprietary Anticalin proteins are a novel class of protein therapeutics validated in the clinic and by partnerships with leading pharmaceutical companies.
Anticalin proteins are a class of low molecular-weight therapeutic proteins derived from lipocalins, which are naturally occurring, low-molecular weight human proteins typically found in blood plasma and other bodily fluids. Anticalin proteins function similarly to monoclonal antibodies, or mAbs, by binding tightly and specifically to a diverse range of targets. An antibody is a large protein used by the immune system that recognizes a unique part of a foreign target molecule, called an antigen. We believe Anticalin proteins possess numerous advantages over antibodies in certain applications. For example, Anticalin proteins are small in size and are monomeric, meaning they consist of single protein units rather than a multi-protein complex. Therefore, we believe Anticalin proteins are generally more stable biophysically than tetrameric monoclonal antibodies, which are composed of four protein subunits. The greater biophysical stability of Anticalin proteins potentially enable unique routes of drug administration such as pulmonary delivery. Higher-molecular-weight entities, such as antibodies, are often too large to be delivered effectively through these methods. In addition, Anticalin proteins are monovalent in structure, which means they bind to a single cell surface receptor, which may avoid the risk of cross-linking of cell surface receptors where such receptors are a therapeutic target. While our basic Anticalin proteins have only a single binding site and are not subject to such cross-linking, the Anticalin technology is also modular, which allows us to design Anticalin protein constructs to bind with specificity to multiple targets at the same time. This multispecificity offers advantages in biological settings where binding to multiple targets can enhance the ability of a drug to achieve its desired effects, such as killing cancer cells. Moreover, unlike antibodies, the pharmacokinetic, or PK, profile of Anticalin proteins can be adjusted to potentially enable program-specific optimal drug exposure. Moreover, no immunogenicity has been observed to date with Anticalin proteins. Such differentiating characteristics suggest that Anticalin proteins have the potential, in certain cases, to become first-in-class drugs. We believe that the drug-like properties of the Anticalin drug class were demonstrated in various clinical trials with different Anticalin-based drug candidates, including PRS-050, PRS-080, and others.
We have collaboration arrangements with major multi-national pharmaceutical companies headquartered in the United States Europe, and Japan. These include existing agreements with Seattle Genetics, Inc., or Seattle Genetics, AstraZeneca AB, or AstraZeneca, Les Laboratoires Servier and Institut de Recherches Internationales Servier, or Servier, Daiichi Sankyo Company Limited, or Daiichi, Sanofi Group, or Sanofi, and F.Hoffmann-La Roche Ltd. and Hoffmann-La Roche Inc., or Roche. We also entered into an exclusive option agreement with ASKA Pharmaceutical Co., Ltd., or ASKA for rights to PRS-080 in Japan and certain other Asian territories. We also have discovery and preclinical collaboration and service agreements with both academic institutions and private firms across the globe, though Pieris Pharmaceuticals, Inc., Pieris Pharmaceuticals GmbH and Pieris Australia Ptd Ltd.
1
Our Drug Candidates
Our current development plans focus on two core pillars, immuno-oncology and respiratory diseases. In addition, we are developing PRS-080, a non-core asset, for the treatment of functional iron deficiency anemia. Each of our drug candidates is in the early stage of development, and we anticipate that it will likely be several years before any of our drug candidates could be commercialized. The following table summarizes the status of our current drug candidates and programs:
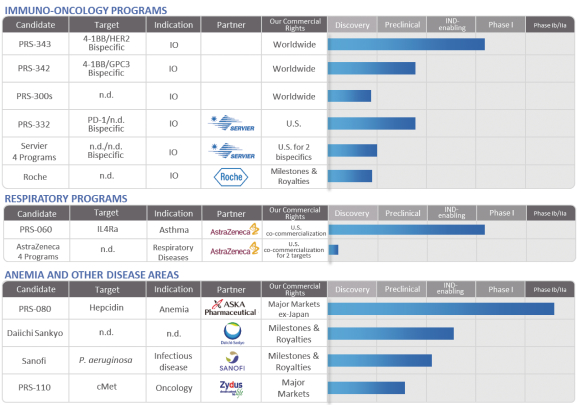
Immuno-Oncology Programs
Current antibody-based therapies targeting tumor cell destruction or immune activation are hampered by, among other factors, low response rates and the induction of immune-related adverse events. The immuno-oncology (IO)-related Anticalin proteins are designed to target checkpoint proteins or, like PRS-343, immune-stimulatory proteins, and consist of a variety of multifunctional biotherapeutics that can combine, via genetic fusion, antibodies with Anticalin proteins or two or more Anticalin proteins to each other. We believe that a tethered Anticalin protein directed at checkpoint inhibitory or costimulatory proteins can preferentially activate the immune system at the site of a tumor microenvironment. We believe that the IO Anticalin proteins represent a “platform within a product” in immuno-oncology since it may be possible to apply a single combined Anticalin-antibody molecule in a number of different cancers. This is based on the shared underlying biology such as checkpoint and costimulatory biology found within tumors arising in different organs.
PRS-343 was designed to target the immune receptor 4-1BB and the tumor target HER2. PRS-343 comprises a genetic fusion of a variant of the HER2-targeting antibody trastuzumab with an Anticalin protein specific for 4-1BB. The mode of action of this 4-1BB/ HER2 bispecific is to promote 4-1BB clustering by bridging
2
4-1BB-positive T cells with HER2-positive tumor cells, thereby providing a potent costimulatory signal to tumor antigen-specific T cells in the tumor microenvironment. PRS-343 is intended to localize 4-1BB activation in the tumor, thereby both increasing efficacy and reducing systemic toxicity compared to 4-1BB-targeting antibodies being developed by third parties in clinical trials. Patient dosing in a multicenter, open-label, Phase I dose escalation study, which will include expansion cohorts, commenced in September 2017. The study is designed to determine the safety, tolerability, and potential anti-cancer activity of PRS-343 in patients with advanced or metastatic HER2-positive solid tumors for which standard treatment options are not available, no longer effective, not tolerated, or for which the patient has refused standard therapy. Elevated HER2 expression is associated with multiple cancers, including gastroesophageal, bladder, gallbladder, gastric, breast, and a range of other tumor types. We expect initial safety and PD data for PRS 343 in the second half of 2018.
Pieris is developing additional fusion proteins in IO that are in the preclinical stage, both as part of its proprietary pipeline and in partnership with Servier. One such program that is part of Pieris’ collaboration with Servier is PRS-332, a bispecific Anticalin-antibody fusion protein comprising an anti-PD-1 antibody genetically fused to an Anticalin molecule targeting an undisclosed checkpoint target. Anti-PD-1 antibodies have demonstrated great clinical benefit in several cancers, including melanoma, non-small cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck carcinomas. However, there are many patients who do not respond, relapse or acquire resistance to PD-1 treatment. Pieris is developing PRS-332 in order to improve on existing PD-1 therapies, with the intent to simultaneously block PD-1 and another immune checkpoint co-expressed on exhausted T cells.
Respiratory Programs—PRS-060
The current standard of care for persistent, moderate to severe allergic asthma is high dose inhaled corticosteroids, or ICS, often in combination with inhaled long-acting beta-adrenergic agonists, or LABA. In very severe allergic asthma, omalizumab (Xolair from Roche) is given to patients in addition to ICS/LABA combinations. Omalizumab has been shown to impact some diseases, such as asthma, that are driven by eosinophils, another important class of immune cells. However, patient response to omalizumab has been shown to be inconsistent.
The next generation of therapies beyond omalizumab target a broader range than just IgE-mediated mechanisms. These approaches target other immune mediators, including IL-5, IL-4, IL-13, thymic stromal lymphopoietin or TSLP, IL-33 (which act in concert on eosinophils, B-cells, epithelial cells, goblet cells and others) and PGD2 (through stimulation of CRTH2 receptors). Asthma is associated with high levels of eosinophils, immune cells that play a role in protecting the body against infection. The creation of eosinophils can be interrupted at the early stages while the cells are still maturing. Multiple products are approved that target eosinophils through IL-5 or its receptor IL-5RA. However, eosinophils are only one of many cell types and immune system components that are involved with the body’s exaggerated inflammation response in asthma. These cells can be seen infiltrating the airways along with eosinophils, leading to the conclusion that more cell types are involved in asthma pathogenesis. We believe that targeting just one of these components is unlikely to be as effective in treating severe asthma as an approach that targets the broader Th2 (cell-mediated) pathway.
PRS-060 binds to the IL-4 receptor alpha-chain (IL-4Ra), thereby inhibiting the actions of IL-4 and IL-13, two cytokines (small proteins mediating signaling between cells within the human body) known to be key mediators in the inflammatory cascade that causes asthma and other inflammatory diseases. The small size and biophysical stability of PRS-060 enables direct delivery to the lungs, through the use of an inhaler, which we believe will enable high pulmonary concentrations of the drug candidate to be achieved at substantially lower doses than would be reached with antibodies that are systemically delivered. Further, an inhaled drug is expected to be better tolerated than systemically administered antibodies. We began a Phase I clinical trial with PRS-060 in Australia in December 2017. PRS-060 is being co-developed with AstraZeneca under our strategic alliance, with additional programs to be initiated in 2018.
3
Anemia and Other Disease Areas
Untreated anemia is associated with chronic fatigue, increased risk of progression of multiple diseases, and death. We believe CKD patients with functional iron deficiency, or FID, -anemia are especially poorly served. These patients have adequate stores of iron but this iron is not efficiently incorporated into red blood cell precursors through rESAs and iron supplements. According to the 2009 publication by Young and Zaritsky in the Clinical Journal of the American Society of Nephrology, this imbalance in iron metabolism is a result of a high level of circulating hepcidin in the blood stream. We believe existing therapies are limited in that they do not have an impact on hepcidin or, in the case of rESAs, patients often become resistant to the therapy.
PRS-080 is a polyethylene glycol (PEG)-conjugated Anticalin protein that binds to hepcidin, a natural regulator of iron levels in the blood. An excess amount of hepcidin can cause FID, which often cannot be treated adequately with iron supplements and can lead to anemia. PRS-080 has been designed to target hepcidin for the treatment of FID in anemic patients with chronic kidney disease, or CKD, particularly in end-stage renal disease patients requiring dialysis. We believe that by blocking the actions of hepcidin, PRS-080 may serve to address anemia by mobilizing iron from the endogenous iron stores in the body for incorporation into red blood cells. With a serum half-life of several days, PRS-080 was designed to inhibit hepcidin sufficiently to mobilize functional serum iron for erythropoiesis, followed by recovery of blood hepcidin levels to prevent iron overload.
PRS-080 has been investigated in two single-ascending dose Phase Ia/Ib trials, first in healthy subjects, then in stage 5 CKD patients requiring hemodialysis, both under governance by the German Federal Institute for Drugs and Medical Devices. Single intravenous PRS-080 administrations were safe and well tolerated up to the tested dose of 16 mg/kg in healthy volunteers and up to the tested dose of 8 mg/kg in end-stage CKD patients. The studies were completed in 2015 and 2017, respectively. Both trials demonstrated dose proportional elevations in both serum iron and transferrin saturation. Based on the Phase Ia/Ib results, a multicenter, randomized, double-blind, placebo-controlled, multiple ascending dose (two cohorts of 4mg/kg and 8 mg/kg, respectively) pilot Phase IIa study in anemic hemodialysis dependent CKD patients commenced in the third quarter of 2017. This study is intended primarily to obtain initial results on the safety, tolerability, and pharmacological activity of five once weekly doses of PRS-080, and, secondarily, to evaluate the effect of repeated PRS-080 administration on hemoglobin levels in this patient population. Completion of dosing of all patients is expected in mid-2018, followed by data unblinding in the second half of 2018.
Recent Developments
Based on information currently available, we estimate that as of December 31, 2017, we had approximately $82.6 million of cash, cash equivalents and investments. These estimates are preliminary and actual results may differ. As such, these estimates should not be viewed as a substitute for our full audited financial statements prepared in accordance with U.S. generally accepted accounting principles. Investors are cautioned not to place undue reliance on this preliminary financial information. We do not undertake any obligation to publicly update or revise this estimate, except as required by law.
Our independent registered public accountants have not audited, reviewed or performed any procedures with respect to such preliminary financial information and accordingly do not express an opinion or any other form of assurance with respect thereto. Complete results as of and for fiscal year ended December 31, 2017 will be included in our Annual Report on Form 10-K.
On February 8, 2018, we entered into a License and Collaboration Agreement, or the Seagen Collaboration Agreement, and a Non-Exclusive Anticalin Platform Technology License Agreement, or the Seagen License Agreement, which we refer to together with the Collaboration Agreement as the Seagen Agreements, with Seattle Genetics, Inc., or Seattle Genetics, pursuant to which the parties will develop multiple targeted bispecific
4
immuno-oncology treatments for solid tumors and blood cancers. Under the terms of the Seagen Agreements, Seattle Genetics will pay Pieris a $30 million upfront fee, tiered royalties on net sales up to the low double-digits, and up to $1.2 billion in total success-based payments across three product candidates. The companies will pursue multiple Antibody-Anticalin fusion proteins during a research phase, and Seattle Genetics has the option to select up to three therapeutic programs for further development. Prior to the initiation of a pivotal trial, Pieris may opt into global co-development and US commercialization of the second program and share in global costs and profits on a 50/50 basis. Seattle Genetics will solely develop, fund and commercialize the other two programs. Seattle Genetics may also decide to select additional candidates from the initial research phase for further development in return for the payment to Pieris of additional fees, milestone payments, and royalties.
Corporate Information
Pieris Pharmaceuticals, Inc. was originally incorporated in the State of Nevada in May 2013 under the name “Marika Inc.”. Pieris Pharmaceuticals, Inc. began operating the business of Pieris Pharmaceuticals GmbH, or Pieris GmbH, through a reverse acquisition on December 17, 2014. Pieris GmbH (formerly Pieris AG, a German company which was founded in 2001) continues as the operating subsidiary of the Company.
Pieris Pharmaceuticals, Inc. is a holding company and the sole stockholder of Pieris GmbH with its corporate headquarters located at located at 255 State Street, 9th Floor, Boston, Massachusetts 02109 and our telephone number is (857) 246-8998. We maintain a website at www.pieris.com, to which we regularly post copies of our press releases as well as additional information about us. The information contained on, or that can be accessed through, our website is not a part of this prospectus supplement. We have included our website address in this prospectus supplement solely as an inactive textual reference.
Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, are available free of charge through the investor relations page of our website as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
Implications of Being an Emerging Growth Company
We are an “emerging growth company” as defined in the JOBS Act enacted in April 2012. As a result, we may take advantage of reduced reporting requirements that are otherwise applicable to public companies, including delaying auditor attestation of internal control over financial reporting and reducing executive compensation disclosures.
We will remain an emerging growth company until the earlier of (i) December 31, 2019, the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement under the Securities Act; (ii) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (iii) the date on which we have issued more than $1.07 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under applicable SEC rules. We expect that we will remain an emerging growth company for the foreseeable future, but cannot retain our emerging growth company status indefinitely and will no longer qualify as an emerging growth company on or before December 31, 2019.
We elected to take advantage of certain of the reduced disclosure obligations in this prospectus supplement, the accompanying prospectus, and the documents incorporated by reference herein and therein and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than what you might receive from other public reporting companies in which you hold equity interests.
5
