Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Theravance Biopharma, Inc. | a18-5540_1ex99d1.htm |
| 8-K - 8-K - Theravance Biopharma, Inc. | a18-5540_18k.htm |
Joint development and commercialization of novel, intestinally-restricted pan-Janus Kinase (JAK) inhibitor for inflammatory intestinal diseases February 7, 2018 Theravance Biopharma and Janssen Biotech, Inc. Global Collaboration for TD-1473
Cautionary Statement Regarding Forward-Looking Statements Under the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, the company cautions investors that any forward-looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward-looking statements or projections. Examples of forward-looking statements in this presentation include statements relating to the company’s business plans and objectives, including financial and operating results, potential partnering transactions and sales targets, the company’s regulatory strategies and timing and results of clinical studies, the potential benefits and mechanisms of action of the company’s product and product candidates (including their potential as components of combination therapies). The company’s forward-looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective (including when our product candidates are studied in combination with other compounds), delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with third parties to discover, develop and commercialize products, risks associated with establishing and maintaining sales, marketing and distribution capabilities. Other risks affecting the company are described under the heading “Risk Factors” and elsewhere in the company’s Form 10-Q filed with the Securities and Exchange Commission (SEC) on November 8, 2017, and other periodic reports filed with the SEC.
Announcing Global Collaboration Agreement TD-1473: novel, oral, intestinally restricted pan-JAK inhibitor Includes related back-up compounds in gastrointestinal restricted JAK program Potential to treat a range of inflammatory intestinal diseases Including ulcerative colitis and Crohn’s disease Impact to Theravance Biopharma and TD-1473 program Up to $1B in potential payments (including $100M upfront) Joint development and commercialization under a profit share structure Apply Janssen expertise in IBD to optimize clinical strategy and execution Accelerate and expand the overall development program Maximize the worldwide commercial opportunity for TD-1473 JAK = janus kinase Joint development and commercialization of TD-1473
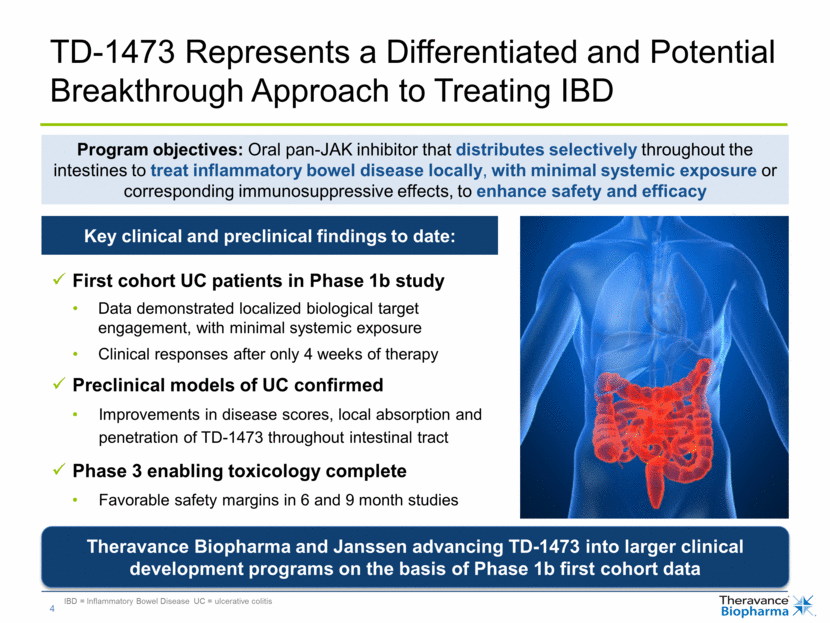
IBD = Inflammatory Bowel Disease UC = ulcerative colitis TD-1473 Represents a Differentiated and Potential Breakthrough Approach to Treating IBD First cohort UC patients in Phase 1b study Data demonstrated localized biological target engagement, with minimal systemic exposure Clinical responses after only 4 weeks of therapy Preclinical models of UC confirmed Improvements in disease scores, local absorption and penetration of TD-1473 throughout intestinal tract Phase 3 enabling toxicology complete Favorable safety margins in 6 and 9 month studies Program objectives: Oral pan-JAK inhibitor that distributes selectively throughout the intestines to treat inflammatory bowel disease locally, with minimal systemic exposure or corresponding immunosuppressive effects, to enhance safety and efficacy Key clinical and preclinical findings to date: Theravance Biopharma and Janssen advancing TD-1473 into larger clinical development programs on the basis of Phase 1b first cohort data
TD-1473 program will benefit from Janssen’s broad expertise in IBD IBD is a key area of focus End-to-end expertise Discovery Translational Development Regulatory Commercial Experienced in multiple IBD programs, across a range of mechanisms Flagship commercial brands in Immunology Janssen Offers Significant Value to TD-1473 A global leader and partner of choice in Immunology
Next Steps in Clinical Development of TD-1473 Large, Phase 2b/3 adaptive design induction and maintenance study in ulcerative colitis Expect to initiate in 2H 2018 Phase 2b/3 design should expedite development path Leveraging Janssen expertise in design and conduct Expect to initiate in 2H 2018 Crohn’s timelines accelerated due to collaboration Enhances commercial opportunity versus going alone Phase 2b/3 Study in Ulcerative Colitis Phase 2 Study in Crohn’s Disease Complete Phase 1b Study Cohorts 2 and 3 in 1H 2018 to inform dose range for next phase of studies Advancing programs in ulcerative colitis and Crohn’s in parallel
Significant economics reflective of the potential value of TD-1473 Up to $1B in payments to Theravance Biopharma, including $100M upfront $200M fee following completion of Phase 2 Crohn’s study and Phase 2b induction portion of UC study, if Janssen elects to enter into exclusive license Up to $700M in potential development and commercialization milestones Profit share structure for US commercialization and Phase 3 program expenses 67% Janssen / 33% Theravance Biopharma Double digit tiered royalties ex-US Leveraging joint expertise in clinical development Janssen would lead development in Phase 3 Crohn’s program Theravance Biopharma will lead completion of Phase 2b/3 UC study If approved, Theravance Biopharma may elect to co-commercialize in US under profit share; Janssen solely responsible for TD-1473 commercialization ex-US Key Terms of TD-1473 Collaboration with Janssen
Global Collaboration Agreement for TD-1473 Shared belief in TD-1473 as a localized medicine with potential to transform the treatment landscape in IBD Meaningful program enhancements for TD-1473 Accelerate clinical development; plan to advance UC and Crohn’s in parallel Apply Janssen expertise in IBD to optimize clinical strategy and execution Maximize worldwide commercial opportunity of TD-1473 Attractive deal economics reducing overall financial risk Potential to maximize value of TD-1473 for Theravance Biopharma Important milestone for TD-1473, the value of our internally discovered pipeline, and our strategy to design localized medicines to make a difference for patients