Attached files
| file | filename |
|---|---|
| 8-K - HUMANIGEN, INC | d1261858k.htm |
Exhibit 99.1

Developing Leading-Edge Immunotherapy, Oncology Treatments OTCQB: HGENwww.humanigen.com 1 With Goal of Potential Significant Near-Term Value Inflection | Corporate Overview - January 29, 2018

Forward-Looking Statements 2 This presentation contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements reflect management’s current knowledge, assumptions, judgment and expectations regarding future performance or events. Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct, and actual results could differ materially from the forward-looking statements.Words such as “will,” “expect,” “intend,” “plan,” “predict,” “potential,” “possible,” and similar expressions identify forward-looking statements, including, without limitation, statements related to the scope, progress, expansion, and costs of developing and commercializing the Company’s product candidates; opportunity to benefit from anticipated regulatory incentives for product candidates; and anticipated expenses related to development activities, clinical trials and the development and potential commercialization of product candidates.Forward-looking statements are subject to risks and uncertainties including, but not limited to, the Company’s ability to complete the restructuring transactions announced on December 21, 2017 on the terms described, the Company’s lack of revenues, history of operating losses, limited cash reserves and ability to obtain additional capital to develop and commercialize its product candidates, including the additional capital which will be necessary to complete the clinical trials that the Company has initiated or plans to initiate, and continue as a going concern; the Company’s ability to execute its strategy and business plan; the potential timing and outcomes of clinical studies of lenzilumab, ifabotuzumab or any other product candidates and the uncertainties inherent in clinical testing; the ability of the Company to timely source adequate supply of its development products from third-party manufacturers on which the Company depends; the potential, if any, for future development of any of its present or future products; the Company's ability to successfully progress, partner or complete further development of its programs; the ability of the Company to identify and develop additional products; the Company's ability to attain market exclusivity or to protect its intellectual property; competition; changes in the regulatory landscape that may prevent the Company from pursuing or realizing any of the expected benefits from the various regulatory incentives at the center of its strategy, or the imposition of regulations that affect the Company's products; and the various risks described in the "Risk Factors" and elsewhere in the Company's periodic and other filings with the Securities and Exchange Commission.You are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this presentation. The company has no obligation, and expressly disclaims any obligation to update, revise or correct any of the forward-looking statements, whether as a result of new information, future events or otherwise.

Investor Overview Cutting-edge science monoclonal antibody biotechnology company – with improving financial profileDeveloping key asset (lenzilumab) for prevention of side effects associated with CAR-T – one of the hottest areas in biotechWorking with leading KOLs to start phase 1/2 trials Lenzilumab has the potential to make CAR-T: Safer, more effective, and a more routine out-patient procedureMore shots on goal in portfolio: Lenzilumab phase 1 trial in chronic myelomonocytic leukemia (CMML) underway to inform potential work in JMMLIfabotuzumab phase 1 radio-imaging trial in glioblastoma multiforme (GBM) underwayIfabotuzumab is a potential CAR constructModest capital requirements anticipated to reach value inflection point in early 2019 – and significant upside potential 3
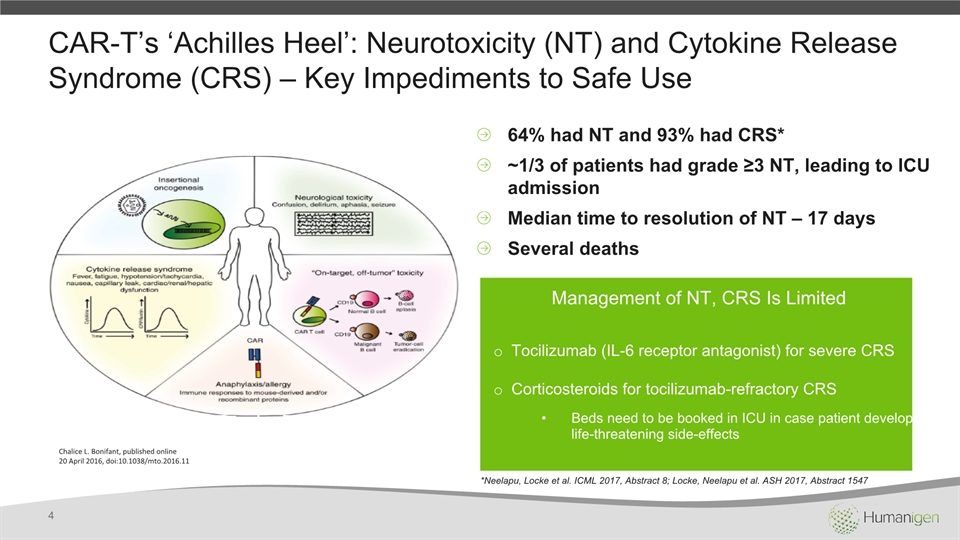
CAR-T’s ‘Achilles Heel’: Neurotoxicity (NT) and Cytokine Release Syndrome (CRS) – Key Impediments to Safe Use 4 64% had NT and 93% had CRS*~1/3 of patients had grade ≥3 NT, leading to ICU admissionMedian time to resolution of NT – 17 daysSeveral deaths Management of NT, CRS Is Limited Tocilizumab (IL-6 receptor antagonist) for severe CRSCorticosteroids for tocilizumab-refractory CRSBeds need to be booked in ICU in case patient develops life-threatening side-effects Chalice L. Bonifant, published online20 April 2016, doi:10.1038/mto.2016.11 *Neelapu, Locke et al. ICML 2017, Abstract 8; Locke, Neelapu et al. ASH 2017, Abstract 1547
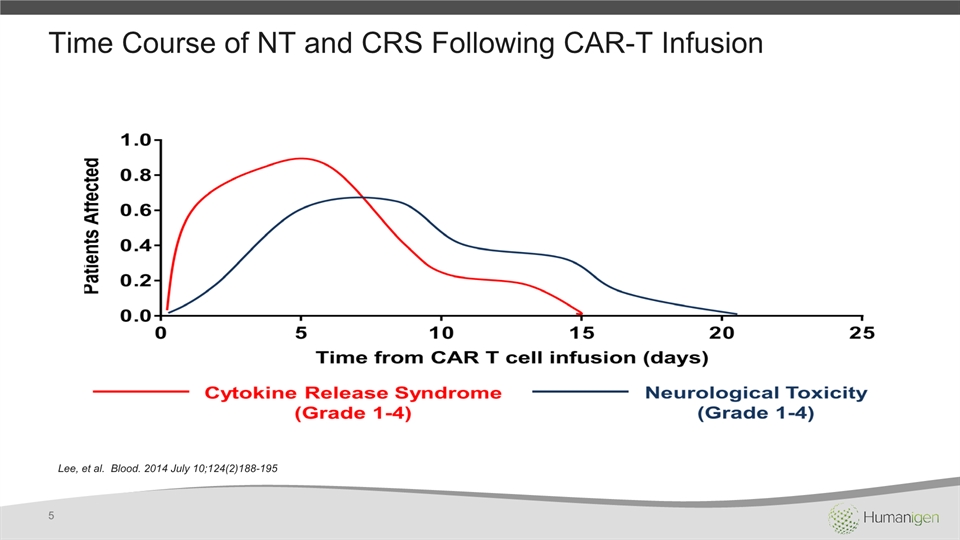
Time Course of NT and CRS Following CAR-T Infusion Lee, et al. Blood. 2014 July 10;124(2)188-195 5
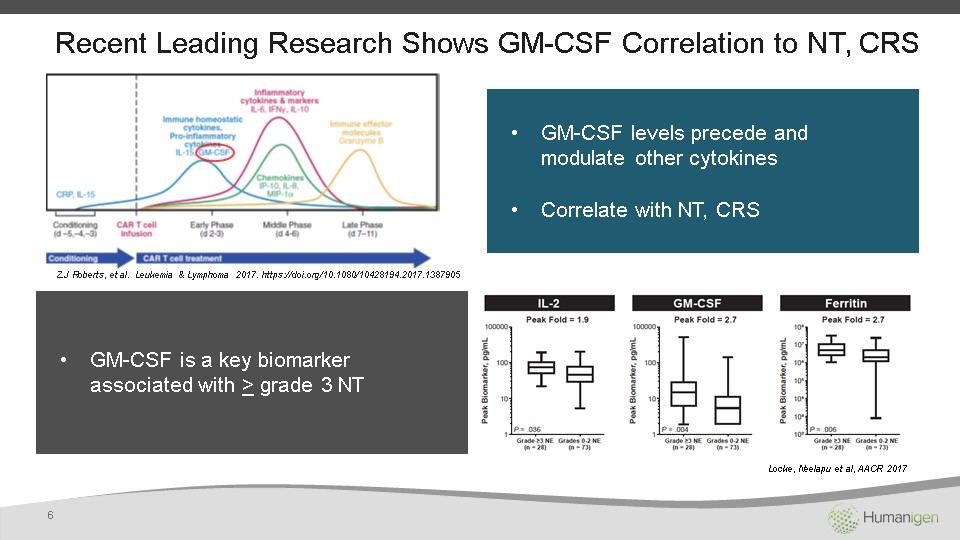
Z.J Roberts, et al. Leukemia & Lymphoma 2017. https://doi.org/10.1080/10428194.2017.1387905 Recent Leading Research Shows GM-CSF Correlation to NT, CRS 6 GM-CSF levels precede and modulate other cytokinesCorrelate with NT, CRS GM-CSF is a key biomarker associated with > grade 3 NT Locke, Neelapu et al, AACR 2017
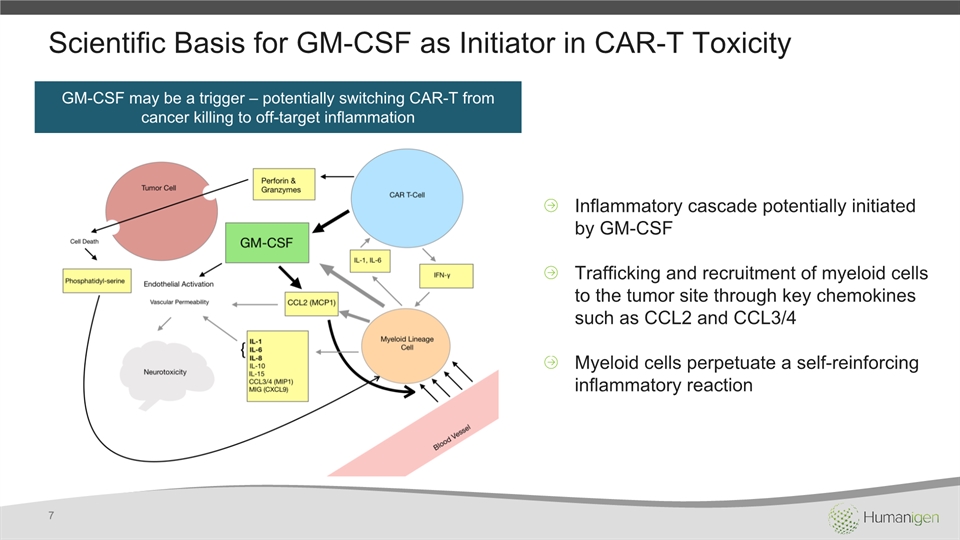
Scientific Basis for GM-CSF as Initiator in CAR-T Toxicity 7 GM-CSF may be a trigger – potentially switching CAR-T from cancer killing to off-target inflammation Inflammatory cascade potentially initiated by GM-CSFTrafficking and recruitment of myeloid cells to the tumor site through key chemokines such as CCL2 and CCL3/4Myeloid cells perpetuate a self-reinforcing inflammatory reaction
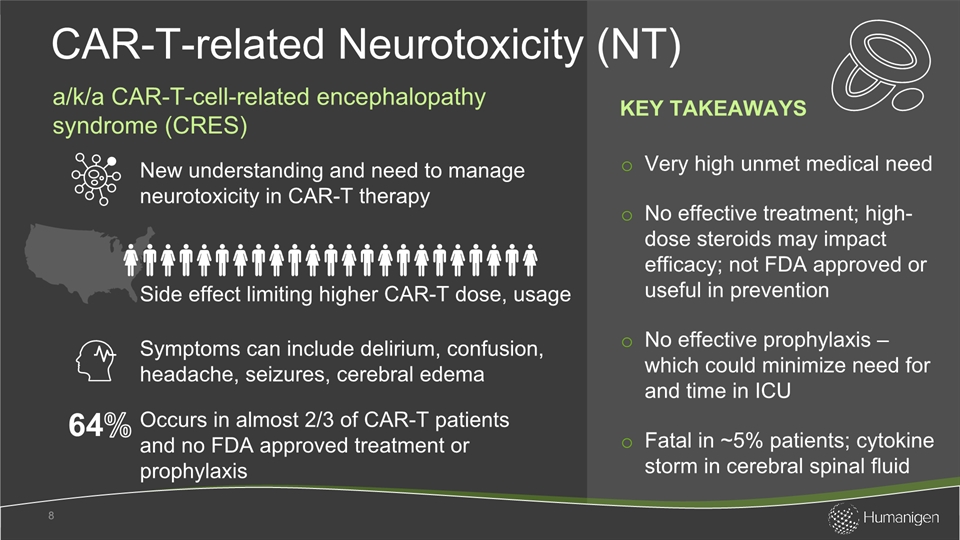
Very high unmet medical need No effective treatment; high-dose steroids may impact efficacy; not FDA approved or useful in preventionNo effective prophylaxis – which could minimize need for and time in ICU Fatal in ~5% patients; cytokine storm in cerebral spinal fluid CAR-T-related Neurotoxicity (NT) 8 New understanding and need to manage neurotoxicity in CAR-T therapy Occurs in almost 2/3 of CAR-T patients and no FDA approved treatment or prophylaxis Side effect limiting higher CAR-T dose, usage Symptoms can include delirium, confusion, headache, seizures, cerebral edema a/k/a CAR-T-cell-related encephalopathy syndrome (CRES) KEY TAKEAWAYS 64%
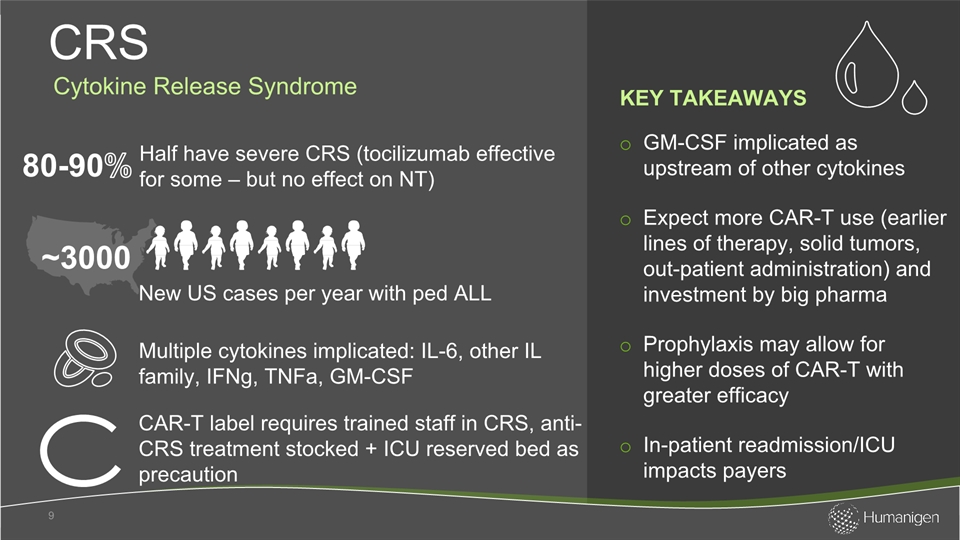
GM-CSF implicated as upstream of other cytokinesExpect more CAR-T use (earlier lines of therapy, solid tumors, out-patient administration) and investment by big pharmaProphylaxis may allow for higher doses of CAR-T with greater efficacy In-patient readmission/ICU impacts payers CRS 9 CAR-T label requires trained staff in CRS, anti-CRS treatment stocked + ICU reserved bed as precaution Multiple cytokines implicated: IL-6, other IL family, IFNg, TNFa, GM-CSF ~3000 KEY TAKEAWAYS New US cases per year with ped ALL Half have severe CRS (tocilizumab effective for some – but no effect on NT) Cytokine Release Syndrome 80-90%

Kymriah® and Yescarta® Have Black-Box Warnings for NT, CRS 10 Kymriah® label Yescarta® label
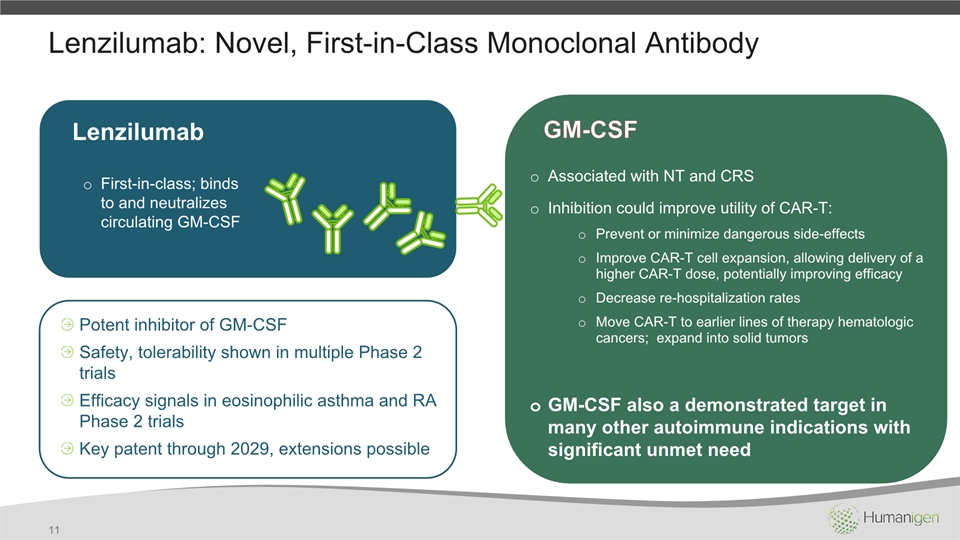
Lenzilumab: Novel, First-in-Class Monoclonal Antibody 11 Lenzilumab First-in-class; binds to and neutralizes circulating GM-CSF Potent inhibitor of GM-CSF Safety, tolerability shown in multiple Phase 2 trialsEfficacy signals in eosinophilic asthma and RA Phase 2 trialsKey patent through 2029, extensions possible Associated with NT and CRSInhibition could improve utility of CAR-T:Prevent or minimize dangerous side-effectsImprove CAR-T cell expansion, allowing delivery of a higher CAR-T dose, potentially improving efficacyDecrease re-hospitalization ratesMove CAR-T to earlier lines of therapy hematologic cancers; expand into solid tumorsGM-CSF also a demonstrated target in many other autoimmune indications with significant unmet need GM-CSF
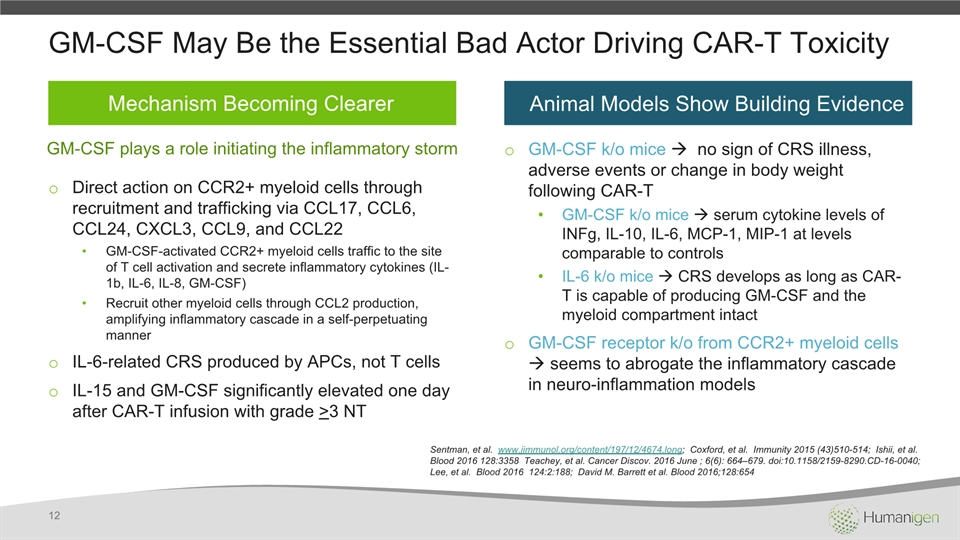
Sentman, et al. www.jimmunol.org/content/197/12/4674.long; Coxford, et al. Immunity 2015 (43)510-514; Ishii, et al. Blood 2016 128:3358 Teachey, et al. Cancer Discov. 2016 June ; 6(6): 664–679. doi:10.1158/2159-8290.CD-16-0040; Lee, et al. Blood 2016 124:2:188; David M. Barrett et al. Blood 2016;128:654 GM-CSF May Be the Essential Bad Actor Driving CAR-T Toxicity Direct action on CCR2+ myeloid cells through recruitment and trafficking via CCL17, CCL6, CCL24, CXCL3, CCL9, and CCL22GM-CSF-activated CCR2+ myeloid cells traffic to the site of T cell activation and secrete inflammatory cytokines (IL-1b, IL-6, IL-8, GM-CSF)Recruit other myeloid cells through CCL2 production, amplifying inflammatory cascade in a self-perpetuating mannerIL-6-related CRS produced by APCs, not T cells IL-15 and GM-CSF significantly elevated one day after CAR-T infusion with grade >3 NT GM-CSF k/o mice no sign of CRS illness, adverse events or change in body weight following CAR-TGM-CSF k/o mice serum cytokine levels of INFg, IL-10, IL-6, MCP-1, MIP-1 at levels comparable to controlsIL-6 k/o mice CRS develops as long as CAR-T is capable of producing GM-CSF and the myeloid compartment intactGM-CSF receptor k/o from CCR2+ myeloid cells seems to abrogate the inflammatory cascade in neuro-inflammation models 12 Mechanism Becoming Clearer Animal Models Show Building Evidence GM-CSF plays a role initiating the inflammatory storm
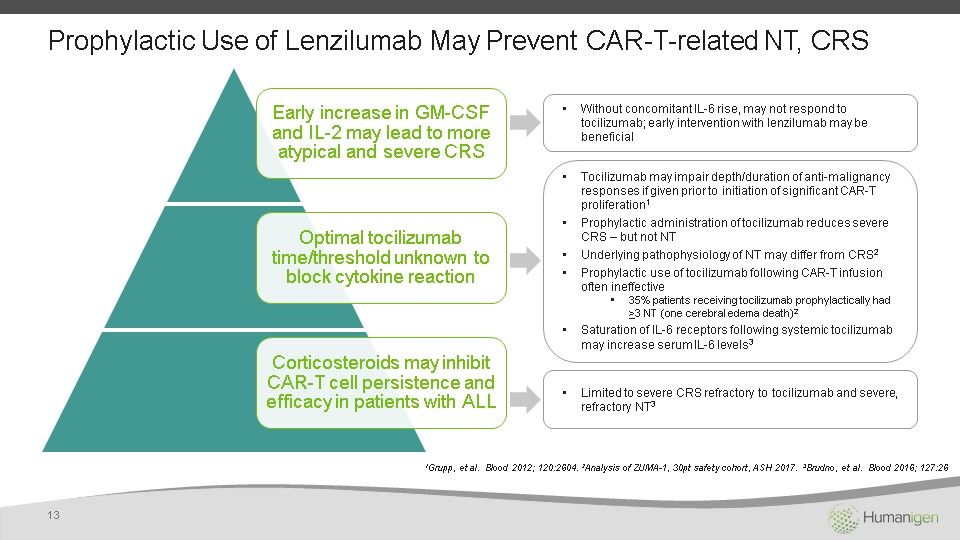
Prophylactic Use of Lenzilumab May Prevent CAR-T-related NT, CRS 13 1Grupp, et al. Blood 2012; 120:2604. 2Analysis of ZUMA-1, 30pt safety cohort, ASH 2017. 3Brudno, et al. Blood 2016; 127:26 Without concomitant IL-6 rise, may not respond to tocilizumab; early intervention with lenzilumab may be beneficial Tocilizumab may impair depth/duration of anti-malignancy responses if given prior to initiation of significant CAR-T proliferation1Prophylactic administration of tocilizumab reduces severe CRS – but not NTUnderlying pathophysiology of NT may differ from CRS2Prophylactic use of tocilizumab following CAR-T infusion often ineffective35% patients receiving tocilizumab prophylactically had >3 NT (one cerebral edema death)2Saturation of IL-6 receptors following systemic tocilizumab may increase serum IL-6 levels3 Limited to severe CRS refractory to tocilizumab and severe, refractory NT3
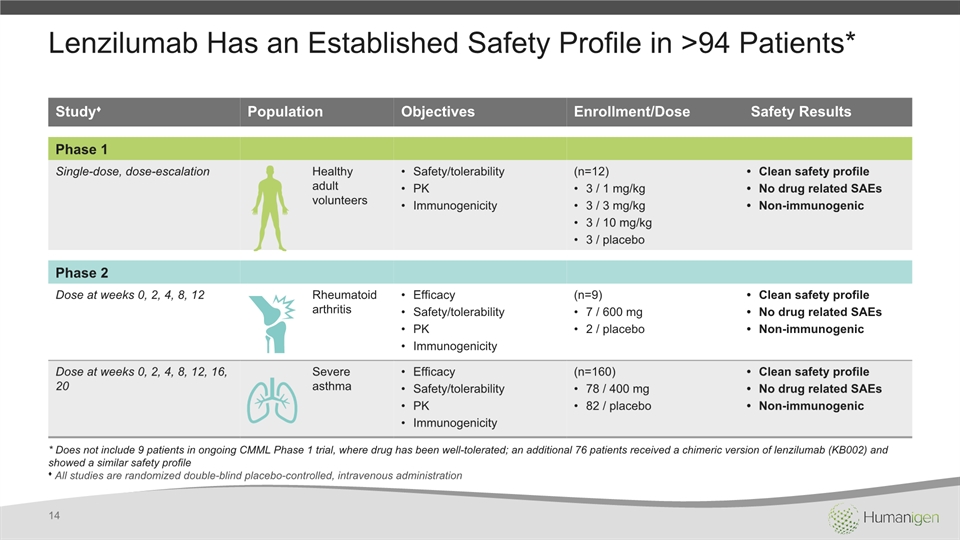
Lenzilumab Has an Established Safety Profile in >94 Patients* 14 Study♦ Population Objectives Enrollment/Dose Safety Results Phase 1 Single-dose, dose-escalation Healthy adult volunteers Safety/tolerabilityPKImmunogenicity (n=12)3 / 1 mg/kg3 / 3 mg/kg3 / 10 mg/kg3 / placebo Clean safety profileNo drug related SAEsNon-immunogenic Phase 2 Dose at weeks 0, 2, 4, 8, 12 Rheumatoid arthritis EfficacySafety/tolerabilityPKImmunogenicity (n=9)7 / 600 mg2 / placebo Clean safety profileNo drug related SAEsNon-immunogenic Dose at weeks 0, 2, 4, 8, 12, 16, 20 Severe asthma EfficacySafety/tolerabilityPKImmunogenicity (n=160)78 / 400 mg82 / placebo Clean safety profileNo drug related SAEsNon-immunogenic * Does not include 9 patients in ongoing CMML Phase 1 trial, where drug has been well-tolerated; an additional 76 patients received a chimeric version of lenzilumab (KB002) and showed a similar safety profile♦ All studies are randomized double-blind placebo-controlled, intravenous administration
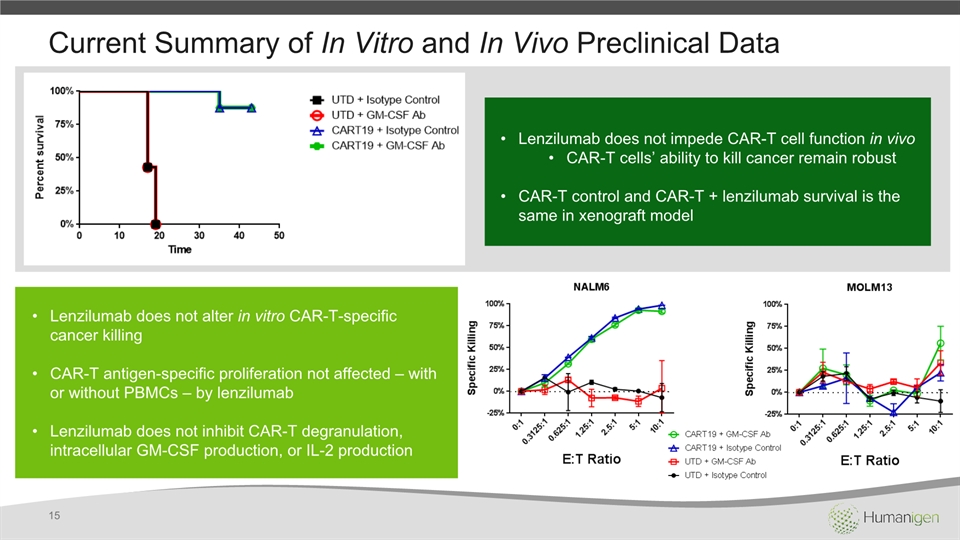
Current Summary of In Vitro and In Vivo Preclinical Data 15 Lenzilumab does not impede CAR-T cell function in vivoCAR-T cells’ ability to kill cancer remain robust CAR-T control and CAR-T + lenzilumab survival is the same in xenograft model Lenzilumab does not alter in vitro CAR-T-specific cancer killingCAR-T antigen-specific proliferation not affected – with or without PBMCs – by lenzilumabLenzilumab does not inhibit CAR-T degranulation, intracellular GM-CSF production, or IL-2 production
Lenzilumab Prophylaxis in CAR-T NT Development Plan 16 1 Thought Leader Validation GM-CSF target, preclinical and clinical development plan developed in collaboration with world’s leading CAR-T experts Scientific Advisory Board meeting convened at ASH 2017Significant input and feedback before, during and after SAB Continue to seek cutting-edge KOL insights; SAB report produced
Lenzilumab Prophylaxis in CAR-T NT Development Plan 17 Preclinical Development Completed by Dr. Saad Kenderian (Mayo Clinic) using validated animal model Initial data positive Final preclinical data expected Jan/Feb 2018 2
Lenzilumab Prophylaxis in CAR-T NT Development Plan 18 Clinical Development Phase 1/2 study design developed by Dr. Sattva Neelapu at MD Anderson – lead investigator for Gilead/Kite’s Yescarta CAR-T Lenzilumab to be studied as prophylactic therapy prior to CAR-T infusion to prevent NTStudy initiation expected Q2 2018 Target primary completion date Q4 2018 3
Lenzilumab Prophylaxis in CAR-T NT Development Plan 19 Regulatory Submission Potential adaptive study design to allow roll-over of Phase 1/2 study into potential pivotal registration/approval studyPrecedents for flexibility in approach: tocilizumab was approved with 29 patients, case reports, no control arm and no formal study, due to high unmet needTargeting FDA Orphan, Breakthrough and Fast Track designations 4
Lenzilumab Prophylaxis in CAR-T NT Development Plan 20 Value Proposition for Strategic Partner, Deal Differentiate a CAR-T offering from competitorsPotentially improve safety, allow for higher CAR-T dosesAssist hospitals/payers with fewer ICU days, readmissionsMove to true out-patient CAR-T treatment and follow-upPotentially increase market share penetration and sales of companion CAR-TDeliver possible standalone lenzilumab salesAid in the evolution of CAR-T therapyEnable possible CAR-T use in solid tumorsCombo therapy with 4-1BB agonists, CPIs, other mAbs 5
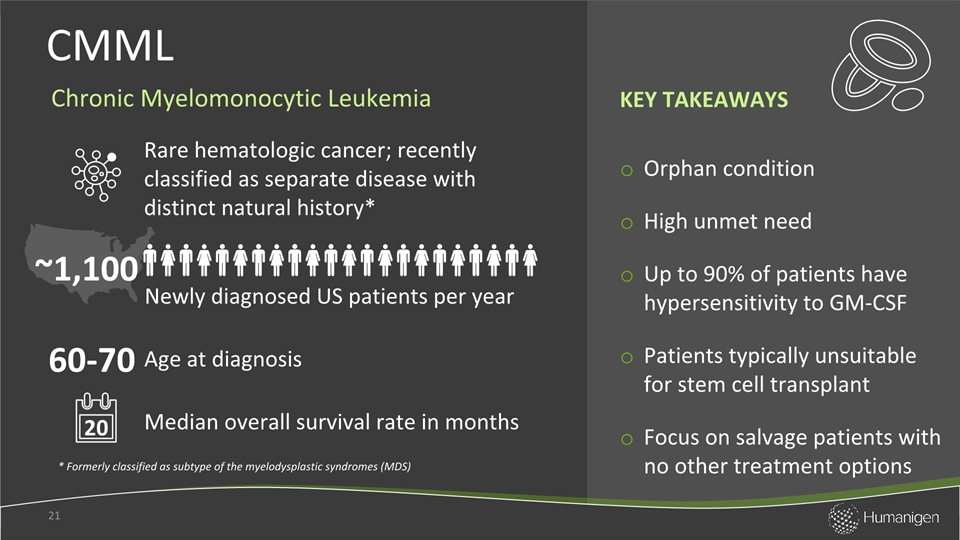
Orphan conditionHigh unmet needUp to 90% of patients have hypersensitivity to GM-CSFPatients typically unsuitable for stem cell transplantFocus on salvage patients with no other treatment options CMML 21 Rare hematologic cancer; recently classified as separate disease with distinct natural history* Median overall survival rate in months Newly diagnosed US patients per year Age at diagnosis ~1,100 * Formerly classified as subtype of the myelodysplastic syndromes (MDS) 60-70 Chronic Myelomonocytic Leukemia 20 KEY TAKEAWAYS
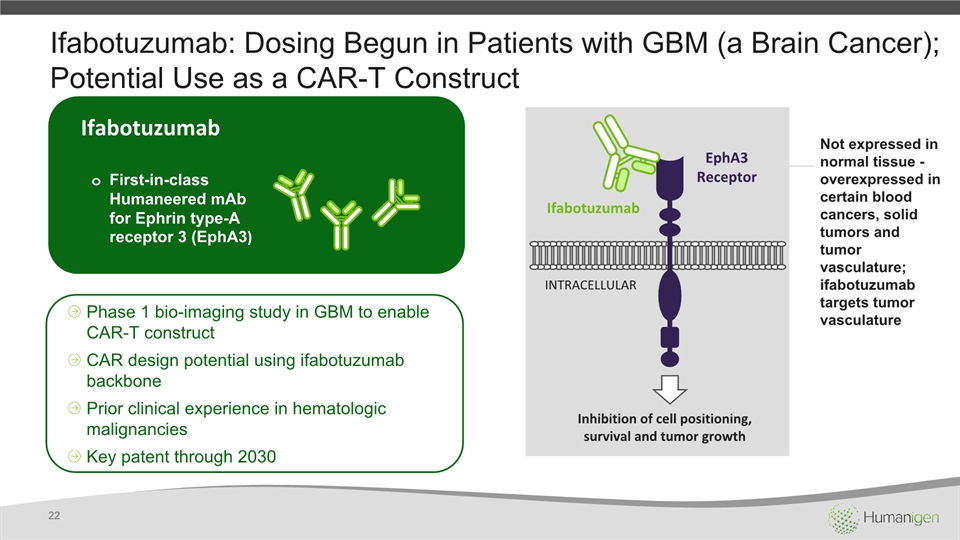
Ifabotuzumab: Dosing Begun in Patients with GBM (a Brain Cancer); Potential Use as a CAR-T Construct 22 Ifabotuzumab First-in-class Humaneered mAb for Ephrin type-A receptor 3 (EphA3) Phase 1 bio-imaging study in GBM to enable CAR-T constructCAR design potential using ifabotuzumab backbonePrior clinical experience in hematologic malignanciesKey patent through 2030 EphA3 Receptor Ifabotuzumab INTRACELLULAR Inhibition of cell positioning, survival and tumor growth Not expressed in normal tissue - overexpressed in certain blood cancers, solid tumors and tumor vasculature; ifabotuzumab targets tumor vasculature
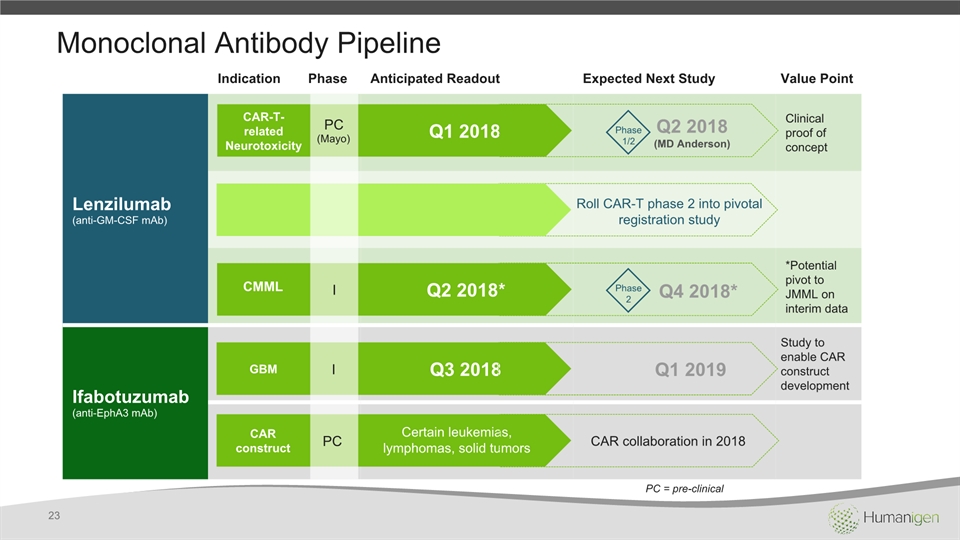
Monoclonal Antibody Pipeline Indication Phase Anticipated Readout Expected Next Study Value Point Lenzilumab(anti-GM-CSF mAb) Clinical proof of concept *Potential pivot to JMML on interim data Ifabotuzumab(anti-EphA3 mAb) Study to enable CAR construct development Q1 2018 Q2 2018* Q3 2018 Q1 2019 CMML GBM I I PC(Mayo) Q2 2018 (MD Anderson) Phase1/2 Q4 2018* Phase2 Roll CAR-T phase 2 into pivotal registration study CAR-T-relatedNeurotoxicity 23 PC = pre-clinical PC CAR construct Certain leukemias, lymphomas, solid tumors CAR collaboration in 2018
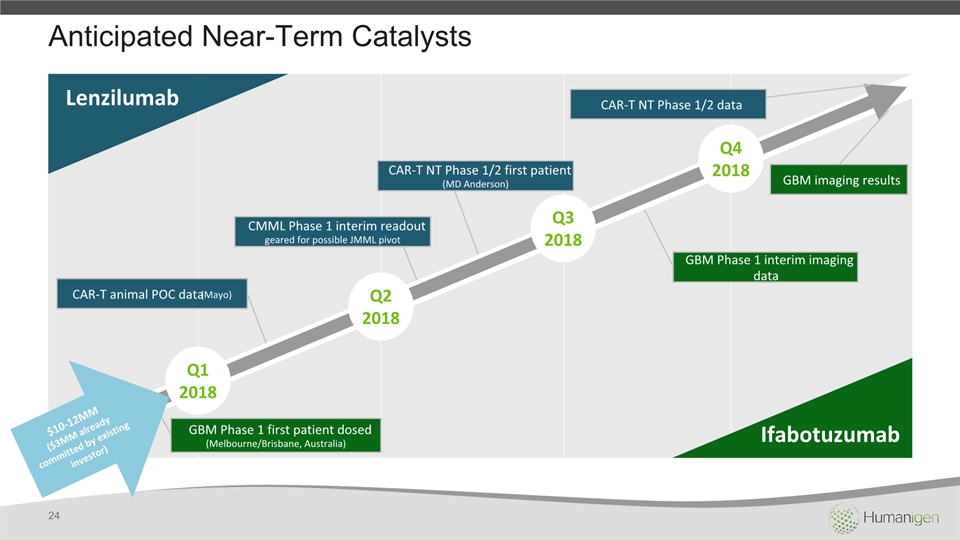
Anticipated Near-Term Catalysts Q12018 Q22018 Q32018 Q42018 Ifabotuzumab Lenzilumab GBM Phase 1 first patient dosed (Melbourne/Brisbane, Australia) GBM imaging results GBM Phase 1 interim imaging data CAR-T animal POC data (Mayo) CAR-T NT Phase 1/2 first patient(MD Anderson) CMML Phase 1 interim readout geared for possible JMML pivot CAR-T NT Phase 1/2 data $10-12MM ($3MM already committed by existing investor) 24
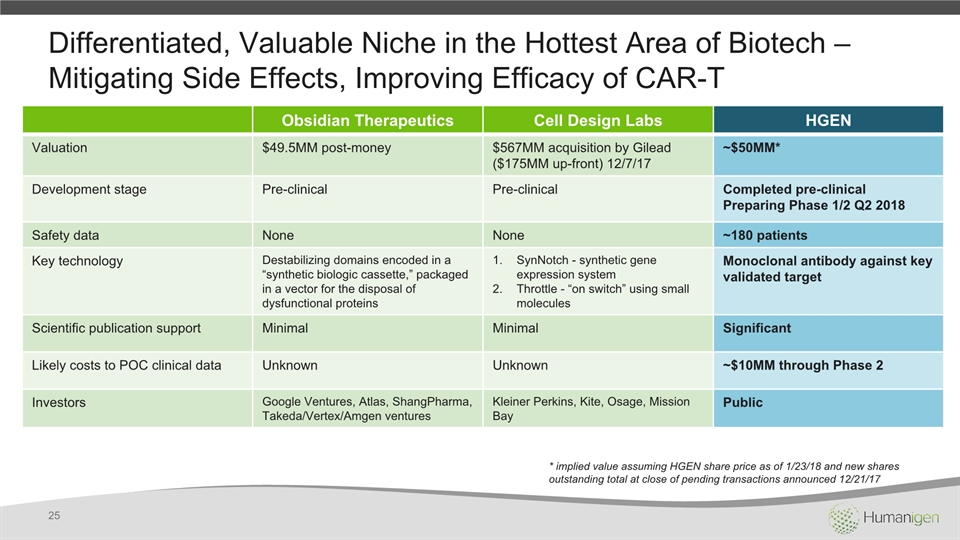
Differentiated, Valuable Niche in the Hottest Area of Biotech – Mitigating Side Effects, Improving Efficacy of CAR-T Obsidian Therapeutics Cell Design Labs HGEN Valuation $49.5MM post-money $567MM acquisition by Gilead($175MM up-front) 12/7/17 ~$50MM* Development stage Pre-clinical Pre-clinical Completed pre-clinicalPreparing Phase 1/2 Q2 2018 Safety data None None ~180 patients Key technology Destabilizing domains encoded in a “synthetic biologic cassette,” packaged in a vector for the disposal of dysfunctional proteins SynNotch - synthetic gene expression system Throttle - “on switch” using small molecules Monoclonal antibody against key validated target Scientific publication support Minimal Minimal Significant Likely costs to POC clinical data Unknown Unknown ~$10MM through Phase 2 Investors Google Ventures, Atlas, ShangPharma, Takeda/Vertex/Amgen ventures Kleiner Perkins, Kite, Osage, Mission Bay Public 25 * implied value assuming HGEN share price as of 1/23/18 and new shares outstanding total at close of pending transactions announced 12/21/17

HGEN Summary CAR-T is one of the hottest fields in biotech; Neurotoxicity the “Achilles Heel’ of CAR-TPositioned to deliver significant value with strong scientific rationale for lenzilumab as prophylaxis to CAR-T NT Working with leading KOLs/CAR-T treatment centers to start lenzilumab phase 1/2 trials for NT in Q2 2018Lenzilumab has the potential to: Make CAR-T safer, more effective, and a more routine out-patient procedureOffer significant strategic value, sales acceleration for CAR-T treatment, plus generate standalone revenue stream More shots on goal in attractive CAR-T/oncology pipeline with highly efficient R+D spend: Lenzilumab phase 1 trial in CMML almost fully recruited – study structured to enable possible JMML pivotIfabotuzumab phase 1 radio-imaging trial in glioblastoma multiforme (GBM) underwayIfabotuzumab is a potential backbone for novel CAR constructComparable valuations are highly attractive and potential for significant transactions near term 26
