Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Evofem Biosciences, Inc. | d460573dex991.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported) January 17, 2018
EVOFEM BIOSCIENCES, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-36754 | 20-8527075 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
12400 High Bluff Drive, Suite 600
San Diego, CA 92130
(Address of Principal Executive Offices and Zip Code)
Registrant’s telephone number, including area code (858) 550-1900
Neothetics, Inc.
9171 Towne Centre Drive, Suite 250
San Diego, CA 92122
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
| Item 5.07. | Submission of Matters to a Vote of Security Holders. |
A special meeting (the “Special Meeting”) of the stockholders of Evofem Biosciences, Inc. (f.k.a. Neothetics, Inc.) (the “Company”) was held on January 17, 2018 at 8:00 a.m., local time, at the offices of DLA Piper LLP (US) located at 4365 Executive Drive, Suite 1100, San Diego, CA 92121. At the Special Meeting, 8,193,531 shares of common stock, or approximately 59% of the outstanding common stock entitled to vote, were represented by proxy or in person.
As previously reported on the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission (the “SEC”) on October 17, 2017 (the “Prior 8-K”), the Company, Nobelli Merger Sub, Inc., a Delaware corporation and a wholly owned subsidiary of the Company (“Merger Sub”), and Evofem Biosciences Operations, Inc., a privately-held Delaware corporation (f.k.a. Evofem Biosciences, Inc.) (“Evofem Operations” or “Evofem”), entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”), pursuant to which, among other things, subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub would merge with and into Evofem Operations, with Evofem Operations becoming a wholly-owned subsidiary of the Company and the surviving corporation of the merger (the “Merger”). As disclosed in the Prior 8-K and concurrently with the execution of the Merger Agreement, the Company also entered into a Securities Purchase Agreement with Evofem Operations and certain investors of Evofem Operations (the “Securities Purchase Agreement”) pursuant to which, conditioned upon and immediately following the Merger, the Company would issue and sell in a private placement transaction (the “Financing”) $20 million of Company common stock.
On December 12, 2017, the Company filed a prospectus/proxy statement/information statement with the SEC (as amended, the “Proxy Statement”) seeking approval of Proposal Nos. 1 through 7 described below. The approval of the Merger Agreement, the Merger and the issuance of the Company’s common stock pursuant to the Merger Agreement (Proposal No. 1) and the approval of the issuance of shares of the Company’s common stock in connection with the Financing (Proposal No. 5) required the affirmative vote of the holders of a majority of the shares of the Company’s common stock having voting power present in person or represented by proxy at the Special Meeting. The approval of the Reverse Stock Split, the change of the Company’s corporate name and the amendment of the Company’s amended and restated certificate of incorporation to cause the Company not to be subject to Section 203 of the DGCL (Proposal Nos. 2, 3 and 4, respectively) required the affirmative vote of the holders of a majority of the shares of the Company’s common stock having voting power outstanding on the record date for the Special Meeting. The approval, on a non-binding advisory vote basis, of Merger-related executive compensation arrangements (Proposal No. 6) and the approval of the adjournment of the Special Meeting, if necessary, to solicit additional proxies (Proposal No. 7) required the affirmative vote of the holders of a majority of the shares of the Company’s common stock having voting power present in person or represented by proxy at the Special Meeting.
The final voting results for each of these proposals is set forth below. Brokers did not have discretionary authority to vote for Proposal Nos. 1, 2, 3, 4, 5 and 6 for the shares of the Company’s common stock held in street name. For more information on these proposals, please refer to the Proxy Statement.
The final voting results for each matter submitted to a vote of the Company’s stockholders are as follows:
Proposal 1. Approval of the Merger Agreement, the Merger and the Issuance of Common Stock in the Merger
| For |
Against |
Abstain |
Broker Non-Votes | |||
| 8,143,368 |
42,201 | 7,962 | 0 |
Proposal 2. Approval of the Certificate of Amendment to the Amended and Restated Certificate of Incorporation of the Company Effecting the Reverse Stock Split
| For |
Against |
Abstain |
Broker Non-Votes | |||
| 8,109,794 |
51,098 | 32,639 | 0 |
Proposal 3. Approval of Name Change
| For |
Against |
Abstain |
Broker Non-Votes | |||
| 8,111,715 |
44,033 | 37,783 | 0 |
Proposal 4. To Approve the Certificate of Amendment to the Amended and Restated Certificate of Incorporation Causing the Company as the Post-Merger Combined Entity Not to be Subject to Section 203 of the DGCL
| For |
Against |
Abstain |
Broker Non-Votes | |||
| 8,131,874 |
43,502 | 18,155 | 0 |
Proposal 5. Approval of the Issuance of Common Stock in the Financing
| For |
Against |
Abstain |
Broker Non-Votes | |||
| 8,107,800 |
71,183 | 14,548 | 0 |
Proposal 6. Advisory Non-Binding Vote on Merger-Related Executive Compensation Arrangements
| For |
Against |
Abstain |
Broker Non-Votes | |||
| 8,086,290 |
62,589 | 44,652 | 0 |
Proposal 7. Approval of Possible Adjournment of the Special Meeting
| For |
Against |
Abstain |
Broker Non-Votes | |||
| 8,062,370 |
60,153 | 71,008 | 0 |
Item 8.01. Other Events.
On January 17, 2018, the Company issued the press release attached as Exhibit 99.1 to this Current Report on Form 8-K. The contents of the press release are hereby incorporated by reference.
Below is information regarding the business and risk factors for the post-merger combined company.
EVOFEM BUSINESS
Overview
Evofem is a clinical-stage specialty biopharmaceutical company committed to improving the health and well-being of women throughout the world by addressing women’s unmet medical needs through the discovery, development and commercialization of innovative, next generation women’s healthcare products. Evofem’s multipurpose prevention technology (“MPT”) is a vaginal gel being developed for multiple indications, including contraception, sexually transmitted infections (“STIs”), and bacterial vaginosis (“BV”).
Evofem’s lead product candidate, Amphora, is a hormone-free, on demand, woman-controlled vaginal gel currently in a Phase 3 clinical trial as a contraceptive and in a Phase 2b/3 trial for the prevention of certain STIs. In addition, Evofem recently completed a Phase 1 trial of its MPT vaginal gel for the reduction of recurrence of BV and is currently designing a Phase 2b/3 trial for this indication.
Based on Evofem’s market research, Evofem believes a majority of women seeking birth control are concerned about exposure to hormones. Hormone-based contraception is the current standard in female birth control. This research also indicates that women are actively seeking alternative methods of contraception, but have limited options to reduce exposure to hormones. As a result, women are dissatisfied with current products on the market. Amphora is designed to empower women by offering a hormone-free, on demand, woman-controlled contraceptive.
Evofem’s MPT vaginal gel has also demonstrated a broad spectrum of antimicrobial activity in vitro, including on chlamydia, gonorrhea, and bacterial vaginosis causing microbes, the three most common causes of reproductive tract infection. There are currently no products indicated for the prevention of chlamydia or gonorrhea, or the reduction of recurrence of BV. Evofem believes its MPT vaginal gel offers a significant opportunity to address this important unmet medical need for women.
Evofem’s MPT vaginal gel has been granted designation as a Qualified Infectious Disease Product (“QIDP”), by the Food and Drug Administration (the “FDA”) for two separate indications: prevention of urogenital gonorrhea infection in women and reduction of recurrence of BV. A drug that receives QIDP designation may qualify for the FDA’s fast-track program, which is intended to expedite review of drugs and facilitate development so an approved product can reach the market expeditiously. QIDP designations also provide an additional five years of marketing exclusivity for an approved product.
Evofem has an exclusive worldwide license to its MPT vaginal gel from Rush University, a nationally recognized research institution. Evofem’s MPT vaginal gel was initially developed by the Program for Topical Prevention of Conception and Disease, an organization led by Rush University dedicated to the discovery and creation of topical products that can prevent pregnancy and the spread of STIs.
Evofem’s Strategy
Evofem is committed to providing women with direct control and management of their sexual and reproductive health. Key elements of Evofem’s strategy include:
| • | Rapid and timely completion of its current Phase 3 clinical trial to seek approval and subsequently commercialize Amphora for contraception. Evofem’s initial focus is the development and commercialization of Amphora as a hormone-free, on demand, woman-controlled contraceptive. Evofem believes this will create a platform for Evofem to advance its supplemental indications and allow Evofem to effectively deploy investor capital for the benefit of all stakeholders. |
| • | Leverage its MPT vaginal gel technology platform to develop and commercialize novel, first-in-class products for women. Evofem intends to expand on its contraceptive indication by being the first company to market a contraceptive product with an additional indication for the prevention of chlamydia and gonorrhea. In addition, Evofem intends to develop a product for the reduction of recurrence of BV. |
| • | Expand its intellectual property position by pursuing opportunities to extend the exclusivity of its highly differentiated and proprietary MPT vaginal gel. Evofem intends to aggressively pursue additional and new patent applications to broaden its intellectual property portfolio. Evofem will continue to seek to obtain domestic and international patent protection and endeavor to promptly file patent applications for new commercially valuable inventions, as well as obtain additional technologies from third-parties. |
| • | Expand its product pipeline. Evofem intends to opportunistically acquire additional products or product candidates that enhance its offerings and complement its core competencies in women’s healthcare. |
| • | Build a world class organization committed to the discovery, development and commercialization of products that address unmet needs in women’s sexual and reproductive health. Evofem has assembled a world class team with industry-recognized expertise in the development and commercialization of products in women’s healthcare. Evofem intends to continue to build on its leadership position and grow a culture dedicated to the development and commercialization of medicines that continue to address the unmet medical needs of women. |
The Contraceptive Market Overview
In 2016, the global revenue for contraceptive products was $21.2 billion and projected to grow at 6.8% per annum to $35.8 billion by 2024, making contraception a substantial and growing subset of the overall healthcare market. This growth is expected to continue to be driven by the United States and Europe because favorable government policies aimed at preventing unwanted pregnancies are in place. The number of women using contraception is projected to grow through 2030.
Current contraceptive options include devices designed to prevent pregnancy through physical means such as condoms, diaphragms and intrauterine devices (“IUDs”), and pharmaceutical options such as a variety of hormonal-based approaches, including oral contraceptives, vaginal rings containing hormones, intramuscular injections, subcutaneous implants and transdermal patches.
Existing contraceptive options can have significant side effects or other limitations. Long-acting options such as IUDs, hormonal injections and implants require medical procedures and are not quickly or easily reversible. Hormonal approaches can be associated with undesirable side-effects such as weight gain and mood changes, which may lead women to seek alternative contraceptive technologies or decide to not use any form of the contraceptive options currently available. Several spermicidal products currently available over-the-counter for use as vaginal contraceptives are based on surfactants, which can cause genital irritation and inflammation that may increase the risk of contracting human immunodeficiency virus (“HIV”), from an infected partner. In addition, spermicides containing the active ingredient nonoxynol-9 (“N-9”) have been required by the FDA to carry a label warning for the risk of contracting HIV. Unlike other vaginal contraceptives currently on the market, Amphora is free of surfactants such as N-9.
The unmet medical needs of the contraception market and the shift away from traditional methods of contraception such as oral contraceptives makes the entry of a non-hormonal contraceptive option such as Amphora timely and desirable. Currently, the only non-hormonal prescription contraceptive method approved in the U.S. market are a copper IUD, which is intrusive and could remain in the user’s body for up to 10 years or a diaphragm, which can be difficult to insert and must be used with contraceptive gel.
Additionally, Evofem believes that growing concern associated with the increasing prevalence of sexually transmitted diseases along with growing demand for new innovative contraception options will further drive global contraceptives market growth.
Market Opportunity
Evofem believes its key market strengths are as follows:
| • | Evofem’s MPT vaginal gel is potentially disruptive to the existing contraceptive landscape and is designed to address underserved and unmet needs in the women’s healthcare market; |
| • | Evofem expects to benefit from favorable trends away from the daily use of oral forms of hormonal contraception to more innovative technologies that underpin the large and growing global contraceptive market; |
| • | Evofem has robust proprietary technology protected by an intellectual property portfolio currently extended to 2033; and |
| • | Evofem intends to add indications to its lead product candidate, Amphora, and to add complementary products or product candidates to its pipeline expected to provide future growth opportunities within the global contraceptive market. |
Evofem believes its product candidates are well positioned to fulfill unmet needs within the existing contraceptive market and to compete with existing contraceptive options. Market penetration requires development and implementation of a tailored strategy, involving healthcare policy officials and healthcare providers for each country or territory.
Innovation and new product introduction in women’s reproductive healthcare and contraception has been limited when compared to other leading therapeutic categories. There have been no approvals in women’s contraception for the year to date 2017 as compared to, for example, oncology, where there have been more than 40 approvals in the same period, demonstrating a unique opportunity in this underdeveloped field.
According to the Centers for Disease Control and Prevention (the “CDC”), reducing the percentage of all pregnancies that are unintended has been one of the National Health Promotion Objectives since they were first established in 1980. Despite the efforts to reduce unintended pregnancies, over 2.0 million of these pregnancies occur in the U.S. annually. Following decades of minimal change or increase, the percentage of pregnancies in the U.S. that are unintended decreased slightly in the period from 2008-2011. Despite this recent decrease, 45% of pregnancies in the U.S. are still unintended. Nearly all sexually experienced women in the United States have used contraception at some time in their lives, but many women may not use contraception consistently or correctly and subsequently become pregnant when not intending to have a child at that time. According to research conducted by the CDC, approximately 40% of women surveyed after giving birth to a child resulting from an unintended pregnancy who were not using contraception noted one of the following three reasons for nonuse: did not expect to have sex, worried about side effects of birth control, or male partner did not want to use birth control.
Hundreds of millions of women worldwide seek contraceptive products during an average 30 plus years of fertility. As such, women utilizing contraception should consider the most appropriate methods for their purposes and intended use, including the use of innovative methods due to concerns associated with traditional offerings. The table below shows expected trends in contraceptive usage in different regions of the world:
| Women of Reproductive Age (millions) (2016 Projected) | U.S. | EU | *BRIC | |||||||||
| All females, age 18-49 |
61.0 | 63.8 | 420.9 | |||||||||
| At risk for pregnancy |
70.4 | % | 67.0 | % | 55.0 | % | ||||||
| Relevant population for contraception |
43.0 | 43.1 | 231.5 | |||||||||
| * | Brazil, Russia, India, China |
The U.S. Contraceptive Market
The total U.S. contraceptive market was valued at $5.5 billion in 2016 with the prescription contraceptive market expected to grow at a compound annual growth rate of 5.4% from 2013 to 2024 and reach a value of approximately $8.4 billion in 2024. The U.S. contraceptive market represented the largest segment of the global contraceptives market in 2016 at 29.4% and is currently dominated by hormonal methods including birth control pills and other reversible methods such as IUDs and injectables. Approximately four of every five sexually experienced women in the United States have used the pill at least one time and this percentage has remained stable since 1995.
More than 12 million women in the United States rely on condoms, or some other form of non-hormonal (e.g. copper IUD, diaphragm, rhythm, withdrawal) contraception as their method of choice.
Evofem conducted market research with reproductive age women ages 18 to 49 and healthcare providers in the United States to evaluate potential interest in Amphora. Of the more than 1,400 women Evofem surveyed, approximately one-third of all women expressed interest in learning more about Amphora. Amphora’s most motivating attributes for consumers included lack of hormones, ease of use and on demand use. Physicians also expressed interest in Amphora, indicating they see many patients for whom they would recommend use of Amphora.
Additionally, this market research also indicated that an Amphora user would receive approximately seven refills of Amphora per year based on reported frequency of intercourse.
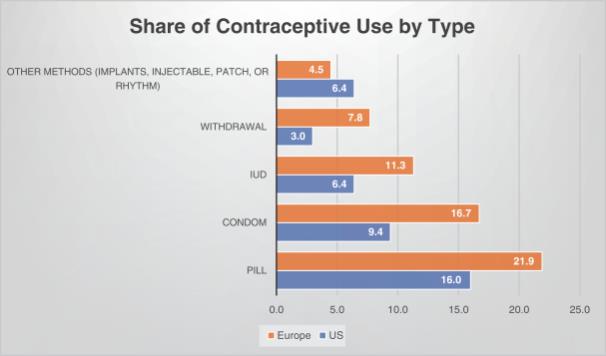
The European Union (EU) Contraceptive Market
The EU contraceptive market was valued at approximately $5.0 billion in 2016, or 24.5% of the global market, and is expected to grow at an average compound annual growth rate of 5.4% from 2013 to 2024, to reach an estimated value of approximately $7.6 billion in 2024. The EU accounted for the second largest market share in the global contraceptives market in 2016. Contraceptive use in the EU varies from region to region. As the table below shows, approximately 30% of women use no contraception and the use of male condoms is significantly higher than the U.S. population (9.4%). Permanent sterilization is also substantially lower than the U.S. (female and male sterilization rates of 14.3% and 4.5%, respectively) and only IUDs are in double digit market share among newer innovations:
Product Candidates
Amphora as a Contraceptive
Evofem believes Amphora, its lead product candidate, addresses significant gaps in the contraceptive market. If approved by the FDA, Amphora will be the only hormone-free, on demand, women-controlled contraceptive drug product available by prescription in the United States that does not require in-office placement by a healthcare provider.
Evofem believes Amphora has significant attributes that will make it an attractive contraceptive choice for women:
| Key Attributes | Potential Benefits | |
| Hormone-free | Amphora is hormone-free and, therefore, avoids known side effects of hormonal-based contraceptives, which include weight gain, headaches, sore breasts, irregular periods, mood changes, decreased sexual desire, acne and nausea. These side effects have been shown to discourage women from continuing to use hormonal contraception on a long-term basis, leading them to seek alternative methods or decide to use nothing at all. | |
| On Demand /Woman-controlled | Amphora can be used as needed; no daily, weekly, or monthly routine. Amphora may be used up to one hour before intercourse at a woman’s discretion. | |
| No Surgical Procedures | No physician insertion or removal required. The use of Amphora is private and discrete and avoids the need for recurring doctor appointments, clinical or surgical procedures. | |
| Cost Effective | Evofem anticipates coverage in the United States under the Affordable Care Act (the “ACA”). Amphora is only used when needed thus eliminating cost for daily use methods. | |
| Surfactant-free | Amphora can be used by women who experience allergy, sensitivity, or side effects to nonoxynol-9. | |
| Personal Lubricant Properties | Amphora has benefits for vaginal use, as a personal lubricant, beyond the primary contraceptive function. Amphora reduces friction and eases penetration. | |
| Bioadhesive Properties | Amphora has bioadhesive and viscosity-retaining properties to form a long-lasting layer of gel over the vaginal and cervical surfaces, which ensures reduced leakage from the vagina. | |
| Ease of Use | The Amphora applicator is designed for convenience and to be stored at room temperature for ease of handling and use. | |
The CDC’s recommendations for use of combined hormonal contraception, as shown below, defines numerous conditions that create unacceptable health risks if hormonal contraception is used. The number of women impacted by these conditions is significant. Evofem believes Amphora, if approved by the FDA, will provide women the desired solution to avoid hormones and certain other negative side effects from current prescription contraception.
Category 4 (a condition that represents an unacceptable health risk if the
contraceptive method is used)
| • | Postpartum < 21 days |
| • | Deep venous thrombosis (current or history with higher risk of recurrence) |
| • | Pulmonary embolism (current or history with higher risk of recurrence) |
| • | Cardiovascular disease or multiple CV risk factors (preexisting) |
| • | Uncontrolled Hypertension |
| • | Major surgery with prolonged immobilization |
| • | Known thrombogenic mutations |
| • | Migraine headaches with aura or without aura in women >/= 35 |
| • | Viral Hepatitis (acute or flare) |
| • | Cirrhosis (decompensated) |
| • | Age > 35 years and smoke 15 cigarettes or more per day |
| • | Valvular heart disease (complicated) |
| • | Impaired cardiac function (moderate or severe) |
| • | Systemic lupus erythematosus with positive or unknown antiphospholipid antibodies |
| • | Ischemic heart disease (current or history) |
| • | Stroke (history) |
| • | Diabetes (complicated) |
| • | Breast cancer (current) |
| • | Certain liver tumors |
| • | Solid organ transplantation (complicated) |
Mechanism of Action in Contraception
A normal vaginal pH of 3.5 to 4.5 is important for maintaining good vaginal health. At this optimum pH level, the vagina contains a balance of necessary healthy bacteria. Additionally, a vaginal pH in this range is inhospitable to spermatozoa (“sperm”) as well as certain viral and bacterial pathogens. Amphora was developed to have acid-buffering (pH 3.5), bioadhesive, and viscosity-retaining properties to provide effective acidification of the male ejaculate in the vagina and to form a long-lasting layer of gel over the vaginal and cervical surfaces. Typically, the introduction of semen (pH = 7.2-8.0) into the vagina causes a rise in pH above 6.0 due to the alkalinity of the ejaculate, which neutralizes the normally acidic vaginal environment and allows the survival of sperm. Amphora acts as a vaginal contraceptive by maintaining the vaginal pH (pH = 3.5-4.5) even in the presence of semen, inhibiting sperm from reaching the ovum to form a zygote. This buffering capacity is due to Amphora’s active pharmaceutical ingredients. Other properties contributing to the contraceptive effect of Amphora are its capacity to reduce/inhibit cervical mucus penetration, to maintain sufficient viscosity even upon dilution with the introduction of semen into the vagina and its bioadhesive strength.
The diagram below shows the respective pH levels of the vagina and semen.
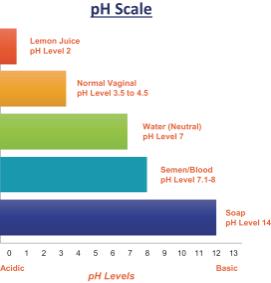
In addition to maintaining an acidic pH after semen deposition, Amphora maintains good viscosity upon dilution, suggesting that deposition of semen in the vagina will cause neither a decrease in thickness nor excessive leakage. Amphora was formulated to allow for placement up to an hour before anticipated intercourse. After proper use of Amphora, postcoital testing shows Amphora remains protective for up to 10 hours based on a lack of progressively motile sperm.
Amphora Clinical Trials
The table below summarizes key clinical milestones achieved to date for Amphora:
| Date |
Trial Phase |
Trial Description |
Publication | |||
| 1999 |
1 | Safety/vaginal tolerance | Amaral E Et al. Contraception. 1999 | |||
| 2004 |
1 | Contraceptive effectiveness | Amaral E Et al. Contraception. 2004 | |||
| 2006 |
1 | Safety trial of Amphora vs. N-9 | Amaral E Et al. Contraception | |||
| 2007 |
1 | Safety and feasibility of diaphragm with Amphora vs. KY Jelly |
Williams et al. Sexually Transmitted Diseases. 2007 | |||
| 2010 |
2 | Acceptability/feasibility of Amphora + diaphragm vs. KY Jelly |
von Mellendorf CE et al. Contraception. 2010. | |||
| 2011 |
3 | Initiation of Phase 3 (AMP001) contraceptive study versus N-9 |
N/A | |||
| 2014 |
3 | Completion of Phase 3 contraceptive trial |
N/A | |||
| 2015 |
N/A | Submission of NDA to the FDA | N/A | |||
| 2016 |
N/A | Received a complete response letter | N/A | |||
| 2017 |
3 | Initiation of Phase 3 (AMPOWER) single-arm contraceptive trial |
N/A | |||
Amphora: Phase 3 Clinical Trial (AMP001)
A key stage in the development of Amphora was the completion of a large-scale Phase 3 clinical trial comparing the contraceptive effectiveness, safety and acceptability of Amphora with that of Conceptrol®, a surfactant-based spermicidal gel containing 4% nonoxynol-9, which is currently on the market for use as a vaginal contraceptive. The primary endpoint of the trial was the rate of pregnancy among trial participants. Secondary endpoints included local and systemic signs and symptoms reported by participants or observed upon medical examination, such as itching, burning, irritation, inflammation or lesions to the cervical or vaginal epithelia and vaginal infections.
AMP001 Trial Design and Implementation
During 2011 through 2014, the AMP001 Amphora Phase 3 clinical trial enrolled 3,389 women throughout approximately 70 research centers located within the United States and Russia. It was an open-label, randomized, non-inferiority trial of repeated use of Amphora compared to Conceptrol over seven cycles of use. After completing the first 7 cycles, some of the women randomized to Amphora continued for up to a total of 13 cycles. In a subset of women (75 in each treatment arm) the lower genital tract (cervix, vagina, and vulva) was observed and photographed by colposcopy. The subset was blinded to avoid possible observer bias. A second subset was also examined microbiologically to document any changes in the vaginal flora, particularly the onset of any infection by Escherichia coli or yeast.
Results of AMP001 Phase 3 Clinical Trial
Full enrollment of the trial was achieved in July 2013 and the trial was completed during the first half of 2014. The six-month cumulative pregnancy rates for perfect use (defined as trial subjects who used the product correctly at every episode of coitus within a given cycle), and typical use (defined as trial subjects who had at least one episode of coitus without using the product correctly during the study and without any backup or emergency contraception) were approximately 4% and 10%, respectively. The observed 10% six-month and 15% one year pregnancy rates among typical users falls within the range expected for the typical use of user-dependent and barrier methods such as condoms or contraceptive gels.
The trial’s primary focus was to evaluate whether Amphora was non-inferior to Conceptrol. As the following table shows, the upper bound of the confidence interval for the difference in pregnancy percentages for typical use of Amphora or Conceptrol at six-months is less than or equal to 5.5%, which met the non-inferiority margin criteria. At one year, typical use failure rates for the male condom and the birth control pill are 18% and 9%, respectively.
| Six months Amphora |
Six months Conceptrol |
Difference | ||||||||||
| PERFECT USE |
4.1 | % | 4.2 | % | -0.1 | % | ||||||
| (95% Confidence Interval) |
(2.7% - 5.4%) | (2.8% - 5.6%) | (-2.1%, 1.8%) | |||||||||
| Number of subjects |
1,153 | 1,158 | ||||||||||
| Number of pregnancies |
36 | 36 | ||||||||||
| TYPICAL USE |
10.5 | % | 10.0 | % | 0.5 | % | ||||||
| (95% Confidence Interval) |
(8.6% - 12.3%) | (8.1% - 11.9%) | (-2.2%, 3.2%) | |||||||||
| Number of subjects |
1,259 | 1,281 | ||||||||||
| Number of pregnancies |
111 | 100 | ||||||||||
AMP001 Safety data
Of the 30 subjects who experienced at least one serious adverse events (“SAE”), 11 were treated with Amphora (0.8%) and 19 were treated with Conceptrol (1.3%). The adverse event (“AE”) reporting for the 13-cycle extension did not identify additional SAEs; therefore, no subject treated with Amphora experienced an SAE with an additional 6 cycles of exposure to Amphora. Significantly more subjects liked Amphora than Conceptrol and significantly more Amphora users would use the product again if it were available (p<0.05 for both comparisons).
The table below sets out the adverse events in the AMP001 Phase 3 clinical trial.
Adverse events in greater than 2% of Amphora gel treated subjects in the Phase 3 Clinical Trial:
| Amphora | Conceptrol | All Subjects | ||||||||||
| System organ class |
(N=1458 | ) | (N=1477) | (N=2935 | ) | |||||||
| Preferred term |
n(%) | n(%) | n(%) | |||||||||
| Total number (%) of subjects with at least one AE |
833 (57.1) | 857 (58.0) | 1690 (57.6) | |||||||||
| Urinary tract infection |
160 (11.0) | 193 (13.1) | 353 (12.0) | |||||||||
| Vaginitis bacterial |
176 (12.1) | 170 (11.5) | 346 (11.8) | |||||||||
| Vulvovaginal mycotic infection |
169 (11.6) | 168 (11.4) | 337 (11.5) | |||||||||
| Headache |
104 (7.1) | 80 (5.4) | 184 (6.3) | |||||||||
| Vulvovaginal pruritus |
60 (4.1) | 76 (5.1) | 136 (4.6) | |||||||||
| Nasopharyngitis |
79 (5.4) | 48 (3.2) | 127 (4.3) | |||||||||
| Vulvovaginal discomfort |
48 (3.3) | 53 (3.6) | 101 (3.4) | |||||||||
| Vulvovaginal candidiasis |
49 (3.4) | 46 (3.1) | 95 (3.2) | |||||||||
| Vulvovaginal burning sensation |
52 (3.6) | 41 (2.8) | 93 (3.2) | |||||||||
| Vaginal discharge |
44 (3.0) | 46 (3.1) | 90 (3.1) | |||||||||
| Dysmenorrhea |
34 (2.3) | 34 (2.3) | 68 (2.3) | |||||||||
| Influenza |
39 (2.7) | 20 (1.4) | 59 (2.0) | |||||||||
Summary of Initial NDA Submission (Contraceptive Indication)
On July 2, 2015, pursuant to section 505(b)(2) of the FDCA, Evofem submitted an NDA for Amphora to the FDA for the proposed indication of prevention of pregnancy. The submission included, among other things, data from the initial Phase 3 clinical trial (AMP001) as well as other safety and efficacy information.
A Complete Response Letter (“CRL”) was issued by the FDA on April 28, 2016. A CRL is issued if the agency determines that the application cannot be approved in its present form and will describe all the specific deficiencies identified by the agency. A CRL will also recommend actions that the applicant might take to place the application or abbreviated application in condition for approval.
The primary approvability issue was the difference in results between the U.S. and Russian cohorts. Although the study met its primary endpoint for the U.S. and Russian data when analyzed combined per the statistical plan, and separately as an ad hoc analysis, because the Russian data showed a higher level of efficacy for Amphora than for the U.S. subjects the data from Russian participants (approximately 20% of the study population) was considered non-generalizable to the U.S. population. Therefore, that data had to be excluded from the efficacy analysis (the Russian Data).
A Type A meeting was held on October 31, 2016 with the FDA who indicated a confirmatory efficacy trial focused on participants in North America would be required. After further consultation with the FDA, the FDA confirmed that a single-arm trial (non-comparative) would be sufficient to address the CRL deficiency. All feedback received from the FDA was incorporated into a protocol for a single-arm trial which was submitted to the FDA on June 30, 2017.
Amphora: AMP002 Confirmatory Phase 3 Trial (AMPOWER)
Evofem has begun a confirmatory, single-arm, Phase 3 trial entitled “A Single-Arm, Phase III, Open Label, Multicenter, Study in Women Aged 18-35 Years of the Contraceptive Efficacy and Safety of Amphora Contraceptive Vaginal Gel.” Evofem refers to this trial as AMPOWER. Evofem expects to enroll approximately 1,350 women aged 18 to 35 in up to 115 sites in the United States. The first subject was enrolled in this trial on July 28, 2017 and Evofem is currently anticipating top-level data will be available in 2019.
The primary endpoint for this trial is a seven cycle cumulative pregnancy rate. In addition to Evofem’s primary outcome for efficacy and secondary safety outcomes, Evofem has also included an exploratory endpoint of sexual satisfaction. Since Amphora also has lubricant properties, Evofem anticipates a positive result for the sexual satisfaction outcome, which could be further explored in future studies and potentially utilized in its labeling and marketing materials. Evofem believes this is the first contraception registration trial to include sexual satisfaction as an outcome.
Evofem plans to resubmit the NDA for Amphora as a prescription contraceptive by mid-2019.
Scientific Advice Process in the European Union:
Evofem previously conducted a regulatory gap analysis with Pharmalex GmbH to determine how the EU regulatory bodies were likely to view its marketing authorization application (“MAA”) upon submission to the EU. Scientific advice was previously sought in April 2016 from the Medical Products Agency of Sweden, and the Agency of Medicine and Sanitary Products of Spain, but an MAA was not pursued due to a lack of resources to support a filing at that time. Evofem plans to reinitiate the scientific advice process and seek marketing authorization for Amphora in the EU through a decentralized procedure.
Amphora for STI Prevention
In the United States, the CDC reports that there were 1.6 million new cases of chlamydia and approximately 468 thousand new cases of gonorrhea in 2016. Evofem believes this represents a significant commercial opportunity for its MPT vaginal gel.
Pre-clinical tests conducted in the early developmental stages by Rush University, and later by Evofem, suggest that Evofem’s MPT vaginal gel has the potential to suppress many of the pathogens responsible for sexually transmitted and commonly occurring bacterial infections. Evofem is advancing its MPT vaginal gel into a pivotal Phase 2b/3 trial to determine the extent to which the gel prevents sexual transmission of two common STIs, chlamydia (primary endpoint) and gonorrhea (secondary endpoint) and intends to conduct additional clinical trials to determine whether the microbicide potential shown in pre-clinical results translates into protection for women. Should this trial meet its primary endpoint, the FDA has indicated that it can be considered one of two pivotal trials required for approval.
Evofem’s MPT vaginal gel has been designated as a QIDP by the FDA for the prevention of urogenital gonorrhea infection in women. A drug that receives QIDP designation may qualify for an additional five years of marketing exclusivity and is eligible for the FDA’s fast-track program, intended to facilitate development and expedite review of drugs so that an approved product can reach the market expeditiously. An additional benefit is that the program allows for a priority review, with a goal of FDA action on the NDA within six months.
MPT Vaginal Gel for Recurrent Bacterial Vaginosis
The prevalence of BV in the United States is estimated to affect 21 million women, or 29.2% of women ages 14 to 49, and is considered to be the most common reproductive tract infections for women ages 15 to 44. There are currently no FDA approved products indicated for the reduction of recurrent BV.
Pre-clinical tests have shown Evofem’s MPT vaginal gel kills many microbes responsible for the recurrence of BV while not affecting lactobacilli, a normal and beneficial bacterium found in a healthy human vagina. The inhibitory mechanism comprises the gel’s buffered acidity and the presence of active pharmaceutical ingredients in the gel. Clinical studies are on-going to determine whether the anti-pathogen potential shown in the laboratory translates into protection for women.
Evofem filed an Investigational New Drug (“IND”) with the FDA in March 2016 to study the ability of its MPT vaginal gel for the reduction of recurrence of BV. Following submission of the IND, Evofem conducted a Phase 1 trial (EVO-002) examining the ability of a single vaginal administration of the vaginal gel at three different doses to reduce vaginal pH. The trial was completed in late 2016 and revealed the highest dose of the gel (5-gram) reduced vaginal pH up to seven days following a single administration compared to placebo gel or no gel. Evofem is currently designing a Phase 2b/3 trial to examine the ability of a 5-gram dose of its gel compared to placebo gel to reduce the recurrence of BV over a 16-week intervention period.
Evofem’s MPT vaginal gel has also been designated as a QIDP by the FDA for the reduction of recurrent episodes of BV.
Commercialization Strategy
Evofem intends to implement a global strategy to commercialize Amphora. In the United States, Evofem’s plan is to build its own integrated sales and marketing infrastructure. Outside of the United States, Evofem expects to leverage global pharmaceutical companies or other qualified potential partners to license commercialization rights or enter collaborations for the commercialization and distribution of Amphora.
While awaiting the decision from the FDA as to the approval of Amphora, Evofem plans to conduct pre-commercialization activities including:
| • | the selection of commercial suppliers, which includes agency of record for the Amphora brand, hiring of sales and sales support personnel to support the anticipated Amphora launch, initiation of payer programs including the addition of medical science liaisons and national/key account managers, and the selection of third-party logistic provider(s); and |
| • | optimizing manufacturing capabilities to include the installation of new equipment into manufacturers’ facilities, planning and preparing for all requisite inspections and planning for process validation and registration batch quantities. |
United States
Evofem estimates that the U.S. market is the largest commercial opportunity for its product candidates. If Amphora is approved for commercialization by the FDA, Evofem intends to establish a commercial sales force to market Amphora directly to obstetricians and gynecologists (“OB/GYNs”) who write the majority of prescriptions for contraceptive products.
The American Congress of Obstetricians and Gynecologists reports that there are approximately 36,000 fellows currently practicing in the United States. However, the top 30% of this group represents 85% of the contraceptive prescription volume. Evofem intends to target the top 30% by deploying a sales force of approximately 85 sales representatives. Evofem’s direct sales force will be complemented by print and digital advertising, social media campaigns, access programs, educational campaigns, and non-personal promotion campaigns targeting both consumers and healthcare providers.
Successful prescription drug market launches require comprehensive and integrated pre-launch activities. During the pre-launch phase for Amphora, Evofem intends to assemble an experienced team of key account managers and medical science liaisons expected to focus on ensuring key payer accounts, pharmacy benefit managers, key opinion leaders and medical associations who are educated about the need to offer a wider set of options to women seeking non-hormonal, woman-controlled contraceptive methods. Evofem expects these educational activities will be supported by presentation of clinical data at key national congresses (such as the American Congress of Obstetricians and Gynecologists and the Society of Family Planning), clinical publications, and additional market development activities. Launch and post-launch commercial activities are expected to include multi-channel marketing campaigns to raise brand awareness, including direct to consumer and health care professional campaigns. These key initiatives will be supported by awareness campaigns in social media, online and print advertisements, paid and earned social media support, and public relations efforts. Evofem expects these campaigns to encourage patients to consult their healthcare providers and ensure payer and healthcare provider strategies are implemented.
Ex-U.S. Markets
In markets outside of the United States, if a product candidate is approved for marketing in an individual market, Evofem intends to establish regional and/or global partnerships by either sublicensing the commercialization rights or to entering into distribution agreements with one or more third parties for the commercialization of the applicable product candidate in that market.
Payer and Reimbursement Strategy
United States
Evofem has conducted market research with 45 different healthcare plans that covered approximately 70% of covered lives within the United States to better understand viable access and pricing strategies for Amphora. Overall, a majority of respondents were positive about the introduction of a new contraception. These respondents cited the many unintended pregnancies, high costs associated with unwanted pregnancies, and the underlying limitations in the contraceptive category (i.e. the lack of non-hormonal options) as reasons a new contraceptive option is desirable. Evofem desires to have approximately 60% of all commercial healthcare plans offering full access and complete coverage of Amphora for all their reproductive aged women’s lives they are managing at the end of the first year of commercialization of Amphora. This coverage is expected to build to approximately 85% to 90% at peak sales.
Pricing strategy
Overall, healthcare plans appear receptive to the idea of pricing Amphora like that of branded oral contraceptives. Healthcare plans interviewed during market research expected Amphora to be priced between $100 to $200 for a monthly supply of a 12-applicator box (comparable to branded contraceptives), believing Amphora would ultimately offset other costs that the payer may incur (i.e. unwanted pregnancies).
Third-party Payers
Evofem expects that any sales of its product candidates will depend, in part, on the extent to which the costs of the applicable product candidates will be covered by healthcare plans, including government health programs in the United States such as Medicare and Medicaid. The process for determining whether a healthcare plan will provide coverage for a product is separate from the process for setting the price or reimbursement rate that the plan will pay for the product once coverage is approved. Evofem is also aware that many healthcare plans may limit coverage to specific products on an approved list, or formulary, which might not include all the approved products for a particular indication. Evofem intends to target those healthcare plans managing the largest number of lives to achieve optimal access for its product portfolio.
In March 2010 the ACA became law with the goals of broadening access to health insurance, reducing or constraining the growth of healthcare spending, enhancing remedies against fraud and abuse, adding new transparency requirements for health care and health insurance industries and imposing additional health policy reforms. The ACA mandates that certain preventative services that have strong scientific evidence of health benefits, including contraception, must be fully covered and reimbursement plans may no longer require a patient co-payment, coinsurance or deductible (i.e., no patient out-of-pocket expenses) for these services when they are delivered by an in-network provider. Since its enactment, there have been judicial and Congressional challenges to certain aspects of the ACA, including the contraceptive coverage mandate. Congress and President Trump have expressed their intentions to repeal or replace the ACA. The President issued an Executive Order and both chambers of Congress passed bills all with the goal of fulfilling their intentions, however, to date the Executive Order has had limited effect and Congressional activities have not resulted in passage of a law. If a law is enacted, many if not all of the provisions of the ACA may no longer apply.
European Union
In Evofem’s market research, it was found that EU consumers were interested in the unique benefits of Amphora product profiles, especially since Amphora is non-hormonal. Contraceptive products are not reimbursed in all the European Union member countries. For example, in Italy there is no coverage for contraceptives, in France and Spain, only oral contraceptives are generally covered, and in Germany, individual reimbursement policies apply.
Pricing and reimbursement
In the EU, pricing and reimbursement strategies vary widely from country to country. Some countries mandate that drug products may be marketed only after a reimbursement price has been agreed, while others may require the completion of additional studies that compare the cost-effectiveness of a product candidate to currently available therapies. For example, the EU provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. EU member states may approve a specific price for a drug product or it may instead adopt a system of direct or indirect controls on the profitability of offering a drug product on the market. Other member states allow companies to fix their own prices for drug products, but monitor and control company profits. The downward pressure on healthcare costs in general, particularly prescription drugs, has become intense creating increasingly high barriers for entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. Therefore, the development of new drug launch strategies has become very challenging to meet both patient need/demand while ensuring products are commercially viable in those markets.
Amphora Manufacturing
Evofem intends to outsource the manufacturing of Amphora (and its other potential product candidates) to third parties. Currently, Evofem has contracted with Swiss-American CDMO, LLC in Carrollton, Texas (“Swiss American”) to manufacture its clinical supplies of Amphora. Swiss-American has agreed to manufacture Amphora and potential other product candidates in accordance with cGMP regulations, as well as in compliance with all applicable laws and other relevant regulatory agency requirements for manufacture of pharmaceutical drug products.
Competition
As shown below, the contraception market was established in 1960, with the introduction of “the pill,” the first oral contraceptive widely available to women in the U.S. This high-dose hormonal option remained the primary form of available contraception on the market until 1988 when the copper IUD was introduced and offered the first non-hormonal option for birth control. As shown in the time line below, there was no notable innovation providing additional options in women’s reproductive health until 30 years after the introduction of “the pill,” when pharmaceutical companies introduced synthetic hormonal products with different hormonal delivery systems, including the hormonal IUD, implants, the patch, and vaginal ring.
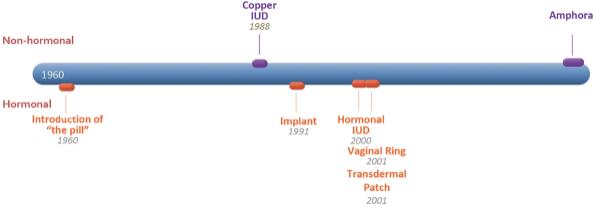
If approved, Amphora would compete for market share in at least four competitive categories: 1) oral contraception, 2) Long-Acting Reversible Contraception (“LARC”) comprised of IUDs, implants, and injectables, 3) short-term non-oral contraceptives, comprised of the weekly or monthly synthetic hormonal options including the patch and vaginal ring, and 4) over-the-counter (“OTC”) methods, dominated primarily by the condom.
Oral Contraceptives (the “pill”)
The pill is the most commonly used form of birth control in the U.S. today. Birth control pills are marketed under a variety of brand names. There are two main kinds of oral contraceptives — combination birth control pills, which contain estrogen and progestin, and the mini pill, which contains only progestin. Oral contraceptives typically must be taken on a regular or daily basis in order to be effective.
Long-Acting Reversible Contraception (LARC)
Implants
The contraception implant (principally marketed in the United States as Nexplanon® by a subsidiary of Merck & Co. must be implanted under the skin and removed by a qualified healthcare provider, requiring a medical procedure provides contraception by releasing hormones over a three year period. The implant has realized an increase in market share over the past five years, outpacing the overall contraceptive category year-over-year, with annual sales in the United States of approximately $141 million.
Injectables
The primary injectable hormonal contraceptive on the market is Depo-Provera® offered by Teva Pharmaceutical Industries Ltd. Each injection provides protection for up to 12 to 14 weeks, but patients must receive injections once every 12 weeks to get full contraceptive protection. Depo Provera was introduced to the market in 1992 and has annual sales in the U.S. of approximately $211 million.
IUDs
The copper IUD was introduced to the market in 1988 and provides protection by disrupting sperm motility and damaging sperm so that they are prevented from joining with an ovum. Today, the copper IUD is principally marketed by Cooper Surgical, Inc. as Paragard® and has annual sales in the U.S. of approximately $290 million. The hormonal IUD is principally offered under the brand names, Kyleena®, Skyla® and Mirena®, a family of products from Bayer Pharmaceuticals, and has annual sales in the U.S. of approximately $1.2 billion. All IUDs must be inserted or removed by a physician.
The LARCs are not dependent on user adherence, thus making this method appealing to those who benefit from a passive form of birth control with no daily requirement to take a pill, however many women have decided to remove their LARC due to the hormonal side effects they experience.
Short-term hormonal, non-oral
Contraceptive Patch
The weekly contraceptive patch was introduced in 2000 by Johnson & Johnson’s Janssen division; however, deaths resulting from venous thromboembolism, (“VTE”), due to hormonal exposure had a significant negative impact on the patch and led to label changes restricting utilization. Following the loss of exclusivity, Johnson & Johnson’s Janssen division exited women’s healthcare and contraception as a promotional category.
Vaginal Ring
The hormonal vaginal ring by Merck & Co. was introduced to the market in 2001 and has annual sales in the U.S. of approximately $650 million. The ring is used for three weeks and then removed for a week during menses and a new hormonal vaginal ring is inserted. The efficacy for the vaginal ring is similar to hormonal oral contraception. Users of the vaginal ring report the same incidence of hormonal related side-effects as those using oral hormonal contraception.
Non-prescription Over-the-Counter (OTC)
Condoms are the dominate product offering in OTC sales. They are manufactured primarily by Trojan® (Church & Dwight) and Durex® (Reckitt Benckiser) brands, with approximately six million women who depend on condom use as their only method of birth control. The market size in the U.S. for male condoms in 2016 was over $900 million.
Global Sales by Leading Contraceptive Companies:
| Bayer |
Merck |
Allergan |
Cooper Surgical |
Church & Dwight | ||||||
| Oral Contraceptive |
Natazia |
Lo Loestrin® Fe |
||||||||
| Short-Term Non-Oral |
Nuvaring |
|||||||||
| IUD/Implant |
Kyleena, Mirena, Skyla |
Nexplanon |
Liletta |
Paragard |
||||||
| OTC |
Trojan Condoms |
The adoption of Evofem’s Amphora, if approved, is expected to come equally from each category discussed, as interest in Amphora falls into two distinct segments: 1) those women seeking an alternative to hormonal contraception; and 2) those women who are expected to utilize Amphora as added protection to their current form of birth control. Evofem’s market research has indicated that the hormone-free, on demand, woman-controlled aspect of Amphora makes it an attractive option across the entire competitive set.
Rush License
As discussed above, Evofem and Rush University entered into an Amended and Restated License Agreement, dated March 27, 2014 (the “Rush License Agreement”), pursuant to which Rush University granted Evofem an exclusive, worldwide license of certain patents and know-how (the “Rush Licensed IP”) related to its MPT vaginal gel authorizing Evofem to make, distribute and commercialize products and processes for any and all therapeutic, prophylactic and/or diagnostic uses, including, without limitation, use for female vaginal health and/or contraception. The Rush License Agreement amended and restated in its entirety the License Agreement, dated February 6, 2012, as amended, entered into between Evofem’s predecessor-in-interest, Instead, Inc. and Rush University’s predecessor-in-interest, Rush-Presbyterian-St. Luke’s Medical Center.
As further described in the Rush License Agreement, Evofem is under an obligation to make royalty payments to Rush University based on net sales of products and/or processes that are claimed in the patents or the know-how licensed to Evofem under the Rush License Agreement. To the extent an Evofem product is not claimed in a licensed patent but does utilize the licensed know-how, the applicable royalty rate applicable to such product and/or processes half the royalty rate would be reduced.
In addition, if during the three years after an Evofem product or process has received regulatory approval and is introduced to the market, if the amounts paid to Rush University as royalties or sublicensing fees do not total a minimum royalty amount, then Evofem must pay a minimum annual royalty to Rush University. If Evofem has to pay a royalty or other payment to a third party in order for Evofem to avoid infringement of third party rights, Evofem may offset up to 50% owed to such third party from up to 50% of the amounts owed to Rush University under the Rush License. The above-described royalty payments expire upon termination of the Rush License Agreement in accordance with its terms.
Evofem also has the right to sub-license its rights to affiliates (without the prior approval of Rush University) and to third parties (with the prior written approval of Rush University, not to be unreasonably delayed or conditioned). To the extent Rush University approves of a third-party sub-license, in lieu of any royalty payment obligation under the Rush License Agreement, Evofem would then be under an obligation to pay Rush University a sub-license fee equal to a percentage of any sublicensing revenue received from any third party sub-licensee.
Pursuant to the Rush License Agreement, Rush University, its affiliates and/or its sublicensees have the right in the form of a royalty free, non-exclusive license from Evofem under the applicable patents and know-how to use the technology embodied by such patents and know-how for non-commercial research purposes.
The Rush License Agreement provides that Evofem must use its best efforts to bring one or more products or processes based on the licensed patents to market, and to continue diligent marketing efforts for one or more of such products or processes during the term of the agreement. Additionally, within one month of the end of each fiscal quarter until the date of first commercial sale of a product, Evofem must provide Rush University with a written development report summarizing its product development activities since the prior such report, as well as any necessary adjustments to the plan of development.
The Rush License Agreement contains additional customary representations and warranties, insurance and confidentiality provisions and is governed by the laws of the State of Illinois, except that questions affecting the licensed patents will be determined in accordance with the national law of the country in which the applicable patent was granted. Evofem has the first right, but not the obligation, to pursue potential infringers of the licensed patents technology and know-how and the prior written approval of Rush University is required to settle any related claim.
Evofem has agreed to defend, indemnify and hold harmless Rush University, its employees and certain other related parties from and against any and all liabilities, damages, settlements, penalties, fines, costs or expenses arising out of any claim, complaint, suit, proceeding or cause of action brought against the relevant indemnity by a third party alleging damage arising from or occurring as a result of the activities performed by or under the authority of Evofem, its affiliates or sub-licensees in connection with the exercise of Evofem’s licenses and rights under the Rush License Agreement, except to the extent caused by Rush University’s negligence or willful misconduct.
Unless terminated in accordance with its terms, the term of the Rush License Agreement continues until the expiration, revocation or invalidation of the last of the patents or the abandonment of the last patent application included within the licensed patents and technology, which includes any patent claiming an improvement made within the term of the Rush License Agreement in the course of research supported or developed by Rush University utilizing the technology.
The Rush License Agreement may be terminated upon mutual written consent of both parties or by a non-breaching party if the other party commits a breach or default of any covenant in the agreement and fails to cure such breach within thirty (30) days after receiving written notice of such breach or default.
If Evofem is in default of its obligations under the Rush License Agreement and such default has not been cured within thirty (30) days, Rush University has the option to: (a) terminate the Rush License Agreement; or (b) convert the exclusive license to a non-exclusive license (subject to the rights of any pre-approved sub-licensee under any pre-approved sub-license). Termination of the Rush License Agreement or conversion to a non-exclusive license shall give Rush University the right to terminate all sub-licenses granted by Evofem that were not approved by Rush University. If Rush University declines to terminate any such sub-license agreement (or such sub-license agreement was approved by Rush University) then: (a) in the case of termination of the Rush License Agreement, the sub-license agreement shall become a direct agreement between Rush University and the relevant sub-licensee; and (b) in the case of conversion of the Rush License Agreement license to a non-exclusive license, such license shall continue in full force and effect in accordance with its terms.
In addition, Rush University may terminate the agreement: (i) upon thirty (30) days’ notice in the event that the aggregate royalties paid under such agreement in any calendar year following March 27, 2017 do not equal a minimum of at least $50,000, except that Evofem may pay to Rush University the difference between the royalties actually paid and $50,000 to prevent Rush University from so terminating the Rush License Agreement, and under such circumstances the Rush License Agreement will continue for an additional two (2) years beyond March 27, 2017; and (ii) in a given country as regards Evofem’s rights in such country, upon sixty (60) days’ notice if, prior to March 27, 2022, Evofem has not, in such country, engaged in certain specified activities in such country in an effort to exploit the products and processes covered by the licensed patents and technology in such country.
Intellectual Property
Evofem believes it has a strong and growing intellectual property portfolio. Evofem strives to protect the proprietary technology that Evofem believe is important to its business, including seeking and maintaining patents intended to cover its product candidates, and their methods of use, as well as any other inventions that are commercially important to the development of its business. Evofem seeks to obtain domestic and international patent protection, and endeavor to promptly file patent applications for new commercially valuable inventions. Evofem also relies on trade secrets to protect aspects of its business that are not amenable to, or that it does not consider appropriate for, patent protection.
Evofem’s success will depend on its ability to obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know-how related to business, defend and enforce its patents, and other intellectual property rights, preserve the confidentiality of its trade secrets and operate without infringing the valid and enforceable patents and other proprietary rights of third parties. Evofem will also rely on continuing technological innovation and in-licensing opportunities to develop and maintain its proprietary position.
As of October 2017, Evofem owns or has exclusively licensed approximately 25 issued patents in U.S. and other countries and jurisdictions, and have approximately 30 applications pending in U.S. and other countries and jurisdictions. Furthermore, Evofem owns two pending Patent Cooperation Treaty applications that can be converted into national stage applications in U.S. and other countries and jurisdictions.
Evofem has an exclusive worldwide license to a portfolio of licensed patents held by Rush University, which provide general protection for Amphora, which expire in 2021 and could be eligible for extensions to at least 2024 in the United States and to 2026 in certain European jurisdictions, if granted by those regulatory bodies. Further, Evofem solely owns multiple patent families relating to the composition and therapeutic use of Amphora, which, upon grant, would expire at the earliest in 2033. Evofem believes that its licensed and solely owned non-hormonal contraceptive gel patent filings, combined with its substantial know-how in this field, will continue to provide opportunities for Evofem to establish a significant barrier to competitor entry into the market.
In addition, as Amphora is a product that acts locally in the vagina, Evofem believes that a generic version of Amphora gel cannot be evaluated for bioequivalence with the comparative pharmacokinetic blood testing that is commonly used to establish bioequivalence of systemic generic drugs. The comparative clinical endpoint studies that are generally conducted to establish bioequivalence of a locally-acting generic drug would not likely be adequately sensitive for detecting differences in performance between the generic drug and its reference listed drug.
In addition to patents, Evofem expects to rely on trade secrets and know-how to develop and maintain its competitive positions. For example, certain aspects of the composition, manufacturing, and use of Amphora are protected by unpatented trade secrets and know-how. Although trade secrets and know-how can be difficult to protect Evofem seeks to protect its proprietary technology and processes, in part, by confidentiality agreements with its employees, consultants, scientific advisors, collaborators, and contractors. Evofem also seeks to preserve the integrity and confidentiality of its data and trade secrets by maintaining physical security of its premises and physical and electronic security of its information technology systems. While Evofem has confidence in these individuals, organizations and systems, agreements or security measures may be breached and Evofem may not have adequate remedies for any breach. In addition, Evofem’s trade secrets and know-how may otherwise become known or may be independently discovered by competitors. To the extent that Evofem’s consultants, contractors or collaborators use intellectual property owned by third parties in their work for us, disputes may arise as to the rights in related or resulting intellectual property, including trade secret, know-how and inventions.
Trademark Basics and Strategy
Evofem owns or has rights to various trademarks, copyrights and trade names used in its business, including Evofem and Amphora. Evofem’s logos and trademarks are the property of Evofem Biosciences, Inc. All other brand names or trademarks appearing in this report are the property of their respective holders. Use or display by Evofem of other parties’ trademarks, trade dress, or products in this report is not intended to, and does not, imply a relationship with, or endorsement or sponsorship of us, by the trademark or trade dress owners.
Healthcare Laws and Regulations
Healthcare providers and third-party payers play a primary role in the recommendation and prescription of drug products that are granted marketing approval. Arrangements with third-party payers and customers are subject to broadly applicable fraud and abuse and other healthcare laws and regulations. Such restrictions under applicable federal and state healthcare laws and regulations, include the following:
Anti-Kickback Statute — the federal Anti-Kickback Statute, among other things, prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federally funded healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate the statute in order to have committed a violation. In addition, the government may assert that a claim that includes items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act;
False Claims Act — the federal False Claims Act imposes criminal and civil penalties, which can be enforced by private citizens through civil whistleblower and qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
Health Insurance Portability and Accountability Act of 1996 — the federal Health Insurance Portability and Accountability Act of 1996 (“ HIPAA”), imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or for making any false statements relating to healthcare matters; as in the case of the federal healthcare Anti-Kickback Statutes, a person or entity does not need to have actual knowledge of the statute or specific intent to violate the statute in order to have committed a violation;
False Statements Statute — the federal False Statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services;
Stark Law — the federal ban on physician self-referrals, which prohibits, subject to certain exceptions, physician referrals of Medicare or Medicaid patients to an entity providing certain “designated health services” if the physician or an immediate family member of the physician has any financial relationships with the entity;
Sunshine Act — the federal transparency or “sunshine” requirements of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, requires manufacturers of drugs, devices, biologics and medical supplies to report to the DHHS information related to physician payments and other transfers of value and physician ownership and investment interests;
State Transparency Laws
Some U.S. state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to healthcare providers and other healthcare providers or marketing expenditures, and some state laws require pharmaceutical companies to implement compliance programs and to track and report gifts, compensation and other remuneration provided to physicians, in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures and pricing information.
State and Foreign Regulatory Concerns
Analogous State and foreign laws and regulations, such as State Anti-Kickback and False Claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payers, including private insurers.
State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not pre-empted by HIPAA, thus complicating compliance efforts.
Government Regulation and Product Approval
United States — FDA Process
The research, development, testing, manufacture, labeling, promotion, advertising, distribution and marketing, among other things, of Evofem’s products are subject to extensive regulation by governmental authorities in the United States and other countries. In the United States, the FDA regulates drugs under the FDCA and its implementing regulations. Failure to comply with the applicable United States requirements may subject Evofem to administrative or judicial sanctions, such as FDA refusal to approve pending NDA warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions and/or criminal prosecution. Medical products containing a combination of new drugs, biological products or medical devices are regulated as
“combination products” in the United States. A combination product generally is defined as a product comprised of components from two or more regulatory categories (e.g., drug/device, device/biologic, drug/biologic). Each component of a combination product is subject to the requirements established by the FDA for that type of component, whether a new drug, biologic or device. To facilitate pre-market review of combination products, the FDA designates one of its centers to have primary jurisdiction for the pre-market review and regulation of the overall product based upon a determination by the FDA of the primary mode of action of the combination product. Amphora is subject to review by the FDA, and it is anticipated that Amphora will be a drug/device combination product under NDA standards.
FDA Drug Approval Process
Amphora may not be marketed in the United States until the product has received FDA approval. The steps to be completed before a drug may be marketed in the United States include:
| • | preclinical laboratory tests, animal studies, and formulation studies, all performed in accordance with the FDA’s Good Laboratory Practice (“GLP”) regulations; |
| • | submission to the FDA of an IND for human clinical testing; |
| • | adequate and well-controlled human clinical trials to establish the safety and efficacy of the drug for each indication to the FDA’s satisfaction; |
| • | submission to the FDA of an NDA; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with cGMP regulations; and |
| • | FDA review and approval of the NDA. |
Preclinical tests include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies. The results of the preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND, which must become effective before human clinical trials in the U.S. may begin and is required to be updated annually. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions about issues such as the conduct of the trials as outlined in the IND. In such a case, the IND sponsor and the FDA must resolve any outstanding FDA concerns or questions before clinical trials can proceed. Evofem’s first IND submitted in 2011 relates to Amphora for the prevention of pregnancy (AMP001). Evofem’s second IND relates to the MPT vaginal gel for the prevention of recurrence of BV (EVO-002). Evofem has also been allowed to conduct a clinical trial relating to prevention of chlamydia and gonorrhea (AMPREVENCE) under this IND.
Clinical trials involve the administration of the investigational drug to human subjects under the supervision of qualified investigators. Clinical trials are conducted under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND. Clinical trials necessary for product approval are typically conducted in three sequential phases, but the phases may overlap. The trial protocol and informed consent information for trial subjects in clinical trials must also be approved by an Institutional Review Board (IRB) for each institution where the trials will be conducted, and each IRB must monitor the trial until completion. Trial subjects must sign an informed consent form before participating in a clinical trial. Clinical testing also must satisfy extensive good clinical practice regulations and regulations for informed consent and privacy of individually identifiable information.
Assuming successful completion of the required clinical testing, the results of the preclinical studies and of the clinical trials, together with other detailed information, including information on the manufacture and composition of the drug, are submitted to the FDA in the form of an NDA requesting approval to market the product for one or more indications. Section 505(b)(1) and Section 505(b)(2) of the FDCA are the provisions governing the type of NDAs that may be submitted under the FDCA. Section 505(b)(1) is the traditional pathway for new chemical entities when no other new drug containing the same active pharmaceutical ingredient or active moiety, which is the molecule or ion responsible for the action of the drug substance, has been approved by the FDA. As an alternate pathway to FDA approval for new or improved formulations of previously approved products, a company may file a Section 505(b)(2) NDA. Section 505(b)(2) permits the submission of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The FDA reviews any NDA submitted to ensure that it is sufficiently complete for substantive review before the FDA accepts the NDA for filing. The FDA may request additional information rather than accept the NDA for filing. Even if the NDA is filed, companies cannot be sure that any approval will be granted on a timely basis, if at all. The FDA may also refer the application to an appropriate advisory committee, typically a panel of clinicians, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendations of the advisory committee, but it typically follows such recommendations. Evofem submitted its NDA for Amphora on July 2, 2015 via the 505(b)(2) regulatory pathway. No advisory committee was convened by the FDA on the first-round review and no advisory committee is expected upon resubmission of Evofem’s NDA.
The FDA may require that certain contraindications, warnings or precautions be included in the product labeling, or may condition the approval of an NDA on other changes to the proposed labeling, development of adequate controls and specifications, or a commitment to conduct post-marketing testing or clinical trials and surveillance programs to monitor the safety of approved products that have been commercialized.
Post-Approval Requirements
Oftentimes, even after a drug has been approved by the FDA for sale, the FDA may require that certain post-approval requirements be satisfied, including the conduct of additional clinical trials. If such post-approval conditions are not satisfied, the FDA may withdraw its approval of the drug. In addition, holders of an approved NDA are required to (i) report certain adverse reactions to the FDA, (ii) comply with certain requirements concerning advertising and promotional labeling for their products, and (iii) continue to have quality control and manufacturing procedures conform to cGMP regulations after approval. The FDA periodically inspects the sponsor’s records related to safety reporting and/or manufacturing facilities. This latter effort includes assessment of ongoing compliance with cGMP regulations. Evofem has used and intends to continue to use third-party manufacturers to produce active pharmaceutical ingredients, for its products in clinical and commercial quantities, and for final, finished product, and future FDA inspections may identify compliance issues at the facilities of its contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of problems with a product after approval may result in restrictions on a product, including withdrawal of the product from the market.
Hatch-Waxman Act
As part of the Drug Price Competition and Patent Term Restoration Act of 1984, Section 505(b)(2) of the FDCA was enacted, otherwise known as the Hatch-Waxman Amendments. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The Hatch-Waxman Amendments permit the applicant to rely upon certain preclinical or clinical studies conducted for an approved product. The FDA may also require companies to perform additional studies or measurements to support the change from the approved product. The FDA may then approve the new product for all or some of the label indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant.
To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an already approved product, which is referred to as the Reference Listed Drug, the applicant is required to certify to the FDA concerning any listed patents in the FDA’s Orange Book publication that relate to the Reference Listed Drug. Specifically, the applicant must certify for all listed patents one of the following certifications: (i) the required patent information has not been filed by the original applicant; (ii) the listed patent already has expired; (iii) the listed patent has not expired, but will expire on a specified date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the manufacture, use or sale of the new product.
If a Paragraph I or II certification is filed, the FDA may make approval of the application effective immediately upon completion of its review. If a Paragraph III certification is filed, the approval may be made effective on the patent expiration date specified in the application, although a tentative approval may be issued before that time. If an application contains a Paragraph IV certification, a series of events will be triggered, the outcome of which will determine the effective date of approval of the 505(b)(2) application. The Section 505(b)(2) application also will not be approved until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the Referenced Listed Drug has expired.
A certification that the new product will not infringe the Reference Listed Drug’s listed patents or that such patents are invalid is called a Paragraph IV certification. If the applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders for the Reference Listed Drug once the applicant’s NDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a legal challenge to the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of their receipt of a Paragraph IV certification automatically prevents the FDA from approving the Section 505(b)(2) NDA by imposing a 30-month automatic statutory injunction, which may be shortened by the court in a pending patent case if either party fails to reasonably cooperate in expediting the case. The 30-month stay terminates if a court issues a final order determining that the patent is invalid, unenforceable or not infringed. Alternatively, if the listed patent holder does not file a patent infringement lawsuit within the required 45-day period, the applicant’s NDA will not be subject to the 30-month stay.
The Hatch-Waxman Act provides five years of data exclusivity for new chemical entities which prevents the FDA from accepting Abbreviated New Drug Applications and 505(b)(2) applications containing the protected active ingredient. The Hatch-Waxman Act also provides three years of exclusivity for applications containing the results of new clinical investigations (other than bioavailability studies) essential to the FDA’s approval of new uses of approved products such as new indications, delivery mechanisms, dosage forms, strengths, or conditions of use.
Pricing and Reimbursement
Sales of products that Evofem markets in the future, and its ability to generate revenues on such sales, are dependent, in significant part, on the availability and level of reimbursement from third-party payers such as state and federal governments, managed care providers and private insurance plans. If Evofem’s products are approved by the FDA, Evofem intends to work with payers to demonstrate the clinical benefits of its products over other delivery modalities to secure adequate and commercially favorable pricing and reimbursement levels.
Other Governmental Regulations, Healthcare Laws and Environmental Matters
The FDA regulates all advertising and promotion activities for products under its jurisdiction both prior to and after approval. A company can make only those claims relating to safety and efficacy that are approved by the FDA. Failure to comply with applicable FDA requirements may subject a company to adverse publicity, enforcement action by the FDA, corrective advertising, consent decrees and the full range of civil and criminal penalties available to the FDA.
In addition, under the Pediatric Research Equity Act (the “PREA:), an NDA or supplement to an NDA must contain data that are adequate to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA has indicated that Amphora is covered by the PREA, but the FDA may, on its own initiative or at the request of an applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Evofem has requested a partial waiver of the PREA in its NDA.
Although Evofem currently does not have any products on the market, it may be subject to additional healthcare regulation and enforcement by the federal government and by authorities in the United States and foreign jurisdictions in which Evofem conducts business. Such laws include, without limitation, state and federal fraud and abuse laws such as anti-kickback statutes, physician self-referral prohibitions, and false claims laws, privacy and security, and the Sunshine Act, many of which may become more applicable to Evofem if its product candidates are approved for commercialization. If Evofem’s operations are found to be in violation of any of such laws or any other governmental regulations that apply to it, Evofem may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, the curtailment or restructuring of its operations, exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect its ability to operate its business and its financial results.
If Evofem establishes international operations, it will be subject to compliance with the Foreign Corrupt Practices Act (the “FCPA”), which prohibits corporations and individuals from paying, offering to pay, or authorizing the payment of anything of value to any foreign government official, government staff member, political party, or political candidate to obtain or retain business or to otherwise influence a person working in an official capacity. Evofem also may be implicated under the FCPA for activities by its partners, collaborators, contract research organizations, vendors or other agents.
Evofem’s present and future business has been and will continue to be subject to various other laws and regulations. Various laws, regulations and recommendations relating to safe working conditions, laboratory practices, the experimental use of animals, and the purchase, storage, movement, import and export and use and disposal of hazardous or potentially hazardous substances used in connection with Evofem’s research work are or may be applicable to its activities. Certain agreements involving exclusive license rights, if any, or acquisitions, if any, may be subject to national or supranational antitrust regulatory control, the effect of which cannot be predicted. The extent of government regulation, which might result from future legislation or administrative action, cannot accurately be predicted.
Review and Approval of Drug Products in the European Union
Evofem is currently assessing how Amphora is going to be regulated in the European Union, and it is expected that Amphora is going to be regulated as a drug. Pursuant to the European Clinical Trials Directive, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the member states. Under this system, an applicant must obtain approval from the competent national authority of a European Union member state in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial after a competent ethics committee has issued a favorable opinion. Clinical trial applications must be accompanied by an investigational medicinal product dossier with supporting information prescribed by the European Clinical Trials Directive and corresponding national laws of the member states and further detailed in applicable guidance documents.
To obtain marketing approval of a drug in the European Union, an applicant must submit a MAA, either under a centralized or decentralized procedure. The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid for all European Union member states, Iceland, Lichtenstein and Norway. The centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy products and products with a new active substance indicated for the treatment of certain diseases. For products with a new active substance indicated for the treatment of certain diseases and products that are highly innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional.
The decentralized procedure is available to applicants who wish to market a product in specific European Union member states where such product has not received marketing approval in any European Union member states before. The decentralized procedure provides for an applicant to apply to one member state to assess the application (the reference member state) and specifically list other member states in which it wishes to obtain approval (concerned member states). Under this procedure, an applicant submits an application based on identical dossiers and related materials, including a draft summary of product characteristics, and draft labelling and package leaflet, to the reference member state and each concerned member state. The reference member state prepares a draft assessment report and drafts of the related materials within 210 days after receipt of a valid application which is then reviewed and approved commented on by the concerned member states. Within 90 days of receiving the reference member state’s assessment report and related materials, each concerned member state must decide whether to approve the assessment report and related materials.
In the European Union, only products for which marketing authorizations have been granted may be promoted. Even if authorized, prescription-only medicines may only be promoted to healthcare professionals, not the general public. All promotion should be in accordance with the particulars listed in the summary of product characteristics. Promotional materials must also comply with various laws, and codes of conduct developed by pharmaceutical industry bodies in the European Union which govern (amongst other things) the training of sales staff, promotional claims and their justification, comparative advertising, misleading advertising, endorsements, and (where permitted) advertising to the general public. Failure to comply with these requirements could lead to the imposition of penalties by the competent authorities of the European Union member states. The penalties could include warnings, orders to discontinue the promotion of the medical device, seizure of promotional materials, fines and possible imprisonment.
RISK FACTORS
An investment in Evofem’s common stock involves a high degree of risk. Before deciding whether to invest in Evofem’s common stock, you should carefully consider the risks described below. If any of these risks actually occurs, Evofem’s business, financial condition, results of operations or cash flow could be harmed. This could cause the trading price of Evofem’s common stock to decline, resulting in a loss of all or part of your investment.
Risks Related to Evofem’s Financial Condition and Capital Requirements
Evofem is heavily reliant on its ability to access funding through capital market transactions. Due to its small public float, limited operating history and lack of revenue, it may be difficult and expensive for Evofem to raise additional funds.
Evofem is heavily reliant on its ability to raise funds through the issuance of shares of its common stock or securities linked to its common stock. Evofem’s ability to raise these funds may be dependent on a number of factors, including the risk factors further described herein and the low trading volume and volatile trading price of its shares of common stock. The stocks of small cap companies in the biotechnology sector similar to Evofem tend to be highly volatile. Evofem expects that the price of its common stock will be highly volatile for the next several years. Even if Evofem expands its portfolio of products and product candidates, Evofem may never successfully commercialize or monetize its current product candidate or any future product candidate that it may seek to develop.
As a result, Evofem may be unable to access funding through sales of its common stock or other equity-linked securities. Even if Evofem is able to access funding, the cost of capital may be substantial. The terms of any funding Evofem is able to obtain may not be favorable to it and may be highly dilutive to its stockholders. Evofem may be unable to access capital due to unfavorable market conditions or other market factors outside of its control. There can be no assurance that Evofem will be able to raise additional capital when needed. The failure to obtain additional capital when needed would have a material adverse effect on its business.
Evofem has incurred losses since its inception and anticipates that it will continue to incur significant losses for the foreseeable future.
Evofem is a specialty clinical development-stage biopharmaceutical company with a limited operating history. Evofem has incurred net losses in each year since its inception in 2009, including net losses of $66.7 million and $32.6 million for the years ended December 31, 2016 and 2015, respectively. As of December 31, 2016, Evofem had an accumulated deficit of $202.0 million. Negative cash flows from its operations are expected to continue for the foreseeable future. Evofem’s utilization of cash has been and will continue to be highly dependent on its product development programs, particularly its programs for the development of its MPT vaginal gel and its lead product candidate, Amphora. Evofem’s cash expenses will be highly dependent on the product development programs it chooses to pursue, the progress of these product development programs, the results of its preclinical studies and clinical
trials, the cost, timing and outcomes of regulatory decisions regarding potential approval for its product candidate or any future product candidate it may choose to develop, the terms and conditions of its contracts with service providers and license partners, and the rate of recruitment of patients in its clinical trials. In addition, the continuation of Evofem’s clinical trials, and quite possibly its entire business, will depend on results of upcoming clinical data analyses and its financial resources at the time. Failure to raise capital as and when needed, on favorable terms or at all, would have a negative impact on Evofem’s financial condition and its ability to develop its product candidates.
Evofem has devoted substantially all of its financial resources to develop its product candidates, including conducting clinical trials and providing general and administrative support for its operations. To date, Evofem has financed its operations primarily through the sale of equity securities. The amount of its future net losses will depend, in part, on the rate of its future expenditures and its ability to obtain funding through equity or debt financings, strategic collaborations or grants. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk.
Evofem expects to continue to incur significant expenses and increasing operating losses for the foreseeable future and its expenses will increase substantially if and as Evofem:
| • | continues the clinical development of its MPT vaginal gel and its lead product candidate, Amphora; |
| • | continues efforts to discover new product candidates; |
| • | undertakes the manufacturing of its product candidates or increases volumes manufactured by third parties; |
| • | advances its programs into larger, more expensive clinical trials; |
| • | initiates additional preclinical, clinical, or other trials or studies for its product candidate or any product candidates Evofem may choose to develop in the future; |
| • | seeks regulatory and marketing approvals and reimbursement for its product candidate or any product candidates Evofem may choose to develop in the future; |
| • | establishes a sales, marketing, and distribution infrastructure to commercialize any products for which Evofem may obtain marketing approval and market for itself; |
| • | seeks to identify, assess, acquire, and/or develop other product candidates; |
| • | makes milestone, royalty or other payments under third-party license agreements; |
| • | seeks to maintain, protect, and expand its intellectual property portfolio; |
| • | seeks to attract and retain skilled personnel; and |
| • | experiences any delays or encounters issues with the development and potential for regulatory approval of its clinical candidates such as safety issues, clinical trial accrual delays, longer follow-up for planned studies, additional major studies or supportive studies necessary to support marketing approval. |
Further, the net losses Evofem incurs may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of its results of operations may not be a good indication of its future performance.
The opinion of Evofem’s accountant assumed Evofem’s ability to continue as a going concern, and Evofem must raise additional funds to finance its operations to remain a going concern.
Based on its cash balances, recurring losses since inception and inadequacy of existing capital resources to fund planned operations for a twelve-month period, Evofem’s independent auditors have included an emphasis-of-matter paragraph in its report on Evofem’s financial statements as of and for the years ended December 31, 2016 and December 31, 2015 assuming Evofem’s ability to continue as a going concern. Evofem will, during the remainder of 2018, require significant additional funding to continue operations. If Evofem is unable to raise additional funds when needed, it will not be able to continue development of its MPT vaginal gel or its lead product candidate, Amphora, or Evofem will be required to delay, scale back or eliminate some or all of its development programs or cease operations. Any additional equity or debt financing that Evofem is able to obtain may be dilutive to its current stockholders and debt financing, if available, may involve restrictive covenants or unfavorable terms. If Evofem raises funds through collaborative or licensing arrangements, it may be required to relinquish, on terms that are not favorable to Evofem, rights to some of its technologies or product candidates that it would otherwise seek to develop or commercialize. Moreover, if Evofem is unable to continue as a going concern, it may be forced to liquidate its assets and the values it receives for its assets in liquidation or dissolution could be significantly lower than the values reflected in its financial statements.
Evofem has never generated any revenue from product sales and may never be profitable.
Evofem has no products approved for commercialization and has never generated any material amount of revenue from product sales. Evofem’s ability to generate revenue and achieve profitability depends on its ability, alone or with strategic collaborators, to successfully complete the development of, and obtain the regulatory and marketing approvals necessary to commercialize one or more of its current or future product candidates. Evofem does not anticipate generating revenue from product sales for the foreseeable future. Evofem’s ability to generate future revenue from product sales depends heavily on its success in many areas, including, but not limited to:
| • | completing research and development of its MPT vaginal gel, Amphora, its lead product candidate, and one or more of its current or future product candidates; |
| • | obtaining regulatory and marketing approvals for one or more of its current or future product candidates; |
| • | manufacturing one or more product candidates and establishing and maintaining supply and manufacturing relationships with third parties that are commercially feasible, meet regulatory requirements and Evofem’s supply needs in sufficient quantities to meet market demand for its product candidates, if approved; |
| • | marketing, launching and commercializing one or more product candidates for which Evofem obtains regulatory and marketing approval, either directly or with a collaborator or distributor; |
| • | gaining market acceptance of one or more of its product candidates as treatment options; |
| • | addressing any competing products; |
| • | protecting, maintaining and enforcing its intellectual property rights, including patents, trade secrets and know-how; |
| • | negotiating favorable terms in any collaboration, licensing or other arrangements into which Evofem may enter; |
| • | obtaining reimbursement or pricing for its MPT vaginal gel, its lead product candidate, Amphora, or one or more of its current or future product candidates that supports profitability; and |
| • | attracting, hiring and retaining qualified personnel. |
Even if one or more of the product candidates that Evofem develops is approved for commercial sale, Evofem anticipates incurring significant costs associated with launching and commercializing any approved product candidate. Evofem also will have to develop or acquire manufacturing capabilities or continue to contract with contract manufacturers in order to continue development and potential commercialization of its product candidates. If Evofem is not able to generate revenue from the sale of any approved products, Evofem may never become profitable.
Raising additional capital may cause dilution to Evofem’s stockholders, restrict its operations or require Evofem to relinquish rights.
In order to complete the development of its MPT vaginal gel and its lead product candidate, Amphora, Evofem must raise significant additional capital. To the extent that Evofem raises additional capital through the sale of equity, convertible debt or other securities convertible into equity, the ownership interest of its stockholders will be diluted, and the terms of these new securities may include liquidation or other preferences that adversely affect rights of Evofem’s stockholders. Debt financing, if available at all, would likely involve agreements that include covenants limiting or restricting Evofem’s ability to take specific actions, such as incurring additional debt, making capital expenditures, making additional product acquisitions or declaring dividends. If Evofem raises additional funds through strategic collaborations or licensing arrangements with third parties, Evofem may have to relinquish valuable rights to its product candidates or future revenue streams or grant licenses on terms that are not favorable to Evofem. Evofem does not know if it will be able to obtain additional funding if and when necessary to fund its entire portfolio of product candidates to meet its projected plans. If Evofem is unable to obtain funding on a timely basis, Evofem may be required to delay or discontinue one or more of its development programs or the commercialization of any product candidates or be unable to expand its operations or otherwise capitalize on potential business opportunities, which could materially harm Evofem’s business, financial condition, and results of operations.
Evofem’s limited operating history makes it difficult to evaluate the success of Evofem’s business to date and to assess its future viability.
To date, its activities have been largely limited to staffing, business planning, raising capital, developing its technology, identifying potential products and undertaking pre-clinical and clinical studies of Amphora. Evofem has a limited operating history which makes it difficult to evaluate its business and prospects. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of uncertainty. As a largely development stage company, Evofem has not yet demonstrated its ability to obtain regulatory approvals, generate significant revenue and conduct biopharmaceutical marketing activities necessary for successful product commercialization. In addition, given its limited operating history, Evofem may encounter unforeseen expenses, difficulties, complications, delays, and other known and unknown factors. Evofem’s likelihood of success must be evaluated in light of such challenges and variables associated with a clinical-stage biopharmaceutical product development company and Evofem may not be successful in its commercialization efforts or may incur greater costs than expected, both of which would materially adversely affect Evofem’s business, results of operations or financial condition.
Risks Related to the Development of Evofem’s Product Candidates
Evofem’s success will depend heavily on whether it can develop its lead product candidate, Amphora, as a contraceptive. Failure to develop Amphora as a contraceptive would likely cause its business to fail.
Evofem currently has a single platform technology, its MPT vaginal gel, from which it intends to create multiple product candidates. However, Evofem will rely primarily on its lead product candidate, Amphora, for use as a contraceptive for the company’s commercial success. Amphora is currently the subject of an ongoing Phase 3 clinical trial intended to demonstrate efficacy as a contraceptive. While Evofem believes that its MPT vaginal gel may also be useful in preventing other indications, currently Evofem’s business depends almost entirely on the successful clinical development and regulatory approval of Amphora for use as a contraceptive, which may never occur. Evofem has never received a regulatory approval for any product. Accordingly, even if Evofem is able to successfully complete its clinical trial for Amphora as a contraceptive, it may be unable to obtain regulatory approval for Amphora as a contraceptive which would have a material adverse effect on its business and operations.
Evofem’s ability to develop its MPT vaginal gel for additional indications could have an adverse effect on its business and its ability to successfully market Amphora as a contraceptive.
Evofem believes that Amphora may also be useful in the prevention of certain other indications, and Evofem is designing a Phase 2b/3 clinical trial for the prevention of certain STIs, including gonorrhea and chlamydia. In addition, Evofem is currently designing a Phase 2b/3 trial of its MPT vaginal gel for the reduction of recurrence of BV. Evofem does not know if it will successfully complete either of these clinical trials. Even if Evofem does complete these clinical trials, there is no assurance that it will obtain regulatory approval of Amphora or its MPT vaginal gel for additional indications. Such a failure could impede the ability of the company to market Amphora as a contraceptive because these product candidates are based on the same chemical formulation. Also, any failure to obtain regulatory approvals for additional indications will likely have a material adverse effect on the company’s business and operations.
Indemnity claims from lawsuits or damages against Evofem’s clinical trial sites could cause Evofem to incur substantial liabilities and to limit commercialization of Amphora, and any future product candidate that Evofem may develop.
In connection with its clinical trials, Evofem’s third party clinical sites face inherent risk of liability exposure from patients enrolled in Evofem’s clinical trials. Evofem has entered into indemnification agreements with each of these clinical trial sites obligating Evofem to reimburse these sites should they incur certain liability in connection with Evofem’s clinical trials. If Evofem or its clinical trial sites cannot successfully defend against these product liability and other health related claims, Evofem may incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in decreased demand for its MPT vaginal gel and its lead product candidate, Amphora or, as applicable, any future product candidate Evofem may develop, injury to Evofem’s reputation, negative media attention and the diversion of Evofem management’s time and attention from Evofem’s product development and commercialization efforts to address claim related matters.
The success of Evofem’s business is also expected to depend in part upon its ability to identify, license, discover, develop or commercialize additional product candidates. Failure to identify additional product candidates would have a negative impact on Evofem’s business and operations.
Although a substantial amount of Evofem’s effort will focus on the continued clinical testing, potential approval and commercialization of its MPT vaginal gel as a contraceptive and as a possible preventative for certain STIs and prevention of recurrence of BV, the success of Evofem’s business is also expected to depend in part upon its ability to identify, license, discover, develop or commercialize additional product candidates. Evofem is seeking to license, or otherwise obtain, product and technology rights to a variety of products and product candidates in the field of women’s health, but there can be no assurance it will be able to do so, or do so on favorable terms. Research programs to identify new product candidates require substantial technical, financial and human resources. There are risks, uncertainties and costs associated with identifying, licensing and advancing product candidates through successful clinical development. Evofem may focus its efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful. Evofem’s research programs or licensing efforts may fail to yield additional product candidates for clinical development and commercialization for a number of reasons, including but not limited to the following:
| • | Evofem’s research or business development methodology or search criteria and process may be unsuccessful in identifying potential product candidates; |
| • | Evofem may not be able or willing to assemble sufficient resources to acquire or discover additional product candidates; |
| • | its product candidates may not succeed in preclinical or clinical testing; |
| • | its potential product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval; |
| • | competitors may develop alternatives that render Evofem’s product candidates obsolete or less attractive; |
| • | product candidates Evofem develops may be covered by third parties’ patents or other exclusive rights; |
| • | the market for a product candidate may change during Evofem’s program so that such a product may become unreasonable to continue to develop; |
| • | research and development programs are quite costly and the company may be unable to obtain the financing and resources to do so; |
| • | a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and |
| • | a product candidate may not be accepted as safe and effective by patients, the medical community or third-party payors. |
If any of these events occur, Evofem may be forced to abandon its development efforts for a program or programs, or Evofem may not be able to identify, license, partner, discover, develop or commercialize additional product candidates, which would have a material adverse effect on its business, financial condition or results of operations and could potentially cause Evofem to cease operations. Moreover, even if Evofem were able to obtain the rights to additional product candidates, there can be no assurance that these candidates will ever be advanced successfully through clinical development.
Clinical trials are costly, time consuming and inherently risky, and Evofem may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
Clinical development is expensive, time consuming and involves significant risk. Evofem cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. In addition, the company’s product candidates are targeted toward pregnancy prevention and the prevention of certain infectious diseases. Therefore, it may be especially difficult to recruit patients to participate in its clinical trials when doing so will require that patients refrain from other methods of contraception and disease prevention. A failure of one or more clinical trials can occur at any stage of development. Events that may prevent successful or timely completion of clinical development include but are not limited to:
| • | inability to obtain the funding necessary to initiate or complete any clinical trial; |
| • | inability to generate satisfactory preclinical, toxicology or other in vivo or in vitro data or to develop diagnostics capable of supporting the initiation or continuation of clinical trials; |
| • | delays in reaching agreement on acceptable terms with clinical research organizations (“CROs”) and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites; |
| • | delays or failure in obtaining required institutional review board (“IRB”) approval at each clinical trial site; |
| • | failure to obtain or delays in obtaining a permit from regulatory authorities to conduct a clinical trial; |
| • | delays in recruiting or failure to recruit sufficient eligible patients in its clinical trials; |
| • | failure by clinical sites or CROs or other third parties to adhere to clinical trial requirements; |
| • | failure by clinical sites, CROs or other third parties to perform in accordance with the good clinical practices requirements of the FDA or applicable foreign regulatory guidelines; |
| • | patients withdrawing from Evofem’s clinical trials; |
| • | adverse events or other issues of concern significant enough for the FDA, or comparable foreign regulatory authority, to put an IND on clinical hold; |
| • | occurrence of adverse events associated with Evofem’s product candidates; |
| • | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; |
| • | the cost of clinical trials of Evofem’s product candidates; |
| • | negative or inconclusive results from Evofem’s clinical trials which may result in Evofem’s deciding, or regulators requiring Evofem, to conduct additional clinical trials or abandon development programs in other ongoing or planned indications for a product candidate; and |
| • | delays in reaching agreement on acceptable terms with third-party manufacturers and the time for manufacture of sufficient quantities of its product candidates for use in clinical trials. |
Any inability to successfully complete clinical development and obtain regulatory approval for one or more of its product candidates could result in additional costs to Evofem or impair its ability to generate revenue. In addition, if Evofem makes manufacturing or formulation changes to its product candidates, Evofem may need to conduct additional nonclinical studies and/or clinical trials to show that the results obtained from such new formulation are consistent with previous results obtained. Clinical trial delays could also shorten any periods during which its products have patent protection and may allow competitors to develop and bring products to market before Evofem does, which could impair its ability to successfully commercialize its product candidates and may harm its business and results of operations.
Contraception is a highly competitive healthcare niche. The success of Amphora and any other future contraceptive product candidate Evofem may pursue will be related to its efficacy and safety outcomes during clinical trials.
Today, there are a variety of hormonal and non-hormonal contraceptive options available to women and men, including oral contraceptive pills and intrauterine devices, newer hormonal contraceptive products including implants, injectables, vaginal rings, patches, and hormonal intrauterine systems, and non-hormonal methods such as female condoms, novel diaphragms, and new methods of female sterilization. Based on Evofem’s market research, clinical testing of Amphora may need to demonstrate efficacy for typical use of at least 80% to be commercially viable. Should Amphora fail to generate the safety and efficacy data expected, Evofem’s business prospects would be materially damaged.
Due in part to Evofem’s limited financial resources, it may fail to select or capitalize on the most scientifically, clinically or commercially promising or profitable indications or therapeutic areas for its product candidate, and it may be unable to pursue and complete the clinical trials that it would like to pursue and complete.
Evofem has limited financial and technical resources to determine the indications on which it should focus the development efforts for its product candidate and any future candidates it may choose to develop. Due to Evofem’s limited available financial resources, it may be required to curtail clinical development programs and activities that might otherwise have led to more rapid progress of its product candidate, or product candidates that it may in the future choose to develop, through the regulatory and development processes. Evofem may make incorrect determinations with regard to the indications and clinical trials on which to focus the available resources that it does have. The decisions to allocate Evofem’s research, management and financial resources toward particular indications may not lead to the development of viable commercial products and may divert resources from better opportunities. Similarly, Evofem’s decisions to delay or terminate development programs may also cause it to miss valuable opportunities.
Risks Related to Regulatory Approval of Evofem’s Product Candidates and Other Legal Compliance Matters
Evofem must obtain regulatory approval prior to marketing or commercializing its products and product candidates. In order to obtain regulatory approval, Evofem must complete its clinical and pre-clinical trials in compliance with the regulatory approval requirements of the FDA and any applicable and comparable foreign regulators. If clinical trials of Evofem’s product candidates fail to satisfactorily demonstrate safety and efficacy to the FDA and other comparable foreign regulators, Evofem may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of its product candidates.
Evofem is not permitted to commercialize, market, promote or sell any product candidate in the United States without obtaining marketing approval from the FDA. Comparable foreign regulatory authorities impose similar restrictions. Evofem may never receive such approvals, and must complete extensive preclinical development and clinical trials to demonstrate the safety and efficacy of its product candidates before Evofem will be able to obtain these approvals.
Any inability to complete preclinical and clinical development successfully could result in additional costs to Evofem, and impair its ability to generate revenues. Moreover, if (1) Evofem is required to conduct additional clinical trials or other testing of its product candidates beyond the trials and testing that it currently contemplates (2) Evofem is unable to successfully complete clinical trials of its product candidates or other testing, (3) the results of these clinical trials or tests are unfavorable, uncertain or are only modestly favorable or (4) there are unacceptable safety concerns associated with its product candidates, Evofem may:
| • | be delayed in obtaining marketing approval for its product candidates; |
| • | not obtain marketing approval at all; |
| • | obtain approval with labeling that includes significant use or distribution restrictions or significant safety warnings, including boxed warnings; |
| • | be subject to additional post-marketing testing or other requirements; or |
| • | be required to remove the product from the market after obtaining marketing approval. |
Amphora is a drug/device combination and the process for obtaining regulatory approval for Amphora in the United States will require compliance with requirements of two divisions of the FDA. A change in the FDA’s primary oversight responsibility would adversely impact Evofem’s development timeline and significantly raise its costs.
Amphora is comprised of both drug and device components and is considered a combination product by the FDA. It is a method of self-applied contraception that uses a pre-filled applicator to apply a semi-solid topical gel. The key active ingredient has been shown to be an active anti-inflammatory and anti-infective and works in combination with other active ingredients to stabilize the pH levels
in the vagina without altering the vaginal microbiome, which results in both the inhibition and the immobilization of spermatozoa. Other properties contributing to the contraceptive effect of Amphora are its capacity to reduce/inhibit cervical mucus penetration, to maintain sufficient viscosity even on dilution, and its bioadhesive strength. The FDA has different divisions responsible for assessing and approving devices and drugs. The Center for Drug Evaluation and Research (the “CDER”) has responsibility for drug products, while the Center for Devices and Radiological Health (the “CDRH”) has oversight responsibility for medical devices. Amphora previously underwent a request for designation process with the FDA that determined that CDER would lead the review. If the designation were to be changed to CDRH, or if either division were to institute additional requirements for the approval of Amphora, Evofem could be required to complete clinical studies with more patients and over longer periods of time than is currently anticipated. This would likely require Evofem to raise additional funds and would cause it to miss anticipated timelines. The impact of either a change in review agency or the imposition of additional requirements for approval would be significant to it and would have a material adverse effect on the prospects for the development of Amphora, its business and its financial condition.
Serious adverse events arising during clinical studies of Evofem’s MPT vaginal gel and its lead product candidate, Amphora, or post marketing could have a material, adverse effect on Evofem’s product development timeline or its ability to develop and market its MPT vaginal gel and its lead product candidate, Amphora, at all.
If serious adverse events or undesirable side effects occur during the clinical investigation of Evofem’s MPT vaginal gel or its lead product candidate, Amphora, or post marketing, the following events could materially and adversely affect Evofem’s business:
| • | regulatory authorities may impose a clinical hold which could result in substantial delays and adversely impact Evofem’s ability to continue development of its MPT vaginal gel and Amphora; |
| • | regulatory authorities may require the addition of specific warnings or contraindications to product labeling or field alerts to physicians and pharmacies; |
| • | Evofem may be required to change the way the MPT vaginal gel and/or Amphora is administered or the labeling of the MPT vaginal gel and/or Amphora; |
| • | Evofem may be required to conduct additional clinical studies with more patients or over longer periods of time than anticipated; |
| • | Evofem may be required to implement a risk minimization action plan, which could result in substantial cost increases and have a negative impact on its ability to commercialize its MPT vaginal gel and/or Amphora; |
| • | Evofem may be required to limit the patients who can receive its MPT vaginal gel and/or Amphora; |
| • | Evofem may be subject to promotional and marketing limitations on its MPT vaginal gel and/or Amphora; |
| • | sales of its MPT vaginal gel and/or Amphora may decrease significantly; |
| • | regulatory authorities may require Evofem to take an approved product off the market; |
| • | Evofem may be subject to litigation or product liability claims; and |
| • | Evofem’s reputation may suffer. |
Any of these events could prevent Evofem from achieving or maintaining market acceptance of its MPT vaginal gel or its lead product candidate, Amphora, or any future product candidate Evofem may seek to develop, or could substantially increase commercialization costs and expenses, which in turn could delay or prevent Evofem from generating significant revenues from its MPT vaginal gel or Amphora sales or the sales from any future product candidate.
If FDA approval is received for Evofem’s MPT vaginal gel, its lead product candidate, Amphora, or any other future product candidate Evofem may develop, serious adverse events or side effects could require the product to be taken off of the market, may require the product to be packaged with safety warnings or may otherwise limit Evofem’s sales of the product.
Even if Evofem obtains regulatory approval for a product, Evofem will remain subject to ongoing regulatory requirements.
If Evofem’s lead product candidate, Amphora, is approved or if its MPT vaginal gel product candidate is approved in additional indications, Evofem will be subject to ongoing regulatory requirements with respect to manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing clinical trials and submission of safety, efficacy and other post-approval information, including both federal and state requirements in the United States and requirements of comparable foreign regulatory authorities.
Manufacturers and manufacturers’ facilities are required to continuously comply with FDA and comparable foreign regulatory authority requirements, including ensuring that quality control and manufacturing procedures conform to current good manufacturing practices (“cGMP”), regulations and corresponding foreign regulatory manufacturing requirements. As such, Evofem and its contract manufacturers will be subject to continual review and inspections to assess compliance with cGMP and adherence to commitments made in any FDA new drug application (“NDA”) or marketing authorization application.
Any regulatory approvals that Evofem receives for any of its product candidates may be subject to limitations on the approved indicated uses for which the product candidate may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase IV clinical trials, and surveillance to monitor the safety and efficacy of the product candidate. Evofem will be required to report adverse reactions and production problems, if any, to the FDA and comparable foreign regulatory authorities. Any new legislation addressing drug safety issues could result in delays in product development or commercialization, or increased costs to assure compliance. If its original marketing approval for a product candidate was obtained through an accelerated approval pathway, Evofem could be required to conduct a successful post-marketing clinical trial in order to confirm the clinical benefit for its products. An unsuccessful post-marketing clinical trial or failure to complete such a trial could result in the withdrawal of marketing approval.
If a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, or disagrees with the promotion, marketing or labeling of a product, the regulatory agency may impose restrictions on that product or Evofem, including requiring withdrawal of the product from the market. If Evofem fails to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may, among other things:
| • | issue warning letters; |
| • | impose civil or criminal penalties; |
| • | suspend or withdraw regulatory approval; |
| • | suspend any of Evofem’s ongoing clinical trials; |
| • | refuse to approve pending applications or supplements to approved applications submitted by Evofem; |
| • | impose restrictions on Evofem’s operations, including closing its contract manufacturers’ facilities; or |
| • | require a product recall. |
Any government investigation of alleged violations of law would require Evofem to expend significant time and resources in response and could generate adverse publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect Evofem’s ability to develop and commercialize its products and the value of Evofem and its operating results would be adversely affected.
Even if Evofem receives approval from the FDA in the United States to market its MPT vaginal gel, its lead product candidate, Amphora, or a future product candidate Evofem may seek to develop, it may fail to receive similar approval outside the United States.
In order to market a new product outside the United States, Evofem must obtain separate marketing approvals in each jurisdiction and comply with numerous and varying regulatory requirements of other countries, including clinical trials, commercial sales, pricing manufacture distribution and safety requirements. The time required to obtain approval in other countries might differ from, and be longer than, that required to obtain FDA approval. The marketing approval process in other countries may include all of the risks associated with obtaining FDA approval in the United States, as well as other risks. Further, Evofem may be unable to obtain rights to the necessary clinical data and may be required to develop its own. In addition, in many countries outside the United States, a new product must receive pricing and reimbursement approval prior to commercialization. This can result in substantial delays in these countries. Additionally, the product labeling requirements outside the United States may be different and inconsistent with the United States labeling requirements, negatively affecting the ability of Evofem to market its products in countries outside the United States.
In addition, if Evofem fails to comply with applicable foreign regulatory requirements, Evofem may be subject to fines, suspension or withdrawal of marketing approvals, product recalls, seizure of products, operating restrictions and criminal prosecution. In such an event, Evofem’s ability to market to its full target market will be reduced and Evofem’s ability to realize the full market potential of its product candidate will be harmed, which could have a materially adverse effect on its business, financial condition, results of operation and prospects.
Evofem’s development and commercialization strategy for its MPT vaginal gel and its lead product candidate, Amphora, depends, in part, on published scientific literature and the FDA’s prior findings regarding the safety and efficacy of approved products based on data developed by others that the FDA may rely on in reviewing Evofem’s NDA.
The Drug Price Competition and Patent Term Restoration Act added section 505(b)(2) to the Federal Food, Drug, and Cosmetic Act (as amended) (the “FDCA”). Section 505(b)(2) permits the filing of a NDA where at least some of the information required for approval comes from investigations that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use from the person by or for whom the investigations were conducted. The FDA interprets section 505(b)(2) of the FDCA, for the purposes of approving an NDA, to permit the applicant to rely, in part, upon published literature or the FDA’s previous findings of safety and efficacy for an approved product. The FDA may also require the applicant to perform additional clinical trials or measurements to support any deviation from the previously approved product. The FDA may then approve the new product candidate for all or some of the label indications for which the referenced product candidate has been approved, as well as for any new indication sought by the section 505(b)(2) applicant. The applicant’s product label, however, may require all or some of the limitations, contraindications, warnings or precautions included in the reference product’s label, including a black box warning, or may require additional limitations, contraindications, warnings or precautions. Evofem has submitted a NDA for Amphora under section 505(b)(2) of the FDCA and as such the NDA relied, in part, on the FDA’s previous findings of safety and efficacy from investigations for approved products and published scientific literature for which Evofem has not received a right of reference. In addition, notwithstanding the approval of many products by the FDA pursuant to section 505(b)(2) of the FDCA, over the last few years some pharmaceutical companies and others have objected to the FDA’s interpretation of section 505(b)(2) of the FDCA. If the FDA changes its interpretation of section 505(b)(2) of the FDCA, or if the FDA’s interpretation is successfully challenged in court, this could delay or even prevent the FDA from approving any section 505(b)(2) NDAs that Evofem submits. Such a result could require Evofem to conduct additional testing and costly clinical trials, which could substantially delay or prevent the approval and launch of its product candidates.
Product liability lawsuits against Evofem could cause Evofem to incur substantial liabilities and to limit commercialization of its MPT vaginal gel, its lead product candidate, Amphora, and any future product candidate that Evofem may develop.
Evofem faces an inherent risk of product liability exposure should it commercialize its MPT vaginal gel or its lead product candidate, Amphora. Evofem will face similar risks with any other future indications for its MPT vaginal gel or other product candidates that it may develop or commercialize. If Evofem cannot successfully defend itself against these product liability claims, it may incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in decreased demand for its MPT vaginal gel, Amphora or, as applicable, any future product candidate Evofem may develop, injury to Evofem’s reputation, negative media attention and the diversion of Evofem management’s time and attention from Evofem’s product development and commercialization efforts to address claim related matters.
Evofem will need to maintain liability insurance coverage as it seeks to conduct and continues to conduct clinical trials for its MPT vaginal gel and Amphora. Such insurance may become increasingly expensive and difficult to procure. In the future, such insurance may not be available to Evofem at all or may only be available at a very high cost and, if available, may not be adequate to cover all liabilities that Evofem may incur. In addition, Evofem may need to increase its liability insurance coverage in connection with the commercialization of its MPT vaginal gel, Amphora or any other product candidate it may commercialize. If it is not able to obtain and maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise, Evofem’s business could be harmed, possibly materially.
If Evofem fails to comply with environmental, health and safety laws and regulations, Evofem could become subject to fines or penalties or incur costs that could have a material adverse effect on its business, financial condition or results of operations.
Evofem’s research and development activities and its third-party manufacturers’ and suppliers’ activities involve the controlled storage, use, and disposal of hazardous materials, including the components of its product candidates and other hazardous compounds. Evofem and its manufacturers and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling, and disposal of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at Evofem’s and its manufacturers’ facilities pending their use and disposal. Evofem cannot eliminate the risk of contamination, which could cause an interruption of its commercialization efforts, research and development efforts and business operations; environmental damage resulting in costly clean-up; and liabilities under applicable laws and regulations governing the use, storage, handling, and disposal of these materials and specified waste products. Although Evofem believes that the safety procedures utilized by it and its third-party manufacturers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, Evofem cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, Evofem may be held liable for any resulting damages and such liability could exceed its resources and state or federal or other applicable authorities may curtail Evofem’s use of specified materials and/or interrupt its business operations. Furthermore, environmental laws and regulations are complex, change frequently, and have tended to become more stringent. Evofem cannot predict the impact of such changes and cannot be certain of its future compliance. Evofem does not currently carry biological or hazardous waste insurance coverage.
Risks Related to Evofem’s Intellectual Property
If Evofem is unable to obtain and maintain patent protection for its MPT vaginal gel, its lead product candidate, Amphora, and other proprietary technologies it develops, or if the scope of the patent protection it has or will obtain is not sufficiently broad, Evofem’s competitors could develop and commercialize products and technology similar or identical to Evofem’s, and Evofem’s ability to successfully commercialize its MPT vaginal gel, Amphora, and other proprietary technologies Evofem may develop may be adversely affected.
Evofem’s success depends in large part on its ability to obtain and maintain patent protection in the United States and other countries with respect to its MPT vaginal gel, its lead product candidate, Amphora, and other proprietary technologies it may develop. Evofem seeks to protect its proprietary position by in-licensing intellectual property and filing patent applications in the United States and abroad relating to its MPT vaginal gel, its Amphora product candidate and other proprietary technologies it may develop. If Evofem or its licensors are unable to obtain or maintain patent protection with respect to its MPT vaginal gel, its Amphora product candidate and other proprietary technologies it may develop, Evofem’s business, financial condition, results of operations, and prospects could be materially harmed.
Changes in either the patent laws or their interpretation in the United States and other countries may diminish Evofem’s ability to protect its inventions, obtain, maintain, and enforce its intellectual property rights and, more generally, could affect the value of its intellectual property or narrow the scope of its owned and licensed patents. With respect to both in-licensed and owned intellectual property, Evofem cannot predict whether the patent applications Evofem and its licensors are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient protection from competitors or other third parties.
The patent prosecution process is expensive, time-consuming, and complex, and Evofem may not be able to file, prosecute, maintain, enforce, or license all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that Evofem will fail to identify patentable aspects of its research and development output in time to obtain patent protection. Although Evofem enters into non-disclosure and confidentiality agreements with parties who have access to confidential or patentable aspects of its research and development output, such as its employees, corporate collaborators, outside scientific collaborators, CROs, contract manufacturers, consultants, advisors, and other third parties, any of these parties may breach the agreements and disclose such output before a patent application is filed, thereby jeopardizing Evofem’s ability to seek patent protection. In addition, Evofem’s ability to obtain and maintain valid and enforceable patents depends on whether the differences between its inventions and the prior art allow Evofem’s inventions to be patentable over the prior art. Furthermore, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, Evofem cannot be certain that Evofem or its licensors were the first to make the inventions claimed in any of its owned or licensed patents or pending patent applications, or that Evofem or its licensors were the first to file for patent protection of such inventions.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions, and has been the subject of much litigation in recent years. As a result, the issuance, scope, validity, enforceability, and commercial value of Evofem’s patent rights are highly uncertain. Evofem’s owned or in-licensed pending and future patent applications may not result in patents being issued which protect Amphora product candidate and other proprietary technologies Evofem may develop or which effectively prevent others from commercializing competitive technologies and product candidates.
Moreover, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Even if patent applications Evofem licenses or owns currently or in the future issue as patents, they may not issue in a form that will provide Evofem with any meaningful protection, prevent competitors or other third parties from competing with Evofem, or otherwise provide Evofem with any competitive advantage. Any patents that Evofem owns or in-licenses may be challenged, narrowed, circumvented, or invalidated by third parties. Consequently, Evofem does not know whether its MPT vaginal gel, Amphora product candidate and other proprietary technology will be protectable or remain protected by valid and enforceable patents. Evofem’s competitors or other third parties may be able to circumvent Evofem’s patents by developing similar or alternative technologies or products in a non-infringing manner which could materially adversely affect Evofem’s business, financial condition, results of operations and prospects.
The issuance of a patent is not conclusive as to its inventorship, scope, validity, or enforceability, and Evofem’s patents may be challenged in the courts or patent offices in the United States and abroad. Evofem or its licensors may be subject to a third party preissuance submission of prior art to the United States Patent and Trademark Office (the “USPTO”) or become involved in opposition, derivation, revocation, reexamination, post-grant and inter partes review, or interference proceedings or other similar proceedings challenging Evofem’s owned or licensed patent rights. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate or render unenforceable, Evofem’s owned or in-licensed patent rights, allow third parties to commercialize its MPT vaginal gel, Amphora product candidate and other proprietary technologies Evofem may develop and compete directly with Evofem, without payment to Evofem, or result in Evofem’s inability to manufacture or commercialize
products without infringing third-party patent rights. Moreover, Evofem, or one of its licensors, may have to participate in interference proceedings declared by the USPTO to determine priority of invention or in post-grant challenge proceedings, such as oppositions in a foreign patent office, that challenge Evofem or its licensor’s priority of invention or other features of patentability with respect to Evofem’s owned or in-licensed patents and patent applications. Such challenges may result in loss of patent rights, loss of exclusivity, or in patent claims being narrowed, invalidated, or held unenforceable, which could limit Evofem’s ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of its MPT vaginal gel, Amphora product candidate and other proprietary technologies Evofem may develop. Such proceedings also may result in substantial cost and require significant time from Evofem’s scientists and management, even if the eventual outcome is favorable to Evofem.
In addition, given the amount of time required for the development, testing, and regulatory review of Evofem’s MPT vaginal gel and Amphora product candidate, patents protecting such product candidates might expire before or shortly after such product candidates are commercialized. As a result, Evofem’s intellectual property may not provide Evofem with sufficient rights to exclude others from commercializing products similar or identical to Evofem’s. Moreover, some of Evofem’s owned and in-licensed patents and patent applications are, and may in the future be, co-owned with third parties. If Evofem is unable to obtain an exclusive license to any such third party co-owners’ interest in such patents or patent applications, such co-owners may be able to license their rights to other third parties, including Evofem’s competitors, and Evofem’s competitors could market competing products and technology. In addition, Evofem may need the cooperation of any such co-owners of its patents in order to enforce such patents against third parties, and such cooperation may not be provided to Evofem. Furthermore, Evofem’s owned and in-licensed patents may be subject to a reservation of rights by one or more third parties. Any of the foregoing could have a material adverse effect on Evofem’s competitive position, business, financial conditions, results of operations, and prospects.
Evofem’s rights to develop and commercialize its MPT vaginal gel and lead product candidate, Amphora, are subject, in part, to the terms and conditions of licenses granted to Evofem by others.
Evofem is reliant upon licenses to certain patent rights and proprietary technology from third parties that are important or necessary to the development of Amphora product candidate. For example, Evofem’s license agreement with Rush University includes intellectual property rights to its MPT vaginal gel and its Amphora product candidate. This agreement requires Evofem, as a condition to the maintenance of Evofem’s license and other rights, to make milestone and royalty payments and satisfy certain performance obligations. Evofem’s obligations under this in-license agreement impose significant financial and logistical burdens upon Evofem’s ability to carry out its business plan. Furthermore, if Evofem does not meet such obligations in a timely manner, and, in the case of milestone payment requirements, if Evofem were unable to obtain an extension of the deadlines for meeting such payment requirements, Evofem could lose the rights to this proprietary technology, which would have a material adverse effect on Evofem’s business, financial condition and results of operations.
There is no assurance that the existing Rush University license agreement covering the rights related to its MPT vaginal gel or its Amphora product candidate will not be terminated due to a material breach of the underlying agreement. This would include a failure on Evofem’s part to make the milestone and royalty payments, Evofem’s failure to obtain applicable approvals from governmental authorities, or the loss of rights to the underlying intellectual property by any such licensors. There is no assurance that Evofem will be able to renew or renegotiate a license agreement on acceptable terms if the agreement is terminated. Evofem cannot guarantee that any license agreement will be enforceable. The termination of this license agreement or Evofem’s inability to enforce its rights under this license agreement would materially and adversely affect Evofem’s ability to commercialize its MPT vaginal gel and its Amphora product candidate.
In addition, with respect to the MPT vaginal gel and Evofem’s Amphora product candidate, Rush University has the right, in certain instances, to control the defense against any infringement litigation arising from the manufacture or development (but not the sale) of the MPT vaginal gel and Evofem’s Amphora product candidate. While Evofem’s license agreement with Rush University requires Rush University to indemnify Evofem for certain losses arising from these claims, this indemnification may not be sufficient to adequately compensate Evofem for any related losses or the potential loss of Evofem’s ability to manufacture and develop its MPT vaginal gel or Amphora product candidate.
In addition, the agreements under which Evofem currently licenses intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what Evofem believes to be the scope of Evofem’s rights to the relevant intellectual property or technology, or increase what Evofem believes to be its financial or other obligations under the relevant agreement, either of which could have a material adverse effect on Evofem’s business, financial condition, results of operations, and prospects. Moreover, if disputes over intellectual property that Evofem has licensed prevent or impair its ability to maintain its current licensing arrangements on commercially acceptable terms, Evofem may be unable to successfully develop and commercialize the affected product candidate, which could have a material adverse effect on Evofem’s business, financial conditions, results of operations, and prospects.
Evofem’s licensors may have relied on third party consultants or collaborators or on funds from third parties such that Evofem’s licensors are not the sole and exclusive owners of the patents Evofem in-licensed. If other third parties have ownership rights to Evofem’s in-licensed patents, they may be able to license such patents to Evofem’s competitors, and Evofem’s competitors could market competing products and technology. This could have a material adverse effect on Evofem’s competitive position, business, financial conditions, results of operations, and prospects.
Evofem may not be able to protect its intellectual property and proprietary rights throughout the world.
Filing, prosecuting, and defending patents on Evofem’s MPT vaginal gel, its Amphora product candidate and other proprietary technologies Evofem may develop in all countries throughout the world would be prohibitively expensive, and the laws of foreign countries may not protect Evofem’s rights to the same extent as the laws of the United States. Consequently, Evofem may not be able to prevent third parties from practicing Evofem’s inventions in all countries outside the United States, or from selling or importing products made using Evofem’s inventions in and into the United States or other jurisdictions. Competitors may use Evofem’s technologies in jurisdictions where Evofem has not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where Evofem has patent protection but enforcement is not as strong as that in the United States. These products may compete with Evofem’s products, and Evofem’s patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for Evofem to stop the infringement of its patents or marketing of competing products in violation of its intellectual property and proprietary rights generally. In addition, some jurisdictions, such as Europe, Japan, and China, may have a higher standard for patentability than in the U.S., including for example the requirement of claims having literal support in the original patent filing and the limitation on using supporting data that is not in the original patent filing. Under those heightened patentability requirements, Evofem may not be able to obtain sufficient patent protection in certain jurisdictions even though the same or similar patent protection can be secured in U.S. and other jurisdictions.
Proceedings to enforce Evofem’s intellectual property and proprietary rights in foreign jurisdictions could result in substantial costs and divert Evofem’s efforts and attention from other aspects of its business, could put Evofem’s patents at risk of being invalidated or interpreted narrowly, could put Evofem’s patent applications at risk of not issuing, and could provoke third parties to assert claims against Evofem. Evofem may not prevail in any lawsuits that it initiates, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, Evofem’s efforts to enforce its intellectual property and proprietary rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that Evofem develops or licenses.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If Evofem or any of its licensors are forced to grant a license to third parties with respect to any patents relevant to Evofem’s business, Evofem’s competitive position may be impaired, and its business, financial condition, results of operations, and prospects may be adversely affected.
Obtaining and maintaining Evofem’s patent protection depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by government patent agencies, and its patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees, and various other government fees on patents and applications will be due to be paid to the USPTO and various government patent agencies outside of the United States over the lifetime of Evofem’s owned or licensed patents and applications. In certain circumstances, Evofem relies on its licensing partners to pay these fees due to U.S. and non-U.S. patent agencies. The USPTO and various non-U.S. government agencies require compliance with several procedural, documentary, fee payment, and other similar provisions during the patent application process. Evofem is also dependent on its licensors to take the necessary action to comply with these requirements with respect to its licensed intellectual property. In some cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. There are situations, however, in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in a partial or complete loss of patent rights in the relevant jurisdiction. In such an event, potential competitors might be able to enter the market with similar or identical products or technology, which could have a material adverse effect on Evofem’s business, financial condition, results of operations, and prospects.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing Evofem’s ability to protect its products.
Changes in either the patent laws or interpretation of the patent laws in the United States could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. Assuming that other requirements for patentability are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America Invents Act (the “America Invents Act”) enacted in September 2011, the United States transitioned to a first inventor to file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. A third party that files a patent application in the USPTO after March 2013, but before Evofem could therefore be awarded a patent covering an invention of Evofem’s even if Evofem had made the invention before it was made by such third party. This will require Evofem to be cognizant going forward of the time from invention to filing of a patent application. Since patent applications in the United States and most other countries are confidential for a period of time after filing or until issuance, Evofem cannot be certain that it or its licensors were the first to either (i) file any patent application related to its MPT vaginal gel, its Amphora product candidate and other proprietary technologies Evofem may develop or (ii) invent any of the inventions claimed in Evofem’s or its licensor’s patents or patent applications.
The America Invents Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate Evofem’s patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. Therefore, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of Evofem’s owned or in-licensed patent applications and the enforcement or defense of Evofem’s owned or in-licensed issued patents, all of which could have a material adverse effect on Evofem’s business, financial condition, results of operations, and prospects.
In addition, the patent positions of companies in the development and commercialization of biologics and pharmaceuticals are particularly uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on Evofem’s existing patent portfolio and Evofem’s ability to protect and enforce its intellectual property in the future.
Issued patents covering Evofem’s MPT vaginal gel, its Amphora product candidate and other proprietary technologies Evofem may develop could be found invalid or unenforceable if challenged in court or before administrative bodies in the United States or abroad.
If Evofem or one of its licensors initiated legal proceedings against a third party to enforce a patent covering Evofem’s MPT vaginal gel, its Amphora product candidate and other proprietary technologies Evofem may develop, the defendant could counterclaim that such patent is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Third parties may raise claims challenging the validity or enforceability of Evofem’s owned or in-licensed patents before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post-grant review, inter partes review, interference proceedings, derivation proceedings, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in the revocation of, cancellation of, or amendment to Evofem’s patents in such a way that they no longer cover its MPT vaginal gel, its Amphora product candidate and other proprietary technologies Evofem may develop. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, Evofem cannot be certain that there is no invalidating prior art, of which Evofem or its licensing partners and the patent examiner were unaware during prosecution. If a third party were to prevail on a legal assertion of invalidity or unenforceability, Evofem would lose at least part, and perhaps all, of the patent protection on Amphora product candidate and other proprietary technologies Evofem may develop. Such a loss of patent protection would have a material adverse impact on Evofem’s business, financial condition, results of operations, and prospects.
If Evofem does not obtain patent term extension and data exclusivity for its MPT vaginal gel and its Amphora product candidate, Evofem’s business may be materially harmed.
Depending upon the timing, duration and specifics of any FDA marketing approval of any product candidate Evofem may develop, one or more of Evofem’s owned or in-licensed U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Action of 1984 (the “Hatch-Waxman Amendments”). The Hatch-Waxman Amendments permit a patent extension term (“PTE”) of up to five years as compensation for patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent may be extended and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. Similar patent term restoration provisions to compensate for commercialization delay caused by regulatory review are also available in certain foreign jurisdictions, such as in Europe under Supplemental Protection Certificate (the “SPC”).
An important part of Evofem’s patent strategy is reliant on its ability to obtain patent term extension on the patents licensed from Rush University. However, Evofem may not be granted an extension, such as PTE for the U.S. patent and SPC for the European patents because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents, or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than Evofem requests. If Evofem is unable to obtain patent term extension or the term of any such extension is shorter than what Evofem requests, its competitors may obtain approval of competing products following Evofem’s patent expiration, and Evofem’s business, financial condition, results of operations, and prospects could be materially harmed.
The patent protection and patent prosecution for Evofem’s MPT vaginal gel and Amphora product candidate is dependent on third parties.
While Evofem normally seeks to obtain the right to control prosecution, maintenance and enforcement of the patents relating to Evofem’s MPT vaginal gel and Amphora product candidate, there may be times when the filing and prosecution activities for patents relating to Evofem’s product candidate are controlled by Evofem’s licensors or collaboration partners. If any of Evofem’s current or future licensing or collaboration partners fail to prosecute, maintain and enforce such patents and patent applications in a manner consistent with the best interests of Evofem’s business, including by payment of all applicable fees for patents covering its product candidate, Evofem could lose its rights to the intellectual property or its exclusivity with respect to those rights, its ability to develop and commercialize its product candidate may be adversely affected and Evofem may not be able to prevent competitors from making, using and selling competing products. In addition, even where Evofem has the right to control patent prosecution of patents and patent applications Evofem has licensed to and from third parties, Evofem may still be adversely affected or prejudiced by actions or inactions of its licensees, its licensors and their counsel that took place prior to the date upon which Evofem assumed control over patent prosecution.
Evofem may be subject to claims challenging the inventorship of its patents and other intellectual property.
Evofem or its licensors may be subject to claims that former employees, collaborators or other third parties have an interest in its owned or in-licensed patents, trade secrets, or other intellectual property as an inventor or co-inventor. For example, Evofem or its licensors may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing Amphora product candidate and other proprietary technologies Evofem may develop. Litigation may be necessary to defend against these and other claims challenging inventorship or Evofem’s or its licensor’s ownership of its owned or in-licensed patents, trade secrets or other intellectual property. If Evofem or its licensors fail in defending any such claims, in addition to paying monetary damages, Evofem may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property that is important to Amphora product candidate and other proprietary technologies Evofem may develop. Even if Evofem is successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. Any of the foregoing could have a material adverse effect on Evofem’s business, financial condition, results of operations and prospects.
If Evofem is unable to protect the confidentiality of its trade secrets, Evofem’s business and competitive position would be harmed.
In addition to seeking patents for its MPT vaginal gel, its Amphora product candidate and other proprietary technologies Evofem may develop, Evofem also relies on trade secrets and confidentiality agreements to protect its unpatented know-how, technology, and other proprietary information and to maintain its competitive position. With respect to its MPT vaginal gel and its Amphora product candidate, Evofem considers trade secrets and know-how to be one of its important sources of intellectual property. Trade secrets and know-how can be difficult to protect. In particular, the trade secrets and know-how in connection with its MPT vaginal gel and its Amphora product candidate and other proprietary technology Evofem may develop may over time be disseminated within the industry through independent development, the publication of journal articles describing the methodology, and the movement of personnel with scientific positions in academic and industry.
Evofem seeks to protect these trade secrets and other proprietary technology, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as Evofem’s employees, corporate collaborators, outside scientific collaborators, CROs, contract manufacturers, consultants, advisors, and other third parties. Evofem also enters into confidentiality and invention or patent assignment agreements with its employees and consultants. Evofem cannot guarantee that it has entered into such agreements with each party that may have or have had access to Evofem’s trade secrets or proprietary technology and processes. Despite these efforts, any of these parties may breach the agreements and disclose Evofem’s proprietary information, including its trade secrets, and Evofem may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of Evofem’s trade secrets were to be lawfully obtained or independently developed by a competitor or other third party, Evofem would have no right to prevent them from using that technology or information to compete with Evofem. If any of Evofem’s trade secrets were to be disclosed to or independently developed by a competitor or other third party, Evofem’s competitive position would be materially and adversely harmed.
Evofem may be subject to claims that third parties have an ownership interest in its trade secrets. For example, Evofem may have disputes arise from conflicting obligations of its employees, consultants or others who are involved in developing its product candidate. Litigation may be necessary to defend against these and other claims challenging ownership of Evofem’s trade secrets. If Evofem fails in defending any such claims, in addition to paying monetary damages, it may lose valuable trade secret rights, such as exclusive ownership of, or right to use, trade secrets that are important to Amphora product candidate and other proprietary technologies Evofem may develop. Such an outcome could have a material adverse effect on Evofem’s business. Even if Evofem is successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Evofem may not be successful in obtaining necessary rights to any product candidate it may develop through acquisitions and in-licenses.
Evofem currently has rights to intellectual property, covering its MPT vaginal gel, its Amphora product candidate and other proprietary technologies it may develop. Other pharmaceutical companies and academic institutions may also have filed or are planning to file patent applications potentially relevant to Evofem’s business. In order to avoid infringing these third party patents, Evofem may find it necessary or prudent to obtain licenses to such patents from such third party intellectual property holders. However, Evofem may be unable to secure such licenses or otherwise acquire or in-license any compositions, methods of use, processes, or other intellectual property rights from third parties that Evofem identifies as necessary for its MPT vaginal gel, its Amphora product candidate and other proprietary technologies Evofem may develop. The licensing or acquisition of third party intellectual property rights is a competitive area, and several more established companies may pursue strategies to license or acquire third party intellectual property rights that Evofem may consider attractive or necessary. These established companies may have a competitive advantage over Evofem due to their size, capital resources and greater clinical development and commercialization capabilities. In addition, companies that perceive Evofem to be a competitor may be unwilling to assign or license rights to Evofem. Evofem also may be unable to license or acquire third party intellectual property rights on terms that would allow it to make an appropriate return on Evofem’s investment or at all. If Evofem is unable to successfully obtain rights to required third party intellectual property rights or maintain the existing intellectual property rights it has, it may have to abandon development of the relevant program or product candidate, which could have a material adverse effect on Evofem’s business, financial condition, results of operations, and prospects.
Evofem may be subject to claims that its employees, consultants, or advisors have wrongfully used or disclosed alleged trade secrets of their current or former employers or claims asserting ownership of what Evofem regards as its own intellectual property.
Many of Evofem’s employees, consultants, and advisors are currently or were previously employed at universities or other biotechnology or pharmaceutical companies, including Evofem’s competitors or potential competitors. Although Evofem tries to ensure that its employees, consultants, and advisors do not use the proprietary information or know-how of others in their work for Evofem, Evofem may be subject to claims that it or these individuals have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s current or former employer. Litigation may be necessary to defend against these claims. If Evofem fails in defending any such claims, in addition to paying monetary damages, Evofem may lose valuable intellectual property rights or personnel. Even if Evofem is successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, while it is Evofem’s policy to require its employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to Evofem, Evofem may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that it regards as its own. The assignment of intellectual property rights may not be self-executing, or the assignment agreements may be breached, and
Evofem may be forced to bring claims against third parties, or defend claims that they may bring against Evofem, to determine the ownership of what Evofem regards as its intellectual property. Such claims could have a material adverse effect on its business, financial condition, results of operations, and prospects.
Third-party claims of intellectual property infringement, misappropriation or other violation against Evofem or its collaborators may prevent or delay the development and commercialization of its MPT vaginal gel, its Amphora product candidate and other proprietary technologies Evofem may develop.
The field of contraceptive and/or anti-STDs vaginal gel is competitive and dynamic. Due to the significant research and development that is taking place by several companies, including Evofem and its competitors, in this field, the intellectual property landscape is in flux, and it may remain uncertain in the future. There may be significant intellectual property related litigation and proceedings, in addition to the ongoing interference proceedings, relating to Evofem’s owned and in-licensed, and other third party, intellectual property and proprietary rights in the future.
Evofem’s commercial success depends in part on Evofem’s and its collaborators’ ability to avoid infringing, misappropriating and otherwise violating the patents and other intellectual property rights of third parties. There is a substantial amount of complex litigation involving patents and other intellectual property rights in the biotechnology and pharmaceutical industries, as well as administrative proceedings for challenging patents, including interference, derivation and reexamination proceedings before the USPTO or oppositions and other comparable proceedings in foreign jurisdictions. As discussed above, recently, due to changes in U.S. law referred to as patent reform, new procedures including inter partes review and post-grant review have been implemented. As stated above, this reform adds uncertainty to the possibility of challenge to Evofem’s patents in the future.
Numerous U.S. and foreign issued patents and pending patent applications owned by third parties exist in the fields in which Evofem is commercializing its MPT vaginal gel, its Amphora product candidate and in which it is developing other proprietary technologies. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that Evofem’s product candidate may give rise to claims of infringement of the patent rights of others. Evofem cannot assure you that its MPT vaginal gel, its Amphora product candidate and other proprietary technologies it may develop will not infringe existing or future patents owned by third parties. Evofem may not be aware of patents that have already been issued and that a third party, for example, a competitor in the fields in which it is developing its product candidate, might assert are infringed by its current or future product candidate, including claims to compositions, formulations, methods of manufacture or methods of use or treatment that cover its product candidate. It is also possible that patents owned by third parties of which Evofem is aware, but which it does not believe are relevant to its MPT vaginal gel, its Amphora product candidate and other proprietary technologies it may develop, could be found to be infringed by its product candidate. In addition, because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that Evofem’s product candidate may infringe.
Third parties may currently have patents or obtain patents in the future, and claim that use of Evofem’s technologies or the manufacture, use or sale of its MPT vaginal gel or its Amphora product candidate infringes upon these patents. In the event that any third party claims that Evofem infringes their patents or that Evofem is otherwise employing their proprietary technology without authorization and initiates litigation against Evofem, even if Evofem believes such claims are without merit, a court of competent jurisdiction could hold that such patents are valid, enforceable and infringed by Evofem’s technologies or product candidate. In this case, the holders of such patents may be able to block Evofem’s ability to commercialize the applicable product candidate or technology unless it obtains a license under the applicable patents, or until such patents expire or are finally determined to be held invalid or unenforceable. Such a license may not be available on commercially reasonable terms or at all. Even if Evofem is able to obtain a license, the license would likely obligate Evofem to pay license fees or royalties or both, and the rights granted to Evofem might be nonexclusive, which could result in its competitors gaining access to the same intellectual property. If Evofem is unable to obtain a necessary license to a third-party patent on commercially reasonable terms, it may be unable to commercialize its product candidate or technologies or such commercialization efforts may be significantly delayed, which could in turn significantly harm Evofem’s business.
Defense of infringement claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and other employee resources from Evofem’s business, and may impact its reputation. In the event of a successful claim of infringement against Evofem, Evofem may be enjoined from further developing or commercializing its infringing products or technologies. In addition, Evofem may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties and/or redesign its infringing products or technologies, which may be impossible or require substantial time and monetary expenditure. In that event, Evofem would be unable to further develop and commercialize its product candidate or technologies, which could harm its business significantly. Further, Evofem cannot predict whether any required license would be available at all or whether it would be available on commercially reasonable terms. In the event that Evofem could not obtain a license, it may be unable to further develop its product candidate and commercialize its product and product candidate, if approved, which could harm its business significantly. Even if Evofem are able to obtain a license, the license would likely obligate Evofem to pay license fees or royalties or both, and the rights granted to it might be nonexclusive, which could result in Evofem’s competitors gaining access to the same intellectual property. Ultimately, Evofem could be prevented from commercializing a product, or be forced to cease some aspect of its business operations, if, as a result of actual or threatened patent infringement claims, Evofem is unable to enter into licenses on acceptable terms.
Engaging in litigation defending Evofem against third parties alleging infringement of patent and other intellectual property rights is very expensive, particularly for a company of Evofem’s size, and time-consuming. Some of Evofem’s competitors may be able to sustain the costs of litigation or administrative proceedings more effectively than Evofem can because of greater financial resources. Patent litigation and other proceedings may also absorb significant management time. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could impair Evofem’s ability to compete in the marketplace. The occurrence of any of the foregoing could have a material adverse effect on Evofem’s business, financial condition or results of operations.
Evofem may become involved in lawsuits to protect or enforce its patents and other intellectual property rights, which could be expensive, time consuming, and unsuccessful.
Competitors may infringe Evofem’s patents or the patents of its licensing partners, or Evofem may be required to defend against claims of infringement. In addition, Evofem’s patents or the patents of its licensing partners also may become involved in inventorship, priority or validity disputes. To counter or defend against such claims can be expensive and time consuming. In an infringement proceeding, a court may decide that a patent owned or in-licensed by Evofem is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that Evofem’s owned and in-licensed patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of Evofem’s owned or in-licensed patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of Evofem’s confidential information could be compromised by disclosure during this type of litigation.
Even if resolved in Evofem’s favor, litigation or other legal proceedings relating to intellectual property claims may cause Evofem to incur significant expenses and could distract Evofem’s personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of Evofem’s common stock. Such litigation or proceedings could substantially increase Evofem’s operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities. Evofem may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of Evofem’s competitors may be able to sustain the costs of such litigation or proceedings more effectively than Evofem can because of their greater financial resources and more mature and developed intellectual property portfolios. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on Evofem’s ability to compete in the marketplace.
If Evofem’s trademarks and trade names are not adequately protected, then Evofem may not be able to build name recognition in its markets of interest and its business may be adversely affected.
Evofem’s registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. During trademark registration proceedings, including those for Amphora, Evofem may receive rejections of its applications by the USPTO or in other foreign jurisdictions. Although Evofem is given an opportunity to respond to those rejections, it may be unable to overcome such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against Evofem’s trademarks, and its trademarks may not survive such proceedings. Moreover, any name Evofem has proposed to use with its product candidate in the United States must be approved by the FDA, regardless of whether Evofem has registered it, or applied to register it, as a trademark. Similar requirements exist in Europe. The FDA typically conducts a review of proposed product names, including an evaluation of potential for confusion with other product names. If the FDA (or an equivalent administrative body in a foreign jurisdiction) objects to any of Evofem’s proposed proprietary product names, it may be required to expend significant additional resources in an effort to identify a suitable substitute name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA. Furthermore, in many countries, owning and maintaining a trademark registration may not provide an adequate defense against a subsequent infringement claim asserted by the owner of a senior trademark.
Evofem may not be able to protect its rights to these trademarks and trade names, which Evofem needs to build name recognition among potential partners or customers in its markets of interest. At times, competitors or other third parties may adopt trade names or trademarks similar to Evofem’s, thereby impeding its ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of Evofem’s registered or unregistered trademarks or trade names. Over the long term, if Evofem is unable to establish name recognition based on its trademarks and trade names, then Evofem may not be able to compete effectively and its business may be adversely affected. Evofem’s efforts to enforce or protect its proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely affect its business, financial condition, results of operations and prospects.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by Evofem’s intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect Evofem’s business or permit Evofem to maintain its competitive advantage. For example:
| • | others may be able to make products that are similar to Evofem’s product candidate or utilize similar technology but that are not covered by the claims of the patents that Evofem licenses or may own; |
| • | Evofem, or its current or future licensors or collaborators, might not have been the first to make the inventions covered by the issued patent or pending patent application that Evofem licenses or may own in the future; |
| • | Evofem, or its current or future licensors or collaborators, might not have been the first to file patent applications covering certain of Evofem’s or their inventions; |
| • | others may independently develop similar or alternative technologies or duplicate any of Evofem’s technologies without infringing its owned or licensed intellectual property rights; |
| • | it is possible that Evofem’s current or future pending owned or licensed patent applications will not lead to issued patents; |
| • | issued patents that Evofem holds rights to may be held invalid or unenforceable, including as a result of legal challenges by its competitors or other third parties; |
| • | Evofem’s competitors or other third parties might conduct research and development activities in countries where Evofem does not have patent rights and then use the information learned from such activities to develop competitive products for sale in Evofem’s major commercial markets; |
| • | Evofem may not develop additional proprietary technologies that are patentable; |
| • | the patents of others may harm Evofem’s business; and |
| • | Evofem may choose not to file a patent in order to maintain certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property. |
Should any of these events occur, they could have a material adverse effect on Evofem’s business, financial condition, results of operations, and prospects.
Risks Related to Evofem’s Reliance on Third Parties
Evofem’s success relies on third party suppliers and manufacturers. Any failure by such third parties, including failure to successfully perform and comply with regulatory requirements, could negatively impact Evofem’s business and its ability to develop and market Amphora and potential future product candidates, and its business could be substantially harmed.
Evofem has a small number of employees and no internal manufacturing capability. Evofem management does not expect to manufacture any products and expects to rely on third parties to make Evofem’s products, and as such it will be subject to inherent uncertainties related to product safety, availability and security. To date, Evofem’s contract manufacturer, Swiss-American Products, Inc., has only produced a small quantity of its MPT vaginal gel for clinical testing. Furthermore, for some of the key raw materials and components of its MPT vaginal gel, Evofem has only a single source of supply, and alternate sources of supply may not be readily available.
Moreover, Evofem does not expect to control the manufacturing processes for the production of its MPT vaginal gel or any of its other future products or product candidates, which must be made in accordance with relevant regulations, and includes, among other things, quality control, quality assurance, compliance with cGMP and the maintenance of records and documentation. In the future, it is possible that Evofem’s suppliers or manufacturers may fail to comply with FDA regulations, the requirements of other regulatory bodies or its own requirements, all of which would result in suspension or prevention of commercialization and/or manufacturing of its products or product candidates, including its MPT vaginal gel and its lad product candidate, Amphora, suspension of ongoing research, disqualification of data or other enforcement actions such as product recall, injunctions, civil penalties or criminal prosecutions against Evofem. Furthermore, Evofem may be unable to replace any supplier or manufacturer with an alternate supplier or manufacturer on a commercially reasonable or timely basis, or at all.
If Evofem were to experience an unexpected loss of supply of, or if any supplier or manufacturer were unable to meet its demand for its product candidates, Evofem could experience delays in research, planned clinical studies or commercialization. Evofem might be unable to find alternative suppliers or manufacturers with FDA approval, of acceptable quality, in the appropriate volumes and at an acceptable cost. The long transition periods necessary to switch manufacturers and suppliers, would significantly delay Evofem’s timelines, which would materially adversely affect its business, financial conditions, results of operation and prospects.
In addition, Evofem’s reliance on third-party manufacturers exposes Evofem to the following additional risks:
| • | Evofem may be unable to identify manufacturers on acceptable terms or at all; |
| • | Evofem’s third-party manufacturers might be unable to timely formulate and manufacture Evofem’s product or produce the quantity and quality required to meet Evofem’s clinical and commercial needs, if any; |
| • | Contract manufacturers may not be able to execute Evofem’s manufacturing procedures appropriately; |
| • | Evofem’s future third-party manufacturers may not perform as agreed or may not remain in the contract manufacturing business for the time required to supply its clinical trials or to successfully produce, store and distribute its products; |
| • | Manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state agencies to ensure strict compliance with cGMPs and other government regulations and corresponding foreign standards, and Evofem does not have control over third-party manufacturers’ compliance with these regulations and standards; |
| • | Evofem may not own, or may have to share, the intellectual property rights to any improvements made by Evofem’s third-party manufacturers in the manufacturing process for its product candidates; and |
| • | Evofem’s third-party manufacturers could breach or terminate their agreement with Evofem. |
Each of these risks could delay Evofem’s clinical trials, the approval, if any of its product candidates by the FDA or the commercialization of its product candidates or result in higher costs or deprive Evofem of potential product revenue. In addition, Evofem relies on third parties to perform release testing on its product candidates prior to delivery to patients. If these tests are not appropriately conducted and test data are not reliable, patients could be put at risk of serious harm, which could result in product liability suits.
The manufacture of medical products is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of medical products often encounter difficulties in production, particularly in scaling up and validating initial production and absence of contamination. These problems include difficulties with production costs and yields, quality control, including stability of the product, quality assurance testing, operator error, shortages of qualified personnel, as well as compliance with strictly enforced federal, state and foreign regulations. Furthermore, if contaminants are discovered in Evofem’s supply of its product candidates or in the manufacturing facilities, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. Evofem cannot be assured that any stability or other issues relating to the manufacture of its product candidates will not occur in the future. Additionally, Evofem’s manufacturers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or unstable political environments. If Evofem’s manufacturers were to encounter any of these difficulties, or otherwise fail to comply with their contractual obligations, Evofem’s ability to provide its product candidates to patients in clinical trials would be jeopardized. Any delay or interruption in the supply of clinical trial supplies could delay the completion of clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, require Evofem to commence new clinical trials at additional expense or terminate clinical trials completely.
Evofem has no internal distribution capabilities and intends to engage third party distributors for distribution of products outside the United States. The inability to identify, or enter into an agreement with, any such third party distributor, would likely have a material adverse effect on Evofem’s business and operations.
Although Evofem currently plans to market and sell its lead product candidate, Amphora, directly in the United States, Evofem does intend to enter into distribution agreements with one or more distributors of Amphora outside the United States. Evofem currently has not entered into any such distribution agreement with any such distributor, and Evofem cannot guaranty that it will be able to enter into any such distribution agreement on commercially reasonable terms, or at all. If Evofem were to outsource product distribution, including the distribution of its MPT vaginal gel, Amphora or any future product candidate or product, this outsourcing would also be subject to uncertainties related to such distribution services, including the quality of such distribution services. For example, distributors may not have the capacity to supply sufficient product if demand increases rapidly. Further, Evofem would be dependent on the distributors to ensure that the distribution process accords with relevant regulations, which includes, among other things, compliance with current good documentation practices, the maintenance of records and documentation and compliance with other regulations, including, without limitation, the Foreign Corrupt Practices Act. Failure to comply with these requirements could result in significant remedial action, including improvement of facilities, suspension of distribution or recall of product. Additionally, any failure by Evofem to forecast demand for finished product, including Amphora, and failure by Evofem to ensure its distributors have appropriate capacity to distribute such quantities of finished product, could result in an interruption in the supply of certain products and a decline in sales of that product. Further third-party distributors may not perform as agreed or may terminate their agreements with Evofem. Any significant problem that Evofem’s distributors experience could delay or interrupt Evofem’s sale of products in the applicable jurisdiction until the applicable distributor cures the problem or until Evofem identifies and negotiates an acceptable agreement with an alternative distributor, if one is available. Any failure or delay in distributing products would likely have a negative impact on Evofem’s business and operations.
Evofem relies and intends to rely on third-parties for the execution of its development programs for its MPT vaginal gel, its lead product candidate, Amphora, and its potential future product candidates. Failure of these third parties to provide services of a suitable quality and within acceptable timeframes may cause the delay or failure of Evofem’s development programs.
Evofem employs a business model that relies on the outsourcing of certain functions, tests and services to CROs, medical institutions and other specialist providers, including, without limitation, the conduct, management and monitoring of Evofem’s ongoing and planned clinical trials. As a result, Evofem relies on these third parties for, among other things, quality assurance, clinical monitoring, clinical data management and regulatory expertise. In terms of Amphora, Evofem has engaged PAREXEL International Corporation as CRO to run substantially all aspects of the AMPOWER clinical trial. Evofem also intends to engage a CRO for all future clinical trial requirements needed to file for regulatory approvals. There is no assurance that such organizations or individuals will be able to provide the functions, tests or services as agreed upon, or to the requisite quality. Evofem will rely on the efforts of these organizations and individuals and could suffer significant delays in the development of its product or processes should they fail to perform as expected.
There is also no assurance that these third parties will not make errors in, or simply fail to be effective in, the design, management or retention of Evofem’s data or data systems. Any failures by such third parties could lead to a loss of data, which in turn could lead to delays in clinical development and obtaining regulatory approval. Third parties may not pass FDA or other regulatory audits, which could delay or prohibit regulatory approval. In addition, the cost of such services could significantly increase over time. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, regulatory approval of Evofem’s MPT vaginal gel, its lead product candidate, Amphora, or any future product candidates, may be delayed, prevented or cost significantly more than expected, all of which would have a material adverse effect on Evofem’s business, financial conditions, results of operation and prospects.
If Evofem fails to enter into or maintain strategic relationships or collaborations with respect to future product candidates, or if Evofem is unable to realize the potential benefits from such collaborations, its business, financial condition, commercialization prospects and results of operation may be materially adversely affected.
If Evofem is successful in identifying and in-licensing the rights to additional product candidates, Evofem’s expected strategy with respect to the development of any such future product candidates is to supplement internal efforts with third-party collaborations. Evofem faces significant competition in seeking appropriate collaborators. Collaborations are complex and time-consuming arrangements to negotiate and document.
Evofem’s success in entering into a definitive agreement for any collaboration will depend upon, among other things, its assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design and outcomes of the clinical studies, the likelihood of approval by regulatory authorities, the potential market for the product, the costs and complexities of manufacturing and delivering such products to customers, the potential of competing products, the strength of the intellectual property and industry and market conditions generally. The collaborator may also consider alternative products or technologies for similar indications that may be available to collaborate on and whether such collaboration could be more attractive than the one with Evofem for its products or product candidates.
Any potential collaboration agreement into which Evofem might enter may call for licensing or cross-licensing of potentially blocking patents, know-how or other intellectual property. Due to the potential overlap of data, know-how and intellectual property rights, there can be no assurance that one of Evofem’s collaborators will not dispute its right to use, license or distribute such data, know-how or other intellectual property rights, and this may potentially lead to disputes, liability or termination of the collaboration.
Evofem may also be restricted under existing and future collaboration agreements from entering into agreements on certain terms with other potential collaborators and may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If that were to occur, Evofem may have to curtail the development of a particular product, reduce or delay Evofem’s development program, delay commercialization, reduce the scope of sales or marketing activities, or increase expenditures and undertake development or commercialization activities at its own expense. If Evofem elects to fund development or commercialization activities on its own, it will need to obtain additional capital, which may not be available to Evofem on acceptable terms or at all. Absent sufficient funds, Evofem may not be able to commercialize a product candidate. If Evofem enters into a collaboration agreement regarding a product or product candidate, it could be subject to, among other things, the following risks, each of which may materially harm its business, commercialization prospects and financial condition:
| • | Evofem may not be able to control the amount and timing of resources that the collaborator devotes to the product development program; |
| • | Evofem may experience financial difficulties and thus not commit sufficient financial resources to the product development program; |
| • | Evofem may be required to relinquish important rights to the collaborator such as marketing, distribution and intellectual property rights; |
| • | a collaborator could move forward with a competing product developed either independently or in collaboration with third parties, including its competitors; |
| • | a collaborator could terminate the agreement (for convenience if permitted) or for its breach; or |
| • | business combinations or significant changes in a collaborator’s business strategy may adversely affect Evofem’s willingness to complete its obligations under any arrangement. |
As a result, a collaboration may not result in the successful development or commercialization of Evofem’s product candidates.
Evofem enters into various contracts in the normal course of its business in which Evofem indemnifies the other party to the contract. In the event Evofem has to perform under these indemnification provisions, it could have a material adverse effect on its business, financial condition and results of operations.
In the normal course of business, Evofem periodically enters into academic, commercial, service, collaboration, licensing, consulting and other agreements that contain indemnification provisions. With respect to Evofem’s academic and other research agreements, including the Rush License, Evofem typically indemnifies the institution and related parties from losses arising from claims relating to the products, processes or services made, used, sold or performed pursuant to the agreements for which Evofem has secured licenses, and from claims arising from Evofem’s or its sublicensees’ exercise of rights under the agreement. With respect to collaboration agreements, Evofem may have to indemnify its collaborators from any third-party product liability claims that could result from the production, use or consumption of the product, as well as for alleged infringements of any patent or other intellectual property right owned by a third party. With respect to consultants, Evofem indemnifies them from claims arising from the good faith performance of their services.
If Evofem’s obligations under an indemnification provision exceed applicable insurance coverage or if Evofem were denied insurance coverage, Evofem’s business, financial condition and results of operations could be adversely affected. Similarly, if Evofem is relying on a collaborator to indemnify Evofem and the collaborator is denied insurance coverage or the indemnification obligation exceeds the applicable insurance coverage, and if the collaborator does not have other assets available to indemnify Evofem, its business, financial condition and results of operations could be adversely affected.
Risks Related to Commercialization of Evofem’s Product Candidate
Evofem currently has limited marketing and sales experience. If Evofem is unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell its product candidates, Evofem may be unable to generate any revenue.
Although some of its employees may have marketed, launched and sold other pharmaceutical products in the past while employed at other companies, Evofem has no experience selling and marketing its product candidates, and Evofem currently has no marketing or sales organization. To successfully commercialize any products that may result from its development programs, Evofem will need to find one or more collaborators to commercialize its products or invest in and develop these capabilities, either on its own or with others, which would be expensive, difficult and time consuming. Any failure or delay in the timely development of Evofem’s internal commercialization capabilities could adversely impact the potential for success of its products.
If commercialization collaborators do not commit sufficient resources to commercialize Evofem’s future products and Evofem is unable to develop the necessary marketing and sales capabilities on its own, Evofem will be unable to generate sufficient product revenue to sustain or grow its business. Evofem may be competing with companies that currently have extensive and well-funded marketing and sales operations, particularly in the markets its product candidates are intended to address. Without appropriate capabilities, whether directly or through third-party collaborators, Evofem may be unable to compete successfully against these more established companies.
Evofem faces competition from other medical device, biotechnology and pharmaceutical companies and its operating results will suffer if Evofem fails to compete effectively.
The medical device, biotechnology and pharmaceutical industries are intensely competitive. Significant competition among various contraceptive products already exists. Existing products have name recognition, are marketed by companies with established commercial infrastructures and with greater financial, technical and personnel resources than Evofem. In order to compete and gain market share, any new product will need to demonstrate advantages in efficacy, convenience, tolerability or safety. In addition, new products developed by others could emerge as competitors to Amphora, if approved. Such products could offer an alternative form of non-hormonal contraceptive that provides protection over longer periods of time. If Evofem is not able to compete effectively against its current and future competitors, Evofem’s business will not grow and its financial condition and operations will suffer.
Evofem’s potential competitors include large, well-established pharmaceutical companies and specialty pharmaceutical companies. These companies include Merck & Co., Inc., Allergan plc, Teva Pharmaceutical Industries Ltd., Bayer AG, Johnson & Johnson, Cooper and Mylan Inc. Additionally, several generic manufacturers currently market and continue to introduce new generic contraceptives. There are other contraceptive product candidates in development that, if approved, would potentially compete with Amphora, including hormonal patches and hormonal vaginal rings.
Evofem’s MPT vaginal gel, its lead product candidate, Amphora, and any of its future potential product candidates, may not gain acceptance among physicians, patients or the medical community, thereby limiting Evofem’s potential to generate revenue, which will undermine its future growth prospects.
Even if Evofem’s MPT vaginal gel, Amphora or any of its future product candidates are approved for commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of any new product by physicians, health care professionals and third-party payors will depend on a number of factors, including:
| • | demonstrated evidence of efficacy and safety; |
| • | sufficient third-party insurance coverage or reimbursement; |
| • | effectiveness of Evofem’s or its collaborators’ sales and marketing strategy; |
| • | the willingness of uninsured consumers to pay for the product; |
| • | the willingness of pharmacy chains to stock the products; |
| • | the prevalence and severity of any adverse side effects; and |
| • | availability of alternative products. |
If Evofem’s MPT vaginal gel, Amphora or any product candidate that Evofem may license, develop or sell does not provide a benefit over currently available options, that product is unlikely to achieve market acceptance and Evofem will not generate sufficient revenues to achieve profitability.
The success of Evofem’s MPT vaginal gel, its lead product candidate, Amphora, or any future contraceptive product candidate Evofem may seek to develop, will depend on the availability of contraceptive alternatives and women’s preferences, in addition to the market’s acceptance of this specific method of contraception.
The commercial success of Evofem’s MPT vaginal gel, Amphora, or any other future contraceptive product candidate Evofem may seek to develop, will depend upon the contraceptive market as well as market acceptance of this alternative method. Risks related to market acceptance include, among other things:
| • | minimum acceptable contraceptive efficacy rates; |
| • | perceived safety differences of hormonal and/or non-hormonal contraceptive options; |
| • | changes in healthcare laws and regulations, including the PPCA, and its effect on pharmaceutical coverage, reimbursement and pricing, and the birth control mandate; |
| • | competition from new lower dose hormonal contraceptives with more favorable side effect profiles; and |
| • | new generic contraceptive options including a generic version of Amphora as a contraceptive. |
If one or more of these risks occur it could reduce the market potential for Evofem’s MPT vagina gel, Amphora, or any future contraceptive product Evofem may seek to develop, and place pressure on Evofem’s business, financial condition, results of operation and prospects.
If Evofem suffers negative publicity concerning the safety or efficacy of Evofem’s products in development, Evofem’s reputation could be harmed and Evofem may be forced to cease development of such products.
If concerns should arise about the actual or anticipated clinical outcomes regarding the safety of any of Evofem’s product candidates, such concerns could adversely affect the market’s perception of these candidates. Such concerns could lead to a decline in investors’ expectations and a decline in the price of Evofem’s common stock.
Evofem relies, and continues to expect to rely, on market research conducted on its behalf to evaluate the potential commercial acceptance its MPT vaginal gel, lead product candidate, Amphora and other future product candidates.
Evofem has contracted with and expects to continue to contract with third parties to perform market research on its behalf. Based on the results of its market research to date, Evofem believes that Amphora, if approved, would be an attractive alternative to hormonal birth control to certain women. However, these research findings may not be indicative or predictive of actual or overall market acceptance and any future market research may not be indicative of the acceptance for another product candidate or future product candidate Evofem may develop.
The commercial success of Evofem’s MPT vaginal gel, its lead product candidate, Amphora, and any future Evofem product candidates will depend in significant measure on the label claims that the FDA or other regulatory authorities approve for the product.
The commercial success of Evofem’s MPT vaginal gel, its lead product candidate, Amphora, and any of Evofem’s future product candidates will depend in significant measure upon Evofem’s ability to obtain approval from the FDA or other regulatory authorities of labeling describing a product candidate’s expected features or benefits. Failure to achieve approval from the FDA or other regulatory authorities of product labeling containing adequate information on features or benefits will prevent or substantially limit Evofem’s advertising and promotion of such features in order to differentiate Amphora or any future product candidate from those products that already exist in the market. This failure would have a material adverse impact on Evofem’s business, financial condition, results of operation and prospects.
The proportion of the contraceptive market that is made up of generic products continues to increase, making introduction of a branded contraceptive difficult and expensive.
The proportion of the U.S. market that is made up of generic products has been increasing over time. In 2005, generic contraceptive products held 47% of prescription volume and 34% of sales and, by 2011, those values had risen to 68% and 44%, respectively. For the year ended December 31, 2016, approximately 83% of the prescription volume and approximately 43% of sales of combined hormonal contraceptives in the United States were generated by generic products. If this trend continues, it may be more difficult to introduce Amphora, if approved, or any future approved contraceptive product candidate Evofem may develop, as a branded contraceptive, at a price that will maximize its revenue and profits. Also, there may be additional marketing costs to introduce Amphora in order to overcome the trend towards generics and to gain access to reimbursement by payors. If Evofem is unable to introduce Amphora or any future approved contraceptive product candidate at a price that is commensurate with that of current branded contraceptive products, or it is unable to gain reimbursement from payors for Amphora, or if patients are unwilling to pay any price differential between Amphora and a generic contraceptive, its revenues will be limited.
Changes in healthcare laws and regulations may eliminate current requirements that health insurance plans cover and reimburse FDA-cleared or approved contraceptive products without cost sharing, which could reduce demand for products such as Amphora. Even if Amphora is approved for commercialization, Evofem management expects that Evofem’s success will be dependent on the willingness or ability of patients to pay out-of-pocket should they not be able to obtain third party reimbursement or should such reimbursement be limited.
Evofem cannot be certain that third party reimbursement will be available for Amphora, and if reimbursement is available, the amount of any such reimbursement. The Patient Protection and Affordable Care Act of 2010 (the “PPACA”) and subsequent regulations enacted by the Department of Health and Human Services (the “DHHS”) require health plans to provide coverage for women’s preventive care, including all forms of FDA-cleared or approved contraception, without imposing any cost sharing on the plan beneficiary. These regulations ensure that women who wish to use an approved form of contraception may request it from their doctors and their health insurance plan must cover all costs associated with such products. However, after the 2016 election, the U.S. Federal Government is attempting to repeal the PPACA and corresponding regulations, which would likely eliminate the requirement for health plans to cover women’s preventive care without cost sharing. Even if the PPACA is not repealed, the DHHS regulations to specifically enforce the preventive health coverage mandate could be repealed under the Congressional Review Act. Any repeal or elimination of the preventive care coverage rules would mean that women seeking to use prescribed forms of contraceptives may have to pay some portion of the cost for such products out-of-pocket, which could deter some women from using prescription contraceptive products, such as Amphora, at all. As a result, Evofem expects that its success will be dependent on the willingness of patients to pay out-of-pocket for Amphora in the event that either they do not have insurance or their insurance requires payment of a portion of Amphora by the patient, thus increasing the patient’s overall cost to use Amphora. This could reduce market demand for Amphora or any future product candidates Evofem may seek to develop, if and when they receive FDA approval, which would have a material adverse effect on its business, financial conditions, and prospects.
In the event that Evofem is successful in obtaining regulatory approval to market its MPT vaginal gel, its lead product candidate, Amphora, or a future product in the United States, revenues may be adversely affected if the product fails to obtain insurance coverage or adequate reimbursement from third-party payers and administrators in the United States.
Third-party payers and administrators, including state Medicaid programs and Medicare, have recently been challenging the prices charged for pharmaceutical and medical device products. The United States government and other third-party payers are increasingly limiting both coverage and the level of reimbursement for new drugs and medical devices. Third-party insurance coverage may not be available to patients for Amphora or any future product Evofem may seek to commercialize. If such government and other third-party payers do not provide adequate coverage and reimbursement for Amphora or such products, healthcare providers may not prescribe them or patients may ask their healthcare providers to prescribe competing products with more favorable reimbursement.
Managed care organizations and other private insurers frequently adopt their own payment or reimbursement reductions. Consolidation among managed care organizations has increased the negotiating power of these entities. Private third-party payers, as well as governments, increasingly employ formularies to control costs by negotiating discounted prices in exchange for formulary inclusion. Failure to obtain timely or adequate pricing or formulary placement for Evofem’s MPT vaginal gel, Amphora or any future product Evofem may seek to commercialize, or obtaining such pricing or placement at unfavorable pricing levels, could materially adversely affect Evofem’s business, financial conditions, results of operation and prospects.
The pharmaceutical and medical device industries are highly regulated and subject to various fraud and abuse laws, including, without limitation, the U.S. federal Anti-Kickback Statute, the U.S. federal False Claims Act and the U.S. Foreign Corrupt Practices Act.
Healthcare fraud and abuse regulations are complex, and even minor irregularities can potentially give rise to claims that a statute or prohibition has been violated. The laws that may affect Evofem’s ability to operate include, among other things:
| • | the federal healthcare programs’ anti-kickback law, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs; |
| • | false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent; |
| • | the Health Insurance Portability and Accountability Act of 1996, which created federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; and |
| • | the U.S. Foreign Corrupt Practices Act, which prohibits corrupt payments, gifts or transfers of value to non-U.S. officials. |
The scope and enforcement of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Regulatory authorities might challenge Evofem’s current or future activities under these laws. Any such challenge could have a material adverse effect on Evofem’s reputation, business, results of operations and financial condition. In addition, efforts to ensure that Evofem’s business arrangements with third parties will comply with these laws will involve substantial costs. Any investigation of Evofem or the third parties with whom Evofem contracts, regardless of the outcome, would be costly and time consuming.
Evofem’s business may be adversely affected by unfavorable macroeconomic conditions.
Various macroeconomic factors could adversely affect Evofem’s business, its results of operations and financial condition, including changes in inflation, interest rates and foreign currency exchange rates and overall economic conditions and uncertainties, including those resulting from political instability (including workforce uncertainty) and the current and future conditions in the global financial markets. For example, if inflation or other factors were to significantly increase Evofem’s business costs, it may be unable to pass through price increases to patients. The cost of importing similar products from foreign markets may affect Evofem’s sales in any domestic market.
Interest rates and the ability to access credit markets could also adversely affect the ability of patients, payers and distributors to purchase, pay for and effectively distribute Evofem’s product if and when approved. Similarly, these macroeconomic factors could affect the ability of Evofem’s current or potential future third-party manufacturers, sole source or single source suppliers, licensors or licensees to remain in business, or otherwise manufacture or supply Evofem’s product candidate. Failure by any of them to remain in business could affect Evofem’s ability to manufacture Amphora or any of its future product candidates.
Risks Related to Evofem’s Business Operations
As Evofem matures and expands its sales and marketing infrastructure, it will need to expand the size of its organization. If Evofem experiences difficulties in managing this growth or fails to attract and retain management and other key personnel, it may be unable to successfully commercialize its products, develop any product candidates or otherwise implement its business plan.
As of October 20, 2017, Evofem had a total of 23 full-time employees, and uses third-party consultants to assist with research and development activities, including regulatory filings and clinical trial operations and support, sales and marketing research and programs, as well as general and administrative activities. As Evofem’s development and commercialization plans and strategies develop, Evofem expects that it will expand the size of its employee base for managerial, operational, sales, marketing, financial, regulatory affairs and other resources. Future growth would impose significant added responsibilities on members of management, including the need to identify, recruit, maintain, motivate and integrate additional employees. In addition, management may have to divert a disproportionate amount of its attention away from day-to-day activities and devote a substantial amount of time to managing these growth activities, which would lead to disruptions in Evofem’s operations. Evofem cannot provide assurance that it will be able to retain adequate staffing levels to run its operations and/or to accomplish all of the objectives that it otherwise would seek to accomplish.
Evofem’s ability to compete in the highly competitive pharmaceutical and medical device industries depends upon its ability to attract and retain highly qualified managerial and key personnel. Evofem is highly dependent on its senior management, including its President and Chief Executive Officer, Saundra Pelletier, its Chief Financial Officer, Justin J. File, Kelly Culwell, M.D., its Chief Medical Officer and Russell Barrans, its Chief Commercial Officer. The loss of the services of any of these individuals could impede, delay or prevent the development and commercialization of Evofem’s product candidates, hurt its ability to raise additional funds and negatively impact its ability to implement its business plan. If Evofem loses the services of any of these individuals, it might not be able to find suitable replacements on a timely basis or at all, and its business could be harmed as a result. Evofem does not maintain “key man” insurance policies on the lives of these individuals.
Evofem might not be able to attract or retain qualified management and other key personnel in the future due to the intense competition for qualified personnel among biotechnology, medical device, pharmaceutical and other businesses, particularly in the San Diego area where it is headquartered. As a result, Evofem may be required to expend significant financial resources in its employee recruitment and retention efforts, including the grant of significant equity incentive awards which would be dilutive to stockholders. Many of the other companies within the contraceptive industry with whom Evofem competes for qualified personnel have greater financial and other resources, different risk profiles and longer histories in the industry than Evofem does. They also may provide more diverse opportunities and better chances for career advancement. If Evofem is not able to attract and retain the necessary personnel to accomplish its business objectives or if Evofem is not able to effectively manage any future growth, it may experience constraints that will harm its ability to implement its business strategy and achieve its business objectives.
Evofem’s current or future employees, principal investigators, consultants and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards.
Evofem may become exposed to the risk of employees, independent contractors, principal investigators, consultants, suppliers, commercial partners or vendors engaging in fraud or other misconduct. Misconduct by employees, independent contractors, principal investigators, consultants, suppliers, commercial partners and vendors could include intentional failures such as failures: (i) to comply with FDA or other regulators’ regulations, (ii) to provide accurate information to such regulators or (iii) to comply with manufacturing standards established by Evofem and/or required by law. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws, regulations and industry guidance intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Misconduct by current or future employees, independent contractors, principal investigators, consultants, suppliers, commercial partners and vendors could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory or civil sanctions and serious harm to Evofem’s reputation. It is not always possible to identify and deter misconduct by employees, independent contractors, principal investigators, consultants, suppliers, commercial partners and vendors, and the precautions Evofem takes to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses, or in protecting it from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against Evofem, and it is not successful in defending or asserting Evofem’s rights, those actions could have a significant adverse impact on its business, including the imposition of significant fines or other sanctions, and its reputation.
Evofem may be vulnerable to disruption, damage and financial obligations as a result of information technology system failures.
Despite the implementation of security measures, any of the internal computer systems belonging to Evofem or its third-party service providers are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war, and telecommunication and electrical failure. Any system failure, accident, security breach or data breach that causes interruptions in its own or in third-party service vendors’ operations could result in a material disruption of its product development programs. For example, the loss of clinical study data from future clinical studies could result in delays in its or its partners’ regulatory approval efforts and significantly increase its costs in order to recover or reproduce the lost data. Further, Evofem’s information technology and other internal infrastructure systems, including firewalls, servers, leased lines and connection to the Internet, face the risk of systemic failure, which could disrupt its operations. To the extent that any disruption or security breach results in a loss or damage to Evofem’s data or applications, or inappropriate disclosure of confidential or proprietary information, it may incur resulting liability, its product development programs and competitive position may be adversely affected and the further development of its products may be delayed. Furthermore, Evofem may incur additional costs to remedy the damage caused by these disruptions or security breaches.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
| Exhibit Number |
Description | |
| 99.1 | Press release dated January 17, 2018 | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| EVOFEM BIOSCIENCES, INC. | ||||
| Date: January 17, 2018 | By: | /s/ Saundra Pelletier | ||
| Name: Saundra Pelletier | ||||
| Title: Chief Executive Officer | ||||
