Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - Diffusion Pharmaceuticals Inc. | ex_103105.htm |
| EX-5.1 - EXHIBIT 5.1 - Diffusion Pharmaceuticals Inc. | ex_103038.htm |
| EX-4.11 - EXHIBIT 4.11 - Diffusion Pharmaceuticals Inc. | ex_103052.htm |
| EX-4.10 - EXHIBIT 4.10 - Diffusion Pharmaceuticals Inc. | ex_103126.htm |
| EX-1.1 - EXHIBIT 1.1 - Diffusion Pharmaceuticals Inc. | ex_103060.htm |
As filed with the Securities and Exchange Commission on January 16, 2018
Registration No. 333-222203
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment no. 1 to
FORM S-1
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
DIFFUSION PHARMACEUTICALS INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
2834 |
|
30-0645032 |
|
(State or other jurisdiction of |
|
(Primary Standard Industrial |
|
(I.R.S. Employer |
1317 Carlton Avenue, Suite 200
Charlottesville, VA 22902
(434) 220-0718
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
David G. Kalergis
Chief Executive Officer
1317 Carlton Avenue, Suite 200
Charlottesville, VA 22902
(434) 220-0718
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With a Copy to:
|
David S. Rosenthal, Esq. |
Robert F. Charron, Esq. Ellenoff Grossman & Schole LLP 1345 Avenue of the Americas New York, New York 10105 (212) 370-1300 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended (the “Securities Act”), check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non-accelerated filer ☐ (Do not check if a smaller reporting company) | Smaller reporting company ☒ |
| Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act.☐
CALCULATION OF REGISTRATION FEE
|
Title of each class of securities to be registered |
Proposed maximum |
Amount of |
||||||
|
Common Stock, $0.001 par value per share (2) |
$ | 14,950,000 | $ | 1,861.28 | ||||
|
Warrants to purchase shares of Common Stock (3) |
--- | --- | ||||||
|
Common stock issuable upon exercise of warrants to purchase Common Stock (2) |
14,950,000 | 1,861.28 | ||||||
| Underwriters' Warrant to purchase shares of Common Stock (3) | ||||||||
| Common stock issuable upon exercise of underwriter's warrant to purchase Common Stock (4) | 934,375 | 116.33 | ||||||
|
Total |
$ | 30,834,375 | $ | 3,838.88 | (5) | |||
|
(1) |
Estimated solely for the purpose of calculating the amount of the registration fee in accordance with Rule 457(o) under the Securities Act. Includes the offering price of additional shares of Common Stock and warrants to purchase Common Stock that the underwriter has the option to purchase. |
|
(2) |
Pursuant to Rule 416 under the Securities Act, the securities being registered hereunder include such indeterminable number of additional shares of common stock as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions. |
|
(3) |
No additional registration fee is payable pursuant to Rule 457(g) under the Securities Act. |
| (4) | Represents warrants to purchase a number of shares of common stock equal to 5% of the number of shares of Common Stock being offered at an exercise price equal to 125% of the public offering price. |
|
(5) |
$1,861.28 previously paid by Registrant. |
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment that specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to completion, dated January 16, 2018
PRELIMINARY PROSPECTUS

9,154,930 Shares of Common Stock
Warrants to Purchase up to 9,154,930 Shares of Common Stock
We are offering 9,154,930 shares of our common stock and warrants to purchase up to 9,154,930 shares of common stock and the shares of common stock that are issuable from time to time upon exercise of the warrants. The shares of common stock and the warrants will be sold in fixed combination, with one warrant to purchase one share of common stock accompanying each share of common stock sold. On January 12, 2018, the last reported sale price of our common stock on the Nasdaq Capital Market was $1.42 per share. The public offering price per share of common stock and accompanying warrant will be determined between us and the underwriter at the time of pricing, and may be at a discount to the then current market price.
Each warrant will have an exercise price of $ per share of common stock, will be exercisable upon issuance and will expire five years from the date of issuance. The shares of common stock and the warrants are immediately separable and will be issued separately, but must be purchased together in this offering.
Our common stock is listed on the Nasdaq Capital Market under the symbol “DFFN.” There is no established trading market for the warrants and we do not expect a market to develop. In addition, we do not intend to list the warrants on the Nasdaq Capital Market, any other national securities exchange or any other trading system.
Investing in our securities involves a high degree of risk. See the section entitled “Risk Factors” beginning on page 4 of this prospectus and in the documents incorporated by reference into this prospectus for a discussion of risks that should be considered in connection with an investment in our securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
|
|
|
Per Share and Warrant (1) |
|
Total |
|
|
Public offering price |
|
$ |
|
$ |
|
|
Underwriting discounts and commissions(2) |
|
$ |
|
$ |
|
|
Proceeds, before expenses, to us (3) |
|
$ |
|
$ |
|
_________
| (1) | The purchase price per fixed combination of one share of common stock and one warrant to purchase one share of common stock is allocated as $____ per share and $____ per warrant. |
|
(2) |
In addition, we have agreed to reimburse the underwriter for certain expenses and to issue common stock purchase warrants to the underwriter. See “Underwriting” for additional information. |
|
(3) |
We estimate the total expenses of this offering payable by us, excluding the underwriting discounts and commissions, will be approximately $350,000. |
The offering is being underwritten on a firm commitment basis. We have granted the underwriter an option for a period of 30 days from the date of this prospectus to purchase up 1,373,239 additional shares of our common stock (15% of the number of shares issued in this offering) at a price per share equal to the public offering price per share and/or up to 1,373,239 additional warrants (15% of the warrants issued in the offering), at the public offering price per warrant, in any combination thereof, less the underwriting discounts and commissions.
The securities are expected to be delivered to purchasers on or about , 2018.
Sole Book-Running Manager
H.C. Wainwright & Co.
The date of this prospectus is , 2018
TABLE OF CONTENTS
|
|
|
Page |
||
|
Prospectus Summary |
|
|
6 |
|
|
The Offering |
9 |
|||
|
Risk Factors |
|
|
10 |
|
|
Special Note Regarding Forward-Looking Statements |
|
|
13 |
|
|
Use of Proceeds |
|
|
15 |
|
|
Capitalization |
16 |
|||
|
Dilution |
18 |
|||
|
Description of Capital Stock |
|
|
20 |
|
|
Market Price of our Common Stock |
23 |
|||
|
Underwriting |
|
|
25 |
|
| Material U.S. Federal Income Tax Considerations | 28 | |||
| Executive Compensation | 34 | |||
| Director Compensation | 39 | |||
|
Legal Matters |
|
|
41 |
|
|
Experts |
|
|
41 |
|
|
Where You Can Find Additional Information |
|
|
41 |
|
|
Incorporation of Certain Information by Reference |
|
|
41 |
|
ABOUT THIS PROSPECTUS
You should rely only on the information contained or incorporated by reference in this prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus. You should not assume that the information in this prospectus is accurate at any date other than the date indicated on the cover page of this prospectus or the filing date of any document incorporated by reference, regardless of its time of delivery. We are offering to sell shares of common stock and warrants and seeking offers to buy shares of common stock and warrants only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of the common stock and warrants.
For investors outside the United States: neither we nor the underwriter have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside the United States.
PROSPECTUS SUMMARY
This summary highlights information contained in other parts of this prospectus or incorporated by reference into this prospectus from our filings with the Securities and Exchange Commission (the “SEC”). As it is only a summary, it does not contain all of the information that you should consider before purchasing our securities in this offering and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere or incorporated by reference into this prospectus. You should read the entire prospectus, the registration statement of which this prospectus is a part, and the information incorporated by reference herein in their entirety, including the “Risk Factors” and our financial statements and the related notes contained in and incorporated by reference into this prospectus, before purchasing our securities in this offering. Unless otherwise mentioned or unless the context requires otherwise, all references in this prospectus to “Diffusion,” “we,” “our,” “us” or similar references mean Diffusion Pharmaceuticals Inc.
Diffusion Pharmaceuticals Inc.
Business Overview
We are a clinical stage biotechnology company focused on extending the life expectancy of cancer patients by improving the effectiveness of current standard-of-care treatments, including radiation therapy and chemotherapy. We are developing our lead product candidate, transcrocetinate sodium, also known as trans sodium crocetinate (“TSC”), for use in the many cancer types in which tumor oxygen deprivation (“hypoxia”) is known to diminish the effectiveness of current treatments. TSC is designed to target the cancer’s hypoxic micro-environment, re-oxygenate treatment-resistant tissue and make the cancer cells more susceptible to the therapeutic effects of standard-of-care radiation therapy and chemotherapy.
Our lead development programs target TSC against cancers known to be inherently treatment-resistant, with a focus on brain cancers. A Phase 1/2 clinical program, completed in the fourth quarter of 2015, evaluated 59 patients with newly diagnosed glioblastoma multiforme (“GBM”), a particularly deadly form of primary brain cancer. This open label, historically controlled study demonstrated a favorable safety and efficacy profile for TSC combined with standard of care, including a 36% improvement in overall survival over the control group at two years. A strong efficacy signal was seen in the inoperable patients, where survival of TSC-treated patients at two years was increased by almost four-fold over the controls. The U.S. Food and Drug Administration, or FDA, has provided Diffusion with final protocol guidance for a Phase 3 trial of TSC in this newly diagnosed inoperable GBM patient population. The Company has responded to all outstanding points raised by the FDA and on December 27, 2017 announced the trial has opened for enrollment under the protocol permitted by the FDA. The trial will enroll 236 patients in total, 118 in each arm. Due to its novel mechanism of action, TSC has safely re-oxygenated a range of tumor types in our preclinical and clinical studies. Diffusion believes its therapeutic potential is not limited to one specific tumor type, thereby making it potentially useful to improve standard-of-care treatments of other life-threatening cancers. Given TSC's safety profile and animal data, we can, with appropriate funding, move directly into Phase 2 studies in other cancers. We also believe that TSC has potential application in other indications involving hypoxia, such as neurodegenerative diseases and emergency medicine. For example, a stroke program is now under discussion with doctors from UCLA and the University of Virginia, with whom we have established a joint team dedicated to developing a program to test TSC in the treatment of stroke, with an in-ambulance trial of TSC in stroke under consideration. Planning for such a trial is on-going.
In addition to the TSC programs, we are exploring alternatives regarding how best to capitalize upon our product candidate RES-529, which may include possible out-licensing and other options. RES-529 is a novel PI3K/Akt/mTOR pathway inhibitor which has completed two Phase 1 clinical trials for age-related macular degeneration and has been studied preclinically in oncology, specifically GBM. RES-529 has shown activity in both in vitro and in vivo glioblastoma animal models and has been demonstrated to be orally bioavailable and can cross the blood-brain barrier.
Summary of Current Product Candidate Pipeline
The following tables, as of September 30, 2017, summarize the targeted clinical indications for Diffusion’s lead molecule, TSC:
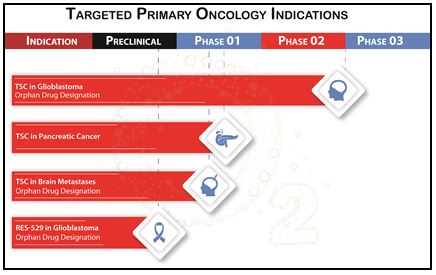

TSC eligible for Phase 2 studies based on preclinical and clinical data from prior Phase 1 and Phase 2 clinical trials, including GBM and Peripheral Artery Disease.
Potential Conversion of Series A Preferred Stock
If we receive at least $10 million in aggregate gross proceeds from this offering, substantially concurrently with the completion of this offering, our Series A Preferred Stock will mandatorily convert into the applicable number of shares of our common stock pursuant to the terms set forth in our Certificate of Incorporation, as amended. Unless otherwise indicated, all information contained in this prospectus assumes the mandatory conversion of all outstanding shares of Series A Preferred Stock into 11,534,649 shares (the “Conversion Shares”) of common stock (including accrued dividends paid in kind and the “make-whole” adjustment feature thereof) pursuant to the terms thereof, which will occur substantially concurrently with the completion of this offering provided that we receive at least $10 million in aggregate gross proceeds from this offering. See “Description of Capital Stock.”
For purposes of calculating an assumed number of Conversion Shares we have assumed a public offering price of $1.42 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on January 12, 2018) and that the Series A Preferred Stock is converted on January 16, 2018.
There is no assurance that we will receive $10 million of aggregate gross proceeds from this offering, in which case the Series A Preferred Stock would not mandatorily convert into shares of common stock. See “Risk Factors -- If we do not receive at least $10 million of aggregate gross proceeds from this offering, the Series A Preferred Stock will remain outstanding.”
Corporate Information
We are a Delaware corporation that was incorporated in June 2015. Prior to June 2015, we were a Nevada corporation. We maintain our principal executive offices at 1317 Carlton Avenue, Suite 200, Charlottesville, VA 22902. Our telephone number there is (434) 220-0718. The address of our website is www.diffusionpharma.com. The information set forth on, or connected to, our website is expressly not incorporated by reference into, and does not constitute a part of, this prospectus.
We are a “smaller reporting company” as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and have elected to take advantage of certain of the scaled disclosure available for smaller reporting companies in this prospectus as well as our filings under the Exchange Act.
THE OFFERING
| Common stock offered by us in this offering | 9,154,930 shares. |
| Warrants offered by us | Warrants to purchase up to 9,154,930 shares, which may be exercised beginning on their date of issuance. The warrants are exercisable until the five year anniversary of the original issuance date. The warrants have an exercise price of $ per share of common stock, subject to adjustment. There is no established trading market for the warrants and we do not expect a market to develop. In addition, we do not intend to list the warrants on the Nasdaq Capital Market, any other national securities exchange or any other trading system. |
| For more information, see the section entitled "Description of Capital Stock" on page 20 of this prospectus. | |
| This prospectus also relates to the offering of the common stock issuable upon the exercise of the warrants. | |
| Option to purchase additional shares and warrants | The underwriter has a 30-day option to purchase up to an amount of additional shares of our common stock equal to 15% of the number of shares and/or 15% of the number of warrants issued in this offering, in any combination thereof, at a price equal to the public offering price per share and per warrant, respectively, less the underwriting discounts and commissions. |
| Use of proceeds | We intend to use the net proceeds from this offering to fund research and development of our lead product candidate, TSC, including clinical trial activities, and for general corporate purposes. See “Use of Proceeds.” |
| Risk factors | You should read the “Risk Factors” section of this prospectus for a discussion of certain of the factors to consider carefully before deciding to purchase any shares of our common stock in this offering. |
| National Securities Exchange Listing | Our common stock is listed on the Nasdaq Capital Market under the symbol “DFFN.” |
The number of shares of our common stock to be outstanding after this offering is based on 14,503,976 shares of common stock outstanding as of September 30, 2017 and excludes as of that date:
|
• |
213,879 shares issuable upon conversion of convertible promissory notes in an aggregate principal amount of $0.6 million; |
|
• |
2,545,989 shares of common stock issuable upon the exercise of outstanding stock options under the Diffusion Pharmaceuticals Inc. 2015 Equity Incentive Plan, as amended (the “2015 Equity Plan”), at a weighted-average exercise price of $7.34 per share; |
|
• |
13,555,887 shares of common stock issuable upon the exercise of outstanding warrants issued in connection with the Series A Preferred Stock private placement, at a weighted-average exercise price of $2.22 per share; |
|
• |
447,721 shares of common stock issuable upon the exercise of other outstanding warrants, at a weighted-average exercise price of $44.52 per share; |
|
• |
114,291 shares of common stock reserved for future issuance under the 2015 Equity Plan: |
|
| • |
shares of common stock issuable upon exercise of the warrants offered hereby; and |
|
| • |
shares of common stock issuable upon the exercise of the underwriter’s warrant. |
|
Unless otherwise indicated, all information contained in this prospectus assumes (i) no exercise by the underwriter of its option to purchase additional shares of common stock and/or warrants and (ii) the mandatory conversion of all outstanding Series A Preferred Stock (including accrued dividends paid in kind and the “make-whole” adjustment feature thereof), which will occur substantially concurrently with the completion of this offering provided that we receive aggregate gross proceeds of at least $10 million from this offering. See “Description of Capital Stock.”
RISK FACTORS
Investing in our common stock and warrants involves a high degree of risk. You should carefully consider the risks described below, together with the other information contained in this prospectus, including our financial statements and the related notes appearing at the end of this prospectus, before making your decision to invest in shares of our common stock and warrants. We cannot assure you that any of the events discussed in the risk factors below will not occur. These risks could have a material and adverse impact on our business, results of operations, financial condition and cash flows, and our future prospects would likely be materially and adversely affected. If that were to happen, the trading price of our common stock and warrants could decline, and you could lose all or part of your investment. In addition, you should also carefully consider the other risks described under the heading “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2016 and our Quarterly Reports on Form 10-Q for the periods ended March 31, 2017, June 30, 2017 and September 30, 2017, which are incorporated herein by reference .
Risks Related to this Offering
We have significant discretion over the use of the net proceeds from this offering.
Our net proceeds from this offering are expected to be approximately $11.5 million. We intend to use the net proceeds of this offering to fund research and development of our lead product candidate, TSC, including clinical trial activities, and for general corporate purposes. Accordingly, our management will have broad discretion as to the application of such proceeds. The proceeds shall be used to carry out our business plan, pay salaries to our employees, and satisfy all our expenses, foreseeable and unforeseeable. As is the case with any business, it should be expected that certain expenses unforeseeable to management at this juncture will arise in the future. There can be no assurance that management’s use of proceeds generated through this offering will prove optimal or translate into revenue or profitability for the Company. Investors are urged to consult with their attorneys, accountants and personal investment advisors prior to making any decision to invest in the Company.
Even if this offering is successful, we will need to raise additional capital in the future to continue operations, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
To date, we have not sold, or received approval to sell, any pharmaceutical products. We do not expect to sell any pharmaceutical products for at least the next several years, if at all. Our net losses were approximately $18.0 million, and $6.7 million for the years ended December 31, 2016 and 2015, respectively. As of September 30, 2017 and December 31, 2016, we had incurred cumulative net losses totaling approximately $63.3 million and $60.2 million, respectively. Moreover, we expect that our net losses will continue and may increase for the foreseeable future. We may not be able to achieve projected results if we generate lower revenues (if any) or receive lower investment income than expected, or we incur greater expenses than expected, or all of the above. We may never generate sufficient product revenue (if any) to become profitable. We also expect to have quarter-to-quarter fluctuations in revenues, expenses, and losses, some of which could be significant.
We estimate that we will receive net proceeds of approximately $11.5 million from the sale of the securities offered by us in this offering, based on the assumed public offering price of $1.42 per share of common stock and warrant (the last reported sale price of our common stock on the NASDAQ Capital Market on January 12, 2018), and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. In the event of a decrease in the net proceeds to us from this offering as a result of a decrease in the assumed public offering price or the number of shares offered by us, we may need to raise additional capital sooner than we anticipate or may need to scale back or eliminate certain of our development programs.
We will need to raise more money to complete our planned clinical trials, continue the research and development necessary to bring our products to market and to establish marketing and additional manufacturing capabilities. We may seek additional funds through public and private stock offerings, government contracts and grants, arrangements with corporate collaborators, borrowings under lines of credit or other sources. However, we may not be able to raise additional funds on favorable terms, or at all. Conditions in the credit markets and the financial services industry may make equity and debt financing more difficult to obtain, and may negatively impact our ability to complete financing transactions. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants, such as limitations on our ability to incur additional indebtedness and other operating restrictions that could adversely impact our ability to conduct our business.
If we are unable to obtain additional funds, we may have to scale back our development of new products, reduce our workforce, license to others products or technologies that we otherwise would seek to commercialize ourselves or, ultimately, cease operations. The amount of money we may need would depend on many factors, including:
|
• |
The costs and progress of our research and development programs; |
|
• |
The scope and results of our preclinical studies and clinical trials; |
|
• |
The amount of our legal expenses and any settlement or damages payments associated with litigation; and |
|
• |
The time and costs involved in: obtaining necessary regulatory approvals; filing, prosecuting and enforcing patent claims; scaling up our manufacturing capabilities; and the commercial arrangements we may establish. |
An investment in our securities is speculative and there can be no assurance of any return on any such investment.
An investment in our securities is speculative and there is no assurance that investors will obtain any return on their investment. Investors will be subject to substantial risks involved in an investment in the Company, including the risk of losing their entire investment.
If you purchase our common stock and warrants in this offering, you will incur immediate and substantial dilution in the book value of your shares, including as a result of the “make-whole” feature of the Series A Preferred Stock.
The public offering price in this offering is substantially higher than the net tangible book value per share of our common stock. Investors purchasing common stock in this offering will pay a price per share that substantially exceeds the book value of our tangible assets after subtracting our liabilities. As a result, investors purchasing common stock in this offering will incur immediate dilution of $0.87 per share, based on the assumed public offering price of $1.42 per share. This dilution also reflects the reclassification of $16.3 million common stock warrant liability to stockholders’ equity as a result of the amendment of our certificate of incorporation on November 1, 2017 with respect to the dividend feature of the Series A Preferred Stock and the issuance of additional shares to the holders of our Series A Preferred Stock pursuant to the “make-whole” feature thereof (assuming the public offering price is less than $2.02 per share). See “Description of Capital Stock.”
As a result of the dilution to investors purchasing shares in this offering, investors may receive significantly less than the purchase price paid in this offering, if anything, in the event of our liquidation. For a further description of the dilution that you will incur as a result of purchasing shares in this offering, see "Dilution."
There may be future sales of our securities or other dilution of our equity, which may adversely affect the market price of our common stock.
We are generally not restricted from issuing additional common stock, including any securities that are convertible into or exchangeable for, or that represent the right to receive, common stock. The market price of our common stock could decline as a result of sales of common stock or securities that are convertible into or exchangeable for, or that represent the right to receive, common stock after this offering or the perception that such sales could occur.
If we cannot continue to satisfy the Nasdaq Capital Market continued listing standards and other Nasdaq rules, our common stock could be delisted, which would harm our business, the trading price of our common stock, our ability to raise additional capital and the liquidity of the market for our common stock.
Our common stock is currently listed on the Nasdaq Capital Market. To maintain the listing of our common stock on the Nasdaq Capital Market, we are required to meet certain listing requirements, including, among others, either: (i) a minimum closing bid price of $1.00 per share, a market value of publicly held shares (excluding shares held by our executive officers, directors and 10% or more stockholders) of at least $1 million and stockholders’ equity of at least $2.5 million; or (ii) a minimum closing bid price of $1.00 per share, a market value of publicly held shares (excluding shares held by our executive officers, directors and 10% or more stockholders) of at least $1 million and a total market value of listed securities of at least $35 million.
There is no assurance that we will continue to meet the minimum closing price requirement and other listing requirements. In the event that our common stock is delisted from Nasdaq and is not eligible for quotation or listing on another market or exchange, trading of our common stock and warrants could be conducted only in the over-the-counter market or on an electronic bulletin board established for unlisted securities such as the Pink Sheets or the OTC Bulletin Board. In such event, it could become more difficult to dispose of, or obtain accurate price quotations for, our common stock and warrants, and there would likely also be a reduction in our coverage by securities analysts and the news media, which could cause the price of our common stock to decline further.
If we do not receive aggregate gross proceeds of at least $10 million from this offering, the Series A Preferred Stock will remain outstanding.
If we receive aggregate gross proceeds of at least $10 million from this offering, substantially concurrently with the completion of this offering, the Series A Preferred Stock will mandatorily convert into the applicable number of shares of our common stock pursuant to the terms set forth in our Certificate of Incorporation, as amended (“Certificate of Incorporation”). See “Description of Capital Stock.” However, if at least $10 million of aggregate gross proceeds are not received from this offering, the Series A Preferred Stock will not mandatorily convert into shares of our common stock and the outstanding shares of Series A Preferred Stock will rank senior to the shares of common stock in the event of a liquidation, dissolution or winding up of the Company. In the event of a Liquidation Event (as defined in the Certificate of Incorporation), the holders of the Series A Preferred Stock shall be entitled to receive, out of the assets of the Company or proceeds thereof legally available therefor, an amount in cash equal to 100% of the stated value of the Series A Preferred Stock before any payment or distribution of the assets of the Company is made or set apart for the holders of the common stock.
The warrants are a risky investment. You may not be able to recover your investment in the warrants, and the warrants may expire worthless.
Whether the warrants will have any value will depend on, among other things, the development of our clinical product candidates, our ability to commercialize our product candidates if approved, our business development efforts and market conditions generally, which conditions will depend on factors related and unrelated to the success of our product candidates and our business development efforts, and cannot be predicted at this time.
If our common stock price does not increase to an amount sufficiently above the exercise price of the warrants during the period the warrants are exercisable, you will be unable to recover any of your investment in the warrants. There can be no assurance that any of the factors that could impact the trading price of our common stock will result in the trading price increasing to an amount that will exceed the exercise price or the price required for you to achieve a positive return on your investment in the warrants.
Holders of the warrants will have no voting rights as common stockholders until they acquire our common stock.
Until you acquire shares of our common stock upon exercise of the warrants, you will have no voting rights with respect to our common stock issuable upon exercise of the warrants. Upon exercise of your warrants, you will be entitled to exercise all the voting rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
Significant holders or beneficial holders of our common stock may not be permitted to exercise warrants that they hold.
The warrants being offered hereby will prohibit a holder from exercising its warrants if doing so would result in such holder (together with such holder's affiliates and any other persons acting as a group together with such holder or any of such holder's affiliates) beneficially owning more than 4.99% of our common stock outstanding immediately after giving effect to the exercise, provided that, at the election of a holder and notice to us, such beneficial ownership limitation shall be 9.99% of our common stock outstanding immediately after giving effect to the exercise. As a result, if you hold a significant amount of our securities, you may not be able to exercise your warrants for shares of our common stock, in whole or in part, at a time when it would be financially beneficial for you to do so.
There is no public market for the warrants to purchase shares of common stock being offered in this offering.
There is no established public trading market for the warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the warrants on any national securities exchange or other trading system, including the Nasdaq Capital Market. Without an active market, the liquidity of the warrants will be limited.
Changes in tax laws and regulations or in our operations may impact our effective tax rate and may adversely affect our business, financial condition and operating results.
Changes in tax laws in any of the jurisdictions in which we operate, or adverse outcomes from tax audits that we may be subject to in any of the jurisdictions in which we operate, could result in an unfavorable change in our effective tax rate, which could adversely affect our business, financial condition and operating results.
In that regard, the U.S. government recently enacted comprehensive tax legislation that includes significant changes to the taxation of business entities, including corporations. These changes include, among others, a permanent reduction to the corporate income tax rate, and a partial limitation on the deductibility of business interest expense. Notwithstanding the reduction in the corporate income tax rate, the overall impact of this tax reform is uncertain, and our business and financial condition could be adversely affected. This prospectus does not discuss any such tax legislation or the manner in which it might affect purchasers of our common stock. We urge our stockholders to consult with their legal and tax advisors with respect to any such legislation and the potential tax consequences of investing in our common stock.
We may be limited in our ability to utilize, or may not be able to utilize, net operating loss carryforwards to reduce our future tax liability as a result of this offering.
As disclosed in our most recent Annual Report on Form 10-K, under Section 382 of Internal Revenue Code of 1986, as amended (the “Code”), if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss (“NOL”) carryforwards and other pre-change tax attributes (such as research tax credits) to offset its post-change income may be limited.
We believe that our recent merger, and other transactions that have occurred, including the offering of our Series A Preferred Stock, triggered an “ownership change” limitation that significantly limited our ability to utilize our NOL carryforwards. This offering may, alone or in conjunction with other changes in our stock ownership that we cannot control, result in an additional ownership change for purposes of Section 382. If we undergo an additional ownership change, our ability to use our NOL carryforwards would become subject to certain limitations, although the extent of such limitations cannot presently be known. We may also experience ownership changes in the future as a result of subsequent changes in our stock ownership, which may be outside of our control. As a result, if we earn net taxable income, our ability to use our NOL carryforwards determined on the date of our ownership change to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us.
Investment in the warrants and our common stock has numerous tax consequences.
There are numerous tax consequences to investors as a result of their investment in the Company. We encourage investors to seek advice from competent tax advisors as to the consequences of an investment in our warrants and common stock (See “Material U.S. Federal Income Tax Consequences”).
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the other information and documents incorporated by reference herein include forward-looking statements. We may, in some cases, use terms such as “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Forward-looking statements appear in and are incorporated by reference into a number of places throughout this prospectus and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned preclinical development and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our intellectual property position, the degree of clinical utility of our products, particularly in specific patient populations, our ability to develop commercial functions, expectations regarding clinical trial data, our results of operations, cash needs, financial condition, liquidity, prospects, growth and strategies, the industry in which we operate and the trends that may affect the industry or us.
By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics and industry change, and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in or incorporated by reference into this prospectus, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in and incorporated by reference into this prospectus. In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in and incorporated by reference into this prospectus, they may not be predictive of results or developments in future periods.
Actual results could differ materially from our forward-looking statements due to a number of factors, including risks related to:
|
• |
our ability to obtain additional financing, including the overhang and restrictive provisions of the Series A convertible preferred stock and related warrants; |
|
• |
our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; |
|
• |
our ability to continue as a going concern; |
|
• |
obtaining and maintaining intellectual property protection for our product candidates and our proprietary technology; |
|
• |
the success and timing of our preclinical studies and clinical trials; |
|
• |
the difficulties in obtaining and maintaining regulatory approval of our products and product candidates, and the labeling under any approval we may obtain; |
|
• |
our failure to recruit or retain key scientific or management personnel or to retain our executive officers; |
|
• |
the accuracy of our estimates of the size and characteristics of the potential markets for our product candidates and our ability to serve those markets; |
|
• |
regulatory developments in the United States and foreign countries; |
|
• |
our ability to operate our business without infringing the intellectual property rights of others; |
|
• |
recently enacted and future legislation regarding the healthcare system; |
|
• |
our ability to satisfy the continued listing requirements of the Nasdaq Capital Market or any other exchange that our securities may trade on in the future; |
|
• |
our plans and ability to develop and commercialize our product candidates; |
|
• |
the rate and degree of market acceptance of any of our product candidates; |
|
• |
the success of competing products that are or may become available; and |
|
• |
the performance of third parties, including contract research organizations and manufacturers. |
You should also read carefully the factors described in the “Risk Factors” section contained in this prospectus and incorporated by reference herein from our Annual Report on Form 10-K filed with the SEC on March 31, 2017, as amended, our Quarterly Reports on Form 10-Q and our other public filings to better understand the risks and uncertainties inherent in our business and underlying any forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements contained in or incorporated by reference into this prospectus will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all.
Any forward-looking statements that we make in or incorporate by reference into this prospectus speak only as of the date of such statement, and, except as required by applicable law, we undertake no obligation to update such statements to reflect events or circumstances after the date of this prospectus or to reflect the occurrence of unanticipated events. Comparisons of results for current and any prior periods are not intended to express any future trends or indications of future performance, unless expressed as such, and should only be viewed as historical data.
For all forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
USE OF PROCEEDS
We estimate that we will receive net proceeds of approximately $11.5 million (or approximately $13.3 million if the underwriter’s option to purchase additional shares of common stock and/or warrants is exercised in full) from the sale of the securities offered by us in this offering, based on the assumed combined public offering price of $1.42 per share of common stock and warrant (the last reported sale price of our common stock on the Nasdaq Capital Market on January 12, 2018), and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
A $0.10 increase (decrease) in the assumed combined public offering price of $1.42 per share of common stock and warrant would increase (decrease) the net proceeds to us from this offering by approximately $1.1 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
Similarly, a one million share increase (decrease) in the number of shares of common stock and accompanying warrants offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us by approximately $1.3 million, assuming the assumed combined public offering price of $1.42 per share of common stock and warrant remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We currently intend to use the net proceeds from this offering to fund research and development of our lead product candidate, TSC, including clinical trial activities, and for general corporate purposes. See “Risk Factors” for a discussion of certain risks that may affect our intended use of the net proceeds from this offering, including that we will need to raise additional capital in the future to complete the development of TSC and our other product candidates.
Our expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot currently allocate specific percentages of the net proceeds that we may use for the purposes specified above, and we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering, or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual use of the net proceeds will vary depending on numerous factors, including our ability to obtain additional financing, the progress, cost and results of our preclinical and clinical development programs, and whether we are able to enter into future licensing or collaboration arrangements. We may find it necessary or advisable to use the net proceeds for other purposes, and our management will have broad discretion in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the net proceeds from this offering.
Pending the use of the net proceeds from this offering, we intend to invest the net proceeds in short-term, investment-grade, interest-bearing securities or certificates of deposit.
CAPITALIZATION
The following table sets forth cash and capitalization as of September 30, 2017:
|
● |
on an actual historical basis for Diffusion; |
|
● |
on a pro forma basis to give effect to the reclassification of $16.3 million common stock warrant liability to stockholders’ equity as a result of the amendment of our certificate of incorporation on November 1, 2017 with respect to the dividend feature of the Series A Preferred Stock and resulting accounting adjustments; |
|
● |
on a pro forma, as adjusted basis to give effect to: |
|
o |
the assumed issuance and sale of shares of common stock and warrants in this offering at the assumed public offering price of $1.42 per share of common stock and warrant (but excluding shares of common stock to be issued and any proceeds received upon exercise of the warrants)(the last reported sale price of our common stock on the Nasdaq Capital Market on January 12, 2018), after deducting estimated underwriting discounts and commissions, and estimated offering expenses payable by us; and |
|
o |
the mandatory conversion of all outstanding shares of Series A Preferred Stock into shares (the “Conversion Shares”) of common stock (including accrued dividends paid in kind and the “make-whole” adjustment feature thereof) pursuant to the terms thereof, which will occur substantially concurrently with the completion of this offering assuming we receive aggregate gross proceeds of at least $10 million, assuming a public offering price of $1.42 per share of common stock and accompanying warrant (the last reported sale price of our common stock on the Nasdaq Capital Market on January 12, 2018) and that this offering is completed on January 16, 2018. |
|
|
Pro Forma |
Pro Forma |
||||||||||
|
Cash, cash equivalents and certificate of deposit |
$ | 11,236,164 | $ | 11,236,164 | $ | 22,716,164 | ||||||
|
Common Stock warrant liability |
$ | 16,316,054 | $ | — | $ | — | ||||||
|
Convertible debt |
$ | 550,000 | $ | 550,000 | $ | 550,000 | ||||||
|
Series A - 13,750,000 shares authorized, 8,324,032 issued and outstanding, actual; 13,750,000 shares authorized, no shares issued or outstanding, as adjusted |
— | — | ||||||||||
| Stockholders' equity: | ||||||||||||
|
Common stock, par value $0.001 per share; 1,000,000,000 authorized, 14,503,976 shares issued and outstanding actual; 1,000,000,000 shares authorized, 35,193,555 shares issued and outstanding pro forma as adjusted |
14,504 | 14,504 | 35,194 | |||||||||
|
Additional paid-in capital |
$ | 69,686,744 | $ | 82,840,268 | $ | 95,125,352 | ||||||
|
Accumulated deficit |
$ | (63,344,552 | ) | $ | (60,182,022 | ) | $ | (60,182,022 | ) | |||
|
Total stockholders' equity |
$ | 6,356,696 | $ | 22,672,750 | $ | 34,978,524 | ||||||
|
Total capitalization |
$ | 23,222,750 | $ | 23,222,750 | $ | 35,528,524 | ||||||
The number of shares of common stock outstanding in the table excludes the following, outstanding as of September 30, 2017:
|
o |
213,879 shares issuable upon conversion of convertible promissory notes in an aggregate principal amount of $0.6 million; |
|
o |
2,545,989 shares of common stock issuable upon the exercise of outstanding stock options under the 2015 Equity Plan, at a weighted-average exercise price of $7.34 per share; |
|
o |
13,555,887 shares of common stock issuable upon the exercise of outstanding warrants issued in connection with the Series A private placement, at a weighted-average exercise price of $2.22 per share; |
|
o |
447,721 shares of common stock issuable upon the exercise of other outstanding warrants, at a weighted-average exercise price of $44.52 per share; and |
|
o |
114,291 shares of common stock reserved for future issuance under the 2015 Equity Plan. |
|
| ○ |
shares of common stock issuable upon exercise of the warrants offered hereby; and |
|
| ○ |
shares of common stock issuable upon the exercise of the underwriter’s warrant. |
The amounts set forth in the table above also assume no exercise by the underwriter of its option to purchase additional shares of common stock and accompanying warrants.
DILUTION
If you invest in our common stock in this offering, your interest will be diluted to the extent of the difference between the public offering price per share of our common stock and accompanying warrant and the net tangible book value per share of our common stock upon consummation of this offering. Dilution results from the fact that the public offering price is substantially in excess of the book value per share attributable to the existing stockholders for the presently outstanding stock.
The historical net tangible book value (deficit) of our common stock as of September 30, 2017 was approximately $(9.2) million, or approximately $(0.64) per share of common stock. Historical net tangible book value (deficit) per share is determined by dividing the number of outstanding shares of common stock into its total tangible assets (total assets less intangible assets) less total liabilities and preferred shares.
On a pro forma basis, after giving effect to the reclassification of our $16.3 million common stock warrant liability to stockholders’ equity as a result of the amendment of our certificate of incorporation on November 1, 2017 with respect to the dividend feature of the Series A Preferred Stock and resulting accounting adjustments, pro forma net tangible book value (deficit) of our common stock as of September 30, 2017 was approximately $7.1 million, or approximately $0.49 per share of common stock. This represents an immediate increase in pro forma net tangible book value of $1.13 per share.
In addition, assuming the aggregate gross proceeds from this offering are at least $10 million, all of the outstanding shares of our Series A Preferred Stock will mandatorily convert into shares of common stock on a one-for-one basis. Further, each holder of Series A Preferred Stock will also receive additional shares of common stock representing (i) paid-in-kind dividends accrued through the date of conversion and (ii) assuming that the public offering price in this offering is less than $2.02 per share, payment in respect of the “make-whole” feature of the Series A Preferred Stock. For purposes of calculating an assumed number of Conversion Shares, we have assumed a combined public offering price of $1.42 per share of common stock and warrant (the last reported sale price of our common stock on the Nasdaq Capital Market on January 12, 2018) and that this offering is completed on January 16, 2018, which would result in the issuance of, in the aggregate, 11,534,649 shares of common stock in respect of the conversion of our Series A Preferred Stock.
Investors purchasing securities in this offering will incur immediate and substantial dilution. On a pro forma as adjusted basis, after giving effect to the conversion of the outstanding shares of Series A Preferred Stock, assuming the combined public offering price in this offering is $1.42 per share of common stock and warrant, the closing price of our common stock on the Nasdaq Capital Market on January 12, 2018, assuming that this offering is closed on January 16, 2018, and to the sale of common stock and warrants offered in this offering assuming a combined public offering price of $1.42 per share of common stock and warrant, the closing price of our common stock on the Nasdaq Capital Market on January 12, 2018 (but excluding any shares of common stock to be issued and any proceeds to be received upon exercise of the warrants), and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2017 would have been approximately $19.4 million, or approximately $0.55 per share of common stock. This represents an immediate increase in pro forma as adjusted net tangible book value of $0.06 per share to existing stockholders, and an immediate dilution in the pro forma as adjusted net tangible book value of $0.87 per share to investors purchasing shares of our common stock and warrants in this offering.
The following table illustrates this per share dilution:
|
Assumed combined public offering price per share of common stock and warrant |
$ | 1.42 | ||
|
Historical net tangible book value (deficit) per share |
$ | (0.64 | ) | |
|
Increase per share attributable to pro forma adjustments |
1.13 | |||
|
Pro forma net tangible book value (deficit) per share at September 30, 2017 |
$ | 0.49 | ||
|
Increase in net tangible book value per share attributable to this offering and mandatory conversion of Series A preferred stock |
0.06 | |||
|
Pro forma as adjusted net tangible book value after this offering |
$ | 0.55 | ||
|
Dilution per share to investors |
$ | 0.87 |
The dilution information discussed above is illustrative only and will change based on the actual public offering price and other terms of this offering determined at pricing. A $1.00 increase or decrease in the assumed combined public offering price of $1.42 per share of common stock and warrant would increase or decrease the as adjusted net tangible book value per share by $0.25 million and the dilution per share to investors participating in this offering by approximately $0.75 per share, assuming the number of shares of common stock and warrants offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The discussion and table above assume no exercise of the underwriter’s option to purchase additional shares of common stock and warrants. If the underwriter fully exercises its option to purchase 1,373,239 additional shares of common stock and 1,373,239 additional warrants in the offering (but excluding any shares of common stock to be issued and any proceeds to be received upon exercise of the warrants), our as adjusted net tangible book value after this offering, calculated in the manner set forth above, would be approximately $20.5 million, our as adjusted net tangible book value per share after this offering would be $0.58 per share, the increase in our as adjusted net tangible book value per share attributable to investors participating in this offering would be $0.09 per share and the as adjusted dilution per share to new investors in this offering would be $0.84 per share.
DESCRIPTION OF CAPITAL STOCK
Company Capitalization
Our authorized capital stock consists of 1,000,000,000 shares of common stock and 30,000,000 shares of preferred stock, $0.001 par value, 13,750,000 of which have been designated as Series A Preferred Stock and the remainder of which remain undesignated. The following summary is qualified in its entirety by reference to our Certificate of Incorporation, as amended, a copy of which is filed as an exhibit to our previous filings with the SEC and incorporated herein by reference.
Common Stock
Authorized. We are authorized to issue 1,000,000,000 shares of common stock, of which 14,503,976 shares were issued and outstanding as of September 30, 2017. We may amend from time to time our Certificate of Incorporation to increase the number of authorized shares of common stock. Any such amendment would require the approval of the holders of a majority of the voting power of the shares entitled to vote thereon.
Voting Rights. For all matters submitted to a vote of stockholders, each holder of common stock is entitled to one vote for each share registered in the holder’s name on our books. Our common stock does not have cumulative voting rights. At all meetings of the stockholders, except where otherwise provided by law, the Certificate of Incorporation or Bylaws, the presence, in person or by proxy duly authorized, of the holders of a majority of the outstanding shares of common stock entitled to vote constitutes a quorum for the transaction of business. Except as otherwise provided by law or by the Certificate of Incorporation or Bylaws, in all matters other than the election of directors, the affirmative vote of the majority of shares of common stock present in person or represented by proxy at the meeting and entitled to vote generally on the subject matter shall be the act of the stockholders. Except as otherwise provided by law, the Certificate of Incorporation or Bylaws, directors are elected by a plurality of the votes of the shares of common stock present in person or represented by proxy at the meeting and entitled to vote generally on the election of directors.
Dividends. Subject to limitations under Delaware law and any preferences that may be applicable to any then outstanding preferred stock, holders of common stock are entitled to receive ratably those dividends, if any, as may be declared by our Board out of legally available funds.
Liquidation. Upon our liquidation, dissolution or winding up, the holders of common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities of our company, subject to any prior rights of any preferred stock then outstanding.
Fully Paid and Non-assessable. All shares of our outstanding common stock are fully paid and non-assessable and any additional shares of common stock that we issue will be fully paid and non-assessable.
Other Rights and Restrictions. Holders of common stock do not have preemptive or subscription rights, and they have no right to convert their common stock into any other securities. There are no redemption or sinking fund provisions applicable to common stock. The rights, preferences and privileges of common stockholders are subject to the rights of the stockholders of any series of preferred stock which we may designate in the future. Our Certificate of Incorporation and Bylaws do not restrict the ability of a holder of common stock to transfer the holder’s shares of common stock.
Listing. Our common stock is quoted on the Nasdaq Capital Market under the symbol “DFFN.”
Transfer Agent and Registrar. The transfer agent and registrar for common stock is Computershare Investor Services, LLC, 250 Royall Street, Canton, Massachusetts, telephone number: 1-800-942-5909.
Warrants
The material terms and provisions of the warrants being issued in this offering are summarized below. The following description is subject to, and qualified in its entirety by, the form of warrant which is filed herewith as an exhibit. You should review the form of warrant for a complete description of the terms and conditions applicable to the warrants. See "Information Incorporated by Reference and Available Information" on page 41.
General. Each purchaser of shares will receive a warrant to purchase one share of common stock for each share purchased in the offering.
Exercisability. The warrants may be exercised at any time on or after their date of issuance, will have an exercise price of $ per share and are exercisable until the fifth year anniversary of the date of issuance. The warrants will be exercisable, at the option of each holder, in whole or in part by delivering a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise. The exercise price of the warrant is subject to adjustment in the event of stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock.
Exercise Limitations. A holder of a warrant will not have the right to exercise any portion of the warrant if the holder (together with its affiliates) would beneficially own in excess of 4.99% (or, at election of holder, 9.99%) of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon notice to us provided that any increase shall not be effective until 61 days following notice to us.
Transferability. Subject to applicable laws, the warrants are separately tradeable immediately after issuance at the option of the holders and may be transferred at the option of the holders.
No Listing. There is no established public trading market for the warrants and we do not expect a market to develop. In addition, we do not intend to apply for listing of the warrants on any securities exchange or trading system. Without an active market, the liquidity of the warrants will be limited.
Fundamental Transactions. In the event of an "fundamental transaction," as defined in the warrant agreement and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the warrants will be entitled to receive upon exercise of the warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the warrants immediately prior to such fundamental transaction.
In addition, in the event of a fundamental transaction (subject to certain exceptions), we or any successor entity shall, at the holder’s option, purchase the holder’s warrants for an amount of cash equal to the value of the warrants as determined in accordance with the Black Scholes option pricing model.
Cashless Exercise. If, at the time a holder exercises its warrant, there is no effective registration statement registering, or the prospectus contained therein is not available for an issuance of the shares underlying the warrant to the holder, and the holder is not able to sell the shares issuable upon exercise of the warrant without limitations on volume pursuant to Rule 144, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of our common stock determined according to a formula set forth in the warrant. In the event of a cashless exercise, if we fail to timely deliver the shares underlying the warrants, we will be subject to certain buy-in provisions.
Rights as a Stockholder. Except as otherwise provided in the warrant or by virtue of a holder's ownership of shares of our common stock, the holders of the warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their warrants.
Amendments. Amendments and waivers of the terms of the warrants require the written consent of the holders of warrants representing not less than two-thirds of the shares issuable upon exercise of the warrants then outstanding and us, as well as any warrant holder that would be disproportionately and materially adversely impacted by the amendment.
No Fractional Shares. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of the warrants. As to any fraction of a share which the holder would otherwise be entitled to purchase upon such exercise, we shall cause the warrant agent to, at our option, pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price of the warrant per whole share or round such fractional share up to the nearest whole share.
Series A Convertible Preferred Stock
As of September 30, 2017, there were 8,324,032 shares of the Series A Preferred Stock issued and outstanding. The following is a summary of the rights, privileges and preferences of the Series A Preferred Stock, which such summary is qualified in its entirety by the Certificate of Designation of Preferences, Rights and Limitations of the Series A Convertible Preferred Stock (the “Series A Certificate of Designation”).
Voting. The holders of the Series A Preferred Stock are entitled to vote with the holders of common stock (and any other class or series that may similarly be entitled to vote with the holders of common stock as the Board may authorize and issue) and not as a separate class, at any annual or special meeting of stockholders of the Company, and may act by written consent in the same manner as the holders of common stock. In the event of any such vote or action by written consent, each holder of shares of Series A Preferred Stock is entitled to that number of votes equal to the whole number of shares of common stock into which the aggregate number of shares of Series A Preferred Stock held of record by such Holder are convertible as of the close of business on the record date fixed for such vote or such written consent based on a conversion price, solely for such purpose, equal to the closing price of our common stock on the date such Series A Preferred Stock was issued. In addition, for as long as 50% of the shares of Series A Preferred Stock outstanding immediately after the final closing remain outstanding, without the consent of holders of at least a majority of the then outstanding shares of Series A Preferred Stock, the Company may not (a) amend the Certificate of Incorporation or Bylaws so as to materially and adversely affect any rights of the holders of the Series A Preferred Stock, (b) increase or decrease (other than by conversion of the Series A Preferred Stock) the authorized number of Series A Preferred Stock to be in excess of the number of shares required to satisfy the Maximum Offering Amount, (c) amend the Series A Certificate of Designation, (d) repay, repurchase or offer to repay, repurchase or otherwise acquire more than a de minimis number of shares of common stock or common stock equivalents or (e) enter into any agreement or understanding with respect to (a) through (d).
Dividends. The Series A Preferred Stock are entitled to an 8.0% cumulative preferred dividend payable semi-annually in shares of common stock or cash (in the Company’s sole discretion) that began accruing on the issue date of the Series A Preferred Stock. The dividend will begin to accrue and be cumulative on the first day of each applicable dividend period and shall remain accumulated dividends with respect to such Series A Preferred Stock until paid; provided, that the first dividend payable with respect to any share of Series A Preferred Stock shall not begin to accrue until the date of original issuance of such share of Series A Preferred Stock. Dividends shall accrue whether or not there are profits, surplus or other funds of the Company legally available for the payment of dividends but shall not be payable until legally permissible, as applicable.
Liquidation. The Series A Preferred Stock ranks senior to the common stock and will rank senior to each other class of capital stock of the Company or series of preferred stock of the Company authorized by the Board in the future that does not expressly provide that such class or series ranks senior to, or on parity with, the Series A Preferred Stock (“Series A Junior Securities”). In the event of a Liquidation Event (as defined in the Series A Certificate of Designation), the holders of the Series A Preferred Stock are entitled to receive, out of the assets of the Company or proceeds thereof legally available therefor, an amount in cash equal to 100% of the stated value of the Series A Preferred Stock before any payment or distribution of the assets of the Company is made or set apart for the holders of Series A Junior Securities. In addition, prior to such Liquidation Event, the holders of Series A Preferred Stock are entitled to notice so that they may exercise their conversion rights prior to such event. If, upon the occurrence of any Liquidation Event, the assets of the Company, or proceeds thereof, distributable after payment in full of the Company’s creditors and any securities senior to the Series A Preferred Stock are insufficient to pay in full the aggregate amount of 100% of the stated value to the holders of the Series A Preferred Stock, the assets of the Company, or the proceeds thereof, shall be distributed ratably among the holders of any Series A Preferred Stock and the holders of any security ranked equally with the Series A Preferred Stock.
Conversion Rate: Each share of Series A Preferred Stock is convertible into a share of common stock at a conversion price equal to the Purchase Price, subject to adjustment as provided in the Certificate of Designation and summarized herein (the “Series A Conversion Price”), at any time after the final closing date at the holder’s sole and absolute discretion. Holders may immediately convert their Series A Preferred Stock prior to the occurrence of certain Liquidation Events. Each share of Series A Preferred Stock will mandatorily convert, initially, into a share of common stock (a) on any date that is more than thirty (30) trading days after the original issued date of such share Series A Preferred Stock that the 30 day moving average of the closing price of the common stock on the Nasdaq Capital Market (or any other exchange where the common stock is traded) exceeds $8.00 per share (subject to adjustment in the event of a stock dividend or split), (b) upon the Company's receipt of aggregate gross proceeds from a financing of at least $10 million or (c) upon the majority vote of the voting power of the then outstanding shares of Series A Preferred Stock. The Company is not required to issue any fractional shares of Series A Preferred Stock or common stock in connection with the conversion of Series A Preferred Stock and may, in each case, at the Company’s discretion, pay the holder such amount in cash or deliver an additional whole share in lieu thereof. If the Company receives aggregate gross proceeds of at least $10 million from this offering, the Series A Preferred Stock will mandatorily convert into the applicable number of shares of our common stock.
Limitations of Conversion: The number of shares of common stock issuable upon a conversion of the Series A Preferred Stock that may be acquired by a holder shall be limited to the extent necessary to ensure that, following such conversion (or other issuance), the total number of shares of common stock then beneficially owned by such holder and its affiliates and any other persons whose beneficial ownership of common stock would be aggregated with the Holder's for purposes of Section 13(d) of the Securities and the Exchange Act, does not exceed 4.99% of the total number of issued and outstanding shares of common stock (including for such purpose the shares of common stock issuable upon such conversion). However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% of the total number of shares of common stock then issued and outstanding provided that such increase in percentage shall not be effective until sixty-one (61) days after notice to the Company.
Dilution Protection. In the event the Company, at any time after the first date of issue of the Series A Preferred Stock and while at least one share of Series A Preferred Stock is outstanding: (a) pays a dividend or otherwise make a distribution or distributions on shares of its common stock or any other equity or equity equivalent securities payable in shares of common stock (which, for avoidance of doubt, shall not include any shares of common stock issued by the Company upon conversion of the Series A Preferred Stock or any debt securities), (b) subdivides outstanding shares of common stock into a larger number of shares, (c) combines (including by way of reverse stock split) outstanding shares of common stock into a smaller number of shares or (d) issues by reclassification of shares of common stock any shares of capital stock of the Company, then in each case the Series A Conversion Price shall be multiplied by a fraction of which (x) the numerator shall be the number of shares of common stock (excluding treasury shares, if any) outstanding immediately before such event and (y) the denominator shall be the number of shares of common stock outstanding immediately after such event. Any adjustment made pursuant to this section shall become effective immediately after the effective date of the applicable event described in subsections (a) through (d) above.
Make-Whole Adjustment. In the event the Company, during the three years immediately following the Initial Closing, subject to certain exceptions, issues in an offering at least $10 million of common stock or securities convertible into or exercisable for common stock at a per share price less than $2.02 per share (the “Make-Whole Price”), the Company will be required to issue to the holders of Series A Preferred Stock a number of shares of common stock equal to the additional number of shares of common stock that such shares of Series A Preferred Stock would be convertible into if the Series A Conversion Price of the Series A Preferred Stock was equal to 105% of the Make-Whole Price (the “Make-Whole Adjustment”). The Company will only be obligated to issue shares with respect to a Make-Whole Adjustment in the first such subsequent offering, if any, following the final closing of the Series A Preferred Stock.
Warrants and Stock Options Issued and Outstanding Prior to this Offering
In connection with the Series A Preferred Stock private placement, we issued 5-year warrants to purchase 12,376,329 shares of common stock at an exercise price equal to $2.22 per share to the holders of the Series A Preferred stock and we issued 5-year warrants to purchase of 1,179,558 shares of common stock at an exercise price equal to $2.22 per share, with such warrants containing a cashless exercise provision to our placement agent in the Series A private placement. As of September 30, 2017, all 13,555,887 warrants issued in connection with the Series A private placement were outstanding and exercisable.
In addition, as of September 30, 2017, other warrants to purchase an aggregate of 447,721 shares of common stock were outstanding and exercisable with per share exercise prices ranging from $20.00 to $750.00 per share, a weighted average exercise price of $44.52 and expiration dates ranging from March 27, 2018 to December 8, 2019.
Market Price for our Common Stock
Our common stock trades publicly on the Nasdaq Capital Market under the symbol “DFFN.” Prior to November 9, 2016, our common stock traded on the OTCQX over-the-counter bulletin board. The following table sets forth the high and low daily sale prices for our common stock, as reported or quoted by the Nasdaq Capital Market or the OTCQX, as applicable, for each calendar quarter during 2018, 2017 and 2016, after giving effect to the 1-for-10 reverse stock split of our common stock on August 17, 2016. The over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
|
For the year ended December 31, 2018 |
High |
Low |
|
First Quarter (through January 12, 2018) |
2.08 |
1.16 |
|
For the year ended December 31, 2017 |
High |
Low |
|
Fourth Quarter |
$2.97 |
$1.06 |
|
Third Quarter |
$3.04 |
$1.34 |
|
Second Quarter |
$4.73 |
$2.26 |
|
First Quarter |
$15.50 |
$2.01 |
|
For the year ended December 31, 2016 |
High |
Low |
|
Fourth Quarter |
$7.00 |
$1.94 |
|
Third Quarter |
9.25 |
5.27 |
|
Second Quarter |
11.50 |
8.00 |
|
First Quarter |
17.00 |
8.50 |
Anti-Takeover Provisions
Delaware Anti-Takeover Law
We are subject to Section 203 of the DGCL. Section 203 generally prohibits a public Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless:
|
• |
prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
|
• |
the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding (a) shares owned by persons who are directors and also officers of the corporation and (b) shares issued under employee stock plans under which employee participants do not have the right to determine whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
|
• |
on or subsequent to the date of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2⁄3% of the outstanding voting stock that is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
|
• |
any merger or consolidation involving the corporation and the interested stockholder; |
|
• |
any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
|
• |
subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
|
• |
any transaction involving the corporation that has the effect of increasing the proportionate share of its stock owned by the interested stockholder; or |
|
• |
the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
UNDERWRITING
We have entered into an underwriting agreement dated , 2018, with H.C. Wainwright & Co., LLC as the sole book-running manager of this offering. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriter and the underwriter has agreed to purchase from us, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, shares of common stock and warrants to purchase shares of common stock.
A copy of the underwriting agreement has been filed as an exhibit to the registration statement of which this prospectus is a part. The shares of common stock and warrants we are offering are being offered by the underwriter subject to certain conditions specified in the underwriting agreement.
We have been advised by the underwriter that it proposes to offer the shares of common stock and warrants directly to the public at the public offering price set forth on the cover page of this prospectus. Any shares of common stock and warrants sold by the underwriter to securities dealers will be sold at the public offering price less a selling concession not in excess of $ per share of common stock and warrant.
The underwriting agreement provides that the underwriter's obligation to purchase the shares of common stock and warrants we are offering is subject to conditions contained in the underwriting agreement. The underwriter is obligated to purchase and pay for all of the shares of common stock and warrants offered by this prospectus.
The underwriter has advised us that it does not intend to confirm sales to any accounts over which it exercises discretionary authority.
Underwriting Discounts, Commissions and Expenses
We have agreed to pay an underwriter discount equal to 8% of the aggregate gross proceeds raised in this offering.
The following table shows the public offering price, underwriting discounts and commissions and proceeds, before expenses to us. These amounts are shown assuming both no exercise and full exercise of the underwriter's option to purchase additional shares of common stock and warrants.
|
Total |
||||||||||||
|
Per Share and Warrant |
Without |
With |
||||||||||
|
Public offering price |
||||||||||||
|
Underwriting discounts and commissions |
||||||||||||
|
Proceeds, before expenses, to us |
||||||||||||
We estimate the total expenses payable by us for this offering to be approximately $1.4 million, which amount includes (i) an underwriting discount of $1.0 million ($1.2 million if the underwriter's option to purchase additional shares of common stock and warrants is exercised in full), (ii) a management fee equal to 1% of the aggregate gross proceeds raised in this offering, (iii) a $35,000 non-accountable expense allowance payable to the underwriter, (iv) reimbursement of the accountable expenses of the underwriter equal to $100,000 including the legal fees of the underwriter being paid by us (none of which has been paid in advance), and (v) other estimated expenses of approximately $100,000 which include legal, accounting, printing costs and various fees associated with the registration and listing of our shares.
Option to Purchase Additional Securities
We have granted the underwriter the option to purchase up to additional shares of common stock and/or additional warrants to purchase up to __ shares of common stock (15% of the shares and 15% of the warrants issued in the offering), in any combination thereof, at the public offering price per share of common stock and per warrant, less the underwriting discounts and commissions. The underwriter may exercise its option at any time, and from time to time, within 30 days from the date of this prospectus. If any additional shares of common stock and/or warrants are purchased pursuant to the option to purchase additional shares of common stock and warrants, the underwriter will purchase these shares of common stock and/or warrants on the same terms as those on which the shares of common stock and warrants are being offered hereby.
Underwriter Warrants
We have agreed to issue to the underwriter warrants to purchase shares of our common stock which represent 5% of the aggregate number of shares of common stock sold in this offering. The underwriter warrants will have a term of five years from the effective date of this prospectus and an exercise price per share equal to $ per share, which represents 125% of the public offering price for the shares sold in this offering. Pursuant to FINRA Rule 5110(g), the underwriter warrants and any shares issued upon exercise of the underwriter warrants shall not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days immediately following the date of effectiveness or commencement of sales of this offering, except the transfer of any security: (i) by operation of law or by reason of our reorganization; (ii) to any FINRA member firm participating in the offering and the officers or partners thereof, if all securities so transferred remain subject to the lock-up restriction set forth above for the remainder of the time period; (iii) if the aggregate amount of our securities held by the underwriter or related persons do not exceed 1% of the securities being offered; (iv) that is beneficially owned on a pro rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund and the participating members in the aggregate do not own more than 10% of the equity in the fund; or (v) the exercise or conversion of any security, if all securities remain subject to the lock-up restriction set forth above for the remainder of the time period.
Right of First Refusal
We have also granted the underwriter, for a period of 12 months from the closing date of this offering, a right of first refusal to act as sole book-running manager for a public offering of equity or debt securities using an underwriter by us or any of our successors or subsidiaries. We have also agreed to a tail fee equal to the cash and warrant compensation in this offering if any investor to which the underwriter introduced us with respect to this offering during the term of its engagement provides us with further capital in a public or private offering or capital raising transaction during the 12-month period following termination of our engagement of the underwriter.
Nasdaq Capital Market Listing
Our common stock is currently traded on the Nasdaq Capital Market under the symbol "DFFN." On January 12, 2018, the last reported sale price of our common stock was $1.42 per share.
Lock-up Agreements
Our officers and directors have agreed with the underwriter to be subject to a lock-up period of 90 days following the date of this prospectus. This means that, during the applicable lock-up period, such persons may not offer for sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly, any shares of our common stock or any securities convertible into, or exercisable or exchangeable for, shares of our common stock. We have also agreed in the underwriting agreement, subject to certain exceptions, to similar lock-up restrictions on the issuance and sale of our securities for 90 days following the closing of this offering. The underwriter may, in its sole discretion and without notice, waive the terms of any of these lock-up agreements.
Stabilization, Short Positions and Penalty Bids
The underwriter may engage in syndicate covering transactions, stabilizing transactions and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of our common stock:
|
● |
Syndicate covering transactions involve purchases of shares in the open market after the distribution has been completed in order to cover syndicate short positions. Such a naked short position would be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriter is concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. |
|
● |
Stabilizing transactions permit bids to purchase the shares so long as the stabilizing bids do not exceed a specific maximum. |
|
● |
Penalty bids permit the underwriter to reclaim a selling concession from a syndicate member when the shares originally sold by the syndicate member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These syndicate covering transactions, stabilizing transactions and penalty bids may have the effect of raising or maintaining the market prices of our shares or preventing or retarding a decline in the market prices of our shares. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. Neither we nor the underwriter make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected on the Nasdaq Capital Market, in the over-the-counter market or on any other trading market and, if commenced, may be discontinued at any time.
In connection with this offering, the underwriter also may engage in passive market making transactions in our common stock in accordance with Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of the distribution. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for that security. However, if all independent bids are lowered below the passive market maker's bid that bid must then be lowered when specific purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither we nor the underwriter make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the prices of our securities. In addition, neither we nor the underwriter make any representation that the underwriter will engage in these transactions or that any transactions, once commenced, will not be discontinued without notice.
Indemnification
We have agreed to indemnify the underwriter against certain liabilities, including certain liabilities arising under the Securities Act, or to contribute to payments that the underwriter may be required to make for these liabilities.
Other Relationships
The underwriter and its respective affiliates may in the future engage in investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. The underwriter has received, or may in the future receive, customary fees and commissions for these transactions.
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following is a general discussion of the material U.S. federal income considerations applicable to the ownership and disposition of shares of our common stock and warrants acquired in this offering. This discussion is for general information only and is not tax advice. Accordingly, all prospective holders of our common stock and warrants should consult their own tax advisors with respect to the U.S. federal, state, local and non-U.S. tax consequences of the purchase, ownership and disposition of our common stock and warrants. This discussion is based on current provisions of the U.S. Internal Revenue Code of 1986, as amended, which we refer to as the Code, existing and proposed U.S. Treasury Regulations promulgated thereunder, current administrative rulings and judicial decisions, all as in effect as of the date of this prospectus, all of which are subject to change or to differing interpretation, possibly with retroactive effect. Any change could alter the tax consequences described in this prospectus. We assume in this discussion that each holder holds shares of our common stock and warrants as capital assets within the meaning of Section 1221 of the Code (generally property held for investment).
This discussion does not address all aspects of U.S. federal income taxation that may be relevant to a particular holder in light of that holder's individual circumstances, does not address the alternative minimum or Medicare contribution taxes, and does not address any aspects of U.S. state, local or non-U.S. taxes or any U.S. federal taxes other than income tax. This discussion also does not consider any specific facts or circumstances that may apply to a non-U.S. holder and does not address aspects of U.S. federal income taxation that may be applicable to investors that are subject to special tax rules, including without limitation:
|
• |
insurance companies; |
|
• |
tax-exempt organizations; |
|
• |
financial institutions; |
|
• |
brokers or dealers in securities; |
|
• |
regulated investment companies; |
|
• |
real estate investment trusts; |
|
• |
pension plans, individual retirement accounts and other tax deferred accounts; |
|
• |
persons that mark their securities to market; |
|
• |
controlled foreign corporations; |
|
• |
passive foreign investment companies; |
|
• |
"dual resident" corporations; |
|
• |
persons that receive our common stock or warrants as compensation for the performance of services; |
|
• |
owners that hold our common stock or warrants as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment; |
|
• |
owners that own, or are deemed to own, more than 5% of our capital stock (except to the extent specifically set forth below); |
|
• |
persons that have a functional currency other than the U.S. dollar; and |
|
• |
certain U.S. expatriates. |
In addition, this discussion does not address the tax treatment of partnerships or other pass-through entities for U.S. federal income tax purposes, or persons who hold our common stock or warrants through partnerships or other pass-through entities for U.S. federal income tax purposes. A partner in a partnership or other pass-through entity that will hold our common stock or warrants should consult his, her or its own tax advisor regarding the tax consequences of acquiring, holding and disposing of our common stock or warrants through a partnership or other pass-through entity, as applicable.
There can be no assurance that the Internal Revenue Service, which we refer to as the IRS, will not challenge one or more of the tax consequences described herein. We have not obtained, nor do we intend to obtain, a ruling from the IRS with respect to the U.S. federal income tax consequences of the purchase, ownership or disposition of our common stock or warrants.
Allocation of Purchase Price Between Common Stock and Warrants to Purchase Our Common Stock
Each share of common stock will be issued together with a warrant, and each holder of our common stock and warrants must allocate the purchase price paid by such holder for our common stock and a warrant between the share of common stock and the warrant based on the relative fair market value of each. This allocation will establish a holder's initial tax basis for U.S. federal income tax purposes in their shares of common stock and warrants. We will not be providing holders with such allocation, and it is possible that different holders will reach different determinations regarding such allocation. A holder's allocation of purchase price between common stock and warrants is not binding on the IRS or the courts, and no assurance can be given that the IRS or the courts will agree with a holder's allocation.
Accordingly, each prospective holder should consult their own tax advisor with respect to the risks associated with an allocation of the purchase price between our common stock and the warrants.
U.S. Holders
This section applies to you if you are a "U.S. holder." For purposes of this discussion, a "U.S. holder" means a beneficial owner of our common stock and warrants who is for U.S. federal income tax purposes: (i) an individual who is a citizen or resident of the United States; (ii) a corporation, or any other organization taxable as a corporation for U.S. federal income tax purposes, created or organized in the United States or under the laws of the United States or of any state thereof or the District of Columbia; (iii) an estate, the income of which is subject to U.S. federal income tax regardless of its source; or (iv) a trust if (1) a U.S. court is able to exercise primary supervision over the trust's administration and one or more U.S. persons have the authority to control all of the trust's substantial decisions or (2) the trust has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a U.S. person.
Distributions on Our Common Stock and Warrants
Distributions, if any, on our common stock generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the U.S. holder's investment, up to such U.S. holder's tax basis in the common stock. Any remaining excess will be treated as capital gain, subject to the tax treatment described below in "—Gain on Sale, Exchange or Other Taxable Disposition of Our Common Stock." Dividends paid by us will generally be eligible for the reduced rates of tax for qualified dividend income allowed to individual U.S. holders and for the dividends received deduction allowed to corporate U.S. holders, in each case assuming that certain holding period and other requirements are satisfied.
While it is not free from doubt, we expect that cash distributions paid on the warrants, on an "as-converted" basis, if any, are subject to the same tax consequences as described in the preceding paragraph for the common stock; however, distributions received in respect of a warrant may not qualify for the lower tax rates applicable to qualified dividend income. U.S. holders should consult their own tax advisors regarding the proper treatment of any distributions paid on the warrants.
Conversion of Warrants into Our Common Stock
Except as discussed below with respect to the cashless exercise of a warrant, a U.S. holder will not recognize gain or loss on the conversion of warrants into our common stock. A U.S. holder's tax basis in the common stock received upon a conversion will equal the sum of (i) the initial tax basis of the warrants converted and (ii) price paid upon exercise. The U.S. holder's holding period for the common stock received upon exercise of the warrants will begin on the date following the date of exercise of the warrants and will not include the period during which the U.S. holder held the warrants.
The tax consequences of a cashless exercise of a warrant are not clear under current U.S. tax law. A cashless exercise may be tax-free, either because the exercise is not a realization event or because the exercise is treated as a recapitalization for U.S. federal income tax purposes. In this case, a U.S. holder's basis in the common stock received would equal the U.S. holder's basis in the warrant. If the cashless exercise were treated as a recapitalization, the holding period of the common stock would include the holding period of the warrant.
It is possible that a cashless exercise could be treated in part as a taxable exchange in which gain or loss would be recognized with respect to the warrants surrendered to pay the exercise price for warrants converted into common stock. In that event, a U.S. holder could be deemed to have surrendered only those warrants used to satisfy the exercise price for an amount equal to the aggregate fair market value of the exercise price of the total number of warrants to be exercised. The U.S. holder would recognize capital gain or loss in an amount equal to the difference between the amount deemed realized and the U.S. holder's tax basis in the warrants surrendered to pay the exercise price. In this case, a U.S. holder's tax basis in the common stock received would equal the sum of the U.S. holder's initial investment in the converted warrants (i.e., the portion of the U.S. holder's purchase price for the shares of common stock that is allocated to the warrants, as described above under "—Allocation of Purchase Price between Common Stock and Warrants") and the exercise price of the warrants converted. A U.S. holder's holding period for the common stock would commence on the date following the date of exercise.
Due to the absence of authority on the U.S. federal income tax treatment of a cashless exercise, there can be no assurance which, if any, of the alternative tax consequences and holding periods described above would be adopted by the IRS or a court of law. Accordingly, U.S. holders should consult their own tax advisors regarding the tax consequences of a cashless exercise.
If a warrant is allowed to lapse unexercised, a U.S. holder generally will recognize a capital loss equal to such holder's tax basis in the warrant.
Sale, Exchange or Other Taxable Disposition of Our Common Stock or Warrants
Upon the sale, exchange, or other taxable disposition of our common stock or warrants, a U.S. holder will recognize gain or loss equal to the difference between the amount realized upon the disposition and the U.S. holder's tax basis in the common stock or warrants sold or exchanged. Any gain or loss generally will be capital gain or loss, and will be long-term capital gain or loss if the U.S. holder's holding period for the common stock or warrants exceeded one year at the time of the disposition. Certain U.S. holders (including individuals) are currently eligible for preferential rates of U.S. federal income taxation in respect of long-term capital gains. The deductibility of capital losses is subject to significant limitations.
Information Reporting and Backup Withholding
In general, information reporting requirements may apply to dividends paid to a U.S. holder on our common stock or the warrants, and to the proceeds of the sale, exchange or other disposition of our common stock and warrants, unless the U.S. holder is an exempt recipient. Backup withholding may apply to such payments if the U.S. holder fails to provide a taxpayer identification number, a certification of exempt status or has been notified by the IRS that it is subject to backup withholding (and such notification has not been withdrawn). Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules will be allowed as a refund or a credit against a U.S. holder's U.S. federal income tax liability provided the required information is timely furnished to the IRS.
Non-U.S. Holders
This section applies to you if you are a "non-U.S. holder". For purposes of this discussion, a "non-U.S. holder" means a person who is not a U.S. holder (as defined above under "U.S. Holders").
Distributions on Our Common Stock and Warrants
Distributions, if any, on our common stock generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the non-U.S. holder's investment, up to such non-U.S. holder's tax basis in the common stock. Any remaining excess will be treated as capital gain, subject to the tax treatment described below in "Gain on Sale, Exchange or Other Taxable Disposition of Our Common Stock."
While it is not free from doubt, we expect that cash distributions paid on the warrants, on an "as-converted" basis, if any, are subject to the same tax consequences as described in the preceding paragraph for the common stock. Non-U.S. Holders should consult their own tax advisors regarding the proper treatment of any distributions paid on the warrants.
Subject to the discussions below regarding backup withholding and the Foreign Account Tax Compliance Act, or FATCA, dividends paid to a non-U.S. holder generally will be subject to withholding of U.S. federal income tax at a rate of 30% of the gross amount or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder's country of residence, unless such dividends are effectively connected with a trade or business conducted by a non U.S. holder within the U.S (as discussed below). A non-U.S. holder of our common stock or warrants who claims the benefit of an applicable income tax treaty between the United States and such holder's country of residence generally will be required to provide a properly executed IRS Form W-8BEN or W-8BEN-E (or successor form), as applicable, and satisfy applicable certification and other requirements. Non-U.S. holders are urged to consult their own tax advisors regarding their entitlement to benefits under a relevant income tax treaty. A non-U.S. holder that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty may be able to obtain a refund or credit of any excess amounts withheld by timely filing the required information with the IRS.
Subject to the discussions below regarding backup withholding and FATCA, dividends that are treated as effectively connected with a trade or business conducted by a non-U.S. holder within the United States and, if an applicable income tax treaty so provides, that are attributable to a "permanent establishment" or a "fixed base" maintained by the non-U.S. holder within the United States, are generally exempt from the 30% withholding tax if the non-U.S. holder satisfies applicable certification and disclosure requirements. U.S. effectively connected income, net of specified deductions and credits, is generally taxed at the same graduated U.S. federal income tax rates applicable to United States persons (as defined in the Code). Any U.S. effectively connected income received by a non-U.S. holder that is a corporation may also be subject to an additional "branch profits tax" at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder's country of residence.
Conversion of Warrants into Our Common Stock
The U.S. federal income tax treatment of a non-U.S. holder's exercise of a warrant, or the lapse of a warrant held by a Non-U.S. Holder, generally will correspond to the U.S. federal income tax treatment of the exercise or lapse of a warrant by a U.S. Holder, as described under "U.S. Holders—Conversion of Warrants into Common Stock" above.
Gain on Sale, Exchange or Other Taxable Disposition of Our Common Stock or Warrants
Subject to the discussions below regarding backup withholding and FATCA, in general, a non-U.S. holder will not be subject to any U.S. federal income tax on any gain realized upon such holder's sale, exchange or other taxable disposition of shares of our common stock or warrants unless:
|
• |
the gain is effectively connected with the non-U.S. holder's conduct of a U.S. trade or business and, if an applicable income tax treaty so provides, is attributable to a "permanent establishment" or a "fixed base" maintained by such non-U.S. holder in the United States, in which case the non-U.S. holder generally will be taxed on such gain at the graduated U.S. federal income tax rates applicable to United States persons (as defined in the Code) and, if the non-U.S. holder is a foreign corporation, the branch profits tax described above in "Distributions on Our Common Stock" also may apply to such gain; |
|
• |
the non-U.S. holder is a nonresident alien individual who is present in the United States for 183 days or more in the taxable year of the taxable disposition and certain other conditions are met, in which case the non-U.S. holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder's country of residence) on the net gain derived from the taxable disposition, which may be offset by certain U.S. source capital losses of the non-U.S. holder, if any; or |
|
• |
we are, or have been, at any time during the five-year period preceding such taxable disposition (or the non-U.S. holder's holding period, if shorter) a "U.S. real property holding corporation," unless our common stock is regularly traded on an established securities market and the non-U.S. holder holds no more than 5% of our outstanding common stock, directly or indirectly, during the shorter of the 5-year period ending on the date of the taxable disposition or the period that the non-U.S. holder held our common stock. If we are determined to be a U.S. real property holding corporation and the foregoing exception does not apply, then a purchaser may withhold 15% of the proceeds payable to a non-U.S. holder from a sale of our common stock or warrants, and the non-U.S. holder generally will be taxed on its net gain derived from the disposition at the graduated U.S. federal income tax rates applicable to United States persons (as defined in the Code). Generally, a corporation is a U.S. real property holding corporation only if the fair market value of its U.S. real property interests equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance, we do not believe that we are, or have been, a U.S. real property holding corporation, or that we are likely to become one in the future. No assurance can be provided that our common stock will be regularly traded on an established securities market for purposes of the rules described above. |
Backup Withholding and Information Reporting
We must report annually to the IRS and to each non-U.S. holder the gross amount of the distributions paid on our common stock or warrants to such holder and the tax withheld, if any, with respect to such distributions. Non-U.S. holders may have to comply with specific certification procedures to establish that the holder is not a United States person (as defined in the Code) in order to avoid backup withholding at the applicable rate with respect to dividends on our common stock or warrants. Dividends paid to non-U.S. holders subject to the U.S. withholding tax, as described above in "Distributions on Our Common Stock and Warrants," generally will be exempt from U.S. backup withholding.
Information reporting and backup withholding generally will apply to the proceeds of a disposition of our common stock and warrants by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a non-U.S. holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker. Non-U.S. holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Copies of information returns may be made available to the tax authorities of the country in which the non-U.S. holder resides or is incorporated under the provisions of a specific treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a non-U.S. holder can be refunded or credited against the non-U.S. holder's U.S. federal income tax liability, if any, provided that an appropriate claim is filed with the IRS.
Withholding and Information Reporting Requirements—FATCA
FATCA may impose a U.S. federal withholding tax at a rate of 30% on payments of dividends on, or gross proceeds from the sale or other disposition of, our common stock or warrants paid to certain foreign entities, unless (i) if the foreign entity is a "foreign financial institution," such foreign entity undertakes certain due diligence, reporting, withholding, and certification obligations, (ii) if the foreign entity is not a "foreign financial institution," such foreign entity either certifies that it does not have any "substantial United States owners" (as defined in the Code) or furnishes identifying information regarding each substantial United States owner, or (iii) the foreign entity otherwise qualifies from an exemption these rules. Under applicable U.S. Treasury Regulations, withholding under FATCA currently applies to payments of dividends paid on our common stock or deemed paid on the warrants and is scheduled to apply to gross proceeds from a sale or other disposition of our common stock or warrants made on or after January 1, 2019. A Non-U.S. holder that is not subject to FATCA withholding generally may certify its exempt status by furnishing a properly executed IRS Form W-8BEN or Form W-8BEN-E (or successor form), as applicable. Under certain circumstances, a non-U.S. holder may be eligible for refunds or credits of the tax. Certain intergovernmental agreements between the United States and other countries may modify these rules. Non-U.S. holders should consult their own tax advisors regarding the possible implications of this legislation on their investment in our common stock and the entities through which they hold our common stock, including, without limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of the 30% withholding tax under FATCA.
Executive Compensation
Summary Compensation Table
The table below provides summary compensation information concerning all compensation for the fiscal years ended December 31, 2017 and 2016 awarded to the individuals that served as our named executive officers during the fiscal year ended December 31, 2017.
SUMMARY COMPENSATION TABLE
|
Name and Principal Position |
Year |
Salary (1) |
Bonus |
Option |
All Other |
Total |
||||||||||||||||
|
David G. Kalergis |
2017 |
$ | 317,677 | $ | 0 | $ | 99,966 | $ | 11,612 | $ | 429,255 | |||||||||||
|
Chief Executive Officer |
2016 |
$ | 244,144 | $ | 0 | $ | 410,240 | $ | 96,000 | $ | 750,384 | |||||||||||
|
John L. Gainer, Ph.D. |
2017 |
$ | 265,958 | $ | 0 | $ | 82,899 | $ | 10,508 | $ | 359,365 | |||||||||||
|
Chief Scientific Officer |
2016 |
$ | 191,740 | $ | 0 | $ | 146,209 | $ | 82,615 | $ | 420,564 | |||||||||||
|
Ben L. Shealy |
2017 |
$ | 178,400 | $ | 0 | $ | 56,323 | $ | 4,311 | $ | 239,034 | |||||||||||
|
Senior Vice President – Finance, Treasurer & Secretary |
2016 |
$ | 152,225 | $ | 0 | $ | 107,892 | $ | 62,359 | $ | 322,746 | |||||||||||
________________________
|
(1) |
Represents cash portion of base salary as described below under "--Employment Agreements." |
|
(2) |
The amounts shown in this column reflect the grant date fair value of option awards granted during the applicable year, calculated in accordance with the provisions of Financial Accounting Standards Board Accounting Standards Codification Topic 718 ("ASC Topic 718") and determined without regard to forfeitures. The assumptions used in the Black-Scholes model for the fiscal years ended December 31, 2017 and 2016 will be and are disclosed in our Annual Reports on Form 10-K for the years ended December 31, 2017 and 2016, respectively. |
|
(3) |
Represents annual cash incentive bonus paid to Messrs. Kalergis, Gainer and Shealy in March 2017 for service in 2016 in the amount of $96,000, $79,000 and $58,000, respectively. Bonuses earned by our named executive officers for the 2017 fiscal year are not known at the time of the filing of this Registration Statement. We expect to know the amount of such bonuses by March 2018. The remaining and other amounts reported in this column for Mr. Shealy and Dr. Gainer represent 401(k) Plan matching contributions by the Company for the applicable year. |
Employment Agreements
David G. Kalergis, Chief Executive Officer. Effective September 6, 2016, we entered into an employment agreement with Mr. Kalergis pursuant to which he serves as our Chief Executive Officer. The employment agreement has an indefinite term. Mr. Kalergis’ annual base salary under the agreement for 2017 was $422,300, and is subject to increase at the discretion of the Board. Mr. Kalergis has the opportunity to earn a target annual bonus of 45 percent of his base salary. The Board may, in its discretion, pay a portion of Mr. Kalergis’ annual salary and annual bonus in the form of equity or equity-based compensation, provided that commencing with the year following the year in which a “change of control” (as defined in the employment agreement) occurs, Mr. Kalergis’ entire base salary and annual bonus will be paid in cash. For 2017, the cash portion of Mr. Kalergis’ base salary was $317,677. The employment agreement contains certain severance and change of control provisions as described in more detail under the heading “—Post-Termination Severance and Change in Control Arrangements.” The employment agreement also contains certain non-competition and non-solicitation provisions (each applicable during employment and for 24 months thereafter), as well as confidentiality and non-disparagement provisions (each applicable during employment and at all times thereafter).
John L. Gainer, Chief Scientific Officer. Effective October 18, 2016, we entered into an employment agreement with Dr. Gainer pursuant to which he serves as our Chief Scientific Officer. The employment agreement has an indefinite term. Dr. Gainer’s annual base salary under the agreement for 2017 was $350,200, and is subject to increase at the discretion of the Board. Dr. Gainer has the opportunity to earn a target annual bonus of 35 percent of his base salary. The Board may, in its discretion, pay a portion of Dr. Gainer’s annual salary and annual bonus in the form of equity or equity-based compensation, provided that commencing with the year following the year in which a “change of control” (as defined in the employment agreement) occurs, Dr. Gainer’s entire base salary and annual bonus will be paid in cash. For 2017, the cash portion of Dr. Gainer’s base salary was $265,958. The employment agreement contains certain severance and change of control provisions as described in more detail under the heading “—Post-Termination Severance and Change in Control Arrangements.” The employment agreement also contains certain non-competition and non-solicitation provisions (each applicable during employment and for 18 months thereafter), as well as confidentiality and non-disparagement provisions (each applicable during employment and at all times thereafter).
Ben L. Shealy, Senior Vice President – Finance, Treasurer and Secretary. Effective October 12, 2016, we entered into an employment agreement with Mr. Shealy pursuant to which he serves as our Senior Vice President – Finance, Treasurer and Secretary. The employment agreement has an indefinite term. Mr. Shealy’s annual base salary under the agreement for 2017 was $237,900, and is subject to increase at the discretion of the Board. Mr. Shealy has the opportunity to earn a target annual bonus of 25 percent of his base salary. The Board may, in its discretion, pay a portion of Mr. Shealy’s annual salary and annual bonus in the form of equity or equity-based compensation, provided that commencing with the year following the year in which a “change of control” (as defined in the employment agreement) occurs, Mr. Shealy’s entire base salary and annual bonus will be paid in cash. For 2017, the cash portion of Mr. Shealy’s base salary was $178,400. The employment agreement contains certain severance and change of control provisions as described in more detail under the heading “—Post-Termination Severance and Change in Control Arrangements.” The employment agreement also contains certain non-competition and non-solicitation provisions (each applicable during employment and for 18 months thereafter), as well as confidentiality and non-disparagement provisions (each applicable during employment and at all times thereafter).
Other Compensatory Arrangements
The Compensation Committee administers the Company’s 2015 Equity Incentive Plan, as amended (the “2015 Equity Plan”), in which our named executive officers participate, the bonus payments made to our named executive officers provided for in the employment agreements discussed above under the heading”—Employment Agreements” and any other compensation-related matters as they otherwise determine in their discretion. The shares underlying the option grants made in 2017 to the named executive officers are scheduled to vest in equal parts each month until fully vested on the third anniversary of the grant date. The award agreements also provide that the awards will vest in full immediately upon a Change of Control, as defined in the 2015 Equity Plan.
We also provide our executive officers with annual bonuses payable in cash and or equity, as described and in the amounts for the fiscal years ended December 31, 2017 and 2016 described above in the Summary Compensation Table. These bonuses are determined in the discretion of our Compensation Committee based upon pre-established targets and performance goals. For the fiscal year ended December 31, 2017, our named executives performance goals included, among other things, strategic growth and financing initiatives, advancement of the Company's clinical program and further protection and expansion of the Company's intellectual property portfolio.
Outstanding Equity Awards at Fiscal Year End
The table below provides information regarding unexercised stock option awards held by each of our named executive officers that remained outstanding at December 31, 2017.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
|
|
|
|
|
Option Awards |
||||||||||||
|
Name |
|
Grant |
|
Number of Securities Options (#) |
|
|
Number of Securities Options (#) |
|
|
Option |
|
Option |
|
|||
|
David G. Kalergis |
|
5/17/2012 |
|
|
29,587 |
|
|
|
0 |
|
|
$ |
2.10 |
|
5/17/2022 |
|
|
|
|
10/9/2012 |
|
|
9,132 |
|
|
|
0 |
|
|
$ |
2.10 |
|
10/9/2022 |
|
|
|
|
10/9/2012 |
|
|
27,395 |
|
|
|
0 |
|
|
$ |
2.10 |
|
10/9/2022 |
|
|
|
|
10/5/2013 |
|
|
12,785 |
|
|
|
0 |
|
|
$ |
3.40 |
|
10/5/2023 |
|
|
|
|
10/5/2013 |
|
|
18,264 |
|
|
|
0 |
|
|
$ |
6.10 |
|
10/5/2023 |
|
|
|
|
12/1/2014 |
|
|
18,264 |
|
|
|
0 |
|
|
$ |
4.10 |
|
12/1/2024 |
|
|
|
|
12/1/2014 |
|
|
12,785 |
|
|
|
0 |
|
|
$ |
4.10 |
|
12/1/2024 |
|
|
|
|
10/30/2015 |
|
|
26,387 |
|
|
|
10,140 |
|
|
$ |
5.40 |
|
10/30/2025 |
|
|
|
|
10/30/2015 |
|
|
23,953 |
|
|
|
9,207 |
|
|
$ |
5.40 |
|
10/30/2025 |
|
|
|
|
5/16/2016 |
|
|
10,778 |
|
|
|
9,639 |
|
|
$ |
9.60 |
|
5/16/2026 |
|
|
|
|
12/8/2016 |
|
|
37,917 |
|
|
|
67,083 |
|
|
$ |
2.74 |
|
12/8/2026 |
|
|
|
|
5/10/2017 |
|
|
9,111 |
|
|
|
31,889 |
|
|
$ |
2.96 |
|
5/10/2027 |
|
|
John L. Gainer, Ph.D. |
|
5/17/2012 |
|
|
22,649 |
|
|
|
0 |
|
|
$ |
2.10 |
|
5/17/2022 |
|
|
|
|
10/9/2012 |
|
|
9,132 |
|
|
|
0 |
|
|
$ |
2.10 |
|
10/9/2022 |
|
|
|
|
10/9/2012 |
|
|
18,264 |
|
|
|
0 |
|
|
$ |
2.10 |
|
10/9/2022 |
|
|
|
|
10/5/2013 |
|
|
9,132 |
|
|
|
0 |
|
|
$ |
3.40 |
|
10/5/2023 |
|
|
|
|
10/5/2013 |
|
|
18,264 |
|
|
|
0 |
|
|
$ |
6.10 |
|
10/5/2023 |
|
|
|
|
12/1/2014 |
|
|
18,264 |
|
|
|
0 |
|
|
$ |
4.10 |
|
12/1/2024 |
|
|
|
|
12/1/2014 |
|
|
10,958 |
|
|
|
0 |
|
|
$ |
4.10 |
|
12/1/2024 |
|
|
|
|
10/30/2015 |
|
|
26,387 |
|
|
|
10,140 |
|
|
$ |
5.40 |
|
10/30/2025 |
|
|
|
|
10/30/2015 |
|
|
15,834 |
|
|
|
6,082 |
|
|
$ |
5.40 |
|
10/30/2025 |
|
|
|
|
5/16/2016 |
|
|
4,407 |
|
|
|
3,927 |
|
|
$ |
9.60 |
|
5/16/2026 |
|
|
|
|
12/8/2016 |
|
|
12,278 |
|
|
|
21,722 |
|
|
$ |
2.74 |
|
12/8/2026 |
|
|
|
|
5/10/2017 |
|
|
7,556 |
|
|
|
26,444 |
|
|
$ |
2.96 |
|
5/10/2027 |
|
|
Ben L. Shealy |
|
5/17/2012 |
|
|
42,006 |
|
|
|
0 |
|
|
$ |
2.10 |
|
5/17/2022 |
|
|
|
|
10/9/2012 |
|
|
20,090 |
|
|
|
0 |
|
|
$ |
2.10 |
|
10/9/2022 |
|
|
|
|
10/5/2013 |
|
|
9,132 |
|
|
|
0 |
|
|
$ |
3.40 |
|
10/5/2023 |
|
|
|
|
12/1/2014 |
|
|
10,958 |
|
|
|
0 |
|
|
$ |
4.10 |
|
12/1/2024 |
|
|
|
|
10/30/2015 |
|
|
17,153 |
|
|
|
6,590 |
|
|
$ |
5.40 |
|
10/30/2025 |
|
|
|
|
5/16/2016 |
|
|
4,731 |
|
|
|
4,228 |
|
|
$ |
9.60 |
|
5/16/2026 |
|
|
|
|
12/8/2016 |
|
|
5,778 |
|
|
|
10,222 |
|
|
$ |
2.74 |
|
12/8/2026 |
|
|
5/10/2017 |
5,133 |
17,967 |
$ |
2.96 |
5/10/2027 |
|||||||||||
________________________
|
(1) |
The unvested shares underlying each option grant are scheduled to vest in equal parts each month over the 36 month period following the grant date. |
401(k) Retirement Plan
We maintain the Diffusion Pharmaceuticals Inc. 401(k) plan pursuant to which all eligible employees, including our named executive officers, are entitled to make pre-tax and after-tax contributions of their compensation. In addition, the Company makes discretionary matching contributions at the rate of 100% for contributions up to 3% of the participant’s eligible compensation and 50% for any additional contributions up to 5% of the participant’s eligible compensation. The matching contributions received by our named executive officers in 2016 and 2017 are reported in the “All Other Compensation” column of the Summary Compensation Table above.
Post-Termination Severance and Change in Control Arrangements
As described under the heading “—Employment Agreements,” we have entered into employment agreements with each of Messrs. Kalergis and Shealy and Dr. Gainer that provide for certain severance and change of control benefits, subject to the execution and non-revocation of a release of claims by the executive or his estate (as applicable). Under Mr. Kalergis’ employment agreement, if his employment is terminated by us other than for “cause,” death or “disability,” or by Mr. Kalergis for “good reason” (as such terms are defined in the employment agreement), Mr. Kalergis will be entitled to any unpaid bonus earned in the year prior to the termination, a pro-rata portion of the bonus earned during the year of termination, continuation of base salary for 12 months, plus 12 months of COBRA premium reimbursement, provided that if such termination occurs within 60 days before or within 24 months following a “change of control” (as defined in the employment agreement), then Mr. Kalergis will be entitled to receive the same severance benefits as provided above, except that he will receive (a) a payment equal to two times the sum of his base salary and the higher of his target annual bonus opportunity and the bonus payment he received for the year immediately preceding the year in which the termination occurred instead of 12 months of base salary continuation, and (b) a payment equal to 36 times the monthly COBRA premium for him and his eligible dependents instead of 12 months of COBRA reimbursements (the payments in clauses (a) and (b) are paid in a lump sum in some cases and partly in a lump sum and partly in installments over 12 months in other cases). In addition, if Mr. Kalergis’ employment is terminated by us without cause or by Mr. Kalergis for good reason, in either case, upon or within 24 months following a change of control, then Mr. Kalergis will be entitled to full vesting of all equity awards received by him from us (with any equity awards that are subject to the satisfaction of performance goals deemed earned at not less than target performance, and with any equity award that is in the form of a stock option or stock appreciation right to remain outstanding and exercisable for 24 months following the termination date (but in no event beyond the expiration date of the applicable option or stock appreciation right)).
Under the employment agreements for Mr. Shealy and Dr. Gainer, in the event that the executive’s employment is terminated by us other than for “cause”, death or "disability” or upon his resignation for “good reason” (as such terms are defined in the applicable employment agreement), the executive will be entitled to any unpaid bonus earned in the year prior to the termination, a pro-rata portion of the bonus earned during the year of termination, continuation of base salary for 9 months, plus 12 months of COBRA premium reimbursement, provided that if such termination occurs within 60 days before or within 24 months following a “change of control” (as defined in the applicable employment agreement), then the executive will be entitled to receive the same severance benefits as provided above, except that he will receive (a) a payment equal to 1.5 times the sum of his base salary and the higher of his target annual bonus opportunity and the bonus payment he received for the year immediately preceding the year in which the termination occurred instead of 9 months of base salary continuation and (b) a payment equal to 18 times the monthly COBRA premium for the executive and his eligible dependents instead of 12 months of COBRA reimbursements (the payments in clauses (a) and (b) are paid in a lump sum in some cases and in installments over 9 or 12 months in other cases). In addition, if the executive’s employment is terminated by the Company without cause or by the executive for good reason, in either case, upon or within 24 months following a change of control, then the executive will be entitled to full vesting of all equity awards received by the executive from us (with any equity awards that are subject to the satisfaction of performance goals deemed earned at not less than target performance, and with any equity award that is in the form of a stock option or stock appreciation right to remain outstanding and exercisable for 24 months following the termination date (but in no event beyond the expiration date of the applicable option or stock appreciation right)).
Under the employment agreements for each of Messrs. Kalergis and Shealy and Dr. Gainer, in the event that the executive’s employment is terminated due to his death or disability, he (or his estate) will be entitled to any unpaid bonus earned in the year prior to the termination, a pro-rata portion of the bonus earned during the year of termination, 12 months of COBRA premium reimbursement and accelerated vesting of (a) all equity awards received in payment of base salary or an annual bonus and (b) with respect to any other equity award, the greater of the portion of the unvested equity award that would have become vested within 12 months after the termination date had no termination occurred and the portion of the unvested equity award that is subject to accelerated vesting (if any) upon such termination under the applicable equity plan or award agreement (with performance goals deemed earned at not less than target performance, and with any equity award that is in the form of a stock option or stock appreciation right to remain outstanding and exercisable for 12 months following the termination date or, if longer, such period as provided under the applicable equity plan or award agreement (but in no event beyond the expiration date of the applicable option or stock appreciation right)).
Further, under the terms of the stock option agreements with our executives, upon a completion of a “change of control” (as defined in the 2015 Equity Plan), options held by our executives will become immediately vested and remain exercisable through their expiration date regardless of whether the holder remains in the employment or service of the Company. Alternatively, in connection with a change of control, the Compensation Committee may, in its sole discretion, cash out the options.
DIRECTOR COMPENSATION
Overview of Director Compensation Program
As described in more detail under the heading “The Board, Its Committees and Corporate Governance—Compensation Committee—Responsibilities,” the Board has delegated to the Compensation Committee the responsibility, among other things, to establish and lead a process for the determination of compensation payable to our non-employee directors. The Compensation Committee makes recommendations regarding compensation payable to our non-employee directors to the entire Board, which then makes final decisions regarding such compensation.
The principal elements of our director compensation program for 2017 included:
|
● |
cash compensation in the form of annual cash retainers; and |
|
● |
long-term equity-based incentive compensation, in the form of stock options. |
We do not compensate our employee directors, Mr. Kalergis and Dr. Gainer, separately for serving on the Board. In addition, pursuant to the option agreement between the Company and Mr. Blech described below, Mr. Blech also does not receive the long-term incentive compensation and annual retainers otherwise payable to our non-employee directors.
Cash Compensation
The non-employee members of the Board were entitled to the following cash retainers for services in 2017:
|
Description |
Annual |
|||
|
Board Member |
$ | 32,500 | ||
|
Lead Independent Director of the Board |
$ | 3,000 | ||
|
Audit Committee Chair |
$ | 15,000 | ||
|
Compensation Committee Chair |
$ | 10,000 | ||
|
Nominating and Corporate Governance Committee Chair |
$ | 7,000 | ||
|
Audit Committee Member (other than Chair) |
$ | 6,000 | ||
|
Compensation Committee Member (other than Chair) |
$ | 5,000 | ||
|
Nominating and Corporate Governance Committee Member (other than Chair) |
$ | 3,000 | ||
The annual cash retainers are paid on a quarterly basis in arrears at the end of each calendar quarter. For example, the retainers paid at the end of the first calendar quarter are for the period from January 1 through March 31. The Compensation Committee has also reserved the right to make all or a portion of such payments in the form of equity rather than cash under certain conditions.
Long-Term Equity-Based Incentive Compensation
In addition to cash compensation, our non-employee directors receive long-term equity-based incentive compensation in the form of options to purchase shares of our common stock. Upon a non-employee director’s initial appointment to the Board, he or she shall receive a stock option award valued at $100,000 vesting in equal annual installments over three years. In addition, each non-employee director receives annually a stock option award valued at $50,000 vesting in equal monthly installments over three years (unless otherwise provided by the Compensation Committee). All such options have a ten-year term and an exercise price equal to the fair market value of our common stock on the grant date.
See the Director Compensation Table under the heading “—Summary Director Compensation Table for Fiscal 2017” for a summary of all options granted to our non-employee directors during the year ended December 31, 2017. See note 3 to the Director Compensation Table under the heading “—Summary Director Compensation Table for Fiscal 2017” for a summary of all options to purchase shares of our common stock held by our non-employee directors as of December 31, 2017.
Blech Option Agreement
In connection with his appointment to the Board on August 11, 2016 and in lieu of the compensation otherwise payable to our non-employee directors as described above, Mr. Blech was granted on August 31, 2016 a one-time stock option grant to purchase 204,907 shares of our common stock, which shares shall vest in equal quarterly installments over 10 years beginning September 30, 2016 (the “Blech Option Grant”). Mr. Blech generally has agreed not to sell any of the shares issuable upon exercise of the Blech Option Grant until August 11, 2021.
Summary Director Compensation Table for Fiscal 2017
The table below provides summary information concerning the compensation of each individual who served as a director of the Company during the year ended December 31, 2017, other than David G. Kalergis, our Chief Executive Officer, and John L. Gainer, Ph.D., our Chief Scientific Officer, whose compensation is described above under “Summary Compensation Table.”
DIRECTOR COMPENSATION TABLE
|
Name |
|
Fees Earned or |
|
|
Option |
|
|
All Other |
|
|
Total ($)(3) |
|
||||
|
Isaac Blech |
|
$ |
0 |
|
|
$ |
0 |
|
|
$ |
0 |
|
|
$ |
0 |
|
|
Mark T. Giles |
|
$ |
58,000 |
|
|
$ |
46,056 |
|
|
$ |
0 |
|
|
$ |
104,056 |
|
|
Alan Levin |
|
$ |
58,500 |
|
|
$ |
46,056 |
|
|
$ |
0 |
|
|
$ |
104,056 |
|
|
Robert Adams |
|
$ |
56,000 |
|
|
$ |
46,056 |
|
|
$ |
0 |
|
|
$ |
104,056 |
|
|
Robert Ruffolo(2) |
$ |
29,000 |
$ |
99,988 |
$ |
0 |
|
|
$ |
128,988 |
||||||
________________________
|
(1) |
The amounts shown in this column reflect the grant date fair value of option awards granted during 2017, calculated in accordance with the provisions of ASC Topic 718 (“ASC Topic 718”), determined without regard to forfeitures. The assumptions used in the Black-Scholes model for will be disclosed in our Annual Report on Form 10-K for the year ended December 31, 2017. |
|
(2) |
As Dr. Ruffolo joined the Board in June 2017, his cash fees were pro-rated for fiscal year 2017. The option awards column includes the initial option grant upon his appointment to the Board as described above under “—Long-Term Equity-Based Compensation.” |
The table below provides information regarding unexercised stock option awards held by each of our directors that remained outstanding at December 31, 2017.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
| Option Awards | ||||||||||||||||
|
Name |
|
Grant |
|
Number of Securities Options (#) |
|
|
Number of Securities Options (#) |
|
|
Option |
|
Option |
|
|||
|
Isaac Blech |
|
1/7/2014 |
|
|
1,745 |
|
|
|
0 |
|
|
$ |
30.00 |
|
1/6/2024 |
|
|
|
|
7/24/2014 |
|
|
8,745 |
|
|
|
0 |
|
|
$ |
39.20 |
|
7/23/2022 |
|
|
|
|
10/21/2014 |
|
|
7,500 |
|
|
|
0 |
|
|
$ |
48.00 |
|
7/10/2018 |
|
|
|
|
8/31/2016 |
|
|
30,738 |
|
|
|
174,169 |
(a) |
|
$ |
8.75 |
|
8/31/2026 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mark T. Giles |
|
5/17/2012 |
|
|
9,863 |
|
|
|
0 |
|
|
$ |
2.10 |
|
5/17/2022 |
|
|
|
|
10/9/2012 |
|
|
9,132 |
|
|
|
0 |
|
|
$ |
2.10 |
|
10/9/2022 |
|
|
|
|
10/5/2013 |
|
|
18,264 |
|
|
|
0 |
|
|
$ |
6.10 |
|
10/5/2023 |
|
|
|
|
12/1/2014 |
|
|
18,264 |
|
|
|
0 |
|
|
$ |
4.10 |
|
12/1/2024 |
|
|
|
|
10/30/2015 |
|
|
26,387 |
|
|
|
10,140 |
(b) |
|
$ |
5.40 |
|
10/30/2025 |
|
|
|
|
7/21/2016 |
|
|
2,770 |
|
|
|
3,078 |
(b) |
|
$ |
9.00 |
|
7/21/2026 |
|
|
|
|
12/8/2016 |
|
|
5,056 |
|
|
|
8,944 |
(b) |
|
$ |
2.74 |
|
12/8/2026 |
|
|
|
|
6/15/2017 |
|
|
11,950 |
|
|
|
11,950 |
(c) |
|
$ |
2.40 |
|
6/15/2027 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alan Levin |
|
6/15/2015 |
|
|
23,014 |
|
|
|
4,600 |
(b) |
|
$ |
4.10 |
|
6/15/2025 |
|
|
|
|
10/30/2015 |
|
|
26,387 |
|
|
|
10,140 |
(b) |
|
$ |
5.40 |
|
10/30/2025 |
|
|
|
|
7/21/2016 |
|
|
2,770 |
|
|
|
3,078 |
(b) |
|
$ |
9.00 |
|
7/21/2026 |
|
|
|
|
12/8/2016 |
|
|
5,056 |
|
|
|
8,944 |
(b) |
|
$ |
2.74 |
|
12/8/2026 |
|
|
|
|
6/15/2017 |
|
|
11,950 |
|
|
|
11,950 |
(c) |
|
$ |
2.40 |
|
6/15/2027 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Robert Adams |
|
5/17/2012 |
|
|
18,629 |
|
|
|
0 |
|
|
$ |
2.10 |
|
5/17/2022 |
|
|
|
|
10/9/2012 |
|
|
9,132 |
|
|
|
0 |
|
|
$ |
2.10 |
|
10/9/2022 |
|
|
|
|
10/5/2013 |
|
|
18,264 |
|
|
|
0 |
|
|
$ |
6.10 |
|
10/5/2023 |
|
|
|
|
12/1/2014 |
|
|
18,264 |
|
|
|
0 |
|
|
$ |
4.10 |
|
12/1/2024 |
|
|
|
|
10/30/2015 |
|
|
26,387 |
|
|
|
10,140 |
(b) |
|
$ |
5.40 |
|
10/30/2025 |
|
|
|
|
7/21/2016 |
|
|
2,770 |
|
|
|
3,078 |
(b) |
|
$ |
9.00 |
|
7/21/2026 |
|
|
|
|
12/8/2016 |
|
|
5,056 |
|
|
|
8,944 |
(b) |
|
$ |
2.74 |
|
12/8/2026 |
|
|
|
|
6/15/2017 |
|
|
11,950 |
|
|
|
11,950 |
(c) |
|
$ |
2.40 |
|
6/15/2027 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Robert Ruffolo |
|
6/29/2017 |
|
|
0 |
|
|
|
52,057 |
(d) |
|
$ |
2.31 |
|
6/29/2027 |
|
________________________
|
(a) |
The unvested shares underlying each option grant are scheduled to vest in equal parts each quarter over the 10-year period following the grant date. |
|
(b) |
The unvested shares underlying each option grant are scheduled to vest in equal parts each month over the 36 month period following the grant date. |
|
(c) |
The unvested shares underlying each option grant are scheduled to vest in equal parts each month over the 12 month period following the grant date. |
|
(d) |
The unvested shares underlying each option grant are scheduled to vest in equal parts each year over the 3-year period following the grant date. |
LEGAL MATTERS
The validity of the securities being offered by this prospectus will be passed upon for us by Dechert LLP, New York, New York. The underwriter is being represented by Ellenoff Grossman & Schole LLP, New York, New York.
EXPERTS
The consolidated financial statements of Diffusion Pharmaceuticals Inc. as of and for the years ended December 31, 2016 and 2015, have been incorporated by reference herein and in the registration statement in reliance upon the report of KPMG LLP, independent registered public accounting firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing. The audit report covering the December 31, 2016 consolidated financial statements contains an explanatory paragraph that states that the Company has suffered recurring losses from operations, has limited resources available to fund current research and development activities, and will require substantial additional financing to continue to fund its research and development activities, which raise substantial doubt about its ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of that uncertainty.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act, with respect to the securities being offered by this prospectus. This prospectus does not contain all of the information in the registration statement and its exhibits. For further information with respect to us and the securities offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
We file annual, quarterly and current reports, proxy statements and other information with the Commission. You may read and copy any of this information at the Commission’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. Please call the Commission at (800) SEC-0330 or (202) 942-8090 for further information on the public reference room. The Commission also maintains an Internet website that contains reports, proxy statements and other information regarding issuers, including us, who file electronically with the Commission. The address of that site is www.sec.gov.
We also maintain an Internet website at www.diffusionpharma.com, which can be used to access free of charge, through the investor relations section, our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, current reports on Form 8-K and any amendments to those reports, as soon as reasonably practicable after we electronically file such material with or furnish it to the Commission and all such reports of ours going forward. The information set forth on, or connected to, our website is expressly not incorporated by reference into, and does not constitute a part of, this prospectus.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The Commission allows us to incorporate by reference information into this prospectus. This means we can disclose information to you by referring you to another document we filed with the Commission. We will make those documents available to you without charge upon your oral or written request. Requests for those documents should be directed to Investor Relations Department, Diffusion Pharmaceuticals Inc., 1317 Carlton Avenue, Suite 200, Charlottesville, Virginia 22902, Attention: Secretary, telephone: (434) 220-0718. This prospectus incorporates by reference the following documents (other than any portion of the respective filings furnished, rather than filed, under the applicable Commission rules) that we have filed with the Commission but have not included or delivered with this prospectus:
|
• |
our Annual Report on Form 10-K for the fiscal year ended December 31, 2016, as amended, filed with the SEC on March 31, 2017. |
|
• |
our Quarterly Reports on Form 10-Q for the fiscal quarters ended March 31, 2017, June 30, 2017 and September 30, 2017, filed with the SEC on May 15, 2017, August 14, 2017 and November 13, 2017, respectively. |
|
• |
our Current Reports on Form 8-K filed with the SEC on January 9, 2017, January 26, 2017 (to the extent not furnished), March 15, 2017, April 3, 2017, April 11, 2017, May 26, 2017, June 16, 2017, July 6, 2017, November 2, 2017, November 20, 2017 and December 29, 2017; |
|
• |
our Definitive Proxy Statement on Schedule 14A filed with the SEC on May 12, 2017; and |
|
• |
the description of our common stock included in our amended registration statements on Form 8-A filed on November 8, 2016 under the Exchange Act, and any amendment or report we may file with the Commission for the purpose of updating such description. |
In addition, all documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, prior to the termination of the offering shall be deemed to be incorporated by reference into this prospectus (other than current reports or portions thereof furnished under Item 2.02 or Item 7.01 of Form 8-K).
You may request a free copy of any of the documents incorporated by reference in this prospectus by writing or telephoning us at the following address:
Diffusion Pharmaceuticals Inc.
1317 Carlton Avenue, Suite 200
Charlottesville, Virginia 22902
(434) 220-0718
Attention: Investor Relations
In accordance with Rule 412 of the Securities Act, any statement contained in a document incorporated by reference herein shall be deemed modified or superseded to the extent that a statement contained herein or in any other subsequently filed document which also is or is deemed to be incorporated by reference herein modifies or supersedes such statement.

9,154,930 Shares of Common Stock
Warrants to Purchase up to 9,154,930 Shares of Common Stock
PROSPECTUS
Sole Book-Running Manager
H.C. Wainwright & Co.
, 2018
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth all costs and expenses, other than underwriting discounts and commissions, paid or payable by us, in connection with the sale of the securities being registered under this registration statement. All amounts shown are estimates except for the SEC registration fee and the Financial Industry Regulatory Authority, Inc., or FINRA, filing fee.
|
Amount |
||||
|
SEC registration fee |
$ | 3,839 | ||
|
FINRA filing fee |
5,125 | |||
|
Blue-sky qualification fees and expenses |
1,500 | |||
|
Legal fees and expenses |
200,000 | |||
|
Accounting fees and expenses |
125,000 | |||
|
Transfer agent and registrar fees and expenses |
2,500 | |||
|
Miscellaneous expenses |
12,036 | |||
|
Total |
$ | 350,000.00 | ||
Item 14. Indemnification of Directors and Officers.
We are incorporated under the laws of the State of Delaware. Section 145 of the Delaware General Corporation Law (“DGCL”), provides that a Delaware corporation may indemnify any persons who are, or are threatened to be made, parties to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person was an officer, director, employee or agent of such corporation, or is or was serving at the request of such person as an officer, director, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided that such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was illegal. A Delaware corporation may indemnify any persons who are, or are threatened to be made, a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person was a director, officer, employee or agent of such corporation, or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit provided such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests except that no indemnification is permitted without judicial approval if the officer or director is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against the expenses which such officer or director has actually and reasonably incurred. Article V of our restated certificate of incorporation provides for indemnification of our directors and officers, and Article X of our amended and restated bylaws provides for indemnification of our directors, officers, employees and other agents, to the maximum extent permitted by the DGCL. We have entered into indemnification agreements with our officers and directors. In addition, we maintain a policy providing directors’ and officers’ liability insurance.
Section 102 of the DGCL permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability:
|
• |
for any breach of the director’s duty of loyalty to the corporation or its stockholders; |
|
• |
for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
|
• |
for acts related to unlawful stock repurchases, redemptions or other distributions or payment of dividends; or |
|
• |
for any transaction from which the director derived an improper personal benefit. |
Our restated certificate of incorporation and amended and restated bylaws include such a provision. Expenses incurred by any officer or director in defending any such action, suit or proceeding in advance of its final disposition shall be paid by us upon delivery to us of an undertaking, by or on behalf of such director or officer, to repay all amounts so advanced if it shall ultimately be determined that such director or officer is not entitled to be indemnified by us.
Item 15. Recent Sales of Unregistered Securities.
Set forth below is information regarding securities issued by us since January 1, 2014 that were not registered under the Securities Act of 1933, as amended, or the Securities Act. Also included is the consideration, if any, received by us, for such securities and information relating to the Securities Act, or rule of the SEC, under which exemption from registration was claimed.
Private Placement
In March 2017, we conducted a private placement (the “Private Placement”), pursuant to which we issued 12,376,329 shares of Series A Preferred Stock and 5-year warrants to purchase 12,376,329 shares of our common stock at an exercise price equal to $2.22 per share (the “Warrants”). The aggregate gross proceeds from the Private Placement were $25.0 million, prior to deducting placement agent fees and expenses payable by us. Maxim Merchant Capital, a division of Maxim Group LLC (“Maxim”), acted as our sole placement agent in the Private Placement. We issued to Maxim and its designees 5-year warrants, to purchase of 1,179,558 shares of common stock at an exercise price equal to $2.22 per share, with such warrants containing a cashless exercise provision. The securities were issued to accredited investors in reliance upon an exemption pursuant to Section 4(a)(2) of the Securities Act and the rules and regulations promulgated thereunder. Please see “Description of Capital Stock” for a description of our common stock, Series A Preferred Stock and Warrants.
As part of the Private Placement, we entered into registration rights agreements with the investors in the Private Placement and Maxim, pursuant to which we agreed to file a registration statement to register for resale the shares of common stock (i) issued and outstanding as a result of, or issuable upon, the conversion of the shares of Series A Preferred Stock, (ii) issued and outstanding as a result of, or issuable upon, the exercise of the Warrants sold in the Private Placement and (iii) issuable as payment of dividends accruing on the Series A Preferred Stock. Such registration statement was declared effective on June 1, 2017.
Reverse Merger
On January 8, 2016, we completed our merger (the “Merger”) with Diffusion Pharmaceuticals LLC, a Virginia limited liability company (“Diffusion LLC”). In connection with the Merger, we issued to the former equity holders of Diffusion LLC an aggregate number of shares of our common stock such that, immediately following the Merger and on a fully-diluted basis, the former equity holders of Diffusion LLC owned approximately 84.1% of our common stock and our pre-Merger stockholders owned approximately 15.9% of our common stock. The issuance of common stock in connection with the Merger was exempt from registration pursuant to Section 4(a)(2) of the Securities Act and Regulation D promulgated thereunder.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits.
See the Exhibit Index attached to this registration statement, which is incorporated by reference herein.
(b) Financial Statement Schedules.
No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or the notes thereto.
Item 17. Undertakings.
The undersigned Registrant hereby undertakes:
|
(1) |
That, for purposes of determining any liability under the Securities Act of 1933, each filing of the Registrant's annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan's annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in this registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
|
(2) |
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
|
(3) |
That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser (i) any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 promulgated under the Securities Act, (ii) any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant, (iii) the portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and (iv) any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
|
(4) |
For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
|
(5) |
For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
|
(6) |
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue. |
EXHIBIT INDEX
|
Exhibit
|
Description |
Method of Filing |
|
1.1 |
Form of Underwriting Agreement, by and between the Company and H.C. Wainwright & Co., LLC |
Filed herewith |
|
2.1 |
Incorporated by reference to Exhibit 2.1 to the registrant’s current report on Form 8-K filed on June 18, 2015 |
|
|
|
|
|
|
2.2 |
Incorporated by reference to Exhibit 2.1 to the registrant’s current report on Form 8-K filed on December 15, 2015 |
|
|
|
|
|
|
3.1 |
Certificate of Incorporation of Diffusion Pharmaceuticals Inc., as amended |
Incorporated by reference to Exhibit 3.1 to the registrant's annual report on Form 10-K for the year ended December 31, 2016 |
|
|
|
|
|
3.2 |
Incorporated by reference to Exhibit 3.4 to the registrant’s annual report on Form 10-K for the year ended December 31, 2015 |
|
|
|
|
|
|
3.3 |
Incorporated by reference to Exhibit 3.3 to the initial filing of this Registration Statement on Form S-1 on December 20, 2017 |
|
|
3.5 |
Incorporated by reference to Exhibit 3.2 to the registrant’s current report on Form 8-K filed on June 18, 2015 |
|
|
|
|
|
|
4.1 |
Form of Diffusion Pharmaceuticals Inc. Convertible Note Agreement |
Incorporated by reference to Exhibit 4.1 to the registrant’s current report on Form 8-K filed on October 3, 2016 |
|
|
|
|
|
4.2 |
Form of Diffusion Pharmaceuticals LLC Convertible Note Agreement |
Incorporated by reference to Exhibit 4.7 to the registrant’s annual report on Form 10-K for the year ended December 31, 2015 |
|
|
|
|
|
4.3 |
Form of Warrant issued to Investors in the 2017 Private Placement by Diffusion Pharmaceuticals Inc. |
Incorporated by reference to Exhibit 4.1 to the registrant’s current report on Form 8-K filed on March 15, 2017 |
|
4.4 |
Form of Warrant issued to Investors in the 2014 Private Placement by the Company |
Incorporated by reference to Exhibit 4.1 to the registrant’s current report on Form 8-K filed on April 29, 2014 |
|
|
|
|
|
|
|
4.5 |
Warrant, dated October 21, 2014, issued by the Company to Isaac Blech |
Incorporated by reference to Exhibit 4.5 to the registrant’s annual report on Form 10-K for the fiscal year ended December 31, 2014 |
|
|
|
|
|
|
|
4.6 |
Warrant, dated April 29, 2014, issued by the Company to Sol J. Barer, Ph.D. |
Incorporated by reference to Exhibit 4.4 to the registrant’s annual report on Form 10-K for the fiscal year ended December 31, 2014 |
|
|
|
|
|
|
|
4.7 |
Incorporated by reference to Exhibit 4.2 to the registrant’s current report on Form 10-Q for the fiscal quarter ended March 31, 2017 |
||
|
4.8 |
Incorporated by reference to Exhibit 10.2 to the registrant’s current report on Form 8-K filed on April 29, 2014 |
||
|
|
|
|
|
|
4.9 |
Registration Rights Agreement dated November 18, 2013 between the Company and Certain Holders |
Incorporated by reference to Exhibit 10.4 to the registrant’s current report on Form 8-K filed on November 22, 2013 |
|
|
|
|
|
|
| 4.10 | Form of Warrant to be issued to the underwriter in this offering | Filed herewith. | |
| 4.11 | Form of Warrant to be issued to investors in this offering | Filed herewith. | |
|
5.1 |
Filed herewith. |
||
|
10.1 |
Incorporated by reference to Exhibit 10.1 to the registrant’s current report on Form 8-K as filed on September 8, 2016 |
||
|
|
|
|
|
|
10.2 |
Incorporated by reference to Exhibit 10.1 to the registrant’s current report on Form 8-K as filed on October 13, 2016 |
||
|
|
|
|
|
|
10.3 |
Incorporated by reference to Exhibit 10.18 to the registrant’s annual report on Form 10-K for the year ended December 31, 2016 |
||
|
10.4 |
Incorporated by reference to Exhibit 10.2 to the registrant’s current report on Form 8-K as filed on June 18, 2015 |
||
|
|
|
|
|
|
10.5 |
Amendment No. 1 to Diffusion Pharmaceuticals Inc. 2015 Equity Incentive Plan** |
Incorporated by reference to Appendix B to the registrant’s definitive proxy statement on Schedule 14A filed on June 10, 2016 |
|
|
|
|
|
|
|
10.6 |
Form of Diffusion Pharmaceuticals Inc. Stock Option Award Agreement** |
Incorporated by reference to Exhibit 10.5 to the registrant’s annual report on Form 10-K for the year ended December 31, 2016 |
|
|
10.7 |
Form of Diffusion Pharmaceuticals LLC Stock Option Award Agreement** |
Incorporated by reference to Exhibit 10.24 to the registrant’s annual report on Form 10-K for the year ended December 31, 2015 |
|
|
10.8 |
Incorporated by reference to Exhibit 10.3 to the registrant’s current report on Form 8-K filed on June 18, 2015 |
||
|
|
|
|
|
|
10.9 |
Incorporated by reference to Exhibit 10.4 to the registrant’s current report on Form 8-K filed on June 18, 2015 |
||
|
|
|
|
|
|
10.10 |
Form of Stock Option Agreement between the Company and certain former Executive Officers** |
Incorporated by reference to Exhibit 10.12 to the registrant’s annual report on Form 10-K for the fiscal year ended December 31, 2014 |
|
|
|
|
|
|
|
10.11 |
Form of Stock Option Agreement between the Company and certain former Directors** |
Incorporated by reference to Exhibit 10.13 to the registrant’s annual report on Form 10-K for the fiscal year ended December 31, 2014 |
|
|
|
|
|
|
|
10.12 |
Incorporated by reference to Exhibit 10.3 to the registrant’s annual report on Form 10-K for the year ended December 31, 2015 |
||
|
|
|
|
|
|
10.13 |
Incorporated by reference to Exhibit 10.1 to the registrant’s current report on Form 8-K filed on March 15, 2017 |
||
|
10.14 |
Incorporated by reference to Exhibit 10.17 to the registrant’s annual report on Form 10-K for the year ended December 31, 2016 |
||
|
|
|
|
|
|
10.15 |
Incorporated by reference to Exhibit 10.2 to the registrant’s current report on Form 8-K filed on March 15, 2017 |
||
|
|
|
|
|
|
10.16 |
Incorporated by reference to Exhibit 10.1 to the registrant’s current report on Form 8-K filed on October 3, 2016 |
||
|
|
|
|
|
|
10.17 |
Incorporated by reference to Exhibit 10.2 to the registrant’s current report on Form 8-K filed on January 8, 2016 |
||
|
10.18 |
Incorporated by reference to Exhibit 10.4 to the registrant’s current report on Form 10-Q for the fiscal quarter ended March 31, 2017 |
||
|
21.1 |
Incorporated by reference to Exhibit 21.1 to the registrant’s annual report on Form 10-K for the year ended December 31, 2016 |
||
|
|
|
|
|
|
23.1 |
Consent of KPMG LLP, independent registered public accounting firm |
Filed herewith |
|
|
23.2 |
Included in Exhibit 5.1 hereto |
||
|
24.1 |
Included on and incorporated by reference from the signature pages to the initial filing of this Registration Statement on Form S-1 on December 20, 2017. |
||
|
* |
All exhibits and schedules to this agreement have been omitted pursuant to Item 601(b)(2) of Regulation S-K. The registrant will furnish the omitted exhibits and schedules to the SEC upon request by the SEC. |
|
** |
A management contract or compensatory plan or arrangement. |
SIGNATURES
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Charlottesville, Virginia, on the 16th day of January, 2018.
|
Diffusion Pharmaceuticals Inc. By: /s/ David G. Kalergis David G. Kalergis Chief Executive Officer (Principal Executive Officer) |
Pursuant to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
SIGNATURE |
|
TITLE |
|
DATE |
|
|
|
|
|
|
|
/s/ David G. Kalergis |
|
Chief Executive Officer and Chairman of the Board |
|
January 16, 2018 |
|
David G. Kalergis |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
/s/ Ben L. Shealy |
|
Senior Vice President, Finance, Treasurer and Secretary |
|
January 16, 2018 |
|
Ben L. Shealy |
|
(Principal Financial & Accounting Officer) |
|
|
|
|
|
|
|
|
|
* |
|
Vice Chairman of the Board of Directors |
|
January 16, 2018 |
|
Isaac Blech |
|
|
|
|
|
|
|
|
|
|
|
* |
|
Director |
|
January 16, 2018 |
|
Robert Adams |
|
|
|
|
|
|
|
|
|
|
|
* |
|
Director |
|
January 16, 2018 |
|
John L. Gainer, Ph.D. |
|
|
|
|
|
|
|
|
|
|
|
* |
|
Director |
|
January 16, 2018 |
|
Mark T. Giles |
|
|
|
|
|
|
|
|
|
|
|
* |
|
Director |
|
January 16, 2018 |
|
Alan Levin |
|
|
|
|
|
|
|
|
|
|
|
* |
|
Director |
|
January 16, 2018 |
|
Robert R. Ruffolo |
|
|
|
|
| * By: | /s/ David G. Kalergis | ||
| David G. Kalergis | |||
| Attorney in fact |
51
