Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 JPM PRESS RELEASE - HALOZYME THERAPEUTICS, INC. | ex991jpm1918pressrelease.htm |
| 8-K - 8-K JPM 2018 - HALOZYME THERAPEUTICS, INC. | a8-kjpm2018.htm |

Building a Premier Oncology
Biotech
Dr. Helen Torley, President & CEO
January 9, 2018
JP Morgan 36th Annual Healthcare Conference

Forward-Looking Statements
All of the statements in this presentation that are not statements of historical
facts constitute forward-looking statements within the meaning of the Private
Securities Litigation Reform Act of 1995. Examples of such statements include
possible activity, benefits and attributes of PEGPH20, future product
development and regulatory events and goals, anticipated clinical trial
results and strategies, product collaborations, our business intentions and
financial estimates and results, including projected revenue amounts. These
statements are based upon management’s current plans and expectations
and are subject to a number of risks and uncertainties which could cause
actual results to differ materially from such statements. A discussion of the risks
and uncertainties that can affect these statements is set forth in the
Company’s annual and quarterly reports filed from time to time with the
Securities and Exchange Commission under the heading “Risk Factors.” The
Company disclaims any intention or obligation to revise or update any
forward-looking statements, whether as a result of new information, future
events, or otherwise.
1

Halozyme Two Pillar Strategy
2
Removing Biological Barriers to Treatment
Enhancing Delivery
of Treatments
Late Stage Investigational
New Product
ENHANZE® PEGPH20
ENHANZE image from “The 5 Steps to Infuse HYQVIA.” http://www.hyqvia.com/resources/videos/

Significant Value Creation in 2017
ENHANZE®
• 2 new partners: BMS, Alexion
• Roche adds new target
• $175M upfront milestones
• Phase 3 starts: Janssen’s
Darzalex®
3
PEGPH20 - Pancreatic
• Supportive Phase 2
• Companion Diagnostic
• Strong progress in Phase 3
trial: HALO-301
PEGPH20 - Pan-Tumor
• > 20 patients enrolled:
Keytruda®/PEGPH20
• 2 studies in 4 tumors:
Tecentriq®/PEGPH20
Financial
• Cash balance exiting
2017 of $460M-$470M
Capabilities
• Initiating > 200 centers in 22 countries in Phase 3 Pancreas Cancer Trial
• Agreements and onboarding of 2 new partners
• Record number of API lots delivered

ENHANZE® Pillar

2017: Transformative Year for ENHANZE®
Pipeline
June 2017: RITUXAN HYCELA™ U.S. FDA Approval
Aug 2017: Initiate Phase 1 trial
Sept 2017: Landmark 11-target I-O deal
Sept 2017: Expanded collaboration, 1 new target
Nov 2017: Initiate Phase 3 trials, daratumumab SC
Dec 2017: Four-target collaboration
5
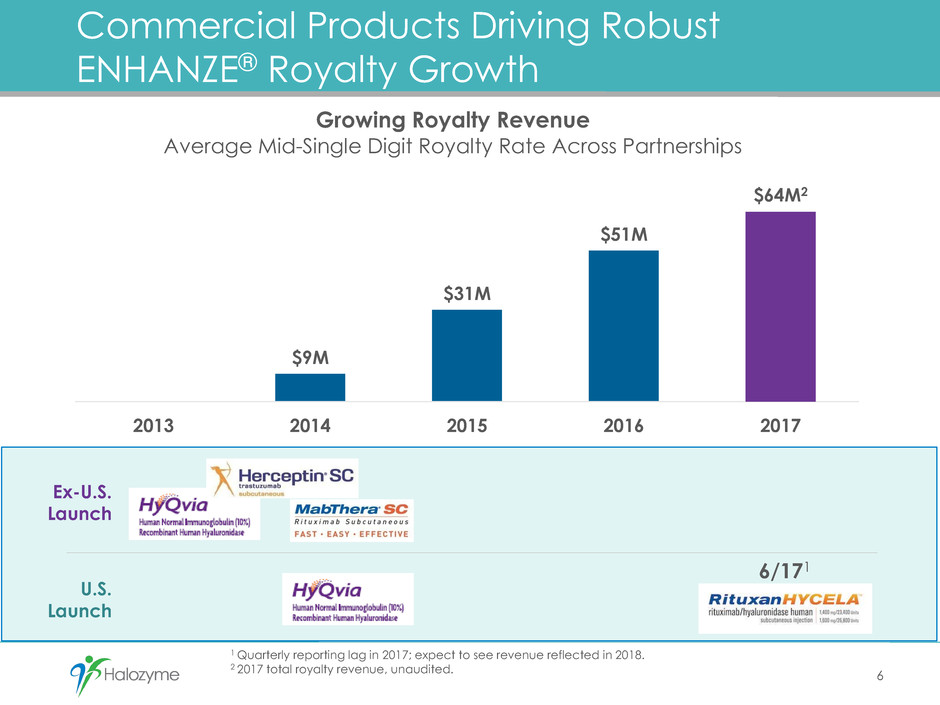
1 Quarterly reporting lag in 2017; expect to see revenue reflected in 2018.
2 2017 total royalty revenue, unaudited.
Commercial Products Driving Robust
ENHANZE® Royalty Growth
$9M
$31M
$51M
$64M2
2013 2014 2015 2016 2017
Growing Royalty Revenue
Average Mid-Single Digit Royalty Rate Across Partnerships
Ex-U.S.
Launch
U.S.
Launch
6
6/171

Approved Products Potential Future Products1,2
Assumes 7 additional products,
including Darzalex® and Opdivo®, are
globally approved and launched in
multiple indications
ENHANZE®: ~$1 Billion Royalty Revenue
Potential in 2027
1 Includes projections for subcutaneous versions of 7 targets not approved or commercially available. Innovator
revenues based on Bloomberg analyst projections. Conversion rates based on Halozyme internal projections.
2 Royalty revenue projection includes targets selected but not yet disclosed.
$1B
$500M
~$1 Billion
2017 2027
7

Approved and Commercialized
Phase 1
Phase 3
ENHANZE® Progress and Value Acceleration
in 2018
2018 2017
1
Darzalex®
2
Perjeta ®/Herceptin®
Anti-IL23
3
4
6 Products 9 products
8
2
3

Potential for Competitive
Differentiation
New Intellectual Property
and Exclusivity
Changing US Reimbursement and
Care Landscape
Reduced Treatment Burden and
Healthcare Costs
ENHANZE® Offers Four Paths for Differentiation
and Value Creation for Partners
9
1
2
3
4

Phase 3 Oncology Asset:
PEGPH20
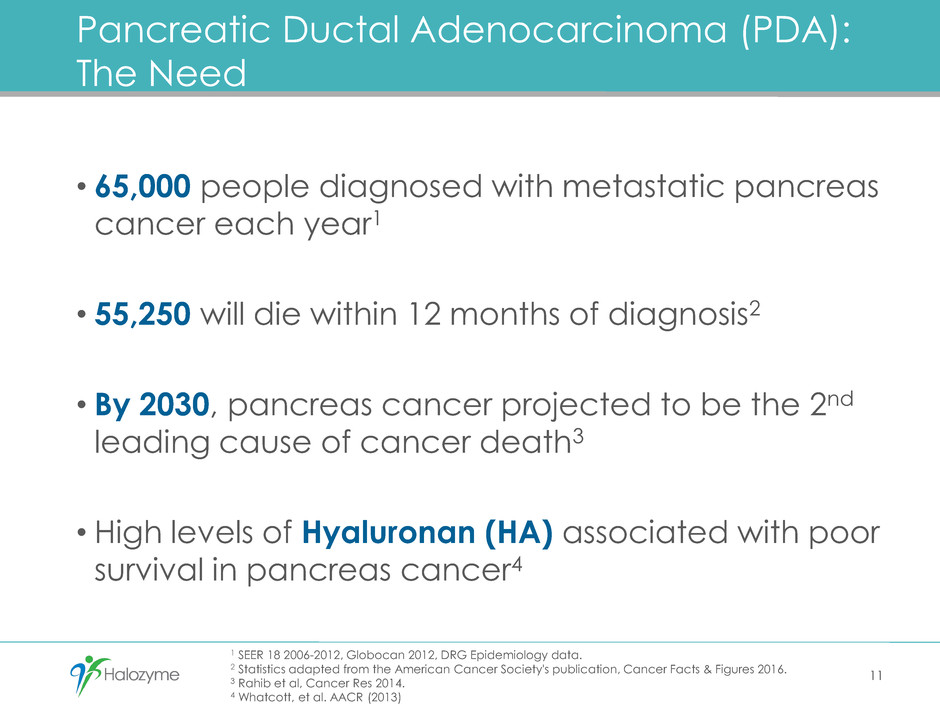
1 SEER 18 2006-2012, Globocan 2012, DRG Epidemiology data.
2 Statistics adapted from the American Cancer Society's publication, Cancer Facts & Figures 2016.
3 Rahib et al, Cancer Res 2014.
4 Whatcott, et al. AACR (2013)
Pancreatic Ductal Adenocarcinoma (PDA):
The Need
• 65,000 people diagnosed with metastatic pancreas
cancer each year1
• 55,250 will die within 12 months of diagnosis2
• By 2030, pancreas cancer projected to be the 2nd
leading cause of cancer death3
• High levels of Hyaluronan (HA) associated with poor
survival in pancreas cancer4
11

1 Provenzano, et al. Cancer Cell. 2012,21(3):418.
PEGPH20 Targets and Degrades Hyaluronan
Before PEGPH201
• Hard
• Fibrotic
• Hypovascular
MPA#1 + Gemcitabine fter PEGPH201
• Soft
• Cellular
• Hypervascular
12

1 SEER 18 2006-2012, Globocan 2012, DRG Epidemiology data.
2 Halozyme estimates for HA-High %.
3 DRG Epidemiology data, Halozyme internal estimates.
~$1B Potential Sales Opportunity in HA-High Metastatic
Pancreatic Ductal Adenocarcinoma (PDA)
13
Diagnosed Metastatic
PDA Annually US and EU 51
Estimated number of
HA-High patients
35-40% of population2
65,000
25,000
~$1B
Projected potential global
sales opportunity for successful
therapy in metastatic PDA3
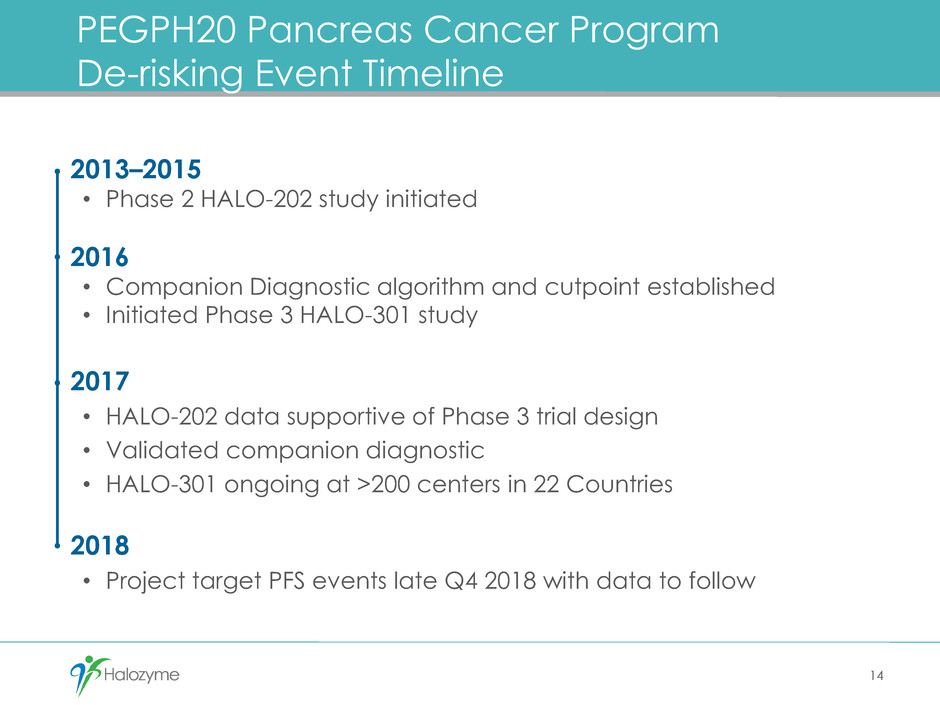
PEGPH20 Pancreas Cancer Program
De-risking Event Timeline
14
2013–2015
• Phase 2 HALO-202 study initiated
2016
• Companion Diagnostic algorithm and cutpoint established
• Initiated Phase 3 HALO-301 study
2017
• HALO-202 data supportive of Phase 3 trial design
• Validated companion diagnostic
• HALO-301 ongoing at >200 centers in 22 Countries
2018
• Project target PFS events late Q4 2018 with data to follow

HALO-301|Pancreatic: Global Phase 3 Trial
Enrolling in 22 Countries
• Randomized (2:1 PAG:AG), double-blind, placebo-controlled, global
• Project to achieve target number of PFS events late Q4 2018,
triggering final data collection, cleaning and interim analysis
PEGPH20 + ABRAXANE®
+ gemcitabine (PAG)
ABRAXANE® +
gemcitabine (AG) +
placebo
1L Metastatic
PDA
High-HA
patients
N=420-570
Primary Endpoints:
• Progression-Free
Survival (PFS)
• Overall Survival (OS)
15
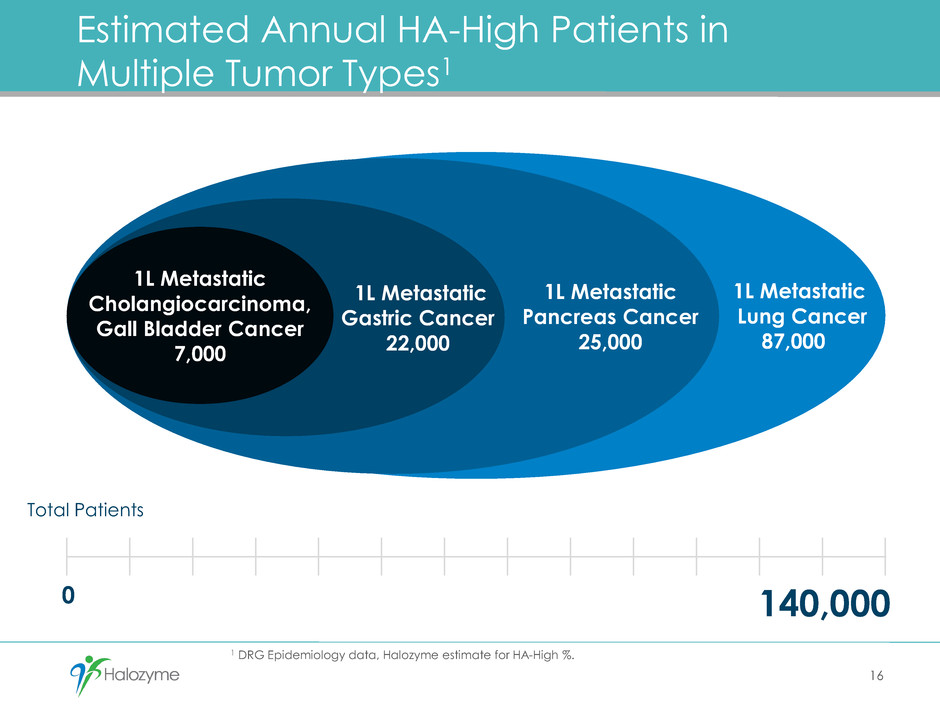
1 DRG Epidemiology data, Halozyme estimate for HA-High %.
1L Metastatic
Lung Cancer
87,000
1L Metastatic
Pancreas Cancer
25,000
1L Metastatic
Gastric Cancer
22,000
Estimated Annual HA-High Patients in
Multiple Tumor Types1
1L Metastatic
Cholangiocarcinoma,
Gall Bladder Cancer
7,000
140,000
Total Patients
0
16

ISTs: SWOG study enrollment closed in March 2017 following futility analysis
in all-comer population. Data collection and analysis ongoing.
Breast Cancer
Gastric Cancer,
NSCLC
Pancreas Cancer,
Gastric Cancer
Gall Bladder Cancer,
Cholangiocarcinoma
Robust Pan-Tumor Testing of PEGPH20
17
1 Pending continued enrollment rate.

Financial Update

Record 2017 Financial Performance
Jan 2017 2017 FY Estimate
Net Revenue $115M to $130M $310M to $320M
New ENHANZE®
agreements: $172M
Operating
Expenses
$240M to $250M $230M to $240M
Disciplined expense
control, flat spending to
2016
Operating Cash
(Burn) / Flow
($75M to $85M) $125M to $135M
Excludes impact of
financing, repayment of
debt principal
Year-end Cash $100M to $110M $460M to $470M
New ENHANZE revenue,
$135M equity raise
19

2018 Financial Guidance
2018
Net Revenue
• Royalty Growth
• Product Sales
$115M to $125M
25% - 30%
API product orders lower as a
result of planned partner
manufacturing transition
• Does not include potential
new ENHANZE® agreements
Operating Expenses $230M to $240M
• Disciplined expense control,
flat to 2017
Operating Cash Burn ($75M) to ($85M)
• Excludes impact of financing,
repayment of debt principal
Debt Repayment ~($95M)
• Includes royalty-backed and
Oxford/SVB loans
Year-end Cash $305M to $315M • Cash runway into 2020
20
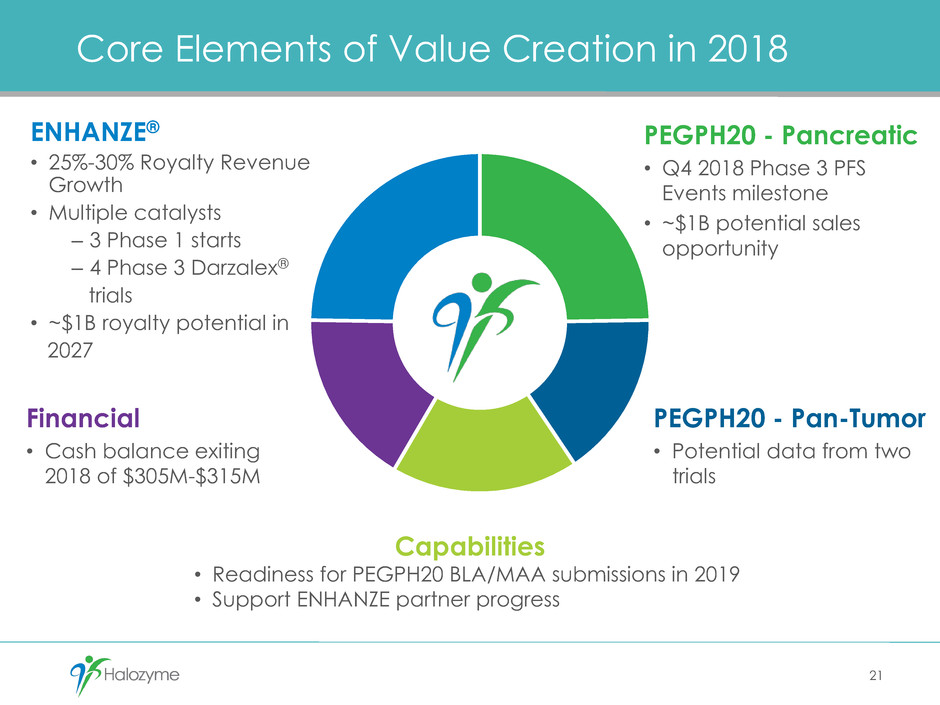
Core Elements of Value Creation in 2018
ENHANZE®
• 25%-30% Royalty Revenue
Growth
• Multiple catalysts
– 3 Phase 1 starts
– 4 Phase 3 Darzalex®
trials
• ~$1B royalty potential in
2027
21
PEGPH20 - Pancreatic
• Q4 2018 Phase 3 PFS
Events milestone
• ~$1B potential sales
opportunity
PEGPH20 - Pan-Tumor
• Potential data from two
trials
Financial
• Cash balance exiting
2018 of $305M-$315M
Capabilities
• Readiness for PEGPH20 BLA/MAA submissions in 2019
• Support ENHANZE partner progress

Building a Premier Oncology
Biotech
Dr. Helen Torley, President & CEO
January 9, 2018
JP Morgan 36th Annual Healthcare Conference
