Attached files
| file | filename |
|---|---|
| 8-K - 8-K - LABORATORY CORP OF AMERICA HOLDINGS | form8-k1918jpmppt.htm |

J.P. MORGAN
HEALTHCARE CONFERENCE
JANUARY 9, 2018 | SAN FRANCISCO, CA

1
FORWARD LOOKING STATEMENT
Cautionary Statement Regarding Forward Looking Statements
This presentation contains forward-looking statements, including statements about the Company’s performance
against strategic objectives and the impact of various factors on operating and financial results. Each of these
forward-looking statements is subject to change based on various risks and uncertainties, including without
limitation, competitive actions and other unforeseen changes and uncertainties in the marketplace, changes in
government regulations, including healthcare reform, changes in payer regulations or policies, other adverse actions
of governmental and third-party payers, failure to maintain or develop customer relationships, changes in testing
guidelines or recommendations, adverse results in material litigation matters, the impact of changes in tax laws and
regulations, and failures in information technology systems or data security.

2
AGENDA
Company Overview
2018 Priorities
Long-term Strategic Initiatives
Financial Strength

3
WHO WE ARE
Our Mission
is to
improve health
and improve lives
LabCorp is
a leading global
life sciences company
that is deeply integrated
in guiding patient care
Our Strategic Objectives are to:
Deliver World-Class Diagnostics
Bring Innovative Medicines to Patients Faster
Use Technology to Improve the Delivery of Care

4
LABCORP OVERVIEW
• Provides diagnostic, drug development
and technology-enabled products and solutions
for ~120 million patient encounters per year
• Operates in two segments – Diagnostics and
Drug Development
• >57,000 mission-driven employees worldwide
• Proprietary data sets with >30 billion lab test results
and >175,000 unique investigators
• Leading scientific and therapeutic expertise, including
>1,800 employed MDs and PhDs
A Leading Global Life Sciences Company
Consolidated Financial Summary(1)
Year Ended
Implied
Growth 2017E(2) 2016
Revenue $10,216 $9,437 8.3%
Adj. EPS $9.50 $8.83 7.6%
Free Cash Flow $990 $897 10.4%
1. Adjusted operating income, margin and earnings per share exclude amortization, restructuring charges
and other special items; dollars in millions, except per share data.
2. Based on the midpoint of guidance issued on October 25, 2017.
5
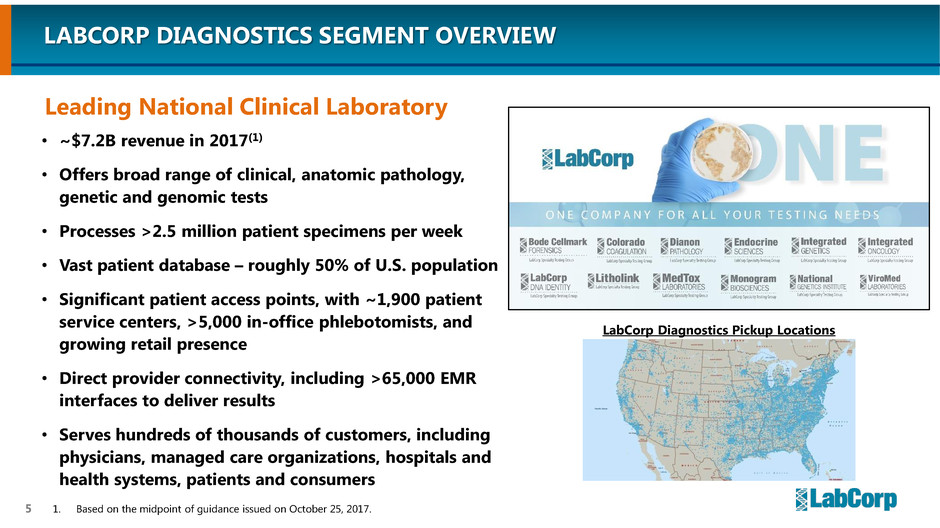
LABCORP DIAGNOSTICS SEGMENT OVERVIEW
• ~$7.2B revenue in 2017(1)
• Offers broad range of clinical, anatomic pathology,
genetic and genomic tests
• Processes >2.5 million patient specimens per week
• Vast patient database – roughly 50% of U.S. population
• Significant patient access points, with ~1,900 patient
service centers, >5,000 in-office phlebotomists, and
growing retail presence
• Direct provider connectivity, including >65,000 EMR
interfaces to deliver results
• Serves hundreds of thousands of customers, including
physicians, managed care organizations, hospitals and
health systems, patients and consumers
1. Based on the midpoint of guidance issued on October 25, 2017.
Leading National Clinical Laboratory
LabCorp Diagnostics Pickup Locations

6
COVANCE DRUG DEVELOPMENT OVERVIEW
• ~$3.0B revenue in 2017(1)
• Market leader in early development, central laboratory,
and Phase I-IV clinical trial management services
• Xcellerate® includes one of the world’s most
comprehensive investigator performance databases
• Collaborated on 86% of the 22 novel drugs approved by
FDA in 2016, including all 4 approved oncology drugs,
and 8 of 9 drugs treating rare and orphan diseases
• Involved in the development of all of the top 50
best-selling drugs on the market(2)
• Acquired Chiltern in 2017, a leading global CRO to
expand scale in Asia-Pacific, expand FSP offering and
strengthen emerging-to-mid biopharma capabilities
Leading CRO / Drug Development Services Provider
1. Based on the midpoint of guidance issued on October 25, 2017, which includes results
from Chiltern as of September 1, 2017.
2. Ranking based on 2016 net sales.
Region
Approximate Number
of Unique Sites
Africa 1,500+
Asia-Pacific 24,000+
Central/Eastern Europe 12,000+
Latin America 9,500+
Middle East 350+
North America 28,000+
Western Europe 21,000+

7
29%
23% 11%
32%
3% 3%
(2)
29%
23% 11%
32%
3%3%
Pharma & Biotech
Other Payers
Medicare & Medicaid
Managed Care (Fee for Service)
Patient
Managed Care (Capitated)
(2)
Attractive Customer Mix and Geographic Presence(1)
EXPANDED REVENUE BASE
1. Based on nine months ended September 30, 2017, which includes results from Chiltern as of September 1, 2017. Does not tie due to rounding.
2. Includes physicians and hospitals, occupational testing services, non-U.S. clinical diagnostic laboratory operations, nutritional chemistry and
food safety operations, and Beacon LBS.
29%
23% 11%
32%
3%3%
Pharma & Biotech
Other Payers
Medicare & Medicaid
Managed Care (Fee for Service)
Patient
Managed Care (Capit ted)
(2)
USA Rest of World
81%
19%
Managed Care (Fee for Service)
Patient
Managed Care (Capitated)
Pharma & Biotech
Other Payers (2)
edicare & Medicaid

8
AGENDA
Company Overview
2018 Priorities
Long-term Strategic Initiatives
Financial Strength

9
OUR 2018 PRIORITIES
Drive Profitable
Growth
Optimize
Enterprise
Margins
Integrate Key
Acquisitions

10
1. Based on nine months ended September 30.
2. Includes $1.0 billion from acquisition of Chiltern.
DRIVING PROFITABLE GROWTH
Diagnostics: Capitalize on Growth Opportunities
• Health systems, large physician
groups and managed care
partnerships
• Expand 23andMe collaboration
• Mitigate pricing impact of PAMA
Drug Development: Build on Existing Momentum
to Exceed Historical Growth Rates
• Convert backlog into profitable revenue growth
• Maintain broad-based strength in net orders
• Capitalize on strategic investments in leadership,
sales force and technology
• Women’s health, genetics and
medical drug monitoring
portfolio and capabilities
• Pursue accretive acquisitions
• Continue focus on quality,
service and innovation
2.0%
1.2%
3.2%
Year Over Year
Organic Volume Growth
2015 2016 2017(1)
(2)

11
• Generates approximately
$500 million in profitable revenue
growth in 2018
• Successful “Best of the Best”
approach to selecting and
retaining talent
• Dedicated and experienced
integration teams, focused on
customer retention and synergies
Maximize Value Through Flawless Integration
INTEGRATING KEY ACQUISITIONS
Mount Sinai
Clinical Outreach
Lab Assets
12
OPTIMIZING ENTERPRISE MARGINS
Continue Value Creation Through the
LaunchPad Business Process Improvement Initiative
Covance LaunchPad
• Applying LaunchPad principles to
Drug Development
• Rightsizing implemented in mid-2017;
$25 million in incremental savings in 2018
• Multi-year initiative, details to be provided
on Q4 earnings call
• Aided by Chiltern capabilities and expertise
Diagnostics LaunchPad
• Ongoing benefit from reengineering projects
• Additional opportunities, including
streamlining delivery of services
Opportunities for Productivity Gains
Automation
Global Service
Delivery Model
Procurement
New Tools
and Technology

13
AGENDA
Company Overview
2018 Priorities
Long-term Strategic Initiatives
Financial Strength

14
Transition to
Value-Based Care
Enhance
Drug Development
Process
Role of the
Consumer
HEALTHCARE IS UNDERGOING A PERIOD OF UNPRECEDENTED CHANGE
• Improving efficiency in care
delivery
• Reducing the overall cost of
patient care
• Utilizing advanced tools and
analytics to deliver better
outcomes via personalized
medicine and population
health
• Dealing with increased trial
complexity, and competition
for patients and investigators
• Greater need for scalable tools
and processes to initiate and
manage trials
• Increased sponsor demand for
data-driven study design and
execution, as well as access to
relevant analytes, biomarkers
and tests
• Increased interest in and
influence over healthcare
decision-making
• Technology advances driving
expectation of convenience
• Consumer satisfaction
increasingly important to
other healthcare stakeholders

15
EXPANDING LABCORP’S ROLE
IN THE HEALTHCARE SYSTEM OF THE FUTURE
• National access
• Comprehensive test menu
• Extensive sales and service organization
• Scientific innovation
• Cost advantages through
economies of scale
Clinical Decision Support
• Programs for key disease states
• Lab reports incorporate care
guidelines
• Developed by physicians
• Data monitoring drives
optimal care management
Leading Laboratory Services
Payer and Provider Collaboration
• Help stakeholders achieve total cost of care
metrics in value-based care contracts
• Actionable lab results
• Global patient results data
• MACRA, HEDIS, and ACO quality metrics
• Care Intelligence® population health
Drug Development Solutions
Value-Based Care Solutions
• Companion diagnostics leadership
• Potential provider revenue stream
from increased participation in
clinical trials
• Cost savings to patients and payers
• “Real World” data

16
EXPANDING LABCORP’S ROLE
IN THE HEALTHCARE SYSTEM OF THE FUTURE
• Xcellerate® suite of informatics
• Companion diagnostics leadership
• Differentiated and growing patient and
investigator database
• Insight through patient engagement
• Dedicated biotech offering through
combination of Covance and Chiltern
• Extended Lab Management Services
• Early Phase Development Solutions
Streamlining Clinical Studies
High quality
data
Proactive risk-based
monitoring
Optimal site
selection
Transparency and
secure collaboration
Efficient product
supply management
Proactive safety
monitoring

17
On pace to
significantly exceed $100 million
in cumulative new CDx-related revenue from
the acquisition of Covance through 2018
• Dedicated CDx organization with capabilities
across development, validation, testing,
regulatory support, commercialization and
market access
• Opened purpose built, state of the art CDx
laboratory, with focus on genomics and
molecular pathology
• Supported ~70% of CDx on the market(1)
• Customizable offering -- in vitro diagnostic
(IVD) partnerships and single site pathway
• Collaborated with over 35 clients on more
than 150 CDx projects in 2017(2)
EXPANDING LABCORP’S ROLE
IN THE HEALTHCARE SYSTEM OF THE FUTURE
Increasing Our Leadership in
Companion and Complementary Diagnostics (CDx)
1. As of January 1, 2018.
2. Based on results through November 2017.
3. As of September 30, 2017.
$114
$153
$276
2015 2016 2017
CDx-Related Backlog
(Dollars in millions)
(3)

18
LabCorp Data is a Key Competitive Advantage for
Patient Recruitment, Site Selection and Protocol Design
EXPANDING LABCORP’S ROLE
IN THE HEALTHCARE SYSTEM OF THE FUTURE
LabCorp Diagnostics Patient Data
• Our patient data is identifiable
• Represents the “real world”
• Billions of data points
• Lab values essential for
inclusion/exclusion criteria
Covance Drug Development
Investigator Data
• Our investigator data is global
• Filter at therapeutic and protocol level
• 175,000+ investigators across
~100,000 sites
• Insight into site location,
performance and study saturation

19
EXPANDING LABCORP’S ROLE
IN THE HEALTHCARE SYSTEM OF THE FUTURE
Incorporating Patient Intelligence
into Clinical Research and Drug Development • Powered by survey responses from
patients that want to be contacted
about clinical trials
• Supported by LabCorp patient
flow and increased emphasis on
scaling opt-in database

20
EXPANDING LABCORP’S ROLE
IN THE HEALTHCARE SYSTEM OF THE FUTURE
Developing a Broad Consumer Platform to Create Deep Relationships
• Organizing around the empowered
consumer
• Creating a convenient, seamless
experience
• Providing easy access to lab test results
and personalized content
• Offering price transparency to highlight
access to highest quality, low-cost
diagnostics

21
EXPANDING LABCORP’S ROLE
IN THE HEALTHCARE SYSTEM OF THE FUTURE
• Patient Service Centers opened inside Walgreens stores
• Strong patient volume, net promoter scores, and patient
feedback
• Start to roll out to new markets in 2018
• Launch new at-home, self-collection offering built on LabCorp’s
10 years of experience with dried blood spot testing
• Invest in expanded capacity and enhanced automation to
support 23andMe strategic collaboration
• Collaborate on new delivery models, such as telehealth and
on-demand phlebotomy
Bringing our High-Quality Offering to Consumers

22
2016
>$200 million
2017
~$500 million
OUR DIFFERENTIATED SOLUTIONS ARE RESONATING WITH CUSTOMERS
Value-Based Care
Solutions
Streamlining
Clinical Studies
Consumer Platform
Completed
3 marquee transactions
in 2017
Cumulative new orders won through
the combination of LabCorp patient
data and Covance capabilities:
On track to deliver
$150 million
in cumulative new revenue
from the acquisition of Covance
through 2018
41%
28%
Patients Seen in Denver
LabCorp at Walgreens
Patients not
seen in past
12 months
Patients
new to
LabCorp
LabCorp PSCs in
Walgreens stores are
attracting new patients

23
AGENDA
Company Overview
2018 Priorities
Long-term Strategic Initiatives
Financial Strength
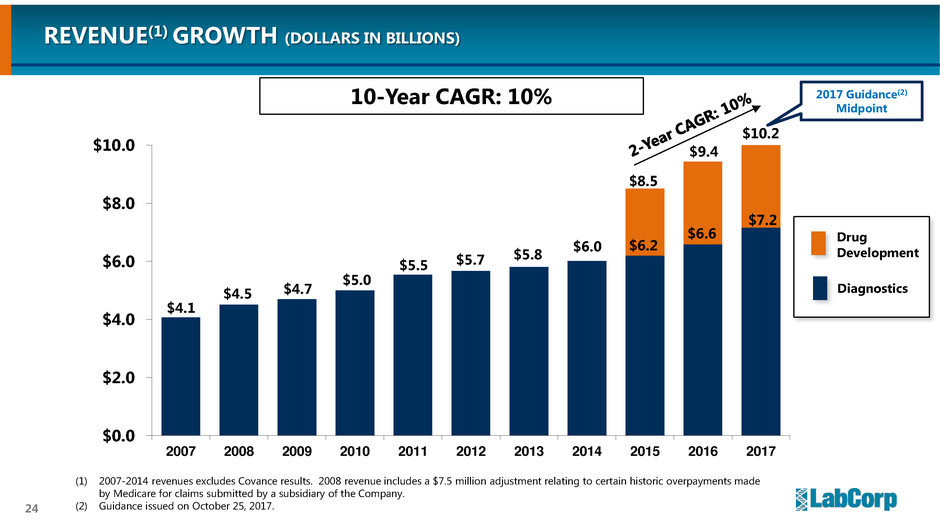
24
$4.1
$4.5 $4.7
$5.0
$5.5 $5.7
$5.8
$6.0
$0.0
$2.0
$4.0
$6.0
$8.0
$10.0
2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017
$8.5
$9.4
$10.2
$6.2
$6.6
$7.2
REVENUE(1) GROWTH (DOLLARS IN BILLIONS)
(1) 2007-2014 revenues excludes Covance results. 2008 revenue includes a $7.5 million adjustment relating to certain historic overpayments made
by Medicare for claims submitted by a subsidiary of the Company.
(2) Guidance issued on October 25, 2017.
2017 Guidance(2)
Midpoint
Drug
Development
Diagnostics
10-Year CAGR: 10%

25
ADJUSTED EPS(1)(2) GROWTH
(1) EPS, as presented, represents adjusted, non-GAAP financial measures (excludes amortization, restructuring and other special charges). Diluted EPS, as
reported in the Company’s Annual Report were: $3.93 in 2007; $4.16 in 2008; $4.98 in 2009; $5.29 in 2010; $5.11 in 2011; $5.99 in 2012; $6.25 in 2013;
$5.91 in 2014; $4.35 in 2015; and $7.02 in 2016.
(2) 2007-2014 figures exclude Covance results, and other items discussed in the Appendix.
(3) Guidance issued on October 25, 2017.
2017 Guidance(3)
Midpoint
$4.45
$4.91
$5.24
$5.98
$6.37
$6.82 $6.95 $6.80
$7.91
$8.83
$9.50
$0.00
$2.00
$4.00
$6.00
$8.00
$10.00
2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017
10-Year CAGR: 8%

26
STRONG FREE CASH FLOW(1) (DOLLARS IN MILLIONS)
(1) Free Cash Flow represents Operating Cash Flow less Capital Expenditures in each of the years presented.
2008-2014 figures exclude Covance results, and other items discussed in the Appendix.
(2) Guidance issued on October 25, 2017.
10 Year Average Free Cash Flow: $732 million
2017 Guidance(2)
Midpoint
$624
$748 $758
$759
$668
$617
$536
$727
$897
$990
$0
$200
$400
$600
$800
$1,000
2008 2009 2010 2011 2012 2013 2014 2015 2016 2017

27
TRACK RECORD OF EFFECTIVE AND BALANCED
CAPITAL DEPLOYMENT TO BUILD SHAREHOLDER VALUE
Approximately $2.3 Billion in
Capital Deployment in 2017(1)
Chiltern Acquisition
$1.2 Billion (52%)
LabCorp
Diagnostics
Acquisitions
$0.6 Billion (26%)
1. Based on nine months ended September 30, 2017.
2018 Free Cash Flow
M&A Priorities
• Diagnostics “tuck-in” transactions
Return of Capital to Shareholders
• Continue share repurchases
Debt Reduction
• Pay down debt to reduce leverage

28
KEY TAKEAWAYS
Global leader in life sciences with leading
diagnostics and drug development businesses
strategically positioned to improve health and lives
Relentless focus on execution in 2018
Driving long-term growth through innovation
in value-based care, the drug development process,
and our consumer platform

J.P. MORGAN
HEALTHCARE CONFERENCE
JANUARY 9, 2018 | SAN FRANCISCO, CA
30
Appendix

31
FOOTNOTES TO “ADJUSTED EPS GROWTH” SLIDE
1) EPS, as presented, represents adjusted, non-GAAP financial measures (excludes amortization, restructuring and other special charges). Diluted EPS, as reported in the Company’s
Annual Report were: $3.24 in 2006; $3.93 in 2007; $4.16 in 2008; $4.98 in 2009; $5.29 in 2010; $5.11 in 2011; $5.99 in 2012; $6.25 in 2013; $5.91 in 2014; $4.34 in 2015; and $7.02 in
2016.
2) 2006-2014 figures exclude Covance results. Excluding the $0.06 per diluted share impact of restructuring and other special charges and the $0.23 per diluted share impact from
amortization in 2006; excluding the $0.25 per diluted share impact of restructuring and other special charges and the $0.27 per diluted share impact from amortization in 2007;
excluding the $0.44 per diluted share impact of restructuring and other special charges and the $0.31 per diluted share impact from amortization in 2008; excluding the ($0.09) per
diluted share impact of restructuring and other special charges and the $0.35 per diluted share impact from amortization in 2009; excluding the $0.26 per diluted share impact of
restructuring and other special charges and the $0.43 per diluted share impact from amortization in 2010; excluding the $0.72 per diluted share impact of restructuring and other
special charges, the $0.03 per diluted share impact from a loss on the divestiture of assets and the $0.51 per diluted share impact from amortization in 2011; excluding the $0.29 per
diluted share impact of restructuring and other special charges and the $0.54 per diluted share impact from amortization in 2012; excluding the $0.15 per diluted share impact of
restructuring and other special charges and the $0.55 per diluted share impact from amortization in 2013; excluding the $0.34 per diluted share impact of restructuring and other
special charges and the $0.55 per diluted share impact from amortization in 2014; excluding the $2.44 per diluted share impact of restructuring and other special charges and the
$1.13 per diluted share impact from amortization in 2015; and excluding the $0.64 per diluted share impact of restructuring and other special charges and the $1.17 per diluted
share impact from amortization in 2016.
3) Guidance issued on October 25, 2017.

32
FOOTNOTES TO “STRONG FREE CASH FLOW” SLIDE(1)
(1) 2007-2014 figures exclude Covance results.
(2) Operating Cash Flow and Free Cash Flow in 2011 exclude the $49.5 million Hunter Labs settlement.
LABORATORY CORPORATION OF AMERICA HOLDINGS
Reconciliation of Non-GAAP Financial Measures
(in millions)
Free Cash Flow: 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016
Operating Cash Flow 710$ 781$ 862$ 884$ 905$ 841$ 819$ 739$ 982$ 1,176$
Less: Capital Expenditures (143) (157) (115) (126) (146) (174) (202) (204) (256) (279)
Free Cash Flow 567$ 624$ 748$ 758$ 759$ 668$ 617$ 536$ 727$ 897$
(2)
(2)
