Attached files
| file | filename |
|---|---|
| 8-K - 8-K - INVESTOR DECK 2018 - Orexigen Therapeutics, Inc. | orex-8k_20180108.htm |

January, 2018 Business Update Exhibit 99.1

Forward Looking Statements This presentation contains forward-looking statements about Orexigen Therapeutics, Inc. and its Contrave® product. Words such as “believes,” “anticipates,” “plans,” “expects,” “indicates,” “will,” “should,” “intends,” “potential,” “suggests,” “assuming,” “designed” and similar expressions are intended to identify forward‐looking statements. These statements are based on the Company‘s current beliefs and expectations. These forward‐looking statements include statements regarding: the potential success of marketing and commercialization efforts for Contrave in the United States; the potential for Contrave and MysimbaTM to achieve commercial success globally, including through potential partnership arrangements outside the United States, and the potential timing of related launch plans and regulatory filings; the Company’s future financial and sales projections, including its sales growth projections through 2018; and the status of various strategic plans and initiatives. The inclusion of financial modeling, forward‐looking statements and potential financing and transaction plans and terms should not be regarded as a representation by Orexigen that any of its plans will be achieved. Actual results may differ materially from those expressed or implied in this presentation due to the risk and uncertainties inherent in the Orexigen business, including, without limitation: the potential that the marketing and commercialization of Contrave/Mysimba will not be successful; the Company’s ability to obtain and maintain partnerships and the ability of it or its partners to maintain marketing authorization globally; the Company’s ability to adequately inform consumers about Contrave; the Company‘s ability to successfully commercialize Contrave with a specialty sales force in the United States; the capabilities and performance of various third parties on which it relies for a number of activities related to the manufacture, development and commercialization of Contrave/Mysimba; the estimates of the capacity of manufacturing and the Company’s ability to secure additional manufacturing capabilities; the Company’s ability to successfully complete the post-marketing requirement studies for Contrave; the therapeutic and commercial value of Contrave/Mysimba; competition in the global obesity market, particularly from existing therapies; the Company’s failure to successfully acquire, develop and market additional product candidates or approved products; the Company’s ability to obtain and maintain global intellectual property protection for Contrave and Mysimba; the potential for an appeals court to determine in our patent litigation matter with Actavis that one or more of the Company’s patents is not valid or that Actavis' proposed generic product is not infringing each of the patents at issue; other legal or regulatory proceedings against Orexigen, as well as potential reputational harm, as a result of misleading public claims about Orexigen; the Company’s ability to maintain sufficient capital to fund its operations for the foreseeable future; the Company’s ability to satisfy covenants in the indentures for its outstanding indebtedness, including one requirement that the Company generate consolidated net product sales of least $100 million for fiscal year 2017; the Company’s ability to satisfy the applicable listing standards of the NASDAQ Global Market; and other risks described in Orexigen’s filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward‐looking statements, which speak only as of the date hereof, and Orexigen undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. Further information regarding these and other risks is included under the heading "Risk Factors" in Orexigen's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2017 and its other reports, which are available from the SEC's website (www.sec.gov) and on Orexigen's website (www.orexigen.com) under the heading "Investors." All forward‐looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.

2017 Performance

Demonstrated the growth and profit potential of Contrave® in the U.S. >2,000,000 prescriptions of Contrave in the U.S. since launch >100,000 physicians have prescribed Contrave in the U.S. since launch Achieved all-time highs in 2017 across multiple key prescription metrics, including total prescription volume and market share, with significantly lower spending than our former partner Drove strong volume growth heading into 2018: +50% increase in TRx in 4Q17 compared to 4Q16 Significantly grew revenue per unit sold: ~$92/unit in 3Q17 vs. ~$77/unit in 2016 Launched innovative digital, social media and CRM strategies Generated nearly 3 billion DTC impressions and 700 million digital impressions Launched innovative telemedicine and home delivery solutions, with 9% of total prescriptions delivered via home delivery in September 2017 Signed first innovative, value-based contract with OptumRx 28% of prescriptions for Contrave have 3rd party reimbursement as of 3Q17 Established broad foundation for growth and value creation outside the U.S. Signed partnerships for 28 countries outside the U.S. in 2017, for a total of 67 Launched in 17 countries outside the U.S. in 2017, for a total of 23 Expect to sell $10-$15 million of Contrave and Mysimba™ to OUS partners in 2017 Prevailed in patent case, confirming IP exclusivity through 2030 Reduced long-term debt, bringing the total amount of debt retired to over $50M Ended the year with approximately $46.5M in cash, cash equivalents and short-term investments 2017 Highlights 4
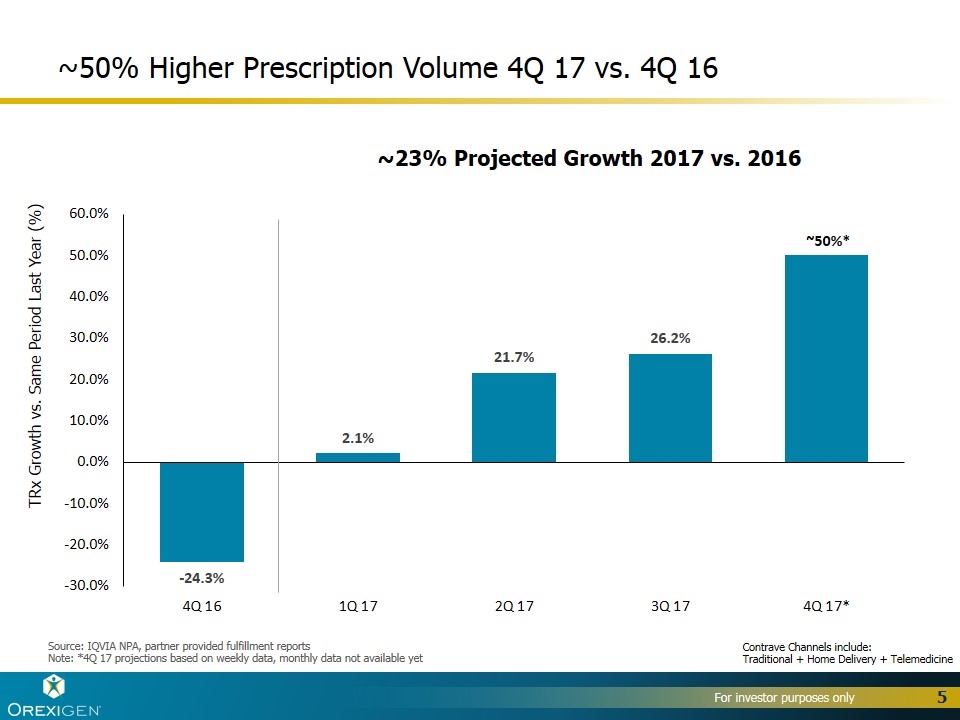
~50% Higher Prescription Volume 4Q 17 vs. 4Q 16 TRx Growth vs. Same Period Last Year (%) ~23% Projected Growth 2017 vs. 2016 Source: IQVIA NPA, partner provided fulfillment reports Note: *4Q 17 projections based on weekly data, monthly data not available yet ~50%* Contrave Channels include: Traditional + Home Delivery + Telemedicine
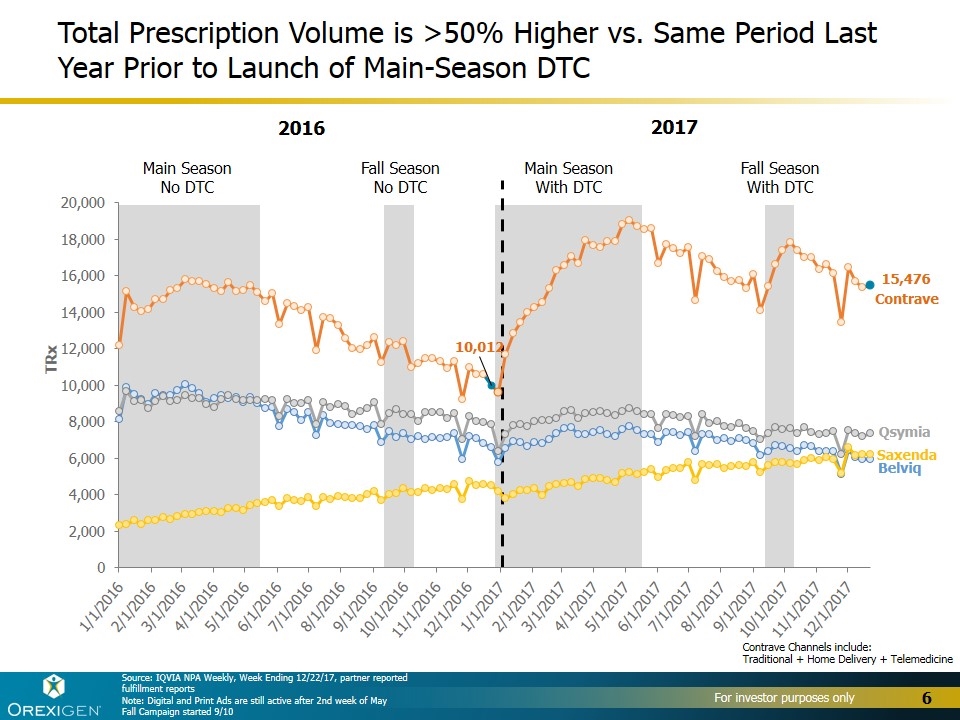
Main Season No DTC Fall Season No DTC Main Season With DTC Fall Season With DTC Total Prescription Volume is >50% Higher vs. Same Period Last Year Prior to Launch of Main-Season DTC Contrave Belviq Qsymia Saxenda Source: IQVIA NPA Weekly, Week Ending 12/22/17, partner reported fulfillment reports Note: Digital and Print Ads are still active after 2nd week of May Fall Campaign started 9/10 2016 2017 Contrave Channels include: Traditional + Home Delivery + Telemedicine
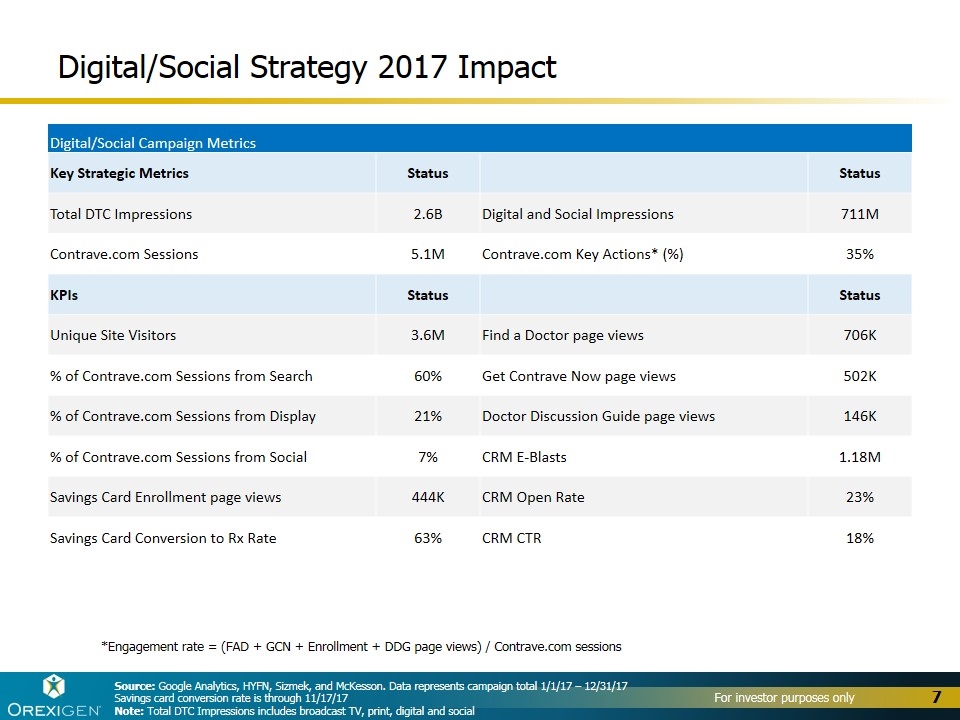
Digital/Social Campaign Metrics Key Strategic Metrics Status Status Total DTC Impressions 2.6B Digital and Social Impressions 711M Contrave.com Sessions 5.1M Contrave.com Key Actions* (%) 35% KPIs Status Status Unique Site Visitors 3.6M Find a Doctor page views 706K % of Contrave.com Sessions from Search 60% Get Contrave Now page views 502K % of Contrave.com Sessions from Display 21% Doctor Discussion Guide page views 146K % of Contrave.com Sessions from Social 7% CRM E-Blasts 1.18M Savings Card Enrollment page views 444K CRM Open Rate 23% Savings Card Conversion to Rx Rate 63% CRM CTR 18% *Engagement rate = (FAD + GCN + Enrollment + DDG page views) / Contrave.com sessions Source: Google Analytics, HYFN, Sizmek, and McKesson. Data represents campaign total 1/1/17 – 12/31/17 Savings card conversion rate is through 11/17/17 Note: Total DTC Impressions includes broadcast TV, print, digital and social Digital/Social Strategy 2017 Impact
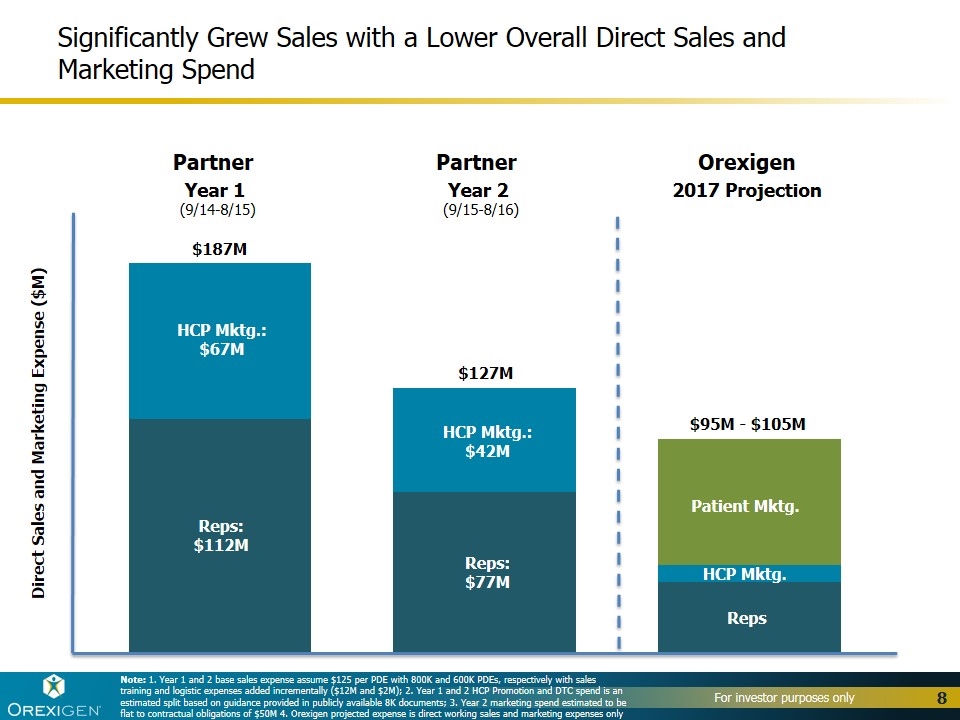
Significantly Grew Sales with a Lower Overall Direct Sales and Marketing Spend $187M $127M $95M - $105M Reps: $112M Reps: $77M Reps HCP Mktg. HCP Mktg.: $67M HCP Mktg.: $42M Patient Mktg. Note: 1. Year 1 and 2 base sales expense assume $125 per PDE with 800K and 600K PDEs, respectively with sales training and logistic expenses added incrementally ($12M and $2M); 2. Year 1 and 2 HCP Promotion and DTC spend is an estimated split based on guidance provided in publicly available 8K documents; 3. Year 2 marketing spend estimated to be flat to contractual obligations of $50M 4. Orexigen projected expense is direct working sales and marketing expenses only Partner Orexigen Direct Sales and Marketing Expense ($M) Year 1 (9/14-8/15) Partner Year 2 (9/15-8/16) 2017 Projection

2018 Initial Performance
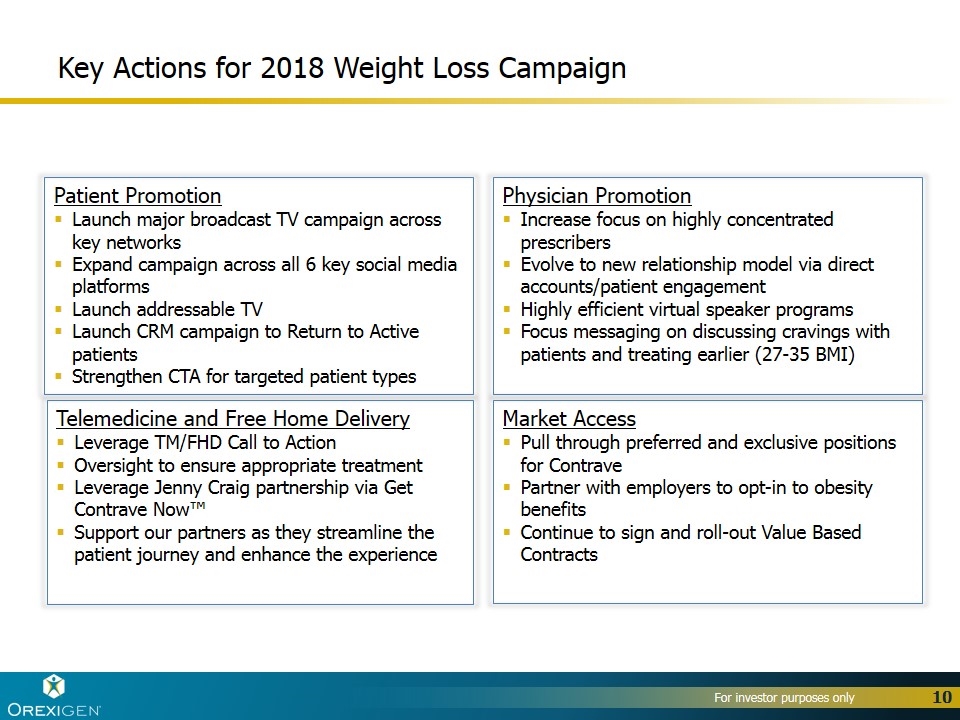
Key Actions for 2018 Weight Loss Campaign Physician Promotion Increase focus on highly concentrated prescribers Evolve to new relationship model via direct accounts/patient engagement Highly efficient virtual speaker programs Focus messaging on discussing cravings with patients and treating earlier (27-35 BMI) Patient Promotion Launch major broadcast TV campaign across key networks Expand campaign across all 6 key social media platforms Launch addressable TV Launch CRM campaign to Return to Active patients Strengthen CTA for targeted patient types Market Access Pull through preferred and exclusive positions for Contrave Partner with employers to opt-in to obesity benefits Continue to sign and roll-out Value Based Contracts Telemedicine and Free Home Delivery Leverage TM/FHD Call to Action Oversight to ensure appropriate treatment Leverage Jenny Craig partnership via Get Contrave Now™ Support our partners as they streamline the patient journey and enhance the experience
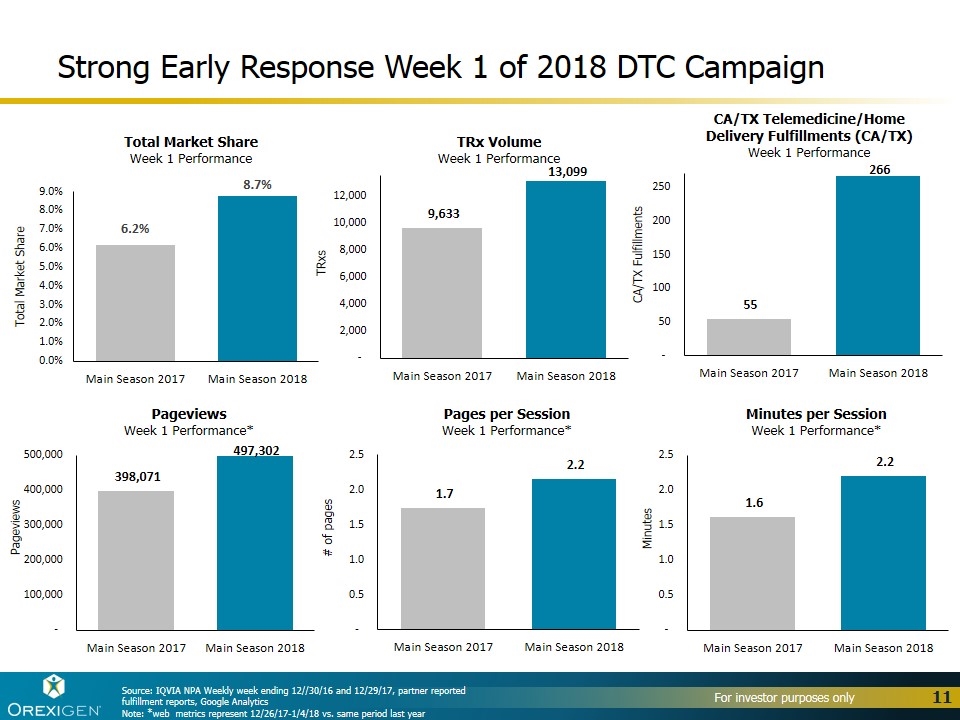
Strong Early Response Week 1 of 2018 DTC Campaign Total Market Share Week 1 Performance CA/TX Fulfillments CA/TX Telemedicine/Home Delivery Fulfillments (CA/TX) Week 1 Performance Total Market Share Pages per Session Week 1 Performance* # of pages TRxs TRx Volume Week 1 Performance Pageviews Week 1 Performance* Pageviews Minutes per Session Week 1 Performance* Minutes Source: IQVIA NPA Weekly week ending 12//30/16 and 12/29/17, partner reported fulfillment reports, Google Analytics Note: *web metrics represent 12/26/17-1/4/18 vs. same period last year
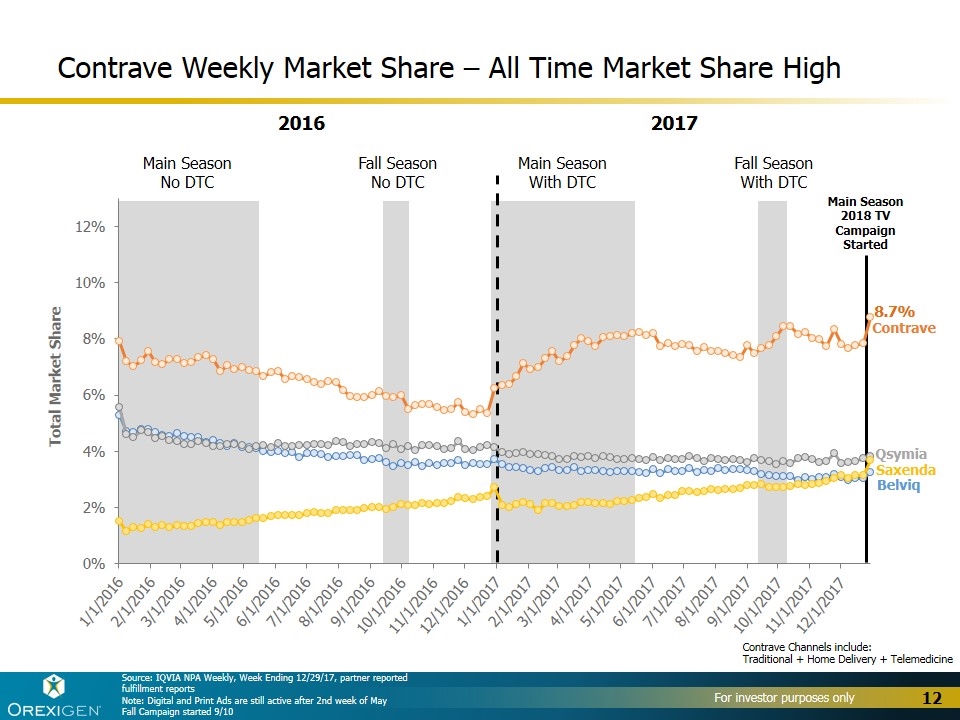
Main Season No DTC Fall Season No DTC Main Season With DTC Fall Season With DTC Contrave Weekly Market Share – All Time Market Share High Contrave Belviq Qsymia Saxenda 2016 2017 Main Season 2018 TV Campaign Started Source: IQVIA NPA Weekly, Week Ending 12/29/17, partner reported fulfillment reports Note: Digital and Print Ads are still active after 2nd week of May Fall Campaign started 9/10 Contrave Channels include: Traditional + Home Delivery + Telemedicine
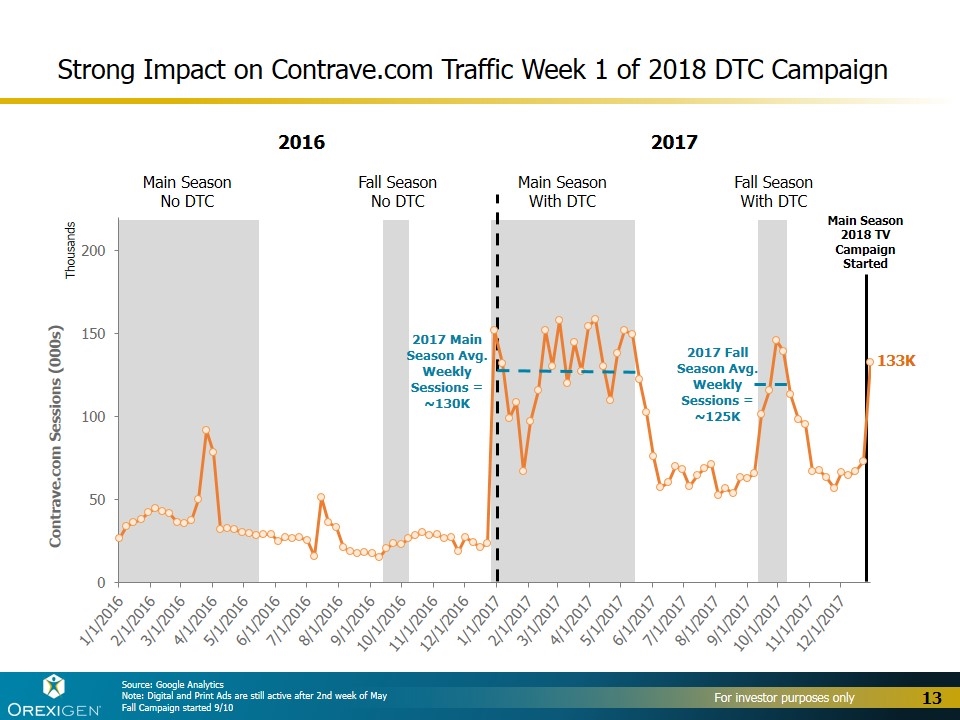
Main Season No DTC Fall Season No DTC Main Season With DTC Fall Season With DTC Strong Impact on Contrave.com Traffic Week 1 of 2018 DTC Campaign Source: Google Analytics Note: Digital and Print Ads are still active after 2nd week of May Fall Campaign started 9/10 2016 2017 Main Season 2018 TV Campaign Started 2017 Main Season Avg. Weekly Sessions = ~130K 2017 Fall Season Avg. Weekly Sessions = ~125K
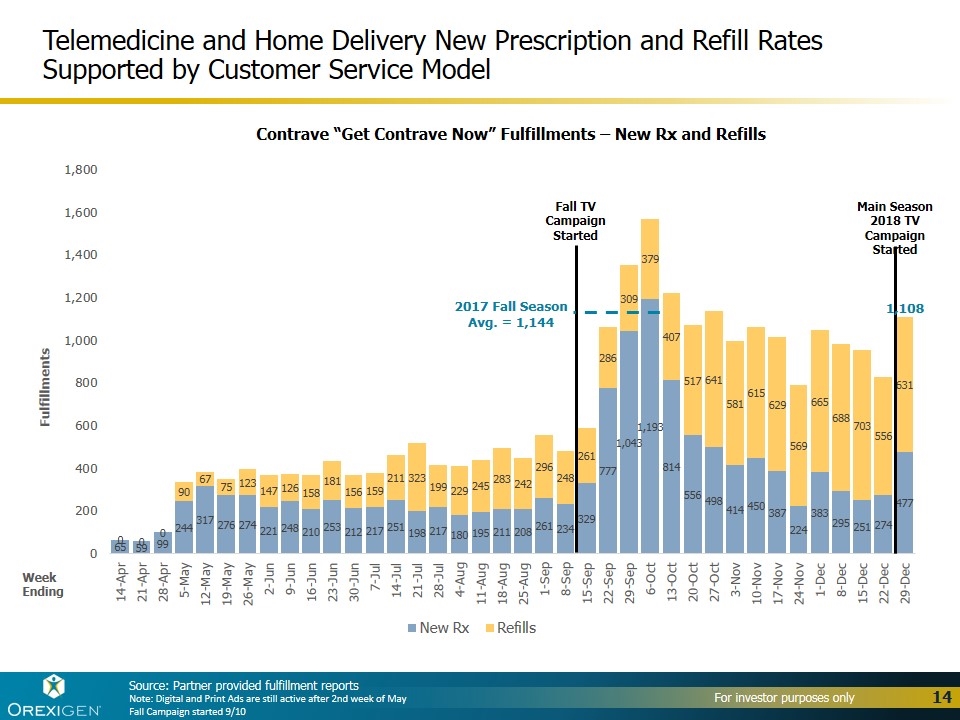
Source: Partner provided fulfillment reports Note: Digital and Print Ads are still active after 2nd week of May Fall Campaign started 9/10 Telemedicine and Home Delivery New Prescription and Refill Rates Supported by Customer Service Model Week Ending Fall TV Campaign Started Contrave “Get Contrave Now” Fulfillments – New Rx and Refills Main Season 2018 TV Campaign Started 2017 Fall Season Avg. = 1,144 1,108
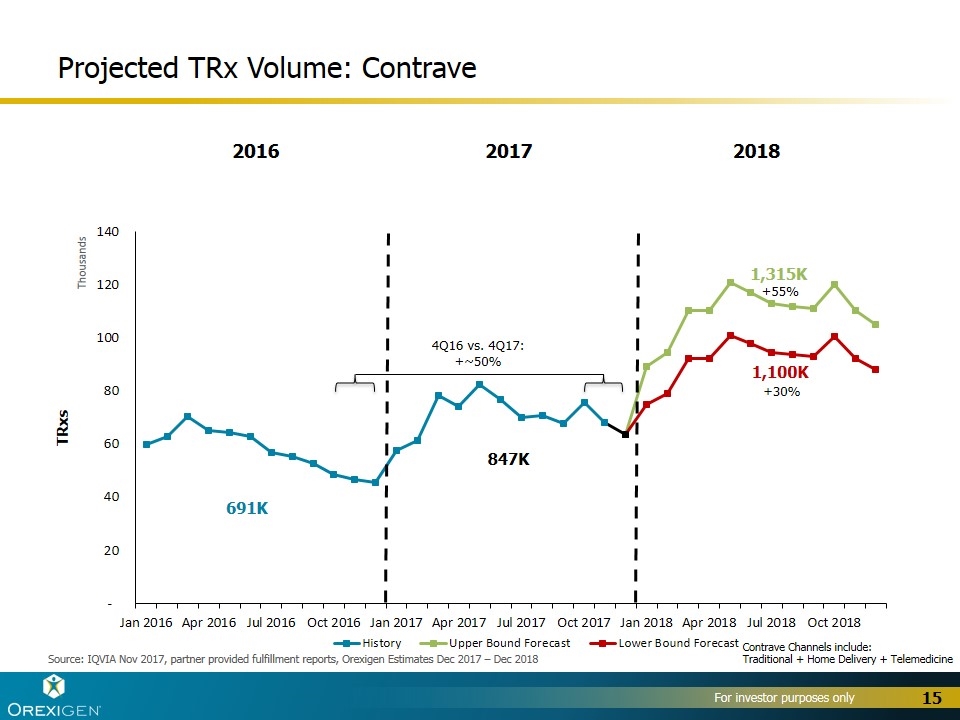
Projected TRx Volume: Contrave TRxs 2016 2017 2018 Source: IQVIA Nov 2017, partner provided fulfillment reports, Orexigen Estimates Dec 2017 – Dec 2018 1,315K 1,100K 691K 847K 4Q16 vs. 4Q17: +~50% +30% +55% Contrave Channels include: Traditional + Home Delivery + Telemedicine
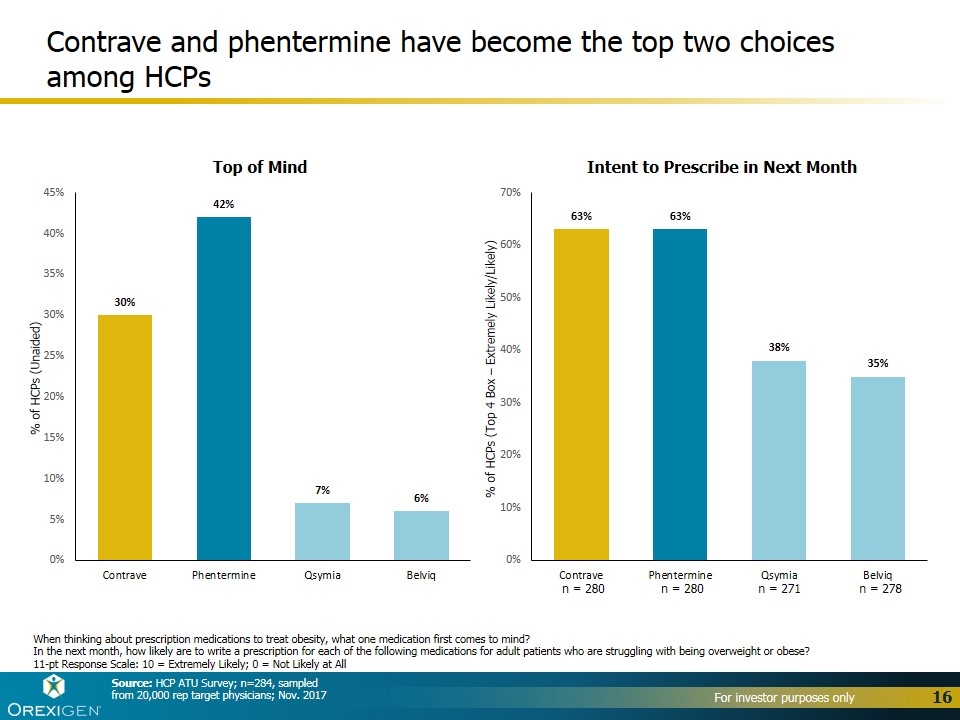
Contrave and phentermine have become the top two choices among HCPs When thinking about prescription medications to treat obesity, what one medication first comes to mind? In the next month, how likely are to write a prescription for each of the following medications for adult patients who are struggling with being overweight or obese? 11-pt Response Scale: 10 = Extremely Likely; 0 = Not Likely at All % of HCPs (Top 4 Box – Extremely Likely/Likely) Source: HCP ATU Survey; n=284, sampled from 20,000 rep target physicians; Nov. 2017 n = 280 n = 280 n = 271 n = 278 % of HCPs (Unaided)
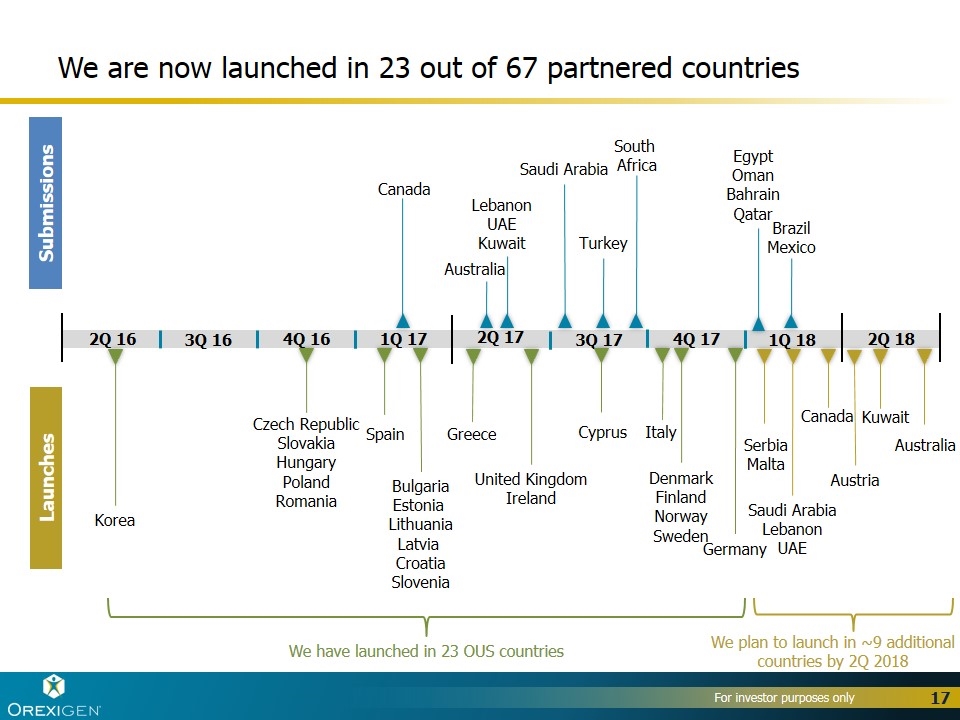
Submissions Launches 1Q 18 4Q 17 3Q 17 2Q 17 1Q 17 4Q 16 3Q 16 2Q 16 Saudi Arabia Lebanon UAE Canada Serbia Malta South Africa Saudi Arabia Australia Canada Lebanon UAE Kuwait Greece United Kingdom Ireland Spain Bulgaria Estonia Lithuania Latvia Croatia Slovenia Korea Czech Republic Slovakia Hungary Poland Romania Turkey We have launched in 23 OUS countries We plan to launch in ~9 additional countries by 2Q 2018 2Q 18 Italy Denmark Finland Norway Sweden We are now launched in 23 out of 67 partnered countries Germany Cyprus Kuwait Austria Australia Egypt Oman Bahrain Qatar Brazil Mexico

Execute 2018 commercial plan in the United States to continue to drive Contrave prescription and revenue growth Support partners’ advancement of launches and regulatory filings in countries outside the United States Continue positive dialog with regulators regarding completion of post-marketing requirements in a cost-efficient manner Work with noteholders and investors to restructure debt and address near-term liquidity needs Advance ongoing discussions related to strategic transactions 2018 Strategic Objectives
Backup
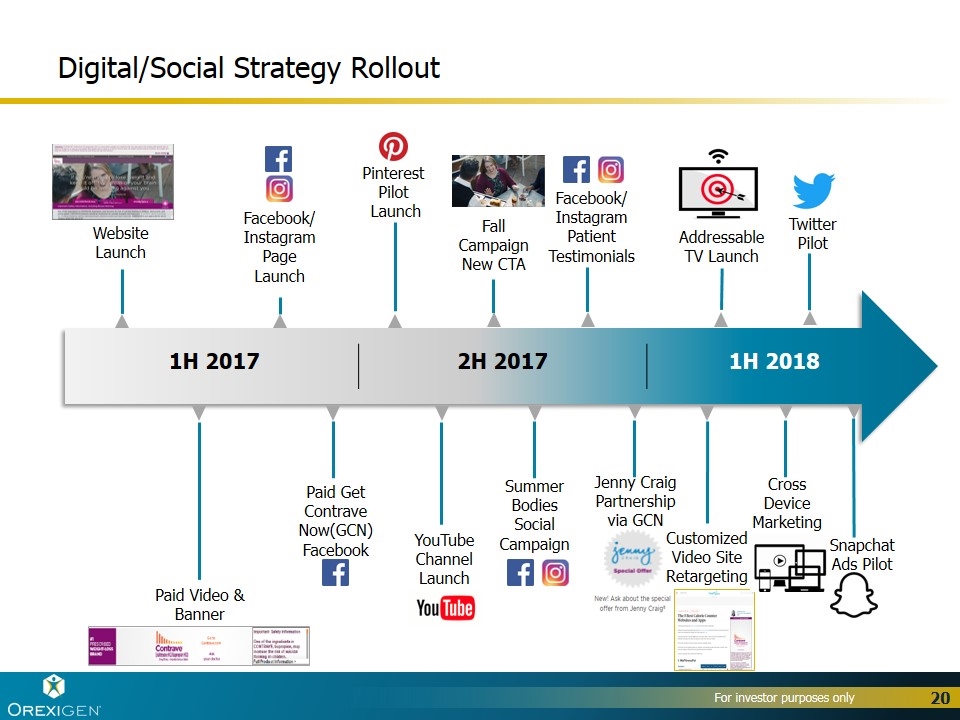
1H 2017 2H 2017 1H 2018 Paid Video & Banner Pinterest Pilot Launch Website Launch Facebook/ Instagram Page Launch Paid Get Contrave Now(GCN) Facebook Digital/Social Strategy Rollout Fall Campaign New CTA Summer Bodies Social Campaign Cross Device Marketing Twitter Pilot Customized Video Site Retargeting Addressable TV Launch YouTube Channel Launch Facebook/ Instagram Patient Testimonials Snapchat Ads Pilot Jenny Craig Partnership via GCN
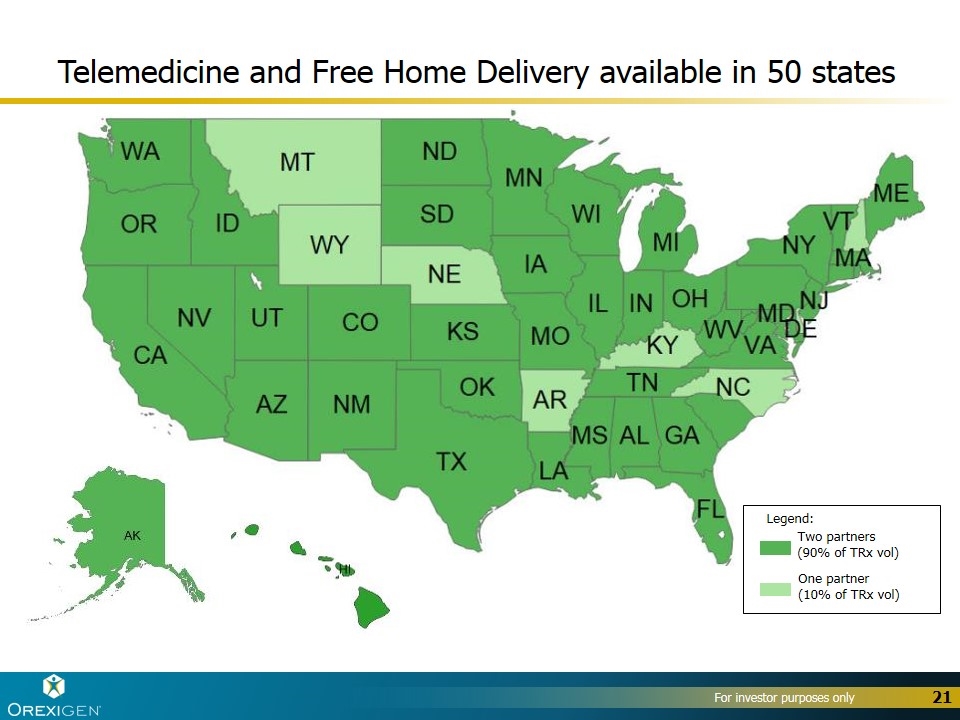
Telemedicine and Free Home Delivery available in 50 states Legend: Two partners (90% of TRx vol) One partner (10% of TRx vol)
