Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Neurotrope, Inc. | tv482845_8k.htm |
Exhibit 99.1

Neurotrope Phase 2 Trial Evidence that Bryostatin Improves Cognitive Function in Advanced Alzheimer’s Patients January 7, 2018 1

Bryostatin Phase 2 Trial Design 2 • Double - blind, randomized, controlled , exploratory trial • Moderate to severe patients (MMSE 4 - 15) • Stable background therapy with cholinesterase inhibitors and/or memantine • Three arms (1:1:1) 20µg, 40µg and control • 7 doses over 12 weeks: 0, 1, 3, 5, 7, 9, 11 • Efficacy evaluated at weeks 5, 9, 13 (primary and secondary endpoints) • Week 15: 30 - day safety & efficacy exploratory endpoint • Post - Hoc endpoints: memantine vs. non - memantine (background therapy ) • All p - values one - tailed as pre - specified in the statistical analysis plan, unless specified otherwise

Scientific Review Summary 3 Top Line Results of 150 Patients Exploratory Phase 2 Trial: • Safe, sustained improvement in SIB (Severe Impairment Battery) in 20µg dosing arm (but not the 40µg*) compared to control group through week 13 • The primary efficacy endpoints with SIB were pre - specified to be tested on the mITT and the Completers ( CAS) population * Exploratory Analyses: • Improvements in SIB sustained at week 15 (30 days after last dose at week 11) Post - Hoc Analyses: • Increased cognition (SIB) observed in the absence of memantine (an NMDA receptor antagonist) as background therapy • Efficacy at week 5 (reported at AAIC 2017) was significantly correlated with week 9, 13 efficacy - evidence of sustained improvement • 20µg dose validated as effective by body surface area (BSA) • Multiple sensitivity analyses reinforce prospective statistical model * 40 µg – ineffective, explained by PKC downregulation

Pre - Clinical Studies: PKC ε Signaling Pathways Integral to Memory and Learning * Increased red, green (yellow) overlapping of presynaptic & postsynaptic staining indicates increased synaptic formation Sen A et al. J Biol Chem. 2012;287(19):15947 - 15958. Sen A et al. J Biol Chem . 2016;291(32):16462 - 16467. Image courtesy of Daniel L. Alkon, MD . Red = Presynaptic staining (Synaptophysin ) Green = Postsynaptic staining ( PSD - 95) Yellow = Merged, Synaptic Formation Treated With Bryostatin & A β Oligomers Treated With A β Oligomers a • Bryostatin/PKC e activity generates new synaptic networks ( s ynaptogenesis) , enhances cognition, prevents neuronal death, and reduces Aβ and hyper - phosphorylated tau Cultured Rat Hippocampal Neuronal Networks 4
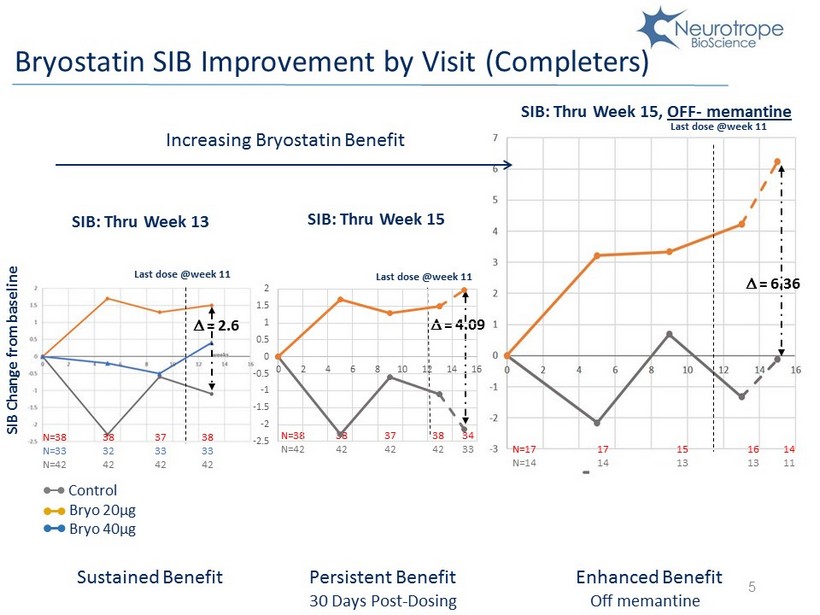
5 Last dose @week 11 D = 6.36 D = 4.09 Control Bryo 20µg Bryo 40µg SIB Change from baseline Last dose @week 11 D = 2.6 SIB: Thru Week 13 SIB: Thru Week 15 SIB: Thru Week 15, OFF - memantine Bryostatin SIB Improvement by Visit (Completers) Increasing Bryostatin Benefit N=38 N=33 N=42 38 32 42 37 33 42 38 33 42 N=38 N=42 38 42 37 42 38 42 34 33 Last dose @week 11 N=17 N=14 17 14 15 13 16 13 14 11 -2.5 -2 -1.5 -1 -0.5 0 0.5 1 1.5 2 0 2 4 6 8 10 12 14 16 Sustained Benefit Persistent Benefit 30 Days Post - Dosing Enhanced Benefit Off memantine

Topline Phase 2: SIB Change From Baseline mITT & Completer (CAS) Analyses at Week 13 6 -2.5 -2 -1.5 -1 -0.5 0 0.5 1 1.5 2 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Completers -2.5 -2 -1.5 -1 -0.5 0 0.5 1 1.5 2 0 1 2 3 4 5 6 7 8 9 10 11 12 13 mITT Control 20µg 40µg Change from baseline in SIB Change from baseline in SIB Week 5 Week 9 Week 13 Difference 20 μ g 3.0 1.0 1.9 1 - sided p - value 0.056 0.290 0.134 Difference 40 μ g 0.6 - 0.6 0.8 1 - sided p - value 0.368 0.638 0. 314 Week 5 Week 9 Week 13 Difference 20 μ g 4 .0 1.9 2.6 1 - sided p - value 0.016 0.165 0.070 Difference 40 μ g 2.1 0.1 1.5 1 - sided p - value 0.137 0.476 0.191 Control 20µg 40µg • Consistent effect for 20 m g vs control across all time points • Lack of effect for 40 m g vs control across all time points Cf. NTRP AAIC 2017 One - tailed; powered p < 0.1

30 Day Post - Dosing Data – Exploratory End Pt. Evidence for Persistent, Enhanced Benefit 7
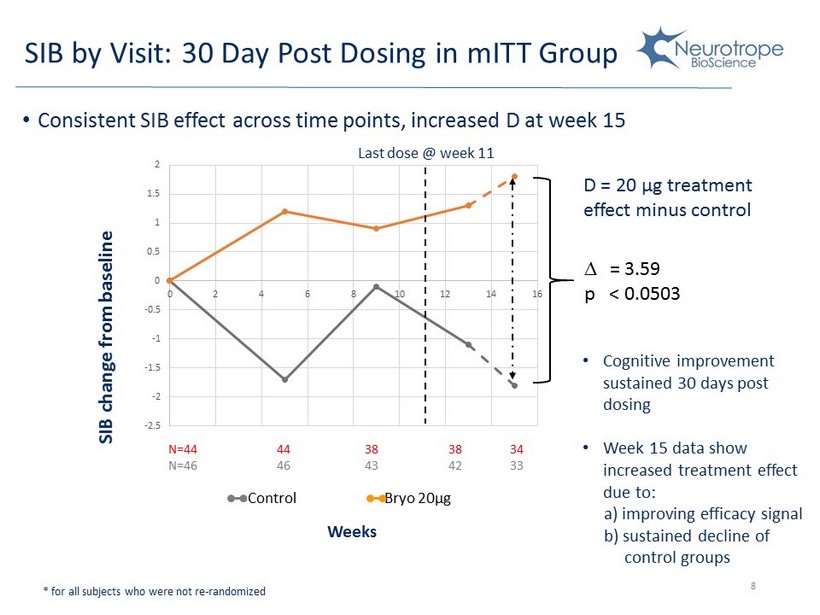
-2.5 -2 -1.5 -1 -0.5 0 0.5 1 1.5 2 0 2 4 6 8 10 12 14 16 44 46 38 43 38 42 34 33 SIB by Visit: 30 Day Post D osing in mITT Group 8 • Consistent SIB effect across time points, increased D at week 15 * for all subjects who were not re - randomized Last dose @ week 11 • Cognitive improvement sustained 30 days post dosing • Week 15 data show increased treatment effect due to: a) improving efficacy signal b) sustained decline of control groups = 3.59 p < 0.0503 SIB change from baseline D = 20 μ g treatment effect minus control Weeks N=44 N=46 Control Bryo 20µg

• Consistent SIB Effect across time points, increasing D at week 15 * SIB by Visit: 30 Days P ost D osing in Completers ( CAS) -2.5 -2 -1.5 -1 -0.5 0 0.5 1 1.5 2 0 2 4 6 8 10 12 14 16 Control Bryo 20ug Control 13-15 Bryo 20ug 13-15 * for all subjects who were not re - randomized Week 15 data show increased treatment effect due to: a) improving efficacy signal b) sustained decline of control groups = 4.09 p < 0.0293 9 Last dose @ week 11 SIB Change from baseline weeks N=38 N=42 38 42 37 42 38 42 34 33 D = 20 μ g treatment effect minus control

Post - Hoc Analyses: Evidence of Memantine’s Negative Impact on Bryostatin’s Therapeutic Benefits 10

Protein kinase C (PKC), which is activated by mGluR5 receptor stimulation, phosphorylates NMDA receptors to increase the cationic conductance of this receptor. PKC can also phosphorylate mGluR5 receptors to modulate their function. PKC, Activated by Bryostatin, Regulates NMDA Receptor Function in Multiple Pathways Pathways Include: • Synaptogenesis • NMDA Receptor Traffic • NMDA Conductance • mGluR5 - NMDA modulation References Pharmaceuticals (Basel). 2013 Feb; 6(2): 251 – 268. Published online 2013 Feb 6., The Journal of Biological Chemistry, 2011 July; 286,25187 - 25200, Nature Neuroscience, 2001, April, Vol. 4, no 4 Sen A et al. J Biol Chem . 2016;291(32):16462 - 16467 11 PKC BDNF, NGF,IGF

SIB By Visit: OFF - Memantine patients in mITT Group at weeks 13 and 15 D = 5.93 p < . 0576 weeks D = 20 μ g treatment effect minus control SIB Change from baseline N=17 N=14 17 14 15 13 16 13 14 11 12 Last dose @ week 11 Control

-2 -1.5 -1 -0.5 0 0.5 0 4 8 12 16 Control Bryo 20ug -3 -2 -1 0 1 2 3 4 5 6 0 2 4 6 8 10 12 14 16 Control Bryo 20ug SIB By Visit: Comparison of OFF vs. ON - Memantine in mITT Group at Weeks 13 and 15 SIB - OFF - Memantine D = 5.93 SIB Change from baseline weeks weeks SIB Change from baseline N=17 N=14 17 14 15 13 16 13 14 11 N=27 N=32 27 32 23 30 22 29 20 22 13 D = 20 μ g treatment effect minus control SIB: ON - Memantine Last dose @ week 11 Last dose @ week 11

-3 -2 -1 0 1 2 3 4 5 6 7 0 2 4 6 8 10 12 14 16 Control Bryo 20ug SIB By Visit: OFF - Memantine Completers (CAS) at Weeks 13 and 15 D = 6.36 p < 0.0488 Last dose @week 11 14 SIB Change from baseline D = 20 μ g treatment effect minus control weeks SIB - OFF - Memantine N=16 N=13 16 13 15 13 16 13 14 11

-3 -2 -1 0 1 0 2 4 6 8 10 12 14 16 Control Bryo 20ug -3 -2 -1 0 1 2 3 4 5 6 7 0 2 4 6 8 10 12 14 16 Control Bryo 20ug SIB By Visit: Comparison of OFF vs. ON - Memantine in Completers at Weeks 13 and 15 *Results from Week 15 are from a model that included all visits, all other results are consistent with the CSR results ( Tables 14.2.1.3 for FAS and 14.2.1.4 for Completers) that did not include Week 15 SIB: OFF - m emantine SIB: ON - memantine SIB Change from baseline SIB Change from baseline D = 20 μ g treatment effect minus control D = 6.36 Last dose @week 11 Weeks N=16 N=13 16 13 15 13 16 13 14 11 N=22 N=29 22 29 22 29 22 29 20 22 15 Weeks

Memantine Blocks Bryostatin SIB Improvement SIB 20 μg bryostatin vs. control mITT Completer (CAS) Off m emantine On m emantine Off m emantine On m emantine Week 5 D 4.48 1.96 5.38 2.94 p - value* 0.0857 0.1973 0.0487 0.1016 Week 9 D 2.08 0.09 2.66 0.90 p - value* 0.2597 0.4847 0.2071 0.3522 Week 13 D 5.11 - 0.14 5.53 0.56 p - value* 0.0437 0.4752 0.0338 0.3988 Week 15 D 5.93 0.79 6.36 1.45 p - value* 0.0576 0.3927 0.0488 0.3120 16 • Larger treatment effects were seen in patients treated with 20μg bryostatin OFF - m emantine vs. ON - memantine in MITT and Completer groups * All p - value are one - tailed as pre - specified unless otherwise denoted
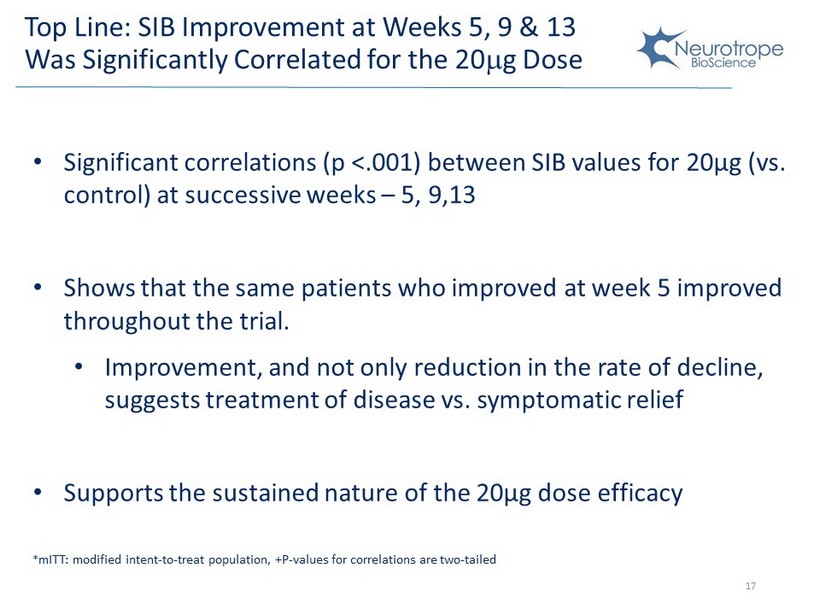
Top Line: SIB Improvement at Weeks 5, 9 & 13 Was Significantly C orrelated for the 20 m g Dose Change from baseline in SIB Change from baseline in SIB • S ignificant correlations (p <.001) between SIB values for 20µg (vs. control) at successive weeks – 5, 9,13 • Shows that the same patients who improved at week 5 improved throughout the trial . • Improvement, and not only reduction in the rate of decline, suggests treatment of disease vs. symptomatic relief • Supports the sustained nature of the 20µg dose efficacy 17 * mITT : modified intent - to - treat population, +P - values for correlations are two - tailed

Bryostatin - 20 μ g Dose Further Validated as Effective by Body Surface Area (BSA) Analysis F - ratio for 20µg vs. 40µg variance of 3.97 and a corresponding 2 - sided p - value of <0.0001, supported a conclusion of unequal variances between the two dosage groups. Narrow Dose Response Distribution around 11.33µg/m 2 for 20 μ g/dose • Normalization of the 20µg dose to each patient’s BSA revealed that the 20µg doses (on a per - patient - basis) were tightly distributed around the 12.5µg/m 2 . The week 13 mean dose adjusted for BSA was 11.33 µg/m 2 in the 20µg dose arm. 18

Concluding Observations: Change from baseline in SIB Change from baseline in SIB 1. 20µg bryostatin showed evidence of safely produced, sustained cognitive improvements (SIB scores) in advanced AD patients 2. These improvements, not just reduction in the rate of decline, persisted at week 15 (30 days after the last dose at week 11 ) – suggesting treatment of disease in addition to symptomatic relief. 3. Greater cognitive improvement in the 20 μ g arm was observed in the absence of memantine – a known partial NMDA receptor antagonist 4. 20 μ g dose validated as an effective, safe dose by Body Surface Area 5. Sensitivity analyses generally agreed with the results of the analysis by Mixed Model for Repeated Measures (MMRM) and included: • ANCOVA – similar to MMRM used here • Imputation of drop outs also similar to MMRM • Adjusting for additional baseline covariates • Pooling sites • Linear vs. quadratic model over time 19

Next Steps in Clinical Development Program A confirmatory t rial in advanced AD, non - memantine p atients 1. Leverage Phase II data by incorporating memantine - free patients into the study 2. Confirm marked improvement in SIB scores among memantine - free patients 3. Draw conclusions on possible long - term effects of bryostatin on SIB improvement from baseline 4. In discussions with leading academic institution to design and conduct the trial • Trial expected to begin in first half of 2018 5. NTRP has the financial resources required to complete this confirmatory trial 20
