Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Karyopharm Therapeutics Inc. | d520326d8k.htm |

Targeting Disease at the Nuclear Pore Exhibit 99.1

6th Annual J.P. Morgan Healthcare Conference January 8-11, 2018

Forward-looking Statements This presentation contains forward-looking statements within the meaning of the “safe harbor” provisions of The Private Securities Litigation Reform Act of 1995. Such forward-looking statements include those regarding the therapeutic potential of and potential clinical development plans and commercialization for Karyopharm’s drug candidates, including the timing of initiation of certain trials and of the reporting of data from such trials, and Karyopharm’s strategic and financial plans and expectations as well as financial projections for Karyopharm. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from Karyopharm’s current expectations. For example, there can be no guarantee that any of Karyopharm’s SINE compounds, including selinexor (KPT-330) and eltanexor (KPT-8602), Karyopharm’s second generation SINE compound, or KPT-9274, Karyopharm’s first-in-class oral dual inhibitor of PAK4 and NAMPT, or any other drug candidate Karyopharm is developing, will successfully complete necessary preclinical and clinical development phases or that development of any of Karyopharm’s drug candidates will continue. Further, there can be no guarantee that any positive developments in Karyopharm’s drug candidate portfolio will result in stock price appreciation. In addition, even if Karyopharm receives marketing approval for selinexor or another drug candidate, there can be no assurance that Karyopharm will be able to successfully commercialize that drug candidate. Management’s expectations and, therefore, any forward-looking statements in this presentation could also be affected by risks and uncertainties relating to a number of other factors, many of which are beyond Karyopharm’s control, including the following: Karyopharm’s results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; the content and timing of decisions made by the FDA and other regulatory authorities, investigational review boards at clinical trial sites and publication review bodies, including with respect to the need for additional clinical studies; Karyopharm’s ability to obtain and maintain requisite regulatory approvals and to enroll patients in its clinical trials; Karyopharm’s partnership with Ono and the potential future financial implications of such partnership; unplanned cash requirements and expenditures; development of drug candidates by Karyopharm’s competitors for diseases for which Karyopharm is currently developing its drug candidates; and Karyopharm’s ability to obtain, maintain and enforce patent and other intellectual property protection for any drug candidates it is developing. These and other risks, including those which may impact management’s expectations, are described in greater detail under the heading “Risk Factors” in Karyopharm’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2017, which is on file with the SEC, and in subsequent filings filed by Karyopharm with the SEC. Any forward-looking statements contained in this presentation are for informational purposes only and speak only as of the date hereof. Other than as is required by law, Karyopharm expressly disclaims any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. Karyopharm’s website is http://www.karyopharm.com. Karyopharm regularly uses its website to post information regarding its business, drug development programs and governance. Karyopharm encourages investors to use www.karyopharm.com, particularly the information in the section entitled “Investors,” as a source of information about Karyopharm. References to www.karyopharm.com in this presentation are not intended to, nor shall they be deemed to, incorporate information on www.karyopharm.com into this presentation by reference. Unless otherwise noted, this presentation contains data that are interim and unaudited based on site reports. In addition, data included in this presentation have not been updated and are as of the cutoff date for the applicable medical conference presentation. Unless otherwise noted, all reference to market sizes are based on reports from Global Data or Karyopharm internal estimates. ©2018 Karyopharm Therapeutics Inc.
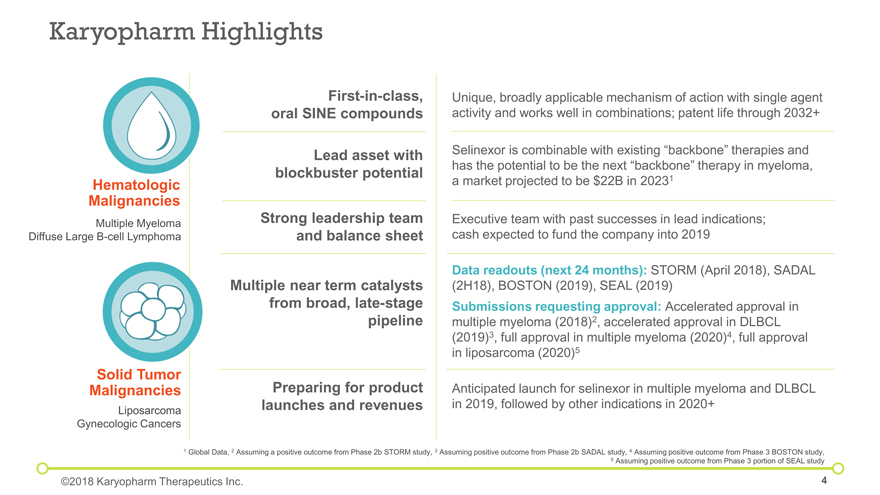
Karyopharm Highlights Strong leadership team and balance sheet First-in-class, oral SINE compounds Lead asset with blockbuster potential 1 Global Data, 2 Assuming a positive outcome from Phase 2b STORM study, 3 Assuming positive outcome from Phase 2b SADAL study, 4 Assuming positive outcome from Phase 3 BOSTON study, 5 Assuming positive outcome from Phase 3 portion of SEAL study Hematologic Malignancies Multiple Myeloma Diffuse Large B-cell Lymphoma Liposarcoma Gynecologic Cancers Solid Tumor Malignancies ©2018 Karyopharm Therapeutics Inc. Multiple near term catalysts from broad, late-stage pipeline Executive team with past successes in lead indications; cash expected to fund the company into 2019 Unique, broadly applicable mechanism of action with single agent activity and works well in combinations; patent life through 2032+ Selinexor is combinable with existing “backbone” therapies and has the potential to be the next “backbone” therapy in myeloma, a market projected to be $22B in 20231 Data readouts (next 24 months): STORM (April 2018), SADAL (2H18), BOSTON (2019), SEAL (2019) Submissions requesting approval: Accelerated approval in multiple myeloma (2018)2, accelerated approval in DLBCL (2019)3, full approval in multiple myeloma (2020)4, full approval in liposarcoma (2020)5 Anticipated launch for selinexor in multiple myeloma and DLBCL in 2019, followed by other indications in 2020+ Preparing for product launches and revenues
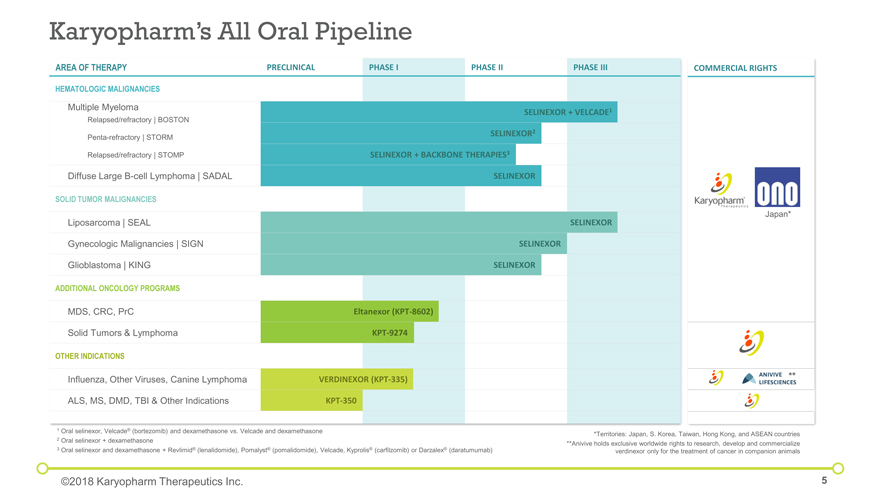
Karyopharm’s All Oral Pipeline COMMERCIAL RIGHTS Japan* Relapsed/refractory | BOSTON Relapsed/refractory | STOMP Penta-refractory | STORM 1 Oral selinexor, Velcade® (bortezomib) and dexamethasone vs. Velcade and dexamethasone 2 Oral selinexor + dexamethasone 3 Oral selinexor and dexamethasone + Revlimid® (lenalidomide), Pomalyst® (pomalidomide), Velcade, Kyprolis® (carfilzomib) or Darzalex® (daratumumab) ©2018 Karyopharm Therapeutics Inc. AREA OF THERAPY PRECLINICAL PHASE I PHASE II PHASE III HEMATOLOGIC MALIGNANCIES Multiple Myeloma SELINEXOR + VELCADE1 SELINEXOR2 SELINEXOR + BACKBONE THERAPIES3 Diffuse Large B-cell Lymphoma | SADAL SELINEXOR SOLID TUMOR MALIGNANCIES Liposarcoma | SEAL SELINEXOR Gynecologic Malignancies | SIGN SELINEXOR Glioblastoma | KING SELINEXOR ADDITIONAL ONCOLOGY PROGRAMS MDS, CRC, PrC Eltanexor (KPT-8602) Solid Tumors & Lymphoma KPT-9274 OTHER INDICATIONS Influenza, Other Viruses, Canine Lymphoma VERDINEXOR (KPT-335) ALS, MS, DMD, TBI & Other Indications KPT-350 Relapsed/refractory | BOSTON Relapsed/refractory | STOMP Penta-refractory | STORM *Territories: Japan, S. Korea, Taiwan, Hong Kong, and ASEAN countries **Anivive holds exclusive worldwide rights to research, develop and commercialize verdinexor only for the treatment of cancer in companion animals **
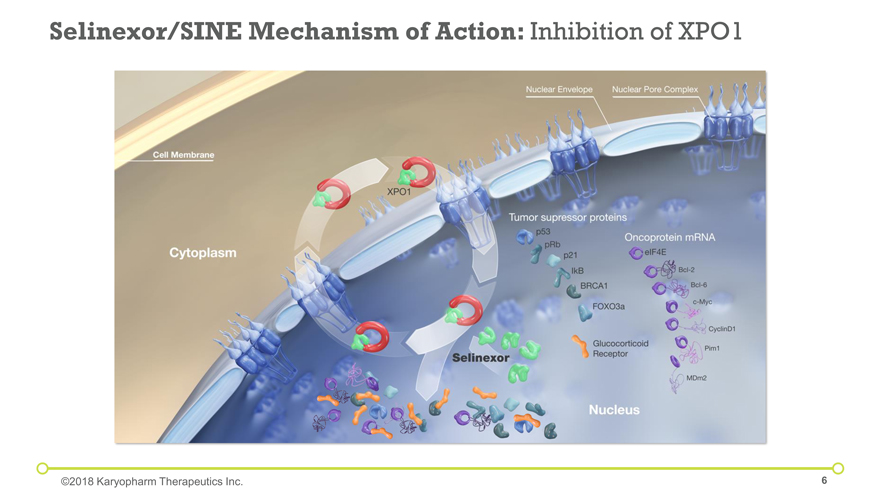
Selinexor/SINE Mechanism of Action: Inhibition of XPO1 ©2018 Karyopharm Therapeutics Inc.
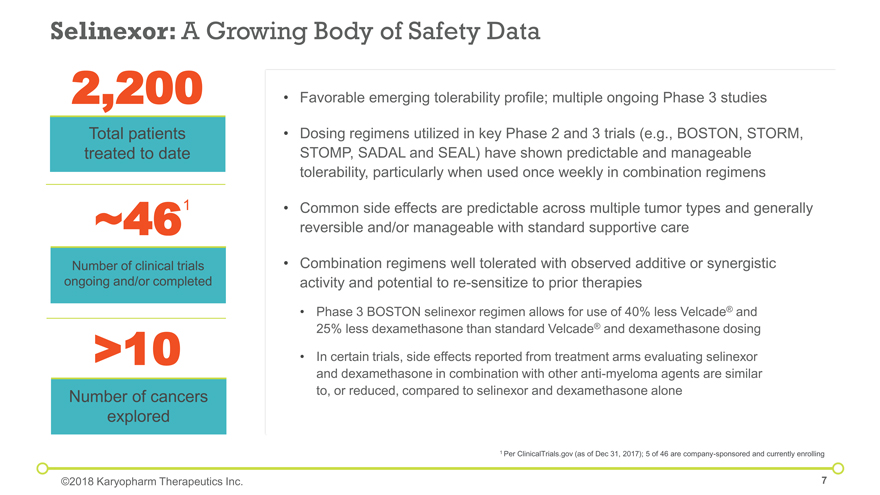
Selinexor: A Growing Body of Safety Data 2,200 Total patients treated to date Favorable emerging tolerability profile; multiple ongoing Phase 3 studies Dosing regimens utilized in key Phase 2 and 3 trials (e.g., BOSTON, STORM, STOMP, SADAL and SEAL) have shown predictable and manageable tolerability, particularly when used once weekly in combination regimens Common side effects are predictable across multiple tumor types and generally reversible and/or manageable with standard supportive care Combination regimens well tolerated with observed additive or synergistic activity and potential to re-sensitize to prior therapies Phase 3 BOSTON selinexor regimen allows for use of 40% less Velcade® and 25% less dexamethasone than standard Velcade® and dexamethasone dosing In certain trials, side effects reported from treatment arms evaluating selinexor and dexamethasone in combination with other anti-myeloma agents are similar to, or reduced, compared to selinexor and dexamethasone alone ~46 Number of clinical trials ongoing and/or completed ©2018 Karyopharm Therapeutics Inc. 1 Per ClinicalTrials.gov (as of Dec 31, 2017); 5 of 46 are company-sponsored and currently enrolling >10 Number of cancers explored 1

Selinexor in Hematologic Malignancies Multiple Myeloma
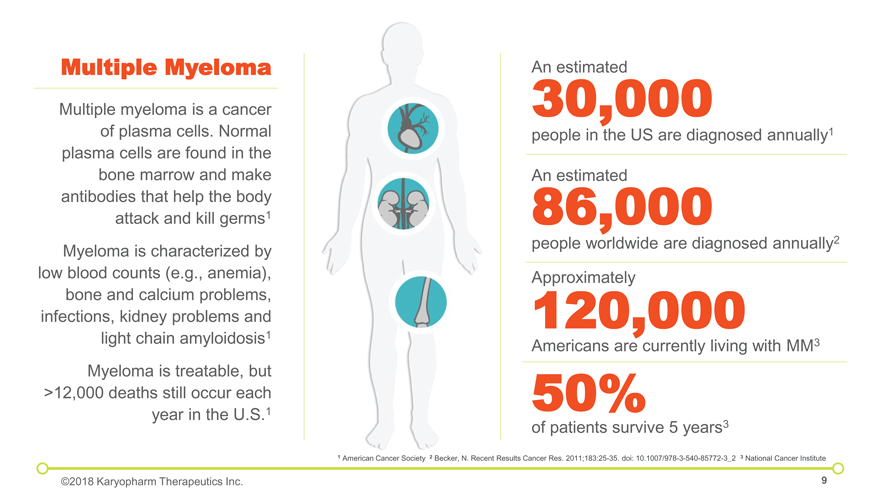
of patients survive 5 years3 50% Americans are currently living with MM3 120,000 Approximately people worldwide are diagnosed annually2 86,000 An estimated 30,000 people in the US are diagnosed annually1 An estimated 1 American Cancer Society 2 Becker, N. Recent Results Cancer Res. 2011;183:25-35. doi: 10.1007/978-3-540-85772-3_2 3 National Cancer Institute Multiple Myeloma Multiple myeloma is a cancer of plasma cells. Normal plasma cells are found in the bone marrow and make antibodies that help the body attack and kill germs1 Myeloma is characterized by low blood counts (e.g., anemia), bone and calcium problems, infections, kidney problems and light chain amyloidosis1 Myeloma is treatable, but >12,000 deaths still occur each year in the U.S.1 ©2018 Karyopharm Therapeutics Inc.
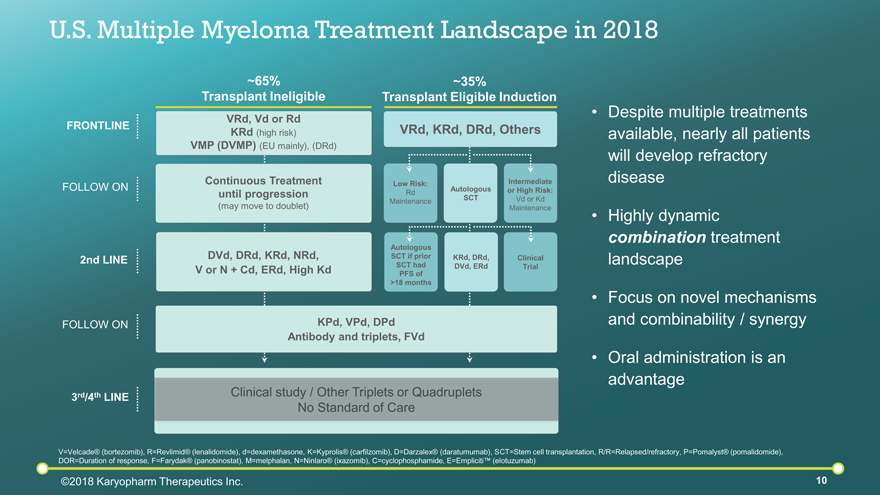
U.S. Multiple Myeloma Treatment Landscape in 2018 FOLLOW ON 2nd LINE FOLLOW ON 3rd/4th LINE FRONTLINE V=Velcade® (bortezomib), R=Revlimid® (lenalidomide), d=dexamethasone, K=Kyprolis® (carfilzomib), D=Darzalex® (daratumumab), SCT=Stem cell transplantation, R/R=Relapsed/refractory, P=Pomalyst® (pomalidomide), DOR=Duration of response, F=Farydak® (panobinostat), M=melphalan, N=Ninlaro® (ixazomib), C=cyclophosphamide, E=Empliciti™ (elotuzumab) Clinical study / Other Triplets or Quadruplets No Standard of Care Despite multiple treatments available, nearly all patients will develop refractory disease Highly dynamic combination treatment landscape Focus on novel mechanisms and combinability / synergy Oral administration is an advantage ~35% Transplant Eligible Induction VRd, KRd, DRd, Others Autologous SCT Intermediate or High Risk: Vd or Kd Maintenance Low Risk: Rd Maintenance KRd, DRd, DVd, ERd Clinical Trial Autologous SCT if prior SCT had PFS of >18 months ~65% Transplant Ineligible VRd, Vd or Rd KRd (high risk) VMP (DVMP) (EU mainly), (DRd) Continuous Treatment until progression (may move to doublet) DVd, DRd, KRd, NRd, V or N + Cd, ERd, High Kd ©2018 Karyopharm Therapeutics Inc. KPd, VPd, DPd Antibody and triplets, FVd
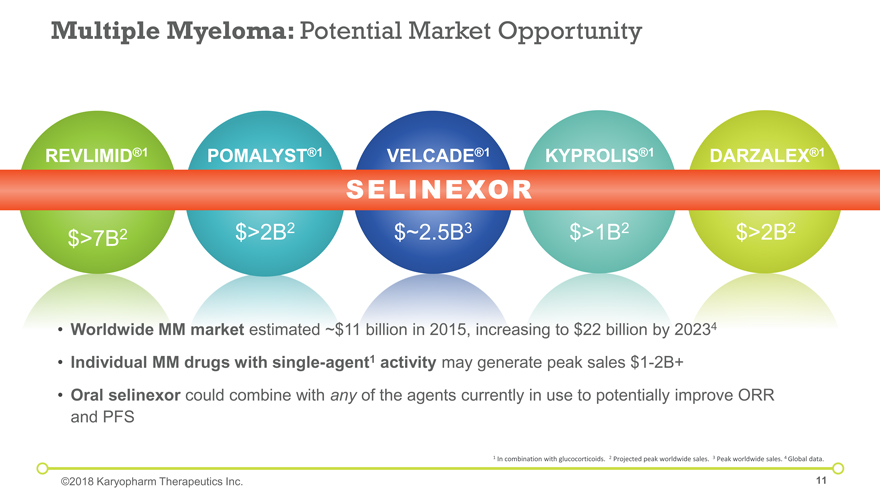
Worldwide MM market estimated ~$11 billion in 2015, increasing to $22 billion by 20234 Individual MM drugs with single-agent1 activity may generate peak sales $1-2B+ Oral selinexor could combine with any of the agents currently in use to potentially improve ORR and PFS VELCADE®1 $>7B2 REVLIMID®1 $>2B2 POMALYST®1 $~2.5B3 DARZALEX®1 $>1B2 KYPROLIS®1 $>2B2 Multiple Myeloma: Potential Market Opportunity SELINEXOR 1 In combination with glucocorticoids. 2 Projected peak worldwide sales. 3 Peak worldwide sales. 4 Global data. ©2018 Karyopharm Therapeutics Inc.
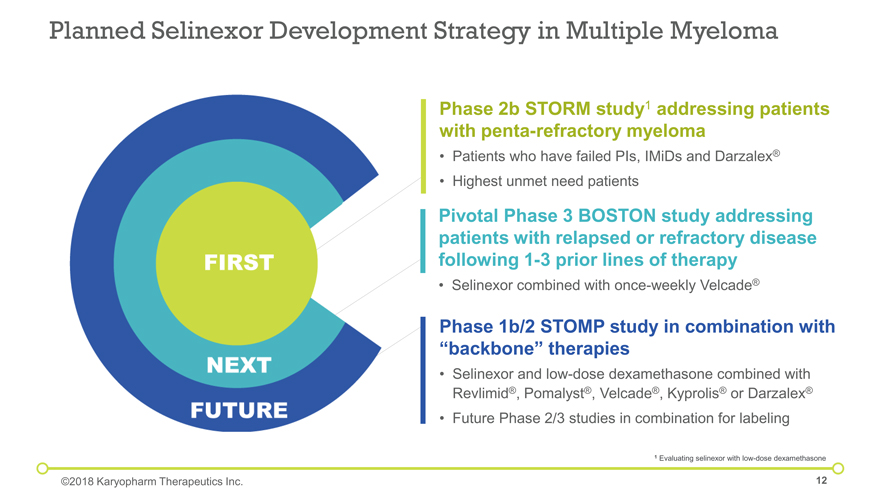
Planned Selinexor Development Strategy in Multiple Myeloma ©2018 Karyopharm Therapeutics Inc. Phase 2b STORM study1 addressing patients with penta-refractory myeloma Patients who have failed PIs, IMiDs and Darzalex® Highest unmet need patients 1 Evaluating selinexor with low-dose dexamethasone Pivotal Phase 3 BOSTON study addressing patients with relapsed or refractory disease following 1-3 prior lines of therapy Selinexor combined with once-weekly Velcade® Phase 1b/2 STOMP study in combination with “backbone” therapies Selinexor and low-dose dexamethasone combined with Revlimid®, Pomalyst®, Velcade®, Kyprolis® or Darzalex® Future Phase 2/3 studies in combination for labeling
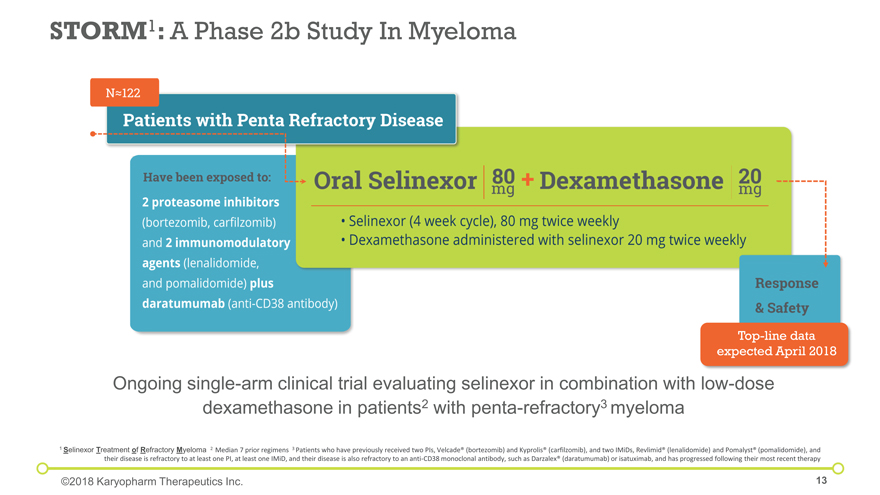
Ongoing single-arm clinical trial evaluating selinexor in combination with low-dose dexamethasone in patients2 with penta-refractory3 myeloma STORM1: A Phase 2b Study In Myeloma 1 Selinexor Treatment of Refractory Myeloma 2 Median 7 prior regimens 3 Patients who have previously received two PIs, Velcade® (bortezomib) and Kyprolis® (carfilzomib), and two IMiDs, Revlimid® (lenalidomide) and Pomalyst® (pomalidomide), and their disease is refractory to at least one PI, at least one IMiD, and their disease is also refractory to an anti-CD38 monoclonal antibody, such as Darzalex® (daratumumab) or isatuximab, and has progressed following their most recent therapy ©2018 Karyopharm Therapeutics Inc. Top-line data expected April 2018
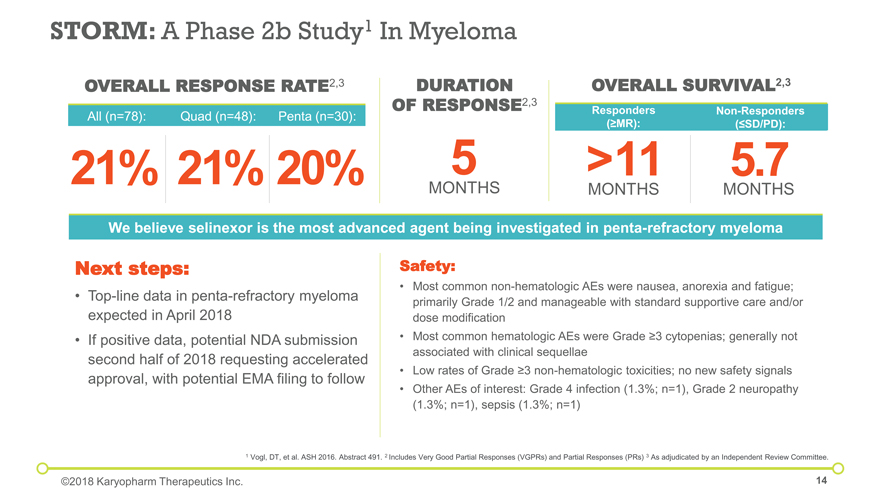
STORM: A Phase 2b Study1 In Myeloma Safety: Most common non-hematologic AEs were nausea, anorexia and fatigue; primarily Grade 1/2 and manageable with standard supportive care and/or dose modification Most common hematologic AEs were Grade ³3 cytopenias; generally not associated with clinical sequellae Low rates of Grade ³3 non-hematologic toxicities; no new safety signals Other AEs of interest: Grade 4 infection (1.3%; n=1), Grade 2 neuropathy (1.3%; n=1), sepsis (1.3%; n=1) Next steps: Top-line data in penta-refractory myeloma expected in April 2018 If positive data, potential NDA submission second half of 2018 requesting accelerated approval, with potential EMA filing to follow 21% OVERALL RESPONSE RATE2,3 DURATION OF RESPONSE2,3 OVERALL SURVIVAL2,3 >11 All (n=78): Quad (n=48): 21% Penta (n=30): 20% Non-Responders (£SD/PD): Responders (³MR): 5 5.7 MONTHS MONTHS We believe selinexor is the most advanced agent being investigated in penta-refractory myeloma MONTHS 1 Vogl, DT, et al. ASH 2016. Abstract 491. 2 Includes Very Good Partial Responses (VGPRs) and Partial Responses (PRs) 3 As adjudicated by an Independent Review Committee. ©2018 Karyopharm Therapeutics Inc.
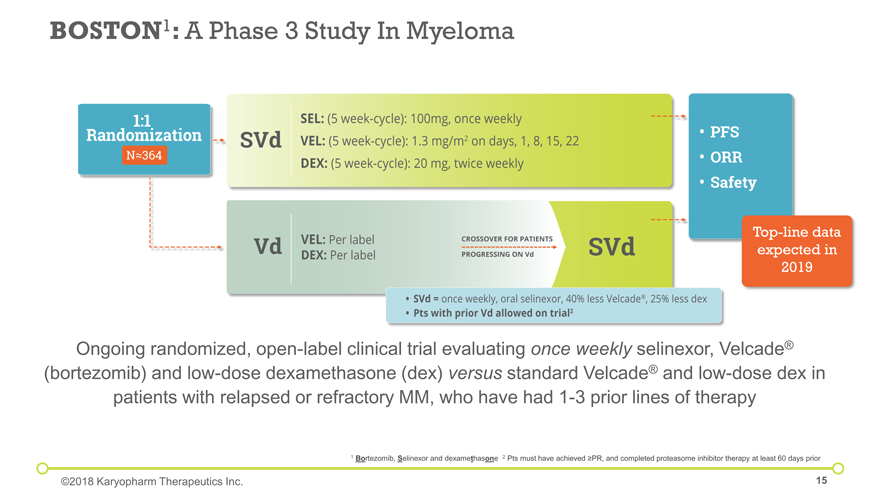
Ongoing randomized, open-label clinical trial evaluating once weekly selinexor, Velcade® (bortezomib) and low-dose dexamethasone (dex) versus standard Velcade® and low-dose dex in patients with relapsed or refractory MM, who have had 1-3 prior lines of therapy BOSTON1: A Phase 3 Study In Myeloma 1 Bortezomib, Selinexor and dexamethasone 2 Pts must have achieved ³PR, and completed proteasome inhibitor therapy at least 60 days prior ©2018 Karyopharm Therapeutics Inc. Top-line data expected in 2019
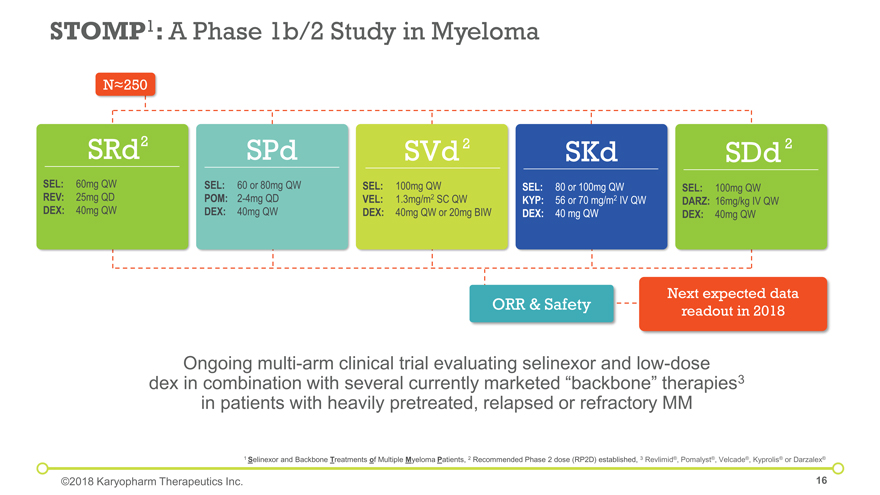
STOMP1: A Phase 1b/2 Study in Myeloma Ongoing multi-arm clinical trial evaluating selinexor and low-dose dex in combination with several currently marketed “backbone” therapies3 in patients with heavily pretreated, relapsed or refractory MM 1 Selinexor and Backbone Treatments of Multiple Myeloma Patients, 2 Recommended Phase 2 dose (RP2D) established, 3 Revlimid®, Pomalyst®, Velcade®, Kyprolis® or Darzalex® ©2018 Karyopharm Therapeutics Inc. SRd SEL:60mg QW REV:25mg QD DEX:40mg QW SPd SEL:60 or 80mg QW POM: 2-4mg QD DEX: 40mg QW SVd SEL:100mg QW VEL:1.3mg/m2 SC QW DEX:40mg QW or 20mg BIW SKd SEL:80 or 100mg QW KYP:56 or 70 mg/m2 IV QW DEX:40 mg QW SDd SEL:100mg QW DARZ:16mg/kg IV QW DEX:40mg QW ORR & Safety Next expected data readout in 2018 Nâ‰^250 2 2 2
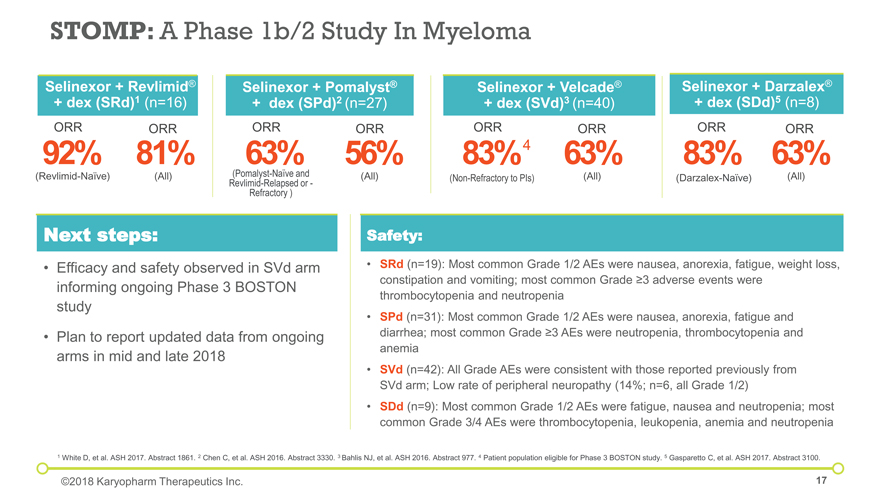
Next steps: Efficacy and safety observed in SVd arm informing ongoing Phase 3 BOSTON study Plan to report updated data from ongoing arms in mid and late 2018 Safety: SRd (n=19): Most common Grade 1/2 AEs were nausea, anorexia, fatigue, weight loss, constipation and vomiting; most common Grade ³3 adverse events were thrombocytopenia and neutropenia SPd (n=31): Most common Grade 1/2 AEs were nausea, anorexia, fatigue and diarrhea; most common Grade ³3 AEs were neutropenia, thrombocytopenia and anemia SVd (n=42): All Grade AEs were consistent with those reported previously from SVd arm; Low rate of peripheral neuropathy (14%; n=6, all Grade 1/2) SDd (n=9): Most common Grade 1/2 AEs were fatigue, nausea and neutropenia; most common Grade 3/4 AEs were thrombocytopenia, leukopenia, anemia and neutropenia STOMP: A Phase 1b/2 Study In Myeloma Selinexor + Velcade® + dex (SVd)3 (n=40) Selinexor + Pomalyst® + dex (SPd)2 (n=27) 1 White D, et al. ASH 2017. Abstract 1861. 2 Chen C, et al. ASH 2016. Abstract 3330. 3 Bahlis NJ, et al. ASH 2016. Abstract 977. 4 Patient population eligible for Phase 3 BOSTON study. 5 Gasparetto C, et al. ASH 2017. Abstract 3100. Selinexor + Revlimid® + dex (SRd)1 (n=16) Selinexor + Darzalex® + dex (SDd)5 (n=8) ©2018 Karyopharm Therapeutics Inc. 83% ORR (Non-Refractory to PIs) ORR 63% (All) 63% ORR (Pomalyst-Naïve and Revlimid-Relapsed or -Refractory ) ORR 56% (All) 92% ORR (Revlimid-Naïve) 83% ORR (Darzalex-Naïve) ORR 63% (All) 4 ORR 81% (All)
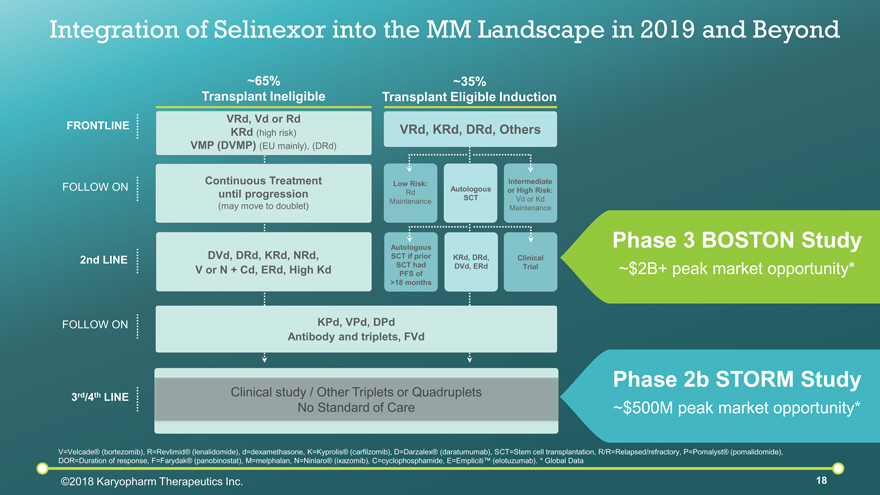
FOLLOW ON 2nd LINE FOLLOW ON 3rd/4th LINE FRONTLINE V=Velcade® (bortezomib), R=Revlimid® (lenalidomide), d=dexamethasone, K=Kyprolis® (carfilzomib), D=Darzalex® (daratumumab), SCT=Stem cell transplantation, R/R=Relapsed/refractory, P=Pomalyst® (pomalidomide), DOR=Duration of response, F=Farydak® (panobinostat), M=melphalan, N=Ninlaro® (ixazomib), C=cyclophosphamide, E=Empliciti™ (elotuzumab). * Global Data Clinical study / Other Triplets or Quadruplets No Standard of Care ~35% Transplant Eligible Induction VRd, KRd, DRd, Others Autologous SCT Intermediate or High Risk: Vd or Kd Maintenance Low Risk: Rd Maintenance KRd, DRd, DVd, ERd Clinical Trial Autologous SCT if prior SCT had PFS of >18 months ~65% Transplant Ineligible VRd, Vd or Rd KRd (high risk) VMP (DVMP) (EU mainly), (DRd) Continuous Treatment until progression (may move to doublet) DVd, DRd, KRd, NRd, V or N + Cd, ERd, High Kd ©2018 Karyopharm Therapeutics Inc. KPd, VPd, DPd Antibody and triplets, FVd Phase 3 BOSTON Study ~$2B+ peak market opportunity* Phase 2b STORM Study ~$500M peak market opportunity* Integration of Selinexor into the MM Landscape in 2019 and Beyond
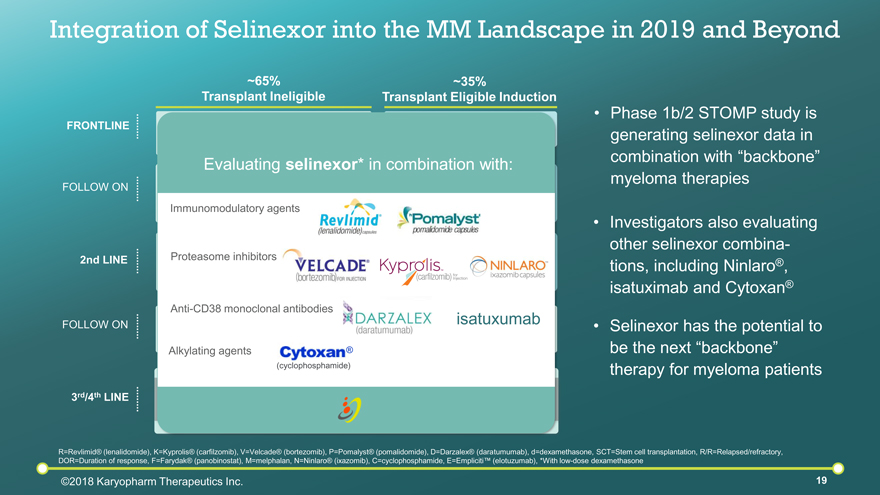
Integration of Selinexor into the MM Landscape in 2019 and Beyond R=Revlimid® (lenalidomide), K=Kyprolis® (carfilzomib), V=Velcade® (bortezomib), P=Pomalyst® (pomalidomide), D=Darzalex® (daratumumab), d=dexamethasone, SCT=Stem cell transplantation, R/R=Relapsed/refractory, DOR=Duration of response, F=Farydak® (panobinostat), M=melphalan, N=Ninlaro® (ixazomib), C=cyclophosphamide, E=Empliciti™ (elotuzumab), *With low-dose dexamethasone Phase 1b/2 STOMP study is generating selinexor data in combination with “backbone” myeloma therapies ©2018 Karyopharm Therapeutics Inc. FOLLOW ON 2nd LINE FOLLOW ON 3rd/4th LINE FRONTLINE Clinical study / Other Triplets or Quadruplets No Standard of Care ~35% Transplant Eligible Induction VRd, KRd, DRd, Others Autologous SCT Intermediate or High Risk: Vd or Kd Maintenance Low Risk: Rd Maintenance KRd, DRd, DVd, ERd Clinical Trial Autologous SCT if prior SCT had PFS of >18 months ~65% Transplant Ineligible VRd, Vd or Rd KRd (high risk) VMP (DVMP) (EU mainly), (DRd) Continuous Treatment until progression (may move to doublet) DVd, DRd, KRd, NRd, V or N + Cd, ERd, High Kd KPd, VPd, DPd Antibody and triplets, FVd The STOMP Study Evaluating selinexor* in combination with: Immunomodulatory agents Proteasome inhibitors Anti-CD38 monoclonal antibodies isatuxumab (cyclophosphamide) ® Alkylating agents Investigators also evaluating other selinexor combina-tions, including Ninlaro®, isatuximab and Cytoxan® Selinexor has the potential to be the next “backbone” therapy for myeloma patients

Selinexor in Hematologic Malignancies Diffuse Large B-Cell Lymphoma
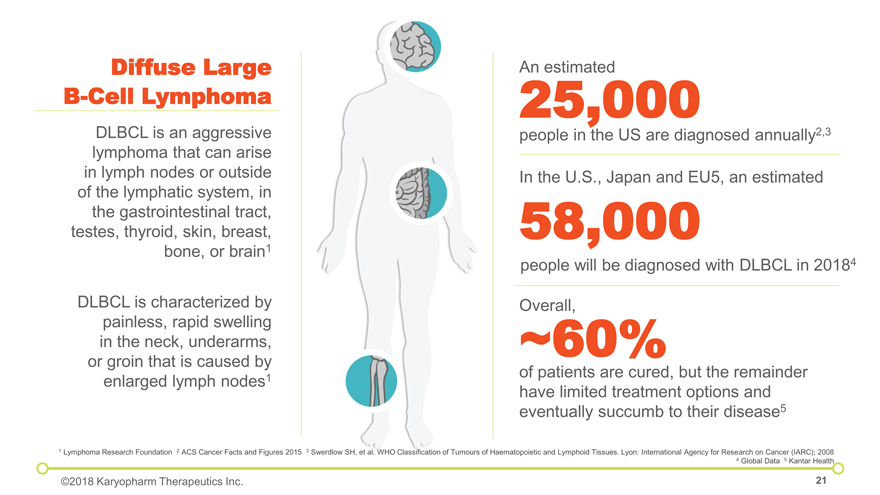
of patients are cured, but the remainder have limited treatment options and eventually succumb to their disease5 ~60% Overall, 25,000 people in the US are diagnosed annually2,3 An estimated In the U.S., Japan and EU5, an estimated 58,000 people will be diagnosed with DLBCL in 20184 Diffuse Large B-Cell Lymphoma DLBCL is an aggressive lymphoma that can arise in lymph nodes or outside of the lymphatic system, in the gastrointestinal tract, testes, thyroid, skin, breast, bone, or brain1 DLBCL is characterized by painless, rapid swelling in the neck, underarms, or groin that is caused by enlarged lymph nodes1 1 Lymphoma Research Foundation 2 ACS Cancer Facts and Figures 2015 3 Swerdlow SH, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer (IARC); 2008 4 Global Data 5 Kantar Health ©2018 Karyopharm Therapeutics Inc.
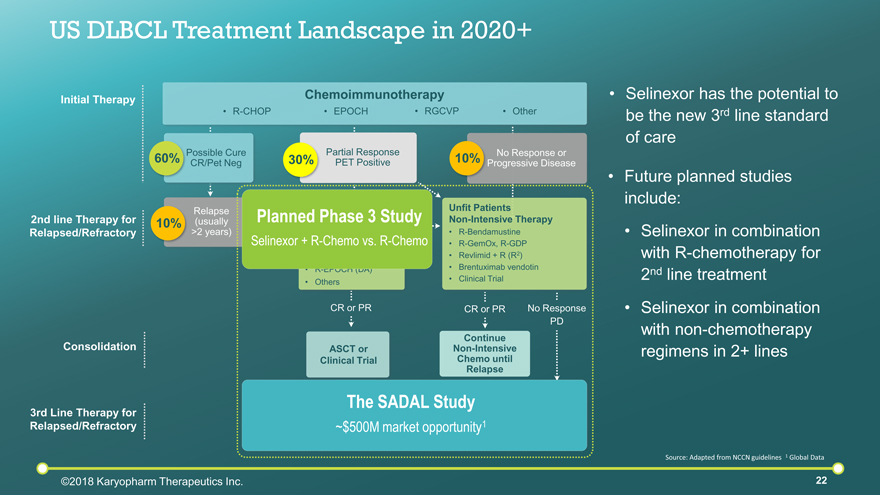
Fit Patients Intensive ChemoTx R-ICE R-DHAP R-ESHAP R-EPOCH (DA) Others No Response PD CR or PR 2nd line Therapy for Relapsed/Refractory Consolidation US DLBCL Treatment Landscape in 2020+ Partial Response PET Positive Chemoimmunotherapy Possible Cure CR/Pet Neg 30% Unfit Patients Non-Intensive Therapy R-Bendamustine R-GemOx, R-GDP Revlimid + R (R2) Brentuximab vendotin Clinical Trial Continue Non-Intensive Chemo until Relapse No Response or Progressive Disease 10% EPOCH R-CHOP RGCVP Other 60% 10% ASCT or Clinical Trial 3rd Line Therapy for Relapsed/Refractory Initial Therapy Source: Adapted from NCCN guidelines 1 Global Data CAR-T Clinical Trial Different Second Line Option Clinical Trial Different Second Line Option Relapse (usually >2 years) CR or PR Selinexor has the potential to be the new 3rd line standard of care The SADAL Study ~$500M market opportunity1 Future planned studies include: Selinexor in combination with R-chemotherapy for 2nd line treatment Planned Phase 3 Study Selinexor + R-Chemo vs. R-Chemo Selinexor in combination with non-chemotherapy regimens in 2+ lines ©2018 Karyopharm Therapeutics Inc.
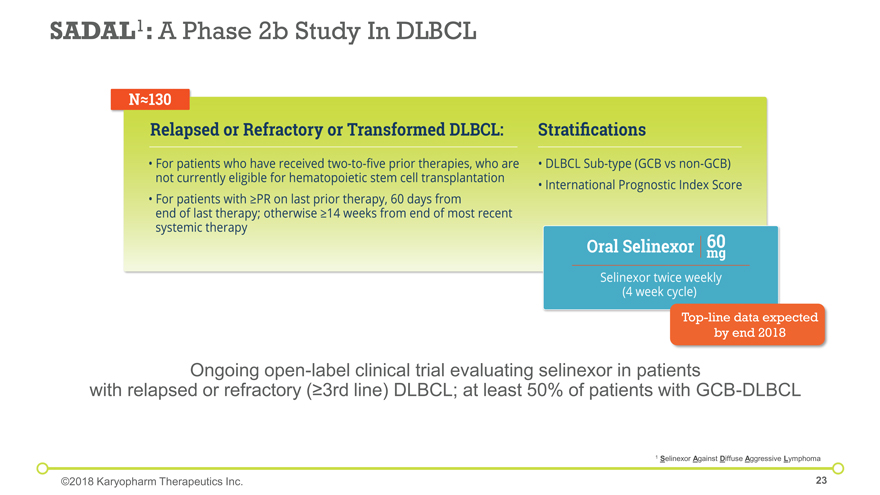
SADAL1: A Phase 2b Study In DLBCL Ongoing open-label clinical trial evaluating selinexor in patients with relapsed or refractory (³3rd line) DLBCL; at least 50% of patients with GCB-DLBCL 1 Selinexor Against Diffuse Aggressive Lymphoma ©2018 Karyopharm Therapeutics Inc. Top-line data expected by end 2018
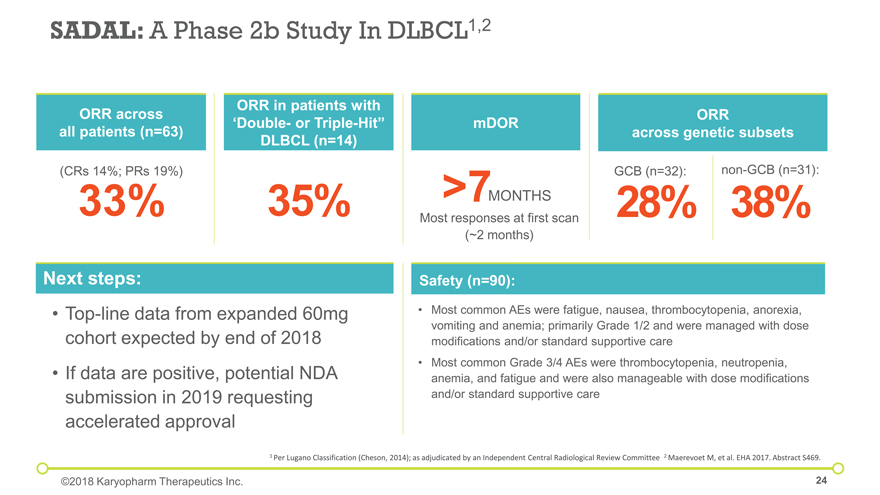
Next steps: Top-line data from expanded 60mg cohort expected by end of 2018 If data are positive, potential NDA submission in 2019 requesting accelerated approval Most common AEs were fatigue, nausea, thrombocytopenia, anorexia, vomiting and anemia; primarily Grade 1/2 and were managed with dose modifications and/or standard supportive care Most common Grade 3/4 AEs were thrombocytopenia, neutropenia, anemia, and fatigue and were also manageable with dose modifications and/or standard supportive care Safety (n=90): SADAL: A Phase 2b Study In DLBCL1,2 35% 38% non-GCB (n=31): GCB (n=32): 28% 33% (CRs 14%; PRs 19%) >7 MONTHS Most responses at first scan (~2 months) 1 Per Lugano Classification (Cheson, 2014); as adjudicated by an Independent Central Radiological Review Committee 2 Maerevoet M, et al. EHA 2017. Abstract S469. ORR across all patients (n=63) ORR in patients with ‘Double- or Triple-Hit” DLBCL (n=14) mDOR ORR across genetic subsets ©2018 Karyopharm Therapeutics Inc.

Selinexor in Solid Tumor Malignancies Liposarcoma Gynecologic Cancers
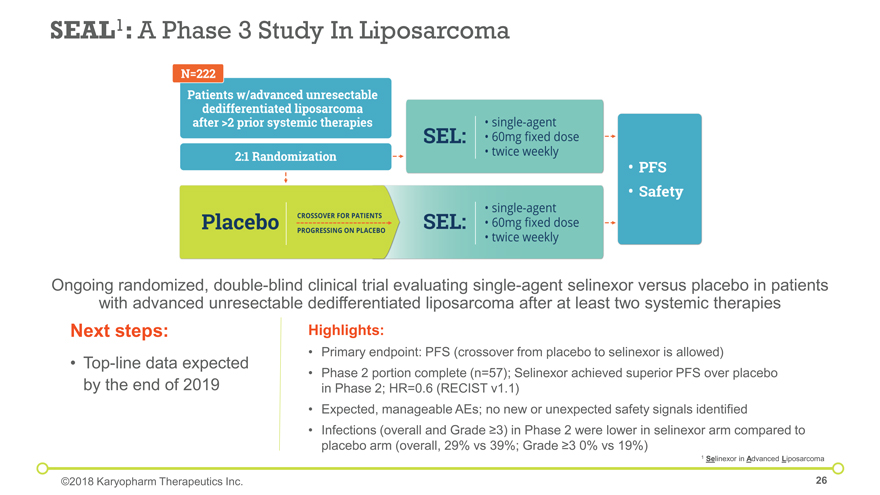
Next steps: Top-line data expected by the end of 2019 Ongoing randomized, double-blind clinical trial evaluating single-agent selinexor versus placebo in patients with advanced unresectable dedifferentiated liposarcoma after at least two systemic therapies SEAL1: A Phase 3 Study In Liposarcoma Highlights: Primary endpoint: PFS (crossover from placebo to selinexor is allowed) Phase 2 portion complete (n=57); Selinexor achieved superior PFS over placebo in Phase 2; HR=0.6 (RECIST v1.1) Expected, manageable AEs; no new or unexpected safety signals identified Infections (overall and Grade ³3) in Phase 2 were lower in selinexor arm compared to placebo arm (overall, 29% vs 39%; Grade ³3 0% vs 19%) 1 Selinexor in Advanced Liposarcoma ©2018 Karyopharm Therapeutics Inc.
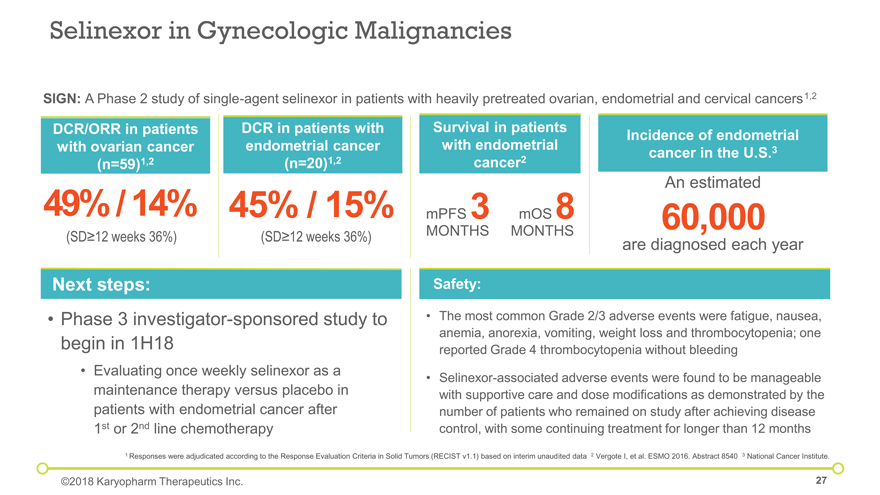
Next steps: Phase 3 investigator-sponsored study to begin in 1H18 Evaluating once weekly selinexor as a maintenance therapy versus placebo in patients with endometrial cancer after 1st or 2nd line chemotherapy The most common Grade 2/3 adverse events were fatigue, nausea, anemia, anorexia, vomiting, weight loss and thrombocytopenia; one reported Grade 4 thrombocytopenia without bleeding Selinexor-associated adverse events were found to be manageable with supportive care and dose modifications as demonstrated by the number of patients who remained on study after achieving disease control, with some continuing treatment for longer than 12 months Safety: Selinexor in Gynecologic Malignancies DCR in patients with endometrial cancer (n=20)1,2 Survival in patients with endometrial cancer2 Incidence of endometrial cancer in the U.S.3 mPFS 3 MONTHS 1 Responses were adjudicated according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1) based on interim unaudited data 2 Vergote I, et al. ESMO 2016. Abstract 8540 3 National Cancer Institute. SIGN: A Phase 2 study of single-agent selinexor in patients with heavily pretreated ovarian, endometrial and cervical cancers1,2 An estimated 60,000 are diagnosed each year 45% / 15% mOS 8 MONTHS DCR/ORR in patients with ovarian cancer (n=59)1,2 49% / 14% (SD³12 weeks 36%) ©2018 Karyopharm Therapeutics Inc. (SD³12 weeks 36%)
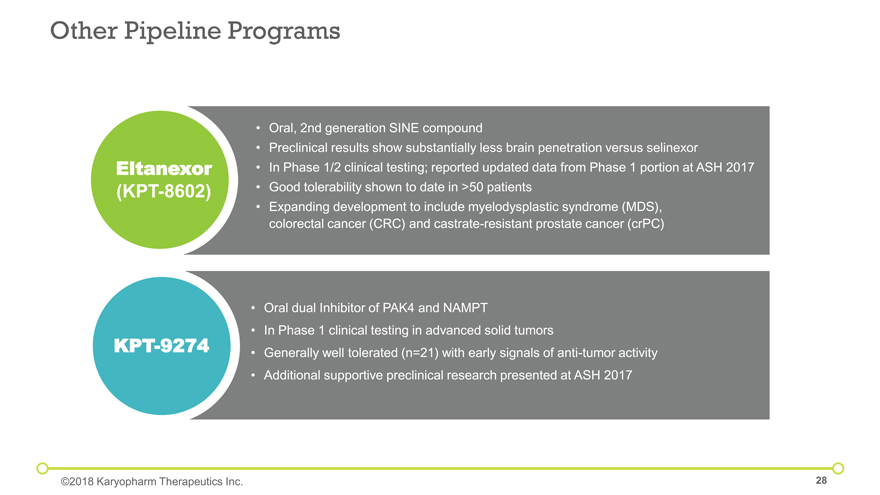
Other Pipeline Programs Eltanexor (KPT-8602) Oral dual Inhibitor of PAK4 and NAMPT In Phase 1 clinical testing in advanced solid tumors Generally well tolerated (n=21) with early signals of anti-tumor activity Additional supportive preclinical research presented at ASH 2017 KPT-9274 ©2018 Karyopharm Therapeutics Inc. Oral, 2nd generation SINE compound Preclinical results show substantially less brain penetration versus selinexor In Phase 1/2 clinical testing; reported updated data from Phase 1 portion at ASH 2017 Good tolerability shown to date in >50 patients Expanding development to include myelodysplastic syndrome (MDS), colorectal cancer (CRC) and castrate-resistant prostate cancer (crPC)

Licensed selinexor and eltanexor rights to Ono for oncology indications in the Territory Total deal value: up to US$193M plus royalties Receive US $22M upfront from Ono Eligible to receive up to US $170M in development and future commercial milestones, plus royalties Karyopharm retains all rights outside of the Territory The Territory: Japan, South Korea, Taiwan, Hong Kong and ASEAN countries Note: US$ value is based on the JPY/US$ exchange rate as of October 11, 2017 Partnerships Karyopharm-Ono Partnership Provides Market Access in Asia for Selinexor and Eltanexor ©2018 Karyopharm Therapeutics Inc.

Michael Kauffman Chief Executive Officer Jatin Shah VP, Clinical Strategy Significant Regulatory and Commercial Experience Mike Falvey Chief Financial Officer Chris Primiano SVP, Operations, Business Development and General Counsel Bill Hatfield SVP, Product Strategy & Market Development Joan Wood Chief Human Resources Officer Ran Frankel Chief Development Operations Officer Kevin Malobisky SVP, Regulatory Affairs, Quality, & Pharmacovigilance Ronit Milstein VP Operations Brian Austad VP, Head of Pharmaceutical Sciences ©2018 Karyopharm Therapeutics Inc. Sharon Shacham President and Chief Scientific Officer
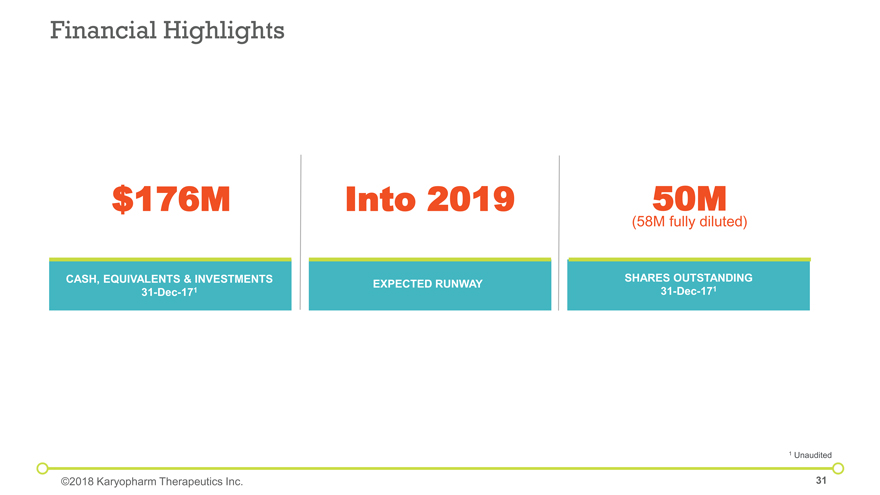
$176M Into 2019 50M (58M fully diluted) CASH, EQUIVALENTS & INVESTMENTS 31-Dec-171 SHARES OUTSTANDING 31-Dec-171 EXPECTED RUNWAY Financial Highlights ©2018 Karyopharm Therapeutics Inc. 1 Unaudited
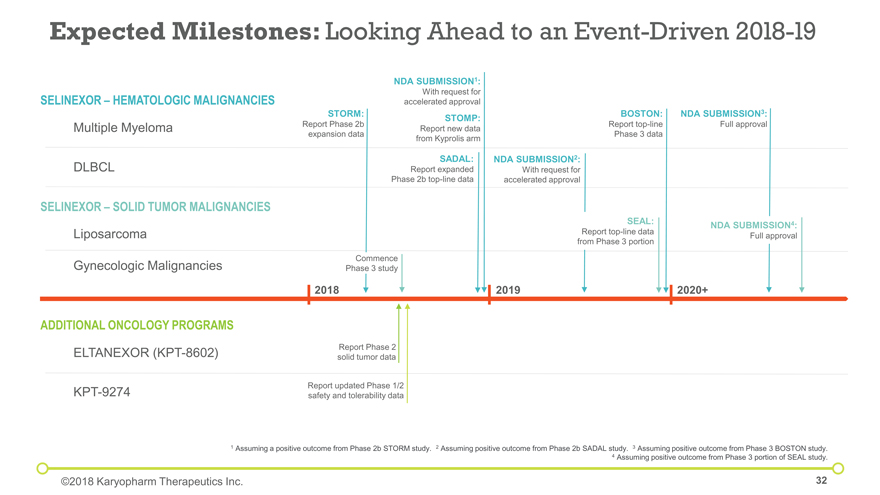
Liposarcoma SELINEXOR – HEMATOLOGIC MALIGNANCIES Expected Milestones: Looking Ahead to an Event-Driven 2018-19 ADDITIONAL ONCOLOGY PROGRAMS STORM: Report Phase 2b expansion data BOSTON: Report top-line Phase 3 data Report updated Phase 1/2 safety and tolerability data 2018 2019 2020+ Report Phase 2 solid tumor data Multiple Myeloma DLBCL SELINEXOR – SOLID TUMOR MALIGNANCIES ELTANEXOR (KPT-8602) KPT-9274 Gynecologic Malignancies NDA SUBMISSION1: With request for accelerated approval 1 Assuming a positive outcome from Phase 2b STORM study. 2 Assuming positive outcome from Phase 2b SADAL study. 3 Assuming positive outcome from Phase 3 BOSTON study. 4 Assuming positive outcome from Phase 3 portion of SEAL study. NDA SUBMISSION2: With request for accelerated approval NDA SUBMISSION3: Full approval SEAL: Report top-line data from Phase 3 portion NDA SUBMISSION4: Full approval STOMP: Report new data from Kyprolis arm SADAL: Report expanded Phase 2b top-line data Commence Phase 3 study ©2018 Karyopharm Therapeutics Inc.
Karyopharm Highlights Strong leadership team and balance sheet First-in-class, oral SINE compounds Lead asset with blockbuster potential 1 Global Data, 2 Assuming a positive outcome from Phase 2b STORM study, 3 Assuming positive outcome from Phase 2b SADAL study, 4 Assuming positive outcome from Phase 3 BOSTON study, 5 Assuming positive outcome from Phase 3 portion of SEAL study Hematologic Malignancies Multiple Myeloma Diffuse Large B-cell Lymphoma Liposarcoma Gynecologic Cancers Solid Tumor Malignancies ©2018 Karyopharm Therapeutics Inc. Near term catalysts from broad, late-stage pipeline Executive team with past successes in lead indications; cash expected to fund the company into 2019 Unique, broadly applicable mechanism of action that works well in combination regimens; patent life through 2032+ Selinexor is combinable with existing “backbone” therapies and has the potential to be the next “backbone” therapy in myeloma, a market projected to be $22B in 20231 Data readouts (next 24 months): STORM (April 2018), SADAL (2H18), BOSTON (2019), SEAL (2019) Submissions requesting approval: Accelerated approval in multiple myeloma (2018)2, accelerated approval in DLBCL (2019)3, full approval in multiple myeloma (2020)4, full approval in liposarcoma (2020)5 Anticipated launch for selinexor in multiple myeloma and DLBCL in 2019, followed by other indications in 2020+ Preparing for product launches and revenues

Thank You
