Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - EXACT SCIENCES CORP | a18-2337_1ex99d1.htm |
| 8-K - 8-K - EXACT SCIENCES CORP | a18-2337_18k.htm |
36th Annual J.P. Morgan Healthcare Conference 1 Kevin Conroy, Chairman and CEO January 9, 2018

This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended that are intended to be covered by the "safe harbor" created by those sections. Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, can generally be identified by the use of forward-looking terms such as "believe," "expect," "may," "will," "should," "could," "seek," "intend," "plan," “goal,” "estimate," "anticipate" or other comparable terms. All statements other than statements of historical facts included in this presentation regarding our strategies, prospects, financial condition, operations, costs, plans and objectives are forward-looking statements. Examples of forward-looking statements include, among others, statements we make regarding expected future operating results, anticipated results of our sales and marketing efforts, expectations concerning payor reimbursement and the anticipated results of our product development efforts. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: our ability to successfully and profitably market our products and services; the acceptance of our products and services by patients and healthcare providers; the willingness of health insurance companies and other payors to cover Cologuard and reimburse us for our performance of the Cologuard test; the amount and nature of competition from other cancer screening products and services; the effects of any healthcare reforms, including the Affordable Care Act, or changes in healthcare pricing, coverage and reimbursement; recommendations, guidelines and/or quality metrics issued by various organizations such as the U.S. Preventive Services Task Force, the American Cancer Society and the National Committee for Quality Assurance regarding cancer screening or our products and services; our ability to successfully develop new products and services; our success establishing and maintaining collaborative licensing and supplier arrangements; our ability to maintain regulatory approvals and comply with applicable regulations; and the other risks and uncertainties described in the Risk Factors and in Management's Discussion and Analysis of Financial Condition and Results of Operations sections of our most recently filed Annual Report on Form 10-K and our subsequently filed Quarterly Report(s) on Form 10-Q. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Exact Sciences has not completed preparation of its financial statements for the fourth quarter or full year of 2017. The revenue ranges presented for Q4 2017 and for the year ended December 31, 2017 are preliminary and unaudited and are thus inherently uncertain and subject to change as we complete our financial results for Q4 2017. We are in the process of completing our customary year-end close and review procedures as of and for the year ended December 31, 2017, and there can be no assurance that our final results for this period will not differ from these estimates. During the course of the preparation of our consolidated financial statements and related notes as of and for the year ended December 31, 2017, we or our independent registered public accountants may identify items that could cause our final reported results to be materially different from the preliminary financial estimates presented herein. 2 Safe harbor statement

Our Vision Exact Sciences is committed to helping win the war on cancer through early detection. 3

4 Pathway to success in 2018 and future
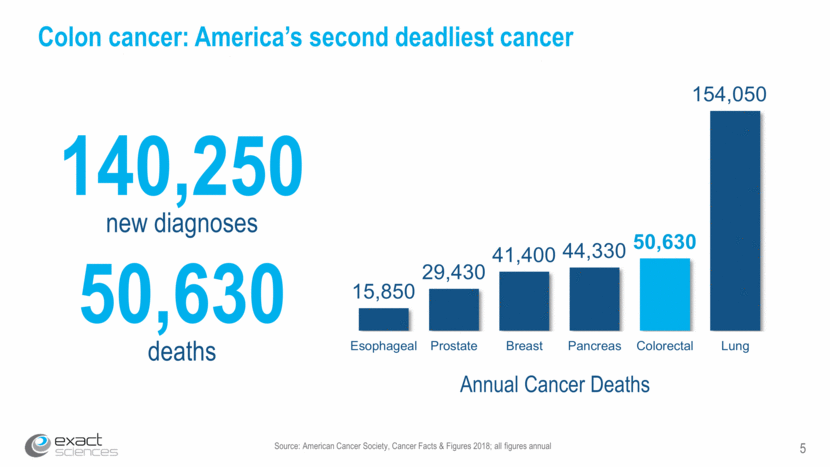
Source: American Cancer Society, Cancer Facts & Figures 2018; all figures annual Colon cancer: America’s second deadliest cancer Source: American Cancer Society, Cancer Facts & Figures 2017; all figures annual new diagnoses 140,250 deaths 50,630 Annual Cancer Deaths 5 15,850 29,430 41,400 44,330 50,630 154,050 Esophageal Prostate Breast Pancreas Colorectal Lung
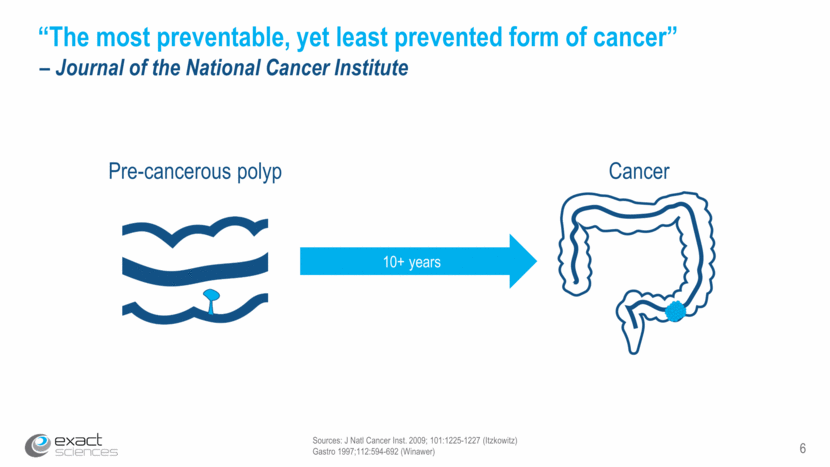
Sources: J Natl Cancer Inst. 2009; 101:1225-1227 (Itzkowitz) Gastro 1997;112:594-692 (Winawer) 6 “The most preventable, yet least prevented form of cancer” – Journal of the National Cancer Institute 10+ years Pre-cancerous polyp Cancer
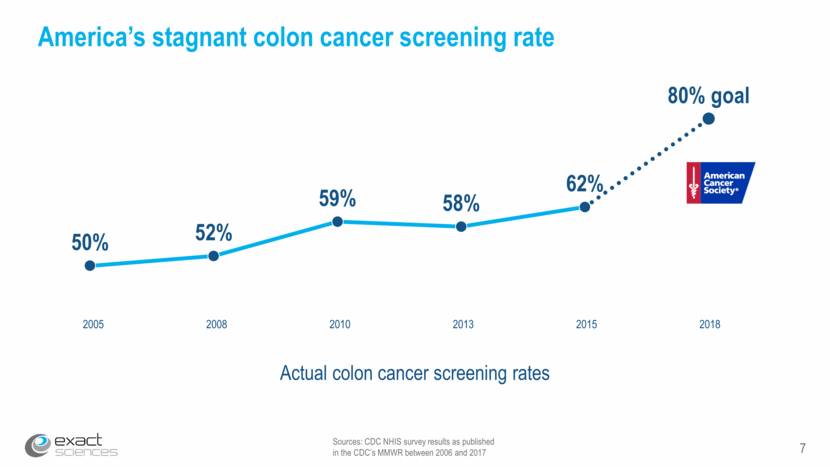
7 Sources: CDC NHIS survey results as published in the CDC’s MMWR between 2006 and 2017 America’s stagnant colon cancer screening rate Actual colon cancer screening rates 2005 2008 2010 2013 2015 2018 50% 52% 59% 58% 62% 80% goal
8 Source: Imperiale TF et al., N Engl J Med (2014) *For stage I and II cancers; 87% specificity Cologuard: Addressing the colon cancer challenge 94% early stage cancer sensitivity* developed with Easy to use Non-invasive No preparation No sedation No time off work
9 Cologuard is changing lives every day “My positive Cologuard test last year led to a colonoscopy that revealed stage I cancer. I’m grateful to now be active and healthy as I travel the country with my husband.” - Brenda Savannah, GA
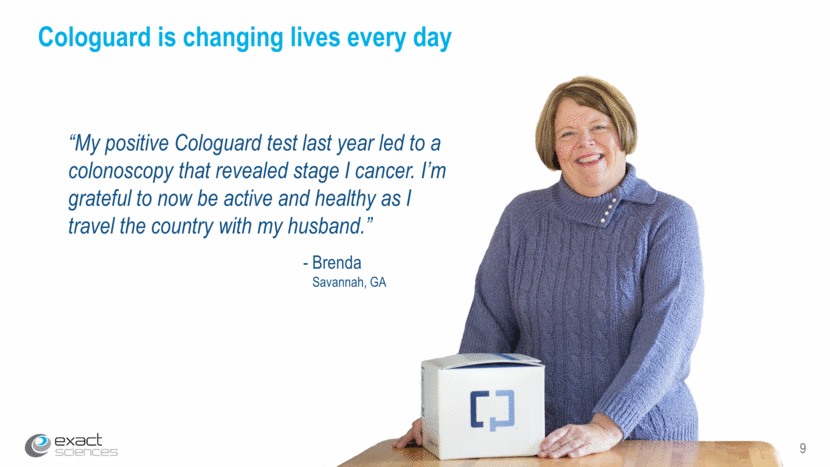
*Estimates based on DeeP-C pivotal trial findings: Imperiale TF et al., N Engl J Med (2014) 10 Impact of Cologuard since launch People screened 923,000 ~4,300 Early-stage cancers detected *
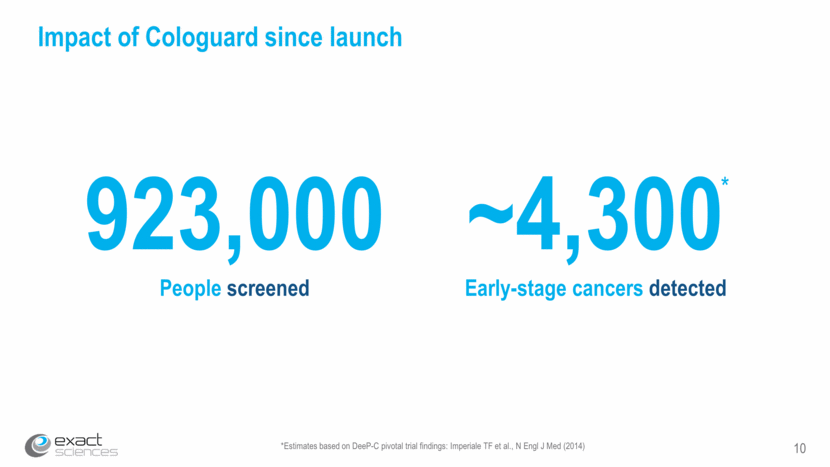
11 Strong Cologuard revenue growth 2015 2016 Quarterly Cologuard revenue ($ Millions) 2017 * *Midpoint of preliminary, unaudited fourth quarter revenue range $1.5 $4.3 $8.1 $12.6 $14.4 $14.8 $21.2 $28.1 $35.2 $48.4 $57.6 $72.6 $87.4
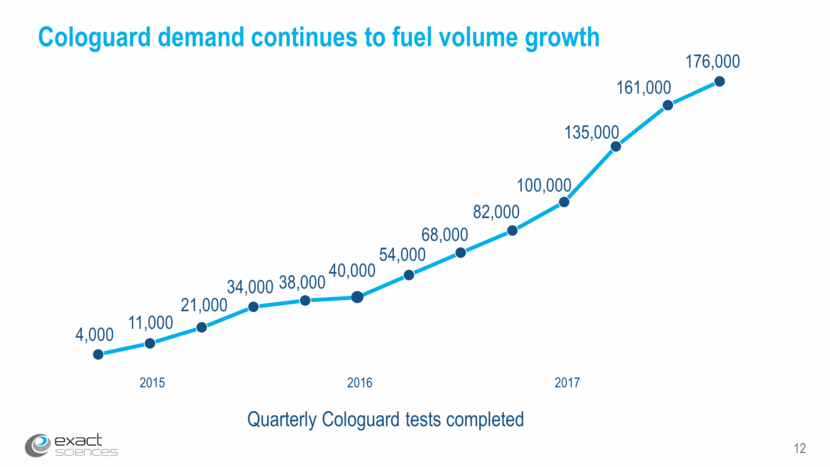
12 Cologuard demand continues to fuel volume growth 2015 2016 Quarterly Cologuard tests completed 2017 4,000 11,000 21,000 34,000 38,000 40,000 54,000 68,000 82,000 100,000 135,000 161,000 176,000
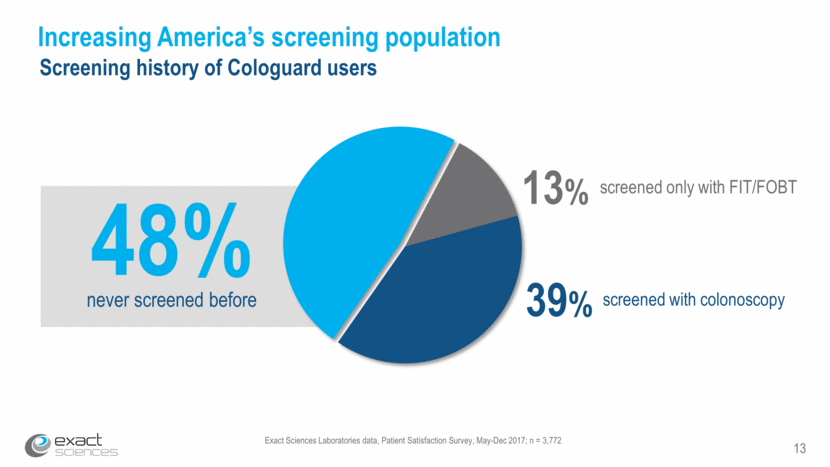
Exact Sciences Laboratories data, Patient Satisfaction Survey, May-Dec 2017; n = 3,772 13 Increasing America’s screening population Screening history of Cologuard users 39% screened with colonoscopy 13% screened only with FIT/FOBT 48% never screened before
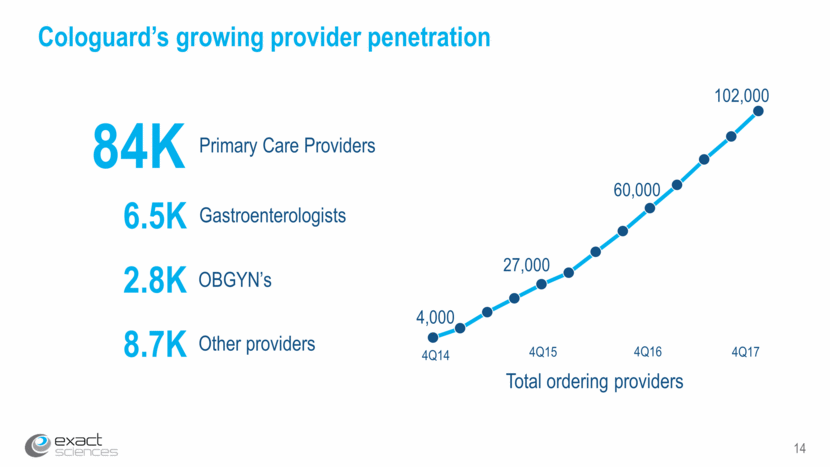
14 Cologuard’s growing provider penetration Total ordering providers 84K Primary Care Providers 4Q15 4Q17 4Q16 4Q14 6.5K Gastroenterologists 2.8K OBGYN’s 8.7K Other providers
15 Exact Sciences’ unique dataset addresses critical needs Improve quality measures and outcomes Payers Improve quality measures and reporting capabilities Providers Increase compliance and repeat screening Patients
16 A multi-billion dollar U.S. market opportunity *85 million average-risk, asymptomatic people ages 50-85, **Assumes revenue per test of $500-525 and 3-year interval for Cologuard, ***(176,000 completed tests * 4 to annualize * 3 to account for interval) / 85M 85M+ 2.5% Potential U.S. screening market for Cologuard* >$14B Total Addressable Market** market share***
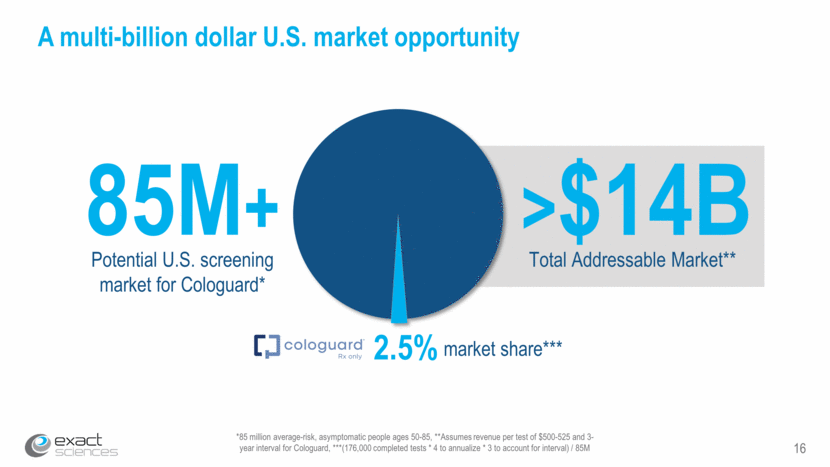
17 Clinical value of Cologuard: comparing numbers needed to screen/treat 166 to find 1 colorectal cancer* 217 to prevent 1 heart attack*** Statins *Imperiale TF et al., N Engl J Med (2014) **Hendrick R et al., AJR (2012) – for ages 40-49 ***Chou R, Dana T, Blazina I, Daeges M, Jeanne TL., JAMA (2016) Mammography 746 to prevent 1 breast cancer-related death**
18 Building infrastructure for long-term growth Lab Operations Sales Teams Customer Care & Billing Medical Affairs Clinical & Regulatory Health Systems
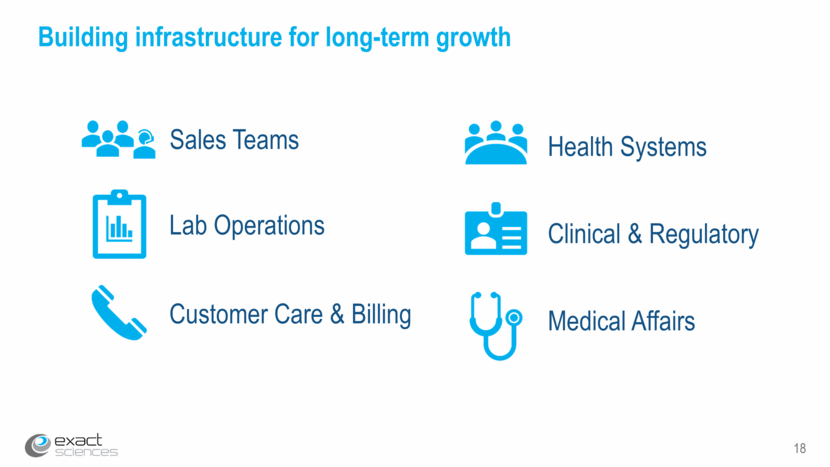
19 Investing in Exact Sciences’ nationwide sales force Primary care sales force Educate physicians & office staff and improve repeat ordering of Cologuard Extend reach of sales force coverage and new physician outreach and pull through Inside sales force ~250 ~100 350 200 2017 2018 (est.)
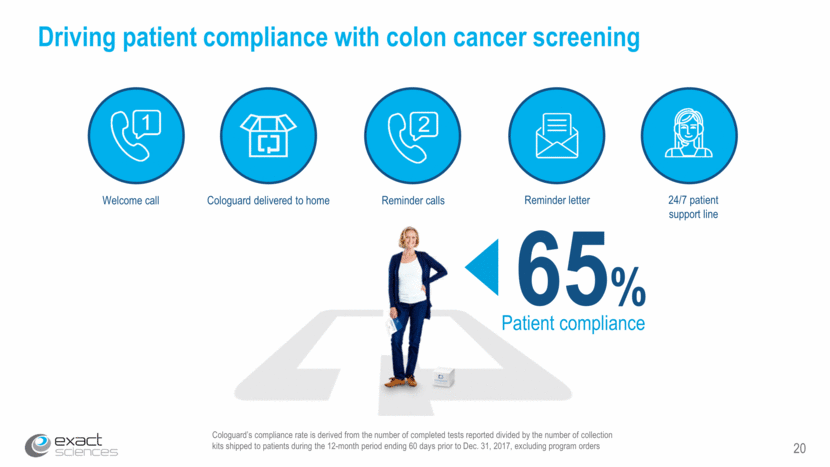
20 Cologuard’s compliance rate is derived from the number of completed tests reported divided by the number of collection kits shipped to patients during the 12-month period ending 60 days prior to Dec. 31, 2017, excluding program orders Driving patient compliance with colon cancer screening 65% Patient compliance Welcome call 24/7 patient support line Cologuard delivered to home Reminder calls Reminder letter
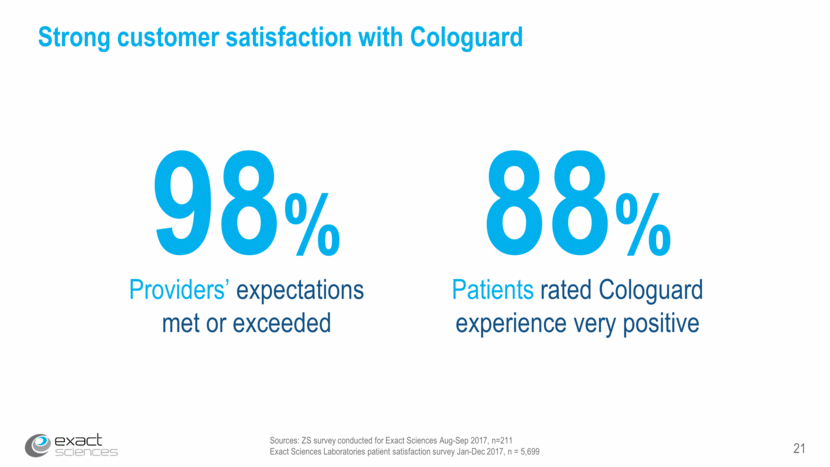
21 Sources: ZS survey conducted for Exact Sciences Aug-Sep 2017, n=211 Exact Sciences Laboratories patient satisfaction survey Jan-Dec 2017, n = 5,699 Strong customer satisfaction with Cologuard Providers’ expectations met or exceeded Patients rated Cologuard experience very positive 88% 98%
22 Broad marketing strategy increasing Cologuard awareness National TV campaign Social & digital media National partnerships Feb 28 – Mar 4 National celebrity spokesperson TBA

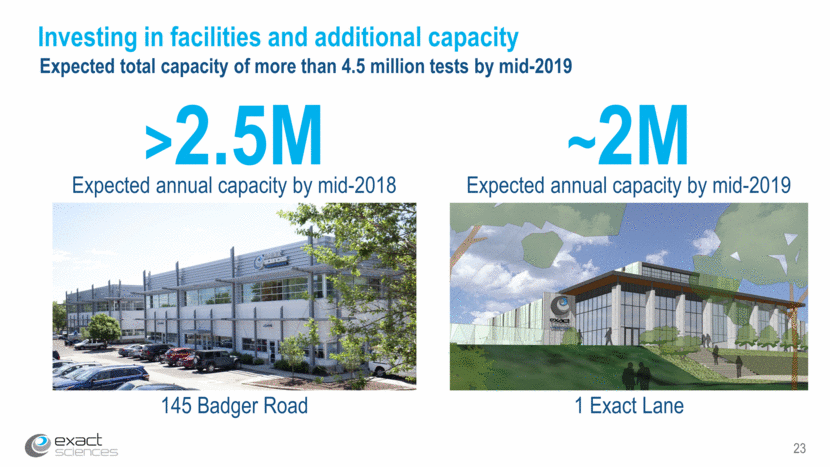
23 Investing in facilities and additional capacity ~2M Expected annual capacity by mid-2019 >2.5M Expected annual capacity by mid-2018 145 Badger Road 1 Exact Lane Expected total capacity of more than 4.5 million tests by mid-2019
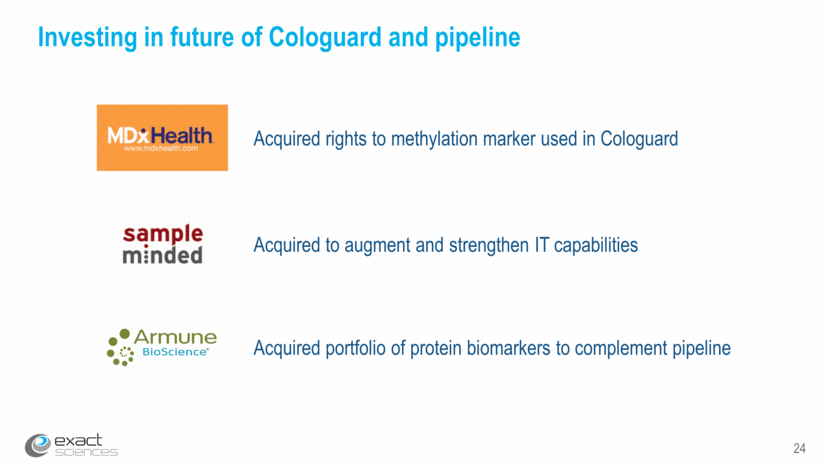
24 Investing in future of Cologuard and pipeline Acquired rights to methylation marker used in Cologuard Acquired to augment and strengthen IT capabilities Acquired portfolio of protein biomarkers to complement pipeline
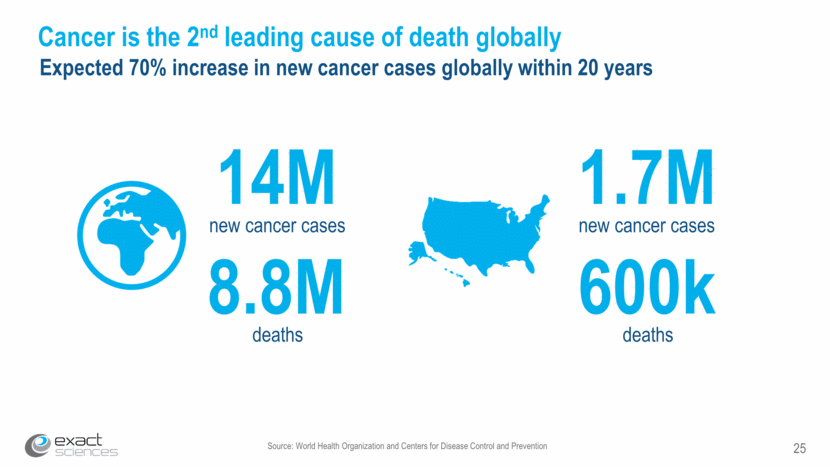
Source: World Health Organization and Centers for Disease Control and Prevention 25 Cancer is the 2nd leading cause of death globally Expected 70% increase in new cancer cases globally within 20 years 1.7M new cancer cases 600k deaths 14M new cancer cases 8.8M deaths
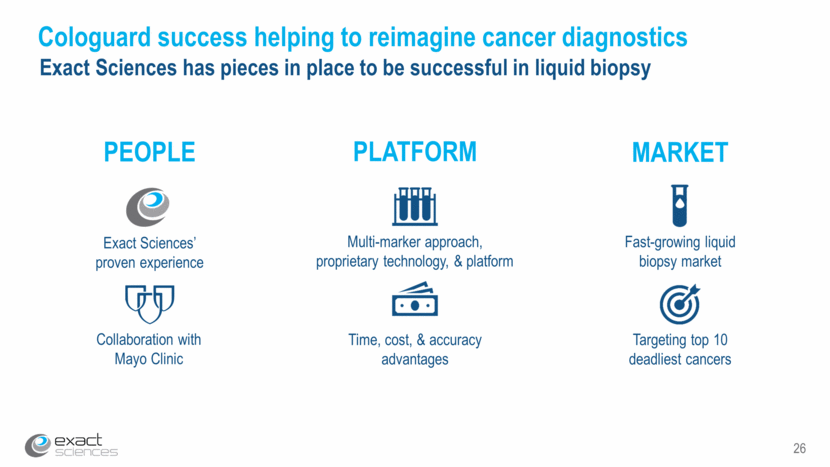
26 Cologuard success helping to reimagine cancer diagnostics Exact Sciences has pieces in place to be successful in liquid biopsy PEOPLE PLATFORM MARKET Collaboration with Mayo Clinic Exact Sciences’ proven experience Multi-marker approach, proprietary technology, & platform Time, cost, & accuracy advantages Fast-growing liquid biopsy market Targeting top 10 deadliest cancers
27 Unique collaboration between Exact Sciences and Mayo Clinic

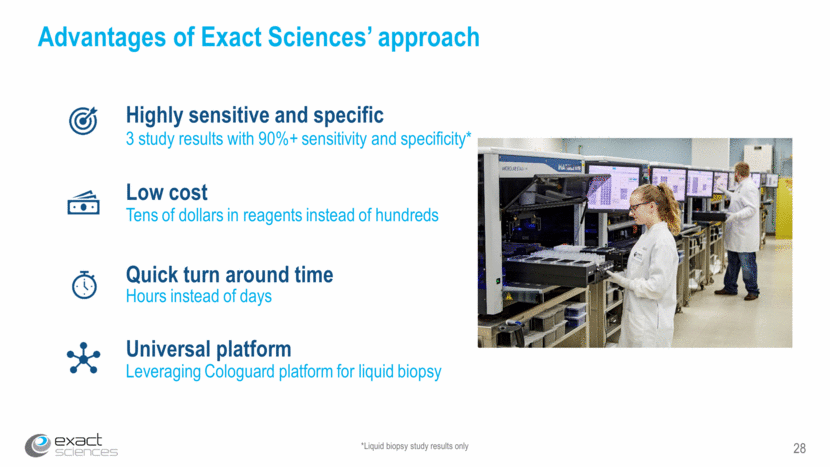
28 *Liquid biopsy study results only Advantages of Exact Sciences’ approach Highly sensitive and specific 3 study results with 90%+ sensitivity and specificity* Low cost Tens of dollars in reagents instead of hundreds Quick turn around time Hours instead of days Universal platform Leveraging Cologuard platform for liquid biopsy
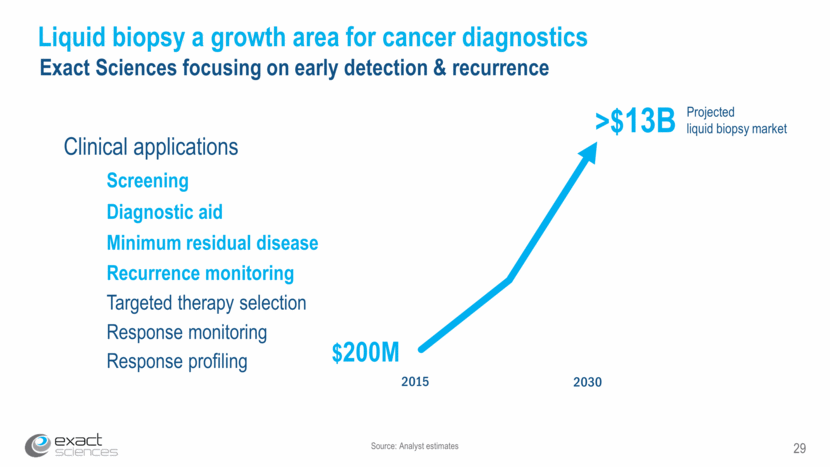
Source: Analyst estimates 29 Liquid biopsy a growth area for cancer diagnostics Exact Sciences focusing on early detection & recurrence Projected liquid biopsy market >$13B $200M Screening Minimum residual disease Recurrence monitoring Response monitoring Targeted therapy selection Clinical applications Diagnostic aid Response profiling 2015 2030
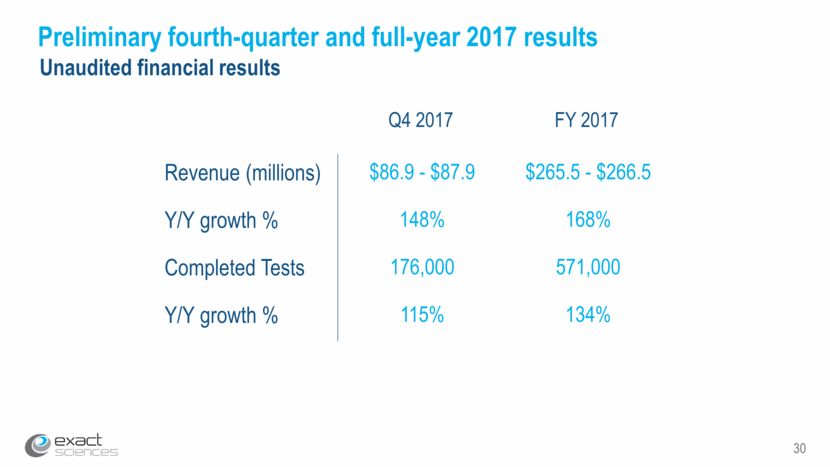
30 Preliminary fourth-quarter and full-year 2017 results Q4 2017 Revenue (millions) $86.9 - $87.9 $265.5 - $266.5 Y/Y growth % 148% 168% Completed Tests 176,000 571,000 Y/Y growth % 115% 134% FY 2017 Unaudited financial results
31

