Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Aclaris Therapeutics, Inc. | ex-99d2.htm |
| 8-K - 8-K - Aclaris Therapeutics, Inc. | f8-k.htm |
Exhibit 99.1
|
|
Company Overview Dr. Neal Walker President and CEO January 2018 © Copyright 2018 Aclaris Therapeutics. All rights reserved. A-1 Illuminating Science Empowering Patients |
|
|
Any statements contained in this presentation that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as "believe", "expect", "may", "plan," "potential," "will," and similar expressions, and are based on Aclaris' current beliefs and expectations. These forward-looking statements include expectations regarding Aclaris’ use of cash through the second half of 2019, development programs in skin and hair conditions, and the clinical development of JAK inhibitors. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the conduct of clinical trials, Aclaris' reliance on third parties over which it may not always have full control, and other risks and uncertainties that are described in the Risk Factors section of Aclaris' Annual Report on Form 10-K for the year ended December 31, 2016, Form 10-Q filed for the quarter ended September 30, 2017, and other filings Aclaris makes with the U.S. Securities and Exchange Commission from time to time. These documents are available under the "Financial Information" section of the Investors page of Aclaris' website at http://www.aclaristx.com. Any forward-looking statements speak only as of the date of this presentation and are based on information available to Aclaris as of the date of this release, and Aclaris assumes no obligation to, and does not intend to, update any forward-looking statements, whether as a result of new information, future events or otherwise This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Cautionary Note Regarding Forward-Looking Statements 2 |
|
|
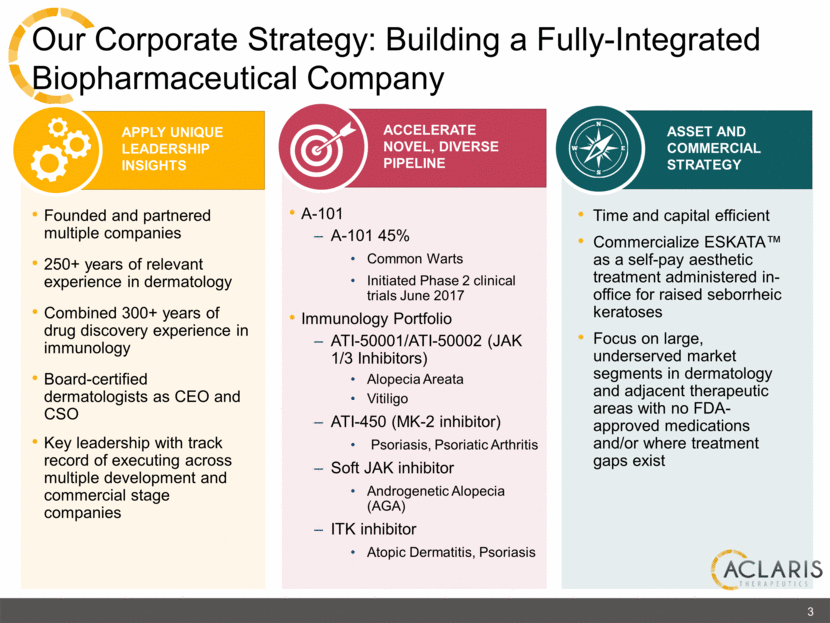
APPLY UNIQUE LEADERSHIP INSIGHTS Our Corporate Strategy: Building a Fully-Integrated Biopharmaceutical Company A-101 A-101 45% Common Warts Initiated Phase 2 clinical trials June 2017 Immunology Portfolio ATI-50001/ATI-50002 (JAK 1/3 Inhibitors) Alopecia Areata Vitiligo ATI-450 (MK-2 inhibitor) Psoriasis, Psoriatic Arthritis Soft JAK inhibitor Androgenetic Alopecia (AGA) ITK inhibitor Atopic Dermatitis, Psoriasis Founded and partnered multiple companies 250+ years of relevant experience in dermatology Combined 300+ years of drug discovery experience in immunology Board-certified dermatologists as CEO and CSO Key leadership with track record of executing across multiple development and commercial stage companies Time and capital efficient Commercialize ESKATA™ as a self-pay aesthetic treatment administered in-office for raised seborrheic keratoses Focus on large, underserved market segments in dermatology and adjacent therapeutic areas with no FDA-approved medications and/or where treatment gaps exist ACCELERATE NOVEL, DIVERSE PIPELINE ASSET AND COMMERCIAL STRATEGY 3 |
|
|
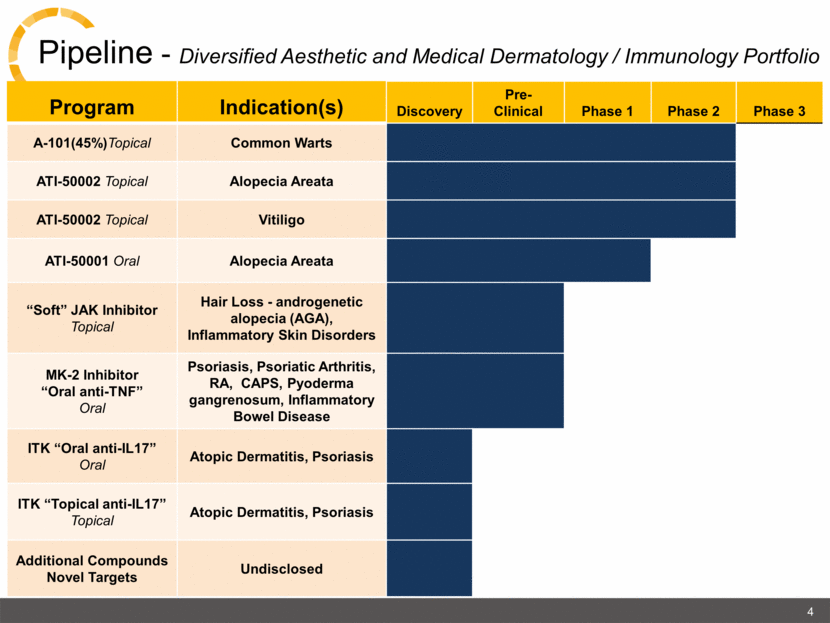
4 Pipeline - Diversified Aesthetic and Medical Dermatology / Immunology Portfolio Program Indication(s) Discovery Pre-Clinical Phase 1 Phase 2 Phase 3 A-101(45%)Topical Common Warts ATI-50002 Topical Alopecia Areata ATI-50002 Topical Vitiligo ATI-50001 Oral Alopecia Areata “Soft” JAK Inhibitor Topical Hair Loss - androgenetic alopecia (AGA), Inflammatory Skin Disorders MK-2 Inhibitor “Oral anti-TNF” Oral Psoriasis, Psoriatic Arthritis, RA, CAPS, Pyoderma gangrenosum, Inflammatory Bowel Disease ITK “Oral anti-IL17” Oral Atopic Dermatitis, Psoriasis ITK “Topical anti-IL17” Topical Atopic Dermatitis, Psoriasis Additional Compounds Novel Targets Undisclosed |
|
|
...and non-surgical procedures increased by 650% from 1997-20163 Global aesthetic market expected to reach $13.29 billion by 2021, growing at CAGR of 10.8%2 Global dermatology market valued at $20 billion in 2015, growing at CAGR of 7.7%1 “The types of procedures being performed is changing. Most of the growth has been in minimally invasive cosmetic procedures.” - The Washington Post, March 2, 2016 1 Global Dermatology Market to 2022 GBI Research. http://www.gbiresearch.com/report-store/market-reports/therapy-analysis/global-dermatology-market-to-2022-innovative-pipeline-and-increasing-uptake-of-biologics-to-diversify-treatment-options-and-d Last accessed November 21, 2017. 2 Medical Aesthetics Market Report. MarketsandMarkets, 2015. http://www.marketsandmarkets.com/PressReleases/medical-aesthetics.asp. Last accessed November 21, 2017. 3 2016 ASAPS Statistics: Complete Charts. American Society for Aesthetic Plastic Surgery. http://www.surgery.org/sites/default/files/ASAPS-Stats2016.pdf. Last accessed November 21, 2017. 5 Markets Poised for Continued Growth: Self-Pay Aesthetic and Medical Dermatology |
|
|
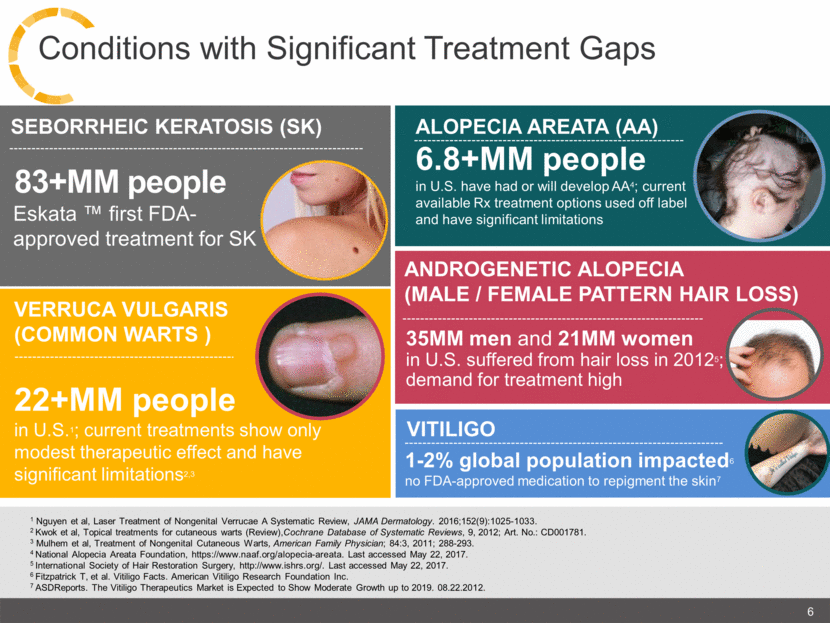
22+MM people in U.S.1; current treatments show only modest therapeutic effect and have significant limitations2,3 ANDROGENETIC ALOPECIA (MALE / FEMALE PATTERN HAIR LOSS) VITILIGO 1-2% global population impacted6 no FDA-approved medication to repigment the skin7 1 Nguyen et al, Laser Treatment of Nongenital Verrucae A Systematic Review, JAMA Dermatology. 2016;152(9):1025-1033. 2 Kwok et al, Topical treatments for cutaneous warts (Review),Cochrane Database of Systematic Reviews, 9, 2012; Art. No.: CD001781. 3 Mulhem et al, Treatment of Nongenital Cutaneous Warts, American Family Physician; 84:3, 2011; 288-293. 4 National Alopecia Areata Foundation, https://www.naaf.org/alopecia-areata. Last accessed May 22, 2017. 5 International Society of Hair Restoration Surgery, http://www.ishrs.org/. Last accessed May 22, 2017. 6 Fitzpatrick T, et al. Vitiligo Facts. American Vitiligo Research Foundation Inc. 7 ASDReports. The Vitiligo Therapeutics Market is Expected to Show Moderate Growth up to 2019. 08.22.2012. VERRUCA VULGARIS (COMMON WARTS ) 6.8+MM people in U.S. have had or will develop AA4; current available Rx treatment options used off label and have significant limitations ALOPECIA AREATA (AA) 35MM men and 21MM women in U.S. suffered from hair loss in 20125; demand for treatment high 83+MM people SEBORRHEIC KERATOSIS (SK) Eskata ™ first FDA-approved treatment for SK 6 Conditions with Significant Treatment Gaps |
|
|
ESKATATM Approval and Launch Illuminating Science Empowering Patients |
|
|
8 8 ESKATA: First FDA-Approved Treatment for Raised SK (hydrogen peroxide) topical solution, 40% (w/w) NDC: 71180-001-01 One Unit Rx Only Store at 20-25oC Single use applicator/0.7 mL. For Topical Use Only. |
|
|
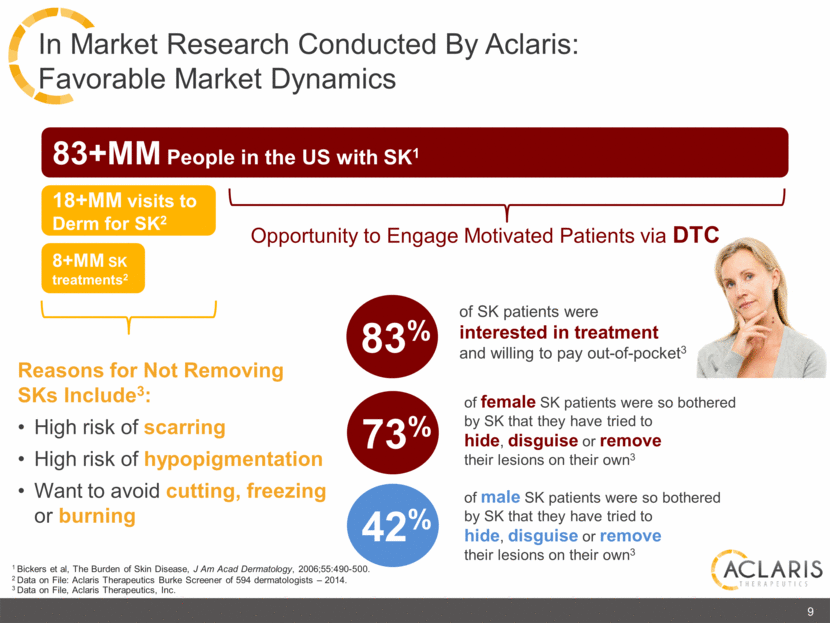
83+MM People in the US with SK1 18+MM visits to Derm for SK2 8+MM SK treatments2 83% of SK patients were interested in treatment and willing to pay out-of-pocket3 1 Bickers et al, The Burden of Skin Disease, J Am Acad Dermatology, 2006;55:490-500. 2 Data on File: Aclaris Therapeutics Burke Screener of 594 dermatologists – 2014. 3 Data on File, Aclaris Therapeutics, Inc. of female SK patients were so bothered by SK that they have tried to hide, disguise or remove their lesions on their own3 Opportunity to Engage Motivated Patients via DTC Reasons for Not Removing SKs Include3: High risk of scarring High risk of hypopigmentation Want to avoid cutting, freezing or burning 73% of male SK patients were so bothered by SK that they have tried to hide, disguise or remove their lesions on their own3 42% 9 In Market Research Conducted By Aclaris: Favorable Market Dynamics |
|
|
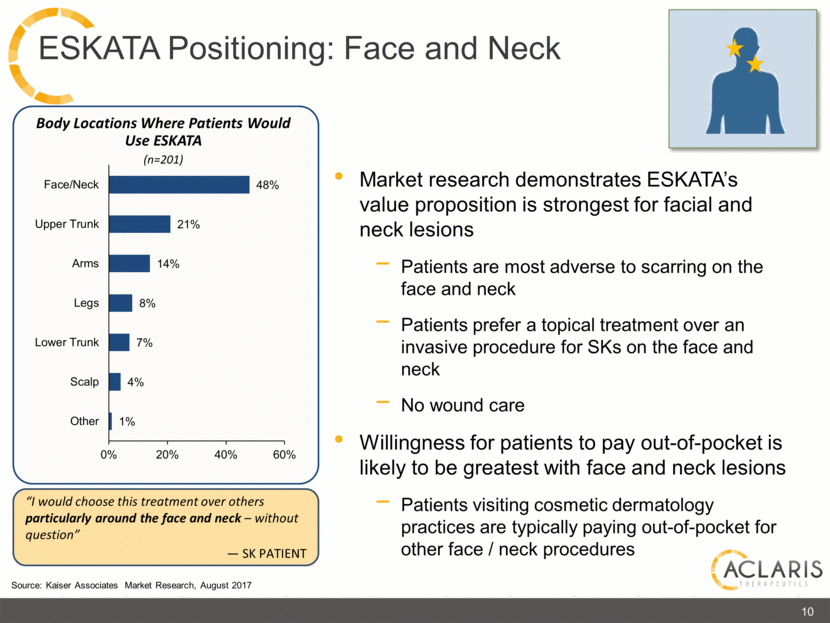
Body Locations Where Patients Would Use ESKATA (n=201) Market research demonstrates ESKATA’s value proposition is strongest for facial and neck lesions Patients are most adverse to scarring on the face and neck Patients prefer a topical treatment over an invasive procedure for SKs on the face and neck No wound care Willingness for patients to pay out-of-pocket is likely to be greatest with face and neck lesions Patients visiting cosmetic dermatology practices are typically paying out-of-pocket for other face / neck procedures “I would choose this treatment over others particularly around the face and neck – without question” — SK PATIENT Source: Kaiser Associates Market Research, August 2017 10 ESKATA Positioning: Face and Neck |
|
|
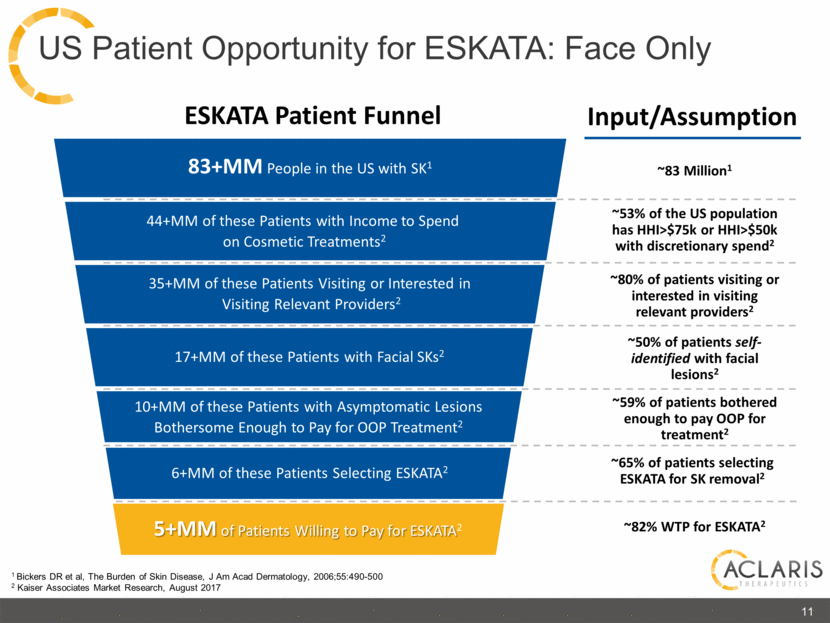
83+MM People in the US with SK1 17+MM of these Patients with Facial SKs2 44+MM of these Patients with Income to Spend on Cosmetic Treatments2 ESKATA Patient Funnel 5+MM of Patients Willing to Pay for ESKATA2 35+MM of these Patients Visiting or Interested in Visiting Relevant Providers2 10+MM of these Patients with Asymptomatic Lesions Bothersome Enough to Pay for OOP Treatment2 6+MM of these Patients Selecting ESKATA2 Input/Assumption ~83 Million1 ~53% of the US population has HHI>$75k or HHI>$50k with discretionary spend2 ~80% of patients visiting or interested in visiting relevant providers2 ~50% of patients self-identified with facial lesions2 ~59% of patients bothered enough to pay OOP for treatment2 ~65% of patients selecting ESKATA for SK removal2 ~82% WTP for ESKATA2 US Patient Opportunity for ESKATA: Face Only 1 Bickers DR et al, The Burden of Skin Disease, J Am Acad Dermatology, 2006;55:490-500 2 Kaiser Associates Market Research, August 2017 11 |
|
|
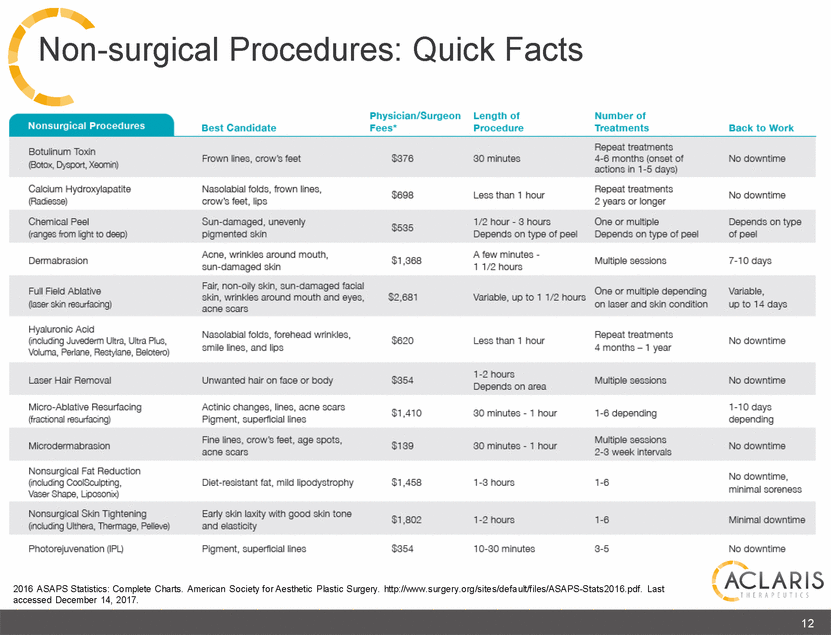
Non-surgical Procedures: Quick Facts Physician/Surgeon Fees* Length of Procedure Number of Treatments Nonsurgical Procedures Best Candidate Back to Work Repeat treatments 4-6 months (onset of actions in 1-5 days) Botulinum Toxln (Botox, Dyspoi, Xeonin) Frown Ones, craw's feet $376 30 minutes No downtime Calcium Hydroxylapatite (Radiesse) Nasolabial folds, frown lines, crow's feet, lips Repeat treatments 2 years or longer $698 Less than 1 hour No downtime Chemical Peel (ranges from light to deep) Sun-damaged, unevenly pigmented skin 1/2 hour - 3 hours Depends on type of peel One or multiple Depends on type of peel Depends on type of peel $535 Acne, wrinkles around mouth, sun-damaged skin Fair, non-oily skin, sun-damaged facial skin, wrinkles around mouth and eyes, acne scars A few minutes - 1 1/2 hours Dermabrasion $1,368 Multiple sessions 7-10 days Full Field Ablative (laser skin resurfacing) One or multiple depending on laser and skin condition Variable, up to 14 days $2,681 Variable, up to 1 1/2 hours Hyaluronic Acid (including Juvederm Ultra, Ultra Plus, Voluma, Perlane, restylane, Belotero) Nasolabial folds, forehead wrinkles, smile lines, and lips Repeat treatments 4 months - 1 year $620 Less than 1 hour No downtime 1-2 hours Depends on area Laser Hair Removal Unwanted hair on face or body $354 Multiple sessions No downtime Micro-Ablative Resurfacing (fractional resurfacing) Actinic changes, lines, acne scars Pigment, superficial lines 1-10 days depending $1,410 30 minutes-1 hour 1-6 depending Fine lines, crow's feet, age spots, acne scars Multiple sessions 2-3 week Intervals Microdermabrasion $139 30 minutes - 1 hour No downtime Nonsurgical Fat Reduction (including CoolSculpting, Vaser Shape, Liposonix) No downtime, minimal soreness Diet-resistant fat, mild lipodystrophy $1,458 1-3 hours 1-6 Nonsurgical Skin Tightening (including Ulthera, Thermage, Pelieve) Early skin laxity with good skin tone and elasticity $1,802 1-2 hours 1-6 Minimal downtime , Photorejuvenation (IPL) Pigment, superficial lines $354 10-30 minutes 3-5 No downtime I ACLARIS THERAPEUTICS 2016 ASAPS Statistics: Complete Charts. American Society for Aesthetic Plastic Surgery. http:/www.surgery.org/sites/default/files/ASAPS-Stats2016.pdf. Last '- accessed December 14, 2017.
|
|
|
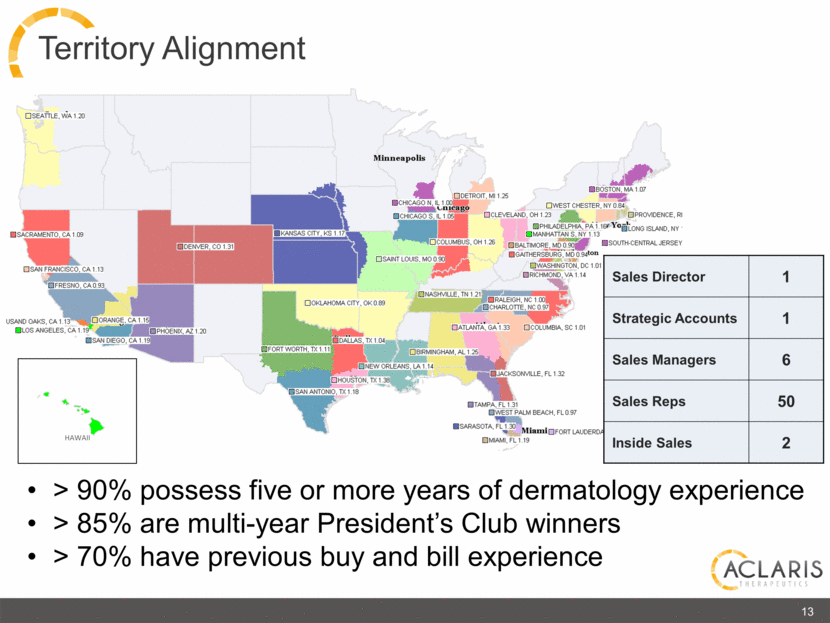
Territory Alignment Sales Director 1 Strategic Accounts 1 Sales Managers 6 Sales Reps 50 Inside Sales 2 HAWAII 13 > 90% possess five or more years of dermatology experience > 85% are multi-year President’s Club winners > 70% have previous buy and bill experience SEATTLE, WA 1.20 SACRAMENTO, CA 1.09 SAN FRANCISCO, CA 1.13 FRESNO, CA 0.93 USAND OAKS, CA 1.13 LOS ANGELES, CA 1.19 ORANGE, CA 1.15 SAN DIEGO, CA 1.19 PHOENIX, AZ 1.20 DENVER, CO 1.31 HAWAII KANSAS CITY, KS 1.17 OKLAHOMA CITY, OK 0.89 DALLAS, TX 1.04 FORT WORTH, TX 1.11 NEW ORLEANS, LA 1.14 HOUSTON, TX 1.38 SAN ANTONID, TX 1.18 CHICAGO N, IL 1.00 CHICAGO S, IL 1.05 COLUMBUS, OH 1.26 SAINT LOUIS, MO 0.90 NASHVILLE, TN 1.21 ATLANTA, GA 1.33 BIRMINGHAM, AL 1.25 JACKSONVILLE, FL 1.32 TAMPA, FL 1.31 WEST PALM BEACH, FL 0.97 SARASOTA, FL 1.30 FORT LAUDERDA MIAMI, FL 1.19 Miami RALEIGH, NC 1.00 CHARLOTT, NC 0.97 COLUMBIA, SC 1.01 DETROIT, MI 1.25 Minneapolis Chicago BOSTON, MA 1.07 WEST CHESTER, NY 0.84 CLEVELAND, OH 1.23 PHILADEPHIA, PA 1.16 MANHATTANS, NY 1.13 BALTIMORE, MD 0.90 GAITHERSBURG, MD 0.94 WASHINGTON, DC 1.01 RICHMOND, VA 1.14 PROVIDENCE, RI LONG ISLAND, NY SOUTH-CENTRAL JERSEY |
|
|
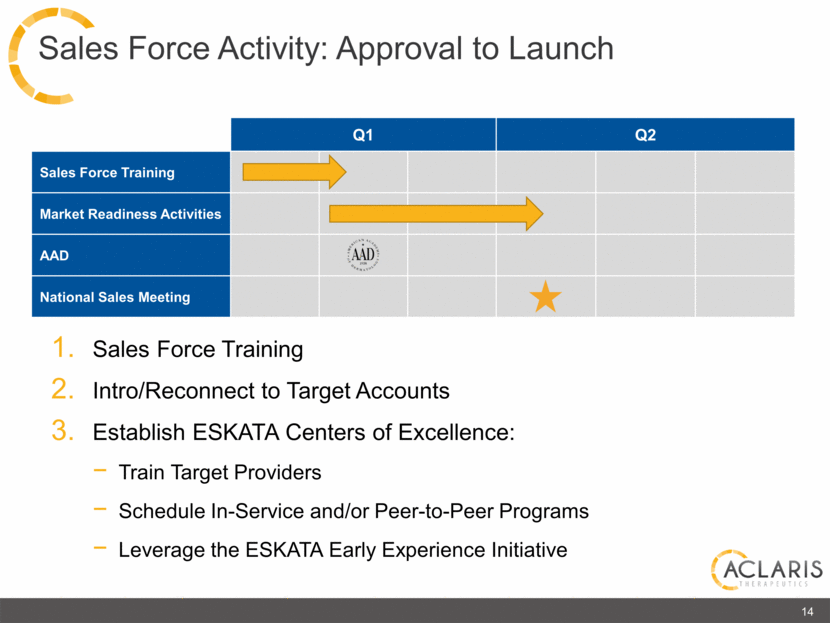
Q1 Q2 Sales Force Training Market Readiness Activities AAD National Sales Meeting 14 Sales Force Activity: Approval to Launch Sales Force Training Intro/Reconnect to Target Accounts Establish ESKATA Centers of Excellence: Train Target Providers Schedule In-Service and/or Peer-to-Peer Programs Leverage the ESKATA Early Experience Initiative |
|
|
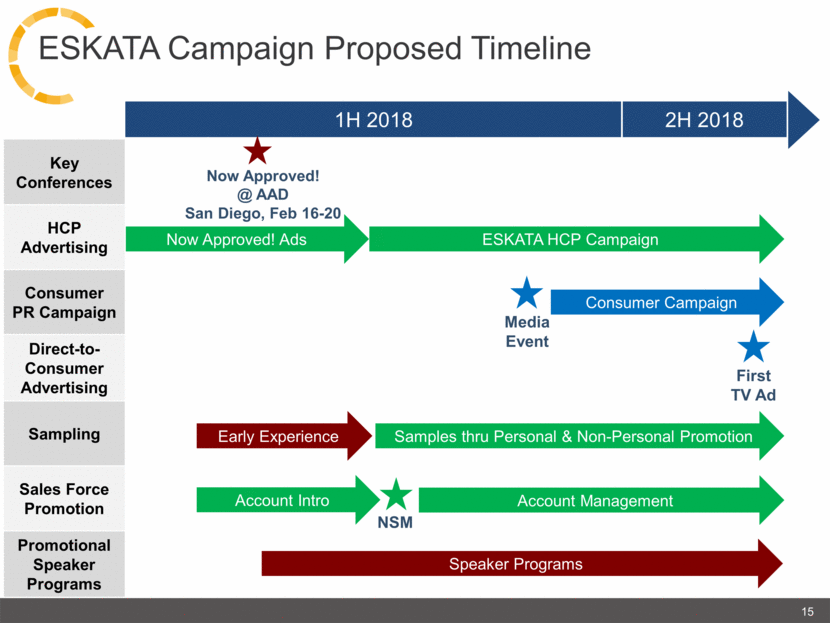
1H 2018 2H 2018 ESKATA Campaign Proposed Timeline Now Approved! Ads Account Intro Early Experience Speaker Programs Now Approved! @ AAD San Diego, Feb 16-20 ESKATA HCP Campaign Key Conferences HCP Advertising Consumer PR Campaign Direct-to-Consumer Advertising Sampling Sales Force Promotion Promotional Speaker Programs Samples thru Personal & Non-Personal Promotion NSM Account Management Consumer Campaign Media Event First TV Ad 15 |
|
|
A-101 45% Topical Solution Candidate For Common Warts |
|
|
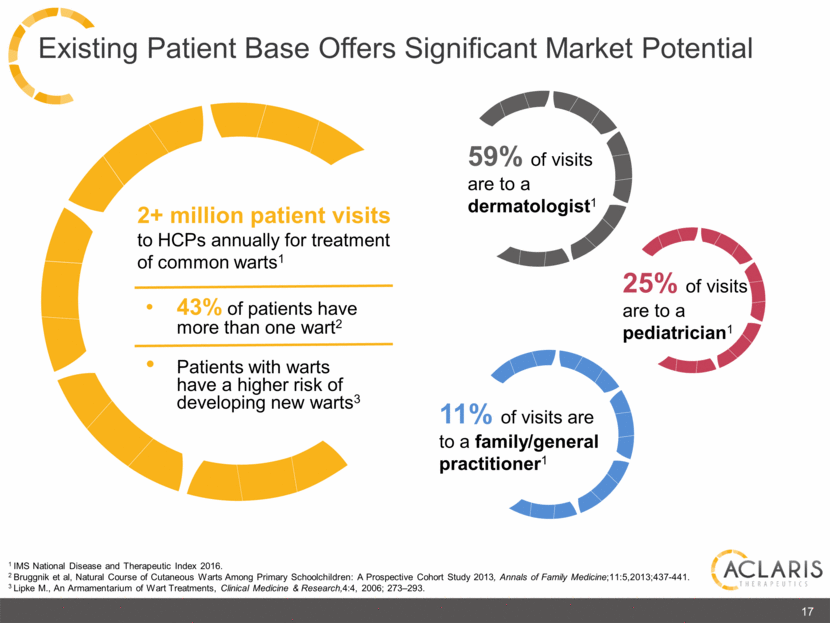
1 IMS National Disease and Therapeutic Index 2016. 2 Bruggnik et al, Natural Course of Cutaneous Warts Among Primary Schoolchildren: A Prospective Cohort Study 2013, Annals of Family Medicine;11:5,2013;437-441. 3 Lipke M., An Armamentarium of Wart Treatments, Clinical Medicine & Research,4:4, 2006; 273–293. 43% of patients have more than one wart2 Patients with warts have a higher risk of developing new warts3 2+ million patient visits to HCPs annually for treatment of common warts1 59% of visits are to a dermatologist1 25% of visits are to a pediatrician1 11% of visits are to a family/general practitioner1 17 Existing Patient Base Offers Significant Market Potential |
|
|
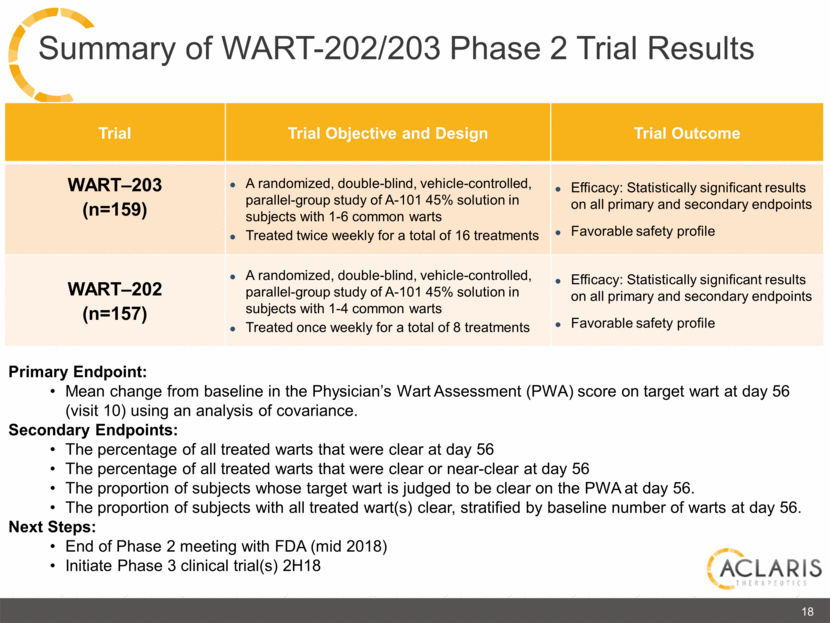
Primary Endpoint: Mean change from baseline in the Physician’s Wart Assessment (PWA) score on target wart at day 56 (visit 10) using an analysis of covariance. Secondary Endpoints: The percentage of all treated warts that were clear at day 56 The percentage of all treated warts that were clear or near-clear at day 56 The proportion of subjects whose target wart is judged to be clear on the PWA at day 56. The proportion of subjects with all treated wart(s) clear, stratified by baseline number of warts at day 56. Next Steps: End of Phase 2 meeting with FDA (mid 2018) Initiate Phase 3 clinical trial(s) 2H18 18 Summary of WART-202/203 Phase 2 Trial Results Trial Trial Objective and Design Trial Outcome WART–203 (n=159) A randomized, double-blind, vehicle-controlled, parallel-group study of A-101 45% solution in subjects with 1-6 common warts Treated twice weekly for a total of 16 treatments Efficacy: Statistically significant results on all primary and secondary endpoints Favorable safety profile WART–202 (n=157) A randomized, double-blind, vehicle-controlled, parallel-group study of A-101 45% solution in subjects with 1-4 common warts Treated once weekly for a total of 8 treatments Efficacy: Statistically significant results on all primary and secondary endpoints Favorable safety profile |
|
|
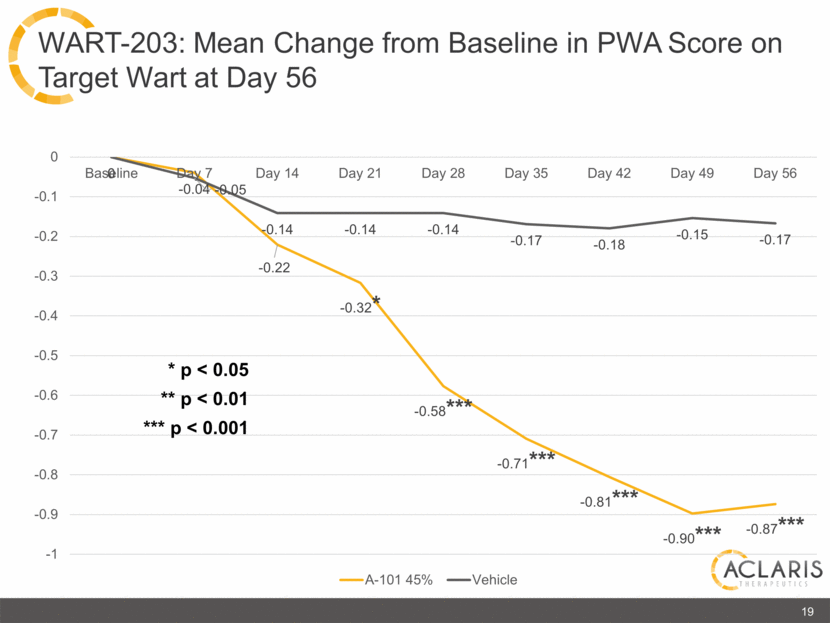
19 WART-203: Mean Change from Baseline in PWA Score on Target Wart at Day 56 * p < 0.05 ** p < 0.01 *** p < 0.001 0 -0.1 -0.2 -0.3 -0.4 -0.5 -0.6 -0.7 -0.8 -0.9 -1 Baseline Day 7 Day 14 Day 21 Day 28 Day 35 Day 42 Day 49 Day 56 0 -0.04-0.05 -0.14 -0.14 -0.17 -0.18 -0.15 -0.17 -0.22 -0.32* -0.58*** -0.71*** -0.81*** -0.90*** -0.87*** A-101 45% Vehicle |
|
|
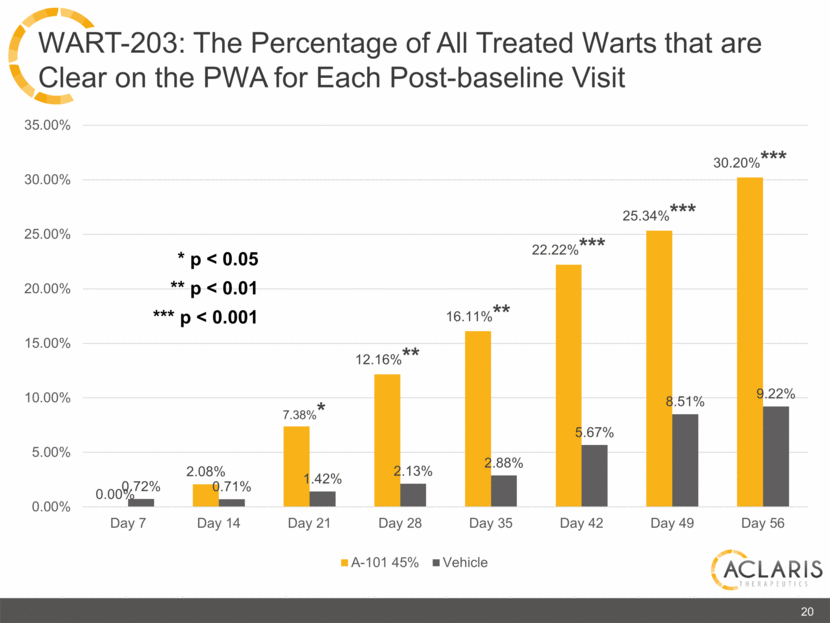
20 WART-203: The Percentage of All Treated Warts that are Clear on the PWA for Each Post-baseline Visit * p < 0.05 ** p < 0.01 *** p < 0.001 35.00% 30.00% 25.00% 20.00% 15.00% 10.00% 5.00% 0.00% Day7 Day 14 Day 21 Day 28 Day 25 Day 42 Day 49 Day 56 0.00% 0.71% 1.42% 2.13% 2.88% 5.67% 8.51% 9.22% 0.72% 2.08% 7.38%* 12.16%** 16.11* 22.22%*** 25.34%*** 30.20%*** |
|
|
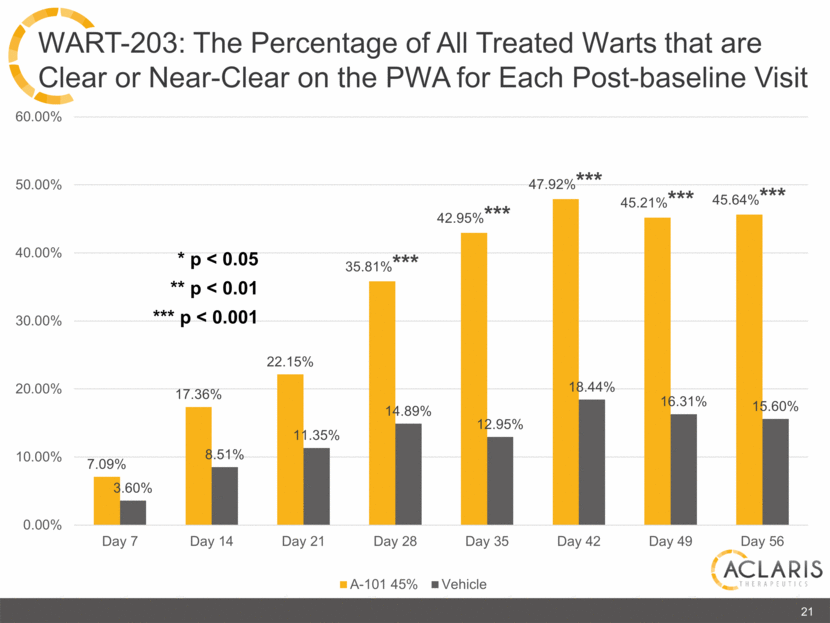
7.09% 17.36% 22.15% 35.81%*** 42.95%*** 47.92%*** 45.21%*** 45.64%*** 3.60% 8.51% 11.35% 14.89% 12.95% 18.44% 16.31% 15.60% 0.00% 10.00% 20.00% 30.00% 40.00% 50.00% 60.00% Day 7 Day 14 Day 21 Day 28 Day 35 Day 42 Day 49 Day 56 A-101 45% Vehicle 21 WART-203: The Percentage of All Treated Warts that are Clear or Near-Clear on the PWA for Each Post-baseline Visit * p < 0.05 ** p < 0.01 *** p < 0.001
|
|
|
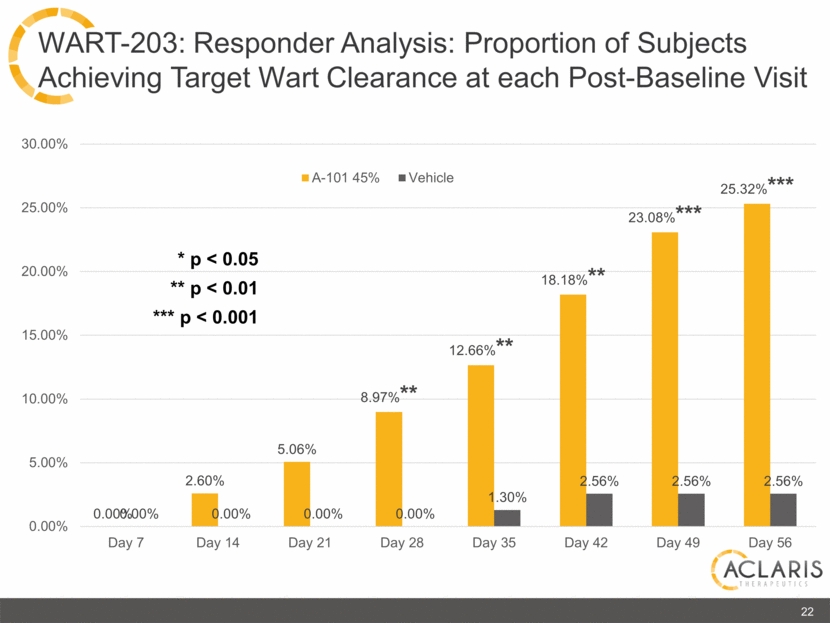
0.00% 2.60% 5.06% 8.97%** 12.66%** 18.18%** 23.08%*** 25.32%*** 0.00% 0.00% 0.00% 0.00% 1.30% 2.56% 2.56% 2.56% 0.00% 5.00% 10.00% 15.00% 20.00% 25.00% 30.00% Day 7 Day 14 Day 21 Day 28 Day 35 Day 42 Day 49 Day 56 A-101 45% Vehicle 22 WART-203: Responder Analysis: Proportion of Subjects Achieving Target Wart Clearance at each Post-Baseline Visit * p < 0.05 ** p < 0.01 *** p < 0.001
|
|
|
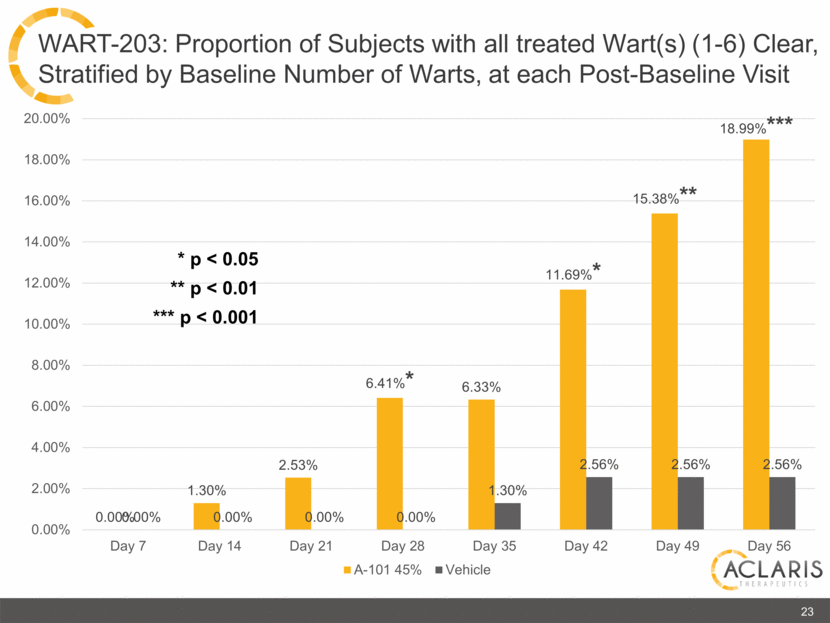
0.00% 1.30% 2.53% 6.41%* 6.33% 11.69%* 15.38%** 18.99%*** 0.00% 0.00% 0.00% 0.00% 1.30% 2.56% 2.56% 2.56% 0.00% 2.00% 4.00% 6.00% 8.00% 10.00% 12.00% 14.00% 16.00% 18.00% 20.00% Day 7 Day 14 Day 21 Day 28 Day 35 Day 42 Day 49 Day 56 A-101 45% Vehicle 23 WART-203: Proportion of Subjects with all treated Wart(s) (1-6) Clear, Stratified by Baseline Number of Warts, at each Post-Baseline Visit * p < 0.05 ** p < 0.01 *** p < 0.001 |
|
|
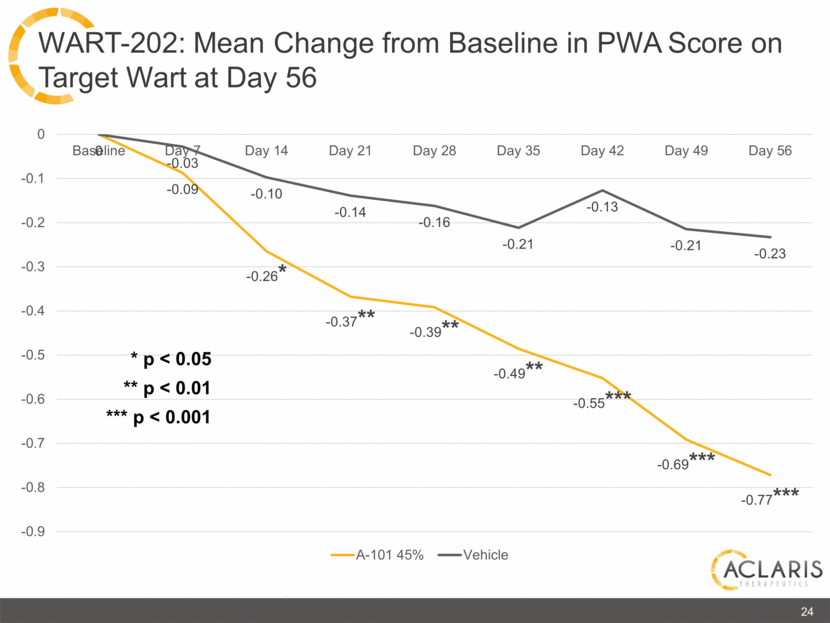
0 -0.09 -0.26* -0.37** -0.39** -0.49** -0.55*** -0.69*** -0.77*** -0.03 -0.10 -0.14 -0.16 -0.21 -0.13 -0.21 -0.23 -0.9 -0.8 -0.7 -0.6 -0.5 -0.4 -0.3 -0.2 -0.1 0 Baseline Day 7 Day 14 Day 21 Day 28 Day 35 Day 42 Day 49 Day 56 A-101 45% Vehicle 24 WART-202: Mean Change from Baseline in PWA Score on Target Wart at Day 56 * p < 0.05 ** p < 0.01 *** p < 0.001 |
|
|
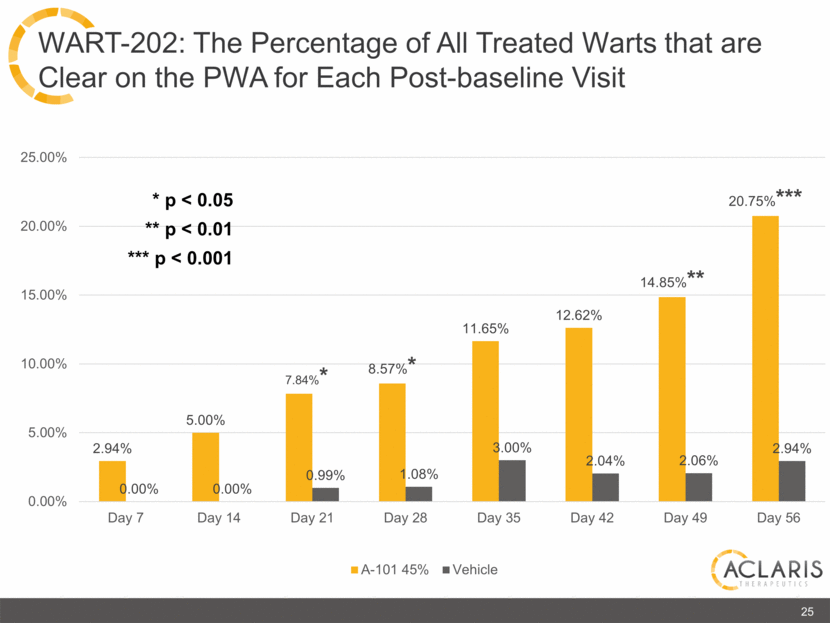
2.94% 5.00% 7.84%* 8.57%* 11.65% 12.62% 14.85%** 20.75%*** 0.00% 0.00% 0.99% 1.08% 3.00% 2.04% 2.06% 2.94% 0.00% 5.00% 10.00% 15.00% 20.00% 25.00% Day 7 Day 14 Day 21 Day 28 Day 35 Day 42 Day 49 Day 56 A-101 45% Vehicle 25 WART-202: The Percentage of All Treated Warts that are Clear on the PWA for Each Post-baseline Visit * p < 0.05 ** p < 0.01 *** p < 0.001 |
|
|
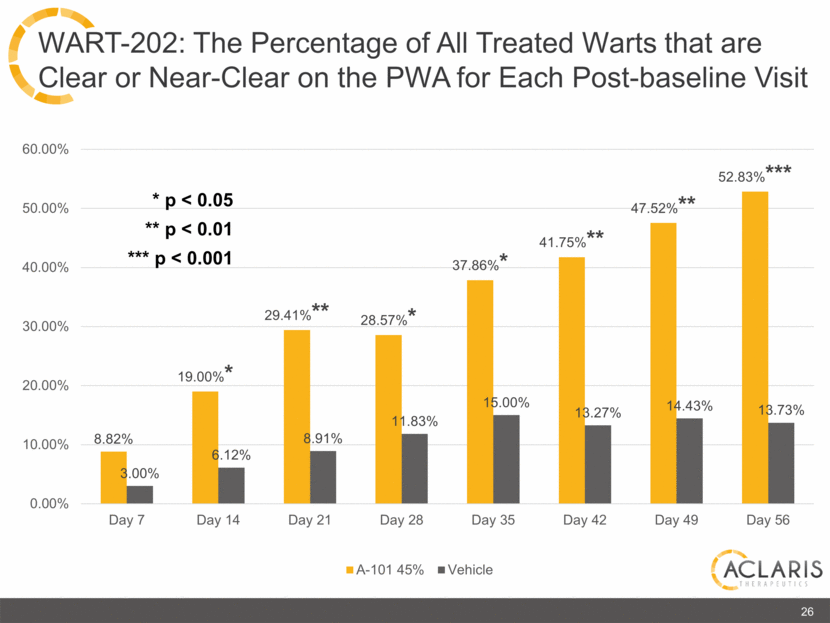
8.82% 19.00%* 29.41%** 28.57%* 37.86%* 41.75%** 47.52%** 52.83%*** 3.00% 6.12% 8.91% 11.83% 15.00% 13.27% 14.43% 13.73% 0.00% 10.00% 20.00% 30.00% 40.00% 50.00% 60.00% Day 7 Day 14 Day 21 Day 28 Day 35 Day 42 Day 49 Day 56 A-101 45% Vehicle 26 WART-202: The Percentage of All Treated Warts that are Clear or Near-Clear on the PWA for Each Post-baseline Visit * p < 0.05 ** p < 0.01 *** p < 0.001 |
|
|
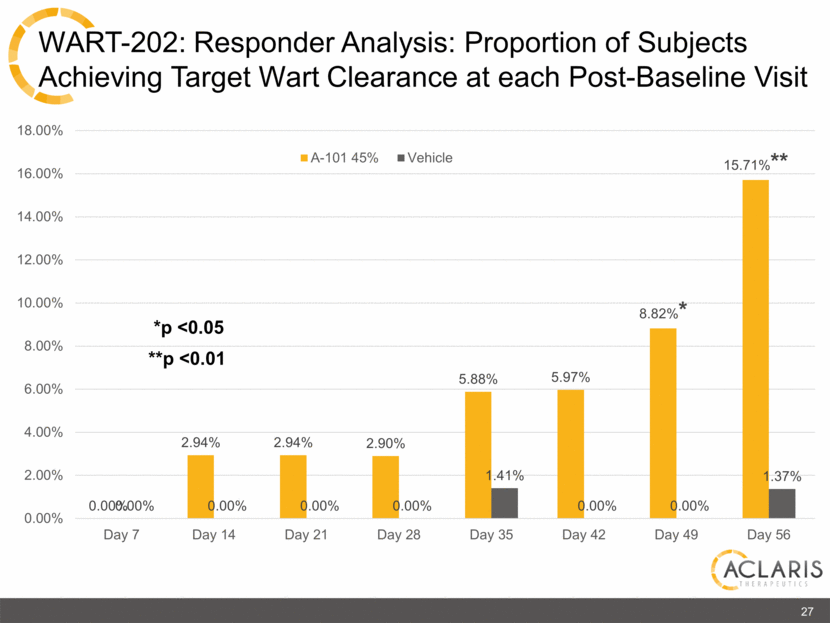
0.00% 2.94% 2.94% 2.90% 5.88% 5.97% 8.82%* 15.71%** 0.00% 0.00% 0.00% 0.00% 1.41% 0.00% 0.00% 1.37% 0.00% 2.00% 4.00% 6.00% 8.00% 10.00% 12.00% 14.00% 16.00% 18.00% Day 7 Day 14 Day 21 Day 28 Day 35 Day 42 Day 49 Day 56 A-101 45% Vehicle *p <0.05 27 **p <0.01 WART-202: Responder Analysis: Proportion of Subjects Achieving Target Wart Clearance at each Post-Baseline Visit
|
|
|
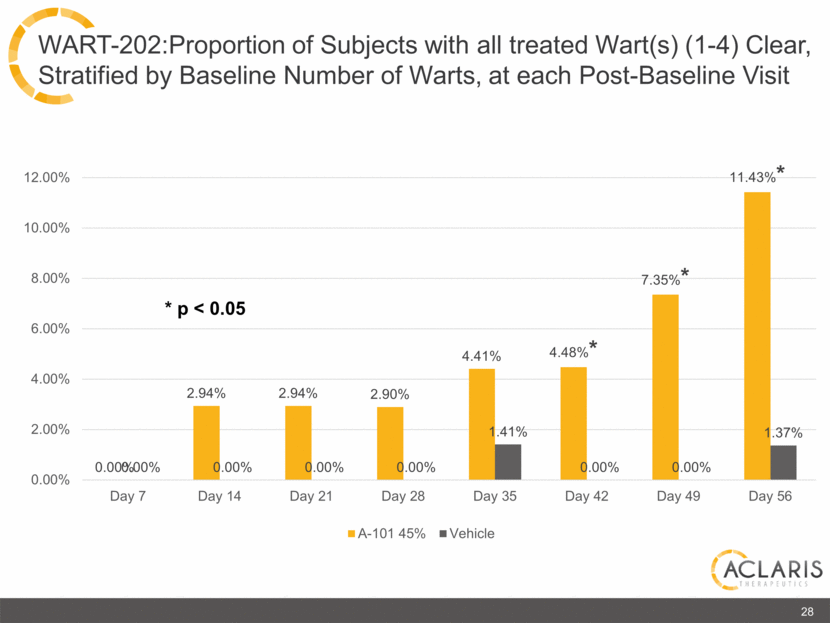
0.00% 2.94% 2.94% 2.90% 4.41% 4.48%* 7.35%* 11.43%* 0.00% 0.00% 0.00% 0.00% 1.41% 0.00% 0.00% 1.37% 0.00% 2.00% 4.00% 6.00% 8.00% 10.00% 12.00% Day 7 Day 14 Day 21 Day 28 Day 35 Day 42 Day 49 Day 56 A-101 45% Vehicle * p < 0.05 28 WART-202:Proportion of Subjects with all treated Wart(s) (1-4) Clear, Stratified by Baseline Number of Warts, at each Post-Baseline Visit
|
|
|
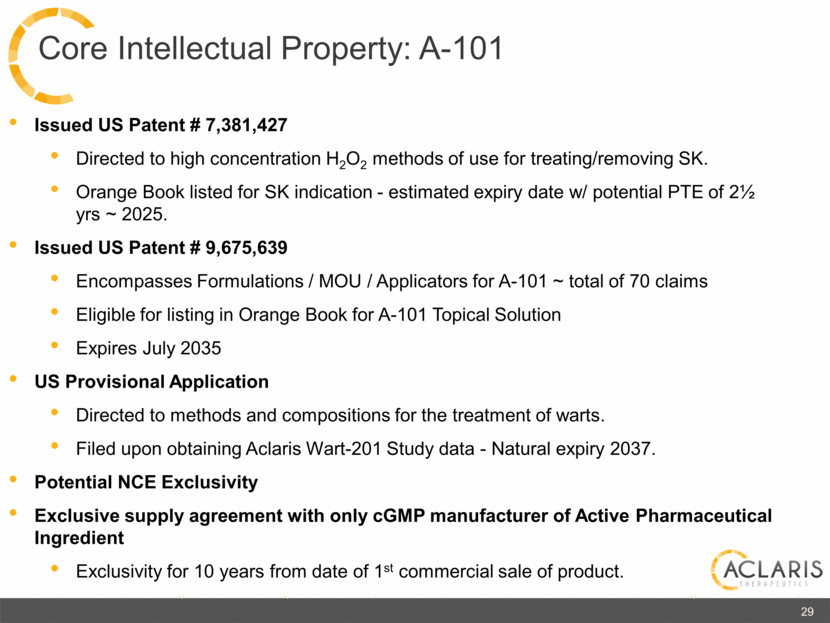
Issued US Patent # 7,381,427 Directed to high concentration H2O2 methods of use for treating/removing SK. Orange Book listed for SK indication - estimated expiry date w/ potential PTE of 2½ yrs ~ 2025. Issued US Patent # 9,675,639 Encompasses Formulations / MOU / Applicators for A-101 ~ total of 70 claims Eligible for listing in Orange Book for A-101 Topical Solution Expires July 2035 US Provisional Application Directed to methods and compositions for the treatment of warts. Filed upon obtaining Aclaris Wart-201 Study data - Natural expiry 2037. Potential NCE Exclusivity Exclusive supply agreement with only cGMP manufacturer of Active Pharmaceutical Ingredient Exclusivity for 10 years from date of 1st commercial sale of product. 29 Core Intellectual Property: A-101 |
|
|
JAK Inhibitor Candidates |
|
|
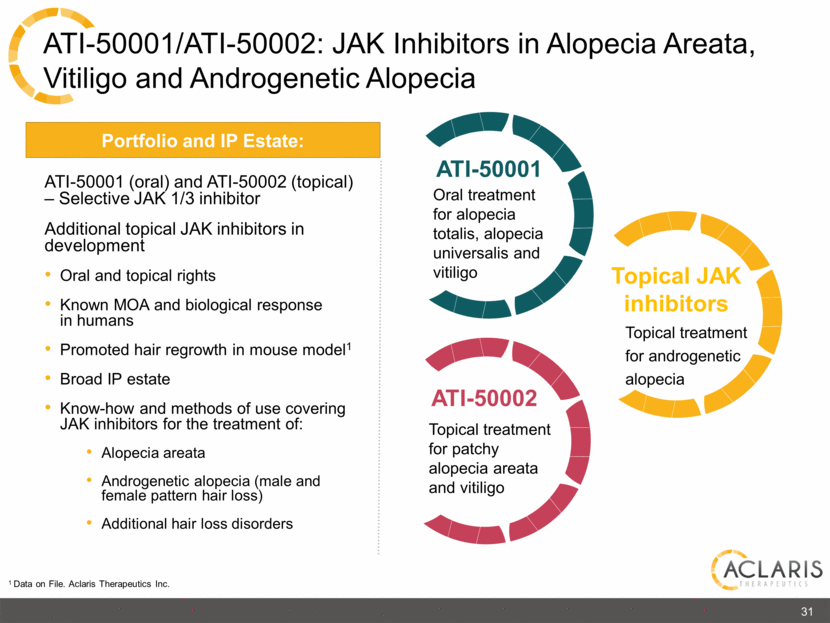
ATI-50001/ATI-50002: JAK Inhibitors in Alopecia Areata, Vitiligo and Androgenetic Alopecia ATI-50001 (oral) and ATI-50002 (topical) – Selective JAK 1/3 inhibitor Additional topical JAK inhibitors in development Oral and topical rights Known MOA and biological response in humans Promoted hair regrowth in mouse model1 Broad IP estate Know-how and methods of use covering JAK inhibitors for the treatment of: Alopecia areata Androgenetic alopecia (male and female pattern hair loss) Additional hair loss disorders Portfolio and IP Estate: ATI-50001 ATI-50002 Topical treatment for patchy alopecia areata and vitiligo Oral treatment for alopecia totalis, alopecia universalis and vitiligo Topical JAK inhibitors Topical treatment for androgenetic alopecia 1 Data on File. Aclaris Therapeutics Inc. 31 |
|
|
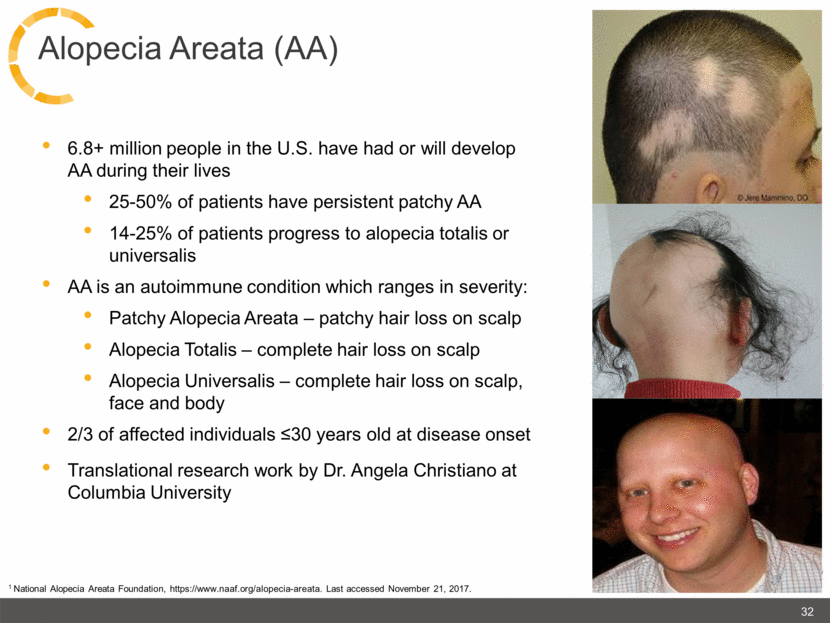
6.8+ million people in the U.S. have had or will develop AA during their lives 25-50% of patients have persistent patchy AA 14-25% of patients progress to alopecia totalis or universalis AA is an autoimmune condition which ranges in severity: Patchy Alopecia Areata – patchy hair loss on scalp Alopecia Totalis – complete hair loss on scalp Alopecia Universalis – complete hair loss on scalp, face and body 2/3 of affected individuals ≤30 years old at disease onset Translational research work by Dr. Angela Christiano at Columbia University 32 Alopecia Areata (AA) 1 National Alopecia Areata Foundation, https://www.naaf.org/alopecia-areata. Last accessed November 21, 2017. |
|
|
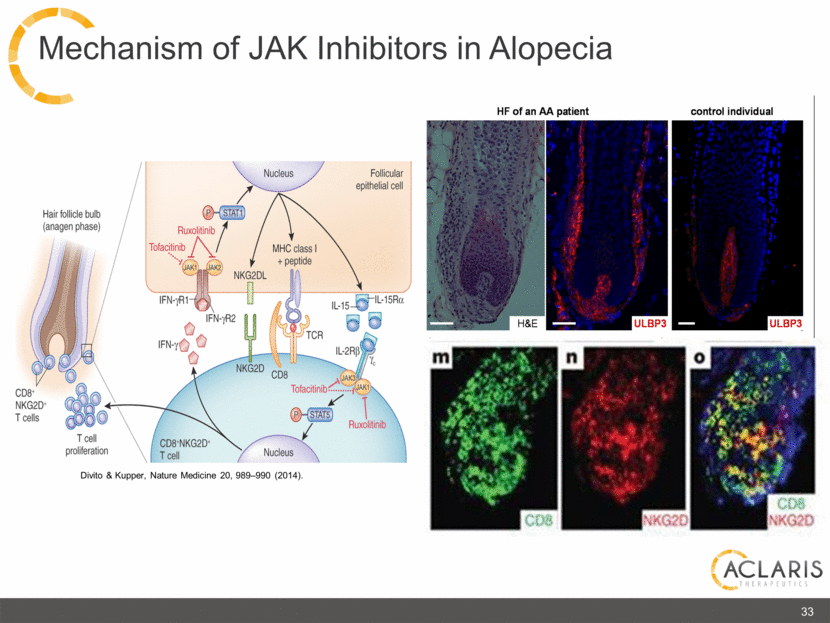
Divito & Kupper, Nature Medicine 20, 989–990 (2014). 33 Mechanism of JAK Inhibitors in Alopecia HF of an AA patient control individual Hair follicle bulb (anagen phase) CD8+ NKG2D+ T cells T cell Proliferation Nucleus Follicular epithelial cell P STAT1 MHC class I +peptide Ruxolitinib Tofacitinib JAK1 JAK2 NKG2DL IFN-γR1 IFN-γR2 IFN-γ NKG2D CD8 TCR IL-15 IL-15Rα IL-2Rβ JAK3 JAK1 P STAT5 Tofacitinib Ruxolitinib Nucleus CD8+NKG2D+ T cell H&E ULBP3 ULBP3 CD8 NKG2D CD8 NKG2D HF of an AA patient control individual ACLARIS THERAPEUTICS |
|
|
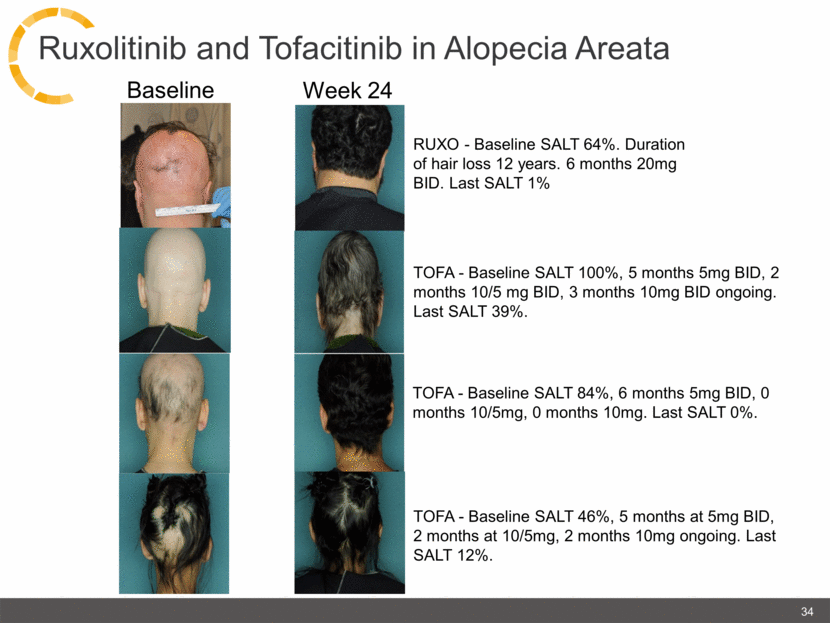
Baseline Week 24 RUXO - Baseline SALT 64%. Duration of hair loss 12 years. 6 months 20mg BID. Last SALT 1% 34 TOFA - Baseline SALT 100%, 5 months 5mg BID, 2 months 10/5 mg BID, 3 months 10mg BID ongoing. Last SALT 39%. TOFA - Baseline SALT 46%, 5 months at 5mg BID, 2 months at 10/5mg, 2 months 10mg ongoing. Last SALT 12%. TOFA - Baseline SALT 84%, 6 months 5mg BID, 0 months 10/5mg, 0 months 10mg. Last SALT 0%. Ruxolitinib and Tofacitinib in Alopecia Areata |
|
|
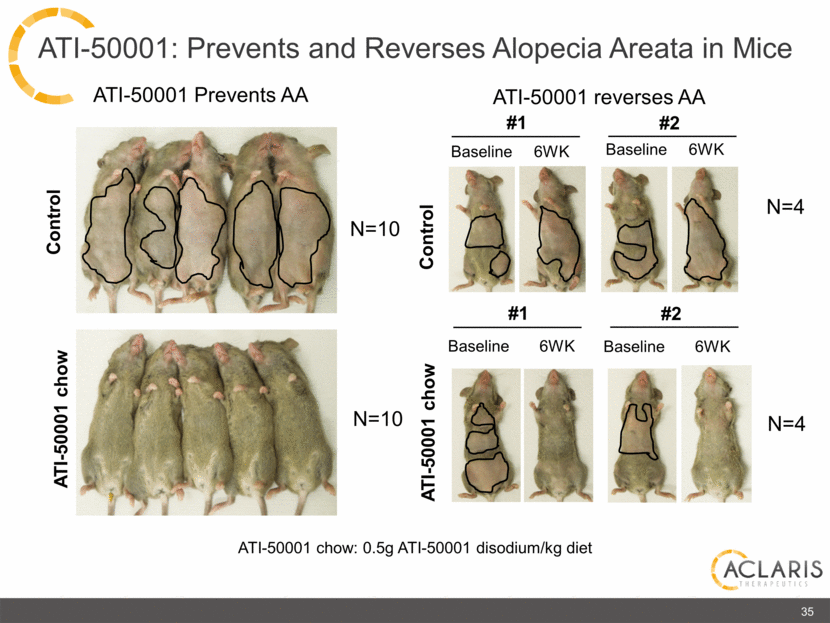
Control ATI-50001 chow ATI-50001 chow: 0.5g ATI-50001 disodium/kg diet N=10 N=10 ATI-50001 reverses AA ATI-50001 Prevents AA #1 Baseline 6WK #2 Baseline 6WK Baseline 6WK ATI-50001 chow Control N=4 N=4 35 Baseline 6WK #1 #2 ATI-50001: Prevents and Reverses Alopecia Areata in Mice |
|
|
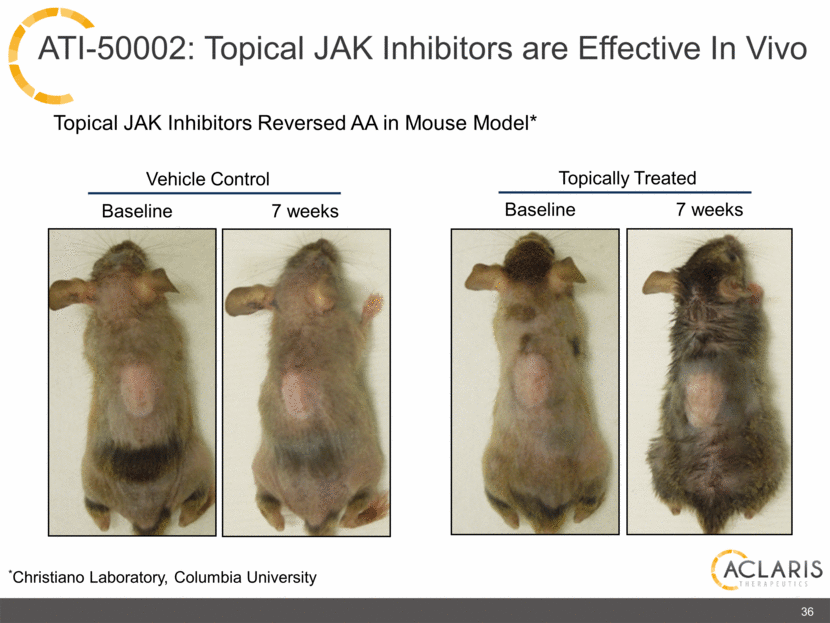
Topical JAK Inhibitors Reversed AA in Mouse Model* Baseline Vehicle Control 7 weeks Topically Treated Baseline 7 weeks *Christiano Laboratory, Columbia University 36 ATI-50002: Topical JAK Inhibitors are Effective In Vivo |
|
|
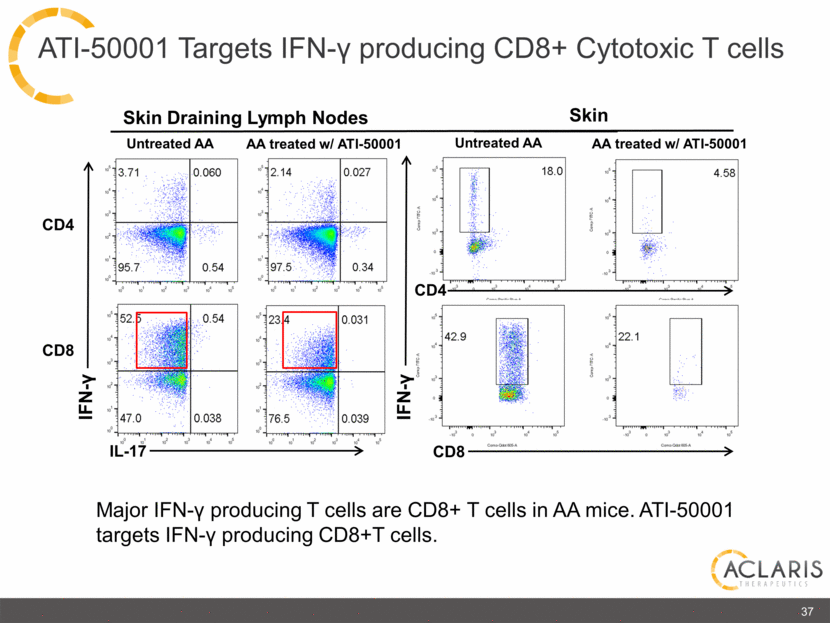
IFN-γ IL-17 IFN-γ CD4 CD4 CD8 Untreated AA AA treated w/ ATI-50001 Skin Draining Lymph Nodes Skin Major IFN-γ producing T cells are CD8+ T cells in AA mice. ATI-50001 targets IFN-γ producing CD8+T cells. 37 CD8 Untreated AA AA treated w/ ATI-50001 ATI-50001 Targets IFN-γ producing CD8+ Cytotoxic T cells 3.71 0.060 95.7 0.54 2.14 0.027 97.5 0.34 18.0 4.58 52.5 0.54 47.0 0.038 23.4 0.031 76.5 0.039 42.9 22.1 |
|
|
Vitiligo is a common autoimmune disease where melanin (pigment) is absent, causing lighter patches of skin to appear on various parts of the body1,2 Vitiligo impacts 1-2% of the global population irrespective of sex, race or age3 Disease onset occurs in about one-half of sufferers between the ages of 10 and 303 Drug candidates: ATI-50001 (oral) and ATI-50002 (topical) JAK inhibitors 38 1 Roddick, J. Autoimmune Diseases. Healthline. 07.22.2015. 2 Oakley, A. Vitiligo. DermnetNZ. 08.2015. 3 Fitzpatrick T., et al. Vitiligo Facts. American Vitiligo Research Foundation Inc. Additional Potential Indications - Vitiligo |
|
|
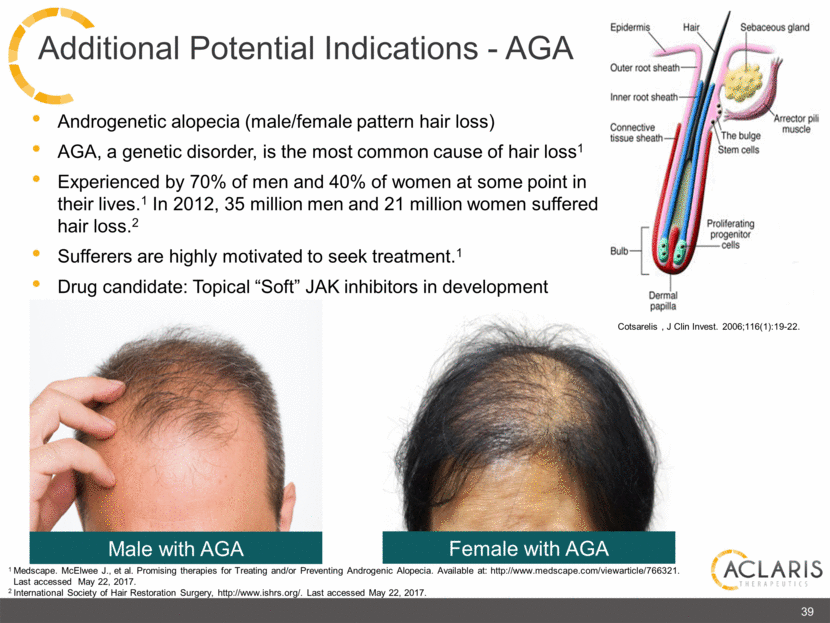
Androgenetic alopecia (male/female pattern hair loss) AGA, a genetic disorder, is the most common cause of hair loss1 Experienced by 70% of men and 40% of women at some point in their lives.1 In 2012, 35 million men and 21 million women suffered hair loss.2 Sufferers are highly motivated to seek treatment.1 Drug candidate: Topical “Soft” JAK inhibitors in development 39 Male with AGA Female with AGA Cotsarelis , J Clin Invest. 2006;116(1):19-22. 1 Medscape. McElwee J., et al. Promising therapies for Treating and/or Preventing Androgenic Alopecia. Available at: http://www.medscape.com/viewarticle/766321. Last accessed May 22, 2017. 2 International Society of Hair Restoration Surgery, http://www.ishrs.org/. Last accessed May 22, 2017. Additional Potential Indications – AGA Epidermis Hair Sebaceous gland Outer root sheath Inner root sheath Connective tissue sheath Bulb Dermal papilla Arrector pili muscle The bulge Stem cells Proliferating progenitor cells |
|
|
40 US & Global JAK IP estate consisting of >150 patents/applications (issued and/or pending) Exclusive license with Rigel Pharmaceuticals for ATI-50001 & ATI-50002 (COM) in dermatology US Natural expiry dates 2030-2034 + potential applicable PTE Corresponding patents & applications in 18 additional jurisdictions (EU, AU, CA, IN, JP, others) - Natural expiry dates 2030 + potential applicable PTE Exclusive license under Columbia University Covers the use of certain JAK inhibitors for the treatment of AA, AGA, and other hair loss disorders and biomarkers to identify potential responders This portfolio includes a recently issued U.S. patent and recently allowed U.S. applications directed to methods of treating AA, AGA and other hair loss disorders by administering ruxolitinib, baricitinib, decernotinib, or tofacitinib, and a recently issued patent in Japan directed to pharmaceutical compositions comprising ruxolitinib, baricitinib, or tofacitinib for use in treating AA, AGA and other hair loss disorders. Natural expiry date 2031 Pending applications in Europe, Japan and Korea Core Intellectual Property: JAK inhibitor |
|
|
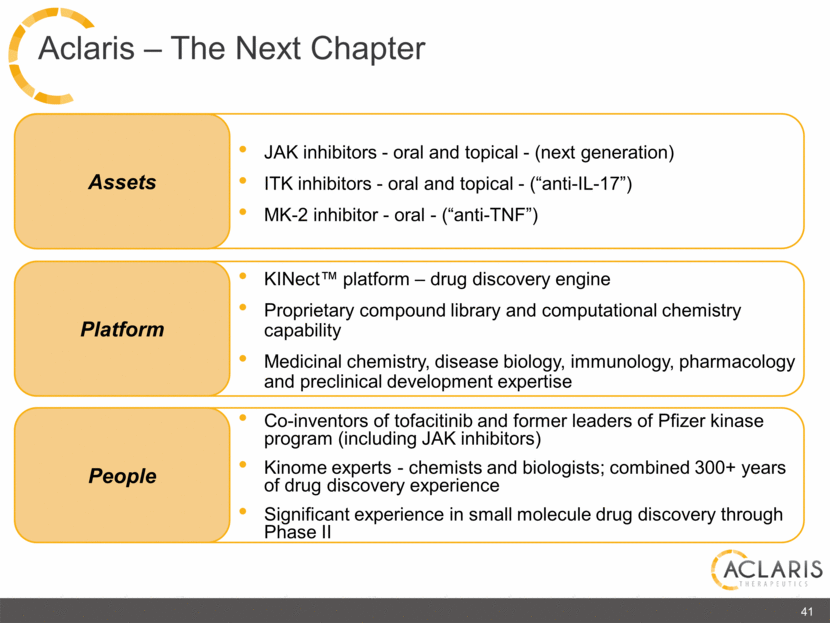
Co-inventors of tofacitinib and former leaders of Pfizer kinase program (including JAK inhibitors) Kinome experts - chemists and biologists; combined 300+ years of drug discovery experience Significant experience in small molecule drug discovery through Phase II People Platform Assets JAK inhibitors - oral and topical - (next generation) ITK inhibitors - oral and topical - (“anti-IL-17”) MK-2 inhibitor - oral - (“anti-TNF”) KINect™ platform – drug discovery engine Proprietary compound library and computational chemistry capability Medicinal chemistry, disease biology, immunology, pharmacology and preclinical development expertise 41 Aclaris – The Next Chapter |
|
|
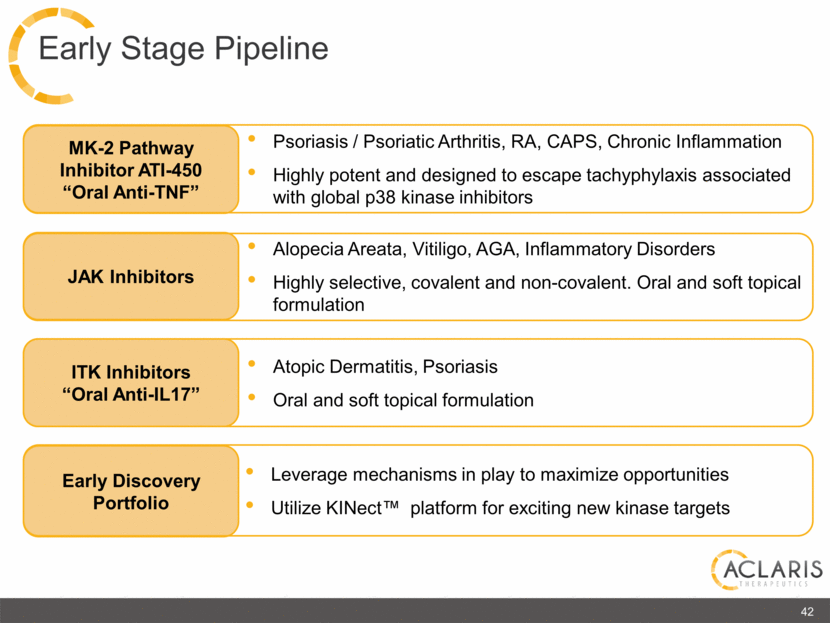
ITK Inhibitors “Oral Anti-IL17” Atopic Dermatitis, Psoriasis Oral and soft topical formulation Alopecia Areata, Vitiligo, AGA, Inflammatory Disorders Highly selective, covalent and non-covalent. Oral and soft topical formulation JAK Inhibitors Psoriasis / Psoriatic Arthritis, RA, CAPS, Chronic Inflammation Highly potent and designed to escape tachyphylaxis associated with global p38 kinase inhibitors MK-2 Pathway Inhibitor ATI-450 “Oral Anti-TNF” 42 Early Discovery Portfolio Leverage mechanisms in play to maximize opportunities Utilize KINect™ platform for exciting new kinase targets Early Stage Pipeline |
|
|
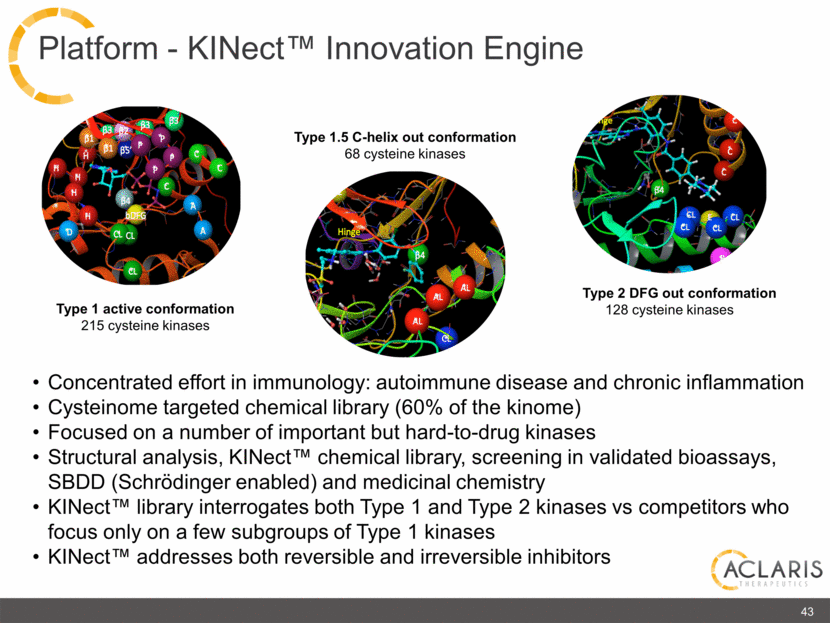
Type 1 active conformation 215 cysteine kinases Type 2 DFG out conformation 128 cysteine kinases Type 1.5 C-helix out conformation 68 cysteine kinases Concentrated effort in immunology: autoimmune disease and chronic inflammation Cysteinome targeted chemical library (60% of the kinome) Focused on a number of important but hard-to-drug kinases Structural analysis, KINect™ chemical library, screening in validated bioassays, SBDD (Schrödinger enabled) and medicinal chemistry KINect™ library interrogates both Type 1 and Type 2 kinases vs competitors who focus only on a few subgroups of Type 1 kinases KINect™ addresses both reversible and irreversible inhibitors 43 Platform - KINect™ Innovation Engine |
|
|
Biomarker Assay Development Clinical Biomarker Assessment In vivo Efficacy and PK Studies PK/PD Relationship Release Assay Validation TRANSLATIONAL RESEARCH COMPUTATIONAL & MEDICINAL CHEMISTRY Leaders in Mechanistic Enzymology Custom Assay Development Compound: Target Interaction Enzyme Inhibitor Mechanisms Direct Binding Kinetics High Throughput Screening CELL & MOLECULAR BIOLOGY BIOCHEMISTRY & ENZYMOLOGY BIOANALYTICAL CHEMISTRY IMMUNOLOGY & IMMUNO-ONCOLOGY Cytokine Expression Th Cell Differentiation/Activation CTL Differentiation and Function B Cell and NK cell Function Ag Specific Cell and In Vivo Models HWB/PBMC/Monocyte Assays Non-GLP Analytical Bioanalytical Method Development Bioanalytical Method Validation Pharmacokinetic/Toxicokinetic Analysis Ab Solubility and Aggregation Schrödinger ™ Enabled Structure Based Drug Design Computational Chemistry Library Design Compound Synthesis Target Clone/Express/Purification Translatable Cellular Assays Target Modulation/Disease Assays Cell Pathway Interrogation Custom Assay Development Multiple Assay Platforms 44 Platform - Research and Development Capabilities |
|
|
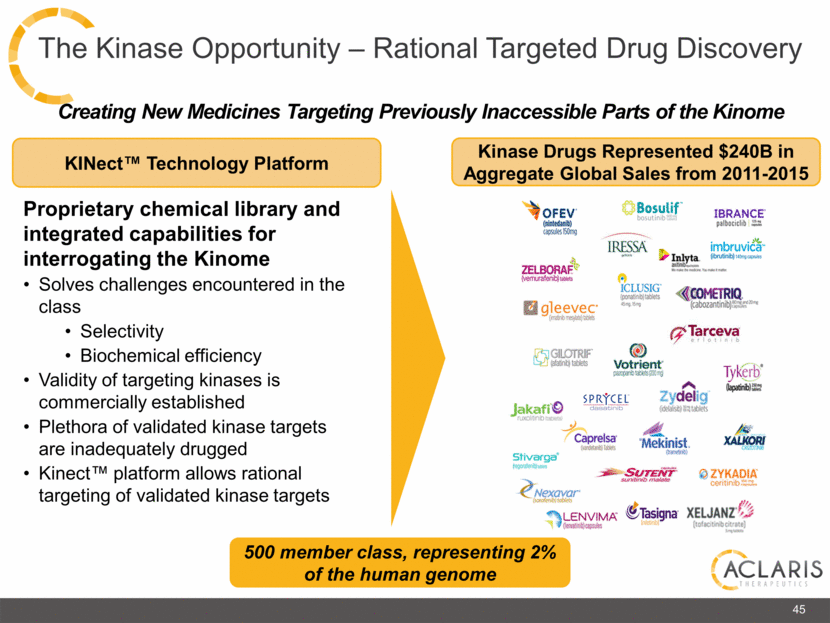
Creating New Medicines Targeting Previously Inaccessible Parts of the Kinome Kinase Drugs Represented $240B in Aggregate Global Sales from 2011-2015 Proprietary chemical library and integrated capabilities for interrogating the Kinome Solves challenges encountered in the class Selectivity Biochemical efficiency Validity of targeting kinases is commercially established Plethora of validated kinase targets are inadequately drugged Kinect™ platform allows rational targeting of validated kinase targets KINect™ Technology Platform 500 member class, representing 2% of the human genome 45 The Kinase Opportunity – Rational Targeted Drug Discovery |
|
|
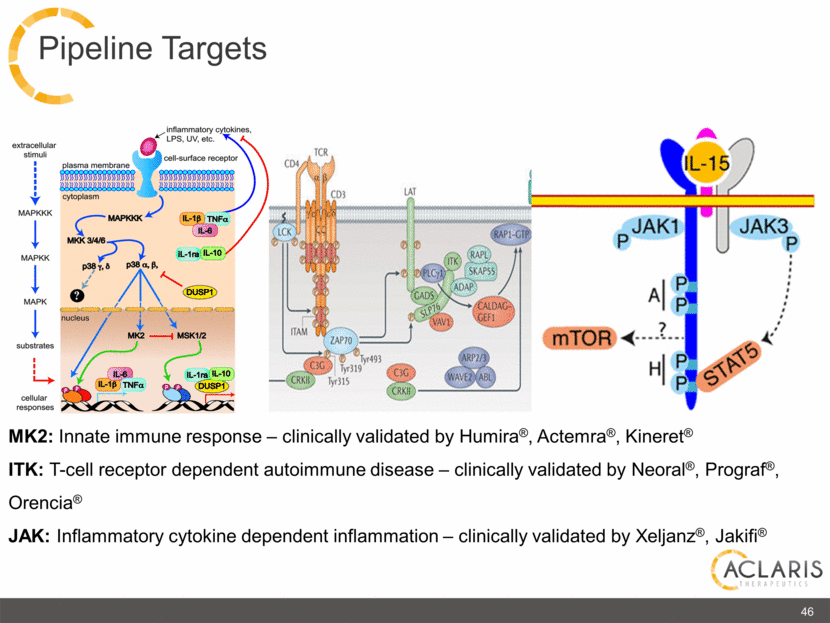
MK2: Innate immune response – clinically validated by Humira®, Actemra®, Kineret® ITK: T-cell receptor dependent autoimmune disease – clinically validated by Neoral®, Prograf®, Orencia® JAK: Inflammatory cytokine dependent inflammation – clinically validated by Xeljanz®, Jakifi® 46 Pipeline Targets extracellular stimuli MAPKKK MAPKK MAPK substrates cellular responses plasma membrane inflammatory cytokines, LPS, UV, etc. cell-surface receptor cytoplasm MAPKKK MKK 3/4/6 p38 nucleus MK2 MSK1/2 IL-6 IL-1ra IL-10 DUSP1 CD4 TCR CD3 LAT LCK ITAM C3G CRKII ZAP70 Tyr315 Tyr319 Tyr493 C3G CRKII WAVW2 AEL ARP2/3 ITK RAPL RAP1-GTP SKAP55 PLCγ1 GADS SLP76 VAV1CALDAG-GEF1 ADAP α β p γ ξ IL-15 JAK1 JAK3 STAT5 mTOR |
|
|
|
|
|
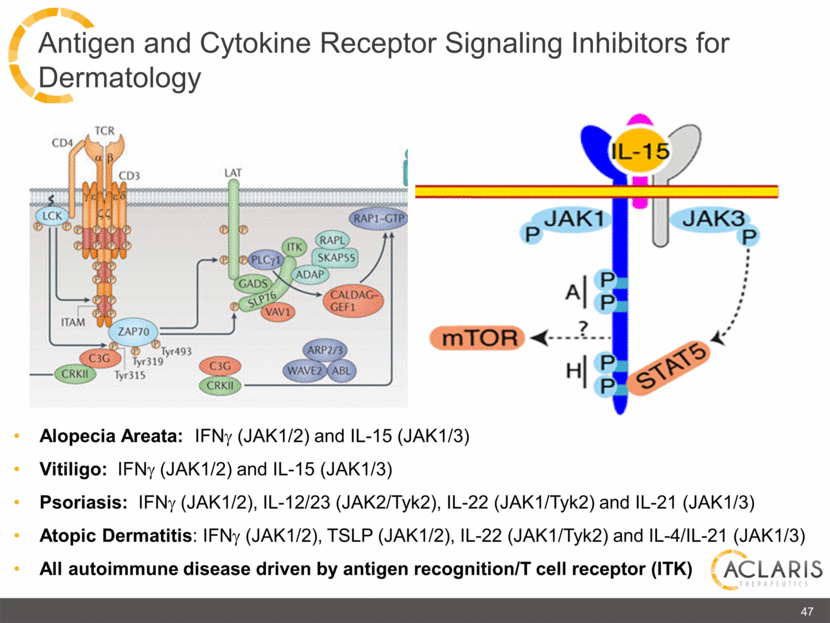
Alopecia Areata: IFNg (JAK1/2) and IL-15 (JAK1/3) Vitiligo: IFNg (JAK1/2) and IL-15 (JAK1/3) Psoriasis: IFNg (JAK1/2), IL-12/23 (JAK2/Tyk2), IL-22 (JAK1/Tyk2) and IL-21 (JAK1/3) Atopic Dermatitis: IFNg (JAK1/2), TSLP (JAK1/2), IL-22 (JAK1/Tyk2) and IL-4/IL-21 (JAK1/3) All autoimmune disease driven by antigen recognition/T cell receptor (ITK) 47 Antigen and Cytokine Receptor Signaling Inhibitors for Dermatology CD4 TCR LCK CD3 ITAM ZAP70 C3G CRKII LAT GADS PLCy1 ITK RAPL SKAP88 ADAP VAV1 SLP76 RAP1-GTP CALDAG-GEF1 ARP2/3 WAVE2 ABL C3G CRKII Tyr493 Tyr319 Tyr315 IL-15 JAK1 P JAK3 P A P P H P P STAT5 mTor |
|
|
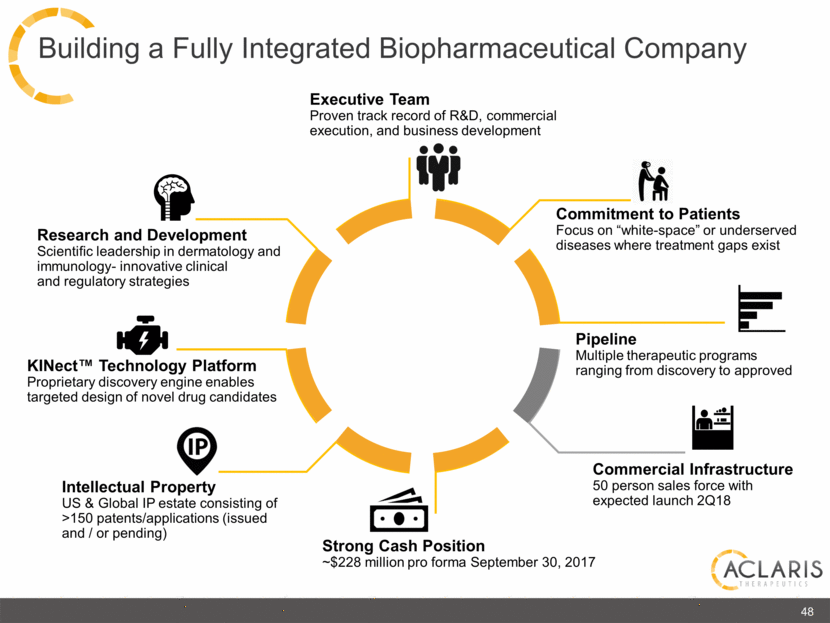
48 Commitment to Patients Focus on “white-space” or underserved diseases where treatment gaps exist Research and Development Scientific leadership in dermatology and immunology- innovative clinical and regulatory strategies Innovative and differentiated clinical and regulatory strategies Executive Team Proven track record of R&D, commercial execution, and business development Pipeline Multiple therapeutic programs ranging from discovery to approved Strong Cash Position ~$228 million pro forma September 30, 2017 Commercial Infrastructure 50 person sales force with expected launch 2Q18 Intellectual Property US & Global IP estate consisting of >150 patents/applications (issued and / or pending) KINect™ Technology Platform Proprietary discovery engine enables targeted design of novel drug candidates Building a Fully Integrated Biopharmaceutical Company |
|
|
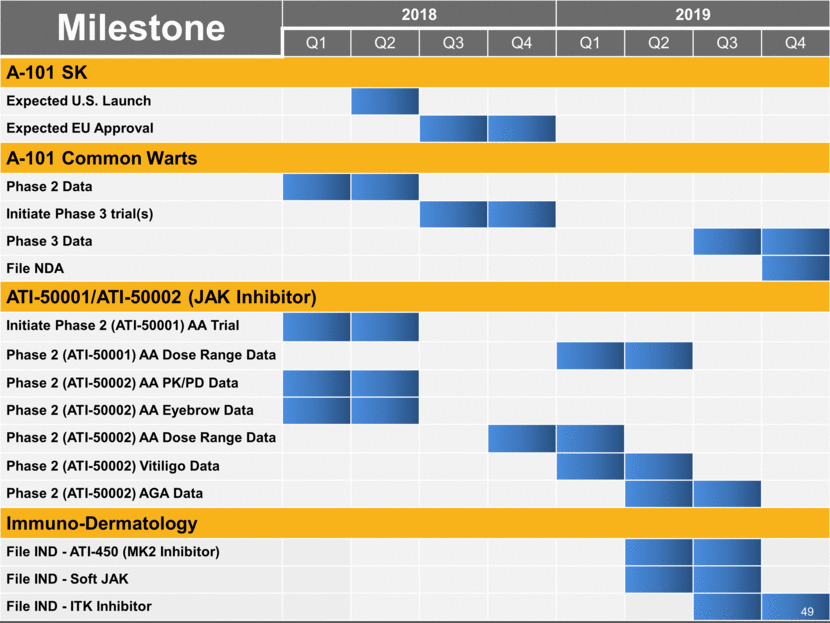
Milestone 2018 2019 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 A-101 SK Expected U.S. Launch Expected EU Approval A-101 Common Warts Phase 2 Data Initiate Phase 3 trial(s) Phase 3 Data File NDA ATI-50001/ATI-50002 (JAK Inhibitor) Initiate Phase 2 (ATI-50001) AA Trial Phase 2 (ATI-50001) AA Dose Range Data Phase 2 (ATI-50002) AA PK/PD Data Phase 2 (ATI-50002) AA Eyebrow Data Phase 2 (ATI-50002) AA Dose Range Data Phase 2 (ATI-50002) Vitiligo Data Phase 2 (ATI-50002) AGA Data Immuno-Dermatology File IND - ATI-450 (MK2 Inhibitor) File IND - Soft JAK File IND - ITK Inhibitor 49 |
|
|
© Copyright 2017 Aclaris Therapeutics. All rights reserved. A-1 THANK YOU www.aclaristx.com |