Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - AMAG PHARMACEUTICALS, INC. | ex991.htm |
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | a2018jpmorgan8-k.htm |

J.P. Morgan
Healthcare Conference
January 2018
© 2018 AMAG Pharmaceuticals, Inc. All rights reserved.
1

Forward-Looking Statements
This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA) and other
federal securities laws. Any statements contained herein which do not describe historical facts, including, among others, expectations regarding
AMAG’s future growth and diversification; expectations regarding Intrarosa’s commercial opportunity and revenue potential, including the number of
women who suffer from dyspareunia in the U.S.; AMAG’s goal to achieve 65% unrestricted commercial coverage by month end; 2018 launch priorities
for Intrarosa, including to broaden its utilization and differentiating it with health care professionals; the expected bremelanotide regulatory timeline
and initiatives in preparation for commercial launch; AMAG’s beliefs regarding the target product profile for bremelanotide, including the presumed
dosing and administration, indication, safety profile and mechanism of action; the breadth of the hypoactive sexual desire disorder (HSDD) market and
bremelanotide’s market potential; beliefs regarding Makena’s position in the market; future revenue drivers for Makena, including securing
reimbursement and distribution for the subcutaneous auto-injector (if FDA approval is received), educating health care professionals on the auto-
injector product, expanding conversion through alternative treatment sites and launching an authorized generic; growth drivers for Cord Blood
Registry (CBR), including plans to grow first time enrollments, optimize first year price, strengthen core audiences, explore new channels and leverage
advancements in stem cell research; growth drivers for Feraheme, including initiating launch activities for the expanded label (if approved) to include
all eligible adult IDA patients, expanding access beyond chronic kidney disease through contracting and relationships in new specialties (if the broader
indication is approved) and continued market share growth; beliefs regarding the size of the current addressable market and expectations that the size
of the addressable market would double if the broader indication is approved; AMAG’s 2018 financial guidance, including forecasted GAAP and non-
GAAP revenue, GAAP operating income, and non-GAAP adjusted EBITDA; plans to issue fourth quarter and full year 2017 financial results in late
February 2018; and AMAG’s 2018 key priorities related to its products and product candidates, portfolio expansion and financial goals are forward-
looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-
looking statements. In addition, AMAG has presented 2018 guidance in this presentation, which guidance is based upon management’s current
assumptions and beliefs about various risks and opportunities, including key milestones whose outcomes may be determined during the first quarter
and first half of 2018. AMAG makes no assurances that such guidance numbers will be indicative of actual results or that any or all of the 2018
guidance assumptions will be realized on the anticipated timelines, or at all, or that the impact on revenues, operating income and adjusted EBITDA
will be as projected, and the company does not undertake any obligation to update such guidance to reflect actual outcomes or revised expectations.
Such risks and uncertainties include, the possibility that the company’s expectations as to key 2018 priorities, goals and expectations will not be
realized, including the results of the pending FDA submissions for the Makena subcutaneous auto-injector and the Feraheme label expansion, as well
as the timing for entrance of generics to the Makena intramuscular formulation, and those other risks identified in AMAG’s Securities and Exchange
Commission (“SEC”) filings, including its Annual Report on Form 10-K for the year ended December 31, 2016 and subsequent filings with the SEC.
AMAG cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. AMAG disclaims
any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on
which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking
statements.
AMAG Pharmaceuticals® and Feraheme® are registered trademarks of AMAG Pharmaceuticals, Inc. MuGard® is a registered trademark of Abeona
Therapeutics, Inc. Makena® is a registered trademark of AMAG Pharma USA, Inc. Cord Blood Registry® and CBR® are registered trademarks of Cbr
Systems, Inc. Intrarosa® is a trademark of Endoceutics, Inc.
2
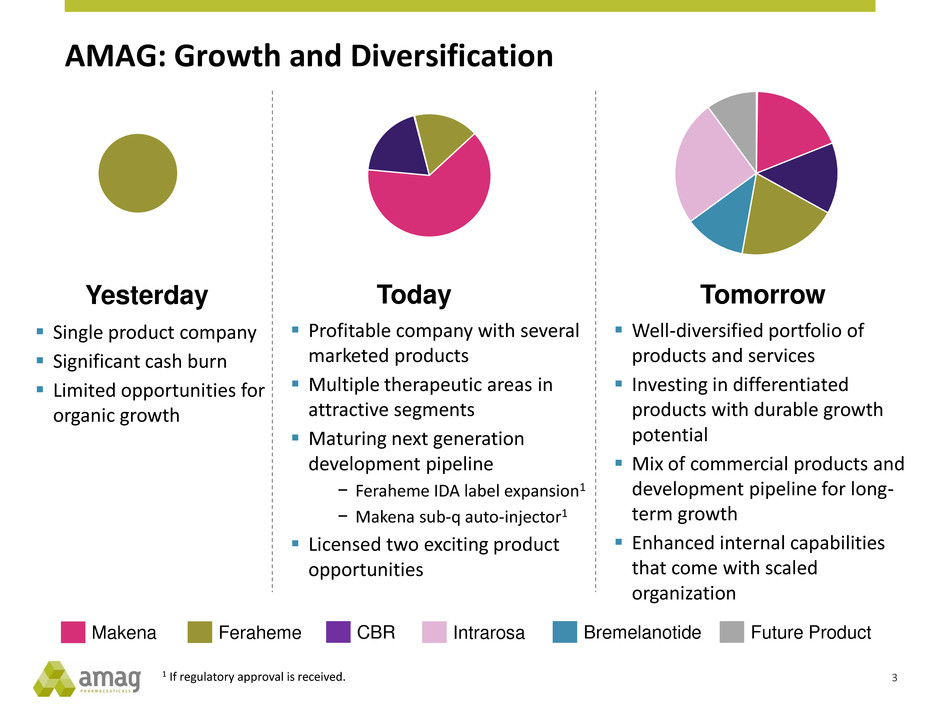
AMAG: Growth and Diversification
Tomorrow
Well-diversified portfolio of
products and services
Investing in differentiated
products with durable growth
potential
Mix of commercial products and
development pipeline for long-
term growth
Enhanced internal capabilities
that come with scaled
organization
Today
Profitable company with several
marketed products
Multiple therapeutic areas in
attractive segments
Maturing next generation
development pipeline
− Feraheme IDA label expansion1
− Makena sub-q auto-injector1
Licensed two exciting product
opportunities
Yesterday
Single product company
Significant cash burn
Limited opportunities for
organic growth
1 If regulatory approval is received.
Makena Feraheme CBR Intrarosa Bremelanotide Future Product
3
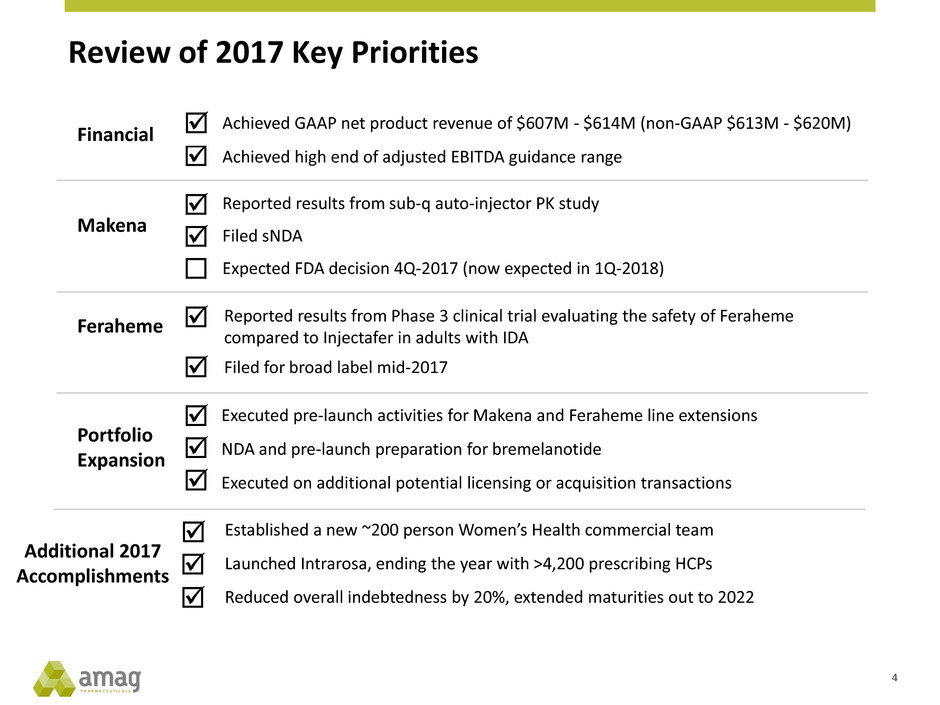
Review of 2017 Key Priorities
Makena
Feraheme
Executed pre-launch activities for Makena and Feraheme line extensions
NDA and pre-launch preparation for bremelanotide
Executed on additional potential licensing or acquisition transactions
Portfolio
Expansion
Financial
Reported results from sub-q auto-injector PK study
Filed sNDA
Expected FDA decision 4Q-2017 (now expected in 1Q-2018)
Reported results from Phase 3 clinical trial evaluating the safety of Feraheme
compared to Injectafer in adults with IDA
Filed for broad label mid-2017
Achieved GAAP net product revenue of $607M - $614M (non-GAAP $613M - $620M)
Achieved high end of adjusted EBITDA guidance range
Established a new ~200 person Women’s Health commercial team
Launched Intrarosa, ending the year with >4,200 prescribing HCPs
Reduced overall indebtedness by 20%, extended maturities out to 2022
Additional 2017
Accomplishments
4

2017 Financial Results
Preliminary and Unaudited
($M)
4Q-2017 Preliminary Results Full Year 2017 Preliminary Results
GAAP Non-GAAP GAAP Non-GAAP
Makena $97 - $102 $97 - $102 $385 - $390 $385 - $390
Feraheme/MuGard $26 - $28 $26 - $28 $106 - $108 $106 - $108
Cord Blood Registry ~$30 ~$311 ~$114 ~$1201
Intrarosa ~$2 ~$2 ~$2 ~$2
Total revenue $155 - $162 $156 - $1631 $607 - $614 $613 - $6201
Operating loss ($16) – ($6) N/A ($302) – ($292) N/A
Adjusted EBITDA2 N/A $58 - $68 N/A $220 - $230
Total GAAP revenue increased 5% and 15% in the fourth
quarter and full year, respectively, over 2016
1 Non-GAAP revenue includes purchase accounting adjustments related to CBR deferred revenue of $1.4M and $5.5M
in the fourth quarter and full year of 2017, respectively.
2 See slide 41 for a reconciliation of GAAP to non-GAAP financials.
5

Treatment of iron
deficiency anemia (IDA)
in adult patients with
chronic kidney disease
(CKD)
The only FDA-approved
therapy to reduce
recurrent preterm birth
in certain at-risk women
World’s largest umbilical
cord stem cell collection
and storage company
Candidate for the
treatment of severe
preeclampsia
An investigational
product for the
treatment of hypoactive
sexual desire disorder
(HSDD) in pre-
menopausal women
Management of oral
mucositis, a common
side effect of radiation or
chemotherapy
Maternal and Women’s HealthHematology /
Oncology Pregnancy
& Birth
Wellness
Post-Menopausal
Health
Velo
Option
Bremelanotide
FDA-approved locally
administered non-
estrogen1 product to
treat moderate to
severe dyspareunia
(pain during sex), a
symptom of VVA, due
to menopause, which
does not carry a boxed
warning in its label
61 Intrarosa is converted by enzymes in the body into androgens and estrogens, though the mechanism of action is not fully established.
AMAG’s Expanding Portfolio of Products

Product Portfolio Overview
•Intrarosa®
• Bremelanotide
• Makena®
• Cord Blood Registry®
• Feraheme®
7
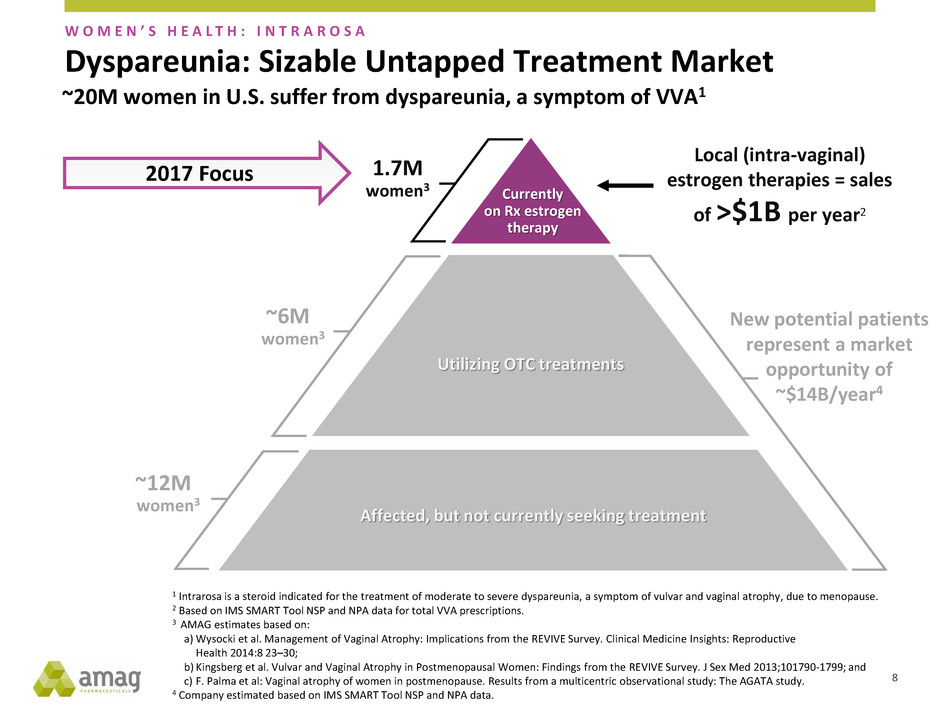
Affected, but not currently seeking treatment
Utilizing OTC treatments
Dyspareunia: Sizable Untapped Treatment Market
8
1 Intrarosa is a steroid indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause.
2 Based on IMS SMART Tool NSP and NPA data for total VVA prescriptions.
3 AMAG estimates based on:
a) Wysocki et al. Management of Vaginal Atrophy: Implications from the REVIVE Survey. Clinical Medicine Insights: Reproductive
Health 2014:8 23–30;
b) Kingsberg et al. Vulvar and Vaginal Atrophy in Postmenopausal Women: Findings from the REVIVE Survey. J Sex Med 2013;101790-1799; and
c) F. Palma et al: Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study.
4 Company estimated based on IMS SMART Tool NSP and NPA data.
Currently
on Rx estrogen
therapy
Local (intra-vaginal)
estrogen therapies = sales
of >$1B per year2
1.7M
women3
~6M
women3
~12M
women3
W O M E N ’ S H E A L T H : I N T R A R O S A
~20M women in U.S. suffer from dyspareunia, a symptom of VVA1
New potential patients
represent a market
opportunity of
~$14B/year4
2017 Focus

9
Intrarosa: Two Key Differentiators
W O M E N ’ S H E A L T H : I N T R A R O S A
Non-Estrogen1 with
No Boxed Warning Differentiated Mechanism of Action
1 Intrarosa is converted by enzymes in the body into androgens and estrogens, though the mechanism of action is not fully established.
®
DHEA levels decrease with age

Launch Priority 3
Increase HCP
prescribing
Launch Priority 2
Increase market
awareness
Intrarosa Launch: Strong Progress in Awareness and Access
10
W O M E N ’ S H E A L T H : I N T R A R O S A
Launch Priority 1
Create affordable
access for all patients
2017: Focused on HCP experience
and patient access to treatment
• Broad use of patient
copay savings card
• ~65% of commercial
lives with unrestricted
formulary access
expected by month end
• 140 sales reps,
>90,0001 calls made
to ~22,0001 HCPs
• 2402 speaker
programs with
>2,5002 HCP
attendees
• NRx market share 2.6%3
• Early adopters with
samples driving higher
NRx share
‒ 9%3 and growing
• >4,2003 HCP prescribers
(>2x since November)
1 SFDC call notes data through 12/22/17.
2 Avant Health Speaker program data.
3 IMS data through w/e 12/22/17.

Performance vs. Previous Osphena® Launch
11
W O M E N ’ S H E A L T H : I N T R A R O S A
Aggregate TRx totals in first 23 weeks of launch – Intrarosa vs. Osphena1
TRx
1 IMS data for the first 23 weeks of each respective launch.
9,002
19,727
-
5,000
10,000
15,000
20,000
25,000
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Week #
Osphena Intrarosa

W O M E N ’ S H E A L T H : I N T R A R O S A
Intrarosa Growth Strategy
12

Affected, but not currently seeking treatment
Utilizing OTC treatments
Dyspareunia: Sizable Untapped Treatment Market
13
1 Intrarosa is a steroid indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause.
2 Based on IMS SMART Tool NSP and NPA data for total VVA prescriptions.
3 AMAG estimates based on:
a) Wysocki et al. Management of Vaginal Atrophy: Implications from the REVIVE Survey. Clinical Medicine Insights: Reproductive
Health 2014:8 23–30;
b) Kingsberg et al. Vulvar and Vaginal Atrophy in Postmenopausal Women: Findings from the REVIVE Survey. J Sex Med 2013;101790-1799; and
c) F. Palma et al: Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study.
4 Company estimated based on IMS SMART Tool NSP and NPA data.
Currently
on Rx estrogen
therapy
Local (intra-vaginal)
estrogen therapies = sales
of >$1B per year2
1.7M
women3
~6M
women3
~12M
women3
W O M E N ’ S H E A L T H : I N T R A R O S A
~20M women in U.S. suffer from dyspareunia, a symptom of VVA1
New potential patients
represent a market
opportunity of
~$14B/year4
2017 Focus
2018
A
d
d
iti
o
n
al
E
ff
ort
s

Develop
condition
awareness
The Digital Patient Journey
W O M E N ’ S H E A L T H : I N T R A R O S A
or
Consult MD
Office visit
or on-line
On-line adherence programs
Seek
treatment
options
14

Product Portfolio Overview
• Intrarosa®
•Bremelanotide
• Makena®
• Cord Blood Registry®
• Feraheme®
15
© 2018 AMAG Pharmaceuticals, Inc. All rights reserved.

Investigational product to treat hypoactive sexual desire disorder (HSDD)
Self-administered auto-injector pen used in anticipation of sexual activity
Novel mechanism of action: melanocortin receptor agonist (MCR4)
Two Phase 3 studies met co-primary, pre-specified endpoints on desire
and distress
Favorable safety profile
On track for NDA submission Q1-2018
Large market opportunity
Significant Opportunity in Area of High Unmet Need
W O M E N ’ S H E A L T H : B R E M E L A N O T I D E
Bremelanotide Overview
16

Treated (Rx)
Presented
to HCP, but
not treated
Not
diagnosed
.5M4
1.6M1,2,4
3.7M
Significant Market Opportunity
~10M pre-menopausal women in U.S. with HSDD1
17
1 Rosen et al, Characteristics of premenopausal and postmenopausal women with acquired, generalized HSDD: the HSDD Registry for women.
2 Patient segmentation market research sponsored by Palatin Technologies, Inc. and conducted by the Burke Institute, September 2016.
3 Survey data from Shifren (2008); 2014 U.S. census data.
4 Dectiva market research report (HSDD registry).
Pre-menopausal
women with HSDD
(primary symptom)
Initial target
patient population
Pre-menopausal
women with HSDD
(not primary symptom)
Post-menopausal
women
with HSDD
W O M E N ’ S H E A L T H : B R E M E L A N O T I D E
4.8M1
4.4M
5.8M2
15M U.S.
Women with HSDD3

Differentiated Target Product Profile
Bremelanotide
Dosing &
Administration
Self-administered subcutaneous auto-injector pen used in anticipation of
sexual activity
Indication The treatment of pre-menopausal women with HSDD
Clinical
Two successful Phase 3 studies completed
Randomized 1,267 pre-menopausal women with primary HSDD
Co-primary efficacy endpoints met
Increase in sexual desire and decrease in distress associated with low
sexual desire
Safety
No known alcohol interaction (alcohol interaction study completed)
Favorable overall safety profile in Phase 3 controlled studies
Most common AEs were nausea, flushing and headache; generally mild-
to-moderate in severity
Only two treatment related SAEs; both occurred in one subject
(nausea/vomiting and headache)
~80% of patients that completed the Phase 3 trials elected to participate
in the open label roll-over safety study
Presumed Mechanism
of Action
Targets pathways (melanocortin receptor agonist) involved in sexual
desire and arousal response
W O M E N ’ S H E A L T H : B R E M E L A N O T I D E
18
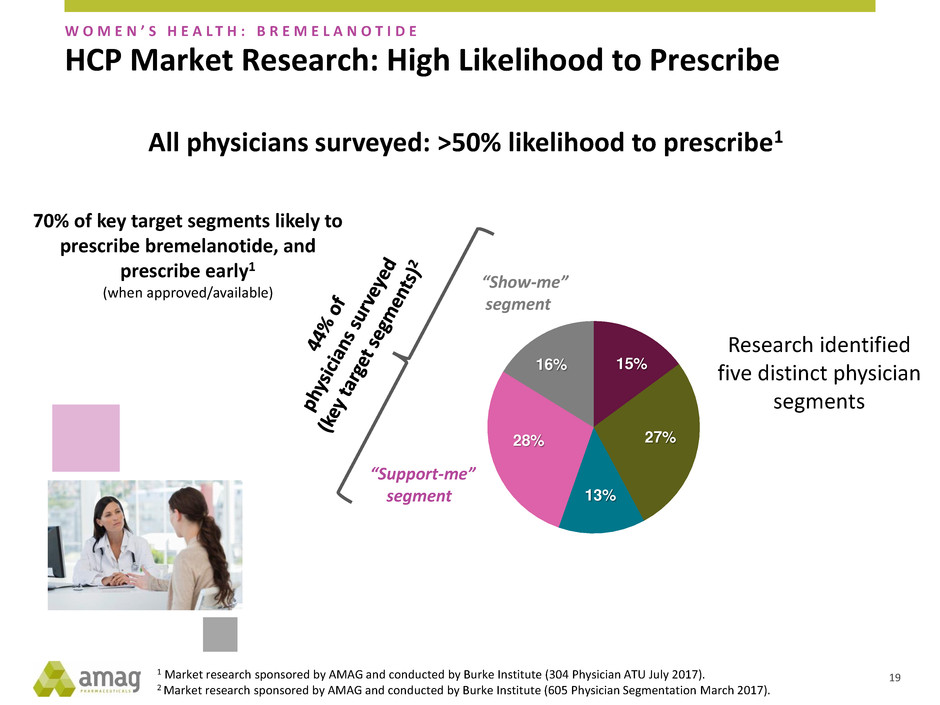
HCP Market Research: High Likelihood to Prescribe
W O M E N ’ S H E A L T H : B R E M E L A N O T I D E
19
15%
27%
13%
28%
16%
All physicians surveyed: >50% likelihood to prescribe1
“Show-me”
segment
“Support-me”
segment
70% of key target segments likely to
prescribe bremelanotide, and
prescribe early1
(when approved/available)
Research identified
five distinct physician
segments
1 Market research sponsored by AMAG and conducted by Burke Institute (304 Physician ATU July 2017).
2 Market research sponsored by AMAG and conducted by Burke Institute (605 Physician Segmentation March 2017).

2016 2017 2018 2019
Estimated Timeline to Approval
20
▪ Targeted FDA approval
▪ Phase 3 studies completed
▪ Completed drug-drug
interaction and safety
pharmacology studies
▪ Planned NDA submission
W O M E N ’ S H E A L T H : B R E M E L A N O T I D E
2018 launching HSDD awareness and education
initiatives in preparation for early 2019 potential approval and launch

Product Portfolio Overview
• Intrarosa®
• Bremelanotide
•Makena®
• Cord Blood Registry®
• Feraheme®
21
© 2018 AMAG Pharmaceuticals, Inc. All rights reserved.
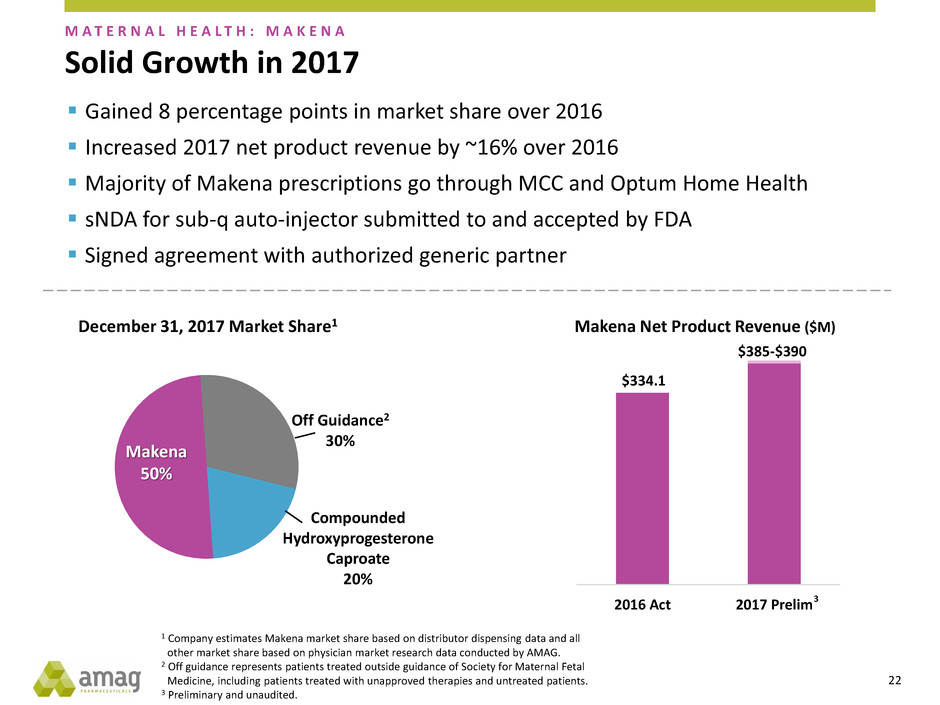
$334.1
2016 Act 2017 Prelim
$385-$390
Solid Growth in 2017
M A T E R N A L H E A L T H : M A K E N A
1 Company estimates Makena market share based on distributor dispensing data and all
other market share based on physician market research data conducted by AMAG.
2 Off guidance represents patients treated outside guidance of Society for Maternal Fetal
Medicine, including patients treated with unapproved therapies and untreated patients.
3 Preliminary and unaudited.
Off Guidance2
30%
Makena
50%
Compounded
Hydroxyprogesterone
Caproate
20%
December 31, 2017 Market Share1
Gained 8 percentage points in market share over 2016
Increased 2017 net product revenue by ~16% over 2016
Majority of Makena prescriptions go through MCC and Optum Home Health
sNDA for sub-q auto-injector submitted to and accepted by FDA
Signed agreement with authorized generic partner
Makena Net Product Revenue ($M)
22
3

M A T E R N A L H E A L T H : M A K E N A
2018 Revenue Drivers
1 If regulatory approval by FDA is received. 23
Secure approval, reimbursement and
distribution of sub-q auto-injector1
Quickly educate HCPs on sub-q auto
injector to facilitate new patient
starts
– Sales force
– Makena Care Connection
Expand conversion activity through
alternate treatment sites (i.e. retail
pharmacies)
Launch authorized generic upon first
generic entrant to capture economics
on residual IM business

Product Portfolio Overview
• Intrarosa®
• Bremelanotide
• Makena®
•Cord Blood Registry®
• Feraheme®
24© 2018 AMAG Pharmaceuticals, Inc. All rights reserved.

$99.6
~$114
2016 Act 2017 Prelim
25
1 Preliminary and unaudited.
2 Non-GAAP CBR revenue includes purchase accounting adjustments related to CBR deferred revenue of $17M and $5.5M for 2016
and 2017, respectively.
M A T E R N A L H E A L T H : C O R D B L O O D R E G I S T R Y
Attractive Recurring Revenue Stream
($M)
$116.6
~$120
2016 Act 2017 Prelim1 1
GAAP CBR Full Year Revenue Non-GAAP CBR Full Year Revenue2

Accomplishments & Growth Drivers
2018 Growth Drivers
Grow ‘first time’ enrollments
Optimize first year price
Strengthen core audiences (HCPs and consumers)
Explore new channels (i.e. employers)
Leverage advancements in stem cell research
M A T E R N A L H E A L T H : C O R D B L O O D R E G I S T R Y
2017 Accomplishments
Reached 700,000 cord blood and cord tissue units stored
‘First time’ enrollments returned to growth
Stabilized new enrollment pricing
Increased annual storage fee on ~1/3 of existing customers
26

Product Portfolio Overview
• Intrarosa®
• Bremelanotide
• Makena®
• Cord Blood Registry®
•Feraheme®
27
© 2018 AMAG Pharmaceuticals, Inc. All rights reserved.

H E M A T O L O G Y / O N C O L O G Y : F E R A H E M E
Feraheme Continued Growth
($M)
$97.1
2016 Act 2017 Prelim
$106-$108
1 Represents revenues from Feraheme only. Excludes revenues from MuGard as reported on financial statements.
2 Preliminary and unaudited numbers.
21
2017 Accomplishments
Completed an additional head-to-head
Phase 3 trial for broader indication well
ahead of schedule
Received FDA acceptance for review of
submission to broaden the current label
5.3% growth in Feraheme grams over 2016
Continued broad patient access strategy,
creating reimbursement predictability
‒ >95% of business is contracted
28

H E M A T O L O G Y / O N C O L O G Y : F E R A H E M E
Initiate launch activities for
potential Q1 expanded label to
include all eligible adult IDA
patients1
Grow market share of Feraheme
through clinical differentiation
Expand access through contracting
beyond CKD with key accounts and
GPOs
Build relationships in new
specialties to maximize expanded
indication
2018 Growth Drivers
1 If regulatory approval is received for expansion of the label to include adult
IDA patients who have an intolerance to iron or have had an unsatisfactory
response to oral iron.
29

Large IV Iron Market Opportunity of $780M1,2
30
1 If regulatory approval is received for broad IDA indication.
2 AMAG estimates market opportunity using ~$600/gram and 1.3M grams (Q2-2017 IMS data annualized).
3 AMAG estimates current market using IMS data and internal analytics.
Feraheme
23%3
Other IV irons
77%3
Current Addressable
IV Iron Market:
$390M3
Additional Addressable
IV Iron Market:
$390M
‒ Iron deficiency anemia
caused by other diseases
– Iron deficiency anemia caused
by chronic kidney disease
Label expansion
doubles
our addressable
market1
Non-dialysis IV iron market
H E M A T O L O G Y / O N C O L O G Y : F E R A H E M E
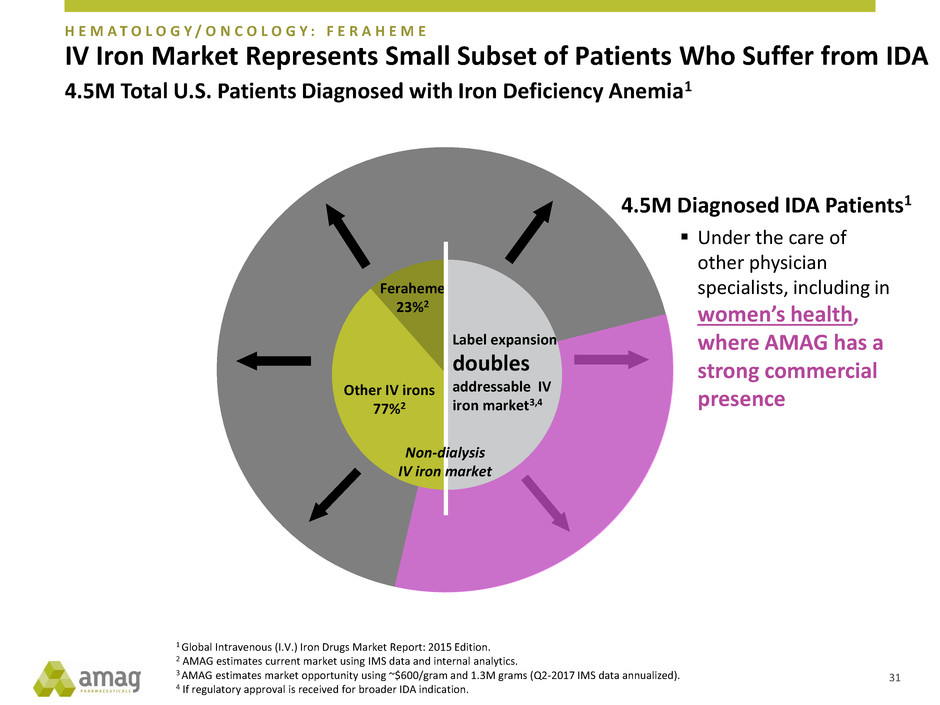
H E M A T O L O G Y / O N C O L O G Y : F E R A H E M E
IV Iron Market Represents Small Subset of Patients Who Suffer from IDA
31
1 Global Intravenous (I.V.) Iron Drugs Market Report: 2015 Edition.
2 AMAG estimates current market using IMS data and internal analytics.
3 AMAG estimates market opportunity using ~$600/gram and 1.3M grams (Q2-2017 IMS data annualized).
4 If regulatory approval is received for broader IDA indication.
4.5M Total U.S. Patients Diagnosed with Iron Deficiency Anemia1
4.5M Diagnosed IDA Patients1
Under the care of
other physician
specialists, including in
women’s health,
where AMAG has a
strong commercial
presence
Feraheme
23%2
Other IV irons
77%2
Label expansion
doubles
addressable IV
iron market3,4
Non-dialysis
IV iron market

Financial Overview
© 2018 AMAG Pharmaceuticals, Inc. All rights reserved. 32

Solid 2017 GAAP Revenue Growth
Q4-2016 Act Q4-2017 Prelim
$151.5 $155-$162
Fourth Quarter Revenue
~5%
($M)
Makena CBRFeraheme/MuGard
2016 Act 2017 Prelim
$532.1
$607-$614
Full Year Revenue
~15%
1 Preliminary and unaudited.
1 1
Intrarosa
33

Solid 2017 Non-GAAP Revenue Growth
Q4-2016 Act Q4-2017 Prelim
$152.9 $156-$163
Fourth Quarter Revenue
~4%
($M)
2016 Act 2017 Prelim
$549.1
$613-$620
Full Year Revenue
~12%
1,2 1,2
1 Preliminary and unaudited.
2 Includes purchase accounting adjustments related to deferred revenue of $1.4M in Q4-2016, $1.4M in Q4-2017, $17M in 2016 and $5.5M
in 2017.
22
Makena CBRFeraheme/MuGardIntrarosa
34

GAAP Operating Income and Non-GAAP Adjusted EBITDA1
$14.6
($11)
$77.4
$63
35
Operating Income (Loss)
$78.9
$265.7
$225
Fourth Quarter Full Year
1 See slide 41 for a reconciliation of GAAP to non-GAAP financials results.
2 Preliminary and unaudited.
3 2017 non-GAAP adjusted EBITDA reflects the mid-point of preliminary, unaudited results. AMAG plans to issue final 2017 financial results in late
February 2018.
2,3 2,3
($M)
Q4-2016 Act Q4-2017 Prelim 2016 Act 2017 Prelim
($297)
Adjusted EBITDA
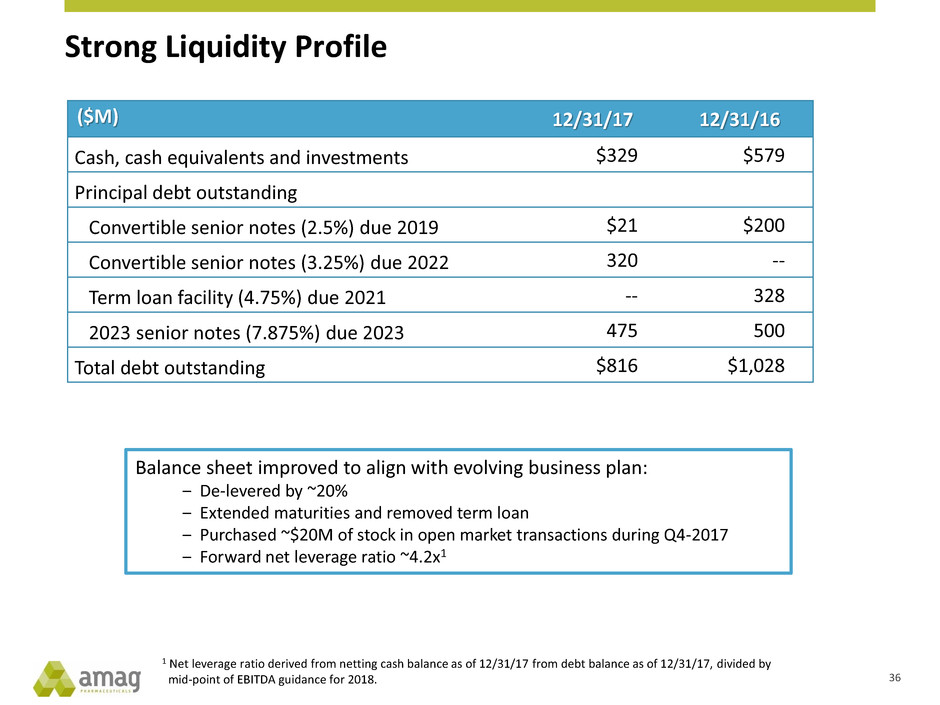
Strong Liquidity Profile
($M) 12/31/17 12/31/16
Cash, cash equivalents and investments $329 $579
Principal debt outstanding
Convertible senior notes (2.5%) due 2019 $21 $200
Convertible senior notes (3.25%) due 2022 320 --
Term loan facility (4.75%) due 2021 -- 328
2023 senior notes (7.875%) due 2023 475 500
Total debt outstanding $816 $1,028
Balance sheet improved to align with evolving business plan:
‒ De-levered by ~20%
‒ Extended maturities and removed term loan
‒ Purchased ~$20M of stock in open market transactions during Q4-2017
‒ Forward net leverage ratio ~4.2x1
36
1 Net leverage ratio derived from netting cash balance as of 12/31/17 from debt balance as of 12/31/17, divided by
mid-point of EBITDA guidance for 2018.
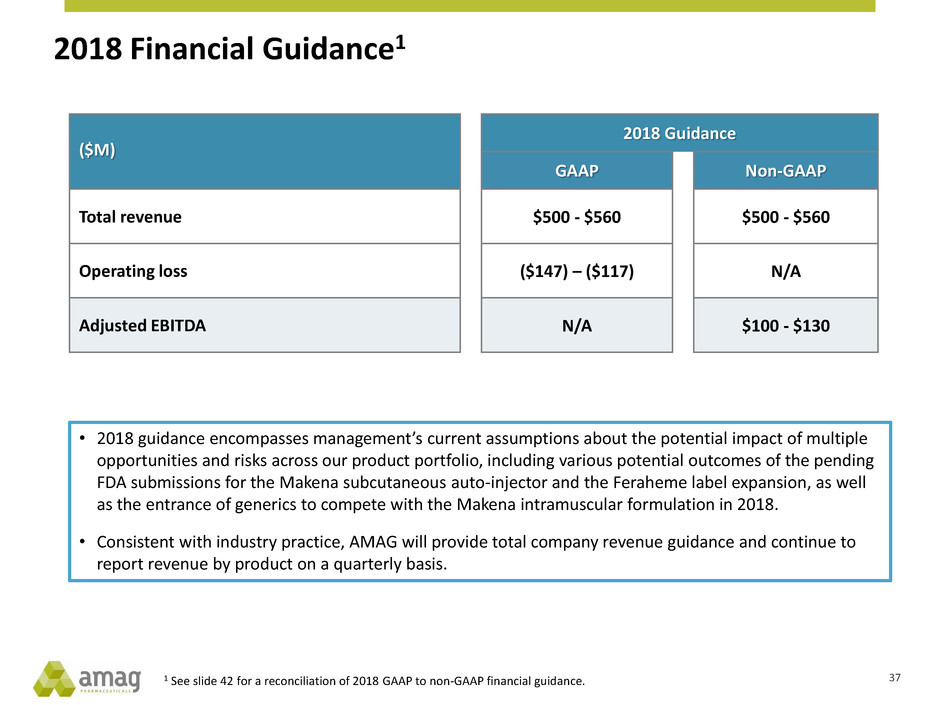
2018 Financial Guidance1
($M)
2018 Guidance
GAAP Non-GAAP
Total revenue $500 - $560 $500 - $560
Operating loss ($147) – ($117) N/A
Adjusted EBITDA N/A $100 - $130
1 See slide 42 for a reconciliation of 2018 GAAP to non-GAAP financial guidance.
• 2018 guidance encompasses management’s current assumptions about the potential impact of multiple
opportunities and risks across our product portfolio, including various potential outcomes of the pending
FDA submissions for the Makena subcutaneous auto-injector and the Feraheme label expansion, as well
as the entrance of generics to compete with the Makena intramuscular formulation in 2018.
• Consistent with industry practice, AMAG will provide total company revenue guidance and continue to
report revenue by product on a quarterly basis.
37

2018 Key Priorities
Intrarosa
Continue to drive awareness and usage of Intrarosa
Initiate digital consumer campaign mid-2018
Invest to expand treatable patient population (i.e. HSDD Phase 3 study)
Makena
Launch subcutaneous auto-injector in Q1-20181
Prepare for potential 2018 competitive generic threat
Authorized generic readiness
CBR Continue to grow ‘first time’ enrollments
Feraheme
Launch expanded IDA label (all causes) in Q1-20181
Increase market share
Bremelanotide
Submit NDA in Q1-2018
Prepare for FDA advisory committee meeting in Q4-2018 (estimated timing)
Portfolio
Expansion
Continue to explore the addition of longer-lived, durable products through
licensing or acquisition transactions
Financial
Drive toward non-GAAP revenue of $530M (midpoint of guidance)
Achieve adjusted EBITDA of $115M (midpoint of guidance)
38
1 If regulatory approval is received.

© 2018 AMAG Pharmaceuticals, Inc. All rights reserved.
39
J.P. Morgan Healthcare
Conference
January 2018

Appendix
40
© 2018 AMAG Pharmaceuticals, Inc. All rights reserved.

Reconciliation of GAAP to Non-GAAP Preliminary Financial Results
41
($M)
GAAP operating income (loss)
Purchase accounting adjustments related to CBR
deferred revenue
Depreciation and intangible asset amortization
Non-cash inventory step-up adjustments
Stock-based compensation
Adjustments to contingent consideration
Impairment charges of intangible assets
Transaction / acquisition related costs
Acquired IPR&D
Restructuring costs
Non-GAAP adjusted EBITDA
Q4-2017
Prelim
Q4-2016
Actual
2017
Prelim
2016
Actual
($16) – ($6) $14.6 ($302) – ($292) $78.9
1 1.4 6 17.0
68 29.1 152 94.2
1 1.0 2 5.7
5 5.7 23 22.5
(1) 20.6 (48) 25.7
-- 3.7 319 19.7
-- 1.3 2 1.3
-- -- 66 --
-- -- -- 0.7
$58 - $68 $77.4 $220 - $230 $265.7

Reconciliation of GAAP to Non-GAAP 2018 Financial Guidance
42
($M) 2018 Financial Guidance
Operating loss ($147) – ($117)
Depreciation & intangible asset amortization 200
Stock-based compensation 23
Non-cash inventory step up and adjustments to contingent consideration 4
Acquired IPR&D 20
Non-GAAP adjusted EBITDA $100 - $130

J.P. Morgan Healthcare
Conference
January 2018
© 2018 AMAG Pharmaceuticals, Inc. All rights reserved. 43
