Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AERIE PHARMACEUTICALS INC | d519591d8k.htm |
 Company Overview Investor Presentation January 2018 Exhibit 99.1 |
 2 Important Information The information in this presentation does not contain all of the information that a potential investor should review before investing in Aerie
shares. The descriptions of Aerie Pharmaceuticals, Inc. (the
“Company” or “Aerie”) in this presentation are qualified in their entirety by reference to reports filed with the SEC. Certain information in this presentation has been obtained from outside sources. While such information is believed to be
reliable for the purposes used herein, no representations are made as to
the accuracy or completeness thereof and we take no responsibility for such
information. Any discussion of the potential use or expected success of Rhopressa ® (netarsudil ophthalmic solution) 0.02%, with respect to foreign approval or additional indications, and our current or any future product candidates is subject to regulatory approval. In addition, any discussion of U.S.
Food and
Drug Administration (“FDA”) approval of Rhopressa ® does not guarantee successful commercialization of Rhopressa ® or FDA approval of Roclatan TM . For more information on Rhopressa ® , refer to the full Rhopressa ® product label at http://investors.aeriepharma.com. The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation
to update this information in light of new information, future events or
otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete. Certain statements in this presentation, including any guidance or timelines presented herein, are “forward-looking statements”
within the meaning of
the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these
forward-looking statements. These statements are based on the
Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. In particular, FDA approval of Rhopressa ® does not constitute approval of Roclatan TM , and there can be no assurance that we will receive FDA approval for Roclatan TM or any future product candidates. Any top line data presented herein is preliminary and based solely on information available to us as of the date of this presentation and additional information about the results may be disclosed at any time. In addition, the preclinical research discussed in this presentation is preliminary and the outcome of such preclinical studies may not be predictive of the outcome of later trials. Any future clinical trial results may not demonstrate
safety and efficacy sufficient to obtain regulatory approval related to
the preclinical research findings discussed in this presentation. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled
“Risk Factors” and “Management’s Discussion and
Analysis of Financial Condition and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new
information, future events or otherwise, except as otherwise required by
law. For Investor Use |
 Aerie IOP–Lowering Products (IP 2030+)
Pipeline Activities • Rhopressa ® • 24-hour IOP lowering, normal tension glaucoma, disease modification, etc. • AR-13503 (ROCK/PKC inhibitor) and AR-1105 (dexamethasone steroid) • Pre-clinical molecules for diseases of the retina • Drug Delivery – Focused on back of the eye (e.g., PRINT ® / DSM) • Rhopressa ® (netarsudil ophthalmic solution) 0.02% • Aerie-owned new chemical entity • FDA Approved December 18, 2017; in launch mode • Roclatan™ (netarsudil/latanoprost
ophthalmic solution) 0.02%/0.005% •
Two P3’s achieved primary efficacy endpoints
• NDA submission expected 2Q 2018 Aerie: Building a Major Ophthalmic Pharmaceutical Company 3 Data on file For Investor Use |
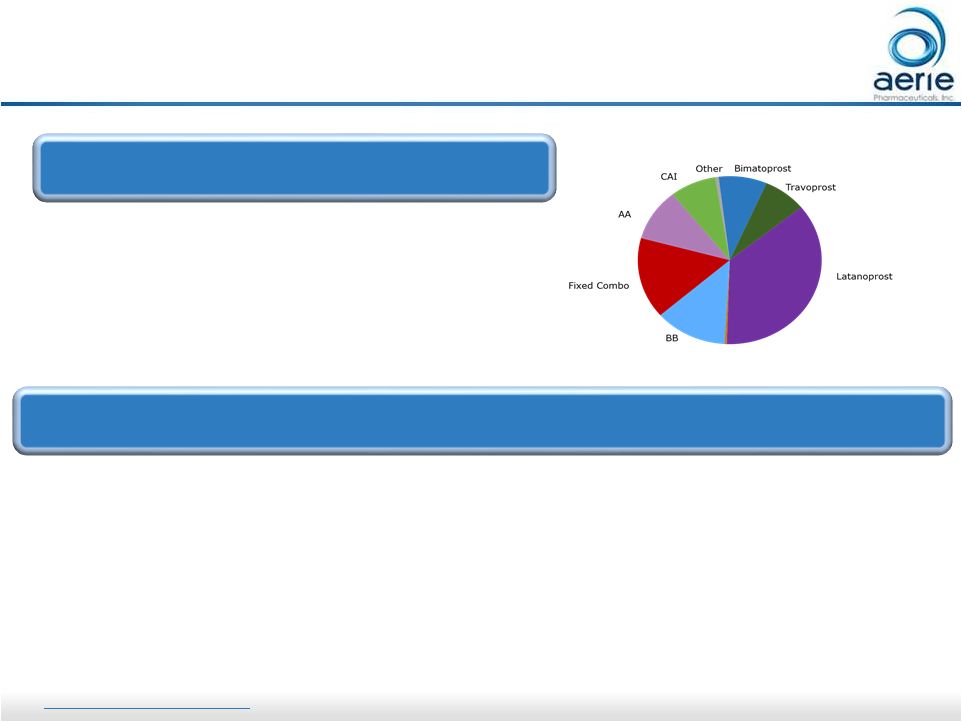 4 4 Rhopressa For Investor Use - ~$3B Market, 36M TRx, 60M bottles - Half of volume first-line (PGAs) - Half of volume 2-4X/Day Adjuncts - Once-daily dosing directed at site of pathology, the trabecular meshwork - Consistent IOP lowering over 12 months and across all IOPs tested, as
demonstrated in clinical trials
Rhopressa ® : Positioning as an Adjunctive Therapy (8%) (36%) (13%) (16%) (10%) (9%) (8%) Graph Source: IMS Data CAI: Carbonic Anhydrase Inhibitor AA: Alpha Agonist BB: Beta Blocker 2016 U.S. Glaucoma Market ® : Market Perspective ® http://investors.aeriepharma.com Refer to the full Rhopressa product label at
|
 5 5 Rhopressa Hired Chief Commercial Officer and VPs of Sales, Marketing, Market Access, Commercial Operations, and Medical Affairs in 2016/2017, and Chief Compliance Officer in 2015 Preparing to hire U.S. salesforce of 100 reps; expected to be fully trained during 2Q 2018 Sales territories finalized and hiring of Sales Regional Directors and District Managers
proceeding
Market access meetings with top Medicare Part D and Commercial Payors under way
- Covered market is ~50/50 Commercial / Part
D -
Part D coverage expected to commence January 2019
We believe awareness of Rhopressa ® is high among ophthalmologists For Investor Use Fully Prepared to Execute Launch Plan ® : Commercialization |
 6 Sales Commercial Operations Market Access Marketing Rhopressa ® : Launch Plan Payer Engagement Field Deployment Commercial and Med D Strategy Drive to Preferred Formulary Positioning Broad Strategy Brand Planning and Messaging KOL Engagement Field Support For Investor Use Hire Reps Sales Training and Deployment Target 12K Prescribers / 80% of Volume Success Metrics and Standards Supply Chain Management Sales Execution Readiness Analytics/Reporting Field Support Programs |
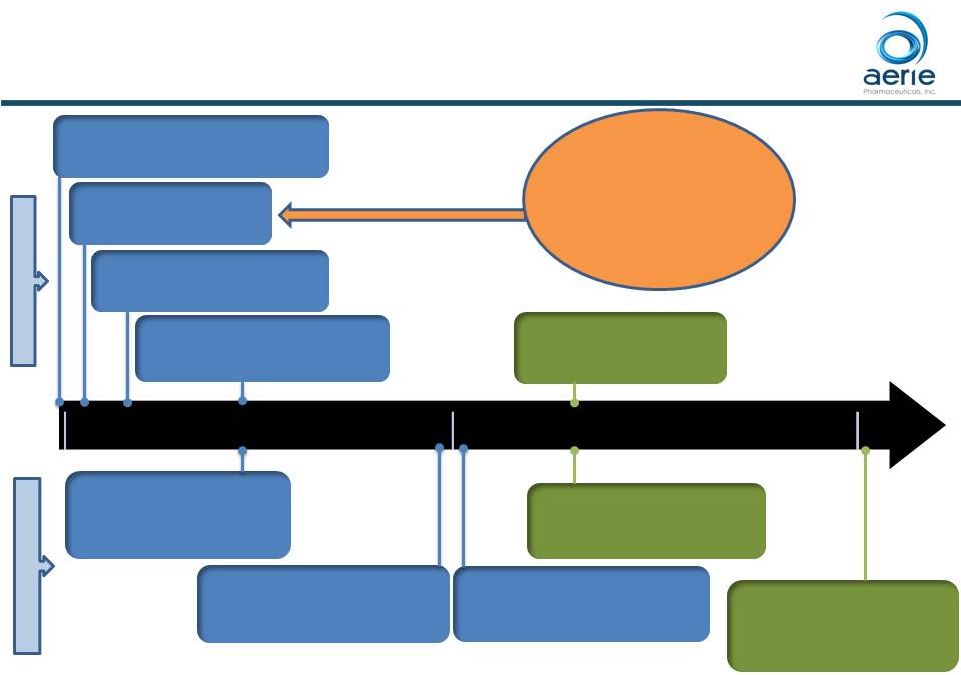 2018 2019 U.S. Launch Plan Timeline January 2020: Roclatan™ Med D Coverage Commences 1H-2019: Roclatan™ Commercial Coverage Commences December 2017: Rhopressa ® FDA Approval 2Q/3Q-2018: Rhopressa ® Sampling and Initial Commercial Sales 1Q-2018: Begin Hiring 100 Sales Reps End of 2018: Rhopressa ® Commercial Coverage Majority Preferred 2Q/3Q-2018: Rhopressa ® Commercial Coverage Majority Non-Preferred 1H-2019: Potential Roclatan TM Launch For Investor Use January 2019: Rhopressa ® Med D Coverage Majority Preferred All dates, except for FDA approval for Rhopressa ® , are estimates. Roclatan TM has not been approved by the FDA. The NDA for Roclatan TM is expected to be submitted in 2Q 2018 7 2Q-2018: Rhopressa ® Commercial Launch Over 1,500 Experienced Applicants To Date S A L E S A C C E S S |
 8 Building a World Class Medical Affairs Team • Hired highly-experienced, successful and recognized leaders in Medical Affairs, Medical Science, Field Medical Affairs and Professional Affairs • Communicating the key medical scientific messages • Responding to technical questions from practitioners and managed care decision makers • Placing speakers at national, regional and local scientific meetings • Active presence at major ophthalmic conferences, including a Medical Affairs Booth Medical Affairs For Investor Use |
 9 American Society of Cataract Refractive Surgeons (ASCRS) May 2017 World Glaucoma Congress Helsinki June 2017 Association of Research in Vision and Ophthalmology (ARVO) May 2017 American Glaucoma Society (AGS) March 2017 Building Aerie’s Presence in the Medical Community: 19 Podium Presentations at Major Eyecare Meetings 4 Presentations, including 24-Hour IOP Lowering of Netarsudil, Peace et al. 5 Posters, including Enhancing Efficacy by Continuous Delivery of AR-13154 in an Animal Model of Proliferative Diabetic Retinopathy, Carbajal et al. 3 Presentations, including 3-Month Interim Results of Mercury 1, Asrani et al., and Aqueous Humor Dynamics of Netarsudil Ophthalmic Solution 0.02% in Healthy Volunteers, Sit et al. Aerie Medical Affairs Booth highlighted, in addition to presentation For Investor Use |
 10 Roclatan TM (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% Positioning as First Line Therapy: • Benefits of Rhopressa ® while also targeting the secondary drain • Achieved statistical superiority to market-leading latanoprost in two P3 trials • Potential to become the most efficacious IOP-lowering medication for glaucoma or ocular hypertension, if approved Roclatan TM Combination Product Candidate Data on file Roclatan TM has not been approved by the FDA For Investor Use NDA Submission Expected 2Q 2018 |
 11 Roclatan TM Efficacy and Safety Efficacy: • Roclatan TM demonstrated statistical superiority over its components (market- leading PGA latanoprost and Rhopressa ® ) in Mercury 1 and 2 Phase 3 trials, at all measured time points • Consistent incremental IOP-lowering over latanoprost and Rhopressa ® in the range of 1 to 3 mmHg Safety: • No treatment-related serious adverse events and minimal evidence of treatment-related systemic effects. The most common adverse event is conjunctival hyperemia with ~60% incidence, majority mild and sporadic and present in 20% of subjects at baseline • Other ocular AEs occurring in ~5-15% of subjects receiving Roclatan TM included: cornea verticillata, conjunctival hemorrhage, eye pruritus, lacrimation
increased, visual acuity reduced, blepharitis and punctate keratitis
Data on
file
For Investor Use |
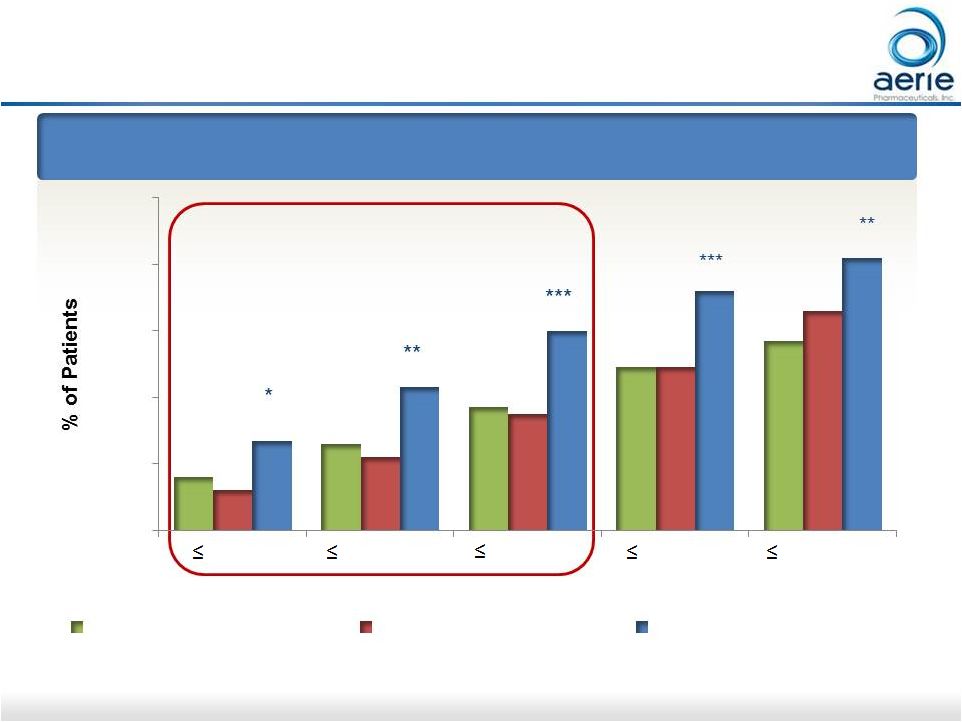 12 Roclatan TM Phase 3 Month 12 Responder Analysis: Goal is to Achieve Lowest IOP Possible At Month 12: % of Patients with IOP Reduced to 18 mmHg or Lower *p<0.05, **p<0.01, ***p<0.0001 ++ Data on File Based on Mercury 1 Interim Analysis 2 For Investor Use ® 16% 26% 37% 49% 57% 12% 22% 35% 49% 66% 27% 43% 60% 72% 82% 0% 20% 40% 60% 80% 100% 14 mmHg 15 mmHg 16 mmHg 17 mmHg 18 mmHg IOP on Treatment Latanoprost (n=203) Roclatan (n=158) Rhopressa (n=148) ™ |
 13 Roclatan™ Next Steps • Roclatan TM NDA submission expected 2Q 2018 • Aerie Ireland plant and 2 contract manufacturers are expected to support Roclatan TM U.S. commercial activities • Mercury 3 commenced in Europe 3Q 2017 - Trial conducted in U.K., France, Germany, Italy, Spain, Belgium and
Austria
- Marketing Authorization Application (MAA) in Europe expected in
2H 2019 For Investor Use |
 14 Expanding Aerie Franchise: Europe and Japan • Europe (2016 Europe “Big 5” Glaucoma Market: 90M units per year, 1.5X
U.S. units)
• Expect to file MAA for Rhopressa ® in 2H 2018 • Current clinical plan expected to satisfy European regulatory requirements (including Rocket 4 for Rhopressa ® and Mercury 3 for Roclatan ) • Mercury 3: 6-month safety and 90-day efficacy registration trial for Europe,
comparing Roclatan for non-inferiority to a fixed-dose combo in Europe (Ganfort ® ) started 3Q 2017. Approximately 250 patients per arm. • Construction of Ireland Plant in process to support worldwide commercial supply • Japan (2016 Glaucoma Market: 52M units per year)
• Plan to advance clinical development on our own • Phases 1 and 2 under way in the U.S. on Japanese and Japanese- Americans, initiated 4Q 2017 • Phase 3 trials expected to be conducted in Japan For Investor Use TM TM |
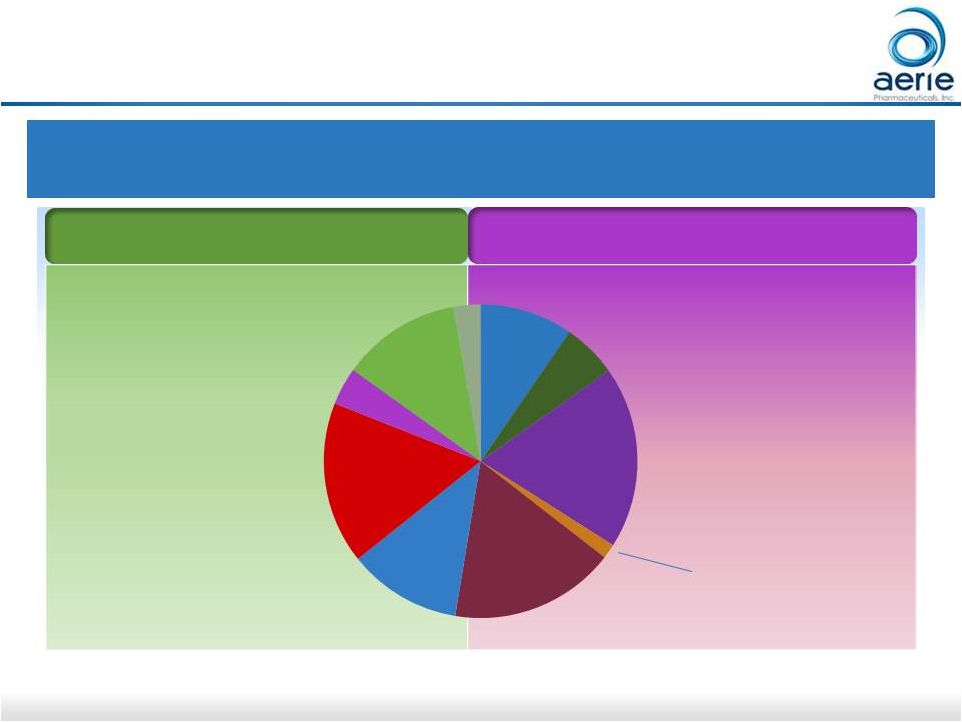 15 Europe Glaucoma Market: Aerie Expects to Commercialize on Its Own (if approved) “Big 5” Europe Glaucoma Market – 2016 $1.0B; 90M TRx* PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic Anhydrase Inhibitor
Sources: IMS Analytics Link at ex-manufacturer price level. *TRx calculated from IMS unit data (1 month = 1 TRx) Non-PGA Market (47%) PGA Market (53%) Bimatoprost Travoprost Latanoprost PGA Fixed Combo BB Non-PGA Fixed Combo AA CAI 17% 12% 12% 4% 19% 10% 6% 2 - 4 Times Daily Once Daily 17% Others 3% For Investor Use Tafluprost 2% |
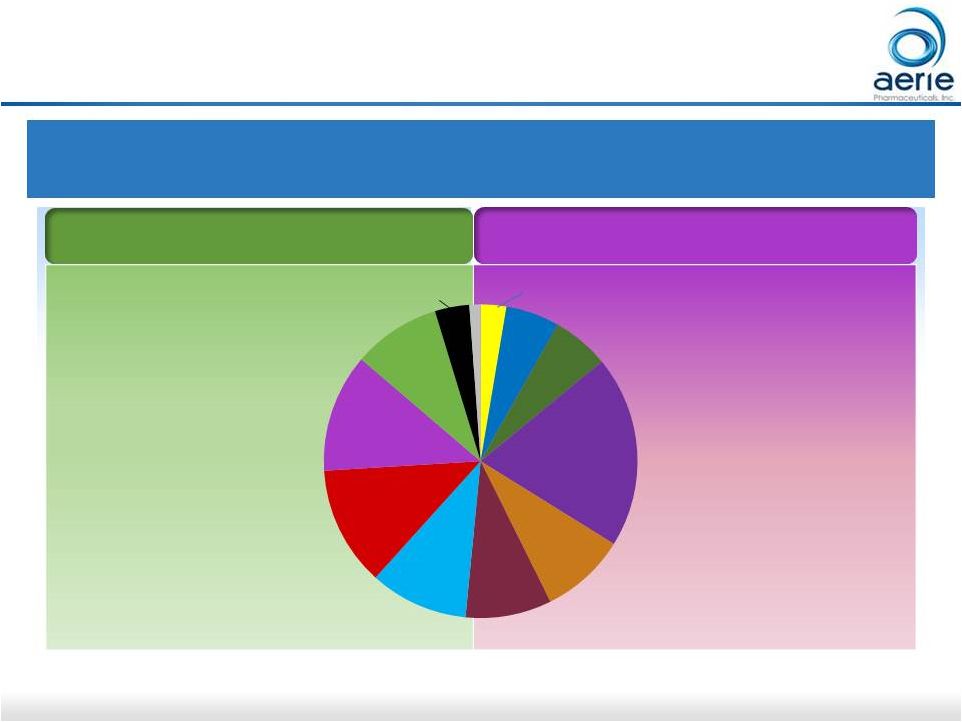 16 Japan Glaucoma Market: Aerie Expects to Partner for Commercialization (if approved) PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic Anhydrase Inhibitor
Sources: IMS Analytics Link at ex-manufacturer price level. *Monthly Units
calculated from IMS SU Data Non-PGA Market (48%)
PGA Market (52%) Unoprostone Bimatoprost Travoprost Latanoprost Tafluprost BB Non-PGA Fixed Combo AA CAI ROCK Inhibitors Others 12% 10% 9% 12% 20% 6% 6% 2 - 4 Times Daily Once Daily (Unoprostone is bid) 9% Japan Glaucoma Market – 2016 $0.8B; 52M Units Annually 9% 4% 3% For Investor Use PGA Fixed Combo |
 17 Advancing the Pipeline AR-13503 and AR-1105 are preclinical stage molecules and have not been approved by the FDA
• Rhopressa ® • Potential for disease modification in glaucoma • 24-hour IOP lowering • Retina Program Opportunities: AR-13503* (ROCK/PKC inhibitor)
potentially for AMD and DME AR-1105 (dexamethasone steroid) potentially for DME • Drug Delivery • Focused on implants for retinal diseases (DSM / PRINT ® ) For Investor Use * Active metabolite of AR-13154 |
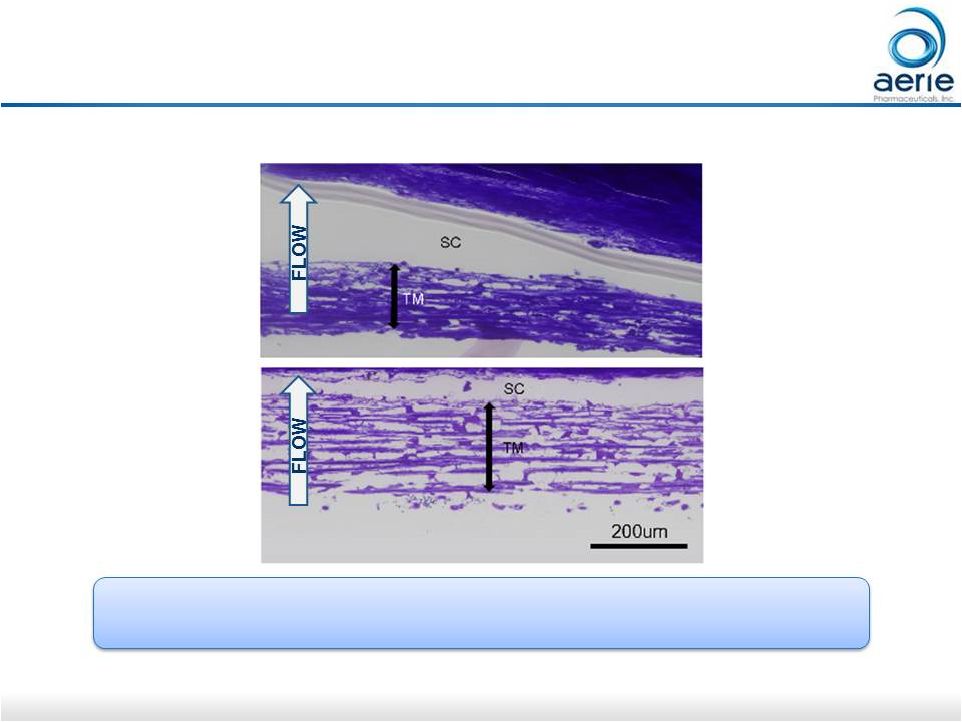 18 Netarsudil* Causes Expansion of TM Tissue, Opening Spaces for Increased Outflow Control + Netarsudil TM: Trabecular Meshwork SC: Schlemm’s Canal Control = buffered saline solution Increasing Trabecular Outflow, Reducing Fibrosis Could Stop Degeneration of Outflow Tissues in Glaucoma Increasing Trabecular Outflow, Reducing Fibrosis Could Stop Degeneration of Outflow Tissues in Glaucoma *Active ingredient of Rhopressa ® Source: Ren R et al. Invest Ophthalmol Vis Sci. 2016; 57(14):6197-6209. For Investor Use 200um |
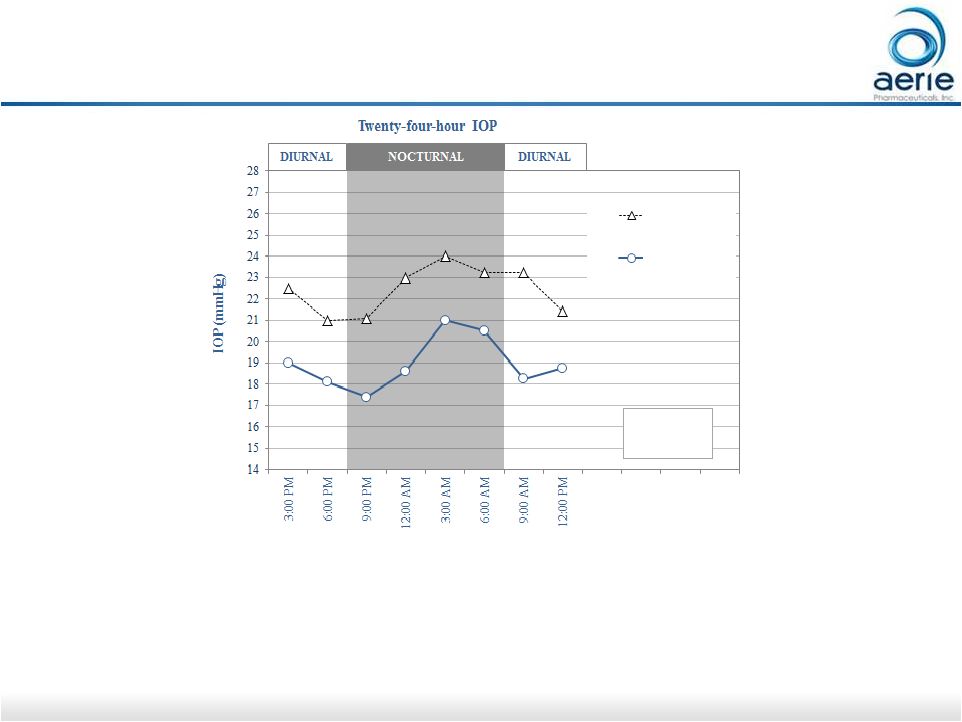 19 Rhopressa ® 24-hour IOP Pilot Study Demonstrates Effective Nocturnal Efficacy Netarsudil (n=8) Baseline (n=8) ** ** ** *** ** ** *** *** ** p<0.01 *** p<0.001 • Netarsudil (active ingredient of Rhopressa ® ) equally effective during nocturnal and diurnal periods • Current glaucoma medications either have no efficacy at night (beta blockers, alpha agonists) or reduced efficacy at night (PGAs, CAIs) 1 - 6 AR-13324-CS204 1. Liu JH, et al. Am J Ophthalmol. 2004; 138:389-395. 2. Gulati V, et al. Arch Ophthalmol. 2012; 130:677-684. 3. Liu JH,
et al. Ophthalmology. 2009; 116:449- 454. 4. Liu JH, et al.
Ophthalmology. 2010; 117:2075-9. 5. Fan S et al. J Glaucoma. 2014; 23:276-81. 6. Liu JH, et al. Am J Ophthalmol. 2016;169:249-257. For Investor Use Pre-dose Post-dose (Day 8/9) |
 20 20 Retinal Diseases – Aerie’s Next Chapter The market for retina eye diseases is twice that of glaucoma with $4.9 billion in the U.S. and $9 billion worldwide per IMS Current treatments for retina eye diseases lose efficacy over time, some have very serious side effects and there are limited surgical options The majority of current treatments for retina eye diseases require repeated injections into the patient’s eye Aerie has two preclinical molecules for the treatment of retinal disease: - AR-13503 (ROCK/PKC Inhibitor) for AMD/DME - AR-1105 (dexamethasone steroid) for DME Aerie also has access to bio-erodible implant technology through DSM collaboration, and also has ophthalmic rights to PRINT ® technology, a fully scalable manufacturing platform for implants For Investor Use |
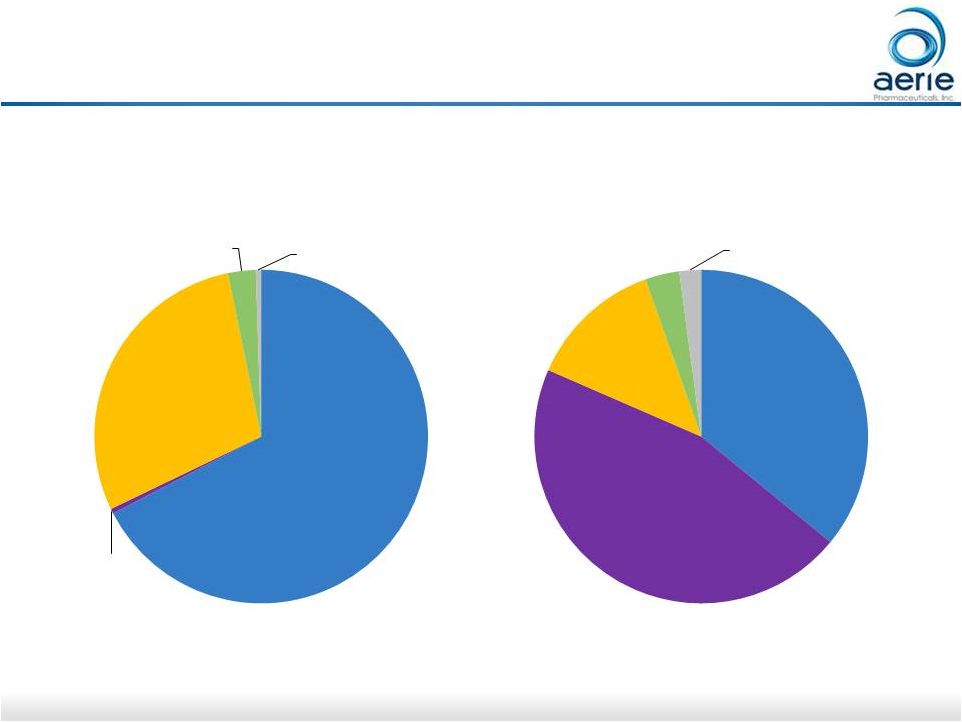 21 21 2016 U.S. Retina Market For Investor Use Eylea, $3.3 Avastin, $0.02 Lucentis, $1.4 steroids, $0.1 others, $0.03 2016 Sales: $4.9B U.S. Unit Sales: 6.5M Eylea, 2.3 Avastin, 3.0 Lucentis, 0.9 steroids, 0.2 others, 0.1 Source: IMS Data |
 22 AR-13503: A First-in-Class ROCK/PKC Inhibitor for the Treatment of Wet AMD and Diabetic Retinopathy • Active metabolite of AR-13154 and netarsudil • Potential to improve outcomes by targeting multiple disease processes • Monotherapy shows strong efficacy in preclinical models • Effective as adjunct to anti-VEGF therapy in preclinical models • Expect durable treatment effect with injection frequency of once every 4 – 6 months For Investor Use |
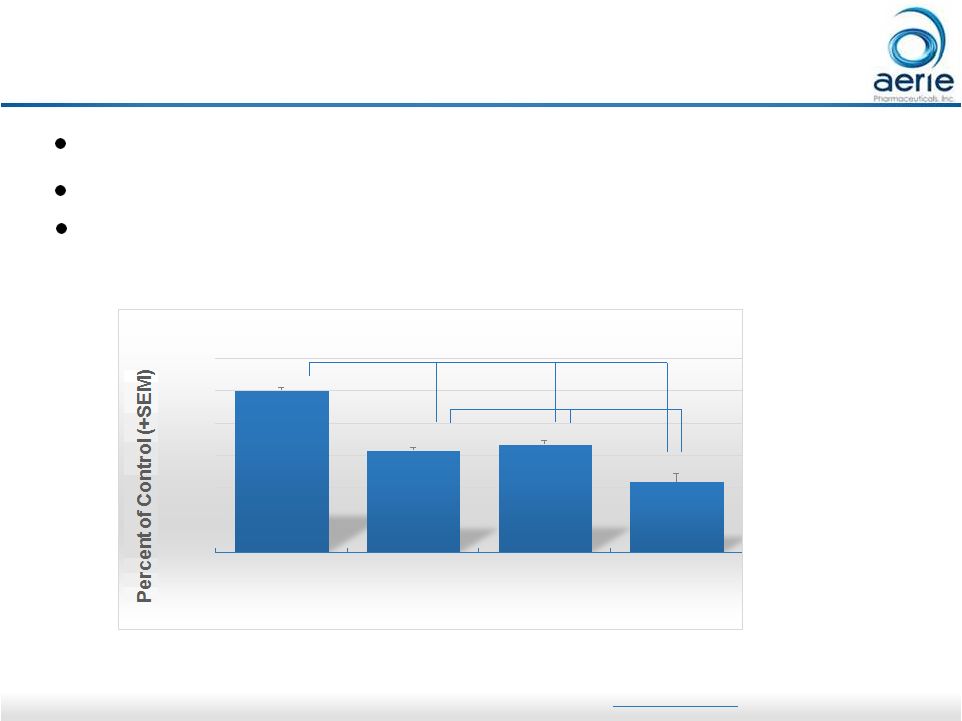 23 Aerie Preclinical Molecule Provides Additive Efficacy to Eylea ® in a Proliferative Diabetic Retinopathy Model 0% 20% 40% 60% 80% 100% 120% Vehicle Control (n=55) AR-13154(S) topical (n=28) Eylea 1mg/kg IP (n=26) AR-13154(S) + Eylea (n=18) Total Neovascular Area -37% -34% -57% ** *** *** *** Oxygen-induced retinopathy model of PDR (mouse) AR-13154(S) is a precursor molecule to AR-13503 Confirms potential as monotherapy and as adjunct to anti-VEGF therapies; not yet tested in humans Data on file; Carbajal, KS et al., Enhancing Efficacy by Continuous Delivery of AR-13154(S) in an Animal Model of Proliferative Diabetic Retinopathy, ARVO 2017, Poster B0481.
For Investor Use For more information on Eylea® please see the product webpage https://www.eylea.us/ |
 24 DSM Collaboration – Implant Delivery Technology • Intravitreal sustained-release, bio-erodible implant technology • Potential for treatment of Wet AMD and Diabetic Retinopathy • Promising results from ongoing feasibility study • Evaluating AR-13503 (ROCK/PKC inhibitor) and related Aerie compounds • Linear sustained elution rates over several months • Achieved target retinal drug concentrations • Executed collaboration/licensing agreement • Continue prototype evaluations and IND-enabling activities For Investor Use Data on File |
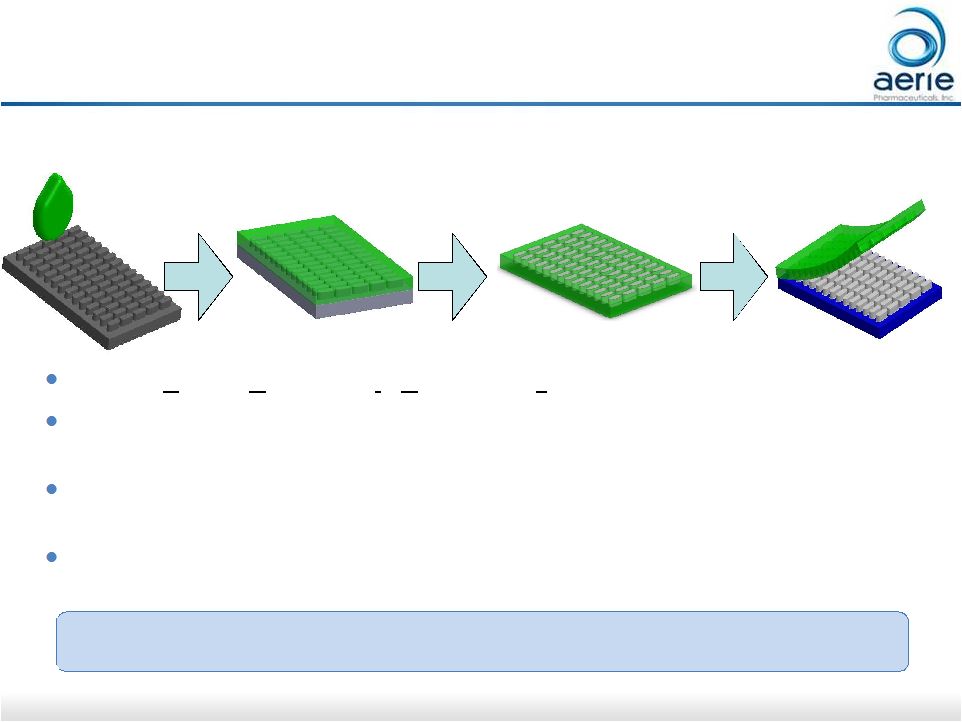 25 PRINT ® Technology for Ophthalmology Best in class control over particle size, shape and formulation Micropatterned Template Mold formation Mold Filling (e.g. drug/polymer) Particle Array PRINT= Particle Replication In Non-wetting Templates Aerie recently acquired the rights to use this technology for ophthalmic applications Proprietary technology capable of creating precisely engineered sustained-release
products using fully scalable manufacturing processes
Expected to accelerate development of Aerie’s retinal disease program, including
pre-clinical AR-13503 and AR-1105
For Investor Use |
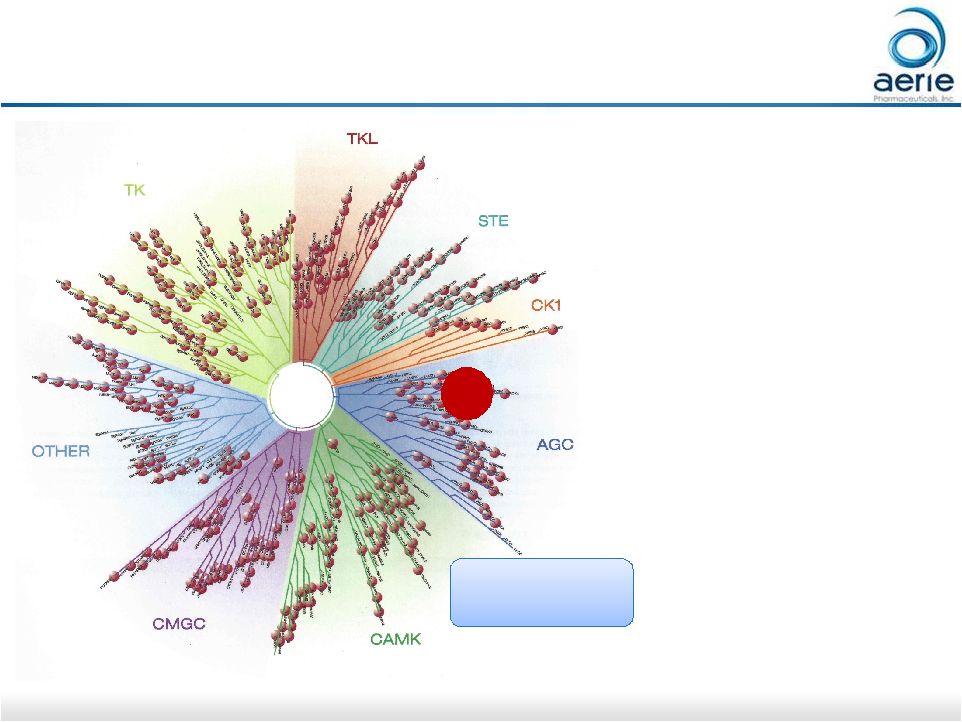 26 Evaluating Aerie’s 3,000+ Owned Molecules ROCK • Commencing screening for additional indications beyond ophthalmology • ROCK inhibition has potential in: • Pulmonary health, including pulmonary fibrosis and bronchial asthma • Dermatology indications • Cancer • Others Relationship tree of human kinases. TK, TKL, STE, CK1, AGC, CAMK, CMGC, Other: Kinase superfamilies
For Investor Use Aerie molecules inhibit both ROCK1 and ROCK2 |
 27 Summary • Lead Product Priorities • Rhopressa ® : Successful launch execution expected in 2Q
2018
• Roclatan™: • Research Initiatives • Rhopressa ® 24-hour IOP lowering, normal tension glaucoma, disease modification
• AR-13503 (ROCK/PKC inhibitor) and AR-1105 (dexamethasone
steroid) with potential for retinal diseases • Evaluating Aerie’s 3,000+ proprietary Rho kinase molecules – beyond ophthalmology • Business Development and Expansion Opportunities • Drug delivery opportunities focused on back of the eye (e.g., PRINT ® / DSM) • EU/JP clinical path and commercialization strategy • Ireland Manufacturing Facility For Investor Use Mercury 3 commenced 3Q 2017 (EU) U.S. NDA submission expected in 2Q 2018 |
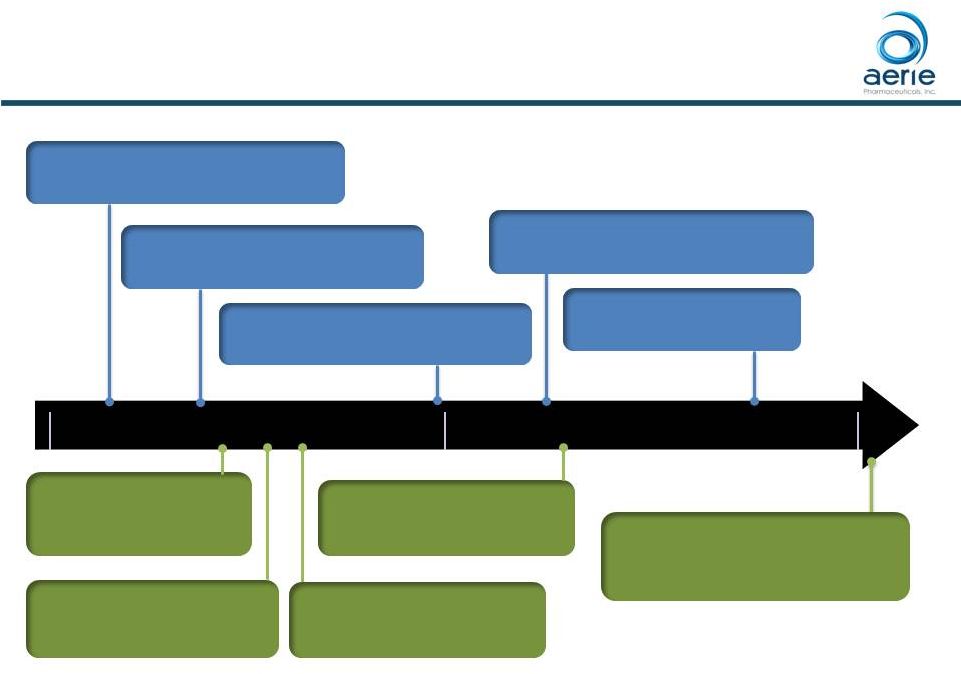 2017 2018 Key Product-Related Milestones 1H-2019: Roclatan™ Potential U.S. Approval and Launch 2Q-2018: Roclatan™ NDA Submission Expected Q1-2017: Rhopressa ® NDA Resubmitted 2Q-2018: Rhopressa ® U.S. Launch Expected Q2-2017: Rhopressa ® Rocket 4 Topline safety (6 mos) Q3-2017: Roclatan™ P3 Mercury 1 12-month Safety Q2-2017: Roclatan™ P3 Mercury 2 Topline Efficacy (3 mos) 2H-2018: Rhopressa® Potential EU MAA Filing For Investor Use Q3-2017: Roclatan™ P3 Mercury 3 (EU) Initiation (6 mos) 2018 and 2019 dates are estimates. Roclatan TM has not been approved by the FDA. 28 December 2017: Rhopressa ® FDA Approval |
