Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - ACCELERON PHARMA INC | xlrn-20180108form8xkex992.htm |
| 8-K - 8-K - ACCELERON PHARMA INC | xlrn-20180108form8xk.htm |

JP Morgan
Healthcare
Conference
January 2018
Exhibit 99.1

Acceleron Forward-Looking Statements
2
THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS ABOUT THE COMPANY’S STRATEGY, FUTURE PLANS
and prospects, including statements regarding the development of the Company's compounds, the timeline for clinical
development and regulatory approval of the Company’s compounds and the expected timing for reporting of data from
ongoing clinical trials. The words “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “may,” “plan,”
“potential,” “project,” “should,” “target,” “will,” “would,” and similar expressions are intended to identify forward-looking
statements, although not all forward-looking statements contain these identifying words.
ACTUAL RESULTS COULD DIFFER MATERIALLY FROM THOSE INCLUDED IN THE FORWARD-LOOKING STATEMENTS DUE to
various risks and uncertainties, including, but not limited to, that preclinical testing of the Company's compounds and
data from clinical trials may not be predictive of the results or success of ongoing or later clinical trials, that the
development of the Company's compounds will take longer and/or cost more than planned, that the Company or its
collaboration partner, Celgene, will be unable to successfully complete the clinical development of the Company’s
compounds, that the Company or Celgene may be delayed in initiating, enrolling or completing any clinical trials, and that
the Company's compounds will not receive regulatory approval or become commercially successful products. These and
other risks and uncertainties are identified under the heading "Risk Factors" included in the Company's most recent
Annual Report on Form 10-K, and other filings that the Company has made and may make with the SEC in the future.
THE FORWARD-LOOKING STATEMENTS CONTAINED IN THIS PRESENTATION ARE BASED ON MANAGEMENT’S CURRENT
views, plans, estimates, assumptions and projections with respect to future events, and the Company does not undertake
and specifically disclaims any obligation to update any forward-looking statements.

3
Our mission
is to transform the lives
of patients with serious
and rare diseases

MUSCLE
PULMONARY
Harnessing the Power of TGF-Beta Biology
4
TGF-Beta
HEMATOLOGY
Strategic Focus in Three Therapeutic Areas
Growth
Repair
Differentiation
C E L L U L A R :
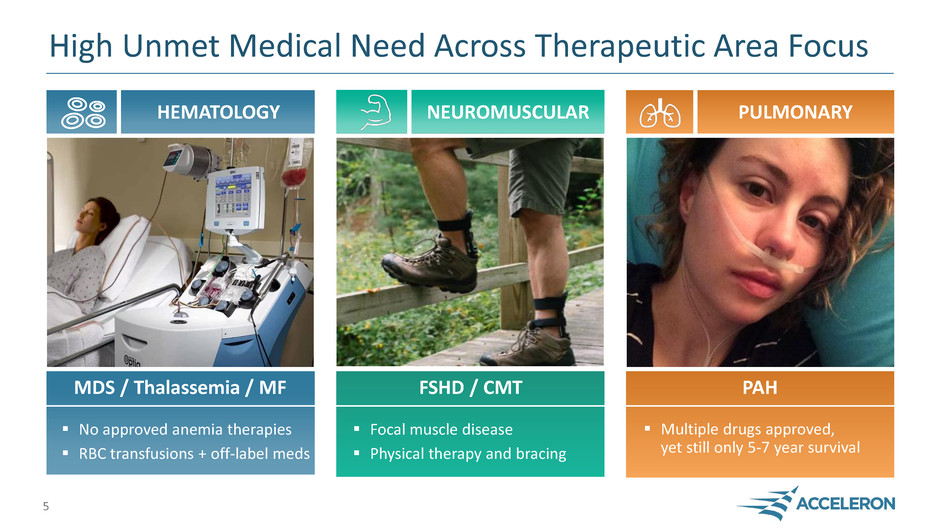
High Unmet Medical Need Across Therapeutic Area Focus
HEMATOLOGY NEUROMUSCULAR PULMONARY
MDS / Thalassemia / MF FSHD / CMT PAH
No approved anemia therapies
RBC transfusions + off-label meds
Focal muscle disease
Physical therapy and bracing
Multiple drugs approved,
yet still only 5-7 year survival
5

Our Vision
6
Leader in neuromuscular and
pulmonary
Complement in-house research
portfolio success with BD efforts
to drive therapeutic area leadership
Drive life-cycle management
Potential multi-billion $ asset
Leader in chronic anemia
HEMATOLOGY
Develop first-in/best-in class assets
NEUROMUSCULAR
PULMONARY
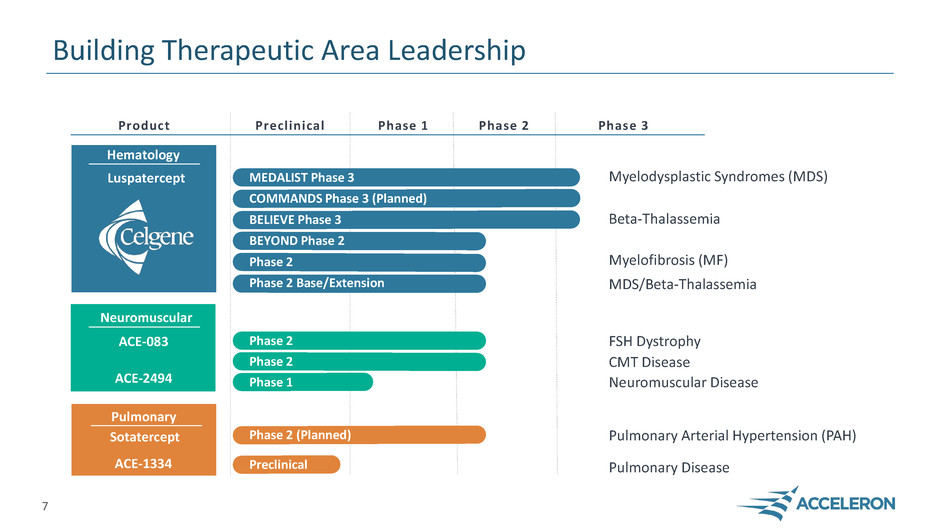
Building Therapeutic Area Leadership
Phase 1
Myelodysplastic Syndromes (MDS)
Beta-Thalassemia
FSH Dystrophy
Neuromuscular Disease
Preclinical Phase 2 Phase 3 Product
Hematology
Luspatercept
Sotatercept
ACE-2494
Neuromuscular
ACE-083
MEDALIST Phase 3
COMMANDS Phase 3 (Planned)
BELIEVE Phase 3
Phase 2 Base/Extension
Myelofibrosis (MF) Phase 2
BEYOND Phase 2
7
Phase 2 CMT Disease
Phase 1
Phase 2
MDS/Beta-Thalassemia
Sotatercept Phase 2 Study (Planned) Pulmonary Arterial Hypertension (PAH)
Pulmonary
ACE-1334 Pulmonary Disease Preclinical
Phase 2 (Planned)

Hematology: Luspatercept

Chronic Anemia Due to Rare Blood Disorders
BETA-THALASSEMIA
Genetic hemoglobin mutation
MYELOFIBROSIS
Fibrotic bone marrow disease
LOWER-RISK MDS
Bone marrow failure disorder
CHRONIC
ANEMIA
9
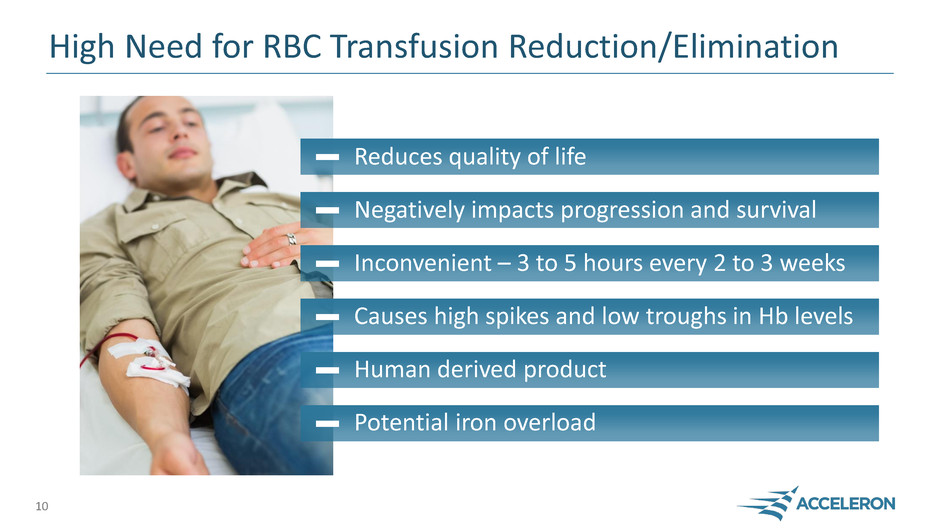
High Need for RBC Transfusion Reduction/Elimination
10
Reduces quality of life
Negatively impacts progression and survival
Inconvenient – 3 to 5 hours every 2 to 3 weeks
Causes high spikes and low troughs in Hb levels
Human derived product
Potential iron overload

Increase and maintain ↑ hemoglobin levels
Reduce or eliminate RBC transfusion burden
LUSPATERCEPT
TREATMENT GOALS:
11

Non-EPO Driven/Ineffective Erythropoiesis Chronic Anemias
12
EPO: erythropoietin
Erythropoiesis-stimulating agent
Erythroid maturation agent (EMA)
Luspatercept-responsive
1 2 3 4 5 6 7
EPO-dependent
ESA
Luspatercept
Hemoglobin (Hb) production
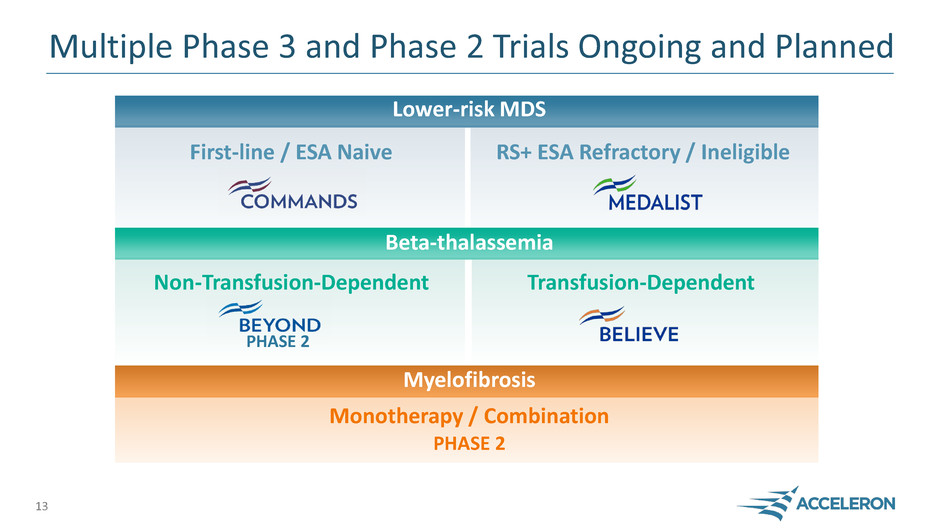
Multiple Phase 3 and Phase 2 Trials Ongoing and Planned
13
Lower-risk MDS
PHASE 2
Beta-thalassemia
Myelofibrosis
First-line / ESA Naive RS+ ESA Refractory / Ineligible
Non-Transfusion-Dependent Transfusion-Dependent
Monotherapy / Combination
PHASE 2

MDS Trials Supported by Luspatercept Phase 2 Results
14
Lower-risk MDS
First-line / ESA Naïve RS+ ESA Refractory / Ineligible
55%1 39%2
RBC Transfusion Independence
1. ASH 2017: ESA naïve LR MDS patients, RBC-TI: 17/31 or 55%
2. ASH 2015: RS+ LR MDS patients, RBC-TI: 12/31 or 39%
RBC Transfusion Independence
Phase 2 Results

Beta-Thalassemia Trials Supported by Luspatercept Phase 2 Results
15
Beta-thalassemia
Non-Transfusion-Dependent Transfusion-Dependent
52%1 41%2
Increase in Mean Hb ≥ 1.5 g/dL
1. EHA 2017: NTD patients, ≥ 1.5 g/dL, 16/31 or 52%, over a 12-week period on treatment versus baseline
2. EHA 2017: TD patients, 12/29 or 41%, in weeks 13-24 fixed interval compared to 12 weeks pre-treatment
RBC Transfusion Reduction ≥ 33%
PHASE 2
Phase 2 Results

MF Trial Supported by Sotatercept Phase 2 MD Anderson IST Results
16
Myelofibrosis
Monotherapy
39%1 30%2
Anemia Response
1. ASH 2017: sotatercept, 7/18 or 39% of evaluable patients
2. ASH 2017: sotatercept, 3/10 or 30% of evaluable patients
Combination Therapy
Anemia Response
Phase 2 Results

>40K PATIENTS
MDS (RS+) ESA
Experienced/Ineligible
NTD Beta-Thalassemia
>20K PATIENTS
Myelofibrosis
>15K PATIENTS
>20K PATIENTS
TD Beta-Thalassemia
MDS First-line
>20K PATIENTS
ADDRESSABLE
US/EU PATIENT
POPULATION
POTENTIAL
NEW INDICATION
>115K
Building a Potential Multi-Billion Dollar Anemia Brand
17

Long-Term Durability of Response (Ongoing Phase 2 Trials)
18
MDS
MF
3
YEARS
2
YEARS
1
YEAR
Median1
19 Months
Median2
14.2 Months
Median3
-thal
1. ASH 2017: Mutational Profile and Analysis of Lower-Risk Myelodysplastic Syndromes (MDS) Patients Treated with Luspatercept: Phase 2 PACE-MDS Study
2. EHA 2017: Luspatercept Increases Hemoglobin and Decreases Transfusion Burden in Adults With Beta-Thalassemia
3. ASH 2017: Sotatercept (ACE-011) Alone and with Ruxolitinib in Patients with MPN-associated Myelofibrosis (MF) and Anemia, 7/18 monotherapy

Collaborating with the Leader in Hematology
19
PROGRAM COSTS
100% Celgene funded
WORLDWIDE ROYALTY
Low- to mid-20%
MILESTONES
$185M outstanding
CO-PROMOTE
North America
Celebrating
10 years
of partnership

Neuromuscular Disease
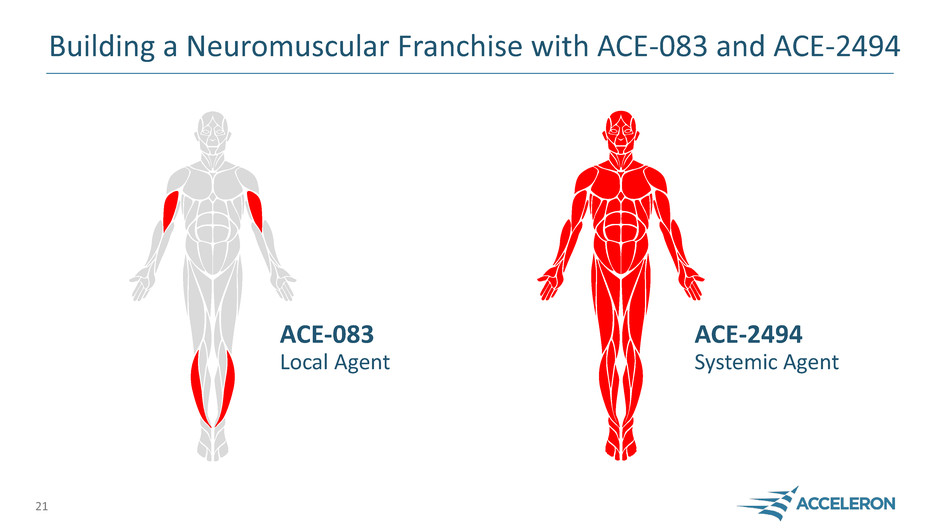
ACE-083
Local Agent
Building a Neuromuscular Franchise with ACE-083 and ACE-2494
21
ACE-2494
Systemic Agent

Administered into target muscle
for concentrated effect
ACE-083: A Locally-Acting Myostatin+ Therapy to Target Focal
Muscle Disease
22
FSHD
MYOPATHY
20,000 US patients
CMT
NEUROPATHY
> 100,000 US patients

Preliminary Results for Phase 2 FSHD Study - Part 1, Cohorts 1
and 2 Pooled
23
N=11 N=12 %
Ch
an
ge
in
T
o
tal
M
u
sc
le
V
o
lu
m
e
Tibialis Anterior Biceps Brachii
12.6% 13.2%
0
5
10
15
20
A
b
sol
u
te
Ch
an
ge
in
F
at
F
rac
tio
n
Tibialis Anterior Biceps Brachii
-5.3%
-0.6%
-10
-5
0
5
N=11 N=12
Safety Summary
No serious adverse events, a majority of adverse events were injection-site related and grades 1-2
One patient with related grade 3 non-serious adverse event of “lower leg intramuscular swelling”
MRI 3 weeks after last dose
Pulmonary Disease

Building a Pulmonary Platform Starting with PAH
25
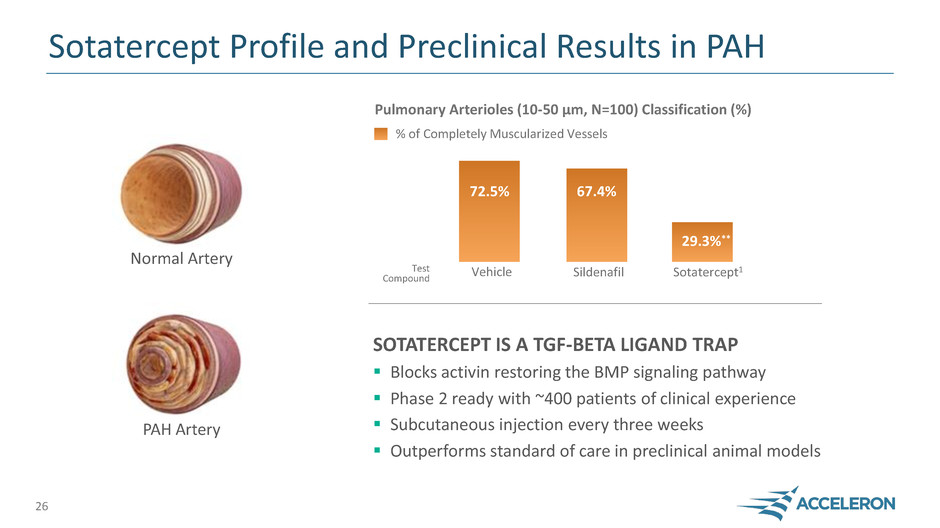
Sotatercept Profile and Preclinical Results in PAH
SOTATERCEPT IS A TGF-BETA LIGAND TRAP
Blocks activin restoring the BMP signaling pathway
Phase 2 ready with ~400 patients of clinical experience
Subcutaneous injection every three weeks
Outperforms standard of care in preclinical animal models
PAH Artery
Normal Artery
Test
Compound
72.5% 67.4%
29.3%**
Pulmonary Arterioles (10-50 µm, N=100) Classification (%)
% of Completely Muscularized Vessels
Vehicle Sildenafil Sotatercept1
26

Where Could Sotatercept Fit in Treatment Paradigm?
27
Endothelin Receptor
Antagonists (ERAs)
PDE-5 Inhibitors
Prostacyclin
Standard of Care Options
MOA: Vasodilation
+
+
+
Goal: Significantly Extend Survival and Quality of Life with Disease Modifying
Combination
sGC Stimulators + SOTATERCEPT
MOA: Vascular Remodeling/
Disease Modifying
Sequential or
Immediate
Combination

Upcoming Corporate Priorities
HEMATOLOGY
Luspatercept
– MEDALIST and BELIEVE Phase 3 trial top-line results expected in mid-2018
– Initiate the COMMANDS Phase 3 trial in 1H 2018
NEUROMUSCULAR
ACE-083
– FSHD and CMT Part 1 of Phase 2 trial preliminary results from all dose-escalation cohorts in 2H 2018
– Initiate FSHD Part 2 of Phase 2 trial Q2 2018
– Initiate CMT Part 2 of Phase 2 trial YE 2018
ACE-2494
– Phase 1 healthy volunteer trial results in 1H 2019
PULMONARY
Sotatercept
– Initiate Phase 2 trial in PAH in 1H 2018
28

Our Commitment to Late-Stage Milestones
29
