Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - INSMED Inc | a18-2170_1ex99d1.htm |
| 8-K - 8-K - INSMED Inc | a18-2170_18k.htm |
Safe Harbor Statement 2 This presentation contains forward looking statements. "Forward-looking statements," as that term is defined in the Private Securities Litigation Reform Act of 1995, are statements that are not historical facts and involve a number of risks and uncertainties. Words herein such as "may," "will," "should," "could," "would," "expects," "plans," "anticipates," "believes," "estimates," "projects," "predicts," "intends," "potential," "continues," and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) may identify forward-looking statements. The forward-looking statements in this press release are based upon the Company’s current expectations and beliefs, and involve known and unknown risks, uncertainties and other factors, which may cause the Company’s actual results, performance and achievements and the timing of certain events to differ materially from the results, performance, achievements or timing discussed, projected, anticipated or indicated in any forward-looking statements. Such factors include, among others: risks that the full six-month data from the CONVERT study or subsequent data from the remainder of the study’s treatment and off-treatment phases will not be consistent with the top-line six-month results of the study; uncertainties in the research and development of our existing product candidates, including due to delays in data readouts, such as the full data from the CONVERT study, patient enrollment and retention or failure of our preclinical studies or clinical trials to satisfy pre-established endpoints, including secondary endpoints in the CONVERT study and endpoints in the CONVERT extension study (the 312 study); risks that subsequent data from the 312 study will not be consistent with the interim results of the study; failure to obtain, or delays in obtaining, regulatory approval from the FDA, Japan’s Ministry of Health, Labour and Welfare, the European Medicines Agency, and other regulatory authorities for our product candidates or their delivery devices, such as the eFlow Nebulizer System, including due to insufficient clinical data, selection of endpoints that are not satisfactory to regulators, complexity in the review process for combination products or inadequate or delayed data from a human factors study required for US regulatory approval; failure to maintain regulatory approval for our product candidates, if received, due to a failure to satisfy post-approval regulatory requirements, such as the submission of sufficient data from confirmatory clinical studies; safety and efficacy concerns related to our product candidates; lack of experience in conducting and managing preclinical development activities and clinical trials necessary for regulatory approval, including the regulatory filing and review process; failure to comply with extensive post-approval regulatory requirements or imposition of significant post-approval restrictions on our product candidates by regulators; uncertainties in the rate and degree of market acceptance of product candidates, if approved; inability to create an effective direct sales and marketing infrastructure or to partner with third parties that offer such an infrastructure for distribution of our product candidates, if approved; inaccuracies in our estimates of the size of the potential markets for our product candidates or limitations by regulators on the proposed treatment population for our product candidates; failure of third parties on which we are dependent to conduct our clinical trials, to manufacture sufficient quantities of our product candidates for clinical or commercial needs, including our raw materials suppliers, or to comply with our agreements or laws and regulations that impact our business; inaccurate estimates regarding our future capital requirements, including those necessary to fund our ongoing clinical development, regulatory and commercialization efforts as well as milestone payments or royalties owed to third parties; failure to develop, or to license for development, additional product candidates, including a failure to attract experienced third-party collaborators; uncertainties in the timing, scope and rate of reimbursement for our product candidates; changes in laws and regulations applicable to our business and failure to comply with such laws and regulations; inability to repay our existing indebtedness or to obtain additional capital when needed; failure to obtain, protect and enforce our patents and other intellectual property and costs associated with litigation or other proceedings related to such matters; restrictions imposed on us by license agreements that are critical for our product development, including our license agreements with PARI Pharma GmbH and AstraZeneca AB, and failure to comply with our obligations under such agreements; competitive developments affecting our product candidates and potential exclusivity related thereto; the cost and potential reputational damage resulting from litigation to which we are a party, including, without limitation, the class action lawsuit pending against us; loss of key personnel; and lack of experience operating internationally. For additional information about the risks and uncertainties that may affect our business, please see the factors discussed in Item 1A, "Risk Factors," in the Company’s Report on Form 10-Q for the quarter ending September 30, 2017. The Company cautions readers not to place undue reliance on any such forward-looking statements, which speak only as of the date of this presentation. The Company disclaims any obligation, except as specifically required by law and the rules of the Securities and Exchange Commission, to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements.

3 Agenda Review interim positive data from Phase 3 INS-212 and INS-312 studies Regulatory and pre-commercial update 2018 Priorities

Treatment of NTM Lung Disease Represents A Significant Unmet Need Current antibiotic regimens are inadequate in treating NTM disease, with no known approved therapies in US and EU 4 1 2 3 4 5 Globally prevalent and growing at approximately 8% annually in the US Up to 33% mortality rate for patients with NTM caused by MAC within 5 years of diagnosis Significant economic burden Declining lung function
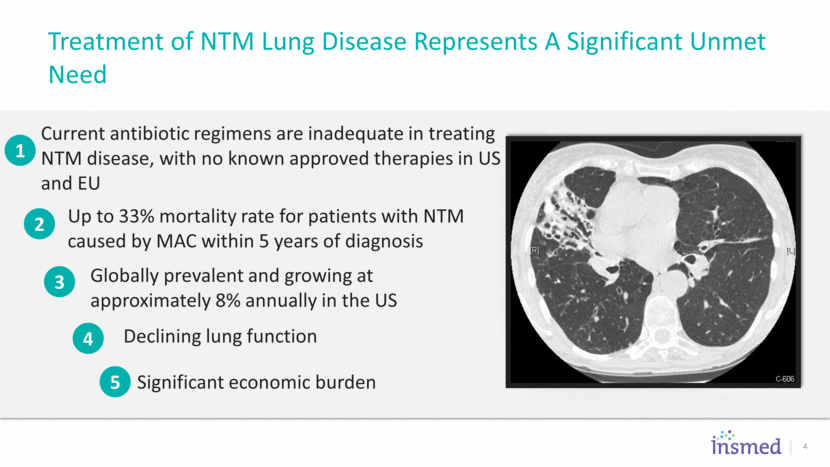
ALIS once daily + Guideline Based Therapy 5 Adult Patients with NTM (MAC) Lung Disease Failing at least 6 months of a multi-drug regimen KEY INCLUSION CRITERIA Guideline Based Therapy R Screening period Month-6 sputum culture PRIMARY ENDPOINT Culture Conversion at 6 months INS-212 non-converters INS-312 ALIS once daily + Guideline Based Therapy Guideline Based Therapy Baseline through Month 6 Up to Month 16 12 months off all treatment FULL APPROVAL ENDPOINT 3 Months Off All Treatment INS-212
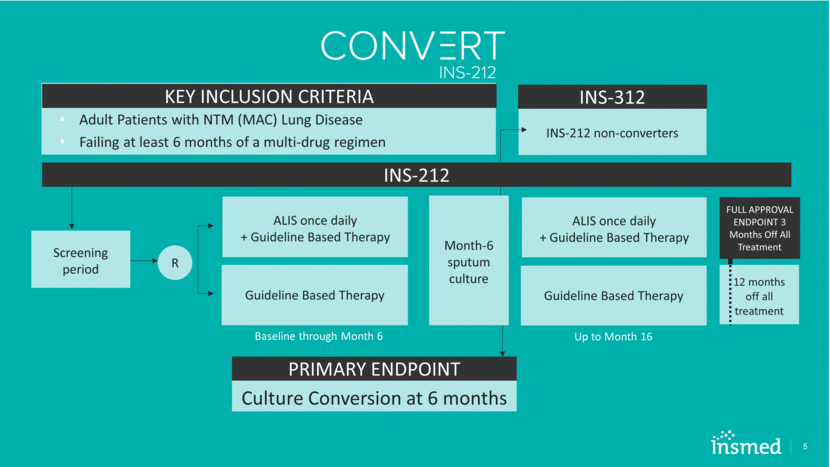
Top-line Data Indicates INS-212 Study Met Primary Endpoint 6 Percentage of Patients with Sputum Culture CONVERSION (3 Consecutive Negative Sputum Cultures) GBT = Guideline Based Therapy Study was 90% powered to show a 15% difference between the treatment arms, assuming that 20% on ALIS + GBT would convert vs. 5% on GBT alone. p <0.0001
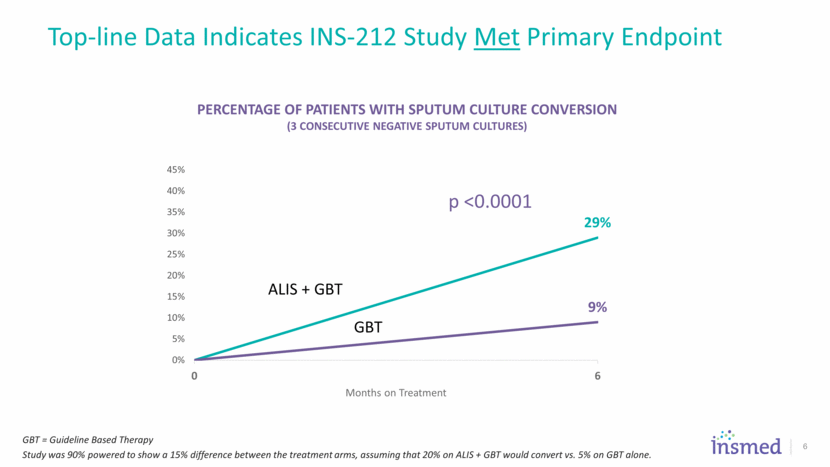
ALIS once daily + Guideline Based Therapy 7 Adult Patients with NTM (MAC) Lung Disease Failing at least 6 months of a multi-drug regimen KEY INCLUSION CRITERIA Guideline Based Therapy R Screening period Month-6 sputum culture PRIMARY ENDPOINT Culture Conversion at 6 months INS-212 non-converters 90 patients from GBT Alone Arm 73 patients from GBT + ALIS Arm INS-312 ALIS once daily + Guideline Based Therapy Guideline Based Therapy Baseline through Month 6 Up to Month 16 12 months off all treatment FULL APPROVAL ENDPOINT 3 Months Off All Treatment INS-212
8 Today’s Interim Data Provide Early Answers to Three Key Questions Are the interim results from INS-312 consistent with what we observed in top line results from INS-212? Do additional patients achieve culture conversion with continued treatment with ALIS beyond six months? Are the effects of ALIS durable?
9 Question 1 Are the interim results from INS-312 consistent with what we observed in top line results from INS-212?

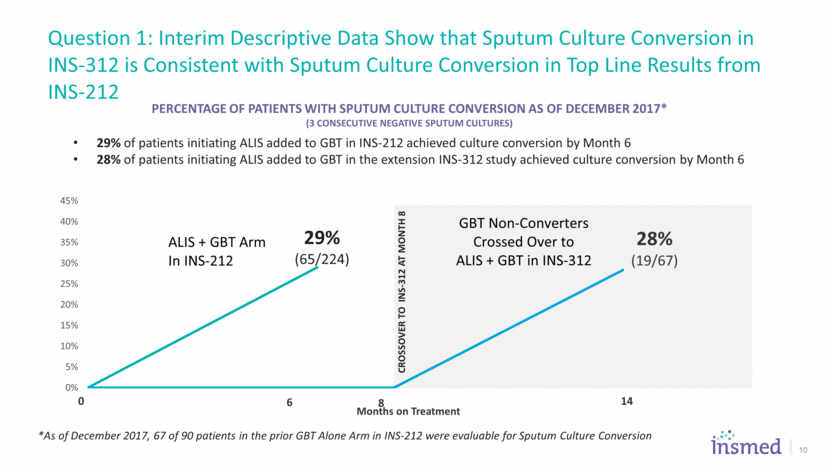
Question 1: Interim Descriptive Data Show that Sputum Culture Conversion in INS-312 is Consistent with Sputum Culture Conversion in Top Line Results from INS-212 10 Percentage of Patients with Sputum Culture CONVERSION AS OF DECEMBER 2017* (3 Consecutive Negative Sputum Cultures) 29% of patients initiating ALIS added to GBT in INS-212 achieved culture conversion by Month 6 28% of patients initiating ALIS added to GBT in the extension INS-312 study achieved culture conversion by Month 6 29% (65/224) *As of December 2017, 67 of 90 patients in the prior GBT Alone Arm in INS-212 were evaluable for Sputum Culture Conversion 28% (19/67) 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% Months on Treatment ALIS + GBT Arm In INS - 212 GBT Non - Converters Crossed Over to ALIS + GBT in INS - 312 CROSSOVER TO INS - 312 AT MONTH 8 0 6 8 14
11 Question 2 Do additional patients achieve culture conversion with continued treatment with ALIS beyond six months?

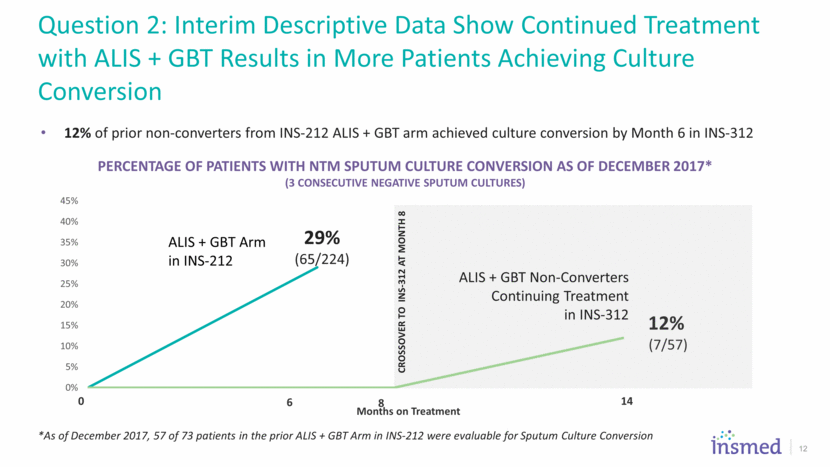
12 29% (65/224) *As of December 2017, 57 of 73 patients in the prior ALIS + GBT Arm in INS-212 were evaluable for Sputum Culture Conversion 12% of prior non-converters from INS-212 ALIS + GBT arm achieved culture conversion by Month 6 in INS-312 Question 2: Interim Descriptive Data Show Continued Treatment with ALIS + GBT Results in More Patients Achieving Culture Conversion ALIS + GBT Non-Converters Continuing Treatment in INS-312 Percentage of Patients with NTM Sputum Culture CONVERSION AS OF DECEMBER 2017* (3 Consecutive Negative Sputum Cultures) 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% Months on Treatment ALIS + GBT Arm in INS - 212 CROSSOVER TO INS - 312 AT MONTH 8 0 6 8 14 12% (7/57)
13 Question 3 Are the effects of ALIS durable? Durability is the percent of patients who complete the treatment cycle and remain culture negative 3 months off all therapy. Durability three months off all treatment is the endpoint required to support full approval.
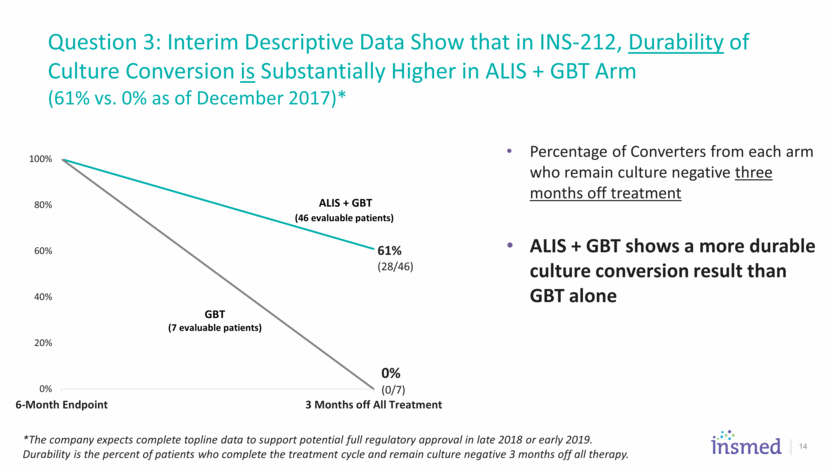
Question 3: Interim Descriptive Data Show that in INS-212, Durability of Culture Conversion is Substantially Higher in ALIS + GBT Arm (61% vs. 0% as of December 2017)* 14 Percentage of Converters from each arm who remain culture negative three months off treatment ALIS + GBT shows a more durable culture conversion result than GBT alone *The company expects complete topline data to support potential full regulatory approval in late 2018 or early 2019. Durability is the percent of patients who complete the treatment cycle and remain culture negative 3 months off all therapy. 61% (28/46) 0% (0/7) 0% 20% 40% 60% 80% 100% 6-Month Endpoint 3 Months off All Treatment ALIS + GBT (46 evaluable patients) GBT (7 evaluable patients)
INS-212 and INS-312 Safety Summary No Change in Overall Safety Assessment for ALIS Based on Interim Data INS-212 Adverse events, consistent with those seen with use of inhaled antibiotics, more frequent in ALIS + GBT arm Serious treatment emergent adverse events were similar between treatment arms No distinctions between treatment arms of hearing loss or renal impairment At this point in time, the overall drop out rate is 18% INS-312 Serious treatment emergent adverse events similar to those in INS-212 As of this point in time, the drop out rate is 24% 15

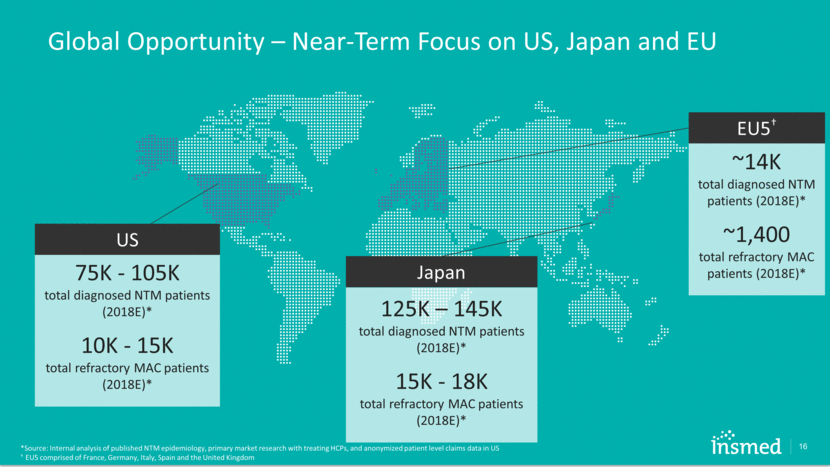
Global Opportunity – Near-Term Focus on US, Japan and EU 16 *Source: Internal analysis of published NTM epidemiology, primary market research with treating HCPs, and anonymized patient level claims data in US † EU5 comprised of France, Germany, Italy, Spain and the United Kingdom 125K – 145K total diagnosed NTM patients (2018E)* 15K - 18K total refractory MAC patients (2018E)* Japan ~14K total diagnosed NTM patients (2018E)* ~1,400 total refractory MAC patients (2018E)* EU5† 75K - 105K total diagnosed NTM patients (2018E)* 10K - 15K total refractory MAC patients (2018E)* US
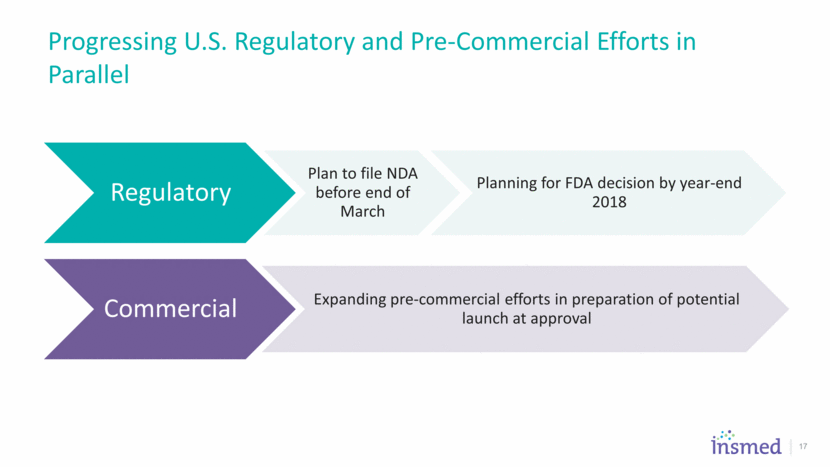
Progressing U.S. Regulatory and Pre-Commercial Efforts in Parallel 17 Regulatory Plan to file NDA before end of March Planning for FDA decision by year-end 2018 Commercial Expanding pre-commercial efforts in preparation of potential launch at approval
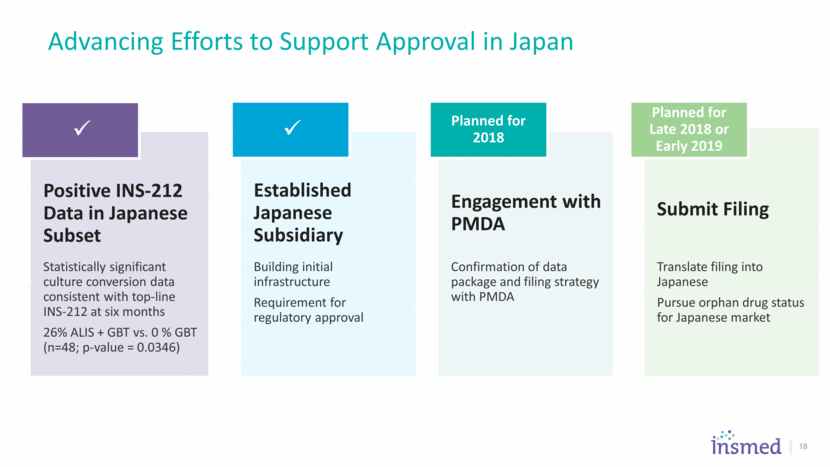
Advancing Efforts to Support Approval in Japan Established Japanese Subsidiary Building initial infrastructure Requirement for regulatory approval Engagement with PMDA Confirmation of data package and filing strategy with PMDA Planned for 2018 Submit Filing Translate filing into Japanese Pursue orphan drug status for Japanese market Planned for Late 2018 or Early 2019 Positive INS-212 Data in Japanese Subset Statistically significant culture conversion data consistent with top-line INS-212 at six months 26% ALIS + GBT vs. 0 % GBT (n=48; p-value = 0.0346) 18
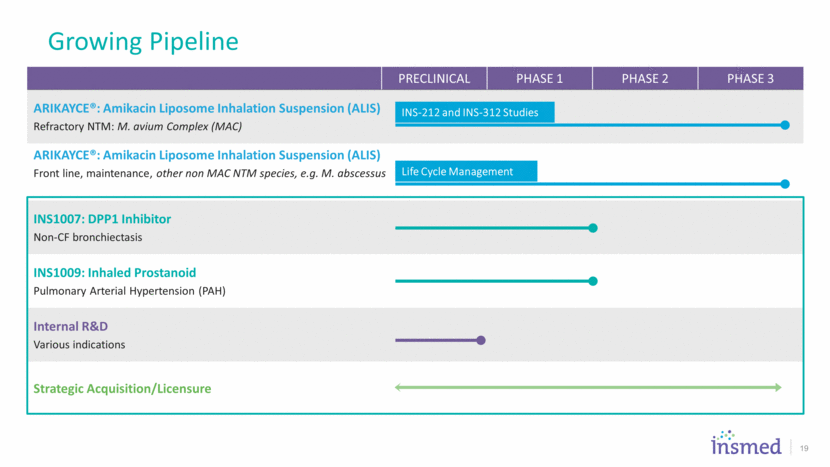
PRECLINICAL PHASE 1 PHASE 2 PHASE 3 ARIKAYCE®: Amikacin Liposome Inhalation Suspension (ALIS) Refractory NTM: M. avium Complex (MAC) ARIKAYCE®: Amikacin Liposome Inhalation Suspension (ALIS) Front line, maintenance, other non MAC NTM species, e.g. M. abscessus INS1007: DPP1 Inhibitor Non-CF bronchiectasis INS1009: Inhaled Prostanoid Pulmonary Arterial Hypertension (PAH) Internal R&D Various indications Strategic Acquisition/Licensure Growing Pipeline 19 Life Cycle Management INS-212 and INS-312 Studies
2018 Priorities 20 PREPARE FOR U.S. LAUNCH GEOGRAPHIC EXPANSION INVESTMENT IN PIPELINE INVEST IN MANUFACTURING CAPACITY
[LOGO]


