Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - STEMLINE THERAPEUTICS INC | a17-28312_3ex99d1.htm |
| 8-K - 8-K - STEMLINE THERAPEUTICS INC | a17-28312_38k.htm |
Stemline Therapeutics, Inc. NASDAQ: STML Analyst and Investor Meeting ASH 2017 December 11, 2017

Forward-Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements.’’ In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “potentially,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations, including our projections regarding our regulatory and commercial timelines for SL-401, our clinical pipeline and market opportunities. You should read carefully our “Special Cautionary Notice Regarding Forward-Looking Statements” and the factors described in the “Risk Factors” sections of our reports on Form 10-K and Form 10-Q filed with the Securities and Exchange Commission to better understand the risks and uncertainties inherent in our business. 2

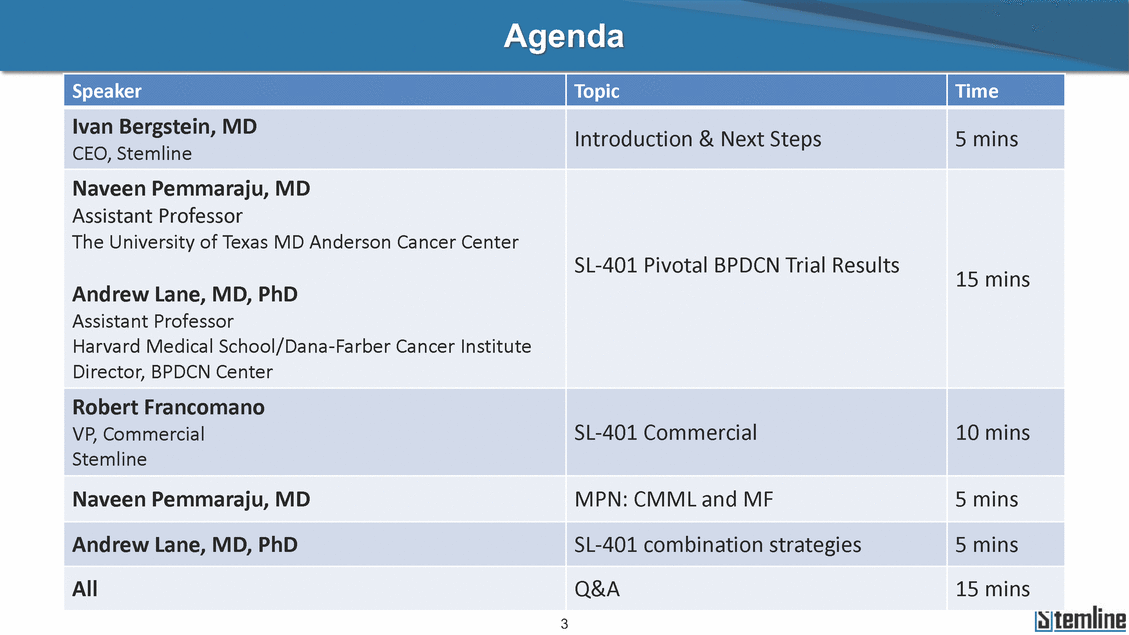
Agenda Introduction & Next Steps 5 mins CEO, Stemline Andrew Lane, MD, PhD 3 Ivan Bergstein, MD Naveen Pemmaraju, MD Assistant Professor The University of Texas MD Anderson Cancer Center SL-401 Pivotal BPDCN Trial Results15 mins Assistant Professor Harvard Medical School/Dana-Farber Cancer Institute Director, BPDCN Center Robert Francomano VP, CommercialSL-401 Commercial10 mins Stemline Naveen Pemmaraju, MDMPN: CMML and MF5 mins Andrew Lane, MD, PhDSL-401 combination strategies5 mins AllQ&A15 mins
SL-401: Introduction & Next Steps

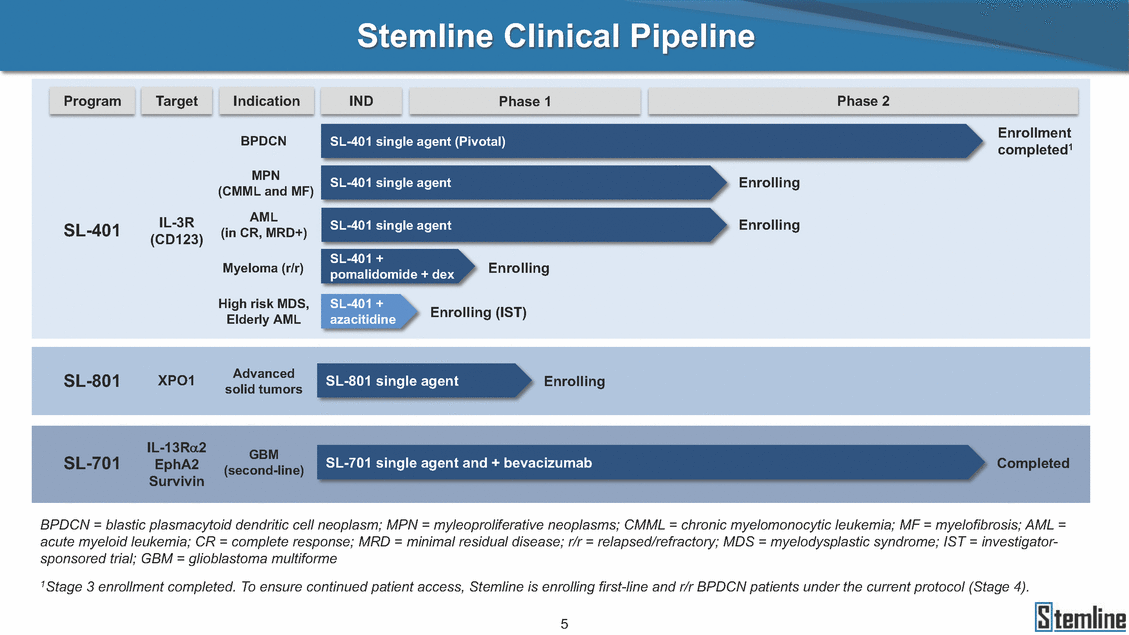
Stemline Clinical Pipeline completed1 (CMML and MF) (in CR, MRD+) (CD123) Enrolling Myeloma (r/r) pomalidomide + dex Enrolling (IST) Elderly AML azacitidine solid tumors GBM SL-701 SL-701 single agent and + bevacizumab Completed EphA2 Survivin BPDCN = blastic plasmacytoid dendritic cell neoplasm; MPN = myleoproliferative neoplasms; CMML = chronic myelomonocytic leukemia; MF = myelofibrosis; AML = acute myeloid leukemia; CR = complete response; MRD = minimal residual disease; r/r = relapsed/refractory; MDS = myelodysplastic syndrome; IST = investigator-sponsored trial; GBM = glioblastoma multiforme 1Stage 3 enrollment completed. To ensure continued patient access, Stemline is enrolling first-line and r/r BPDCN patients under the current protocol (Stage 4). 5 IL-13Ra2 (second-line) SL-801XPO1AdvancedSL-801 single agentEnrolling BPDCNSL-401 single agent (Pivotal)Enrollment MPNSL-401 single agentEnrolling SL-401IL-3RAMLSL-401 single agentEnrolling SL-401 + High risk MDS,SL-401 + Indication Phase 1 IND Phase 2 Target Program
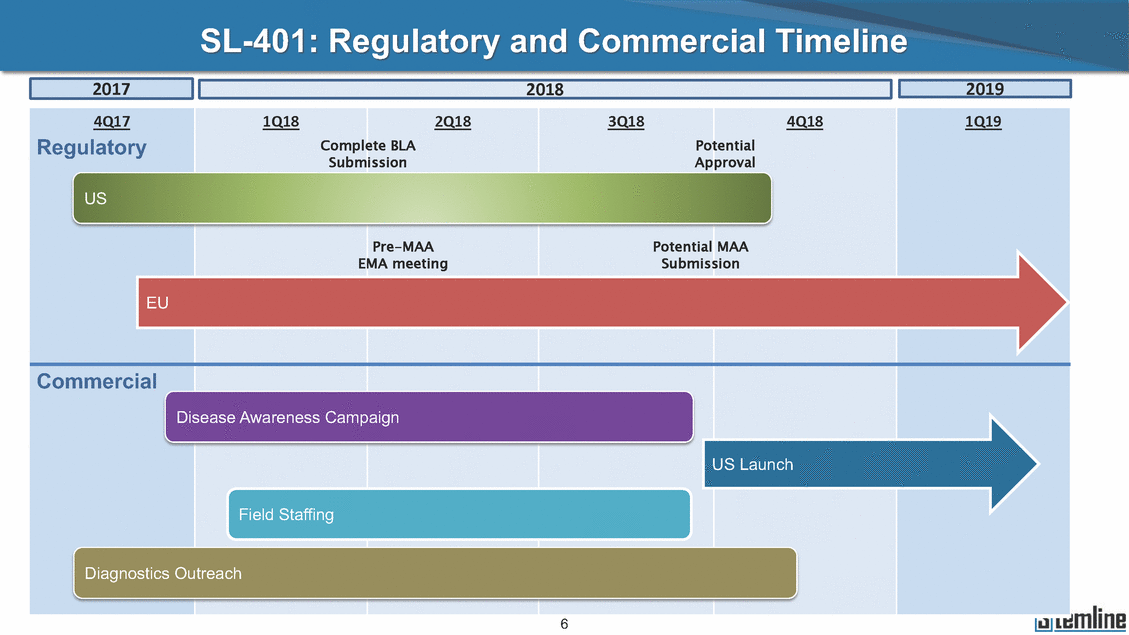
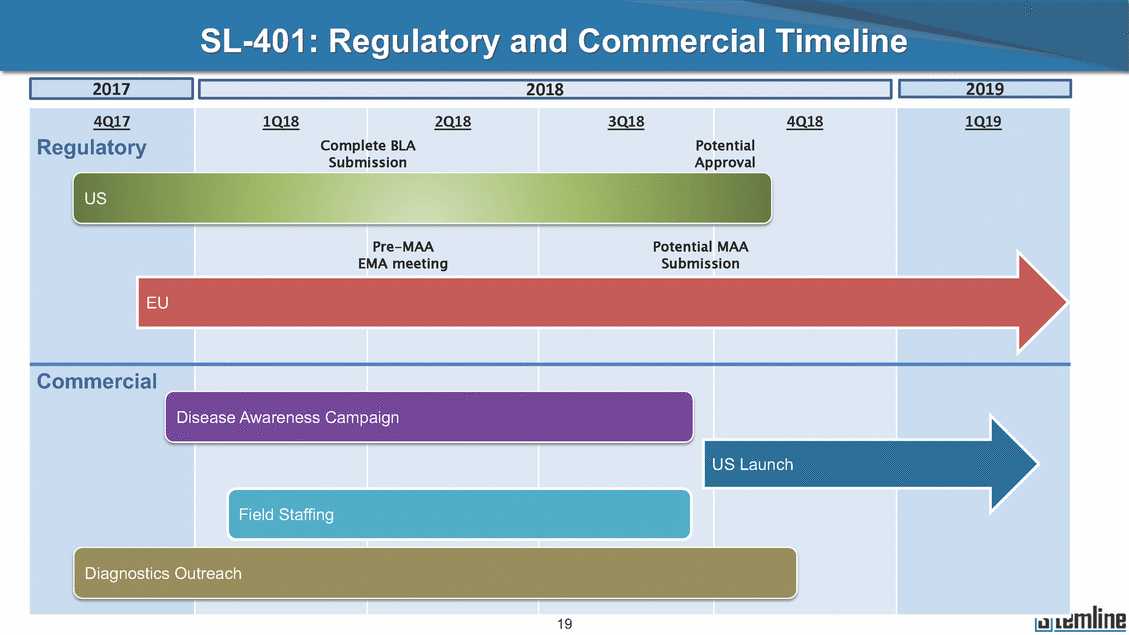
SL-401: Regulatory and Commercial Timeline Submission Approval Pre-MAA EMA meeting Potential MAA Submission EU Commercial US Launch 6 4Q17 Regulatory 1Q18 2Q18 3Q18 4Q18 Complete BLA Potential 1Q19 2019 2018 2017
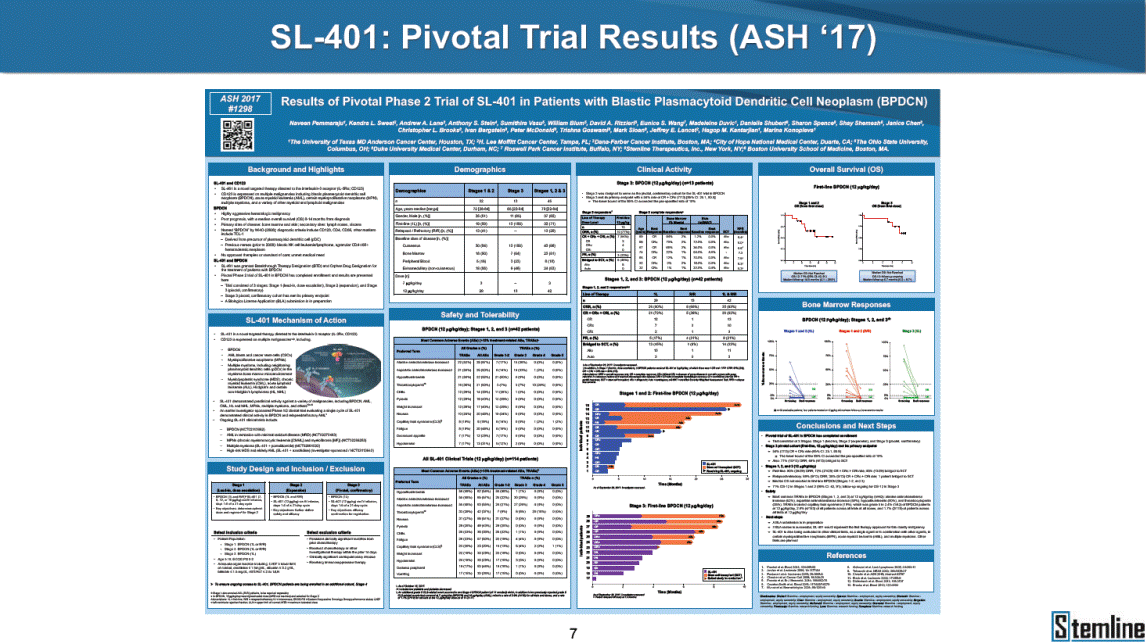
SL-401: Pivotal Trial Results (ASH ‘17) #1298 Naveen Pemmaraju1, Kendra L. Sweet2, Andrew A. Lane3, Anthony S. Stein4, Sumithira Vasu5, William Blum5, David A. Rizzieri6, Eunice S. Wang7, Madeleine Duvic1, Danielle Shubert8, Sharon Spence8, Shay Shemesh8, Janice Chen8, Background and Highlights St • Stage 3 met its primary e neoplasms (MPN), 1L BPDCN, N=13 O(SSta(gfero1-m2, f1i2rsutgd/kog/sdeay) ) O(SSta(fgreo3m, 1f2irusgt/kdgo/dsaey)) BPDCN Age, years median [range] 72 [28-84] 65 [22-84] 70 [22-84] 13 (29) 0% 0% 0% 0% Allo Allo Allo - 6.4+ 6.0+ 5.5+ 7.3 0.50 3 0% 3% Allo Allo 7.5+ 8.3+ 8 (18) Allo 6 0 5 10 15 20 25 30 rug Designation for Time (months) Time (months) Extramedullary (non-cutaneous) 18 (56) 6 (46) 24 (53) 22 CRc 1% 1% 22.0% 0.4% Allo Auto 0 5.3+ the treatment of patients with BPDCN OS-12: 71% (95% CI: 42, 91) OS-12: follow-up ongoing Res n 2 (1L) Stages 1 Common Adverse Events (AEs) (>15% treatment-related AEs, TRAEs)ƾ 100% 100% 100% 14 (33%) TRAEs All AEs Grade 1-2 Grade 3 Grade 4 Grade 5 Auto 3 0 3 75% 75% 75% - Myeloproliferative neoplasms (MPNs) CR + CRc + CRi rate = 33% (1/3). in organs with gross ssment Tool; RFS = relapse 5% CR t response CR 0% CR 0% Screening Best responseScreening Best response 13 CR Allo onclusions and Next Steps u 7 CR primary endpoint rate (95% CI: 25.1, 80.8) -401 • Stages 1, 2, and 3 (12 µg/kg/day) 2 CR Allo Stem cell transplant (SCT) ce As of September 25, 2017. usion, y) Allo • Next steps 0 (0%) 0 (0%) Al mia (AML), and multiple myeloma. Other lanned or R/R) ences 0 (0%) 0 (0%) SL-Stem 4. Chauhan et al. Cancer Cell 2009; 16:309-23 17 Exited study in remission ¥ 11. Black et al. Leukemia 2003; 17:155-9 5. Frovola et al. Br J Haematol. 2014; 166:862-74 12. Diefenbach et al. Blood 2011; 118:3737 0 2 4 6 8 10 7. Munoz ll trials and doses, and a rate ¥ Patient relapsed off study employment, ployment, equity ownership; McDonald: Stemline - employment, equity ownership; Goswami: Stemline - employment, equity mmaraju 7 Individual patients Individual patients % Bone marrow blasts Survival Probability Survival Probability ASH 2017 Results of Pivotal Phase 2 Trial of SL-401 in Patients with Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) Christopher L. Brooks8, Ivan Bergstein8, Peter McDonald8, Trishna Goswami8, Mark Sloan9, Jeffrey E. Lancet2, Hagop M. Kantarjian 1The University of Texas MD Anderson Cancer Center, Houston, TX; 2H. Lee Moffitt Cancer Center, Tampa, FL; 3Dana-Farber Cancer Institute, Boston, MA; 4City of Hope Na Columbus, OH; 6Duke University Medical Center, Durham, NC; 7 Roswell Park Cancer Institute, Buffalo, NY; 8Stemline Therapeutics, Inc., New York, NY;9 Bos 1, Marina Konopleva1 tional Medical Center, Duarte, CA; 5The Ohio State University, ton University School of Medicine, Boston, MA. SL-401 and CD123 • SL-401 is a novel tar • CD123 is expressed neoplasm (BPDCN), multiple myeloma, a geted therapy directed to the interleukin-3 receptor (IL-3 on multiple malignancies including blastic plasmacytoid acute myeloid leukemia (AML), certain myeloproliferativ nd a variety of other myeloid and lymphoid malignancies Demographics Clinical Activity age 3: BPDCN (12 µg/kg/day) (n=13 patients) serve as the pivotal, confirmatory cohort for the SL-401 trial in B endpoint with a 54% rate of CR + CRc (7/13) [95% CI: 25.1, 80.8 the 95% CI exceeded the pre-specified rate of 10% PDCN ] Overall Survival (OS) First-line BPDCN (12 µg/kg/day) Rα; CD123) dendritic cell Demographics Stages 1 & 2 Stage 3 Stages 1, 2 & 3 • Stage 3 was designed to SOtav 1L gereall1Saunrvdiv2al BPDCN, N=16 OvSertaallgSeur3vival n 32 13 45 - The lower bound of • Highly aggressive h • Poor prognosis, with • Primary sites of dise • Named “BPDCN” by include TCL-1 - Derived from prec - Previous names ( hematodermic ne • No approved therapi SL-401 and BPDCN • SL-401 was granted ematologic malignancy a median overall survival (OS) 8-14 months from diagno ase: bone marrow and skin; secondary sites: lymph nod WHO (2008); diagnostic criteria include CD123, CD4, C ursor of plasmacytoid dendritic cell (pDC) prior to 2008): blastic NK cell leukemia/lymphoma, agran oplasm es or standard of care; unmet medical need Breakthrough Therapy Designation (BTD) and Orphan D Gender, Male [n, (%)] sis es, viscera First-line (1L) [n, (%)] D56, other markers Relapsed / Refractory (R Baseline sites of disease ular CD4+/56+ Cutaneous Bone Marrow Peripheral Blood 26 (81) 11 (85) 19 (59) 13 (100) /R) [n, (%)] 13 (41) --[n, (%)] 30 (94) 13 (100) 16 (50) 7 (54) 5 (16) 3 (23) Stage 3 respondersɸ Stage 3 complete respondersɸ 37 (82) 32 (71) Line of Therapy Firs Dose Level 12 µ t-line g/kg Bone Marrow Skin (% Blasts) (mSWAT) Age Best Best Bes (years) Response Baseline response Baseline respo t RFS nse SCT (months) 1.00 0.75 1.00 0.75 0.50 0.25 n 1 ORR, n (%) 10 ( CR + CRc + CRi, n (%) 7 (5 CR 43 (96) CRc CRi 23 (51) PR, n (%) 3 (2 Bridged to SCT, n (%) 6 (4 3 77%) 4%) 69 CR 94% 2% 1.2% 0. 68 CRc 70% 2% 72.0% 0. 4 57 CR 66% 2% 35.0% 0. 0 3%) 74 CRc 22% 1% 54.0% 4. 6%) 65 CR 12% 1% 70.0% 0. 32 CRc 2% 2% 18.0% 0. 0.25 0.00 0.00 0 2 4 6 8 10 • Pivotal Phase 2 trial here - Trial consisted of 3 (pivotal, confirm - Stage 3 pivotal, co - A Biologics Licens of SL-401 in BPDCN has completed enrollment and resu 3 stages: Stage 1 (lead-in, dose escalation), Stage 2 (exp atory) nfirmatory cohort has met its primary endpoint e Application (BLA) submission is in preparation lts are presented ansion), and Stage Dose [n] 7 µg/kg/day 12 µg/kg/day 3 --29 13 3 42 Stages 1 Stages 1, 2, and 3 respond Line of Therapy n ORR, n (%) CR + CRc + CRi, n (%) CR CRc CRi PR, n (%) Bridged to SCT, n (%) Allo , 2, and 3: BPDCN (12 µg/kg/day) (n=42 patie ersɸ,ɣ 1L R/R 29 13 26 (90%)9 (69%) 21 (72%)5 (38%) 12 1 7 3 2 1 5 (17%)4 (31%) 13 (45%)1 (8%) 10 1 nts) 1L & R/R Median OS: Not Reached Median OS: Not Reached Me dian follow up 14.0 months (4.1 – 28.6+) Bone Marrow BPDCN (12 µg/kg/day); S M tages and 2 (R edian follow up 6.7 months (0.2 – 8.7+) ponses 1, 2, and 3ȸ /R) Stage 3 (1L) 42 35 (83%) 26 (62%) 13 10 3 9 (21%) St ages 1 and BPDC Safety and Tolerability N (12 µg/kg/day); Stages 1, 2, and 3 (n=42 patie nts) SL • SL-401 is a novel targ • CD123 is expressed - BPDCN - AML blasts and c -401 Mechanism of Actio eted therapy directed to the interleukin-3 receptor (IL-3Rα; on multiple malignancies1-9, including: ancer stem cells (CSCs) CD123) Most Preferred Term All Grades n (%) TRAEs n (%) 11 - Multiple myelom plasmacytoid de myeloma bone m - Myelodysplastic myeloid leukemi leukemia (ALL), non-Hodgkin’s ly • SL-401 demonstrated CML, HL and NHL, M • An earlier investigator demonstrated clinical a, including neighboring ndritic cells (pDCs) in the arrow microenvironment syndrome (MDS), chronic a (CML), acute lymphoid Hodgkin’s and certain mphomas (HL, NHL) preclinical activity against a variety of malignancies, includi PNs, multiple myeloma, and others10-13 -sponsored Phase 1/2 clinical trial evaluating a single cycle activity in BPDCN and relapsed/refractory AML1 Alanine aminotransferase Aspartate aminotransferas Hypoalbuminaemia ThrombocytopeniaЖ Chills ng BPDCN, AML, Pyrexia Weight increased of SL-401 Nausea increased 22 (52%) 28 (67%) 7 (17%) 15 (36%) e increased 21 (50%) 25 (60%) 6 (14%) 14 (33%) 21 (50%) 22 (52%) 21 (50%) 0 (0%) 16 (38%) 21 (50%) 3 (7%) 3 (7%) 12 (29%) 14 (33%) 11 (26%) 1 (2%) 12 (29%) 18 (43%) 12 (29%) 0 (0%) 12 (29%) 17 (40%) 12 (29%) 0 (0%) 10 (24%) 20 (48%) 10 (24%) 0 (0%) 0 (0%) 0 (0%) ɸ As of September 25, 2017. Investig 1 (2%) 0 (0%) ɣ In addition, in Stage 1 (lead-in, dose Abbreviations: ORR = overall respon 0 (0%) 0 (0%) reduction in cutaneous lesions and re partial response; SCT = stem cell tran 10 (24%) 0 (0%) free survival. 0 (0%) 0 (0%) S 0 (0%) 0 (0%) 0 (0%) 0 (0%) 16 CR 15 CR 0 (0%) 0 (0%) 14 CRc ator-assessed. escalation), 3 BPDCN patients received SL-401 at 7µg/kg/day, of which there was 1 C se rate; CR = complete response; CRc (clinical CR) = absence of gross disease in non-sk sidual microscopic skin disease; CRi = CR with incomplete hematologic recovery; CI = splant; Allo = allogeneic; Auto = autologous; mSWAT = modified Severity-Weighted Asse tages 1 and 2: First-line BPDCN (12 µg/kg/da R and 1 PR: ORR: 67% (2/3),50% 50% 50% confidence interval; PR = y) Auto u Auto 25% 5% 0% ȸ Sc reening Bes 25% PR 5% 25% PR PR n=38 eval uable patients; four patients treated at 12 µg/kg did not have foll ow-up bon e marrow results. • Ongoing SL-401 clinic - BPDCN (NCT021 - AML in remission - MPNs (chronic m - Multiple myeloma - High-risk MDS an al trials include: 13982) with minimal residual disease (MRD) (NCT02270463) yelomonocytic leukemia [CMML] and myelofibrosis [MF]) (N (SL-401 + pomalidomide) (NCT02661022) d elderly AML (SL-401 + azacitidine) (Investigator-sponsore CT02268253) d / NCT03113643) Capillary leak syndrome (C Fatigue Decreased appetite Hypotension All SL LS)ђ8 (19%) 8 (19%) 6 (14%) 0 (0%) 8 (19%) 20 (48%) 8 (19%) 0 (0%) 7 (17%) 12 (29%) 7 (17%) 0 (0%) 7 (17%) 13 (31%) 5 (12%) 2 (5%) -401 Clinical Trials (12 µg/kg/day) (n=114 patien 1 (2%) 1 (2%) 12 PR 0 (0%) 0 (0%) 11 CR 10 CR 0 (0%) 0 (0%) 9 CR 8 CRc 0 (0%) 0 (0%) 6 CRi 5 CRc ts) 4 PR 3 CRi Allo Allo Auto • Pivotal - Tri • Stage - 54 trial of al consi 3 pivot % (7/13 C SL-401 in sted of 3 St al cohort (fir ) CR + CRc BPDCN has completed enrollmen ages: Stage 1 (lead-in), Stage 2 (ex st-line, 12 µg/kg/day) met its t pansio n), and Stage 3 (pivotal, confirmatory) - Als o The l o: 77% ower bound (10/13) OR of the 95% CI exceeded the pre-sp R; 46% (6/13) bridged to SCT ecified rate of 10% Study D esign and Inclusion / Excl usion 10 15 Time (Months) Investigator-assessed. Stage 3: First-line BPDCN (12 µ SL Re 20 g/kg/da iving SL-401, ongoing-Fir 25 30 - Re - M - 71 • Safety - M inc (3 st-line: 9 lapsed/ edian O % OS-1 ost com rease ( 8%). TR 0% (26/29) refractory: S not reach 2 in Stages mon TRAEs 52%), aspa AEs includ ORR; 72% (21/29) CR + CRc + C 69% (9/13) ORR; 38% (5/13) CR + ed in first-line BPDCN (Stages 1-2, 1 and 2 (95% CI: 42, 91); follow-up in BPDCN (Stages 1, 2, and 3) at rtate aminotransferase increase (50 ed capillary leak syndrome (19%), Ri rate; CRc + and 3) ongoin 12 ug/kg %), hyp which w 45% (13/29) bridged to SCT CRi rate; 1 patient bridged to SCT g for OS-12 in Stage 3 /day (n=42): alanine aminotransferase oalbuminemia (50%), and thrombocytopenia as grade 5 in 2.4% (1/42) of BPDCN patients Most Co Preferred Term mmon Adverse Events (AEs) (>15% treatment-related AEs, TRA All Grades n (%) TRAEs TRAEs All AEs Grade 1-2 Grade 3 Es)ƾ n (%) Grade 4 Grade 5 1 CR 0 5 Stage 1 (Lead-in, dose escalati on) (Pivot Stage 3 al, confirmatory) Hypoalbuminaemia Alanine aminotransferase Aspartate aminotransferas 56 (49%) 62 (54%) 55 (48%) 1 (1%) increased 55 (48%) 65 (57%) 25 (22%) 30 (26%) e increased 55 (48%) 63 (55%) 24 (21%) 27 (24%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 4 (4%) 0 (0%) • BPDCN (1L and R/R)ǂ SL-40 9, 12, or 16 µg/kg) via IV inf days 1-5 of a 21-day cycle 1 (7, • BPDCN (1L and R/R) • BPDCN (1 • SL-401 (12 µg/kg) via IV infusion, • SL-401 (1 days 1-5 of a 21-day cycle days 1-5 o L) 2 µg/kg) via IV infusion, f a 21-day cycle • Key objectives: determine o dose and regimen♦ for Stag Select inclusion criter • Patient Population: - Stage 1: BPDCN (1L - Stage 2: BPDCN (1L - Stage 3: BPDCN (1L • Age ≥ 18; ECOG PS 0-2 • Adequate organ function of normal, creatinine ≤ 1. bilirubin ≤ 1.5 mg/dL, AS ptimal • Key objectives: further define • Key object e 2 safety and efficacy confirmati ia Select exclusion crit • Persistent clinically signi or R/R) prior chemotherapy • Received chemotherapy investigational therapy w ) • Clinically significant card • Receiving immunosuppr including: LVEF ≥ lower limit 5mg/dL, albumin ≥ 3.2 g/dL, T/ALT ≤ 2.5x ULN ives: efficacy on for registration ThrombocytopeniaЖ Nausea Pyrexia eria Chills ficant toxicities from Fatigue or other Capillary leak syndrome (C ithin the prior 14 days Weight increased iopulmonary disease essive therapy Hypotension Oedema peripheral Vomiting 33 (29%) 42 (37%) 7 (6%) 6 (5%) 31 (27%) 58 (51%) 31 (27%) 0 (0%) 29 (25%) 49 (43%) 29 (25%) 0 (0%) 26 (23%) 38 (33%) 25 (22%) 1 (1%) 26 (23%) 57 (50%) 22 (19%) 4 (4%) LS)ђ23 (20%) 23 (20%) 15 (13%) 5 (4%) 22 (19%) 33 (29%) 22 (19%) 0 (0%) 20 (18%) 33 (29%) 17 (15%) 3 (3%) 19 (17%) 50 (44%) 18 (16%) 1 (1%) 17 (15%) 30 (26%) 17 (15%) 0 (0%) 20 (18%) 0 (0%) 29 CRc 28 CR 0 (0%) 0 (0%) 27 CR 0 (0%) 0 (0%) 26 CR 25 CRc 0 (0%) 0 (0%) 24 CRc 2 (2%) 1 (1%) 23 CRc 0 (0%) 0 (0%) 22 PR 21 PR 20 SD 0 (0%) 0 (0%) 19 SD 0 (0%) 0 (0%) 18 PR Allo Allo Allo at all 12 µg/kg trials at /day, 2.6% 12 µg/kg/d (4/153) of all patients across all tria ay ls at all doses, and 1.7% (2/119) of patients across Allo lo - A - If - SL ce tri BLA sub BLA revi -401 is rtain my als are p mission is i ew is succe also being eloprolifera n preparation ssful, SL-401 would represent the evaluated in other clinical trials, as tive neoplasms (MPN), acute myel Refer first ther a single oid leuke apy approved for this deadly malignancy agent or in combination with other agents, in 401 cell trans plant (SCT) 1. Franke 2. Jordan 3. Pardan l et al. Bl et al. Le ani et al. ood 2014; 12 ukemia 2000 Leukemia 2 4:385-928. A ; 14:1177-849. T 015; 29:1605-810. C ldinucci ehranchi hristie et et al. Leuk Lymphoma 2005; 46:303-11 et al. NEJM 2010; 363:1025-37 al. ASH 2015; Abstract #3797 Ø To ensure ongoing access ǂ Stage 1 also enrolled AML (R/R) pat ♦ In BPDCN, 12 µg/kg/day was highe Abbreviations: 1L = first-line; R/R = rel = left ventricular ejection fraction; ULN to SL-401, BPDCN patients are being enrolled in an additional ients, to be reported separately st tested dose (MTD not reached) and selected for Stage 2 apsed/refractory; IV = intravenous; ECOG PS = Eastern Cooperative Oncology G = upper limit of normal; MTD = maximum tolerated dose cohort, Stage 4 ƾ As of October 18, 2017. Ж Includes low platelets and plate ђ An additional grade 5 CLS-relate CLS-related events that occurred roup performance status; LVEF of 1.7% (2/119) for all trials at the lets decreased. d event occurred in one Stage 4 BPDCN patient (of 11 enrolled) which, in addition to t at 7 µg/kg/day (BPDCN) and 16 µg/kg/day (AML), reflects a rate of 2.6% (4/153) for a 12 µg/kg/day dose as of 11-21-17. 6. Cousta n-Smith et al. Ha Shubert: equity own et al. Blood 2 ematologica Stemline - em ership; Chen : Stemline-rese 011; 117:6267-627613. B 2001; 86-1261-9 ployment, equity ownership; Spence: Stemlin : Stemline - employment, equity ownership; arch funding; Lane: Stemline-research fundi rooks et e - empl Brooks: St ng; Konop al. Blood 2013; 122:4104 oyment, equity ownership; Shemesh: Stemline - emline - employment, equity ownership; Bergstein: leva: Stemline-research funding wo previously reported grade 5 As of September 25, 2017. I nvestigator-assessed.Time (Months) at 7.3 months. Disclosures: Stemline - em ownership; Pe Stage 2 (Expansion)
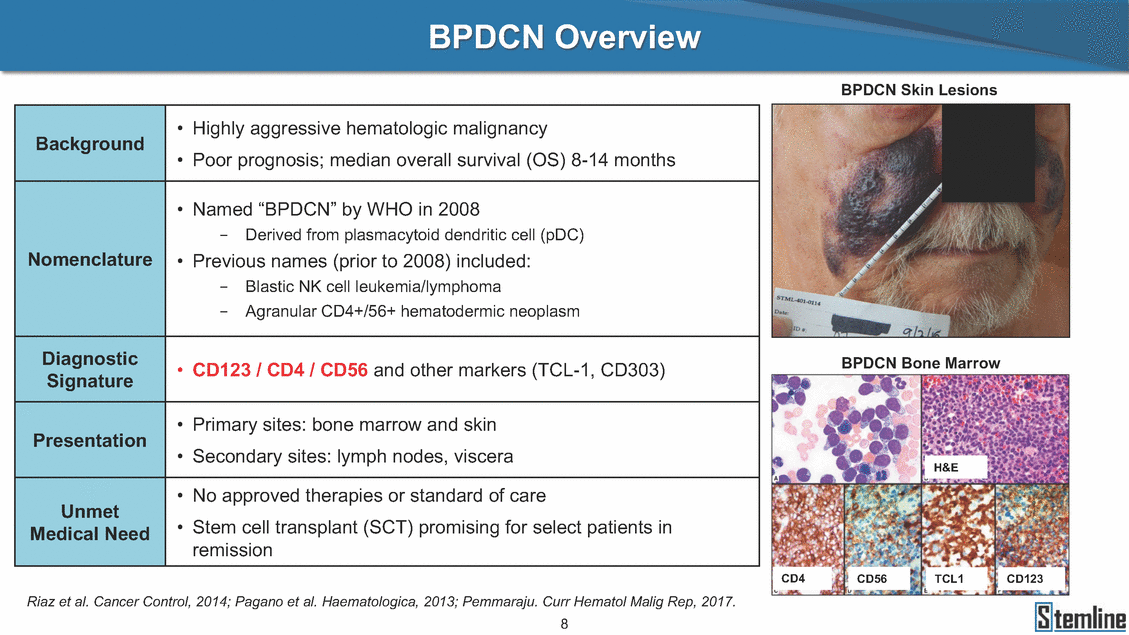
BPDCN Overview BPDCN Skin Lesions • • Highly aggressive hematologic malignancy Poor prognosis; median overall survival (OS) 8-14 months • Named “BPDCN” by WHO in 2008 - Derived from plasmacytoid dendritic cell (pDC) • Previous names (prior to 2008) included: - - Blastic NK cell leukemia/lymphoma Agranular CD4+/56+ hematodermic neoplasm BPDCN Bone Marrow • CD123 / CD4 / CD56 and other markers (TCL-1, CD303) • • Primary sites: bone marrow and skin Secondary sites: lymph nodes, viscera H&E • • No approved therapies or standard of care Stem cell transplant (SCT) promising for select patients in remission CD4 CD56 TCL1 CD123 Riaz et al. Cancer Control, 2014; Pagano et al. Haematologica, 2013; Pemmaraju. Curr Hematol Malig Rep, 2017. 8 Background Nomenclature Diagnostic Signature Presentation Unmet Medical Need
Study Design and Inclusion / Exclusion • • BPDCN (1L and R/R) SL-401 (12 µg/kg) via IV infusion, days 1-5 of a 21-day cycle Key objectives: further define safety and efficacy • • BPDCN (1L) SL-401 (12 µg/kg) via IV infusion, days 1-5 of a 21-day cycle Key objectives: efficacy confirmation for registration BPDCN (1L and R/R)ǂ SL-401 (7, 9, 12, or 16 µg/kg) via IV infusion, days 1-5 of a 21-day cycle Key objectives: determine optimal dose and regimen♦ for Stage 2 • • • • Select inclusion criteria Select exclusion criteria • Patient Population: • Persistent clinically significant toxicities from prior chemotherapy Received chemotherapy or other investigational therapy within the prior 14 days Clinically significant cardiopulmonary disease Receiving immunosuppressive therapy Stage 1: BPDCN (1L or R/R) Stage 2: BPDCN (1L or R/R) Stage 3: BPDCN (1L) - - - • • • • • Age ≥ 18; ECOG PS 0-2 Adequate organ function including: LVEF ≥ lower limit of normal, creatinine ≤ 1.5mg/dL, albumin ≥ 3.2 g/dL, bilirubin ≤ 1.5 mg/dL, AST/ALT ≤ 2.5x ULN Ø To ensure ongoing access to SL-401, BPDCN patients are being enrolled in an additional cohort, Stage 4 ǂ Stage 1 also enrolled AML (R/R) patients, to be reported separately + In BPDCN, 12 µg/kg/day was highest tested dose (MTD not reached) and selected for Stage 2 Abbreviations: 1L = first-line; R/R = relapsed/refractory; IV = intravenous; ECOG PS = Eastern Cooperative Oncology Group performance status; LVEF = left ventricular ejection fraction; ULN = upper limit of normal; MTD = maximum tolerated dose 9 Stage 3 (Pivotal, confirmatory) Stage 1 (Lead-in, dose escalation) Stage 2 (Expansion)
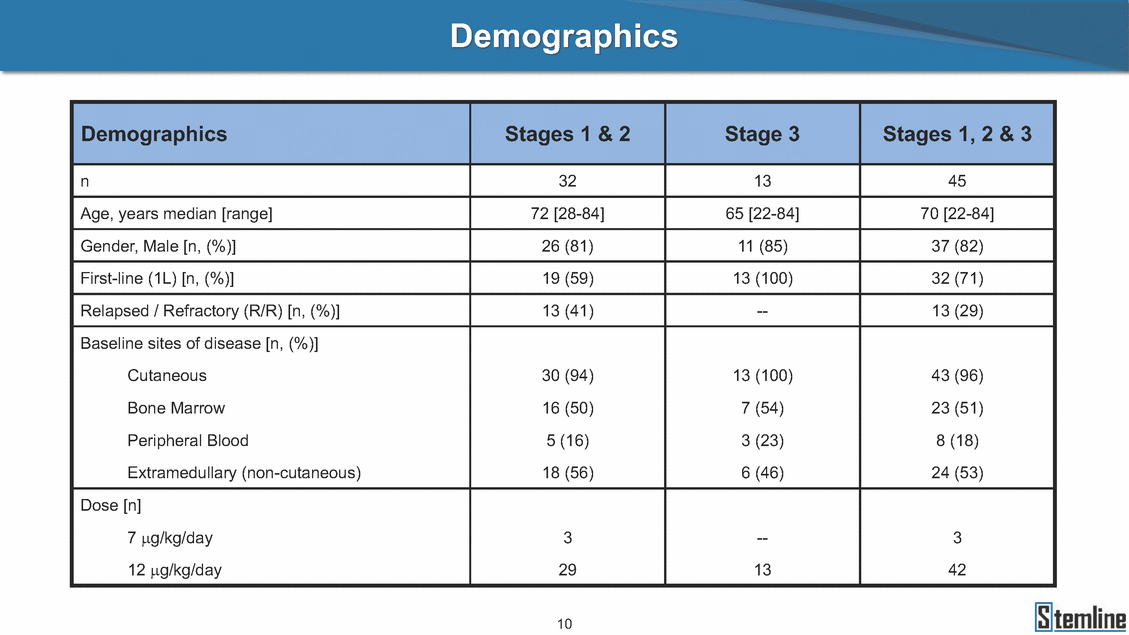
Demographics Demographics Stages 1 & 2 Stage 3 Stages 1, 2 & 3 n 32 13 45 Age, years median [range] 72 [28-84] 65 [22-84] 70 [22-84] Gender, Male [n, (%)] 26 (81) 11 (85) 37 (82) First-line (1L) [n, (%)] 19 (59) 13 (100) 32 (71) Relapsed / Refractory (R/R) [n, (%)] 13 (41) -- 13 (29) Baseline sites of disease [n, (%)] Cutaneous 30 (94) 13 (100) 43 (96) Bone Marrow 16 (50) 7 (54) 23 (51) Peripheral Blood 5 (16) 3 (23) 8 (18) Extramedullary (non-cutaneous) 18 (56) 6 (46) 24 (53) Dose [n] 7 μg/kg/day 3 -- 3 12 μg/kg/day 29 13 42
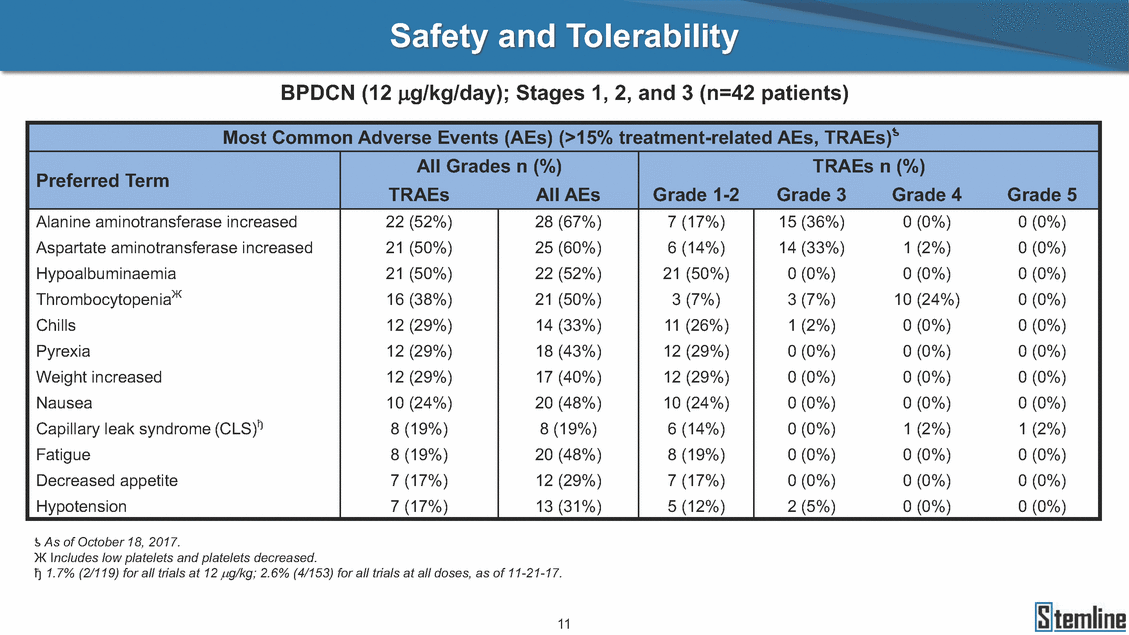
Safety and Tolerability BPDCN (12 µg/kg/day); Stages 1, 2, and 3 (n=42 patients) Preferred Term Alanine aminotransferase increased Aspartate aminotransferase increased Hypoalbuminaemia ThrombocytopeniaЖ Chills Pyrexia Weight increased Nausea Capillary leak syndrome (CLS)ђ Fatigue Decreased appetite Hypotension 22 (52%) 21 (50%) 21 (50%) 16 (38%) 12 (29%) 12 (29%) 12 (29%) 10 (24%) 8 (19%) 8 (19%) 7 (17%) 7 (17%) 28 (67%) 25 (60%) 22 (52%) 21 (50%) 14 (33%) 18 (43%) 17 (40%) 20 (48%) 8 (19%) 20 (48%) 12 (29%) 13 (31%) 7 (17%) 6 (14%) 21 (50%) 3 (7%) 11 (26%) 12 (29%) 12 (29%) 10 (24%) 6 (14%) 8 (19%) 7 (17%) 5 (12%) 15 (36%) 14 (33%) 0 (0%) 3 (7%) 1 (2%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 2 (5%) 0 (0%) 1 (2%) 0 (0%) 10 (24%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 1 (2%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 1 (2%) 0 (0%) 0 (0%) 0 (0%) ƾ As of October 18, 2017. Ж Includes low platelets and platelets decreased. ђ 1.7% (2/119) for all trials at 12 µg/kg; 2.6% (4/153) for all trials at all doses, as of 11-21-17. 11 Most Common Adverse Events (AEs) (>15% treatment-related AEs, TRAEs)ƾ All Grades n (%)TRAEs n (%) TRAEsAll AEsGrade 1-2Grade 3Grade 4Grade 5
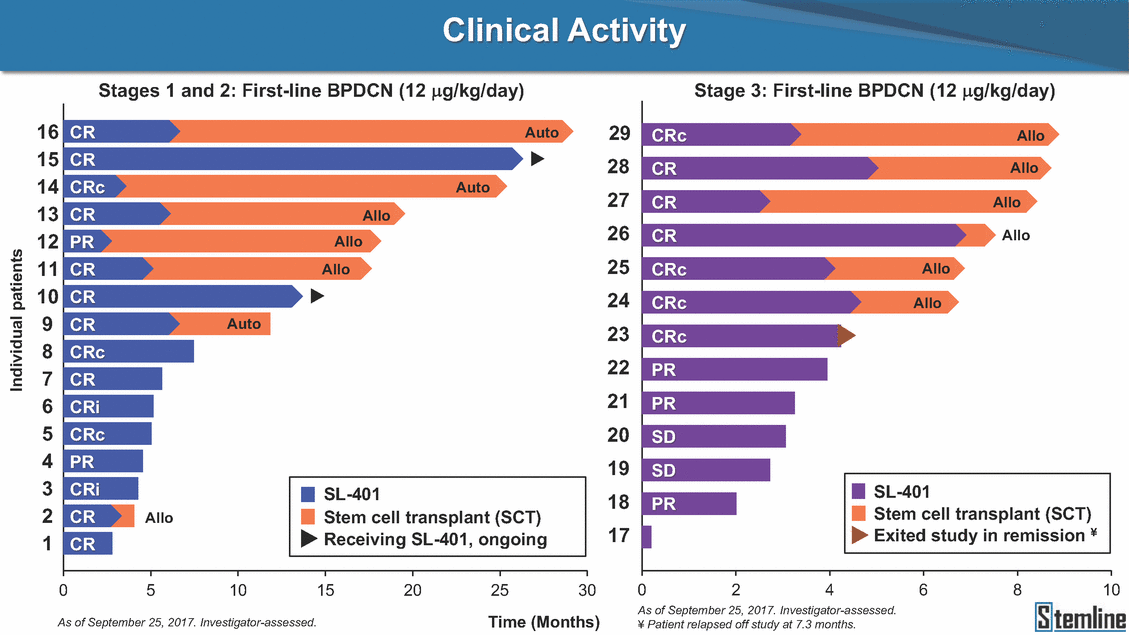
Clinical Activity Stage 3: BPDCN (12 µg/kg/day) (n=13 patients) Stage 3 was designed to serve as the pivotal, confirmatory cohort for the SL-401 trial in BPDCN Stage 3 met its primary endpoint with a 54% rate of CR + CRc (7/13) [95% CI: 25.1, 80.8] • • - The lower bound of the 95% CI exceeded the pre-specified rate of 10% Stage 3 respondersɸ Stage 3 complete respondersɸ n ORR, n (%) CR + CRc + CRi, n (%) CR CRc CRi PR, n (%) Bridged to SCT, n (%) Allo Auto 13 10 (77%) 7 (54%) 3 4 0 3 (23%) 6 (46%) 6 0 69 68 57 74 65 32 22 CR CRc CR CRc CR CRc CRc 94% 70% 66% 22% 12% 2% 1% 2% 2% 2% 1% 1% 2% 1% 1.2% 72.0% 35.0% 54.0% 70.0% 18.0% 22.0% 0.0% 0.0% 0.0% 4.0% 0.0% 0.3% 0.4% Allo Allo Allo - Allo Allo Allo 6.4+ 6.0+ 5.5+ 7.3 7.5+ 8.3+ 5.3+ ɸ As of September 25, 2017. Investigator-assessed. ɣ In addition, in Stage 1 (lead-in, dose escalation), 3 BPDCN patients received SL-401 at 7µg/kg/day, of which there was 1 CR and 1 PR: ORR: 67% (2/3), CR + CRc + CRi rate = 33% (1/3). Abbreviations: ORR = overall response rate; CR = complete response; CRc (clinical CR) = absence of gross disease in non-skin organs with gross reduction in cutaneous lesions and residual microscopic skin disease; CRi = CR with incomplete hematologic recovery; CI = confidence interval; PR = partial response; SCT = stem cell transplant; Allo = allogeneic; Auto = autologous; mSWAT = modified Severity-Weighted Assessment Tool; RFS = relapse free survival. 12 Bone MarrowSkin (% Blasts)(mSWAT) AgeBestBestBestRFS (years)ResponseBaselineresponseBaselineresponseSCT(months) Line of TherapyFirst-line Dose Level12 µg/kg
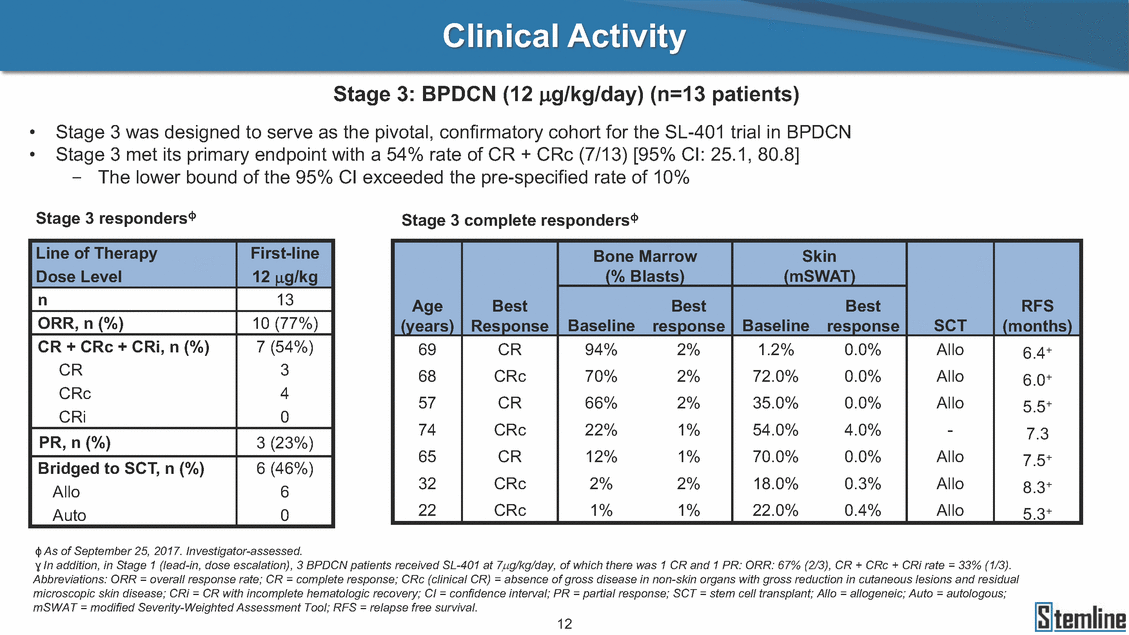
Clinical Activity Stages 1, 2, and Stages 1, 2, and 3 respondersɸ,ɣ 3: BPDCN (12 µg/kg/day) (n=42 patients) n ORR, n (%) CR + CRc + CRi, n (%) CR CRc CRi PR, n (%) Bridged to SCT, n (%) Allo Auto 29 26 (90%) 21 (72%) 12 7 2 5 (17%) 13 (45%) 10 3 13 9 (69%) 5 (38%) 1 3 1 4 (31%) 1 (8%) 1 0 42 35 (83%) 26 (62%) 13 10 3 9 (21%) 14 (33%) 11 3 ɸ As of September 25, 2017. Investigator-assessed. ɣ In addition, in Stage 1 (lead-in, dose escalation), 3 BPDCN patients received SL-401 at 7µg/kg/day, of which there was 1 CR and 1 PR: ORR: 67% (2/3), CR + CRc + CRi rate = 33% (1/3). Abbreviations: ORR = overall response rate; CR = complete response; CRc (clinical CR) = absence of gross disease in non-skin organs with gross reduction in cutaneous lesions and residual microscopic skin disease; CRi = CR with incomplete hematologic recovery; CI = confidence interval; PR = partial response; SCT = stem cell transplant; Allo = allogeneic; Auto = autologous; mSWAT = modified Severity-Weighted Assessment Tool; RFS = relapse free survival. 13 Line of Therapy1LR/R1L & R/R
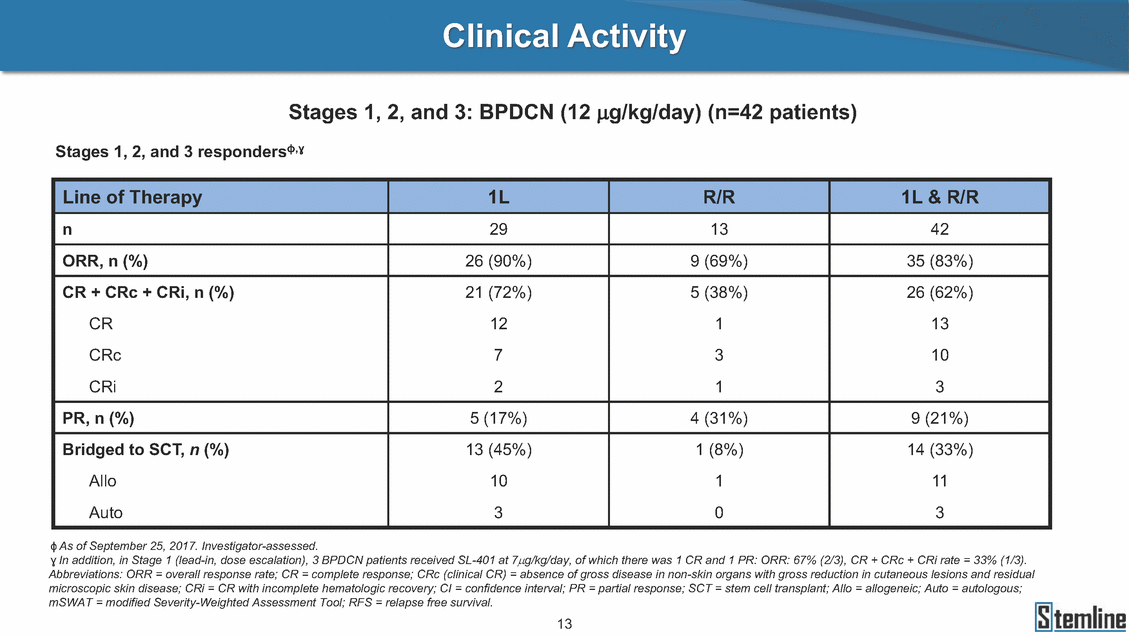
BPDCN (12 JlQ/kg/day); Stages 1, 2, and 3m Stages 1 and 2 {1L) Stages 1 and 2 {RIR) Stage 3 {1L) 100% 100% 100% .en. en 75% 75% 75% .!!! .c 3: 0... 'ca E 50% 50% 50% Q) 1: m0 0 25% PR PR PR 5% 5% CR Best response CR CR 0% 0% 0% Screening Screening Best response Screening Best response cb n=38 evaluable patients; four patients treated at 12 J.!Qikg did not have follow-up bone marrow results. 14 lS1temline 5%
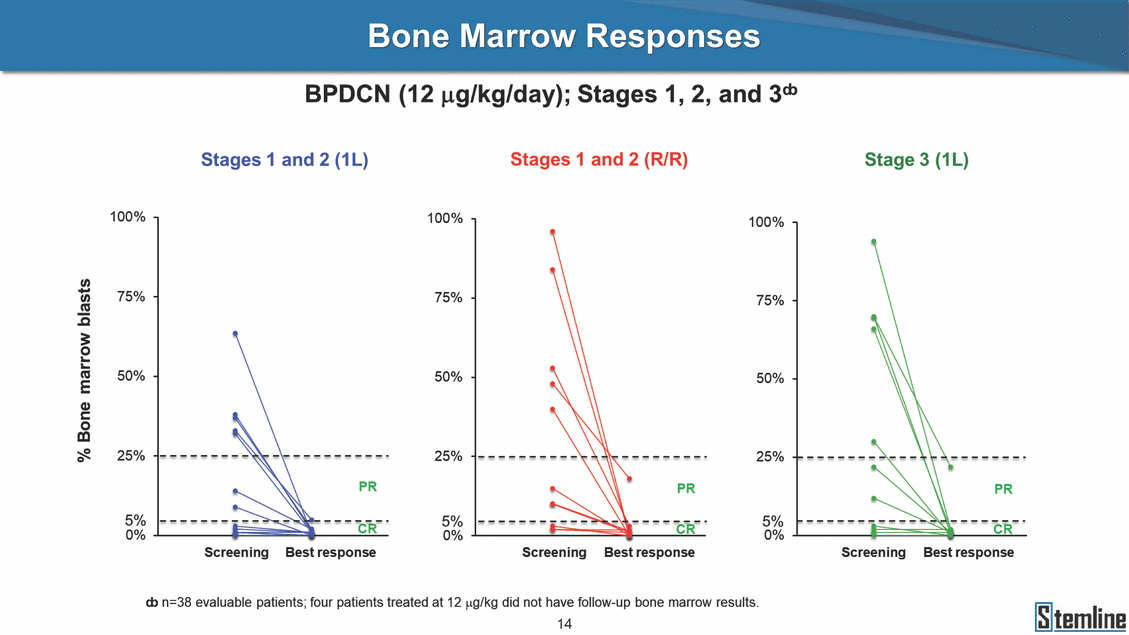
Clinical Activity Stages 1 and 2: First-line BPDCN (12 µg/kg/day) Stage 3: First-line BPDCN (12 µg/kg/day) 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 Allo Allo Allo Allo Allo Allo SL-401 Stem cell transplant (SCT) Exited study in remission ¥ 0 5 10 15 20 25 30 0 2 4 6 8 10 As of September 25, 2017. Investigator-assessed. ¥ Patient relapsed off study at 7.3 months. Time (Months) As of September 25, 2017. Investigator-assessed. Individual patients CR CR CRc CRAllo PRAllo CRAllo CRu CRAuto CRc CR CRi CRc PR CRiSL-401 CRAllo Stem cell Auto 29CRc u28CR Auto 27CR 26CR 25CRc 24CRc 23CRc 22PR 21PR 20SD 19SD 18PR transplant (SCT) CRReceiving SL-401, ongoing 17
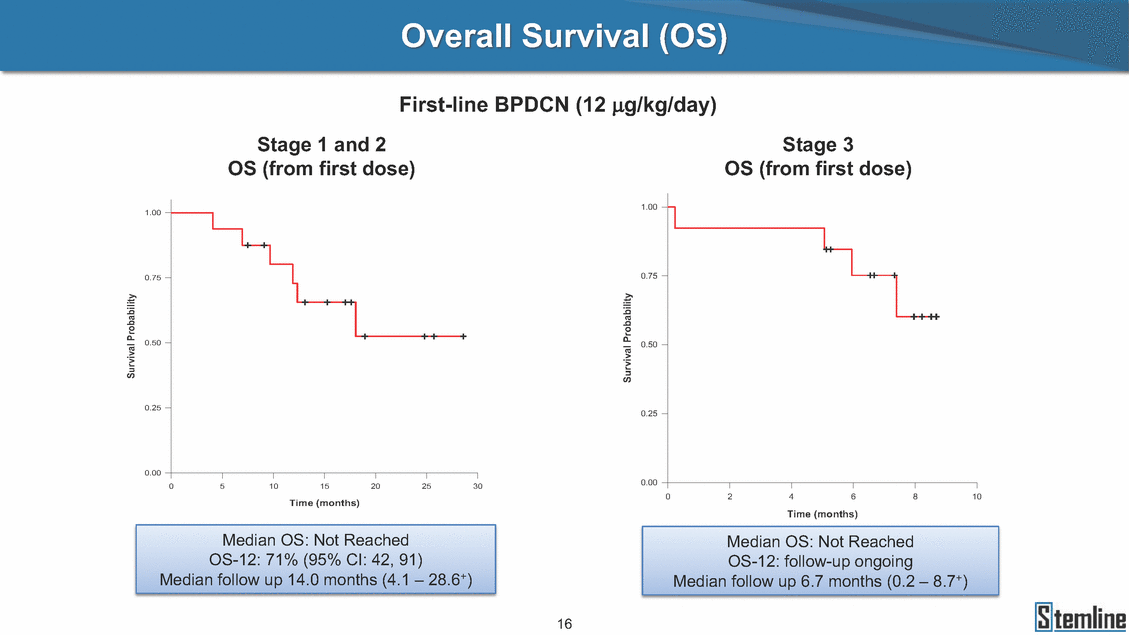
Overall Survival (OS) First-line BPDCN (12 µg/kg/day) Overall Survival Overall Survival 1L SBPtaDgCeN,3N=13 S1tLaBgPeD1CNa,nNd=126 O(SSta(gfero1-m2, 1f2irusgt/kdgo/dsaey)) (Stage 3, 12 ug/kg/day) OS (from first dose) 1.00 1.00 0.75 0.75 0.50 0.50 0.25 0.25 0.00 0.00 0 5 10 15 20 25 30 0 2 4 6 8 10 Time (months) Time (months) Median OS: Not Reached OS-12: 71% (95% CI: 42, 91) Median OS: Not Reached OS-12: follow-up ongoing Median follow up 14.0 months (4.1 – 28.6+) Median follow up 6.7 months (0.2 – 8.7+) 16 Survival Probability Survival Probability
Conclusions and Next Steps • Stage 3 pivotal cohort (first-line, 12 µg/kg/day) met its primary endpoint - 54% (7/13) CR + CRc rate (95% CI: 25.1, 80.8) The lower bound of the 95% CI exceeded the pre-specified rate of 10% o - 77% (10/13) ORR; 46% (6/13) bridged to SCT • Stages 1, 2, and 3 (12 µg/kg/day) - - - - First-line: 90% (26/29) ORR; 72% (21/29) CR + CRc + CRi rate; 45% (13/29) bridged to SCT Relapsed/refractory: 69% (9/13) ORR; 38% (5/13) CR + CRc + CRi rate; 1 patient bridged to SCT Median OS not reached in first-line BPDCN (Stages 1-2, and 3) 71% OS-12 in Stages 1 and 2 (95% CI: 42, 91); follow-up ongoing for OS-12 in Stage 3 • Safety - Most common TRAEs in BPDCN (Stages 1, 2, and 3) at 12 ug/kg/day (n=42): alanine aminotransferase increase (52%), aspartate aminotransferase increase (50%), hypoalbuminemia (50%), and thrombocytopenia (38%). TRAEs included capillary leak syndrome (19%), which was grade 5 in 2.4% (1/42) of BPDCN patients at 12 µg/kg/day, 2.6% (4/153) of all patients across all trials at all doses, and 1.7% (2/119) of patients across all trials at 12 µg/kg/day • Next steps - - A BLA submission is in preparation SL-401 is also being evaluated in other clinical trials, as a single agent or in combination with other agents, in additional malignancies 17
Acknowledgements We would like to thank: • Investigators, co-investigators, and study teams at each participating center: • This study is sponsored by Stemline Therapeutics, Inc. 18 18

SL-401: Regulatory and Commercial Timeline Submission Approval Pre-MAA EMA meeting Potential MAA Submission EU Commercial US Launch 19 4Q17 Regulatory 1Q18 2Q18 3Q18 4Q18 Complete BLA Potential 1Q19 2019 2018 2017
Market Opportunity – BPDCN Universe Trial BPDCN clinical trial sites Centers 2 CT Centers BPDCN (US) TIER 1 Centers Trial BPDCN clinical trial sites Centers 2 CT Centers 12 Tier 1 Centers 5 Tier 2 Centers 1 Tier 3 Center 1 Hospital System Maintenance Therapy (post-SCT) ~40% first-line BPDCN patients on SL-401 bridged to SCT Study Design In Process “BPDCN Signature” (30%) x (16%) x new AML cases (EHA, 2016) Potential upside BPDCN (EU) Similar patient size to US Filing Discussions Underway

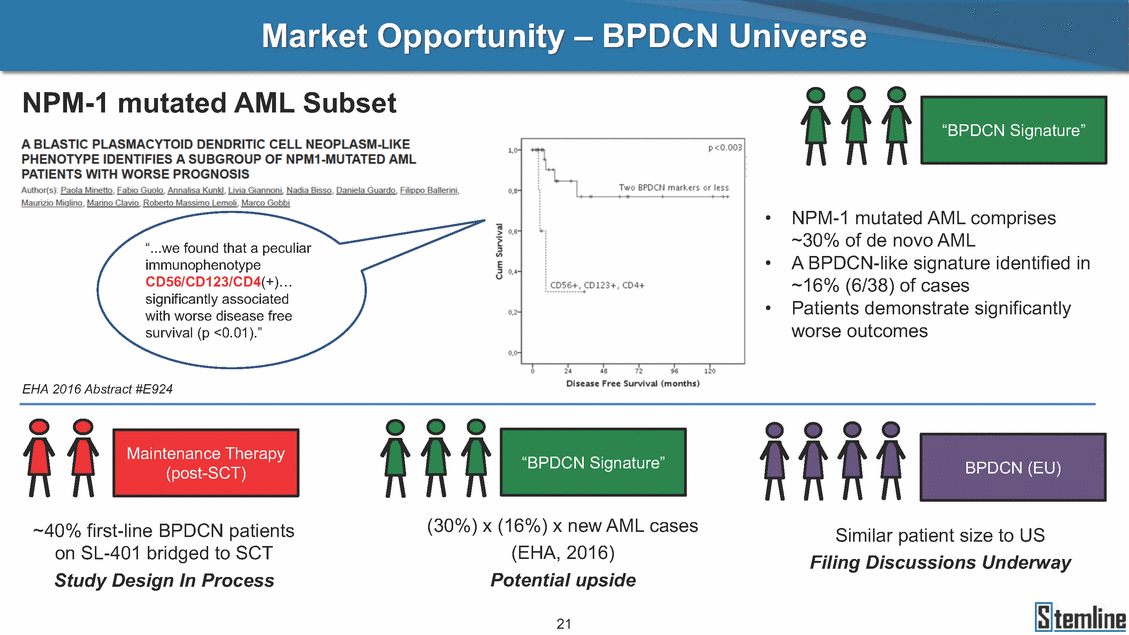
Market Opportunity – BPDCN Universe NPM-1 mutated AML Subset “BPDCN Signature” • NPM-1 mutated AML comprises ~30% of de novo AML “...we found that a peculiar immunophenotype CD56/CD123/CD4(+)… significantly associated with worse disease free survival (p <0.01).” • A BPDCN-like signature identified in ~16% (6/38) of cases • Patients demonstrate significantly worse outcomes nce Therapy “BPDCN Signature” BPDCN (EU) (post-SCT) (30%) x (16%) x new AML cases ~40% first-line BPDCN patients Similar patient size to US (EHA, 2016) on SL-401 bridged to SCT Study Design In Process Filing Discussions Underway Potential upside 21 EHA 2016 Abstract #E924 Maintena
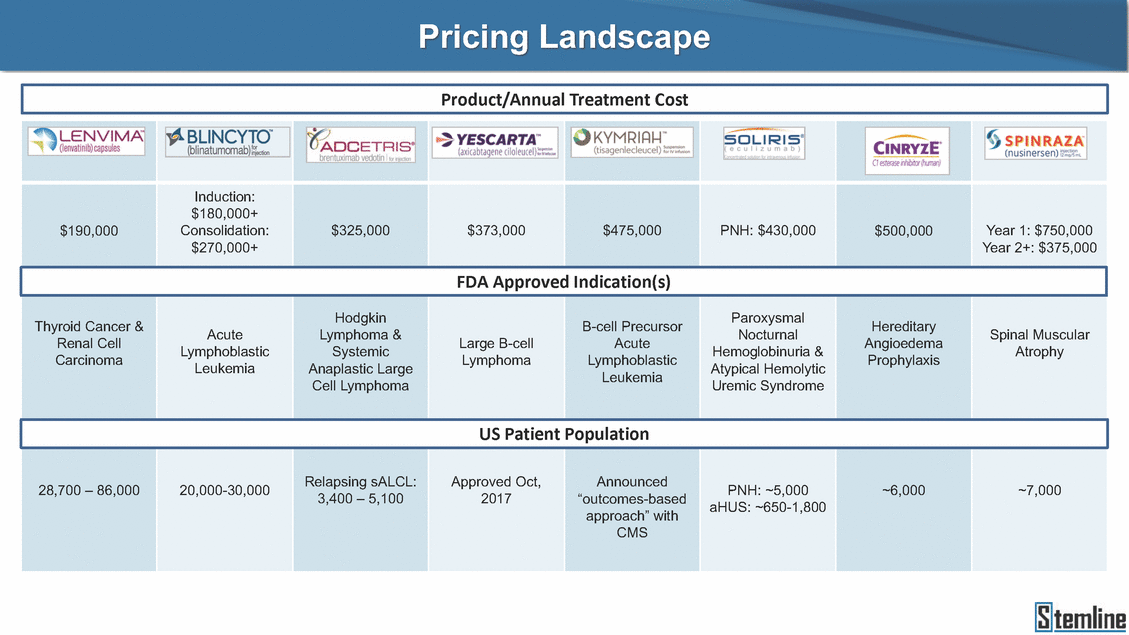
Pricing Landscape Product/Annual Treatment Cost FDA Approved Indication(s) Atrophy Uremic Syndrome US Patient Population aHUS: ~650-1,800 28,700 – 86,000 20,000-30,000 Relapsing sALCL: 3,400 – 5,100 Approved Oct, 2017 Announced “outcomes-based approach” with CMS PNH: ~5,000 ~6,000 ~7,000 Thyroid Cancer & Renal Cell Carcinoma Acute Lymphoblastic Leukemia Hodgkin Lymphoma & Systemic Anaplastic Large Cell Lymphoma Large B-cell Lymphoma B-cell Precursor Acute Lymphoblastic Leukemia Paroxysmal Nocturnal Hemoglobinuria & Atypical Hemolytic Hereditary Angioedema Prophylaxis Spinal Muscular $190,000 Induction: $180,000+ Consolidation: $270,000+ $325,000 $373,000 $475,000 PNH: $430,000 $500,000 Year 1: $750,000 Year 2+: $375,000
B Blastic P D C N The signature marker triad co123, co4, co56 may change diagnosis-for good Neoplasm Cell COMMON MISDIAGNOSES .. ACTUALLY BE • ALL • AML • MDS • NHL • CMML BPDCN? • Leukemia cutis,cutaneous lymphoma,NK cell neoplasms · Marker CD123 CD4 CD56 TCL1 C043 Knowing can be as simple as co123, co4, co56 A signature triad of diagnostic biomarkers, including co123, may help identify an aggressive and deadly hematologic cancer.1* IS]temline 23 CD123 ASSESSMENT SHOULD BE IN ALL HEMATOLOGIC DIAGNOSTI INCLUDED C PANELS. 1 6 Occurrence 85%-100% 80%-100% 90%-100% 80%-100o/o 80%-100% 50%-70% Common Diagnostic Cell Surface Markers in BPDC N INCLUDP-u

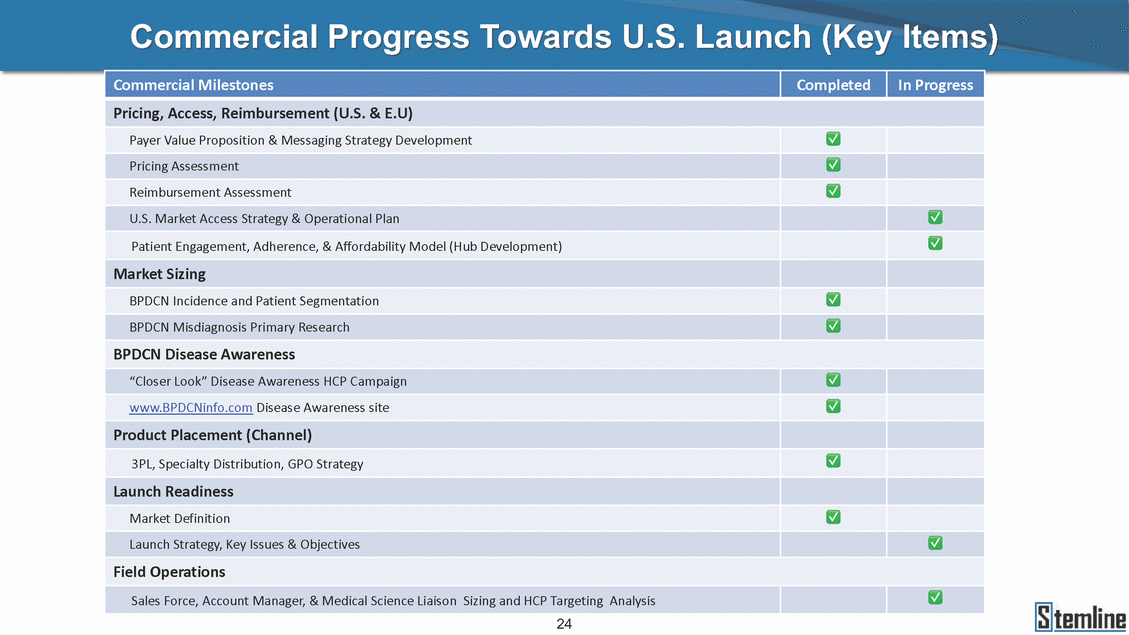
Commercial Progress Towards U.S. Launch (Key Items) 24 Pricing, Access, Reimbursement (U.S. & E.U) Payer Value Proposition & Messaging Strategy Development✅ Pricing Assessment✅ Reimbursement Assessment✅ U.S. Market Access Strategy & Operational Plan✅ Patient Engagement, Adherence, & Affordability Model (Hub Development)✅ Market Sizing BPDCN Incidence and Patient Segmentation✅ BPDCN Misdiagnosis Primary Research✅ BPDCN Disease Awareness “Closer Look” Disease Awareness HCP Campaign✅ www.BPDCNinfo.com Disease Awareness site✅ Product Placement (Channel) 3PL, Specialty Distribution, GPO Strategy✅ Launch Readiness Market Definition✅ Launch Strategy, Key Issues & Objectives✅ Field Operations Sales Force, Account Manager, & Medical Science Liaison Sizing and HCP Targeting Analysis✅
SL-401: Additional Opportunities

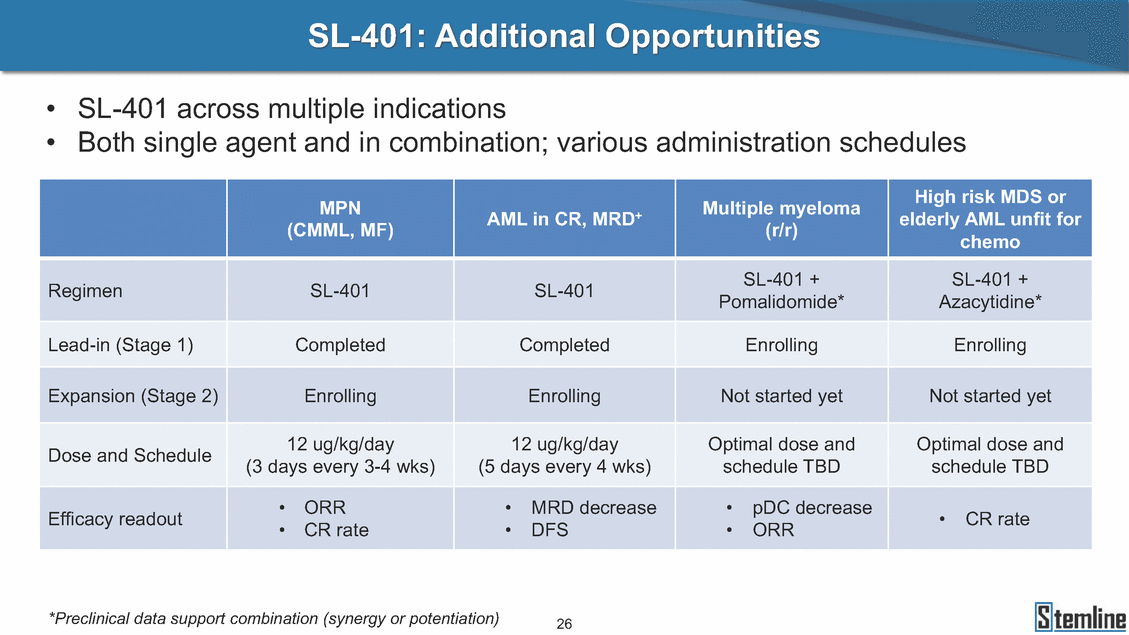
SL-401: Additional Opportunities • • SL-401 across multiple indications Both single agent and in combination; various administration schedules Pomalidomide* Azacytidine* (3 days every 3-4 wks) (5 days every 4 wks) schedule TBD schedule TBD • CR rate • CR rate • DFS • ORR *Preclinical data support combination (synergy or potentiation) 26 chemo RegimenSL-401SL-401SL-401 +SL-401 + Lead-in (Stage 1)CompletedCompletedEnrollingEnrolling Expansion (Stage 2)EnrollingEnrollingNot started yetNot started yet Dose and Schedule12 ug/kg/day12 ug/kg/dayOptimal dose andOptimal dose and Efficacy readout•ORR•MRD decrease•pDC decrease
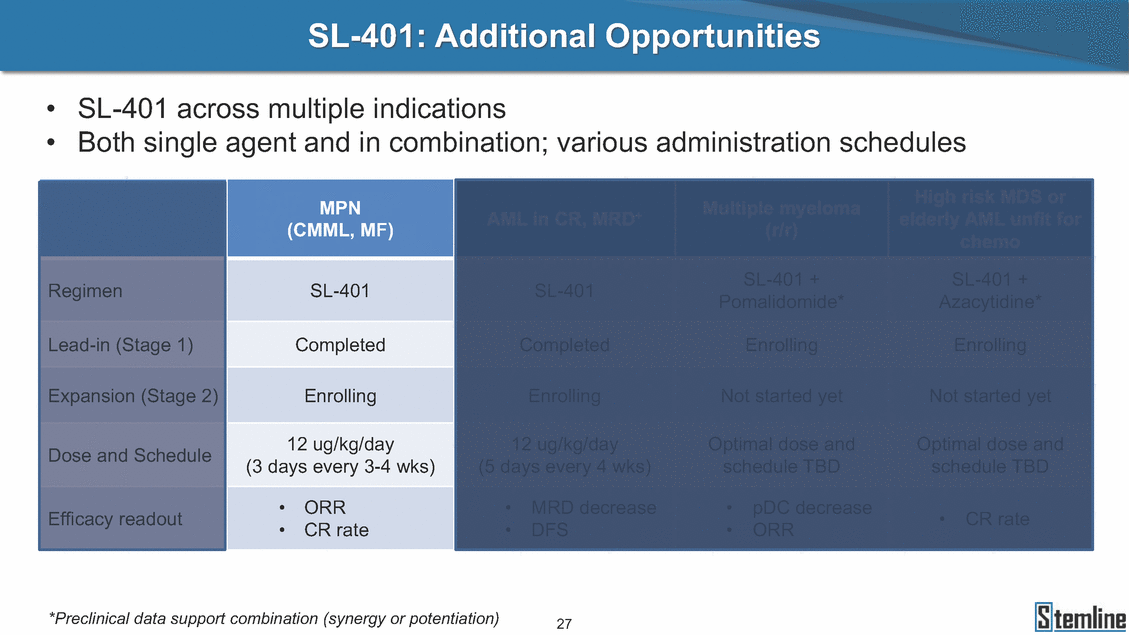
SL-401: Additional Opportunities • • SL-401 across multiple indications Both single agent and in combination; various administration schedules Azacytidine* (5 days every 4 wks) schedule TBD • DFS • ORR *Preclinical data support combination (synergy or potentiation) 27 chemo Regimen Lead-in (Stage 1) Expansion (Stage 2) Dose and Schedule Efficacy readout SL-401 SL-401SL-401 + Pomalidomide* SL-401 + Enrolling Not started yet Optimal dose and schedule TBD •CR rate Completed CompletedEnrolling Enrolling EnrollingNot started yet 12 ug/kg/day (3 days every 3-4 wks) 12 ug/kg/dayOptimal dose and •ORR •CR rate •MRD decrease•pDC decrease
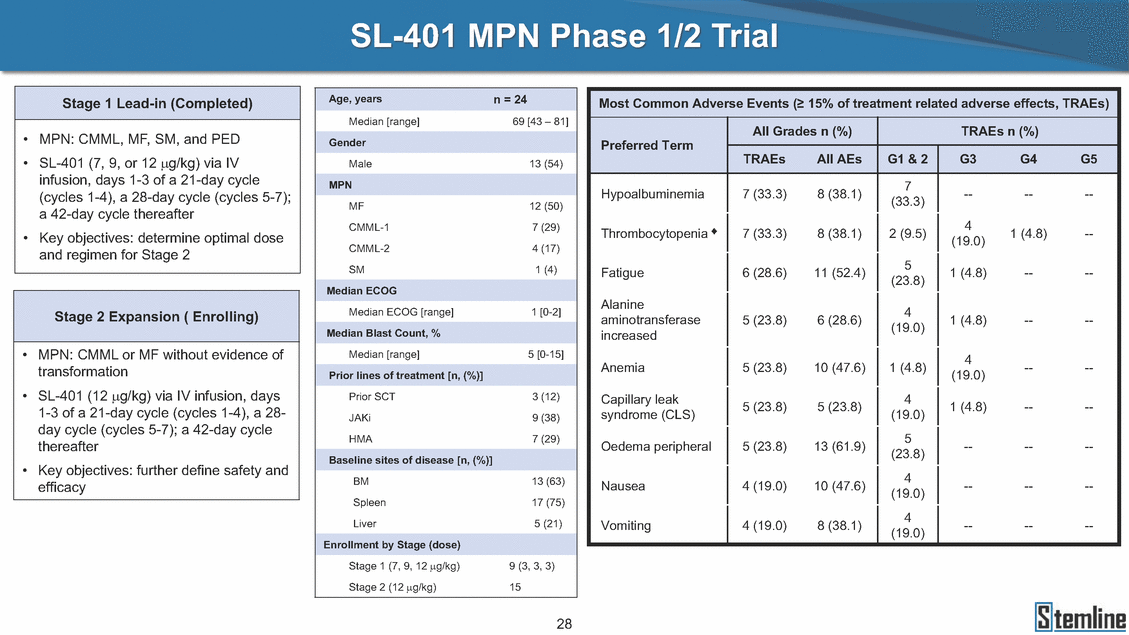
SL-401 MPN Phase 1/2 Trial Median [range] 69 [43 – 81] • • MPN: CMML, MF, SM, and PED SL-401 (7, 9, or 12 µg/kg) via IV infusion, days 1-3 of a 21-day cycle (cycles 1-4), a 28-day cycle (cycles 5-7); a 42-day cycle thereafter Key objectives: determine optimal dose and regimen for Stage 2 Male 13 (54) 7 (33.3) Hypoalbuminemia 7 (33.3) 8 (38.1) ---- --MF CMML-1 CMML-2 SM 12 (50) 7 (29) 4 (17) 1 (4) 4 (19.0) Thrombocytopenia + 7 (33.3) 8 (38.1) 2 (9.5) 1 (4.8) --• 5 (23.8) Fatigue 6 (28.6) 11 (52.4) 1 (4.8) ---- Alanine aminotransferase increased 4 (19.0) Median ECOG [range] 1 [0-2] 5 (23.8) 6 (28.6) 1 (4.8) ---- • MPN: CMML or MF without evidence of transformation SL-401 (12 µg/kg) via IV infusion, days 1-3 of a 21-day cycle (cycles 1-4), a 28-day cycle (cycles 5-7); a 42-day cycle thereafter Key objectives: further define safety and efficacy Median [range] 5 [0-15] 4 (19.0) Anemia 5 (23.8) 10 (47.6) 1 (4.8) ---- • Prior SCT JAKi HMA 3 (12) 9 (38) 7 (29) Capillary leak syndrome (CLS) 4 (19.0) 5 (23.8) 4 (19.0) 4 (19.0) 5 (23.8) 5 (23.8) 1 (4.8) ---- Oedema peripheral 5 (23.8) 13 (61.9) ---- --• BM Spleen Liver 13 (63) 17 (75) 5 (21) Nausea 4 (19.0) 10 (47.6) ---- --Vomiting 4 (19.0) 8 (38.1) ---- --Stage 1 (7, 9, 12 µg/kg) Stage 2 (12 µg/kg) 9 (3, 3, 3) 15 28 Enrollment by Stage (dose) Baseline sites of disease [n, (%)] Prior lines of treatment [n, (%)] Median Blast Count, % Stage 2 Expansion ( Enrolling) Median ECOG MPN Gender Most Common Adverse Events (≥ 15% of treatment related adverse effects, TRAEs) All Grades n (%)TRAEs n (%) Preferred Term TRAEsAll AEsG1 & 2G3G4G5 Stage 1 Lead-in (Completed) Age, yearsn = 24
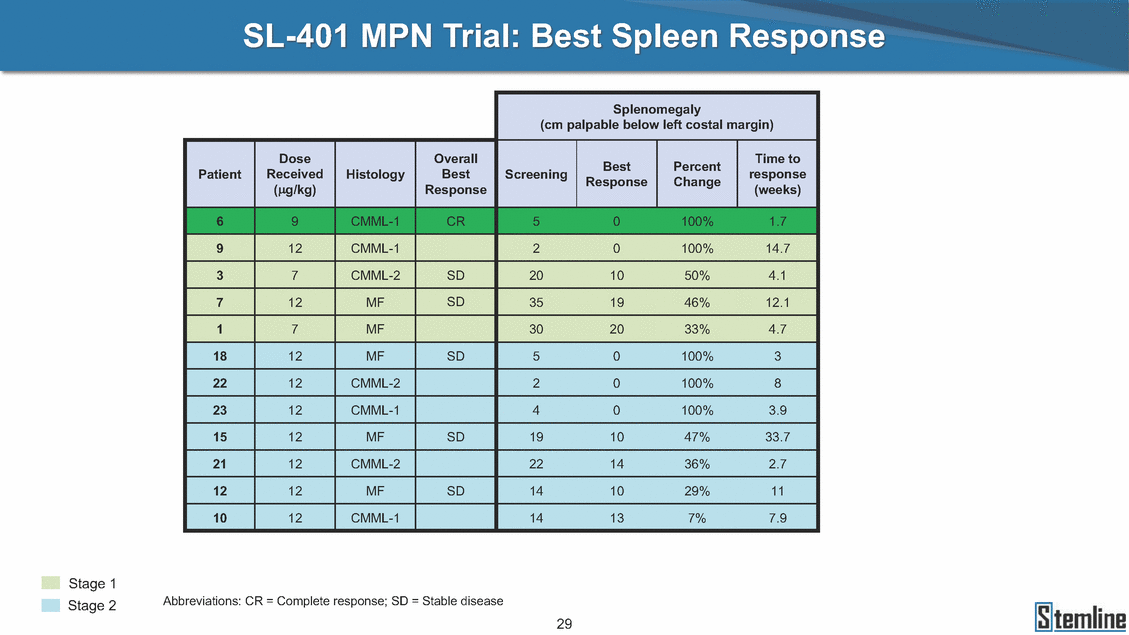
SL-401 MPN Trial: Best Spleen Response Best Percent Patient Received Histology Best Screening response Stage 1 Stage 2 Abbreviations: CR = Complete response; SD = Stable disease 29 Splenomegaly (cm palpable below left costal margin) DoseOverallTime to (µg/kg) ResponseResponseChange(weeks) 69CMML-1CR50100%1.7 912CMML-120100%14.7 37CMML-2SD201050%4.1 712MFSD351946%12.1 17MF302033%4.7 1812MFSD50100%3 2212CMML-220100%8 2312CMML-140100%3.9 1512MFSD191047%33.7 2112CMML-2221436%2.7 1212MFSD141029%11 1012CMML-114137%7.9
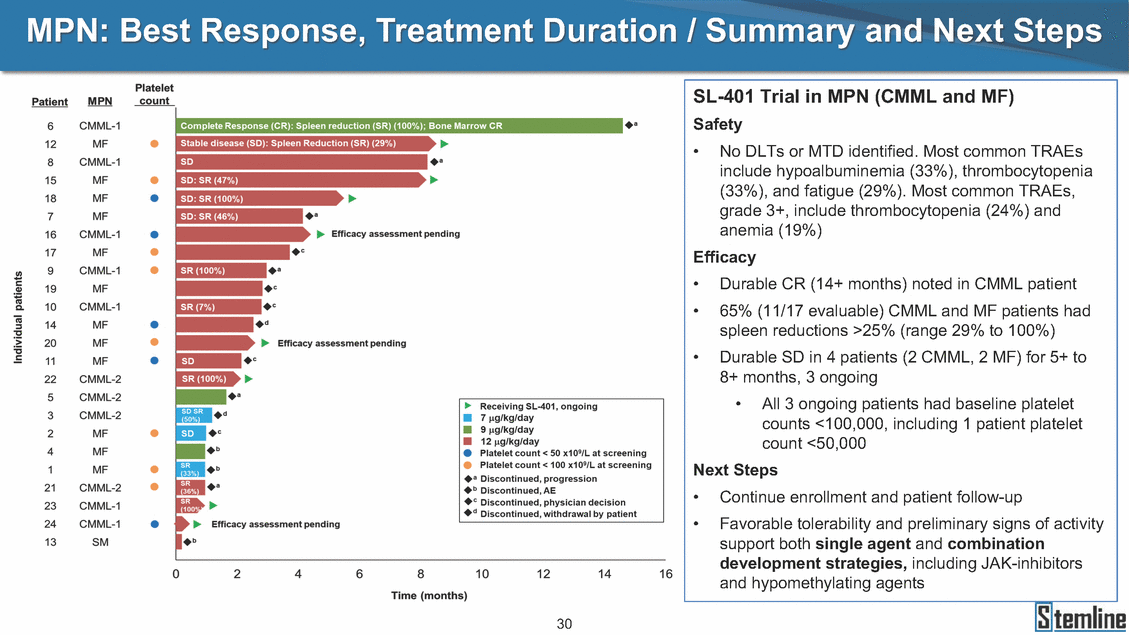
MPN: Best Response, Treatment Duration / Summary and Next Steps SL-401 Trial in MPN (CMML and MF) Safety • No DLTs or MTD identified. Most common TRAEs include hypoalbuminemia (33%), thrombocytopenia (33%), and fatigue (29%). Most common TRAEs, grade 3+, include thrombocytopenia (24%) and anemia (19%) Efficacy • • Durable CR (14+ months) noted in CMML patient 65% (11/17 evaluable) CMML and MF patients had spleen reductions >25% (range 29% to 100%) Durable SD in 4 patients (2 CMML, 2 MF) for 5+ to 8+ months, 3 ongoing • • All 3 ongoing patients had baseline platelet counts <100,000, including 1 patient platelet count <50,000 Next Steps • • Continue enrollment and patient follow-up Favorable tolerability and preliminary signs of activity support both single agent and combination development strategies, including JAK-inhibitors and hypomethylating agents 30
SL-401: Combination Strategies

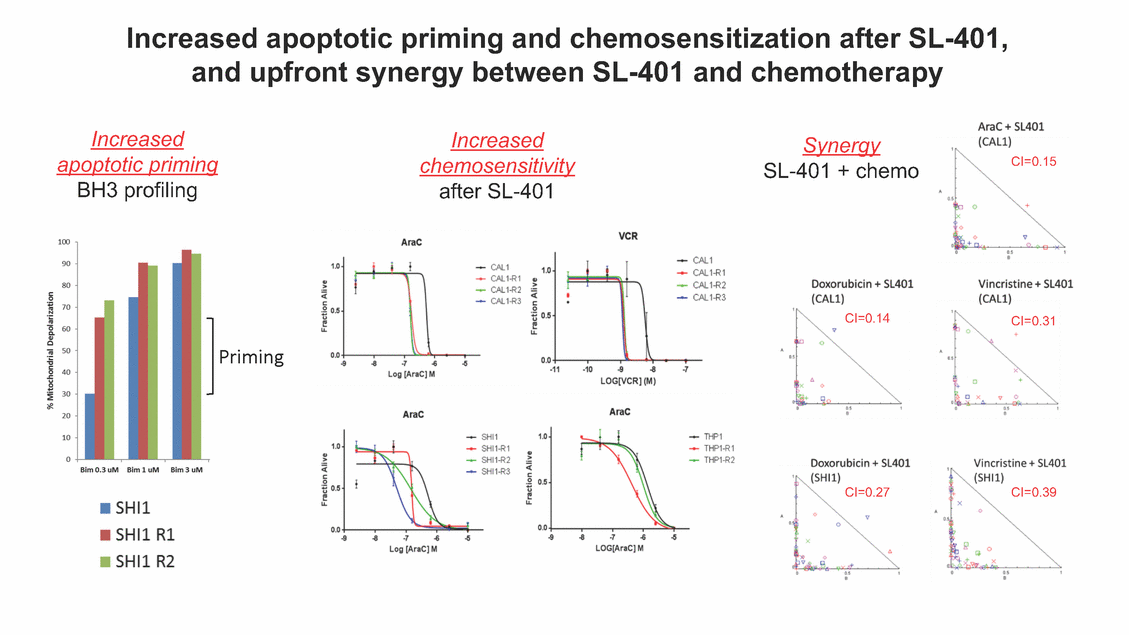
Increased apoptotic priming and chemosensitization after SL-401, and upfront synergy between SL-401 and chemotherapy Increased apoptotic priming Increased chemosensitivity Synergy SL-401 + chemo CI=0.15 BH3 profiling after SL-401 CI=0.14 CI=0.31 Priming CI=0.27 CI=0.39
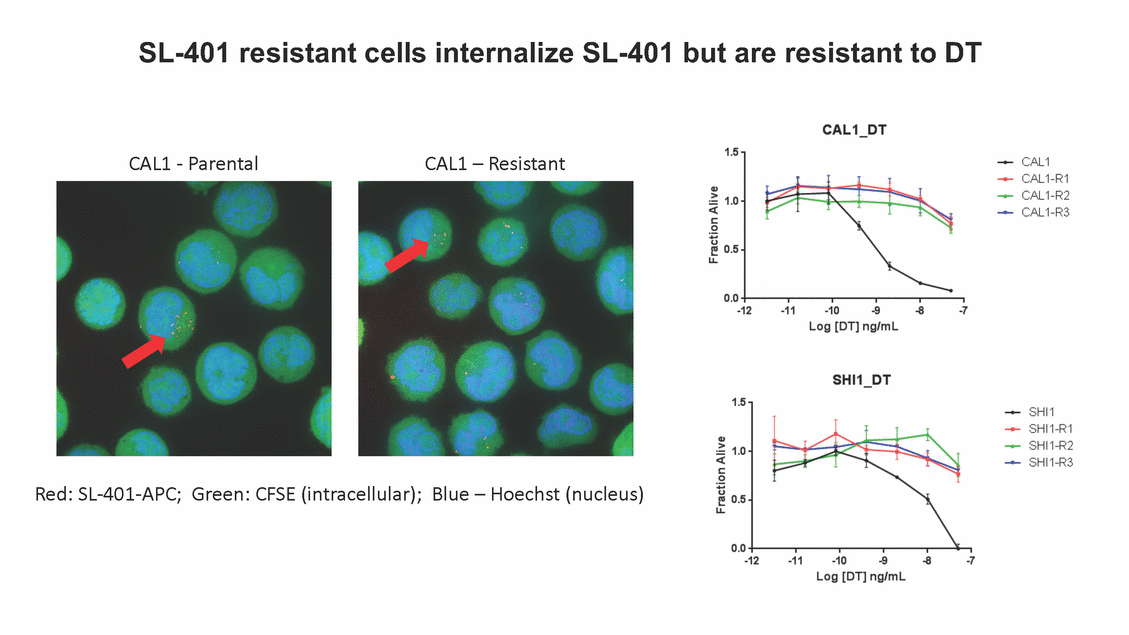
SL-401 resistant cells internalize SL-401 but are resistant to DT CAL1 - Parental CAL1 – Resistant Red: SL-401-APC; Green: CFSE (intracellular); Blue – Hoechst (nucleus)
Loss of DPH1 is sufficient to confer resistance to SL-401 CRISPR targeting DPH1 biotin-NAD+ +/-SL-401 Cell lysate + +/-+ IL-3 DPH1 Actin No drug SL-401 1 0 0 THP1 R1 R2 R3 THP1 R1 R2 R3 N T G 1 8 0 N T G 2 ADP-R-(eEF2) g 2 6 0 g 3 eEF2 4 0 g 5 g 6 2 0 DPH1 0 0 2 4 D a y s p o s t S L - 4 0 1 6 8 Actin % G F P DT

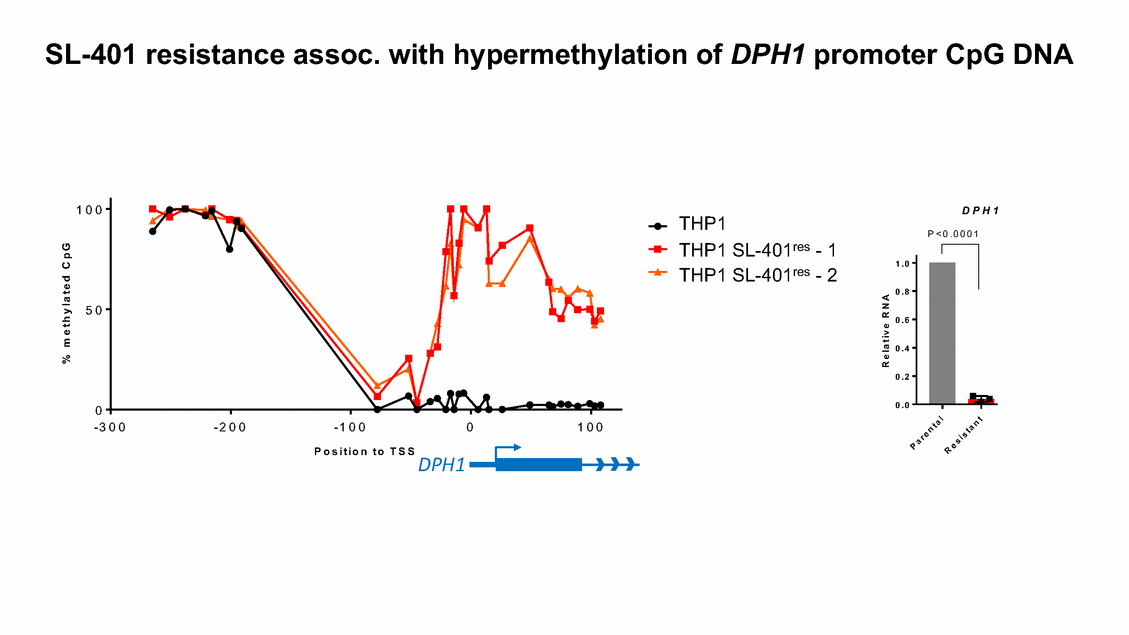
SL-401 resistance assoc. with hypermethylation of DPH1 promoter CpG DNA 1 0 0 D P H 1 THP1 THP1 SL-401res - 1 P < 0 . 0 0 0 1 P = 0 . 0 0 0 3 1 . 0 THP1 SL-401res - 2 0 . 8 5 0 0 . 6 0 . 4 0 . 2 0 . 0 0 - 3 0 0 - 2 0 0 - 1 0 0 0 1 0 0 P o s i t i o n t o T S S DPH1 % m e t h y l a t e d C p G R e l a t i v e R N A
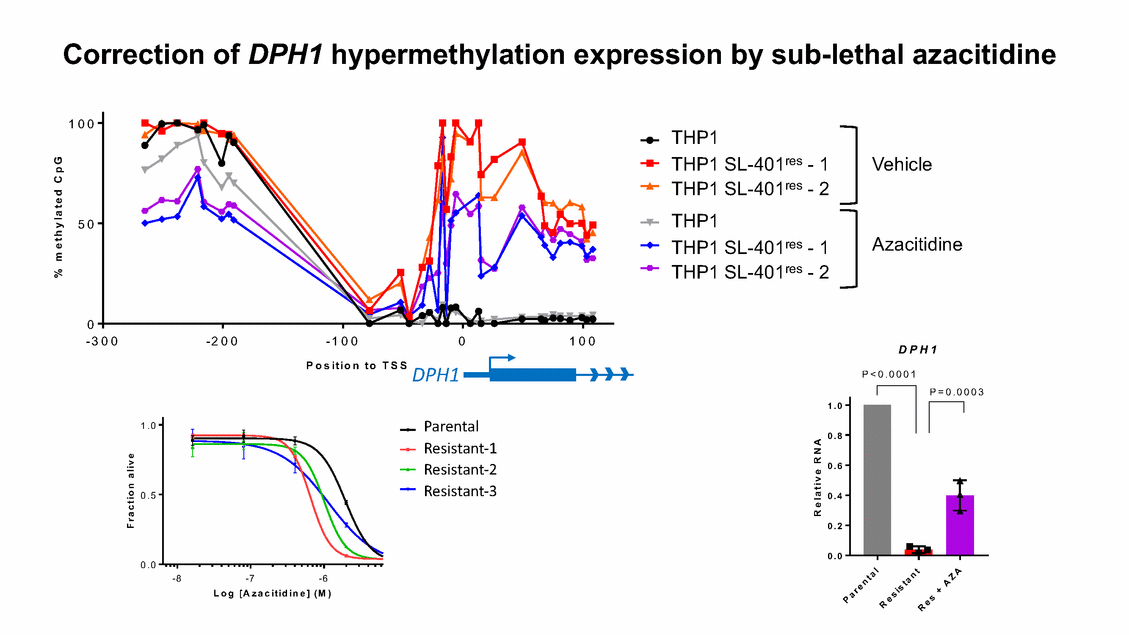
Correction of DPH1 hypermethylation expression by sub-lethal azacitidine 1 0 0 THP1 THP1 SL-401res - 1 THP1 SL-401res - 2 THP1 THP1 SL-401res - 1 THP1 SL-401res - 2 Vehicle 5 0 Azacitidine 0 - 3 0 0 - 2 0 0 - 1 0 0 0 1 0 0 D P H 1 P o s i t i o n t o T S S DPH1 P < 0 . 0 0 0 1 P = 0 . 0 0 0 3 1 . 0 Parental 1 .0 0 . 8 Resistant-1 Resistant-2 Resistant-3 0 . 6 0 .5 0 . 4 0 . 2 0 . 0 0 .0 - 8 - 7 - 6 L o g [ A z a c i t i d i n e ] ( M ) % m e t h y l a t e d C p G F r a c t i o n a l i v e R e l a t i v e R N A
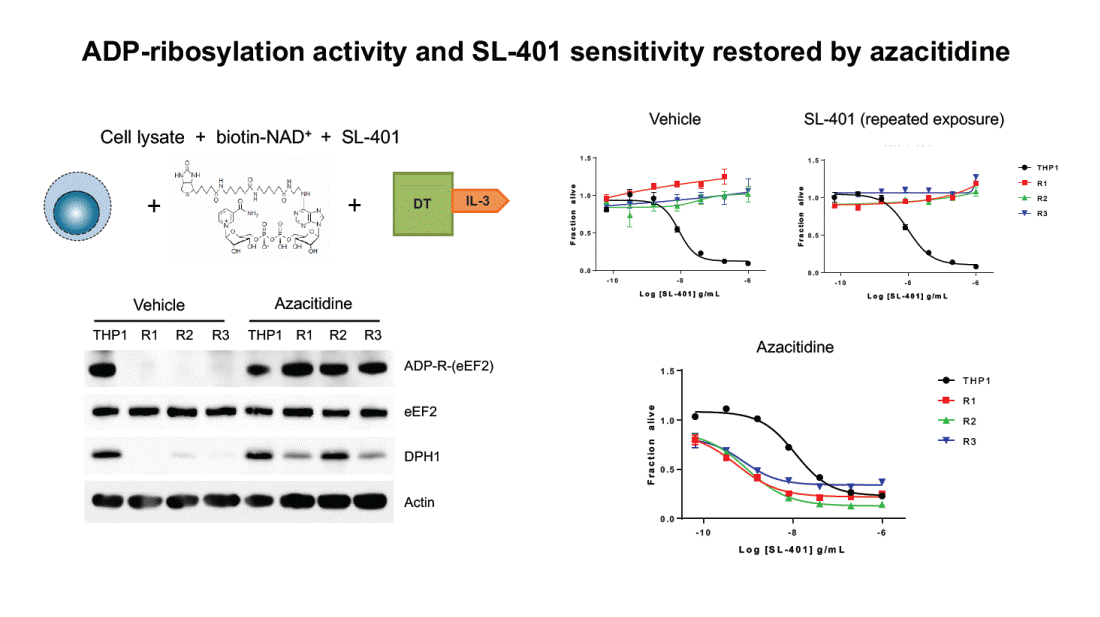
ADP-ribosylation activity and SL-401 sensitivity restored by azacitidine Vehicle SL-401 (repeated exposure) Cell lysate + biotin-NAD+ + SL-401 1 . 5 1 . 5 T H P 1 R 1 + + 1 . 0 IL-3 1 . 0 R 2 R 3 0 . 5 0 . 5 0 . 0 0 . 0 - 1 0 - 8 L o g [ S L - 4 0 1 ] g / m L - 6 - 1 0 - 8 L o g [ S L - 4 0 1 ] g / m L - 6 Azacitidine Vehicle THP1 R1 R2 R3 THP1 R1 R2 R3 Azacitidine ADP-R-(eEF2) 1 . 5 T H P 1 R 1 eEF2 1 . 0 R 2 R 3 DPH1 0 . 5 Actin 0 . 0 - 1 0 - 8 L o g [ S L - 4 0 1 ] g / m L - 6 F r a c t i o n a l i v e F r a c t i o n a l i v e F r a c t i o n a l i v e DT
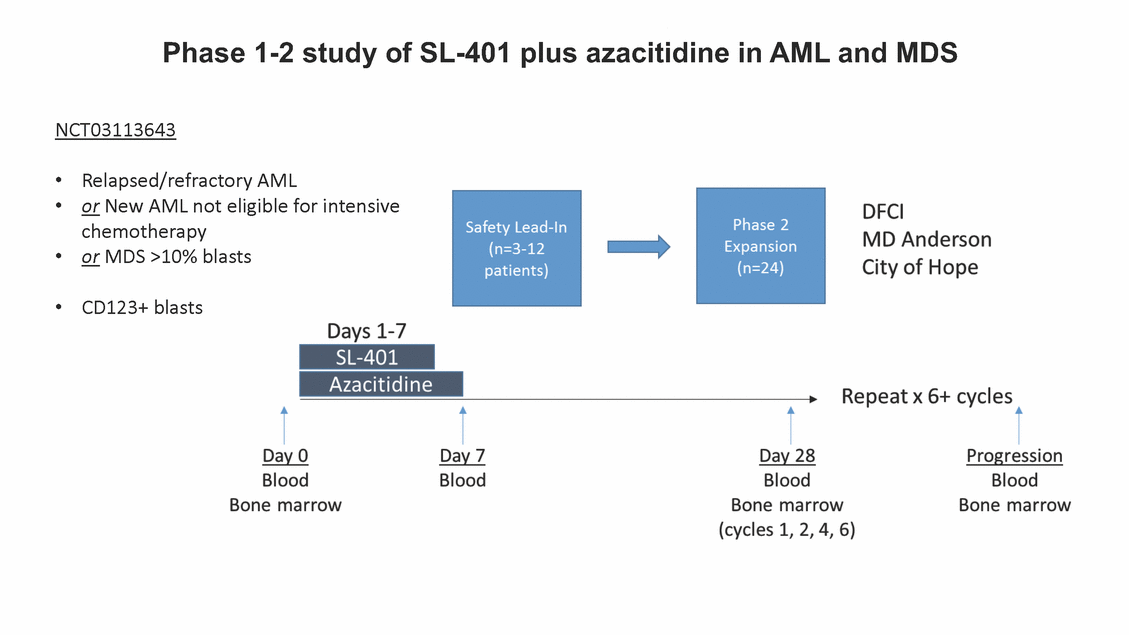
Phase 1-2 study of SL-401 plus azacitidine in AML and MDS NCT03113643 • • Relapsed/refractory AML or New AML not eligible for intensive chemotherapy or MDS >10% blasts • • CD123+ blasts
Upcoming Milestones

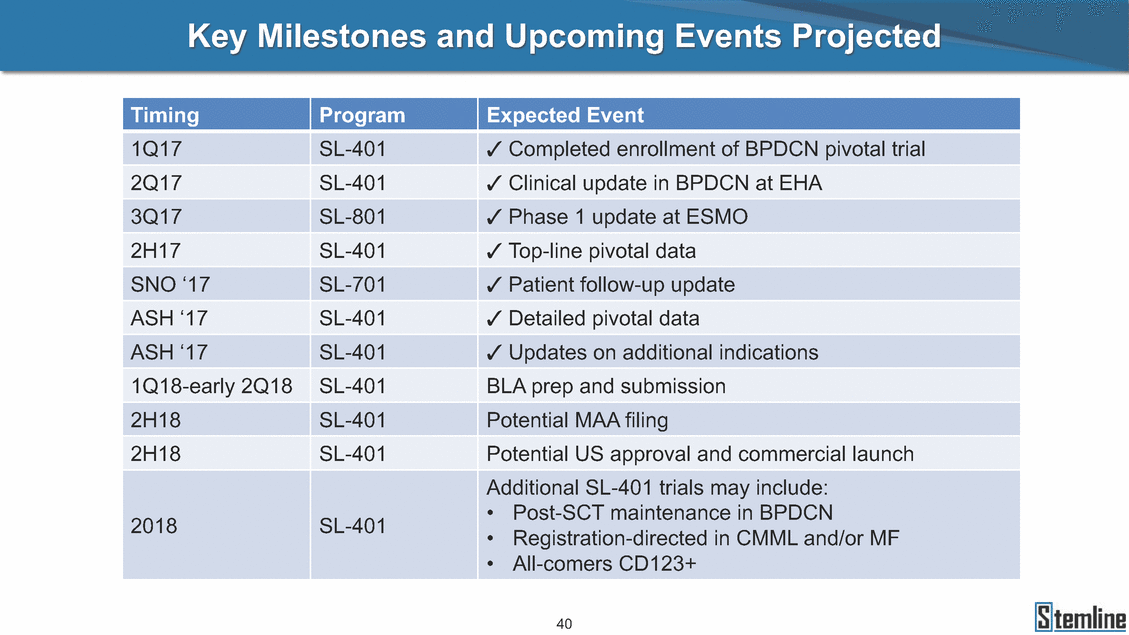
Key Milestones and Upcoming Events Projected 40 Timing Program Expected Event 1Q17 SL-401 Completed enrollment of BPDCN pivotal trial 2Q17 SL-401 Clinical update in BPDCN at EHA 3Q17 SL-801 Phase 1 update at ESMO 2H17 SL-401 Top-line pivotal data SNO ‘17 SL-701 Patient follow-up update ASH ‘17 SL-401 Detailed pivotal data ASH ‘17 SL-401 Updates on additional indications 1Q18-early 2Q18 SL-401 BLA prep and submission 2H18 SL-401 Potential MAA filing 2H18 SL-401 Potential US approval and commercial launch 2018 SL-401 Additional SL-401 trials may include: • Post-SCT maintenance in BPDCN • Registration-directed in CMML and/or MF • All-comers CD123+
Q&A

Stemline Therapeutics, Inc. NASDAQ: STML Analyst and Investor Meeting ASH 2017 December 11, 2017

