Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - Blueprint Medicines Corp | ex-99d3.htm |
| EX-99.1 - EX-99.1 - Blueprint Medicines Corp | ex-99d1.htm |
| 8-K - 8-K - Blueprint Medicines Corp | f8-k.htm |
Exhibit 99.2
|
|
Clinical activity in a Phase 1 study of BLU-285, a potent, highly-selective inhibitor of KIT D816V in advanced systemic mastocytosis Daniel J. DeAngelo, Albert T. Quiery, Deepti Radia, Mark W. Drummond, Jason Gotlib, William A. Robinson, Elizabeth Hexner, Srdan Verstovsek, Hongliang Shi, Terri Alvarez-Diez, Oleg Schmidt-Kittler, Erica Evans, Mary E. Healy, Beni B. Wolf and Michael W. Deininger American Society of Hematology Annual Meeting, Atlanta, GA USA,10 Dec 2017 |
|
|
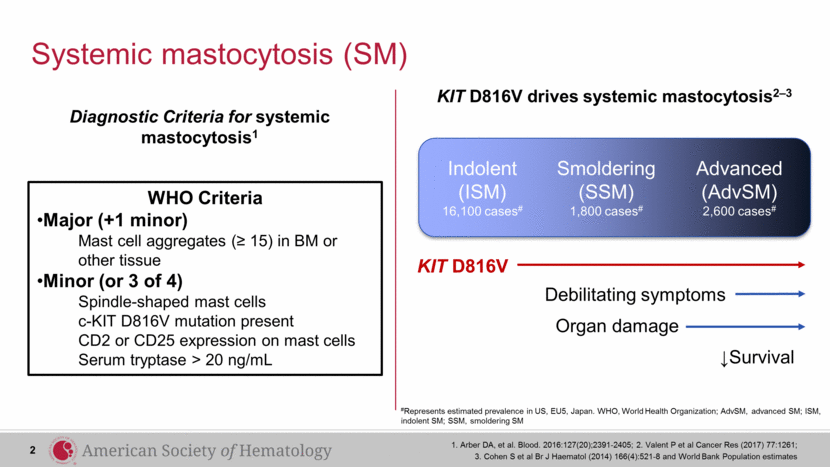
Systemic mastocytosis (SM) #Represents estimated prevalence in US, EU5, Japan. WHO, World Health Organization; AdvSM, advanced SM; ISM, indolent SM; SSM, smoldering SM 1. Arber DA, et al. Blood. 2016:127(20);2391-2405; 2. Valent P et al Cancer Res (2017) 77:1261; 3. Cohen S et al Br J Haematol (2014) 166(4):521-8 and World Bank Population estimates 2 WHO Criteria Major (+1 minor) Mast cell aggregates (≥ 15) in BM or other tissue Minor (or 3 of 4) Spindle-shaped mast cells c-KIT D816V mutation present CD2 or CD25 expression on mast cells Serum tryptase > 20 ng/mL KIT D816V drives systemic mastocytosis2–3 ↓Survival Organ damage Debilitating symptoms KIT D816V Indolent (ISM) 16,100 cases# Smoldering(SSM) 1,800 cases# Advanced (AdvSM) 2,600 cases# Indolent (ISM) 16,100 cases# Smoldering(SSM) 1,800 cases# Advanced (AdvSM) 2,600 cases# Diagnostic Criteria for systemic mastocytosis1 |
|
|
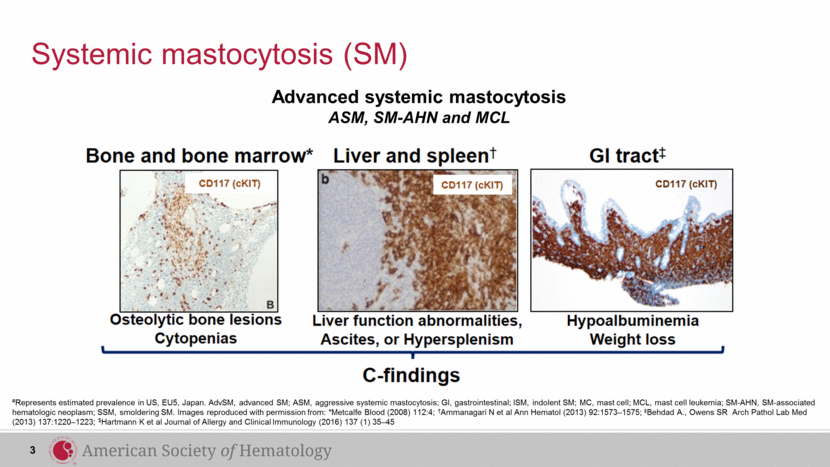
Systemic mastocytosis (SM) Advanced systemic mastocytosis ASM, SM-AHN and MCL 3 #Represents estimated prevalence in US, EU5, Japan. AdvSM, advanced SM; ASM, aggressive systemic mastocytosis; GI, gastrointestinal; ISM, indolent SM; MC, mast cell; MCL, mast cell leukemia; SM-AHN, SM-associated hematologic neoplasm; SSM, smoldering SM. Images reproduced with permission from: *Metcalfe Blood (2008) 112:4; †Ammanagari N et al Ann Hematol (2013) 92:1573–1575; ‡Behdad A., Owens SR Arch Pathol Lab Med (2013) 137:1220–1223; $Hartmann K et al Journal of Allergy and Clinical Immunology (2016) 137 (1) 35–45 |
|
|
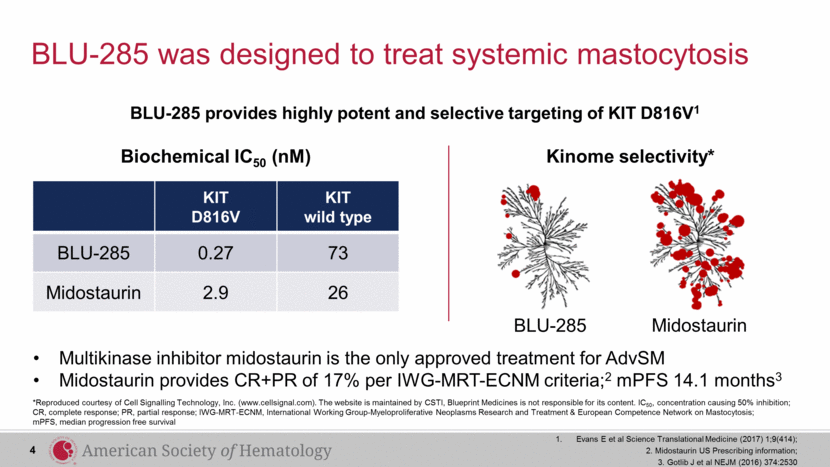
BLU-285 was designed to treat systemic mastocytosis *Reproduced courtesy of Cell Signalling Technology, Inc. (www.cellsignal.com). The website is maintained by CSTI, Blueprint Medicines is not responsible for its content. IC50, concentration causing 50% inhibition; CR, complete response; PR, partial response; IWG-MRT-ECNM, International Working Group-Myeloproliferative Neoplasms Research and Treatment & European Competence Network on Mastocytosis; mPFS, median progression free survival Multikinase inhibitor midostaurin is the only approved treatment for AdvSM Midostaurin provides CR+PR of 17% per IWG-MRT-ECNM criteria;2 mPFS 14.1 months3 KIT D816V KIT wild type BLU-285 0.27 73 Midostaurin 2.9 26 Biochemical IC50 (nM) Kinome selectivity* BLU-285 provides highly potent and selective targeting of KIT D816V1 4 Evans E et al Science Translational Medicine (2017) 1;9(414); 2. Midostaurin US Prescribing information; 3. Gotlib J et al NEJM (2016) 374:2530 BLU-285 Midostaurin |
|
|
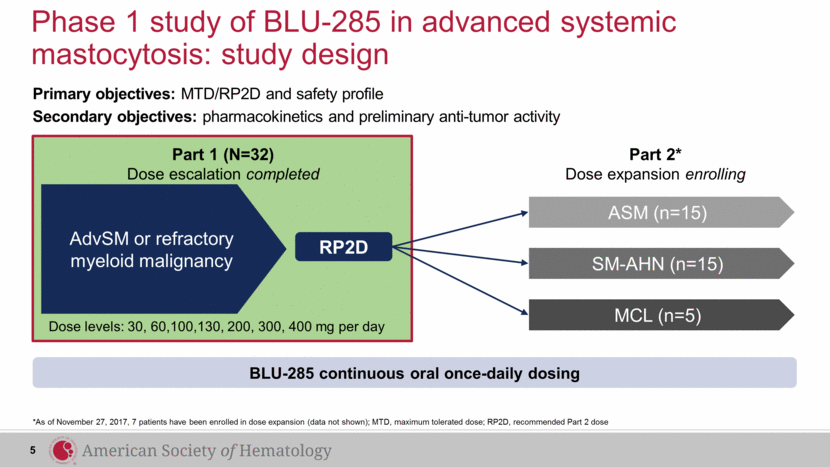
Phase 1 study of BLU-285 in advanced systemic mastocytosis: study design *As of November 27, 2017, 7 patients have been enrolled in dose expansion (data not shown); MTD, maximum tolerated dose; RP2D, recommended Part 2 dose Primary objectives: MTD/RP2D and safety profile Secondary objectives: pharmacokinetics and preliminary anti-tumor activity AdvSM or refractory myeloid malignancy RP2D Part 2* Dose expansion enrolling Part 1 (N=32) Dose escalation completed BLU-285 continuous oral once-daily dosing SM-AHN (n=15) ASM (n=15) MCL (n=5) 5 Dose levels: 30, 60,100,130, 200, 300, 400 mg per day |
|
|
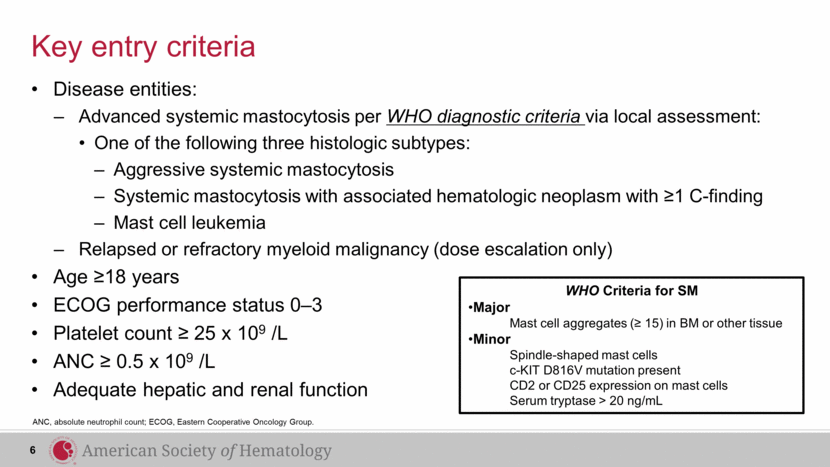
Key entry criteria Disease entities: Advanced systemic mastocytosis per WHO diagnostic criteria via local assessment: One of the following three histologic subtypes: Aggressive systemic mastocytosis Systemic mastocytosis with associated hematologic neoplasm with ≥1 C-finding Mast cell leukemia Relapsed or refractory myeloid malignancy (dose escalation only) Age ≥18 years ECOG performance status 0–3 Platelet count ≥ 25 x 109 /L ANC ≥ 0.5 x 109 /L Adequate hepatic and renal function ANC, absolute neutrophil count; ECOG, Eastern Cooperative Oncology Group. 6 WHO Criteria for SM Major Mast cell aggregates (≥ 15) in BM or other tissue Minor Spindle-shaped mast cells c-KIT D816V mutation present CD2 or CD25 expression on mast cells Serum tryptase > 20 ng/mL |
|
|
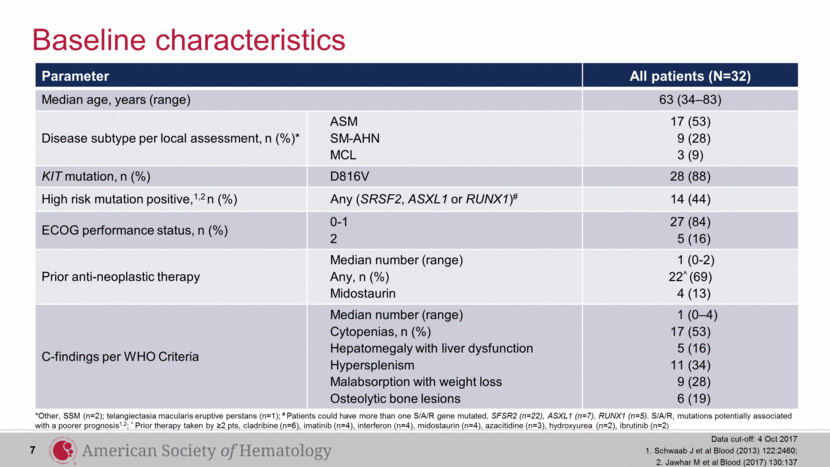
Parameter All patients (N=32) Median age, years (range) 63 (34–83) Disease subtype per local assessment, n (%)* ASM SM-AHN MCL 17 (53) 9 (28) 3 (9) KIT mutation, n (%) D816V 28 (88) High risk mutation positive,1,2 n (%) Any (SRSF2, ASXL1 or RUNX1)# 14 (44) ECOG performance status, n (%) 0-1 2 27 (84) 5 (16) Prior anti-neoplastic therapy Median number (range) Any, n (%) Midostaurin 1 (0-2) 22^ (69) 4 (13) C-findings per WHO Criteria Median number (range) Cytopenias, n (%) Hepatomegaly with liver dysfunction Hypersplenism Malabsorption with weight loss Osteolytic bone lesions 1 (0–4) 17 (53) 5 (16) 11 (34) 9 (28) 6 (19) Baseline characteristics Data cut-off: 4 Oct 2017 1. Schwaab J et al Blood (2013) 122:2460; 2. Jawhar M et al Blood (2017) 130:137 7 *Other, SSM (n=2); telangiectasia macularis eruptive perstans (n=1); # Patients could have more than one S/A/R gene mutated, SFSR2 (n=22), ASXL1 (n=7), RUNX1 (n=5). S/A/R, mutations potentially associated with a poorer prognosis1,2; ^ Prior therapy taken by ≥2 pts, cladribine (n=6), imatinib (n=4), interferon (n=4), midostaurin (n=4), azacitidine (n=3), hydroxyurea (n=2), ibrutinib (n=2) |
|
|
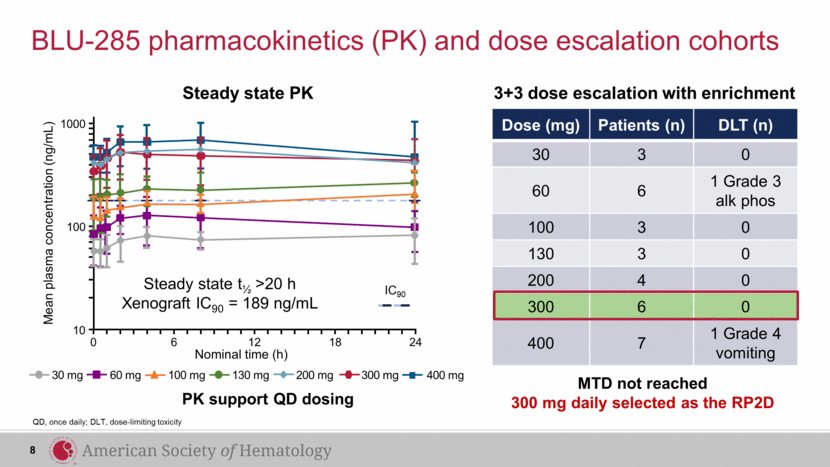
MTD not reached 300 mg daily selected as the RP2D Dose (mg) Patients (n) DLT (n) 30 3 0 60 6 1 Grade 3 alk phos 100 3 0 130 3 0 200 4 0 300 6 0 400 7 1 Grade 4 vomiting BLU-285 pharmacokinetics (PK) and dose escalation cohorts QD, once daily; DLT, dose-limiting toxicity 3+3 dose escalation with enrichment 8 Mean plasma concentration (ng/mL) 24 Steady state t½ >20 h Xenograft IC90 = 189 ng/mL 1000 100 10 0 18 12 Nominal time (h) 6 IC90 PK support QD dosing 400 mg 30 mg 60 mg 100 mg 130 mg 200 mg 300 mg Steady state PK |
|
|
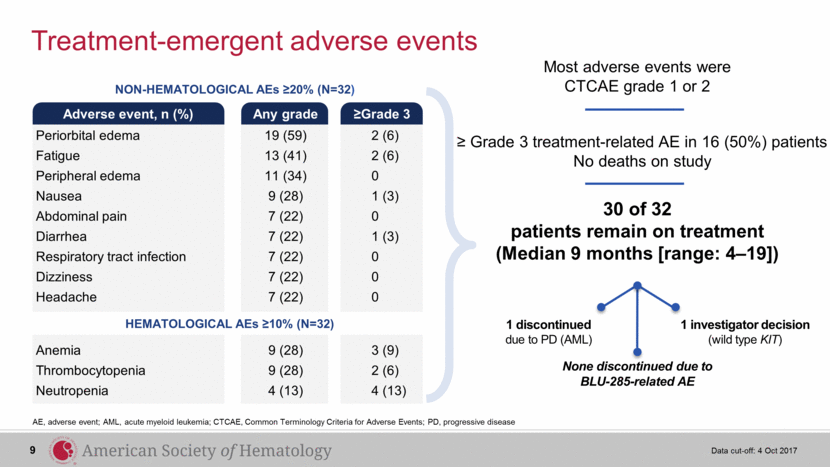
Treatment-emergent adverse events AE, adverse event; AML, acute myeloid leukemia; CTCAE, Common Terminology Criteria for Adverse Events; PD, progressive disease Data cut-off: 4 Oct 2017 NON-HEMATOLOGICAL AEs ≥20% (N=32) Adverse event, n (%) Any grade ≥Grade 3 Periorbital edema 19 (59) 2 (6) Fatigue 13 (41) 2 (6) Peripheral edema 11 (34) 0 Nausea 9 (28) 1 (3) Abdominal pain 7 (22) 0 Diarrhea 7 (22) 1 (3) Respiratory tract infection 7 (22) 0 Dizziness 7 (22) 0 Headache 7 (22) 0 Anemia 9 (28) 3 (9) Thrombocytopenia 9 (28) 2 (6) Neutropenia 4 (13) 4 (13) HEMATOLOGICAL AEs ≥10% (N=32) Most adverse events were CTCAE grade 1 or 2 ≥ Grade 3 treatment-related AE in 16 (50%) patients No deaths on study 30 of 32 patients remain on treatment (Median 9 months [range: 4–19]) 1 discontinued due to PD (AML) 1 investigator decision (wild type KIT) None discontinued due to BLU-285-related AE 9 |
|
|
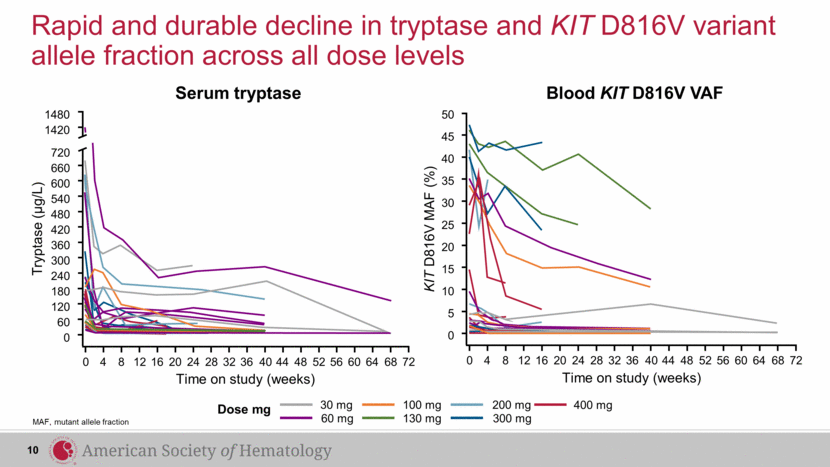
Rapid and durable decline in tryptase and KIT D816V variant allele fraction across all dose levels MAF, mutant allele fraction 400 mg 30 mg 60 mg 100 mg 130 mg 200 mg 300 mg 45 50 40 35 30 25 20 15 10 5 0 Time on study (weeks) KIT D816V MAF (%) 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 Tryptase (µg/L) 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 1420 1480 720 660 600 420 360 300 180 120 0 Time on study (weeks) 540 480 240 60 Serum tryptase Blood KIT D816V VAF 10 Dose mg |
|
|
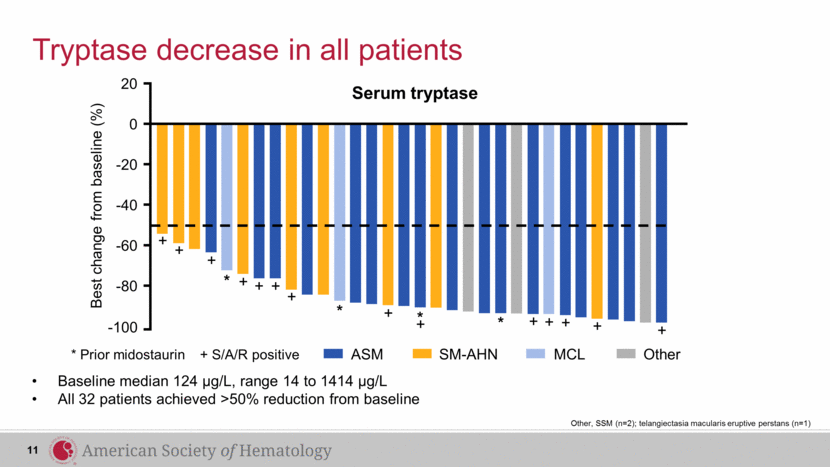
Tryptase decrease in all patients * Prior midostaurin + S/A/R positive Serum tryptase Best change from baseline (%) 0 -20 -40 -60 -80 -100 * * * + + + + + + + + + + + + * + + ASM SM-AHN MCL Other 20 Baseline median 124 µg/L, range 14 to 1414 µg/L All 32 patients achieved >50% reduction from baseline 11 Other, SSM (n=2); telangiectasia macularis eruptive perstans (n=1) |
|
|
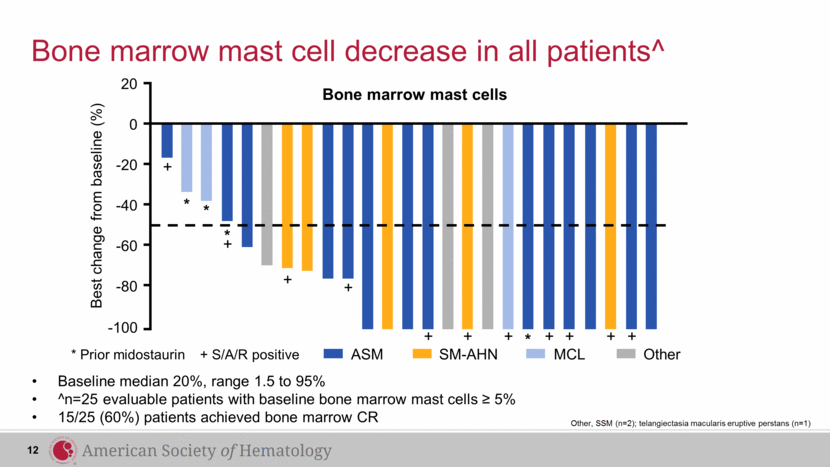
Bone marrow mast cells Bone marrow mast cell decrease in all patients^ Baseline median 20%, range 1.5 to 95% ^n=25 evaluable patients with baseline bone marrow mast cells ≥ 5% 15/25 (60%) patients achieved bone marrow CR + + + + + + 0 -20 -40 -60 -80 -100 * * * * + + + + + 20 Best change from baseline (%) 12 * Prior midostaurin + S/A/R positive ASM SM-AHN MCL Other Other, SSM (n=2); telangiectasia macularis eruptive perstans (n=1) |
|
|
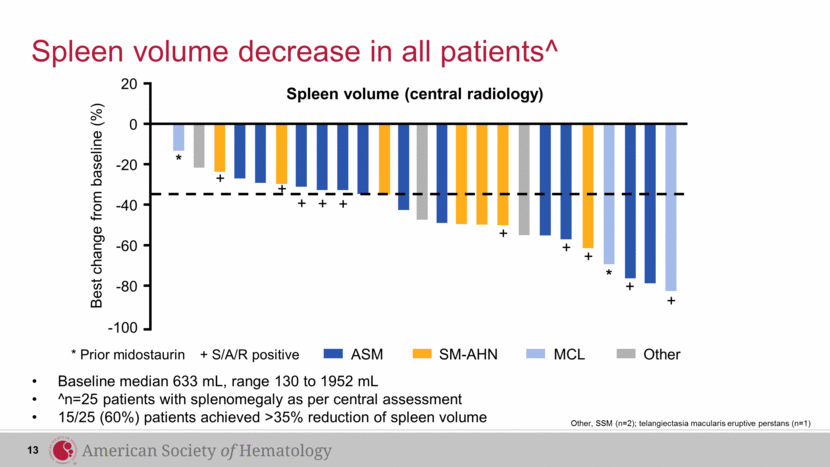
Spleen volume (central radiology) Spleen volume decrease in all patients^ 13 * * + + + + + + + + + + Best change from baseline (%) 0 -20 -40 -60 -80 -100 20 Baseline median 633 mL, range 130 to 1952 mL ^n=25 patients with splenomegaly as per central assessment 15/25 (60%) patients achieved >35% reduction of spleen volume * Prior midostaurin + S/A/R positive ASM SM-AHN MCL Other Other, SSM (n=2); telangiectasia macularis eruptive perstans (n=1) |
|
|
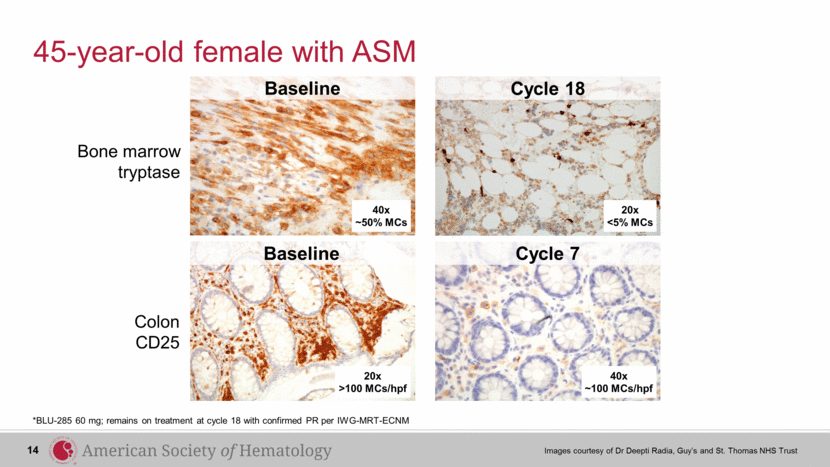
45-year-old female with ASM *BLU-285 60 mg; remains on treatment at cycle 18 with confirmed PR per IWG-MRT-ECNM Images courtesy of Dr Deepti Radia, Guy’s and St. Thomas NHS Trust Bone marrow tryptase Colon CD25 Baseline Cycle 18 40x ~50% MCs Baseline Cycle 7 20x <5% MCs 40x ~100 MCs/hpf 20x >100 MCs/hpf 14 |
|
|
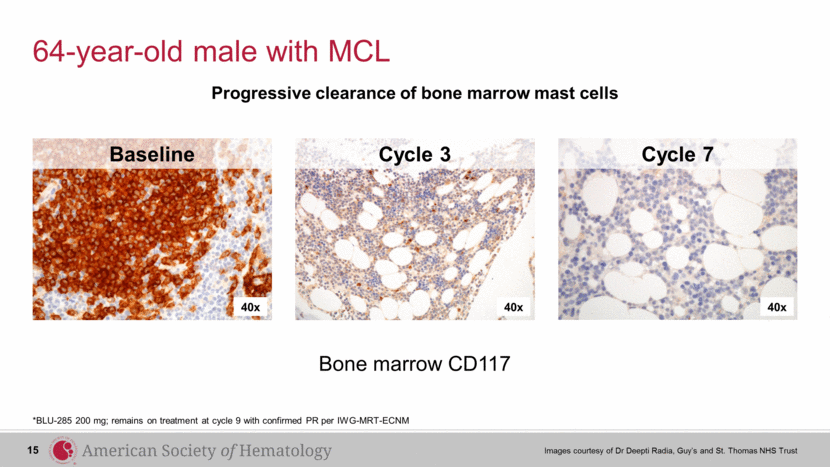
64-year-old male with MCL *BLU-285 200 mg; remains on treatment at cycle 9 with confirmed PR per IWG-MRT-ECNM 40x 40x 40x Baseline Cycle 3 Cycle 7 Bone marrow CD117 Progressive clearance of bone marrow mast cells 15 Images courtesy of Dr Deepti Radia, Guy’s and St. Thomas NHS Trust |
|
|
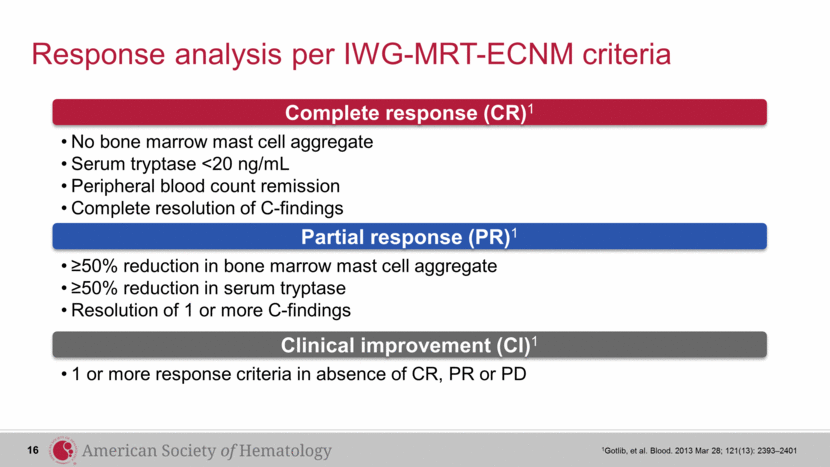
Response analysis per IWG-MRT-ECNM criteria 1Gotlib, et al. Blood. 2013 Mar 28; 121(13): 2393–2401 No bone marrow mast cell aggregate Serum tryptase <20 ng/mL Peripheral blood count remission Complete resolution of C-findings ≥50% reduction in bone marrow mast cell aggregate ≥50% reduction in serum tryptase Resolution of 1 or more C-findings 1 or more response criteria in absence of CR, PR or PD Complete response (CR)1 Partial response (PR)1 Clinical improvement (CI)1 16 |
|
|
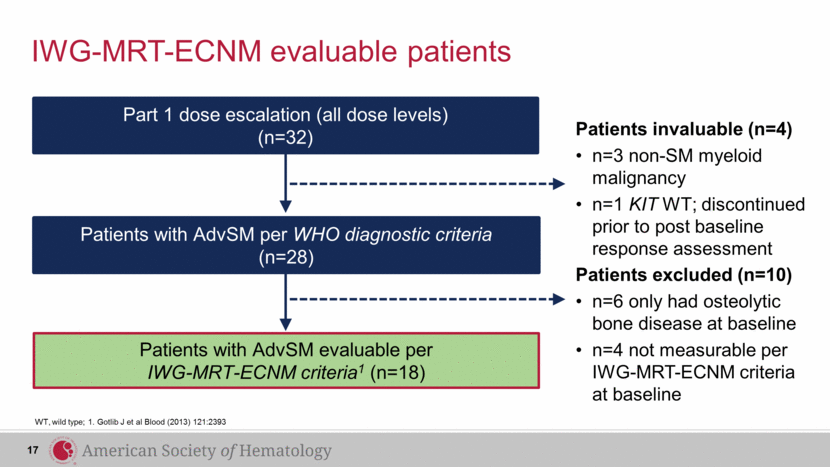
Patients invaluable (n=4) n=3 non-SM myeloid malignancy n=1 KIT WT; discontinued prior to post baseline response assessment Part 1 dose escalation (all dose levels) (n=32) Patients with AdvSM per WHO diagnostic criteria (n=28) IWG-MRT-ECNM evaluable patients WT, wild type; 1. Gotlib J et al Blood (2013) 121:2393 Patients with AdvSM evaluable per IWG-MRT-ECNM criteria1 (n=18) Patients excluded (n=10) n=6 only had osteolytic bone disease at baseline n=4 not measurable per IWG-MRT-ECNM criteria at baseline 17 |
|
|
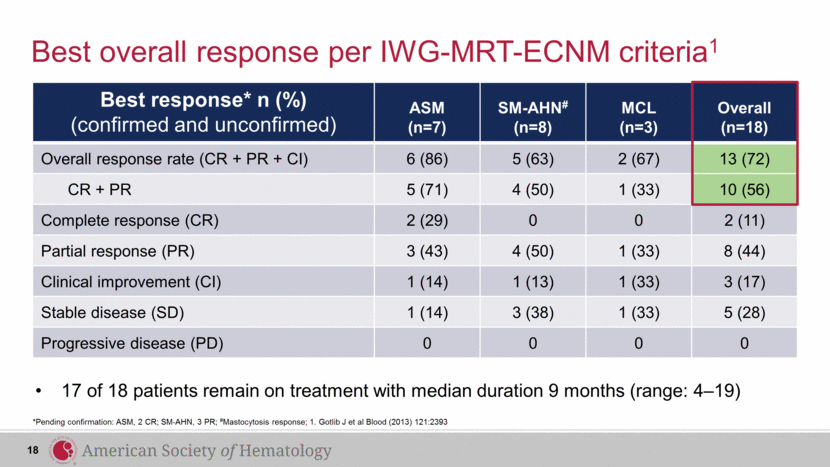
Best response* n (%) (confirmed and unconfirmed) ASM (n=7) SM-AHN# (n=8) MCL (n=3) Overall (n=18) Overall response rate (CR + PR + CI) 6 (86) 5 (63) 2 (67) 13 (72) CR + PR 5 (71) 4 (50) 1 (33) 10 (56) Complete response (CR) 2 (29) 0 0 2 (11) Partial response (PR) 3 (43) 4 (50) 1 (33) 8 (44) Clinical improvement (CI) 1 (14) 1 (13) 1 (33) 3 (17) Stable disease (SD) 1 (14) 3 (38) 1 (33) 5 (28) Progressive disease (PD) 0 0 0 0 Best overall response per IWG-MRT-ECNM criteria1 *Pending confirmation: ASM, 2 CR; SM-AHN, 3 PR; #Mastocytosis response; 1. Gotlib J et al Blood (2013) 121:2393 17 of 18 patients remain on treatment with median duration 9 months (range: 4–19) 18 |
|
|
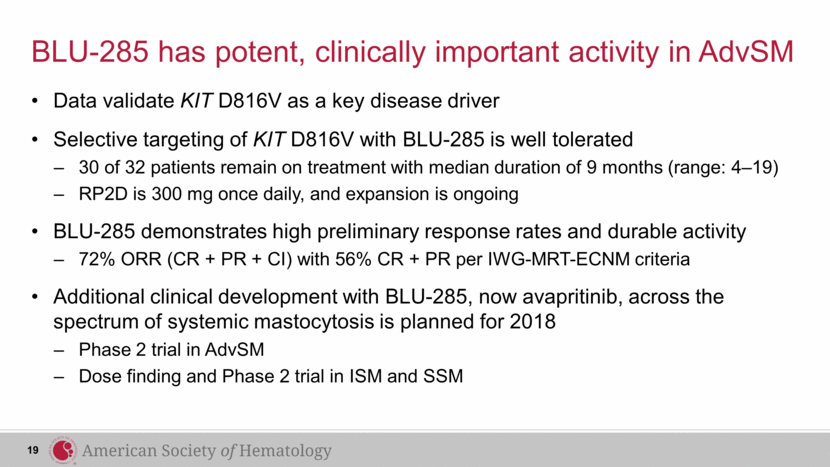
BLU-285 has potent, clinically important activity in AdvSM Data validate KIT D816V as a key disease driver Selective targeting of KIT D816V with BLU-285 is well tolerated 30 of 32 patients remain on treatment with median duration of 9 months (range: 4–19) RP2D is 300 mg once daily, and expansion is ongoing BLU-285 demonstrates high preliminary response rates and durable activity 72% ORR (CR + PR + CI) with 56% CR + PR per IWG-MRT-ECNM criteria Additional clinical development with BLU-285, now avapritinib, across the spectrum of systemic mastocytosis is planned for 2018 Phase 2 trial in AdvSM Dose finding and Phase 2 trial in ISM and SSM 19 |
|
|
Acknowledgments We thank the participating patients, their families, all study co-investigators, and research coordinators at the following institutions: Deepti Radia, Guy's & St Thomas NHS Trust Mark Drummond, Beatson West of Scotland Cancer Centre Elizabeth Hexner, Abramson Cancer Center at the University of Pennsylvania Albert Quiery, University of Michigan Comprehensive Cancer Center Dan DeAngelo, Dana-Farber Cancer Institute Michael Deininger, University of Utah, Huntsman Cancer Institute Srdan Verstovsek, MD Anderson Cancer Center William Robinson, University of Colorado Jason Gotlib, Stanford Cancer Institute We thank Tracy George, Hans Peter Horny, and Maureen Conlan for expert technical analyses We also thank Sarah Jackson, PhD, of iMed Comms, an Ashfield company, who provided editorial writing support funded by Blueprint Medicines 20 |