Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Assertio Therapeutics, Inc | a17-27951_1ex99d1.htm |
| 8-K - 8-K - Assertio Therapeutics, Inc | a17-27951_18k.htm |
Depomed Announces NUCYNTA® Commercialization Agreement with Collegium Pharmaceutical December 4, 2017

Forward Looking Statements 2 This presentation contains forward-looking statements within the meaning established by the Private Securities Litigation Reform Act of 1995 ("Act"). The forward-looking statements are intended to qualify under provisions of the federal securities laws for "safe harbor" treatment established by the Act. Forward-looking statements are based on currently available information, expectations, estimates, assumptions and projections, and management's judgment. Words such as would, expects, intends, plans, believes, estimates, assumes, anticipates, projects, predicts, forecasts or variations of such words or similar expressions are intended to identify forward-looking statements. The forward-looking statements are not guarantees of future performance. They are subject to risks, uncertainty and changes in circumstances. The statements that are not historical facts contained in this presentation are forward-looking statements, including, but not limited to, those related to Depomed’s strategy, plans, objectives, expectations (financial or otherwise) and intentions, future financial results and growth potential, including projected revenues and earnings, expectations regarding periods of exclusivity for the NUCYNTA® franchise, Gralise®, CAMBIA®, Zipsor®, cebranopadol, as well as any other statements that are not historical facts. These forward-looking statements are based on Depomed’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. Factors that may cause a result different than expected or anticipated include, but are not limited to: risks associated with the commercialization of our products, including competitive risks; risks relating to the pending ANDA litigation for Nucynta and Nucynta ER and generic entry into the market; pricing and third party payor reimbursement risks for our products; changes in regulatory policies, procedures, and laws, including FDA regulation of our products and regulation of our promotional practices; risks relating to the successful development of new product candidates; other risks and unforeseen events; as well as other risks related to Depomed's business detailed from time-to-time under the caption "Risk Factors" and elsewhere in Depomed's Securities and Exchange Commission filings and reports, including in its Annual Report on Form 10-K for the year ended December 31, 2016, its most recent Quarterly Report on Form 10-Q. Depomed assumes no duty or obligation to provide public updates of any forward-looking statements. “Safe Harbor” Statement Under the Private Securities Litigation Reform Act of 1995

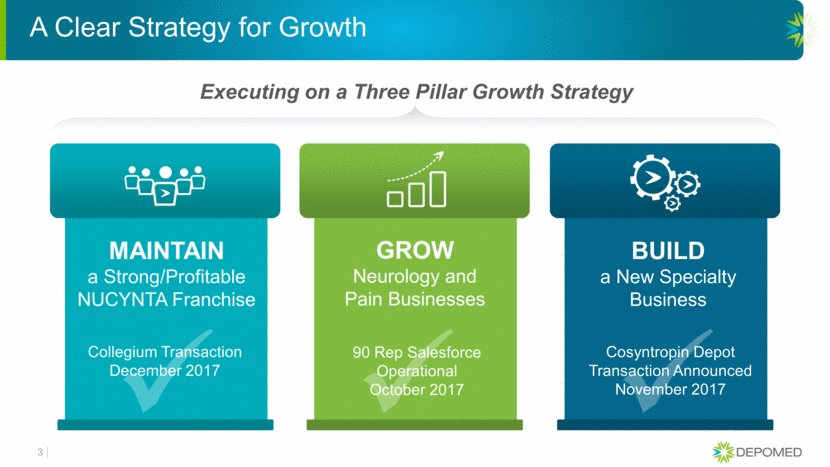
A Clear Strategy for Growth Executing on a Three Pillar Growth Strategy 3 MAINTAIN a Strong/Profitable NUCYNTA Franchise GROW Neurology and Pain Businesses BUILD a New Specialty Business Collegium Transaction December 2017 90 Rep Salesforce Operational October 2017 Cosyntropin Depot Transaction Announced November 2017
Collegium Deal Supports Maintaining a Strong/Profitable NUCYNTA® Franchise Opioid Market Dynamics Demanded Evaluation of Multiple Options: Option 1: Grow into Current Pain Organization through Business Development Activity: Extensive analysis and discussion resulted in conclusion that differentiated/ complementary assets for acquisition/ in-licensing carry too high of an execution risk Option 2: Right Size Current Pain Salesforce from 250 to 130 -150 Representatives: While increasing efficiency, this would have resulted in significant customer disruption and risk of lost sales Option 3: Secure Synergistic Partnership 4 MAINTAIN Partnership with Collegium Supports NUCYNTA® Growth while Enabling Operational Synergies and the Company’s New Strategy

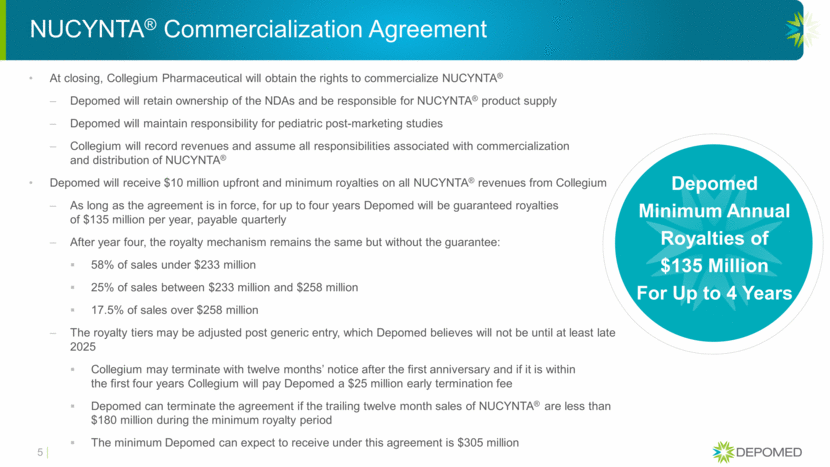
NUCYNTA® Commercialization Agreement At closing, Collegium Pharmaceutical will obtain the rights to commercialize NUCYNTA® Depomed will retain ownership of the NDAs and be responsible for NUCYNTA® product supply Depomed will maintain responsibility for pediatric post-marketing studies Collegium will record revenues and assume all responsibilities associated with commercialization and distribution of NUCYNTA® Depomed will receive $10 million upfront and minimum royalties on all NUCYNTA® revenues from Collegium As long as the agreement is in force, for up to four years Depomed will be guaranteed royalties of $135 million per year, payable quarterly After year four, the royalty mechanism remains the same but without the guarantee: 58% of sales under $233 million 25% of sales between $233 million and $258 million 17.5% of sales over $258 million The royalty tiers may be adjusted post generic entry, which Depomed believes will not be until at least late 2025 Collegium may terminate with twelve months’ notice after the first anniversary and if it is within the first four years Collegium will pay Depomed a $25 million early termination fee Depomed can terminate the agreement if the trailing twelve month sales of NUCYNTA® are less than $180 million during the minimum royalty period The minimum Depomed can expect to receive under this agreement is $305 million 5 Depomed Minimum Annual Royalties of $135 Million For Up to 4 Years
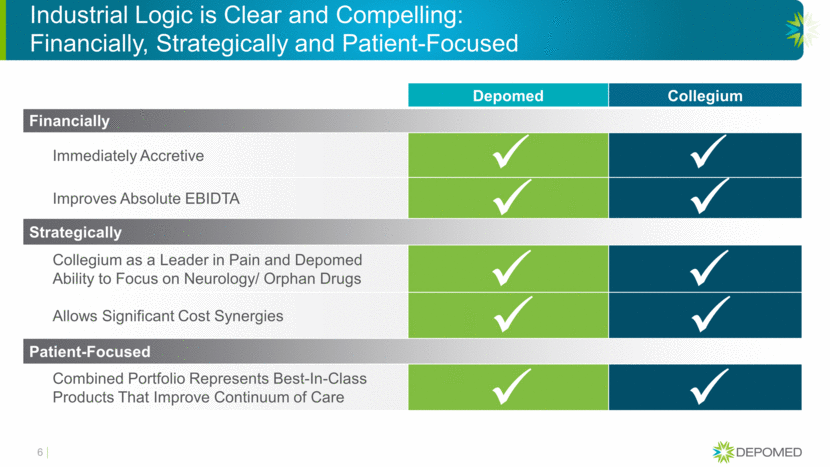
Industrial Logic is Clear and Compelling: Financially, Strategically and Patient-Focused Depomed Collegium Financially Immediately Accretive Improves Absolute EBIDTA Strategically Collegium as a Leader in Pain and Depomed Ability to Focus on Neurology/ Orphan Drugs Allows Significant Cost Synergies Patient-Focused Combined Portfolio Represents Best-In-Class Products That Improve Continuum of Care 6
Collegium: Ideal Partner to Take NUCYNTA® to the Next Level Recognized as an emerging leader in pain management and committed to building a leading pain company Xtampza® ER is the fastest growing Extended Release branded opioid Strong home office, commercial and managed care infrastructures Salesforce focused on Pain Specialists with significant overlap with NUCYNTA customers Collegium desired an Immediate Release product and viewed NUCYNTA® IR as best-in-class Xtampza® ER and NUCYNTA® ER’s different MOAs will be positioned as complementary products: Xtampza® ER: Abuse deterrent oxycodone of choice NUCYNTA® ER: Dual MOA and Diabetic Peripheral Neuropathy (DPN) Offering improves the continuum of care for patients with chronic pain 7 Collegium and Depomed Share a Commitment to Putting the Patient First
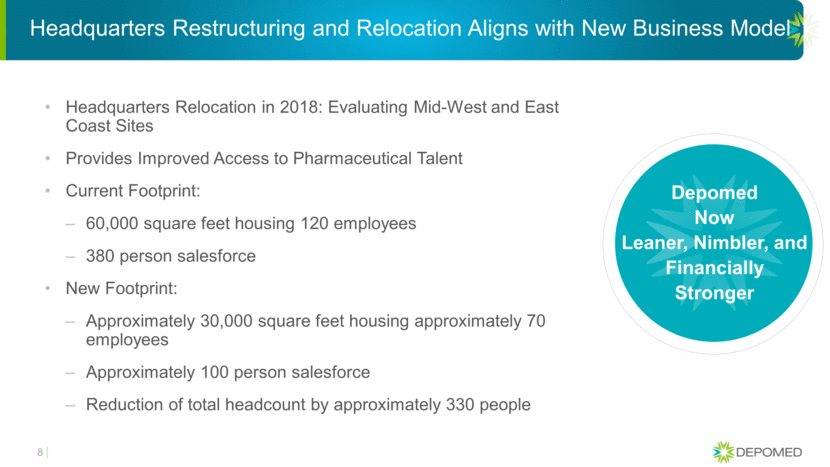
Headquarters Restructuring and Relocation Aligns with New Business Model Headquarters Relocation in 2018: Evaluating Mid-West and East Coast Sites Provides Improved Access to Pharmaceutical Talent Current Footprint: 60,000 square feet housing 120 employees 380 person salesforce New Footprint: Approximately 30,000 square feet housing approximately 70 employees Approximately 100 person salesforce Reduction of total headcount by approximately 330 people Depomed Now Leaner, Nimbler, and Financially Stronger 8
Financial Overview August Moretti SVP Finance and Chief Financial Officer

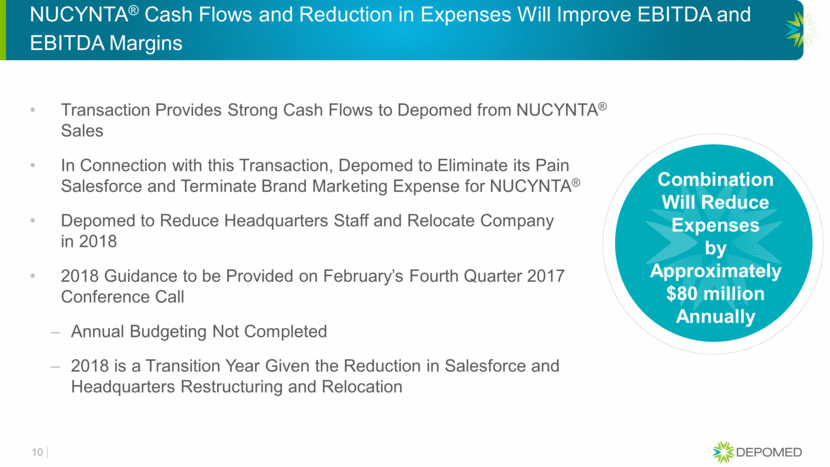
NUCYNTA® Cash Flows and Reduction in Expenses Will Improve EBITDA and EBITDA Margins Transaction Provides Strong Cash Flows to Depomed from NUCYNTA® Sales In Connection with this Transaction, Depomed to Eliminate its Pain Salesforce and Terminate Brand Marketing Expense for NUCYNTA® Depomed to Reduce Headquarters Staff and Relocate Company in 2018 2018 Guidance to be Provided on February’s Fourth Quarter 2017 Conference Call Annual Budgeting Not Completed 2018 is a Transition Year Given the Reduction in Salesforce and Headquarters Restructuring and Relocation Combination Will Reduce Expenses by Approximately $80 million Annually 10
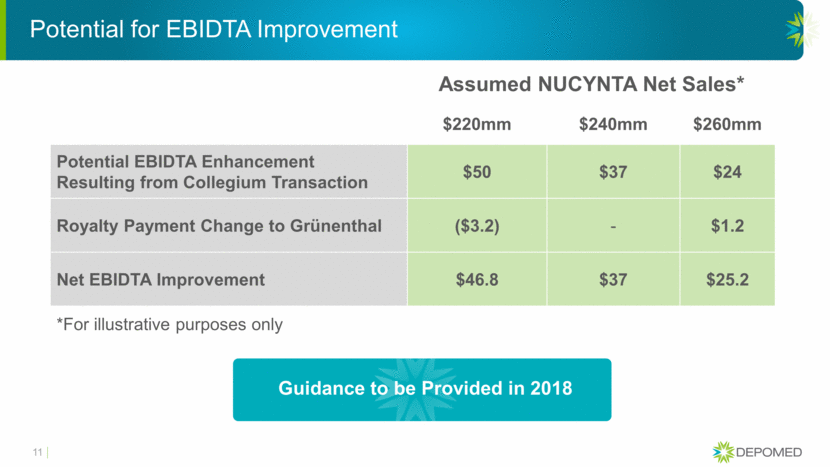
Potential for EBIDTA Improvement Assumed NUCYNTA Net Sales* 11 $220mm $240mm $260mm Potential EBIDTA Enhancement Resulting from Collegium Transaction $50 $37 $24 Royalty Payment Change to Grünenthal ($3.2) - $1.2 Net EBIDTA Improvement $46.8 $37 $25.2 Guidance to be Provided in 2018 *For illustrative purposes only
Questions and Answers

Summary Arthur Higgins President and Chief Executive Officer

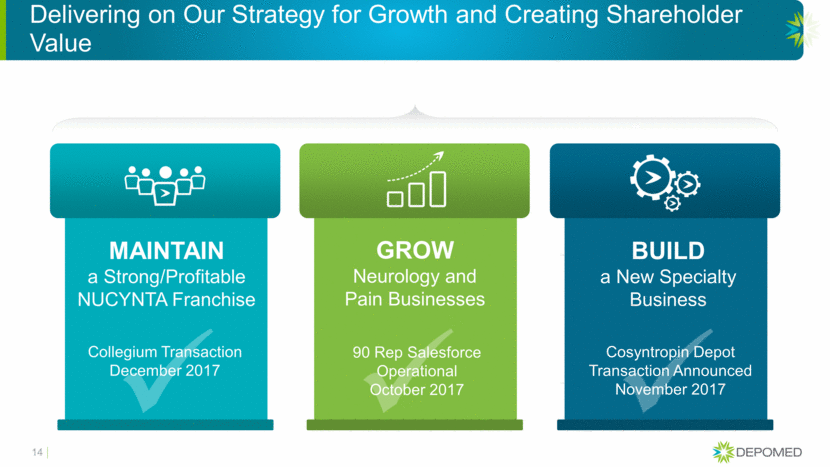
Delivering on Our Strategy for Growth and Creating Shareholder Value 14 MAINTAIN a Strong/Profitable NUCYNTA Franchise GROW Neurology and Pain Businesses BUILD a New Specialty Business Collegium Transaction December 2017 90 Rep Salesforce Operational October 2017 Cosyntropin Depot Transaction Announced November 2017