Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - Fibrocell Science, Inc. | a2233838zex-23_1.htm |
As filed with the Securities and Exchange Commission on November 16, 2017
Registration Statement No. 333-221375
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
FIBROCELL SCIENCE, INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
2834 (Primary Standard Industrial Classification Code Number) |
87-0458888 (I.R.S. Employer Identification Number) |
405 Eagleview Boulevard
Exton, Pennsylvania 19341
(484) 713-6000
(Address, including zip code, and telephone number, including
area code, of registrant's principal executive offices)
John M. Maslowski
President and Chief Executive Officer
Fibrocell Science, Inc.
405 Eagleview Boulevard
Exton, Pennsylvania 19341
(484) 713-6000
(Name, address, including zip code, and telephone number, including area code, of agent for service)
| Copies to: | ||
Steven J. Abrams Hogan Lovells US LLP 1735 Market Street, 23rd Floor Philadelphia, PA 19103 (267) 675-4600 |
Steven M. Skolnick, Esq. Lowenstein Sandler LLP 1251 Avenue of the Americas New York, New York 10020 (212) 262-6700 |
|
Approximate date of commencement of proposed sale to public:
As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ý
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company," and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company ý Emerging growth company o |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. o
|
||||
| Title of Each Class of Securities To Be Registered |
Proposed Maximum Aggregate Offering Price(1) |
Amount of Registration Fee(2)(5) |
||
|---|---|---|---|---|
Common stock, $0.001 par value per share |
$23,322,000.00 | $2,903.59 | ||
Underwriter's warrants to purchase common stock(3) |
— | — | ||
Common stock issuable upon exercise of the underwriter's warrants(4) |
$1,166,100.00 | $145.18 | ||
Total |
$24,488,100.00 | $3,048.77 | ||
|
||||
- (1)
- Estimated
solely for purposes of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. Includes
shares subject to the underwriter's option to purchase additional shares.
- (2)
- Calculated
pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price of the securities registered hereunder.
- (3)
- No
additional registration fee is payable pursuant to Rule 457(g) under the Securities Act.
- (4)
- Represents
warrants to purchase a number of shares of common stock equal to 4% of the number of shares of common stock that are being offered in this offering at an
exercise price equal to 125% of the offering price.
- (5)
- The Registrant previously paid $3,006.68 in connection with the initial filing of the Registration Statement.
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information contained in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED NOVEMBER 16, 2017
PRELIMINARY PROSPECTUS
13,000,000 Shares

Fibrocell Science, Inc.
Common Stock
We are offering 13,000,000 shares of our common stock.
Our common stock is listed on the Nasdaq Capital Market under the symbol "FCSC." The last reported sale price for our common stock on the Nasdaq Capital Market on November 15, 2017 was $1.56 per share. The actual offering price per share will be as determined between us and the underwriter at the time of pricing, and may be at a discount to the current market price.
You should rely only on the information contained herein or incorporated by reference in this prospectus. We have not authorized any other person to provide you with different information.
Investing in our common stock involves a high degree of risk. See "Risk Factors" beginning on page 9 of this prospectus and under similar headings in the documents incorporated by reference into this prospectus.
|
||||
| |
Per Share |
Total |
||
|---|---|---|---|---|
Public offering price |
$ | $ | ||
Underwriting discounts and commissions(1) |
$ | $ | ||
Proceeds, before expenses, to us |
$ | $ | ||
|
||||
- (1)
- See the section titled "Underwriting" for a description of the compensation payable to the underwriter.
We have granted the underwriter the option to purchase up to an additional 1,950,000 shares of common stock to cover over-allotments, if any, at the public offering price less the underwriting discounts and commissions. The underwriter may exercise its option at any time within 30 days from the date of this prospectus. If the underwriter exercises the option in full, the total underwriting discounts and commissions payable by us will be $ , and the total proceeds to us, before expenses, will be $ .
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriter expects to deliver the shares of common stock to purchasers on , 2017.
Sole Book-Running Manager
H.C. Wainwright & Co.
The date of this prospectus is , 2017.
TABLE OF CONTENTS
You should read this prospectus, including the information incorporated by reference herein, and any related free writing prospectus that we have authorized for use in connection with this offering.
You should rely only on the information that we have included or incorporated by reference in this prospectus and any related free writing prospectus that we may authorize to be provided to you. We have not authorized any underwriter, dealer or other person to give any information or to make any representation other than those contained or incorporated by reference in this prospectus or any related free writing prospectus that we may authorize to be provided to you. You must not rely upon any information or representation not contained or incorporated by reference in this prospectus or any related free writing prospectus. This prospectus and any related free writing prospectus do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus or any related free writing prospectus constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
You should not assume that the information contained in this prospectus or any related free writing prospectus is accurate on any date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference herein or therein is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus or any related free writing prospectus is delivered, or securities are sold, on a later date.
This prospectus contains or incorporates by reference summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed or have been incorporated by reference as exhibits to the registration statement of which this prospectus forms a part, and you may obtain copies of those documents as described in this prospectus under the headings "Incorporation by Reference" and "Where You Can Find More Information."
i
This summary highlights information contained in other parts of this prospectus and in the documents we incorporate by reference. Because it is only a summary, it does not contain all of the information that you should consider before investing in shares of our common stock and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere in this prospectus, any applicable free writing prospectus and the documents incorporated by reference herein and therein. You should read all such documents carefully, especially the risk factors and our consolidated financial statements and the related notes included or incorporated by reference herein or therein, before deciding to buy shares of our common stock. Unless the context requires otherwise, references in this prospectus to "Fibrocell," "we," "us" and "our" refer to Fibrocell Science, Inc. and its subsidiaries.
Company Overview
We are an autologous cell and gene therapy company focused on translating personalized biologics into medical breakthroughs for diseases affecting the skin and connective tissue. Our distinctive approach to personalized biologics is based on our proprietary autologous fibroblast technology. Fibroblasts are the most common cell in skin and connective tissue and are responsible for synthesizing extracellular matrix proteins, including collagen and other growth factors, that provide structure and support. Because fibroblasts naturally reside in the localized environment of the skin and connective tissue, they represent an ideal delivery vehicle for proteins targeted to these areas. We target the underlying cause of disease by using fibroblast cells from a patient's skin and genetically modifying them to create localized therapies that are compatible with the unique biology of the patient, which are autologous.
We are focused on discovering and developing localized therapies for diseases affecting the skin and connective tissue, where there are high unmet needs, to improve the lives of patients and their families. In that regard, we commit significant resources to our research and development programs. Currently, all of our research and development operations and focus are on gaining regulatory approvals to commercialize our product candidates in the United States; however, we may seek to expand into international markets in the future.
Our current pipeline consists of the following product candidates which we are developing in collaboration with Intrexon Corporation, or Intrexon:
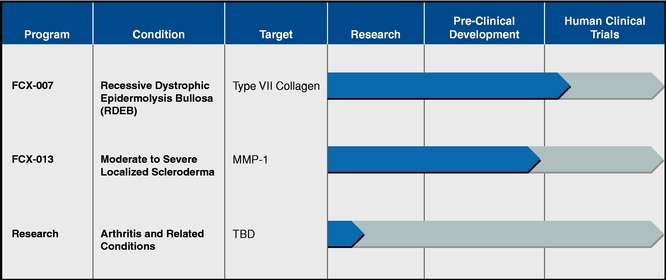
Our most advanced product candidate, FCX-007, is currently in a Phase 1/2 trial for the treatment of recessive dystrophic epidermolysis bullosa, or RDEB. We are also in pre-clinical development of
1
FCX-013, our product candidate for the treatment of moderate to severe localized scleroderma. In addition, we have a third program in the research phase for the treatment of arthritis and related conditions. See further discussion of our gene-therapy product candidates under the heading "Development Programs" below.
Intrexon Collaborations
We collaborate with Intrexon, a related party, through two distinct exclusive channel collaboration agreements, consisting of the Exclusive Channel Collaboration Agreement entered into in October 2012, as amended, or the 2012 ECC, and the Exclusive Channel Collaboration Agreement entered into in December 2015, or the 2015 ECC. Pursuant to these agreements, we engage Intrexon for support services for the research and development of product candidates covered under the respective agreements and reimburse Intrexon for its cost for time and materials for such work. In addition, we are required to pay Intrexon quarterly cash royalties on all products developed under the 2012 ECC in an amount equal to 7% of aggregate quarterly net sales up to $25 million, plus 14% on aggregate quarterly net sales greater than $25 million. Under the 2015 ECC, we are required to pay Intrexon development milestones of up to $30 million for the first product developed under the 2015 ECC (and development milestones of up to $55 million for each subsequent product developed under the 2015 ECC) and commercialization milestones of up to $22.5 million for each product developed, a low double-digit royalty on our net sales of such products and half of any sublicensing revenues we receive from third parties in consideration for sublicenses granted by us with respect to such products but only to the extent such sublicensing revenues are not included in net sales subject to royalties.
We are developing FCX-007 and FCX-013 under the 2012 ECC and we are in the research phase for a gene-therapy treatment for arthritis and related conditions under the 2015 ECC.
Development Programs
FCX-007 for Recessive Dystrophic Epidermolysis Bullosa
RDEB is the most severe form of dystrophic epidermolysis bullosa, or DEB, a congenital, progressive, devastatingly painful and debilitating genetic disorder that often leads to death. RDEB is caused by a mutation of the COL7A1 gene, the gene which encodes for type VII collagen, or COL7, a protein that forms anchoring fibrils. Anchoring fibrils hold together the layers of skin, and without them, skin layers separate causing severe blistering, open wounds and scarring in response to friction, including normal daily activities like rubbing or scratching. Children who inherit this condition are often called "butterfly children" because their skin can be as fragile as a butterfly's wings. We estimate that there are approximately 1,100 - 2,500 RDEB patients in the U.S. Currently, treatments for RDEB address only the sequelae, including daily bandaging (which can cost a patient in excess of $10,000 per month), hydrogel dressings, antibiotics, feeding tubes and surgeries.
Our lead product candidate, FCX-007, is in clinical development for the treatment of RDEB. FCX-007 is a genetically-modified autologous fibroblast that encodes the gene for COL7 for localized treatment of RDEB and is being developed in collaboration with Intrexon. By genetically modifying autologous fibroblasts ex vivo to produce COL7, culturing them and then treating blisters and wounds locally via injection, FCX-007 offers the potential to address the underlying cause of the disease by providing high levels of COL7 directly to the affected areas, thereby avoiding systemic treatment. In addition, we believe the autologous nature of the cells, localized delivery, use of an integrative vector and the low turnover rate of the protein will contribute to long-term persistence of the COL7 produced by FCX-007.
FCX-007 has received Orphan Drug Designation for the treatment of DEB, including RDEB, Rare Pediatric Disease Designation for the treatment of RDEB and Fast Track Designation for the treatment of RDEB from the United States Food and Drug Administration, or FDA.
2
Phase 1/2 Clinical Trial of FCX-007 for RDEB
The primary objective of this open-label trial is to evaluate the safety of FCX-007 in RDEB patients. Additionally, the trial will assess (i) the mechanism of action of FCX-007 through the evaluation of COL7 expression and the presence of anchoring fibrils and (ii) the efficacy of FCX-007 through intra-subject paired analysis of target wound area by comparing FCX-007 treated wounds to untreated wounds in Phase 1 and to wounds administered with sterile saline in Phase 2 through the evaluation of digital imaging of wounds. Twelve patients are targeted to be treated with FCX-007 consisting of six adults in the Phase 1 portion of the trial and six patients in the Phase 2 portion of the trial. Prior to conducting clinical trials on pediatric patients, we are required to obtain allowance from the FDA by submitting evidence of FCX-007 safety and benefit in adult patients and data from its completed pre-clinical toxicology study.
We are actively recruiting adult patients to complete enrollment in the Phase 1 portion of the trial and currently have four of the six adult patients enrolled. The patients in the Phase 1 portion of the trial are divided into two equal cohorts in order to evaluate the safety of FCX-007 in each population type. One cohort is comprised of patients who have positive expression of the non-collagenous portion of the COL7 protein (NC1+) and the other cohort is comprised of patients who do not express the non-collagenous portion of the protein (NC1–). Patients enrolled to date fulfilled the NC1+ cohort and also provided the first patient for the NC1– cohort. Two more patients are required for the NC1– cohort to complete enrollment in the Phase 1 portion of the trial. The clinical trial protocol is designed to allow a cohort to move into the Phase 2 portion of the trial even if the other cohort is still enrolling or in the follow-up evaluation period.
The first adult patient in the NC1+ cohort in the Phase 1 portion of the Phase 1/2 clinical trial was dosed in the first quarter of 2017. In April 2017, the Data Safety Monitoring Board, or DSMB, recommended continuation of the Phase 1/2 clinical trial of FCX-007 for the treatment of RDEB, following a planned review of safety data from the first patient treated in the Phase 1 portion of the trial. No product-related adverse events were reported. Based on the DSMB's recommendation, the remaining two patients in the NC1+ cohort in the Phase 1 portion of the trial were dosed in June 2017.
In September 2017, we reported interim results from the Phase 1 portion of the Phase 1/2 clinical trial of FCX-007. Three adult NC1+ patients were dosed with a single intradermal injection session of FCX-007 in the margins of and across targeted wounds, as well as in separate intact skin sites. Five wounds were treated on the three subjects, ranging in size from 4.4cm2 to 13.1cm2. Data from these patients show FCX-007 was well-tolerated through 12 weeks post-administration. There were no serious adverse events and no product related adverse events reported.
The targeted wounds were evaluated during a monitoring period prior to dosing and were observed to be open for up to eight months. Compared to the baseline measurement collected at Day 0 before the single intradermal injection session of FCX-007, at four weeks post-administration 100% (5/5) of wounds were ³ 75% healed. At 12 weeks post-administration, 80% (4/5) of wounds were ³ 70% healed. The wound that was < 70% healed from the twelve week data set was biopsied by the investigator in the middle of the wound bed rather than on the wound edge, which we believe may have contributed to the wound's instability. We plan to continue to monitor this and other wounds throughout the follow-up visits.
Various pharmacology signals for vector DNA, COL7 mRNA, or COL7 protein expression were detected throughout the data set in each patient for one or more assays up to 12 weeks post-administration (qPCR, electron microscopy or immunofluorescence). Anchoring fibrils have not been detected to date, whereas expressed COL7 mRNA and COL7 protein have been confirmed in multiple patient samples including one that detected linear expression of COL7 at the basement
3
membrane zone. The DSMB for the trial reviewed the interim data and concluded that safety and potential benefit were established, and allowed continuation of enrollment and dosing.
We plan to use the interim data from the Phase 1 portion of the Phase 1/2 clinical trial to support a future filing for Regenerative Medicine Advanced Therapy or Breakthrough Therapy Designation for FCX-007. We also believe the interim data will support an FDA filing to obtain allowance for pediatric enrollment in the Phase 2 portion of the Phase 1/2 clinical trial, which we expect to initiate in the first quarter of 2018. The FDA previously required us to file safety and potential benefit data from adults in the Phase 1 portion of the trial for review prior to enrolling pediatric patients.
With data from the first three patients meeting the primary trial objective of safety, we plan to increase expression and dosing of FCX-007. We expect to perform additional dosing of adult patients in the Phase 1 portion of the trial in the fourth quarter of 2017. In addition, we enrolled an RDEB adult as the first patient of the Phase 2 portion of the Phase 1/2 clinical trial of FCX-007, and we expect to initiate the Phase 2 portion of the trial, through the additional dosing of adult patients, in the fourth quarter of 2017. Furthermore, subject to FDA allowance, we expect to initiate enrollment of pediatric patients in the Phase 2 portion of the trial in the first quarter of 2018.
We have designated our existing, current good manufacturing practices, or cGMP, cell therapy manufacturing facility in Exton, PA as the production site for FCX-007 after incorporation into FCX-007's IND. The facility will be used for the remaining clinical and future commercial manufacture of FCX-007, with capacity to serve the U.S. market for RDEB. The approximately 13,000 square foot facility previously supported our commercial autologous fibroblast manufacturing, with multiple FDA inspections conducted at the site. The facility includes cleanroom cell therapy manufacturing, quality control testing, cryogenic storage, shipping/receiving and warehousing space.
FCX-013 for Moderate to Severe Localized Scleroderma
Localized scleroderma is a chronic autoimmune skin disorder that manifests as excess production of extracellular matrix, specifically collagen, resulting in thickening of the skin and connective tissue. Localized scleroderma encompasses several subtypes which are classified based on the depth and pattern of the lesion(s). The moderate to severe forms of the disorder include linear, generalized, deep, pansclerotic and mixed morphea subtypes. Linear scleroderma is the most common subtype in juvenile localized scleroderma and is associated with high morbidity and lifelong disability. Linear lesions of the limbs may cause limb length discrepancy due to impaired growth, muscle atrophy and joint contractures-orthopedic complications are reported in 30% to 50% of patients. Current treatments for localized scleroderma, which include systemic or topical corticosteroids, UVA light therapy and physical therapy, only address the symptoms of the disorder. We estimate that there are approximately 90,000 patients in the U.S. considered to have moderate to severe localized scleroderma.
Our second gene-therapy product candidate, FCX-013, is in pre-clinical development for the treatment of moderate to severe localized scleroderma. FCX-013 is an autologous fibroblast genetically-modified using lentivirus and encoded for matrix metalloproteinase 1 (MMP-1), the protein responsible for breaking down collagen. FCX-013 incorporates Intrexon's proprietary RheoSwitch Therapeutic System®, or RTS®, a biologic switch activated by an orally administered compound to control protein expression at the site of localized scleroderma lesions. FCX-013 is designed to be injected under the skin at the location of the fibrotic lesions where the genetically-modified fibroblast cells will produce MMP-1 to break down excess collagen accumulation. With the FCX-013 therapy, the patient will take an oral compound to facilitate protein expression. Once the fibrosis is resolved, the patient will stop taking the oral compound which will halt further MMP-1 production.
We have successfully completed a proof-of-concept study for FCX-013 in which the primary objective was to determine whether FCX-013 had the potential to reduce dermal thickness in fibrotic tissue. In this study, FCX-013 was evaluated in a bleomycin-induced scleroderma model utilizing severe
4
combined immunodeficiency, or SCID, mice. Data from the study demonstrated that FCX-013 reduced dermal thickness of fibrotic tissue to levels similar to that of the non-treated control and further reduced the thickness of the sub-dermal muscle layer. Based upon these data and the FDA's feedback to our pre-Investigational New Drug application, or IND, briefing package, we advanced FCX-013 into a pre-clinical dose-ranging study which has been completed. We expect to complete a toxicology/biodistribution study and submit an IND application for FCX-013 to the FDA in the fourth quarter of 2017. In addition, we expect to initiate a human safety clinical trial for FCX-013 in 2018.
FCX-013 has received Orphan Drug Designation from the FDA for the treatment of localized scleroderma and Rare Pediatric Disease Designation for moderate to severe localized scleroderma.
New Gene Therapy Program for Arthritis and Related Conditions
Arthritis is a broad term that covers a group of more than 100 different types of diseases that affect the joints, as well as connective tissues and organs, including the skin. According to the Centers for Disease Control and Prevention, arthritis—characterized by joint inflammation, pain and decreased range of motion—is the United States' most common cause of disability affecting more than 52 million adults as well as 300,000 children at a cost exceeding $120 billion.
Our third gene-therapy program is in the research phase and is focused on the treatment of arthritis and related conditions. Our goal is to deliver a protein therapy locally to the joint to provide sustained efficacy while avoiding key side effects typically associated with systemic therapy.
Risks Associated with Our Business
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the "Risk Factors" section of this prospectus immediately following this prospectus summary and in Part I, Item 1A "Risk Factors" of our Annual Report on Form 10-K filed with the Securities and Exchange Commission, or SEC, on March 9, 2017, in Part II, Item 1A. "Risk Factors" of our Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2017, filed with the SEC on August 9, 2017 and in Part II, Item 1A. "Risk Factors" of our Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2017, filed with the SEC on November 13, 2017, which are incorporated by reference in this prospectus. These risks include the following:
- •
- We have incurred significant losses since our inception, which we anticipate will continue for the foreseeable future. As of
September 30, 2017, we had an accumulated deficit of $181.7 million.
- •
- We have never generated significant revenue from product sales and may never be profitable.
- •
- Our business is highly dependent on the success of FCX-007, our lead product candidate.
- •
- We may encounter difficulties enrolling or retaining subjects in our clinical trials.
- •
- Clinical product development is costly and time consuming and involves uncertain outcomes, and results of earlier studies and trials may not be
predictive of future trial results.
- •
- We may not be able to submit INDs, commence clinical trials or report data on the timelines we expect, and even if we are able to, the FDA may
not permit us to proceed.
- •
- If our product candidates fail to demonstrate quality, safety and efficacy to the satisfaction of regulatory authorities, we may incur
additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
- •
- The net proceeds from this offering will not be sufficient to commercialize any of our product candidates and we will need to obtain additional funding for commercialization. Failure to
5
- •
- There is substantial doubt relating to our ability to continue as a going concern as determined by management and as reflected in the report of
our independent public accounting firm. We will need to raise substantial additional capital to fund our operations.
- •
- We have relied and expect to rely on third parties to conduct aspects of our research and development and clinical trials. If they terminate
our arrangements, fail to meet deadlines or perform in an unsatisfactory manner, our business could be harmed.
- •
- The potential commercial success of any current or future product candidate will depend upon the degree of market acceptance by physicians, patients, third-party payors and others in the medical community.
obtain additional funding when needed may force us to delay, limit or terminate our product development efforts or other operations.
Corporate Information
Our corporate headquarters is located at 405 Eagleview Boulevard, Exton, Pennsylvania 19341. Our phone number is (484) 713-6000. Our corporate website is www.fibrocell.com. We make available free of charge on our website our annual, quarterly and current reports, including amendments to such reports, as soon as reasonably practicable after we electronically file such material with, or furnish such material to, the SEC. Information contained on our website is not incorporated by reference into this prospectus, and you should not consider information contained on our website as part of this prospectus.
6
Common stock offered by us |
13,000,000 shares (14,950,000 shares if the underwriter's option to purchase additional shares is exercised in full). | |
Common stock to be outstanding after this offering |
27,719,987 shares (29,669,987 shares if the underwriter's option to purchase additional shares is exercised in full). |
|
Option to purchase additional shares |
The underwriter has the option to purchase from us up to a maximum of 1,950,000 additional shares of common stock. The underwriter can exercise this option at any time within 30 days from the date of this prospectus. |
|
Use of proceeds |
We estimate that the net proceeds to us from this offering, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, will be approximately $18.4 million ($21.2 million if the underwriter's option to purchase additional shares is exercised in full), assuming an offering price of $1.56 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on November 15, 2017. The actual offering price per share will be as determined between us and the underwriter at the time of pricing, and may be at a discount to the current market price. We currently intend to use the net proceeds from this offering for the continued clinical and pre-clinical development of our product candidates, FCX-007 and FCX-013, and for the research of potential product candidates under the 2015 ECC, and for other general corporate purposes, which may include working capital, research and development expenditures, the funding of in-licensing agreements for product candidates, additional technologies or other forms of intellectual property, expenditures relating to manufacturing infrastructure and other capital expenditures and general and administrative expenses. See "Use of Proceeds" on page 17. |
|
Risk Factors |
An investment in our common stock involves a high degree of risk. See "Risk Factors" beginning on page 9 of this prospectus and the similarly titled sections in the documents incorporated by reference into this prospectus. |
|
Nasdaq Capital Market symbol |
FCSC. |
Outstanding Shares
The number of shares of our common stock to be outstanding after this offering is based on 14,719,987 shares of our common stock outstanding as of September 30, 2017, and excludes:
- •
- 5,515,404 shares of our common stock issuable upon the conversion of our outstanding convertible promissory notes, including accrued interest
thereon, payable in shares of our common stock, outstanding as of September 30, 2017;
- •
- 3,512,000 shares of our common stock issuable upon the conversion of our Series A Convertible Preferred Stock, par value $0.001 per share, or the Series A Preferred Stock, including accrued
7
- •
- 1,116,350 shares of our common stock issuable upon the exercise of stock options outstanding as of September 30, 2017, at a weighted
average exercise price of $13.38 per share, of which stock options to purchase 660,225 shares of our common stock were then exercisable;
- •
- 10,411,177 shares of our common stock issuable upon the exercise of warrants at a weighted average exercise price of $4.85 per share, all of
which warrants were then exercisable;
- •
- an aggregate of 1,403,899 shares of our common stock reserved for future grants of stock options (or other similar equity instruments) under
the Fibrocell Science, Inc. 2009 Equity Incentive Plan, as amended, or the Equity Incentive Plan; and
- •
- 520,000 shares (or 598,000 shares if the underwriter's option to purchase additional shares is exercised in full) of our common stock issuable upon exercise of the warrants being issued to the underwriter in connection with this offering.
dividends thereon, payable in shares of our common stock, outstanding as of September 30, 2017;
Additionally, one of our outstanding warrants, which is currently exercisable for 46,430 shares of our common stock at an exercise price per share of $2.10, contains so-called full-ratchet anti-dilution provisions which may be triggered by the issuance of the shares of our common stock being offered hereby or upon any future issuance by us of shares of our common stock or common stock equivalents at a per share price below the then-exercise price of the warrant, subject to some exceptions. See "Dilution" on page 21 of this prospectus for more information about these possible anti-dilution adjustments.
Except as otherwise indicated herein, all information in this prospectus, including the number of shares that will be outstanding after this offering, does not assume or give effect to the exercise of options or warrants outstanding as of September 30, 2017.
Unless otherwise indicated, all information contained in this prospectus assumes no exercise by the underwriter of its over-allotment option.
8
An investment in our common stock involves a high degree of risk. Before deciding whether to invest in our common stock, you should carefully consider the risks described below and those discussed under the Section captioned "Risk Factors" contained in our Annual Report on Form 10-K for the fiscal year ended December 31, 2016, our Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2017 and our Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2017, which are incorporated by reference in this prospectus, together with the information included in this prospectus and documents incorporated by reference herein, and in any free writing prospectus that we have authorized for use in connection with this offering. If any of these risks actually occurs, our business, financial condition, results of operations or cash flow could be harmed. This could cause the trading price of our common stock to decline, resulting in a loss of all or part of your investment.
Risks Related to this Offering
We have a significant number of outstanding convertible notes, convertible preferred stock, warrants and stock options, and future sales of the underlying shares of common stock could adversely affect the market price of our common stock.
As of September 30, 2017, we had outstanding convertible notes convertible for 5,515,404 shares of our common stock (including accrued interest thereon), outstanding convertible preferred stock convertible for 3,512,000 shares of our common stock (including accrued interest thereon), outstanding warrants exercisable for 10,411,177 shares of our common stock at a weighted average exercise price of $4.85 per share, all of which warrants were then exercisable, and outstanding stock options exercisable for 1,116,350 shares of our common stock at a weighted average exercise price of $13.38 per share, of which stock options to purchase 660,625 shares of our common stock were then exercisable. One of our outstanding warrants, which is currently exercisable for 46,430 shares of our common stock at an exercise price per share of $2.10, contains so-called full-ratchet anti-dilution provisions which may be triggered by the issuance of the shares of our common stock being offered hereby or upon any future issuance by us of shares of our common stock or common stock equivalents at a per share price below the then-exercise price of the warrant, subject to some exceptions. Upon conversion of these notes or preferred stock or exercise of these warrants or stock options, we would issue additional shares of our common stock. As a result, our current stockholders as a group would own a substantially smaller interest in us and may have less influence on our management and policies than they now have. Furthermore, the holders may sell these shares in the public markets from time to time, without limitations on the timing, amount or method of sale. As our stock price rises, the holders may convert more of their notes or preferred stock or exercise more of their warrants or stock options and sell a large number of shares. This could cause the market price of our common stock to decline.
We may be required to raise additional financing by issuing new securities with terms or rights superior to those of our existing securityholders, which could adversely affect the market price of shares of our common stock and our business.
We will require additional financing to fund future operations, including expansion in current and new markets, development and acquisition, capital costs and the costs of any necessary implementation of technological innovations or alternative technologies. We may not be able to obtain financing on favorable terms, if at all. If we raise additional funds by issuing equity securities, the percentage ownership of our current stockholders will be reduced, and the holders of the new equity securities may have rights superior to those of our existing securityholders, which could adversely affect the market price of our common stock and the voting power of shares of our common stock. If we raise additional funds by issuing debt securities, the holders of these debt securities would similarly have some rights senior to those of our existing securityholders, and the terms of these debt securities could impose
9
restrictions on operations and create a significant interest expense for us which could have a materially adverse effect on our business.
Management will have broad discretion as to the use of the proceeds from this offering, and we may not use the proceeds effectively.
Our management will have broad discretion with respect to the use of proceeds of this offering, including for any of the purposes described in the section of this prospectus entitled "Use of Proceeds." You will be relying on the judgment of our management regarding the application of the proceeds of this offering. The results and effectiveness of the use of proceeds are uncertain, and we could spend the proceeds in ways that you do not agree with or that do not improve our results of operations or enhance the value of our common stock. Our failure to apply these funds effectively could harm our business, delay the development of our product candidates and cause the price of our common stock to decline.
You will experience immediate and substantial dilution in the net tangible book value per share of the common stock you purchase.
Since the public offering price for our common stock in this offering is substantially higher than the net tangible book deficit per share of our common stock outstanding prior to this offering, you will suffer immediate and substantial dilution in the net tangible book value of the common stock you purchase in this offering. See the section titled "Dilution" below for a more detailed discussion of the dilution you will incur if you purchase shares in this offering.
Issuances of shares of our common stock or securities convertible into or exercisable for shares of our common stock following this offering, as well as the exercise of options and warrants outstanding, will dilute your ownership interests and may adversely affect the future market price of our common stock.
The issuance of additional shares of our common stock could be dilutive to stockholders if they do not invest in future offerings. We intend to use the net proceeds from this offering for the continued clinical and pre-clinical development of our product candidates, FCX-007 and FCX-013, and for the research of potential product candidates under the 2015 ECC, and for other general corporate purposes, which may include working capital, research and development expenditures, the funding of in-licensing agreements for product candidates, additional technologies or other forms of intellectual property, expenditures relating to manufacturing infrastructure and other capital expenditures and general and administrative expenses. We may seek additional capital through a combination of private and public equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements, which may cause your ownership interest to be diluted.
In addition, we have a significant number of options and warrants to purchase shares of our common stock outstanding. If these securities are converted or exercised, you may incur further dilution. Moreover, to the extent that we issue additional convertible notes, convertible preferred stock, options or warrants to purchase, or securities convertible into or exchangeable for, shares of our common stock in the future and those options, warrants or other securities are exercised, converted or exchanged, stockholders may experience further dilution.
A significant portion of our total outstanding shares are eligible to be sold into the market, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market, either by us or by our current stockholders, or the perception that these sales could occur, could cause a decline in the market price of our securities. Such sales, along with any other market transactions, could adversely affect the market price of our common stock.
10
Upon completion of this offering, based on our shares outstanding as of September 30, 2017, we will have 27,719,987 shares of common stock outstanding based on the issuance and sale of 13,000,000 shares of our common stock in this offering. Of these shares, only 5,548,810 are subject to a contractual lock-up with the underwriter for this offering for a period of 90 days following this offering. These shares can be sold, subject to any applicable volume limitations under federal securities laws, after the earlier of the expiration of, or release from, the 90 day lock-up period. The balance of our outstanding shares of common stock, including any shares purchased in this offering other than shares purchased by our current stockholders who are also subject to the contractual lock-up, may be resold into the public market immediately without restriction, unless owned or purchased by our affiliates. Moreover, some of the holders of our common stock have the right, subject to specified conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders.
As of September 30, 2017, there were approximately 2,511,915 shares subject to outstanding options or that are otherwise issuable under our Equity Incentive Plan, all of which shares we have registered under the Securities Act of 1933, as amended, or the Securities Act, on a registration statement on Form S-8. These shares can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described above, to the extent applicable.
11
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this prospectus or the documents incorporated herein by reference regarding our strategy, future operations, future product research or development, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words "anticipate," "believe," "goals," "estimate," "expect," "intend," "may," "might," "plan," "predict," "project," "target," "potential," "will," "would," "could," "should," "continue" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
The forward-looking statements in this prospectus include, among other things, statements about:
- •
- our expectations related to the use of the proceeds from this offering;
- •
- our expectation that our existing cash resources, plus the net proceeds of this offering, will be sufficient to enable us to fund our
operations into the second quarter of 2019;
- •
- future expenses and capital expenditures;
- •
- our estimates regarding expenses, future revenues, capital requirements and needs for, and ability to obtain, additional financing;
- •
- our plans to address our future capital requirements and the consequences of failing to do so;
- •
- our need to raise substantial additional capital to fund our operations;
- •
- our expectation to initiate the Phase 2 portion of the Phase 1/2 clinical trial of FCX-007 in the fourth quarter of 2017;
- •
- our expectation to initiate enrollment of pediatric patients in the Phase 2 portion of our Phase 1/2 clinical trial of
FCX-007 in the first quarter of 2018;
- •
- our expectation to complete a toxicology/biodistribution study and submit an IND for FCX-013 to the FDA in the fourth quarter of 2017;
- •
- our expectation to initiate a human safety clinical trial for FCX-013 in 2018;
- •
- our product development goals under our collaborations with Intrexon for all of our product candidates;
- •
- the potential benefits of Fast Track, Orphan Drug and Rare Pediatric Disease designations;
- •
- the potential advantages of our product candidates and technologies; and
- •
- the effect of legal and regulatory developments.
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this prospectus, particularly under "Risk Factors" on page 9 of this prospectus and the documents incorporated herein that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures, collaborations or investments we may make.
You should read this prospectus and the documents that we have filed as exhibits to this prospectus completely and with the understanding that our actual future results may be materially different from what we expect.
12
Except as required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or developments. You should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. Before deciding to purchase our securities, you should carefully consider the risk factors discussed and incorporated by reference in this prospectus and the documents incorporated herein.
13
You should read the following selected financial data together with our financial statements and the related notes contained in Item 8 of Part II of our Annual Report on Form 10-K for the fiscal year ended December 31, 2016 and in Item 1 of Part I of our Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2017, which are incorporated by reference into this prospectus, except that share and per share information for the periods ended December 31, 2016 and December 31, 2015 have been revised to reflect the 1-for-3 reverse stock split of our issued and outstanding shares of common stock effective at the close of business on March 10, 2017.
We have derived the statements of operations data for each of the two years ended December 31, 2016 and December 31, 2015 from the audited financial statements contained in Item 8 of Part II of our Annual Report on Form 10-K for the fiscal year ended December 31, 2016. We have derived the statements of operation data for the nine months ended September 30, 2017 and September 30, 2016 from our unaudited historical condensed financial statements and related notes thereto contained in Item 1 of Part I of our Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2017.
The historical financial information set forth below may not be indicative of our future performance and should be read together with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our historical financial statements and notes to those statements included in Item 7 of Part II and Item 8 of Part II, respectively, of our Annual Report on Form 10-K for the fiscal year ended December 31, 2016, and any amendment or update thereto reflected in subsequent filings with the SEC, and all other annual, quarterly and other reports that we file with the SEC after the date of the initial registration statement of which this prospectus forms a part and that also are incorporated herein by reference.
14
Fibrocell Science, Inc.
Statement of Operations Summary
($ in thousands except share and per share data)
| |
Nine Months Ended September 30, (Unaudited) |
(Adjusted for Stock Split) Year Ended December 31, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2017 | 2016 | 2016 | 2015 | |||||||||
Total revenue |
— | 318 | $ | 355 | $ | 492 | |||||||
Total cost of revenue |
— | 697 | 697 | 722 | |||||||||
| | | | | | | | | | | | | | |
Gross loss |
— | (379 | ) | (342 | ) | (230 | ) | ||||||
Operating Expenses |
14,077 | 21,625 | 26,137 | 37,177 | |||||||||
| | | | | | | | | | | | | | |
Operating loss |
(14,077 | ) | (22,004 | ) | (26,479 | ) | (37,407 | ) | |||||
Other income (expense) |
(5,063 | ) | 10,206 | 11,187 | 2,954 | ||||||||
| | | | | | | | | | | | | | |
Loss before income taxes |
(19,140 | ) | (11,798 | ) | (15,292 | ) | (34,453 | ) | |||||
Income taxes |
— | — | — | — | |||||||||
| | | | | | | | | | | | | | |
Net loss |
(19,140 | ) | (11,798 | ) | $ | (15,292 | ) | $ | (34,453 | ) | |||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Per Share Information: |
|||||||||||||
Net loss: |
|||||||||||||
Basic* |
(1.58 | ) | (0.81 | ) | $ | (1.04 | ) | $ | (2.45 | ) | |||
Diluted |
(1.58 | ) | (0.94 | ) | $ | (1.18 | ) | $ | (2.55 | ) | |||
Weighted average number of common shares outstanding: |
|||||||||||||
Basic |
14,702,624 | 14,632,988 | 14,641,528 | 14,059,360 | |||||||||
| | | | | | | | | | | | | | |
Diluted |
14,702,624 | 14,640,996 | 14,647,534 | 14,117,010 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
- *
- Basic and Diluted net loss for the nine months ended September 30, 2017 includes $4,163 in dividends paid in kind and deemed dividends to preferred stockholders.
15
Fibrocell Science, Inc.
Loss per Share Summary
($ in thousands except share and per share data)
| |
Nine Months Ended September 30, (Unaudited) |
(Adjusted for Stock Split) Year Ended December 31, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2017 | 2016 | 2016 | 2015 | |||||||||
Loss per share—Basic: |
|||||||||||||
Numerator for basic loss per share |
(23,303 | ) | (11,798 | ) | $ | (15,292 | ) | $ | (34,453 | ) | |||
| | | | | | | | | | | | | | |
Denominator for basic loss per share |
14,702,624 | 14,632,988 | 14,641,528 | 14,059,360 | |||||||||
| | | | | | | | | | | | | | |
Basic loss per common share |
(1.58 | ) | (0.81 | ) | $ | (1.04 | ) | $ | (2.45 | ) | |||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Loss per share—Diluted: |
|||||||||||||
Numerator for basic loss per share |
(23,303 | ) | (11,798 | ) | $ | (15,292 | ) | $ | (34,453 | ) | |||
Adjust: Change in fair value of dilutive warrants outstanding |
— | 1,958 | 1,958 | 1,529 | |||||||||
| | | | | | | | | | | | | | |
Numerator for diluted loss per share |
(23,303 | ) | (13,756 | ) | $ | (17,250 | ) | $ | (35,982 | ) | |||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Denominator for basic loss per share |
14,702,624 | 14,632,988 | 14,641,528 | 14,059,360 | |||||||||
Plus: Incremental shares underlying "in the money" warrants outstanding |
— | 8,008 | 6,006 | 57,650 | |||||||||
| | | | | | | | | | | | | | |
Denominator for diluted loss per share |
14,702,624 | 14,640,996 | 14,647,534 | 14,117,010 | |||||||||
| | | | | | | | | | | | | | |
Diluted loss per common share |
(1.58 | ) | (0.94 | ) | $ | (1.18 | ) | $ | (2.55 | ) | |||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Shares underlying "in the money" options outstanding |
208,840 | 170,226 | 150,120 | 629,449 | |||||||||
Shares underlying "out of the money" options outstanding |
763,442 | 1,228,224 | 1,218,563 | 391,197 | |||||||||
Shares underlying "in the money" warrants outstanding |
48,979 | — | 17,858 | 299,081 | |||||||||
Shares underlying "out of the money" warrants outstanding |
9,521,152 | 3,534,122 | 4,382,445 | 1,573,803 | |||||||||
Shares underlying convertible notes |
5,297,059 | 5,304,533 | 5,304,533 | — | |||||||||
Shares underlying convertible accrued interest on convertible notes |
177,059 | 13,545 | 40,137 | — | |||||||||
Shares underlying convertible preferred stock |
3,477,333 | — | — | — | |||||||||
16
We estimate that the net proceeds from our issuance and sale of 13,000,000 shares of our common stock in this offering will be approximately $18.4 million, or approximately $21.2 million if the underwriter exercises its over-allotment option in full, assuming a public offering price of $1.56 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on November 15, 2017, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
A $0.50 increase or decrease in the assumed public offering price of $1.56 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on November 15, 2017, would increase or decrease the net proceeds to us from this offering by $6.0 million or $(6.0) million, respectively, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriter discounts and commissions and estimated offering expenses payable by us.
Similarly, each increase or decrease of 1,000,000 shares offered by us would increase or decrease the net proceeds to us by approximately $1.5 million or $(1.5) million, respectively, assuming the assumed public offering price of $1.56 per share remains the same, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
As of September 30, 2017, we had cash and cash equivalents of $11.9 million. We intend to use the net proceeds from this offering to continue to fund the clinical and pre-clinical development of FCX-007 and FCX-013, and for the research of potential product candidates under the 2015 ECC, and for other general corporate purposes, which may include, without limitation:
- •
- working capital;
- •
- research and development expenditures;
- •
- the funding of in-licensing agreements for product candidates, additional technologies or other forms of intellectual property;
- •
- expenditures relating to manufacturing infrastructure and other capital expenditures; and
- •
- general administrative expenses.
This expected use of net proceeds from this offering and our existing cash and cash equivalents represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve. The amounts and timing of our use of the net proceeds from this offering will depend on a number of factors, such as the timing and progress of our research and development efforts, the timing and progress of any partnering efforts, technological advances and the competitive environment for our product candidates. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to us from the sale of the securities offered by us hereunder. Accordingly, our management will have broad discretion in the timing and application of these proceeds. Pending application of the net proceeds as described above, we intend to temporarily invest the proceeds in short-term, interest-bearing instruments.
Although it is difficult to predict future liquidity requirements, we believe that the net proceeds from this offering, together with our existing cash resources, will be sufficient to enable us to fund our operations into the second quarter of 2019. We have based this estimate on assumptions that may prove to be incorrect, and we could use our available capital resources sooner than we currently expect.
17
MARKET PRICE OF OUR COMMON STOCK
Our common stock trades on the Nasdaq Capital Market under the symbol "FCSC." The following table sets forth for the periods indicated the high and low sale prices per share for our common stock, adjusted to reflect the effect of the reverse stock split of our common stock on March 10, 2017, as reported on the Nasdaq Capital Market for the periods indicated:
| |
Market Price | ||||||
|---|---|---|---|---|---|---|---|
| |
High | Low | |||||
First quarter 2015 |
$ | 17.97 | $ | 7.14 | |||
Second quarter 2015 |
$ | 19.20 | $ | 9.75 | |||
Third quarter 2015 |
$ | 22.80 | $ | 11.04 | |||
Fourth quarter 2015 |
$ | 18.54 | $ | 10.50 | |||
First quarter 2016 |
$ | 13.86 | $ | 6.12 | |||
Second quarter 2016 |
$ | 11.34 | $ | 2.73 | |||
Third quarter 2016 |
$ | 4.14 | $ | 2.10 | |||
Fourth quarter 2016 |
$ | 3.15 | $ | 1.56 | |||
First quarter 2017 |
$ | 3.51 | $ | 1.86 | |||
Second quarter 2017 |
$ | 4.64 | $ | 1.80 | |||
Third quarter 2017 |
$ | 4.17 | $ | 2.41 | |||
Fourth quarter 2017 (through November 15, 2017) |
$ | 3.29 | $ | 1.54 | |||
As of November 15, 2017, the closing price of our common stock as reported by the Nasdaq Capital Market was $1.56. As of November 15, 2017, we had approximately 31 holders of record of our common stock. This number does not include beneficial owners whose shares were held in street name.
We have never declared or paid any cash dividends on our common stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. We do not intend to pay cash dividends in respect of our common stock in the foreseeable future. In addition, our outstanding convertible promissory notes and our outstanding Series A convertible preferred stock each restrict our ability to pay cash dividends on our equity securities.
18
The following table sets forth our cash and cash equivalents and capitalization as of September 30, 2017:
- •
- on an actual basis;
- •
- on an as adjusted basis to give further effect to our issuance and sale of 13,000,000 shares of our common stock in this offering at an assumed public offering price of $1.56 per share, the last reported sale price for our common stock on the Nasdaq Capital Market on November 15, 2017, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
Our capitalization following the closing of this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this table together with our consolidated financial statements and the related notes and the "Management's Discussion and Analysis of Financial Condition and Results of Operations" section of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2017, filed with the SEC on November 13, 2017, which is incorporated by reference into this prospectus.
| |
As of September 30, 2017 | ||||||
|---|---|---|---|---|---|---|---|
| |
Actual | As Adjusted(1) | |||||
| |
(in thousands except share data) |
||||||
Cash and cash equivalents |
$ | 11,911 | $ | 30,321 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Warrant liability |
10,735 | 10,735 | |||||
Derivative liability |
1,442 | 1,442 | |||||
Convertible promissory notes, net of debt discount $18,003* |
— | — | |||||
Stockholders' equity (deficit) |
|||||||
Preferred stock, $0.001 par value per share; 5,000,000 shares authorized |
|||||||
Series A nonredeemable convertible preferred stock; 8,000 shares designated, 8,000 shares issued and outstanding as of September 30, 2017; aggregate liquidation preference of $8,182 at September 30, 2017 |
— | — | |||||
Common stock, $0.001 par value per share; 150,000,000 shares authorized, 14,719,987 shares issued and outstanding, actual; 150,000,000 shares authorized; 27,719,987 shares issued and outstanding, as adjusted |
15 | 28 | |||||
Additional paid-in capital |
178,362 | 196,759 | |||||
Accumulated deficit |
(181,703 | ) | (181,703 | ) | |||
| | | | | | | | |
Total stockholders' equity (deficit) |
(3,326 | ) | 15,084 | ||||
| | | | | | | | |
Total capitalization |
$ | 8,851 | $ | 27,261 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
- *
- The
principal value of the convertible promissory notes is $18,003 as of September 30, 2017.
- (1)
- Each $0.50 increase or decrease in the assumed public offering price per share would increase or decrease the amount of cash and cash equivalents, working capital, total assets, and total stockholders' equity by approximately $6.0 million or $(6.0) million, respectively, assuming the number of shares offered by us, as set forth on the cover page
19
of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares of common stock to be issued in this offering. Each increase or decrease of 1,000,000 shares offered by us would increase or decrease the as adjusted amount of cash and cash equivalents, working capital, total assets and total stockholders' equity by approximately $1.5 million or $(1.5) million, respectively, assuming that the assumed public offering price remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering as determined between us and the underwriter at pricing.
The foregoing table and calculations are based on 14,719,987 shares of our common stock outstanding as of September 30, 2017, and excludes:
- •
- 5,515,404 shares of our common stock issuable upon the conversion of our outstanding convertible promissory notes, including accrued interest
thereon, payable in shares of our common stock, outstanding as of September 30, 2017;
- •
- 3,512,000 shares of our common stock issuable upon the conversion of our convertible preferred stock, including accrued dividends thereon,
payable in shares of our common stock, outstanding as of September 30, 2017;
- •
- 1,116,350 shares of our common stock issuable upon the exercise of stock options outstanding as of September 30, 2017, at a weighted
average exercise price of $13.38 per share, of which stock options to purchase 660,625 shares of our common stock were then exercisable;
- •
- 10,411,177 shares of our common stock issuable upon the exercise of warrants at a weighted average exercise price of $4.85 per share, all of
which warrants were then exercisable;
- •
- an aggregate of 1,403,899 shares of our common stock reserved for future grants of stock options (or other similar equity instruments) under
the Equity Incentive Plan; and
- •
- 520,000 shares (or 598,000 shares if the underwriter's option to purchase additional shares is exercised in full) of our common stock issuable upon exercise of the warrants being issued to the underwriter in connection with this offering.
20
If you invest in our common stock in this offering, your ownership interest will be diluted immediately to the extent of the difference between the public offering price per share of our common stock and the as adjusted net tangible book deficit per share of our common stock after this offering.
Our historical net tangible book deficit as of September 30, 2017 was $(3.3) million, or $(0.23) per share of our common stock. Historical net tangible book deficit per share represents the amount of our total tangible assets less total liabilities, divided by the number of shares of our common stock outstanding as of September 30, 2017.
After giving effect to our issuance and sale of 13,000,000 shares of our common stock in this offering at an assumed offering price of $1.56 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on November 15, 2017, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2017 would have been $15.1 million, or $0.54 per share. This represents an immediate increase in net tangible book value per share of $0.77 to existing stockholders and immediate dilution of $1.02 per share to new investors purchasing common stock in this offering. Dilution per share to new investors is determined by subtracting as adjusted net tangible book value per share after this offering from the public offering price per share paid by new investors. The following table illustrates this dilution on a per share basis:
Assumed public offering price per share |
$ | 1.56 | |||||
Historical net tangible book deficit per share as of September 30, 2017 |
$ | (0.23 | ) | ||||
Increase in net tangible book value per share attributable to new investors |
0.77 | ||||||
| | | | | | | | |
As adjusted net tangible book value per share after this offering |
0.54 | ||||||
| | | | | | | | |
Dilution per share to new investors |
$ | 1.02 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Each $0.50 increase or decrease in the assumed public offering price of $1.56 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on November 15, 2017, would increase or decrease our as adjusted net tangible book value per share after this offering by approximately $0.22 or $(0.22), respectively, and the dilution per share to new investors purchasing shares in this offering by $0.28 and $(0.28), respectively, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares to be issued in this offering. Each increase (decrease) of 1,000,000 shares offered by us would increase (decrease) our as adjusted net tangible book value per share by $0.03 and $(0.03), respectively, and the dilution per share to new investors purchasing shares in this offering by $0.03 and $(0.03), respectively, assuming that the assumed public offering price remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering as determined between us and the underwriter at pricing.
If the underwriter exercises its over-allotment option in full, the as adjusted net tangible book value per share after this offering would be $0.60 per share, the increase in net tangible book value per share to existing stockholders would be $0.83 per share and the dilution to new investors purchasing shares in this offering would be $0.96 per share.
21
The foregoing table and calculations are based on 14,719,987 shares of our common stock outstanding as of September 30, 2017, and excludes:
- •
- 5,515,404 shares of our common stock issuable upon the conversion of our outstanding convertible promissory notes, including accrued interest
thereon, payable in shares of our common stock, outstanding as of September 30, 2017;
- •
- 3,512,000 shares of our common stock issuable upon the conversion of our convertible preferred stock, including accrued dividends thereon,
payable in shares of our common stock, outstanding as of September 30, 2017;
- •
- 1,116,350 shares of our common stock issuable upon the exercise of stock options outstanding as of September 30, 2017, at a weighted
average exercise price of $13.38 per share, of which stock options to purchase 660,625 shares of our common stock were then exercisable;
- •
- 10,411,177 shares of our common stock issuable upon the exercise of warrants at a weighted average exercise price of $4.85 per share, all of
which warrants were then exercisable;
- •
- an aggregate of 1,403,899 shares of our common stock reserved for future grants of stock options (or other similar equity instruments) under
the Equity Incentive Plan; and
- •
- 520,000 shares (or 598,000 shares if the underwriter's option to purchase additional shares is exercised in full) of our common stock issuable upon exercise of the warrants being issued to the underwriter in connection with this offering.
Additionally, one of our outstanding warrants, which is currently exercisable for 46,430 shares of our common stock at an exercise price per share of $2.10, contains so-called full-ratchet anti-dilution provisions which may be triggered by the issuance of the shares of our common stock being offered hereby or upon any future issuance by us of shares of our common stock or common stock equivalents at a per share price below the then-exercise price of the warrant, subject to some exceptions. Upon consummation of the offering, we anticipate that the exercise price of this outstanding warrant will be adjusted downward to the public offering price in this offering and the number of shares underlying this warrant will be increased to 62,502, assuming an offering price of $1.56 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on November 15, 2017.
To the extent that any convertible notes, convertible preferred stock, options or warrants are exercised or converted, new options are issued under our Equity Incentive Plan or we otherwise issue additional shares of common stock in the future at a price less than the public offering price, there may be further dilution to new investors purchasing common stock in this offering.
22
The following description of our capital stock and provisions of our Restated Certificate of Incorporation, as amended, or the Certificate of Incorporation, and our Fourth Amended and Restated Bylaws, as amended, or the Bylaws, are summaries and are qualified by reference to the Certificate of Incorporation and the Bylaws. We have filed copies of these documents with the SEC as exhibits to our registration statement of which this prospectus forms a part.
Our authorized capital stock consists of 150,000,000 shares of our common stock, par value $0.001 per share, and 5,000,000 shares of our preferred stock, par value $0.001 per share, 8,000 of which preferred stock is designated as Series A Convertible Preferred Stock, or the Series A Preferred Stock.
As of September 30, 2017, we had 14,719,987 shares of our common stock and 8,000 shares of our Series A Preferred Stock issued and outstanding held by 30 and 5 stockholders of record, respectively. This number does not include beneficial owners whose shares were held in street name.
Common Stock
Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders and do not have cumulative voting rights. Each election of directors by our stockholders will be determined by a plurality of the votes cast by the stockholders entitled to vote on the election. Holders of common stock are entitled to receive proportionately any dividends as may be declared by our board of directors, subject to any preferential dividend rights of then outstanding preferred stock.
In the event of our liquidation or dissolution, the holders of our common stock are entitled to receive proportionately all assets available for distribution to stockholders after the payment of all debts and other liabilities and subject to the prior rights of any of our then outstanding preferred stock. Holders of our common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of our common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of our preferred stock that we may designate and issue in the future.
Preferred Stock
Under the terms of our Certificate of Incorporation, our board of directors is authorized to issue shares of preferred stock in one or more series without stockholder approval. Our board of directors has the discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock.
The purpose of authorizing our board of directors to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions, future financings and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or could discourage a third party from seeking to acquire, a majority of our outstanding voting stock.
Series A Convertible Preferred Stock
On March 7, 2017, we entered into a Securities Purchase Agreement with certain of our existing investors pursuant to which we issued and sold a total of 8,000 units, each a Unit and collectively the Units, for a purchase price of $1,000 per Unit, with each Unit consisting of (i) one share of our Series A Preferred Stock convertible into 429 shares of our common stock and (ii) an accompanying warrant to purchase up to a number of shares of common stock equal to 100% of the conversion
23
shares issuable on March 7, 2017 pursuant to the shares of Series A Preferred Stock purchased by each investor. We refer to the foregoing transactions as the Series A Preferred Stock Offering.
Voluntary Conversions by Holders.
Each holder of Series A Preferred Stock may, at any time, elect to convert shares of Series A Preferred Stock into shares of our common stock at the Conversion Price, subject to certain beneficial ownership limitations described below. The number of shares into which each share of Series A Preferred Stock is determined by dividing the then stated value of the share of Series A Preferred Stock by the Conversion Price. The Conversion Price is defined as $2.32710 (subject to adjustment for stock splits, stock dividends, stock combinations, recapitalizations or similar events).
Fundamental Transactions; Change of Control.
In the event we effect certain mergers, consolidations, sales of substantially all of our assets, tender or exchange offers, reclassifications or share exchanges in which our common stock is effectively converted into or exchanged for other securities, cash or property, we consummate a business combination in which another person acquires 50% of the outstanding shares of our common stock, or any person or group becomes the beneficial owner of 50% of the aggregate ordinary voting power represented by our issued and outstanding common stock, then, upon any subsequent conversion of the Series A Preferred Stock, the holders of such Series A Preferred Stock will have the right to receive any shares of the successor or acquiring corporation and any additional consideration it would have been entitled to receive if it had been a holder of the number of shares of common stock then issuable upon conversion in full (including accrued but unpaid dividends thereon) of the Series A Preferred Stock immediately prior to any of the foregoing transactions.
In addition, we have agreed to have any successor entity in any of the foregoing transactions in which we are not the surviving entity assume in writing all of our obligations under the Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock, or the Certificate of Designations.
Limitations on Conversion and Issuance.
The Series A Preferred Stock may not be converted and shares of our common stock may not be issued under the Certificate of Designation with respect to such Series A Preferred Stock if, after giving effect to the conversion or issuance, a holder together with its affiliates would beneficially own in excess of 4.99% of the outstanding shares of our common stock, or the Ownership Threshold; provided, however, that if a holder, together with its affiliates, otherwise comes to own a percentage of our common stock in excess of the Ownership Threshold, the percentage limitation shall increase to 9.99%. Such increased percentage shall continue to apply until the holder is deemed to own an amount of shares less than or equal to the Ownership Threshold of the outstanding shares of common stock, at which point the Ownership Threshold will apply again.
This limitation on beneficial ownership may be increased, decreased or terminated, in the sole discretion of each holder of the Series A Preferred Stock, upon sixty-one (61) days' written notice to us by such holder of Series A Preferred Stock.
Dividends.
Holders of the Series A Preferred Stock are entitled to receive, and we shall pay, cumulative dividends at a rate per share of 4% per annum (calculated quarterly as a percentage of the applicable stated value per share for each quarterly period). After five years, holders of the Series A Preferred Stock are entitled to receive cumulative dividends at a rate per share of 8% per annum (calculated quarterly as a percentage of the applicable stated value per share for such quarterly period). Dividends
24
on a share of Series A Preferred Stock increase such share of Series A Preferred Stock's stated value and are payable by way of inclusion in the stated value (i) on the applicable conversion date (but only with respect to the shares of Series A Preferred Stock being converted), (ii) upon our liquidation and (iii) upon the occurrence of a fundamental transaction.
If we, at any time while the Series A Preferred Stock is outstanding, pay a stock dividend or otherwise make a distribution or distributions payable in shares of our common stock on shares of our common stock or any other common stock equivalents, each holder of Series A Preferred Stock shall be entitled to receive such dividend or distribution in such amounts as each such holder of Series A Preferred Stock would have been entitled to receive, on a per share basis, if the shares of Series A Preferred Stock held by each such holder were converted into shares of common stock at the time of payment of such stock dividend or distribution.
Liquidation Preference.
Upon our liquidation, dissolution or winding up, the holders of the Series A Preferred Stock shall be entitled to receive out of our assets, whether capital or surplus, an amount equal to such holder's then stated value for each share of Series A Preferred Stock before any distribution to the holders of our common stock, any class or series of preferred stock and all other common stock equivalents other than those securities which are explicitly senior or pari passu to the Series A Preferred Stock in redemption, distribution of assets upon a liquidation or dividends. If there are insufficient assets to pay in full such amounts, then the available assets shall be ratably distributed to the holders of the Series A Preferred Stock in accordance with the respective amounts that would be payable on such shares if all amounts payable thereon were paid in full.
Redemption Rights.
We are not obligated to redeem or repurchase any shares of Series A Preferred Stock. Shares of Series A Preferred Stock are not otherwise entitled to any redemption rights, or mandatory sinking fund or analogous fund provisions.
Voting Rights.
Shares of Series A Preferred Stock will generally have no voting rights, except as required by law; provided, however, that without the prior written consent of the holders of at least 70% of the then outstanding shares of Series A Preferred Stock, we may not: (i) alter or change adversely the powers, preferences or rights given to the Series A Preferred Stock or alter or amend the Certificate of Designation; (ii) amend our Certificate of Incorporation or other charter documents in any manner that adversely affects any rights of a holder of the Series A Preferred Stock; (iii) authorize or create any class of stock ranking as to redemption, distribution of assets upon liquidation or dividends senior to, or otherwise pari passu with, the Series A Preferred Stock; (iv) declare or make any dividends other than dividend payments or other distributions payable solely in the common stock; or (v) enter into any agreement with respect to any of the foregoing.
Transfer of Series A Preferred Stock.
We will register the transfer of any shares of the Series A Preferred Stock in our preferred stock register, upon surrender of the certificates evidencing such shares to be transferred, duly endorsed by a holder of shares of Series A Preferred Stock. Upon any such registration or transfer, a new certificate evidencing the shares of Series A Preferred Stock so transferred will be issued to the transferee and a new certificate evidencing the remaining portion of the shares not so transferred, if any, will be issued to the transferring holder, in each case, within three (3) business days.
25
No Exchange Listing of Preferred Shares.
The Series A Preferred Stock is not currently listed on any national securities exchange or other nationally recognized trading system and we do not plan on making an application to list the Series A Preferred Stock on any national securities exchange or other nationally recognized trading system. Our common stock issuable upon conversion of shares of Series A Preferred Stock is listed on the Nasdaq Capital Market.
Warrants
As of September 30, 2017, we had outstanding:
- •
- warrants held by certain of our investors issued in connection with a preferred stock financing to purchase up to 46,430 shares of our common
stock at an exercise price of $2.10, which will remain exercisable until December 13, 2017;
- •
- warrants held by certain of our investors issued in connection with a 2012 convertibles notes financing to purchase up to 375,194 shares of our
common stock, at an exercise price of $7.50 per share, which will remain exercisable until June 1, 2018;
- •
- warrants held by certain of our investors to purchase up to an aggregate of 523,045 shares of our common stock, at an exercise price of $22.50
per share, which will remain exercisable until December 13, 2018;
- •
- warrants held by certain of our investors, or the 2016 Private Placement Warrants, to purchase up to an aggregate of 6,029,174 shares of our
common stock, at an exercise price of $4.50 per share, which will remain exercisable until September 7, 2021; and
- •
- warrants held by certain of our investors, and issued in connection with the Series A Convertible Preferred Offering to purchase up to an aggregate of 3,437,334 shares of our common stock, at a weighted-average exercise price of $2.54 per share, which will remain exercisable until March 8, 2022.
These warrants provide for adjustments in the event of specified mergers, reorganizations, reclassifications, stock dividends, stock splits or other changes in our corporate structure.
Additionally, one of our outstanding warrants, which is currently exercisable for 46,430 shares of our common stock at an exercise price per share of $2.10, contains so-called full-ratchet anti-dilution provisions which may be triggered by the issuance of the shares of our common stock being offered hereby or upon any future issuance by us of shares of our common stock or common stock equivalents at a per share price below the then-exercise price of the warrant, subject to some exceptions. Upon consummation of the offering, we anticipate that the exercise price of this outstanding warrant will be adjusted downward to the public offering price in this offering and the number of shares underlying this warrant will be increased to 62,502, assuming an offering price of $1.56 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on November 15, 2017.
Options
As of September 30, 2017, options to purchase an aggregate of 1,116,350 shares of our common stock, at a weighted-average exercise price of 13.38 per share, were outstanding.
Delaware Anti-Takeover Law and Certain Charter and Bylaw provisions
Provisions of Delaware law and our Certificate of Incorporation and Bylaws could make the acquisition of our company through a tender offer, a proxy contest or other means more difficult and could make the removal of incumbent officers and directors more difficult. We expect these provisions
26
to discourage coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of our company to first negotiate with our board of directors. We believe that the benefits provided by our ability to negotiate with the proponent of an unfriendly or unsolicited proposal outweigh the disadvantages of discouraging these proposals. We believe the negotiation of an unfriendly or unsolicited proposal could result in an improvement of its terms.
Our Certificate of Incorporation provides for our board of directors to be divided into three classes serving staggered terms. Approximately one-third of the board of directors will be elected each year. The provision for a classified board could prevent a party who acquires control of a majority of the outstanding voting stock from obtaining control of the board of directors until the second annual stockholders' meeting following the date the acquirer obtains the controlling stock interest. The classified board provision could discourage a potential acquirer from making a tender offer or otherwise attempting to obtain control of our company and could increase the likelihood that incumbent directors will retain their positions.
Our Bylaws do not permit stockholders to call a special meeting of stockholders. Our Bylaws provide that special meetings of the stockholders may be called only by a majority of the members of our board of directors, our Chairman of the board of directors, our Chief Executive Officer or our President. Our Bylaws require that all stockholder actions be taken by a vote of the stockholders at an annual or special meeting, and do not permit our stockholders to act by written consent without a meeting. Our Bylaws provide for an advance notice procedure for stockholder proposals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to the board of directors. At an annual meeting, stockholders may only consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of the board of directors. Stockholders may also consider a proposal or nomination by a person who was a stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has given to our Secretary timely written notice, in proper form, of his, her or its intention to bring that business before the meeting. The Bylaws do not give our board of directors the power to approve or disapprove stockholder nominations of candidates or proposals regarding other business to be conducted at a special or annual meeting of the stockholders. However, our Bylaws may have the effect of precluding the conduct of business at a meeting if the proper procedures are not followed. These provisions may also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer's own slate of directors or otherwise attempting to obtain control of our company.
Preferred Stock
Our board of directors has the authority, without action by our stockholders, to designate and issue preferred stock in one or more series. Our board of directors may also designate the rights, preferences and privileges of each series of preferred stock, any or all of which may be greater than the rights of the common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock on the rights of holders of the common stock until our board of directors determines the specific rights of the holders of the preferred stock. However, these effects might include: (a) restricting dividends on the common stock; (b) diluting the voting power of the common stock; (c) impairing the liquidation rights of the common stock; and (d) delaying or preventing a change in control of our company without further action by our stockholders.
Registration Rights
On September 7, 2016, we entered into a registration rights agreement with certain holders of our convertible promissory notes, and warrants in connection with a private placement transaction, pursuant to which we will register under the Securities Act for resale shares of our commons stock issuable upon the conversion of such notes or the exercise of such warrants and any other shares held by the investors
27
in the private placement. The registration rights agreement contains customary terms such as demand and piggyback registration rights.
If we fail, under certain circumstances to file and keep effective a registration statement with respect to the securities covered under the registration rights agreement, we have agreed to pay liquidated damages to each investor in an amount equal to one percent (1.0%) of the aggregate amount invested by each such investor pursuant to the convertible promissory notes then owned by each such investor for each 30-day period or pro rata for any portion thereof during which the failure to file or keep effective continues. The registration rights will terminate with respect to each investor upon the date each such investor ceases to hold registrable securities under the terms of the registration rights agreement.
Transfer Agent and Registrar
Our transfer agent and registrar for our common stock is Broadridge Corporate Issuer Solutions, Inc.
Nasdaq Capital Market
Our common stock is listed on the Nasdaq Capital Market under the symbol "FCSC."
28
CERTAIN RELATIONSHIPS AND TRANSACTIONS WITH RELATED PERSONS
Since January 1, 2014, we have engaged in the following transactions with our directors, executive officers, holders of more than 5% of our voting securities, and affiliates or immediate family members of our directors, executive officers, and holders of more than 5% of our voting securities. We believe that all of these transactions were on terms as favorable as could have been obtained from unrelated third parties.
Intrexon Collaborations
We are a party to two separate exclusive channel collaboration agreements with Intrexon Corporation, or Intrexon, pursuant to which we are Intrexon's exclusive channel collaborator in the development and commercialization of products within certain specified fields. We engage Intrexon for support services for the research and development of product candidates covered under these agreements and reimburse Intrexon for its cost for time and materials for such work.
Our first exclusive channel collaboration agreement with Intrexon was entered into in October 2012, and was subsequently amended in June 2013 and January 2014. We refer to this collaboration as the 2012 ECC. FCX-007 and FCX-013, our gene-therapy product candidates for the treatment of recessive dystrophic epidermolysis bullosa and linear scleroderma, respectively, are being developed under the 2012 ECC. During 2016, we incurred research and development expenses of $3.7 million under the 2012 ECC. As of October 31, 2017, we have incurred research and development expenses of approximately $4.8 million in 2017 under the 2012 ECC.
In December 2015, we entered into our second exclusive channel collaboration agreement with Intrexon, or the 2015 ECC. We are currently in the research phase for a gene-therapy product for arthritis and related conditions under the 2015 ECC. During 2016, we did not incur any research and development expenses under the 2015 ECC, however, we paid Intrexon $10.0 million related to an up-front technology access fee in January 2016. We have not incurred any research and development expenses under the 2015 ECC in 2017, as of September 30, 2017.
Randal J. Kirk is the chairman of the board of directors and chief executive officer of Intrexon. Together with his affiliates, Mr. Kirk owns more than 50% of Intrexon's common stock and approximately 38% of our common stock, as of November 15, 2017. Two of our directors, Julian Kirk (who is the son of Randal J. Kirk) and Marcus E. Smith, are officers of Third Security, which is owned by Randal J. Kirk.
Participation in 2016 Private Placement
On September 7, 2016, we issued an aggregate of approximately $18.1 million in principal of convertible promissory notes, each a Note and collectively, the Notes, and accompanying warrants to purchase an aggregate of 6,029,174 shares of our common stock in a private placement to institutional and accredited investors, or the 2016 Private Placement. The Notes bear interest at 4% per annum and have a stated maturity date of the earlier of (i) September 7, 2026 and (ii) one-hundred and eighty (180) days after the date on which our product candidate, FCX-007, is approved by the FDA for the treatment of RDEB. Each individual Note holder has the right to require us to repay all or any portion of the unpaid principal from time to time on or after September 7, 2021. With respect to accrued and unpaid interest on the Note, each Note holder may elect, at any time and from time to time, to have any accrued and unpaid interest converted into shares of our common stock. In addition, each Note holder may elect to accelerate the repayment of all unpaid principal and accrued interest under such holder's Note upon consummation of a specified change of control transaction or occurrence of certain events of default as specified in the Notes.
29
In addition, upon an event of default, the base interest rate (excluding any additional interest) for the Notes automatically increases to twelve percent (12%) per annum. Subject to any applicable cure period set forth in the Notes, all amounts outstanding with respect to the Notes (principal and accrued interest) would become due and payable immediately upon an event of default. The conversion price of the Notes, the exercise price of the accompanying warrants and the number of shares of our common stock issuable upon conversion of the Notes and exercise of the accompanying warrants are each subject to adjustment upon certain corporate events, including stock dividends, stock splits and distributions of cash or other assets to our stockholders.
Certain of our stockholders who held more than 5% of our voting securities at the time of the transaction and their affiliated entities purchased shares in our 2016 Private Placement. Affiliates of Randal J. Kirk (including Intrexon) participated in the 2016 Private Placement, and were issued an aggregate of $6,762,500 in principal of Notes and 2016 Private Placement Warrants to purchase an aggregate of 2,254,168 shares of common stock.
We have elected to accrue all interest due on the Notes. In June 2017, we issued 14,895 shares of restricted common stock in connection with the conversion of an aggregate amount of $51,609.79 of the Notes, with such amount representing $50,000.00 in unpaid principal under the Notes and $1,609.79 in accrued and unpaid interest under the Notes. In July 2017 we issued 7,195 shares of restricted common stock in connection with the conversion of an aggregate amount of $25,876.87 of the Notes, with such amount representing $25,000 in unpaid principal under the Notes and $876.87 in accrued and unpaid interest under the Notes. In August 2017 we issued 2,821 shares of restricted common stock in connection with the conversion of an aggregate amount of $10,379.04 of the Notes, with such amount representing $10,000 in unpaid principal under the Notes and $379.04 in accrued and unpaid interest under the Notes.
Registration Rights Agreement
On September 7, 2016, we entered into a registration rights agreement with certain holders of the Notes and 2016 Private Placement Warrants in connection with the 2016 Private Placement, pursuant to which we will register under the Securities Act for resale shares of our commons stock issuable upon the conversion of the Notes or the exercise of the 2016 Private Placement Warrants and any other shares of our common stock held by the investors in the 2016 Private Placement. The registration rights agreement contains customary terms such as demand and piggyback registration rights, which are described further in "Description of Capital Stock—Registration Rights," above.
Participation in Series A Preferred Stock Offering
On March 7, 2017, we entered into a Securities Purchase Agreement with certain of our existing investors pursuant to which we issued and sold a total of 8,000 Units for a purchase price of $1,000 per Unit, with each Unit consisting of (i) one share of our Series A Preferred Stock convertible into 429 shares of our common stock and (ii) an accompanying warrant to purchase up to a number of shares of common stock equal to 100% of the conversion shares issuable on March 7, 2017 pursuant to the shares of Series A Preferred Stock purchased by each investor, or the Series A Preferred Stock Offering. Certain of our stockholders who held more than 5% of our voting securities at the time of the transaction and their affiliated entities purchased shares in our Series A Preferred Stock Offering Affiliates of Randal J. Kirk (including Intrexon) participated in the Series A Preferred Stock Offering, and were issued an aggregate of 3,016 shares of Series A Preferred Stock and accompanying warrants to purchase 1,295,875 shares of common stock for aggregate gross proceeds of $3,016,000. See "Description of Capital Stock—Series A Convertible Preferred Stock" for more information about the Series A Preferred Stock.
30
The following table sets forth information with respect to the beneficial ownership of our common stock as of November 15, 2017 by:
- •
- each of our directors;
- •
- each of our named executive officers;
- •
- all of our current directors and executive officers as a group; and
- •
- each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common stock based on currently available Schedules 13D and 13G filed with the SEC.
The percentage ownership information under the column entitled "Before offering" is based on 14,719,987 shares of our common stock outstanding as of November 15, 2017. The percentage ownership information under the column entitled "After offering" gives effect to the sale of 13,000,000 shares of common stock in this offering, and assumes no exercise of the underwriter's over-allotment option.
Beneficial ownership is determined in accordance with the rules and regulations of the SEC and includes voting or investment power with respect to our common stock. Shares of our common stock subject to options and warrants that are currently exercisable or exercisable within 60 days after November 15, 2017 are considered outstanding and beneficially owned by the person holding the options or warrants for the purpose of calculating the percentage ownership of that person but not for the purpose of calculating the percentage ownership of any other person. Except as otherwise noted, the persons and entities in this table have sole voting and investing power with respect to all of the shares of our common stock beneficially owned by them, subject to community property laws, where applicable. Except as otherwise set forth below, the address of each beneficial owner is c/o Fibrocell Science, Inc., 405 Eagleview Blvd., Exton, Pennsylvania 19341.
| |
|
Percentage of shares beneficially owned |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of shares beneficially owned |
|||||||||
Name and Address of Beneficial Owner
|
Before offering |
After offering |
||||||||
5% Stockholders: |
||||||||||
Randal J. Kirk(1) |
5,540,138 | 37.6 | % | 19.99 | % | |||||
FMR LLC(2) |
868,814 | 5.9 | % | 3.1 | % | |||||
Directors and Named Executive Officers: |
||||||||||
John Maslowski(3) |
73,272 | * | * | |||||||
David Pernock(4) |
248,000 | 1.7 | % | * | ||||||
Keith A. Goldan(5) |
667 | * | * | |||||||
Michael F. Marino |
— | * | * | |||||||
Julian Kirk(6) |
40,667 | * | * | |||||||
Marc Mazur(7) |
50,335 | * | * | |||||||
Kelvin Moore(8) |
47,520 | * | * | |||||||
Marcus E. Smith(6) |
40,667 | * | * | |||||||
Christine St.Clare(9) |
44,001 | * | * | |||||||
Douglas J. Swirsky(6) |
40,667 | * | * | |||||||
All Current Executive Officers and Directors as a Group (7 persons)(10) |
337,129 | 2.2 | % | 1.2 | % | |||||
- *
- Less than one percent.
31
- (1)
- Based on the Schedule 13D/A filed by Randal J. Kirk on July 27, 2015, Third Security, LLC, or Third Security, has sole voting and investment power with respect to 10,219,631 shares (3,406,544 on a post-split basis) of Fibrocell common stock held by NRM VII Holdings I, LLC, or NRM VII Holdings, Kapital Joe, LLC, or Kapital Joe, and Mascara Kaboom, LLC, or Mascara Kaboom, and Intrexon has shared voting and investment power with respect to 6,400,783 shares (2,133,594 on a post-split basis) of Fibrocell common stock held by Intrexon.
Based on Schedule 13D/A filed by Third Security on September 9, 2016, or the 2016 13D/A, NRM VII Holdings, Intrexon, Kapital Joe, and Mascara Kaboom acquired an aggregate of $6,762,500 principal amount of convertible promissory notes and accompanying warrants to purchase 6,762,500 shares (2,254,168 on a post-split basis) of our common stock in a private placement transaction that closed on September 7, 2016. Unpaid principal and interest on the notes is convertible into shares of common stock at the option of the note holder at $1.13625 ($3.40875 on a post-split basis), subject to adjustment.
Based on Schedule 13D/A filed by Third Security on March 10, 2017, or the 2017 13D/A, NRM VII Holdings, Intrexon, Kapital Joe, and Mascara Kaboom acquired an aggregate of 3,016 units, comprised of (i) 3,016 shares of Series A Preferred Stock convertible into 3,887,624 shares (1,293,864 on a post-split basis) of common stock and (ii) warrants to purchase 3,887,624 shares (1,295,875 on a post-split basis) of common stock.
According to the 2016 13D/A and 2017 13D/A, the convertible promissory notes, the shares of Series A Preferred Stock and the warrants contain certain conversion and exercise restrictions. If NRM VII Holdings, Intrexon, Kapital Joe and Mascara Kaboom exercised the warrants and converted the principal and accrued interest of the convertible promissory notes and the Series A Preferred Stock, NRM VII Holdings, Intrexon, Kapital Joe and Mascara Kaboom would receive, in the aggregate, (i) 3,550,043 shares (split-effected) of our common stock pursuant to exercise of the warrants, (ii) 1,983,863 (split-effected) of common stock underlying $6,762,500 outstanding principal amount of convertible promissory notes, (iii) 87,999 shares (split-effected) of common stock underlying an estimated $299,969 of accrued interest on the convertible promissory notes and (iv) 1,324,024 shares (split-effected) of common stock underlying the Series A Preferred Stock (inclusive of dividends through November 15, 2017 payable by way of inclusion in the stated value of the Series A Preferred Stock, resulting in the beneficial ownership of approximately 57.6% of our common stock. NRM VII Holdings is managed by an affiliate that is managed by Third Security which is owned by Mr. Kirk. Kapital Joe and Mascara Kaboom are managed by Third Security. Mr. Kirk could be deemed to have indirect beneficial ownership of the shares of common stock directly beneficially owned by NRM VII Holdings, Intrexon, Kapital Joe and Mascara Kaboom. The address for Randal J. Kirk is c/o Third Security, 1881 Grove Avenue, Radford, Virginia 24141.
- (2)
- Based on the Schedule 13-G filed by FMR LLC on February 14, 2017, Fidelity SelectCo, LLC (SelectCo), 1225 17th Street, Suite, 1100, Denver, Colorado 80202, a wholly-owned subsidiary of FMR and an investment adviser registered under Section 203 of the Investment Advisers Act of 1940, is the beneficial owner of 2,606,440 shares (868,814 on a post-split basis) of Fibrocell common stock as a result of acting as investment adviser to various investment companies registered under Section 8 of the Investment Company Act of 1940, or the SelectCo Funds. Abigail P. Johnson and FMR through its control of SelectCo, and the SelectCo Funds each has sole power to dispose of the 2,606,440 shares (868,814 on a post-split basis) owned by the SelectCo Funds. The ownership of one investment company, Fidelity Select Biotechnology Portfolio, amounted
32
to 2,432,240 shares (810,747 on a post-split basis). Fidelity Select Biotechnology Portfolio has its principal business office at 245 Summer Street, Boston, Massachusetts 02210. Members of the family of Abigail P. Johnson, Chairman and Chief Executive Officer of FMR, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR. The Johnson family group and all other Series B stockholders have entered into a stockholders' voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the stockholders' voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, to form a controlling group with respect to FMR. The address for FMR is 245 Summer Street, Boston, Massachusetts 02210.
- (3)
- Consists
of (i) 819 shares of common stock and (ii) options to purchase an aggregate of 72,453 shares of our common stock exercisable within
60 days of November 15, 2017.
- (4)
- Mr. Pernock
resigned in December 2016. The share amounts set forth in the table consist of options to purchase 248,000 shares of common stock exercisable
within 60 days of November 15, 2017.
- (5)
- Mr. Goldan
resigned in January 2017. The share amounts set forth in the table consist of 667 shares of common stock held by Mr. Goldan as of
November 15, 2017.
- (6)
- The
share amounts set forth in the table consist solely of shares underlying one or more outstanding options to purchase our common stock exercisable within
60 days of November 15, 2017.
- (7)
- Consists
of (i) 3,000 shares of our common stock and (ii) options and warrants to purchase an aggregate of 47,335 shares of our common stock
exercisable within 60 days of November 15, 2017.
- (8)
- Consists
of (i) 1,519 shares of our common stock and (ii) options to purchase 46,001 shares of our common stock exercisable within 60 days of
November 15, 2017.
- (9)
- Consists
of (i) 3,334 shares of common stock and (ii) options to purchase 40,667 shares of our common stock exercisable within 60 days of
November 15, 2017.
- (10)
- Consists of (i) 8,672 shares of common stock and (ii) options and warrants to purchase an aggregate of 328,457 shares of our common stock exercisable within 60 days of November 15, 2017.
33
We have entered into an underwriting agreement dated , 2017, with H.C. Wainwright & Co., LLC as the sole book-running manager of this offering. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriter and the underwriter has agreed to purchase from us, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, 13,000,000 shares of our common stock.
A copy of the underwriting agreement has been filed as an exhibit to the registration statement of which this prospectus is a part. The shares of common stock we are offering are being offered by the underwriter subject to certain conditions specified in the underwriting agreement.
We have been advised by the underwriter that it proposes to offer the shares directly to the public at the public offering price set forth on the cover page of this prospectus. Any shares sold by the underwriter to securities dealers will be sold at the public offering price less a selling concession not in excess of $ per share.
The underwriting agreement provides that the underwriter's obligation to purchase the securities we are offering is subject to conditions contained in the underwriting agreement. The underwriter is obligated to purchase and pay for all of the shares offered by this prospectus.
No action has been taken by us or the underwriter that would permit a public offering of the common stock in any jurisdiction where action for that purpose is required. None of the shares included in this offering may be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sales of any of the shares be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who receive this prospectus are advised to inform themselves about and to observe any restrictions relating to this offering of the common stock and the distribution of this prospectus. This prospectus is neither an offer to sell nor a solicitation of any offer to buy the common stock in any jurisdiction where that would not be permitted or legal.
The underwriter has advised us that it does not intend to confirm sales to any accounts over which it exercises discretionary authority.
Underwriting Discounts, Commissions and Expenses
We have agreed to pay an underwriter discount equal to 7% of the aggregate gross proceeds raised in this offering, provided, however, that no discount shall be paid in regards to any gross proceeds raised by sales of securities in this offering to Randal J. Kirk, Intrexon Corporation, Third Security or any of their affiliates.
The following table shows the public offering price, underwriting discounts and commissions and proceeds, before expenses to us. These amounts are shown assuming both no exercise and full exercise of the underwriter's option to purchase additional shares.
| |
|
Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Per Share | Without Option Exercise |
With Option Exercise |
|||||||
Public offering price |
||||||||||
Underwriting discounts and commissions |
||||||||||
Proceeds, before expenses, to us |
||||||||||
We estimate the total expenses payable by us for this offering to be approximately $1.9 million, which amount includes (i) an assumed underwriting discount of $1.4 million ($1.6 million if the
34
underwriter's option to purchase additional shares is exercised in full) based upon the assumed public offering price of $1.56 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on November 15, 2017), (ii) $25,000 non-accountable expense allowance payable to the underwriter, (iii) reimbursement of the accountable expenses of the underwriter equal to $100,000 (none of which has been paid in advance), including the legal fees of the underwriter being paid by us, and (iv) other estimated expenses of approximately $325,000 which include legal, accounting, printing costs and various fees associated with the registration and listing of our shares.
Underwriter Warrants
We have agreed to issue to the underwriter warrants to purchase a number of shares of our common stock equal to 4% of the aggregate number of shares of common stock sold in this offering. The underwriter warrants will have a term of five years from the effective date of this prospectus and an exercise price per share equal to 125% of the public offering price for the shares sold in this offering. Pursuant to FINRA Rule 5110(g), the underwriter warrants and any shares issued upon exercise of the underwriter warrants shall not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days immediately following the date of effectiveness or commencement of sales of this offering, except the transfer of any security: (i) by operation of law or by reason of our reorganization; (ii) to any FINRA member firm participating in the offering and the officers or partners thereof, if all securities so transferred remain subject to the lock-up restriction set forth above for the remainder of the time period; (iii) if the aggregate amount of our securities held by the underwriter or related persons do not exceed 1% of the securities being offered; (iv) that is beneficially owned on a pro rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund and the participating members in the aggregate do not own more than 10% of the equity in the fund; or (v) the exercise or conversion of any security, if all securities remain subject to the lock-up restriction set forth above for the remainder of the time period.
Right of First Refusal
We have also granted the underwriter, for a period of 10 months from the closing date of this offering, a right of first refusal to act as sole book-running manager for each and every future public or private equity offering by us or any of our successors or subsidiaries. We have also agreed to a tail fee equal to the cash and warrant compensation in this offering if any investor to which the underwriter introduced us with respect to this offering during the term of its engagement provides us with further capital in a public or private offering or capital raising transaction, with certain exceptions, during the 12-month period following termination of our engagement of the underwriter.
Option to Purchase Additional Shares
We have granted to the underwriter an option, exercisable not later than 30 days after the date of this prospectus, to purchase up to an additional 1,950,000 shares of common stock at the public offering price, less the underwriting discounts and commissions, set forth on the cover page of this prospectus, to cover over-allotments, if any. If any additional shares of common stock are purchased pursuant to the option to purchase additional shares, the underwriter will offer these shares of common stock on the same terms as those on which the other shares of common stock are being offered hereby.
Nasdaq Capital Market Listing
Our stock is currently traded on the Nasdaq Capital Market under the symbol "FCSC." On November 15, 2017, the last reported sale price of our common stock was $1.56 per share.
35
Lock-up Agreements
Our officers and directors and certain of our stockholders have agreed with the underwriter to be subject to a lock-up period of 90 days following the date of this prospectus. This means that, during the applicable lock-up period, such persons may not offer for sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly, any shares of our common stock or any securities convertible into, or exercisable or exchangeable for, shares of our common stock. Certain limited transfers are permitted during the lock-up period if the transferee agrees to these lock-up restrictions. We have also agreed in the underwriting agreement, subject to certain exceptions, to similar lock-up restrictions on the issuance and sale of our securities for 90 days following the closing of this offering. The underwriter may, in its sole discretion and without notice, waive the terms of any of these lock-up agreements.
Stabilization, Short Positions and Penalty Bids
The underwriter may engage in syndicate covering transactions, stabilizing transactions and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of our common stock:
- •
- Syndicate covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover
syndicate short positions. Such a naked short position would be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriter is concerned
that there could be downward pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in the offering.
- •
- Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specific maximum.
- •
- Penalty bids permit the underwriter to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions.
These syndicate covering transactions, stabilizing transactions and penalty bids may have the effect of raising or maintaining the market prices of our securities or preventing or retarding a decline in the market prices of our securities. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. Neither we nor the underwriter make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected on the Nasdaq Capital Market, in the over-the-counter market or on any other trading market and, if commenced, may be discontinued at any time.
In connection with this offering, the underwriter also may engage in passive market making transactions in our common stock in accordance with Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of the distribution. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for that security. However, if all independent bids are lowered below the passive market maker's bid that bid must then be lowered when specific purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither we nor the underwriter make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the prices of our securities. In addition, neither we nor the underwriter make any representation that the underwriter will engage in these transactions or that any transactions, once commenced, will not be discontinued without notice.
36
Indemnification
We have agreed to indemnify the underwriter against certain liabilities, including certain liabilities arising under the Securities Act, or to contribute to payments that the underwriter may be required to make for these liabilities.
Other Relationships
The underwriter and its respective affiliates have engaged in, and may in the future engage in, investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. The underwriter has received, or may in the future receive, customary fees and commissions for these transactions.
37
The validity of the shares of common stock offered hereby is being passed upon for us by Hogan Lovells US LLP, Philadelphia, Pennsylvania. Lowenstein Sandler LLP, New York, New York is acting as counsel for the underwriter in connection with this offering.
The financial statements incorporated in this Prospectus by reference to the Annual Report on Form 10-K for the year ended December 31, 2016 have been so incorporated in reliance on the report (which contains an explanatory paragraph relating to the Company's ability to continue as a going concern as described in Note 1 to the financial statements) of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
The SEC allows us to "incorporate by reference" information into this prospectus, which means that we can disclose important information to you by referring you to another document filed separately with the SEC. The SEC file number for the documents incorporated by reference in this prospectus is 001-31564. The documents incorporated by reference into this prospectus contain important information that you should read about us.
The following documents are incorporated by reference into this document:
- •
- our Annual Report on Form 10-K for the year ended December 31, 2016, filed on March 9, 2017;
- •
- our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2017, June 30, 2017 and September 30, 2017,
filed on May 10, 2017, August 9, 2017 and November 13, 2017, respectively;
- •
- those portions of our Definitive Proxy Statement on Schedule 14A filed on January 27, 2017 that are deemed "filed" with the SEC;
- •
- those portions of our Definitive Proxy Statement on Schedule 14A filed on April 27, 2017 that are deemed "filed" with the SEC;
and
- •
- our Current Reports on Form 8-K (other than portions thereof furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits accompanying such reports that relate to such items) filed with the SEC on January 9, 2017, January 26, 2017, February 13, 2017, February 23, 2017, March 1, 2017, March 3, 2017, March 8, 2017, March 10, 2017, May 9, 2017, June 27, 2017, September 26, 2017, October 4, 2017 and November 15, 2017.
We also incorporate by reference into this prospectus all documents (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items) that are filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of the initial registration statement of which this prospectus is a part and prior to the effectiveness of such registration statement and all documents that are filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus but prior to the termination of the offering. These documents include periodic reports, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as well as proxy statements.
Any statement contained herein or in a document incorporated or deemed to be incorporated by reference into this document will be deemed to be modified or superseded for purposes of the document to the extent that a statement contained in this document or any other subsequently filed
38
document that is deemed to be incorporated by reference into this document modifies or supersedes the statement.
You may request, orally or in writing, a copy of any or all of the documents incorporated herein by reference. These documents will be provided to you at no cost, by contacting: Fibrocell Science, Inc., Attn: Office of the Corporate Secretary, 405 Eagleview Blvd., Exton, PA 19341. In addition, copies of any or all of the documents incorporated herein by reference may be accessed at our website at http://www.fibrocell.com. The information on such website is not incorporated by reference and is not a part of this prospectus.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of our common stock. This prospectus, which constitutes part of the registration statement, does not include all of the information contained in the registration statement and the exhibits, schedules and amendments to the registration statement. For further information with respect to us and our common stock, we refer you to the registration statement and to the exhibits and schedules to the registration statement. Statements contained in this prospectus about the contents of any contract, agreement or other document are not necessarily complete, and, in each instance, we refer you to the copy of the contract, agreement or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference. You should rely only on information contained in, or incorporated by reference into, this prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus or incorporated by reference in this prospectus.
You may read and copy the registration statement of which this prospectus is a part at the SEC's public reference room, which is located at 100 F Street, N.E., Room 1580, Washington, DC 20549. You can request copies of the registration statement by writing to the SEC and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the SEC's public reference room. In addition, the SEC maintains an Internet website, which is located at http://www.sec.gov, that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. You may access the registration statement of which this prospectus is a part at the SEC's Internet website. Our reports on Forms 10-K, 10-Q and 8-K, and amendments to those reports, are also available for download, free of charge, as soon as reasonably practicable after these reports are filed with the SEC, at our website at http://fibrocell.com. The content contained in, or that can be accessed through, our website is not a part of this prospectus.
39
13,000,000 Shares

Common Stock
PRELIMINARY PROSPECTUS
Sole Book-Running Manager
H.C. Wainwright & Co.
, 2017
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the expenses to be incurred in connection with the offering described in this Registration Statement, other than underwriting discounts and commissions, all of which will be paid by us. All amounts are estimates except the Securities and Exchange Commission, or SEC, registration fee and the Financial Industry Regulatory Authority, Inc., filing fee.
| |
Amount | |||
|---|---|---|---|---|
SEC registration fee |
$ | 3,048.77 | ||
Financial Industry Regulatory Authority, Inc. filing fee |
4,173.22 | |||
Accountant's fees and expenses |
150,000.00 | |||
Legal fees and expenses |
250,000.00 | |||
Transfer agent's fees and expenses |
5,000.00 | |||
Printing and engraving expenses |
10,000.00 | |||
Miscellaneous |
28,000.00 | |||
| | | | | |
Total expenses |
$ | 450,221.99 | ||
| | | | | |
| | | | | |
| | | | | |
Item 14. Indemnification of Directors and Officers.
Section 102 of the Delaware General Corporation Law, or the DGCL, permits a corporation to eliminate the personal liability of its directors or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his or her duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our Certificate of Incorporation provides that no director shall be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except to the extent that the DGCL prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Section 145 of the DGCL provides that a corporation has the power to indemnify a director, officer, employee or agent of the corporation and certain other persons serving at the request of the corporation in related capacities against expenses (including attorneys' fees), judgments, fines and amounts paid in settlements actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he or she is or is threatened to be made a party by reason of such position, if such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnification for such expenses which the Court of Chancery or such other court shall deem proper.
Our Certificate of Incorporation provides that we will indemnify each person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of us), by reason of the fact that he or she is or was, or has agreed to become, our director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee
II-1
of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (all such persons being referred to as an Indemnitee), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys' fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding and any appeal therefrom if such Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful.
Our Certificate of Incorporation also provides that we will indemnify any Indemnitee who was or is a party to an action or suit by or in the right of us to procure a judgment in our favor by reason of the fact that the Indemnitee is or was, or has agreed to become, our director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee or, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys' fees) and, to the extent permitted by law, amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, and any appeal therefrom, if the Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to us, unless a court determines that, despite such adjudication but in view of all of the circumstances, he or she is entitled to indemnification of such expenses. Notwithstanding the foregoing, to the extent that any Indemnitee has been successful, on the merits or otherwise, he or she will be indemnified by us against all expenses (including attorneys' fees) actually and reasonably incurred by him or her or on his or her behalf in connection therewith. If we do not assume the defense, expenses must be advanced to an Indemnitee under certain circumstances.
We have entered into indemnification agreements with our directors and executive officers. In general, these agreements provide that we will indemnify the director or executive officer to the fullest extent permitted by law for claims arising in his or her capacity as a director or officer of our company or in connection with their service at our request for another corporation or entity. The indemnification agreements also provide for procedures that will apply in the event that a director or executive officer makes a claim for indemnification and establish certain presumptions that are favorable to the director or executive officer.
We maintain a general liability insurance policy that covers certain liabilities of our directors and officers arising out of claims based on acts or omissions in their capacities as directors or officers.
The underwriting agreement we will enter into in connection with the offering of securities being registered hereby provides that the underwriter will indemnify, under certain conditions, our directors and officers (as well as certain other persons) against certain liabilities arising in connection with such offering.
Insofar as the forgoing provisions permit indemnification of directors, executive officers, or persons controlling us for liability arising under the Securities Act of 1933, as amended, or the Securities Act, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
II-2
Item 15. Recent Sales of Unregistered Securities.
2016 Private Placement
On September 7, 2016, we issued an aggregate of approximately $18.1 million in principal of Notes and 2016 Private Placement Warrants to purchase an aggregate of 6,029,174 shares of common stock in a private placement to institutional and accredited investors.
The securities sold in this private placement were sold in reliance on the exemption from registration afforded by Section 4(a)(2) of the Securities Act and Rule 506(c) of Regulation D promulgated under the Securities Act. Each of the investors represented that it was an accredited investor, as such term is defined in Rule 501(a) of Regulation D under the Securities Act, and that it was acquiring the shares for investment only and not with a view towards, or for resale in connection with, the public sale or distribution thereof.
Conversion of 2016 Convertible Notes
In June 2017, we issued 14,895 shares of restricted common stock in connection with the conversion of an aggregate amount of $51,609.79 of the Notes, with such amount representing $50,000.00 in unpaid principal under the Notes and $1,609.79 in accrued and unpaid interest under the Notes.
In July 2017 we issued 7,195 shares of restricted common stock in connection with the conversion of an aggregate amount of $25,876.87 of the Notes, with such amount representing $25,000 in unpaid principal under the Notes and $876.87 in accrued and unpaid interest under the Notes.
In August 2017 we issued 2,821 shares of restricted common stock in connection with the conversion of an aggregate amount of $10,379.04 of the Notes, with such amount representing $10,000 in unpaid principal under the Notes and $379.04 in accrued and unpaid interest under the Notes.
These shares of our common stock were issued in reliance on the exemption from registration provided by Section 3(a)(9) of the Securities Act, there was no additional consideration paid upon the conversion of the promissory notes and we did not receive any additional proceeds in connection with the issuance of the unregistered shares of common stock.
Item 16. Exhibits and Financial Statement Schedules.
The exhibits to the registration statement are listed in the Exhibit Index attached hereto and incorporated by reference herein.
(a) The undersigned registrant hereby undertakes that:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price
II-3
represent no more than 20 percent change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned Registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned Registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned Registrant or used or referred to by the undersigned Registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned Registrant or its securities provided by or on behalf of the undersigned Registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned Registrant to the purchaser.
(5) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(6) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(b) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant's annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan's annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(e) The undersigned registrant hereby undertakes to deliver or cause to be delivered with the prospectus, to each person to whom the prospectus is sent or given, the latest annual report to security
II-4
holders that is incorporated by reference in the prospectus and furnished pursuant to and meeting the requirements of Rule 14a-3 or Rule 14c-3 under the Securities Exchange Act of 1934; and, where interim financial information required to be presented by Article 3 of Regulation S-X are not set forth in the prospectus, to deliver, or cause to be delivered to each person to whom the prospectus is sent or given, the latest quarterly report that is specifically incorporated by reference in the prospectus to provide such interim financial information.
(h) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
II-5
II-6
II-7
II-8
- #
- To
be filed by amendment.
- *
- Filed
herewith.
- **
- Previously
filed.
- †
- Indicates
management contract or compensatory plan or arrangement.
- ¨
- Confidential treatment has been granted as to certain portions of this exhibit pursuant to Rule 406 of the Securities Act of 1933, as amended, or Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
II-9
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the city of Exton, Commonwealth of Pennsylvania, on this 16th day of November, 2017.
| FIBROCELL SCIENCE, INC. | ||||||
By: |
/s/ JOHN M. MASLOWSKI |
|||||
| Name: | John M. Maslowski | |||||
| Title: | President and Chief Executive Officer | |||||
Signature
|
Title
|
Date
|
||||
|---|---|---|---|---|---|---|
| /s/ JOHN M. MASLOWSKI John M. Maslowski |
Director, President and Chief Executive Officer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | November 16, 2017 | ||||
* Douglas J. Swirsky |
Chairman of the Board |
November 16, 2017 |
||||
* Kelvin Moore |
Director |
November 16, 2017 |
||||
* Marc Mazur |
Director |
November 16, 2017 |
||||
* Julian Kirk |
Director |
November 16, 2017 |
||||
* Marcus Smith |
Director |
November 16, 2017 |
||||
* Christine St. Clare |
Director |
November 16, 2017 |
||||
By: |
/s/ JOHN M. MASLOWSKI John M. Maslowski Attorney-in-fact |
|||||
II-10
