Attached files
| file | filename |
|---|---|
| 8-K - 8-K - IOVANCE BIOTHERAPEUTICS, INC. | tv479126_8k.htm |
Exhibit 99.1

Novel Cryopreserved Tumor Infiltrating Lymphocytes (LN - 144) Administered to Patients with Metastatic Melanoma Demonstrates Efficacy and Tolerability in a Multicenter Phase 2 Clinical Trial Amod Sarnaik 1 , Jason Chesney 2 , Harriet Kluger 3 , Brendan Curti 4 , Omid Hamid 5 , Jose Lutzky 6 , Maria Fardis 7 , Igor Gorbatchevsky 7 , Sam Suzuki 7 , Bente Larsen 7 , Nancy L. Samberg 7 , John Kirkwood 8 1 Moffitt Cancer Center, Tampa, FL, USA; 2 James Graham Brown Cancer Center, Louisville, KY, USA; 3 Yale Cancer Center, New Haven, CT, USA; 4 Earle A. Chiles Research Institute, Providence Cancer Center, Portland, OR, USA; 5 The Angeles Clinic , Los Angeles , CA, USA; 6 Mount Sinai Comprehensive Cancer Center, Miami, FL, USA; 7 Iovance Biotherapeutics, San Carlos, CA, USA 8 University of Pittsburgh Hillman Cancer Center, Pittsburgh, PA, USA Poster no.: P515 SITC Annual Meeting | November 8 - 12, 2017 | National Harbor, MD, USA © 2017, Iovance Biotherapeutics, Inc.

© 2017, Iovance Biotherapeutics, Inc.
Background 1 Goff, et al. Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor - Infiltr ating Lymphocytes for Patients With Metastatic Melanoma. J Clin Oncol . 2016 Jul 10;34(20):2389 - 97. 2 Sarnaik A, Kluger H, Chesney J, et al. Efficacy of single administration of tumor - infiltrating lymphocytes (TIL) in heavily pre treated patients with metastatic melanoma following checkpoint therapy. J Clin Oncol 2017; 35 [ suppl ; abstr 3045]. © 2017, Iovance Biotherapeutics, Inc. • The safety and efficacy of adoptive cell therapy (ACT) utilizing tumor infiltrating lymphocytes (TIL) has been studied in hun dre ds of patients with metastatic melanoma, and has demonstrated meaningful and durable objective response rates (ORR). 1 • Iovance Biotherapeutics is conducting an ongoing Phase 2 trial, C - 144 - 01, utilizing centralized GMP manufacturing of TIL, assess ing both non - cryopreserved generation - 1 (Gen - 1) and cryopreserved generation - 2 (Gen - 2) TIL manufacturing processes. • Gen - 1 is approximately 5 - 6 week in duration of manufacturing (administered in Cohort 1 of C - 144 - 01 study), while Gen - 2 is 22 day in duration of manufacturing (administered in Cohort 2 of C - 144 - 01 study). • Preliminary data from Cohort 1 patients treated with the Gen - 1 LN - 144 manufactured product, was encouraging in treating post - PD - 1 metastatic melanoma patients as the TIL therapy produced responses. 2 • Benefits of Gen - 2 include: ̶ Reduction in the time patients and physicians wait to infuse TIL to patient ̶ Cryopreservation permits flexibility in scheduling, distribution, and delivery ̶ Reduction of manufacturing costs • Preliminary data from Cohort 2 is presented herein.

Gen - 2 Cryopreserved LN - 144 Manufacturing Process T - 190 o C © 2017, Iovance Biotherapeutics, Inc. Excise tumor Thaw, infuse + IL - 2 administration Fragmentation Expansion ex vivo ex vivo culture IL - 2 + OKT3 Co - culture TIL and feeder cells Rapid Expansion (REP - 11 days) Tumor Fragment Culture (Pre - REP - 11 days) + IL - 2 Bulk TILs LN 2 cryopreserved LN - 144 infusion Harvest NMA - LD preconditioning therapy

Study Design © 2017, Iovance Biotherapeutics, Inc.

A Phase 2, Multicenter Study to Assess the Efficacy and Safety of Autologous Tumor Infiltrating Lymphocytes (LN - 144) for Treatment of Patients with Metastatic Melanoma Key Inclusion Criteria: • Measurable metastatic melanoma and ≥ 1 lesion resectable for TIL generation • Progression on at least one prior line of systemic therapy • Age ≥ 18 • ECOG PS 0 - 1 Treatment Cohorts: 1. Non - Cryopreserved LN - 144 product 2. Cryopreserved LN - 144 product 3. Retreatment with LN - 144 for patients without response or who progress after initial response Endpoints: • Primary: Efficacy defined as ORR • Secondary: Safety and Efficacy Iovance C - 144 - 01 Phase 2 Trial in Metastatic Melanoma © 2017, Iovance Biotherapeutics, Inc. (Current Amendment) Unresectable or Metastatic Melanoma Progressed after Prior Anti - PD - 1 therapy and, if BRAF mutant, after BRAF inhibitor Cohort 1: Non - cryopreserved TIL Product, n=30 Cohort 2: Cryopreserved TIL Product, n=30 Cohort 3: TIL Re - treatment, n=10

Methods © 2017, Iovance Biotherapeutics, Inc.

Results © 2017, Iovance Biotherapeutics, Inc.

Table 1. Comparison Patient Characteristics from Cohort 1( ASCO 2017 ) vs Cohort 2 Cohort 2 has: 4 median prior therapies; all patients have received prior anti - PD - 1 and anti - CTLA - 4 Had higher tumor burden reflected by greater SoD for target lesions and higher mean LDH at Baseline. © 2017, Iovance Biotherapeutics, Inc.
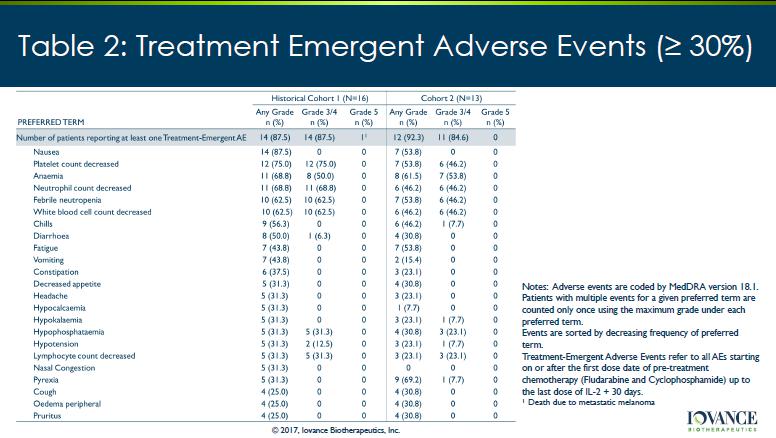
Table 2: Treatment Emergent Adverse Events (≥ 30%) Notes: Adverse events are coded by MedDRA version 18.1. Patients with multiple events for a given preferred term are counted only once using the maximum grade under each preferred term. Events are sorted by decreasing frequency of preferred term. Treatment - Emergent Adverse Events refer to all AEs starting on or after the first dose date of pre - treatment chemotherapy (Fludarabine and Cyclophosphamide) up to the last dose of IL - 2 + 30 days . 1 Death due to metastatic melanoma © 2017, Iovance Biotherapeutics, Inc .

Cohort 2 Gen - 2 Infusion Product and TIL Therapy Characteristics • Mean number of TIL cells infused: 37 x 10 9 • Median number of IL - 2 doses administered was 4.5 © 2017, Iovance Biotherapeutics, Inc. • PR for Patient 6 is unconfirmed as the patient has not reached the second efficacy assessment yet. • One patient (Patient 9) had passed away prior to the first assessment (still considered in the Efficacy Set). Abbreviations: PR, partial response; SD, stable disease, PD, progressive disease Figure 1. Efficacy

Figure 2. Clinical Status of Response Evaluable Patients with SD or a Better Response © 2017, Iovance Biotherapeutics, Inc. Figure 2. Of 9 patients in Efficacy Set, one patient (Patient 9) is not evaluable (NE) due to melanoma - related death prior to first tumor assessment not represented on figure. • Responses are seen in patients treated with Gen - 2 • DCR is: 78% • Time to response is similar to Cohort 1 • One patient (Patient 3) with PD as best response is not included in the swim lane plot.
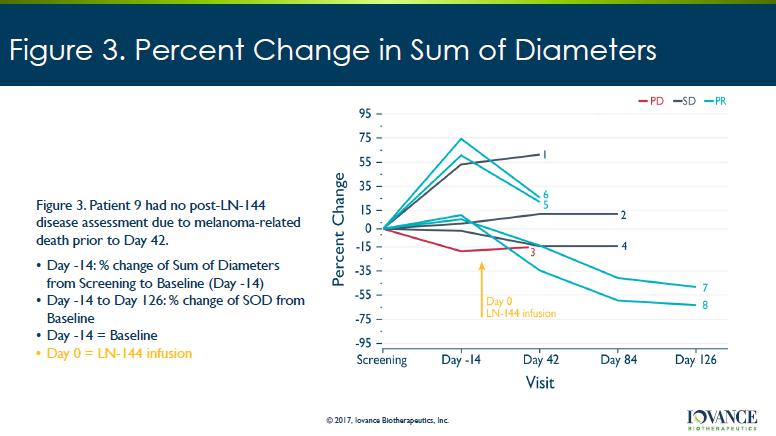
Figure 3. Percent Change in Sum of Diameters © 2017, Iovance Biotherapeutics, Inc. Figure 3. Patient 9 had no post - LN - 144 disease assessment due to melanoma - related death prior to Day 42. • Day - 14: % change of Sum of Diameters from Screening to Baseline (Day - 14) • Day - 14 to Day 126: % change of SOD from Baseline • Day - 14 = Baseline • Day 0 = LN - 144 infusion

© 2017, Iovance Biotherapeutics, Inc. Figure 4. Increase of HMGB1 Upon TIL Treatment HMGB1 Figure 4. Plasma HMGB1 levels were measured using HMGB1 ELISA kit (Tecan US, Inc). Data shown represents fold change in HMGB1 levels pre (Day - 7) and post (Day 4 and Day 14) LN - 144 infusion in Cohort 1 and Cohort 2 patients (p values were calculated using two - tailed paired t - test based on log - transformed data). Sample size ( bold and italicized ) and mean ( italicized ) values are shown in parentheses for each time point. HMGB1 is secreted by activated immune cells and released by damaged tumor cells. The increased HMGB1 levels observed after treatment with LN - 144 are therefore suggestive of an immune - mediated mechanism of anti - tumor activity. Day -7 Day 4 Day 14 Day -7 Day 4 Day 14 0.1 1 10 100 1000 10000 H M G B 1 ( n g / m l ) ( l o g 1 0 ) p=0.0004 p=0.1 (ns) (20, 25.9) (15,11.1) (21, 39.8) (7, 25.1) (7, 5.6) (7, 84.8) p=0.02 p=0.2 (ns) Cohort 1 Cohort 2

© 2017, Iovance Biotherapeutics, Inc. IP - 10 Figure 5. Biomarker of Interest IP - 10 Figure 5 . Plasma IP - 10 levels were measured using Luminex assay . Data shown represents fold change in IP - 10 levels pre (Day - 7 ) and post (Day 4 and Day 14 ) LN - 144 infusion in Cohort 1 and Cohort 2 patients (p values were calculated using two - tailed paired t - test based on log - transformed data) . Sample size (bold and italicized) and mean (italicized) values are shown in parentheses for each time point . The post - LN - 144 infusion increase in IP - 10 is being monitored to understand possible correlation with TIL persistence . Day -7 Day 4 Day 14 Day -7 Day 4 Day 14 10 100 1000 10000 100000 I P - 1 0 ( p g / m l ) ( l o g 1 0 ) (20, 1209) (15, 6827) (21, 4895) (7, 960) (7, 6716) (7, 7146) p=0.004 p<0.0001 p=0.02 p=0.02 Cohort 1 Cohort 2

Conclusions © 2017, Iovance Biotherapeutics, Inc.

Conclusions © 2017, Iovance Biotherapeutics, Inc. • Preliminary results from the existing data demonstrate comparable safety between Gen - 1 and Gen - 2 LN - 144 TIL products. • Administration of Gen - 2 LN - 144 leads to clinical responses seen in advanced disease metastatic melanoma patients; all had progressed on anti - PD - 1 and anti - CTLA - 4 prior therapies. • Disease Control rate for Cohort 2 was 78%. • Preliminary biomarker data is supportive of the cytolytic mechanism of action proposed for TIL therapy. • The Gen - 2 manufacturing takes 22 days. This process significantly shortens the duration of time a patient has to wait to receive their TIL, offers flexibility in the timing of dosing the patients, and leads to a reduction of cost of manufacturing.

Disclosures and Acknowledgements © 2017, Iovance Biotherapeutics, Inc. ACKNOWLEDGEMENTS • All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. • The authors would also like to thank the patients and their families for participation in the study. • The authors acknowledge Toshimi Takamura, Michelle Blaskovich, and Lavakumar Karyampudi from Iovance for their contributions. • The authors would also like to acknowledge all site team members from Moffitt Cancer Center (Allison Richards & Valerie Stark), James Graham Brown Cancer Center, Yale Cancer Center, Earle A. Chiles Research Institute, Providence Cancer Center, The Angeles Clinic, Mount Sinai Comprehensive Cancer Center, and the University of Pittsburgh Hillman Cancer Center for their contributions. DISCLOSURE & FUNDING STATEMENT • This study and poster are sponsored by Iovance Biotherapeutics, Inc. • MF, IG, SS, BL, and NS are employees of Iovance Biotherapeutics, Inc. and have stock options.
