Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Vyant Bio, Inc. | a17-26255_1ex99d1.htm |
| 8-K - 8-K - Vyant Bio, Inc. | a17-26255_18k.htm |
Q3 2017 Earnings Report & Company Updates Thursday – November 9, 2017 08:30am Eastern CEO: Panna Sharma webcast link: http://public.viavid.com/player/index.php?id=127177

Forward-Looking Statements These slides may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements pertaining to future financial and/or operating results, future growth in revenues, margins, research, technology, clinical development and potential opportunities for Cancer Genetics, Inc. tests and services, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management constitute forward-looking statements. Any statements that are not historical fact (including, but not limited to, statements that contain words such as "will," "believes," "plans," "anticipates," "expects," "estimates") should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, risks of cancellation of customer contracts or discontinuance of trials, risks that anticipated benefits from acquisitions will not be realized, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, maintenance of intellectual property rights and other risks discussed in the Cancer Genetics, Inc. Form 10-K for the year ended December 31, 2016 and the Form 10-Q for the Quarter ended September 30, 2017 along with other filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof. Cancer Genetics, Inc. disclaims any obligation to update these forward-looking statements. p.2

Everything we do is focused on 1 KEY idea .. deliver innovation & patient value by providing the most comprehensive capabilities For precision oncology development.
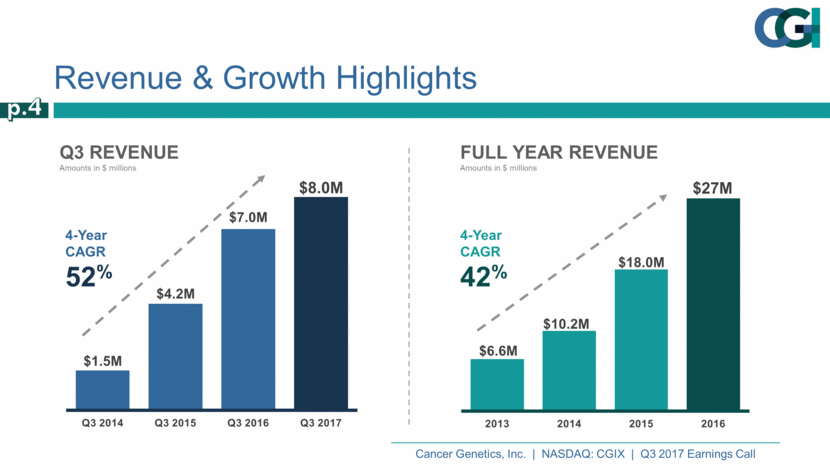
Q3 Revenue Amounts in $ millions Revenue & Growth Highlights p.4 4-Year CAGR 52% $8.0M 4-Year CAGR 42% Full year Revenue Amounts in $ millions $6.6M $10.2M $18.0M $27M 2013 2014 2015 2016 $1.5M $4.2M $7.0M Q3 2014 Q3 2015 Q3 2016 Q3 2017
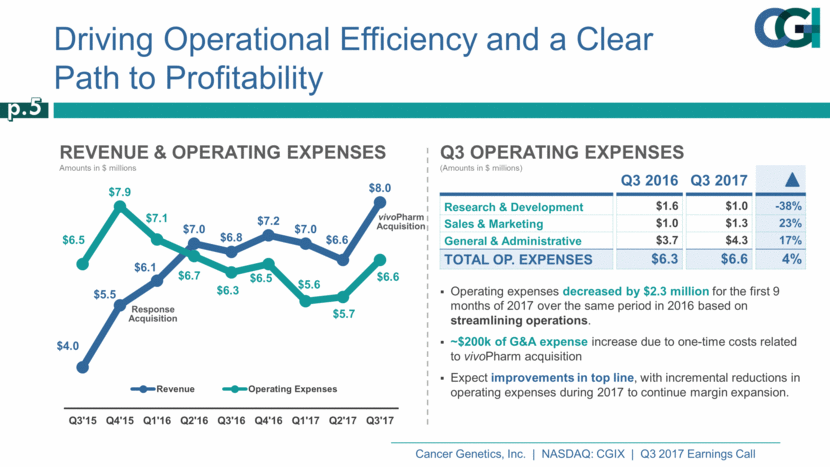
Revenue & Operating Expenses Amounts in $ millions Driving Operational Efficiency and a Clear Path to Profitability Response Acquisition Operating expenses decreased by $2.3 million for the first 9 months of 2017 over the same period in 2016 based on streamlining operations. ~$200k of G&A expense increase due to one-time costs related to vivoPharm acquisition Expect improvements in top line, with incremental reductions in operating expenses during 2017 to continue margin expansion. p.5 Q3 2016 Q3 2017 ▲ Research & Development $1.6 $1.0 -38% Sales & Marketing $1.0 $1.3 23% General & Administrative $3.7 $4.3 17% Total Op. Expenses $6.3 $6.6 4% Q3 Operating Expenses (Amounts in $ millions) vivoPharm Acquisition $4.0 $5.5 $6.1 $7.0 $6.8 $7.2 $7.0 $6.6 $8.0 $6.5 $7.9 $7.1 $6.7 $6.3 $6.5 $5.6 $5.7 $6.6 Q3'15 Q4'15 Q1'16 Q2'16 Q3'16 Q4'16 Q1'17 Q2'17 Q3'17 Revenue Operating Expenses
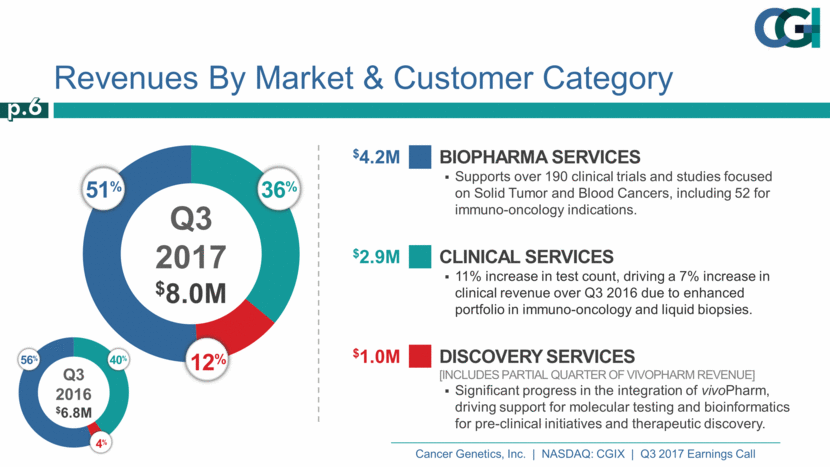
Revenues By Market & Customer Category Biopharma Services Supports over 190 clinical trials and studies focused on Solid Tumor and Blood Cancers, including 52 for immuno-oncology indications. $4.2M Clinical Services 11% increase in test count, driving a 7% increase in clinical revenue over Q3 2016 due to enhanced portfolio in immuno-oncology and liquid biopsies. $2.9M Discovery Services [Includes partial quarter of vivoPharm revenue] Significant progress in the integration of vivoPharm, driving support for molecular testing and bioinformatics for pre-clinical initiatives and therapeutic discovery. $1.0M 51% 36% 12% Q3 2017 $8.0M p.6
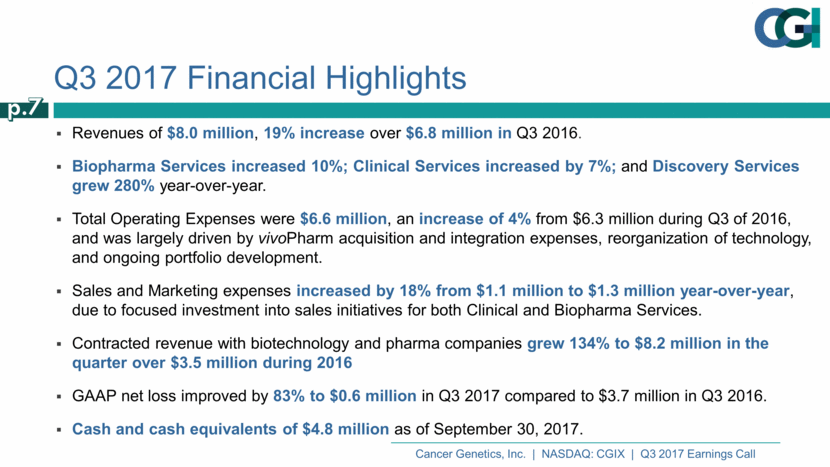
Q3 2017 Financial Highlights Revenues of $8.0 million, 19% increase over $6.8 million in Q3 2016. Biopharma Services increased 10%; Clinical Services increased by 7%; and Discovery Services grew 280% year-over-year. Total Operating Expenses were $6.6 million, an increase of 4% from $6.3 million during Q3 of 2016, and was largely driven by vivoPharm acquisition and integration expenses, reorganization of technology, and ongoing portfolio development. Sales and Marketing expenses increased by 18% from $1.1 million to $1.3 million year-over-year, due to focused investment into sales initiatives for both Clinical and Biopharma Services. Contracted revenue with biotechnology and pharma companies grew 134% to $8.2 million in the quarter over $3.5 million during 2016 GAAP net loss improved by 83% to $0.6 million in Q3 2017 compared to $3.7 million in Q3 2016. Cash and cash equivalents of $4.8 million as of September 30, 2017. p.7
Q3 2017 Business Highlights Frost & Sullivan acclaimed CGI’s advanced genomic & biomarker technologies that drive industry innovation in support of precision cancer treatment. Presented at 2017 Disruptive Growth Company Showcase, The MicroCap Conference, and hosted panel discussion on “The Convergence of Artificial Intelligence and Healthcare – New Developments in Precision Medicine”. Continued successful integration of acquired global oncology and Immuno-Oncology Discovery Services Company, vivoPharm Pty Ltd. Announced 25th contract win to provide testing and diagnostic services for combination Immuno-Oncology clinical trials. Launched AntigenID™ solidifying CGI’s position as precision oncology leader in the field of Immuno-Oncology Therapy & Drug Development. Named one of the Top 10 Drug Discovery & Development Solution Providers by Panel of Industry Experts at Pharma Tech Outlook. Ongoing development of CGI’s Artificial Intelligence program, CGI Match, and gaining select market feedback. p.8

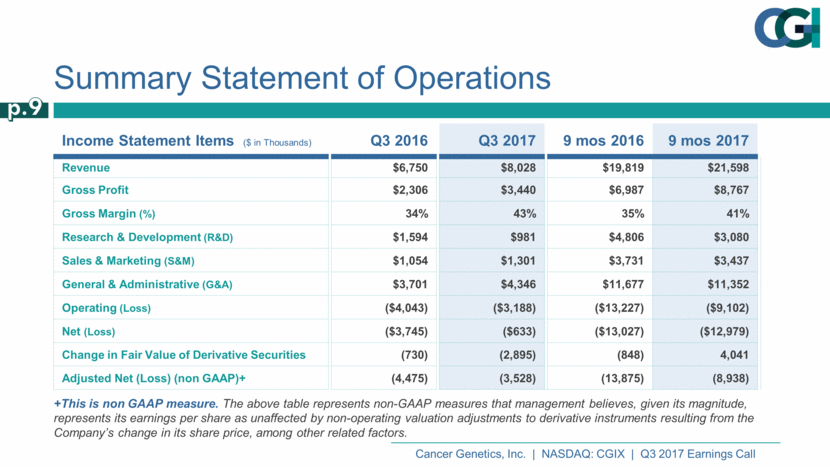
Income Statement Items ($ in Thousands) Q3 2016 Q3 2017 9 mos 2016 9 mos 2017 Revenue $6,750 $8,028 $19,819 $21,598 Gross Profit $2,306 $3,440 $6,987 $8,767 Gross Margin (%) 34% 43% 35% 41% Research & Development (R&D) $1,594 $981 $4,806 $3,080 Sales & Marketing (S&M) $1,054 $1,301 $3,731 $3,437 General & Administrative (G&A) $3,701 $4,346 $11,677 $11,352 Operating (Loss) ($4,043) ($3,188) ($13,227) ($9,102) Net (Loss) ($3,745) ($633) ($13,027) ($12,979) Change in Fair Value of Derivative Securities (730) (2,895) (848) 4,041 Adjusted Net (Loss) (non GAAP)+ (4,475) (3,528) (13,875) (8,938) Summary Statement of Operations +This is non GAAP measure. The above table represents non-GAAP measures that management believes, given its magnitude, represents its earnings per share as unaffected by non-operating valuation adjustments to derivative instruments resulting from the Company’s change in its share price, among other related factors. p.9
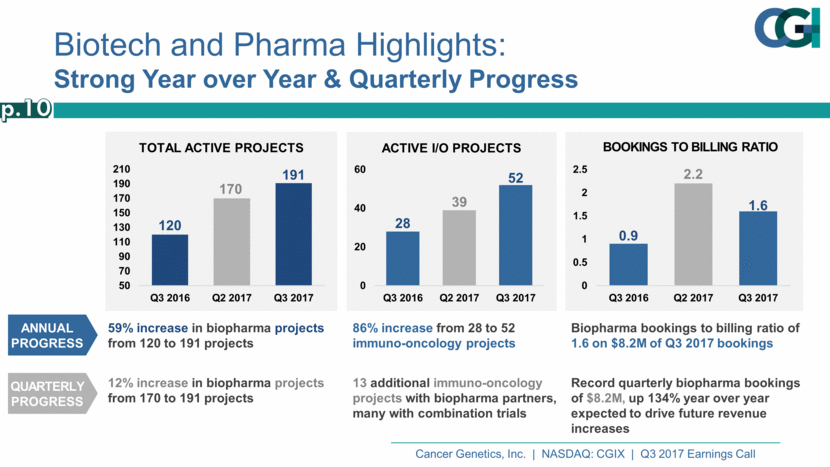
Biotech and Pharma Highlights: Strong Year over Year & Quarterly Progress Biopharma bookings to billing ratio of 1.6 on $8.2M of Q3 2017 bookings Record quarterly biopharma bookings of $8.2M, up 134% year over year expected to drive future revenue increases 59% increase in biopharma projects from 120 to 191 projects 12% increase in biopharma projects from 170 to 191 projects 86% increase from 28 to 52 immuno-oncology projects 13 additional immuno-oncology projects with biopharma partners, many with combination trials Annual Progress Quarterly Progress p.10 0.9 2.2 1.6 0 0.5 1 1.5 2 2.5 Q3 2016 Q2 2017 Q3 2017 BOOKINGS TO BILLING RATIO 120 170 191 50 70 90 110 130 150 170 190 210 Q3 2016 Q2 2017 Q3 2017 TOTAL ACTIVE PROJECTS 28 39 52 0 20 40 60 Q3 2016 Q2 2017 Q3 2017 ACTIVE I/O PROJECTS
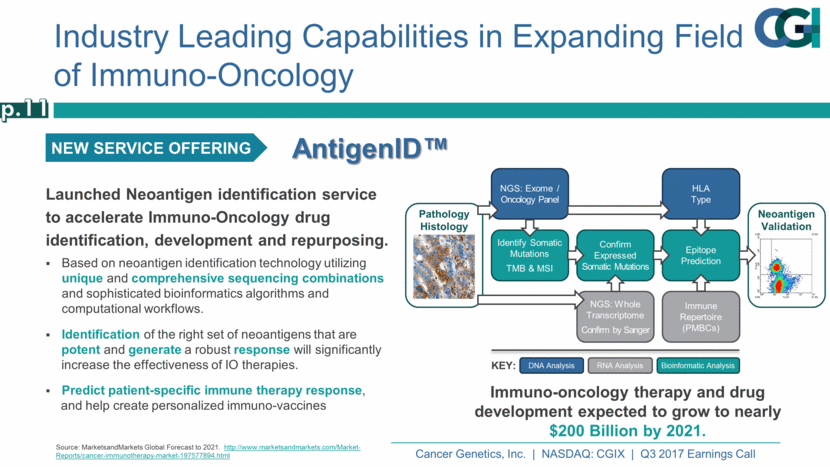
Confirm Expressed Somatic Mutations NGS: Whole Transcriptome Confirm by Sanger NGS: Exome / Oncology Panel Identify Somatic Mutations TMB & MSI HLA Type Epitope Prediction Immune Repertoire (PMBCs) Pathology Histology Neoantigen Validation KEY: DNA Analysis RNA Analysis Bioinformatic Analysis Industry Leading Capabilities in Expanding Field of Immuno-Oncology Launched Neoantigen identification service to accelerate Immuno-Oncology drug identification, development and repurposing. Based on neoantigen identification technology utilizing unique and comprehensive sequencing combinations and sophisticated bioinformatics algorithms and computational workflows. Identification of the right set of neoantigens that are potent and generate a robust response will significantly increase the effectiveness of IO therapies. Predict patient-specific immune therapy response, and help create personalized immuno-vaccines New service offering AntigenID™ Immuno-oncology therapy and drug development expected to grow to nearly $200 Billion by 2021. Source: MarketsandMarkets Global Forecast to 2021. http://www.marketsandmarkets.com/Market-Reports/cancer-immunotherapy-market-197577894.html p.11
2017 Goals Multiple Myeloma NGS Panel [Mayo] Further Expansion in Asia-Pacific Integration of vivoPharm Launch IO capabilities for Drug Discovery & Repurposing Develop Genetic Counselor Network Expand Hereditary Service Offering Liquid Biopsy for Kidney Cancer Bioinformatics Center of Excellence in India Hereditary Cancer Testing Panels Artificial Intelligence Engine to Improve Clinical Trial Matching Expand Biopharma Partnerships Liquid Biopsy for Lung Cancer Immuno-Oncology NGS Panel p.12
www.cgix.com www.cancergenetics.com CGI Headquarters 201 Route 17 North Rutherford, NJ 07070 Phone: +1 201-528-9200 RUTHERFORD, NJ, USA #3-1-135/1A CNR Complex Mallapur Main Road, R.R. Dst. Hyderabad – 500 076, Telangana Phone: +91 040-2717-8178 HYDERABAD, INDIA Research Triangle Park 133 Southcenter Court Morrisville, NC 27569 Phone: +1 919-465-0100 RALEIGH, NC, USA 781 Cai Lun Road, Room 803 Shanghai 201203 P.R. China Phone: +86 21-5049-5700 SHANGHAI, CHINA LOS ANGELES, CA, USA 1640 Marengo Street Fourth Floor Los Angeles, CA 90033 Phone: +1 323-224-3900 Grillparzerstrasse 25 81675 Munich Germany Phone: +49 891-2228-7690 MUNICH, GERMANY HERSHEY, PA, USA 1214 Research Boulevard Hummelstown, PA 17036 Phone: +1 717-798-9990 240 Plenty Rd, Level 3, Suite S29 Bundoora, VIC 3083 Australia Phone: +61 3-9988-1800 MELBOURNE, AUSTRALIA

