Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Salarius Pharmaceuticals, Inc. | flks201709308-k.htm |
| EX-99.2 - EXHIBIT 99.2 - Salarius Pharmaceuticals, Inc. | alsclinicaltrialresults.htm |
| EX-99.1 - EXHIBIT 99.1 - Salarius Pharmaceuticals, Inc. | flks201709308-kpr.htm |

Novel Treatments for Severe Neurological Diseases
NASDAQ: FLKS
6 November 2017

2
Any statements in this presentation and the oral commentary about future expectations, plans and prospects for the company, including statements
about the company’s strategy, future operations, ongoing clinical trials, development of its consumer and drug product candidates, plans for potential
future product candidates and other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,”
“project,” “suggest,” “target,” “potential,” “will,” “approximately,” “development plans,” “would,” “could,” “should,” “continue,” and similar expressions,
constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially
from those indicated by such forward-looking statements as a result of various important factors, including: the status, timing, design, costs, results
and interpretation of the company’s clinical studies; the uncertainties inherent in conducting clinical studies; results from our ongoing and planned
preclinical development; expectations of our ability to make regulatory filings and obtain and maintain regulatory approvals, our ability to commercialize
our consumer products; positioning and product attributes of our consumer products; results of early clinical studies as indicative of the results of
future trials; availability of funding sufficient for the company’s foreseeable and unforeseeable operating expenses and capital expenditure
requirements; other matters that could affect the availability or commercial potential of the company’s consumer or drug product candidates; the
inherent uncertainties associated with intellectual property; and other factors discussed in the Risk Factors set forth in the company’s Annual Report
on Form 10-K filed with the Securities and Exchange Commission (SEC) and in other filings the company makes with the SEC from time to time. We
may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on
our forward-looking statements. The forward-looking statements in this presentation represent our views as of the date of this presentation. We
anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking
statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore,
not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation.
This presentation also contains estimates and other statistical data made by independent parties and by the company relating to market size and
growth and other data about the company’s industry. This data involves a number of assumptions and limitations, and you are cautioned not to give
undue weight to such estimates. In addition, projections, assumptions and estimates of the company’s future performance and the future performance
of the markets in which the company operates are necessarily subject to a high degree of uncertainty and risk.
This presentation contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade
names referred to in this presentation, including logos, artwork and other visual displays, may appear without the ® or TM symbols, but such
references are not intended to indicate, in any way, that their respective owners will not assert, to the fullest extent under applicable law, their rights
thereto. We do not intend our use or display of other companies' trade names or trademarks to imply a relationship with, or endorsement or
sponsorship of us by, any other companies.
Forward-Looking Statements

3
Screening
Run-In
No treatment
Period 1
Cross-
Over
FLX-787
Period 1
Cross-
Over
Placebo
7 day follow
up call
14 days
14 days
14 days
Period 2
Cross-
Over
Placebo
14 days
Period 2
Cross-
Over
FLX-787
14 days14-21 day
washout
Exploratory Cramping/Spasticity Study in MND
Under Australian Clinical Trial Notification (CTN)
Randomization
BID/TID dosing with FLX-787 (19 mg) or Placebo control
• Randomized, Double-blind, Placebo-controlled, Cross-over Study to Evaluate Efficacy
and Tolerability of FLX-787 in Patients with Motor Neuron Disease
• N=8 per protocol subjects. Terminated early to focus resources on larger Phase 2b US trial
• Orally Disintegrating Tablet (ODT) of 19 mg FLX-787 (spicy) or Placebo (sweet)
Inclusion Criteria:
1. Diagnosed with Amyotrophic Lateral Sclerosis (ALS) or Progressive Lateral Sclerosis (PLS) for at least 1 month
2. > 12 cramps per month (approximately 3 per week)
3. Spasticity of at least 3 months duration that is not completely relieved by current therapy

4
• FLX-787 treatment was associated with improvements in cramping and related
symptoms, from pre-treatment baselines, including:
- Decreased Pain Intensity (NRS) associated with most painful cramp (p<0.05)
- Decreased Stiffness (NRS) (p<0.05)
- Greater Percentage Reduction of Cramps from baseline (~30% decrease) (p=0.08)
- Increased Number of Cramp Free Days (p=0.09)
- Improved Patient (p=0.06) and Clinician (p=0.06) Global Impression of Change
• These data, the first in patients with serious neurological disease, indicate the
potential of FLX-787 to alleviate cramps and cramp-related symptoms.
• 19 mg FLX-787 (BID or TID) were generally well tolerated in subjects with MND. No
drug-related SAEs or discontinuations were reported. A few GI-related AEs (abdominal
pain, diarrhea) were reported with FLX-787.
• The comparison of Period 1 and Period 2 results suggest the cross-over results are not
driven by a cross-over bias or unblinding effect. (see appendix)
• Results confirm the potential of the ongoing 100 subject, parallel design, ALS
trial with 4 weeks of run-in and treatment, utilizing a more robust 30 mg TID
dose.
FLX-787 Reduces Pain from Cramping (p<0.05)
and Associated Stiffness (p<0.05) in ALS Patients
Statistically
significant
improvements
Improvements
approaching
statistical
significance

5
Efficacy Endpoint Run-In Flex-787 Control P
Mean Median Mean Median Mean Median
Total Number of Cramps
% change from Baseline
68.72 37.33 46.04
-27.60
21.27
-31.09
71.31
5.92
39.42
-0.11 0.08
Number of Cramp Free Days
# of days change from Baseline
1.60 1.17 4.65
3.05
4.35
2.29
1.66
0.06
0.00
0.00 0.09
Statistical Treatment Comparisons (Medians)
Run-In Flex-787 Control P
Total NRS Pain Intensity Score of Most
Painful Cramp
% Change from Baseline
50.75
-13.65 19.00 <0.05
Total NRS Spasticity Score*
% Change from Baseline
62.46
-14.53 0.00 0.16
Total NRS Stiffness Score
% Change from Baseline
61.38
-9.84 12.07 <0.05
Summary Efficacy Results
(Per Protocol Population; N=8)
Notes: Baseline is the corresponding value from Run-in period. P-values are from Wilcoxon signed rank test of the paired treatment differences,
data are reported as medians.
*Of note, baseline spasticity levels in the patient population were modest, and spasticity assessed by Modified Ashworth and Tardieu scales
were not consistent with a treatment difference.

6
FLX-787 Control
Scale (Score) N=8 N=8
Very Much Improved (1) 0 ( 0.0%) 0 ( 0.0%)
Much Improved (2) 1 (12.5%) 0 ( 0.0%)
Minimally Improved (3) 3 (37.5%) 1 (12.5%)
No Change (4) 4 (50.0%) 5 (62.5%)
Minimally Worse (5) 0 ( 0.0%) 2 (25.0%)
Much Worse (6) 0 ( 0.0%) 0 ( 0.0%)
Very Much Worse (7) 0 ( 0.0%) 0 ( 0.0%)
FLX-787 Control
Scale (Score) N=8 N=8
Very Much Improved (1) 1 (12.5%) 0 ( 0.0%)
Much Improved (2) 1 (12.5%) 0 ( 0.0%)
Minimally Improved (3) 2 (25.0%) 0 ( 0.0%)
No Change (4) 4 (50.0%) 6 (75.0%)
Minimally Worse (5) 0 ( 0.0%) 2 (25.0%)
Much Worse (6) 0 ( 0.0%) 0 ( 0.0%)
Very Much Worse (7) 0 ( 0.0%) 0 ( 0.0%)
Clinical & Patient Global Impression of Change
(Per Protocol Population, N=8)
Patient Global Impression Assessment of Change
(PGI-C)*
P=0.06
Patients evaluated themselves as improved with
FLX-787 treatment 50% of the time, compared
to 12.5% with Control treatment
Clinical Global Impression Assessment of Change
(CGI-C)*
P=0.06
Clinicians blinded to treatments evaluated
50% of patients as improved with FLX-787,
compared to 0% with Control treatment
* For PGI-C “Compared to your condition at baseline (the beginning of this study period), how much has your condition changed?” and for CGI-C “Rate
total change in the subject’s symptoms whether or not, in your clinical judgment, it is due entirely to drug treatment. Compared to his/her condition
since last visit, how much has he/she changed”.
7
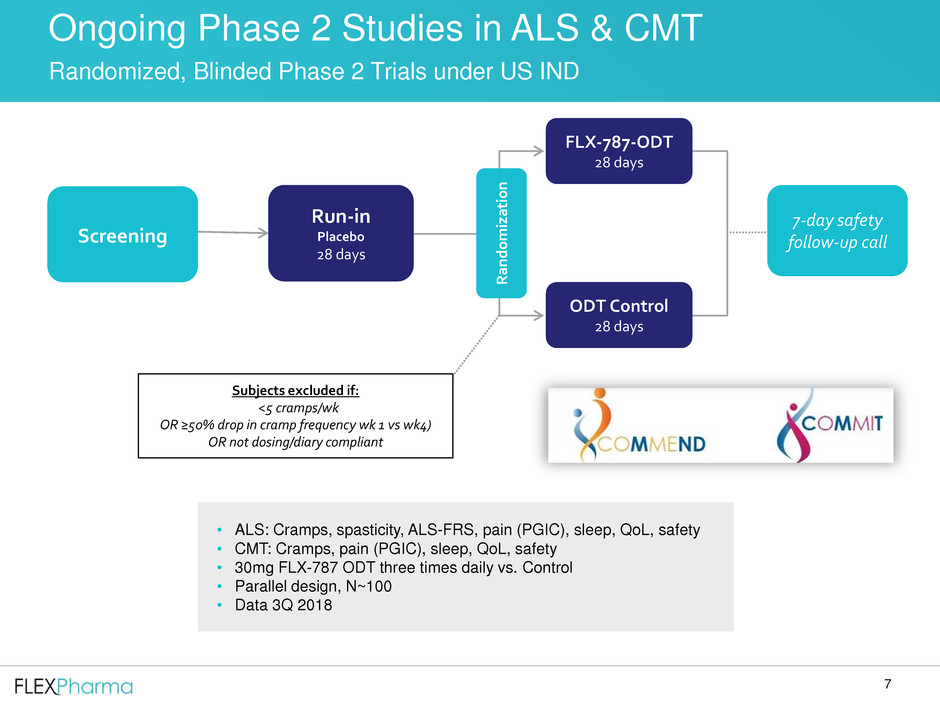
Ongoing Phase 2 Studies in ALS & CMT
Randomized, Blinded Phase 2 Trials under US IND
• ALS: Cramps, spasticity, ALS-FRS, pain (PGIC), sleep, QoL, safety
• CMT: Cramps, pain (PGIC), sleep, QoL, safety
• 30mg FLX-787 ODT three times daily vs. Control
• Parallel design, N~100
• Data 3Q 2018
FLX-787-ODT
28 days
ODT Control
28 days
Subjects excluded if:
<5 cramps/wk
OR ≥50% drop in cramp frequency wk 1 vs wk4)
OR not dosing/diary compliant
Screening
R
an
do
m
iz
at
io
n
Run-in
Placebo
28 days
7-day safety
follow-up call

8
No FDA approved treatment
95% of ALS patients report cramps1
Cramps can be frequent: median of 4 cramps/day 2
55% of ALS patients with pain attributed that pain to muscle cramps 3
57% of ALS patients to seek treatments directed at limiting cramps4
Cramping interferes with sleep and reduces QoL for patients suffering from
degenerative neurological diseases 5
1.Caress, JB, et al, Muscle Nerve. 2016 April;53(4): 513-517/ 2 Stephens HE 2016, Caress JB 2016, Weiss MD 2016, Weber M 2010/ 3 Bedlack RS, 2009 Stephens,
Joyce, Oskarsson. National Study of Muscle Cramps in ALS in the USA, 2016a / 4 Ganzini et al, Correlates of suffering in amyotrophic lateral sclerosis. Neurology
1999 Apr 22;52(7):1434-40 / 5 Hanisch F, Skudlarek A, Berndt J, Kornhuber ME, Brain Behav. 2015 Mar;5(3)
FLX-787 granted Fast Track designation for the treatment of
severe muscle cramps associated with ALS (July 2017)
Severe and Debilitating Muscle Cramps: Significant
Morbidity and Medical Need in Neurological Diseases

9
Baclofen
• Spasticity Tx, Limited cramp efficacy
• Sedating, ataxia (incoordination),
memory problems
Quinine • Malaria Tx, Not approved for NLC• Black Box warning
Mexiletine • Anti-arrhythmia• Black Box warning
Benzodiazepines • Sedating, ataxia• Addictive
Limitations of Current Cramp Treatments
Many patients seek and receive treatments that are ineffective or un-safe1
Fr
eq
ue
nc
y
of
U
se
1 Stephens, Joyce, Oskarsson. National Study of Muscle Cramps in ALS in the USA, 2016a
Mexiletine BLACK BOX
Increased Mortality
excessive mortality or nonfatal
cardiac arrest rate (7.7%
encainide/flecainide vs. 3% placebo)
in asymptomatic non-life-threatening
ventricular arrhythmias w/ MI 6 days -
2 years prior; restrict use to life-
threatening ventricular arrhythmias,
no survival benefit in pts w/o life-
threatening arrhythmias
Quinine BLACK BOX
Hematologic Toxicity
serious and life-threatening toxicity
incl. thrombocytopenia and HUS/TTP
may occur w/ use for nocturnal leg
cramp tx or prevention; TTP-assoc.
chronic renal impairment reported;
risk assoc. w/ nocturnal leg cramp
use in absence of evidence of
efficacy does not outweigh any
potential benefit
10
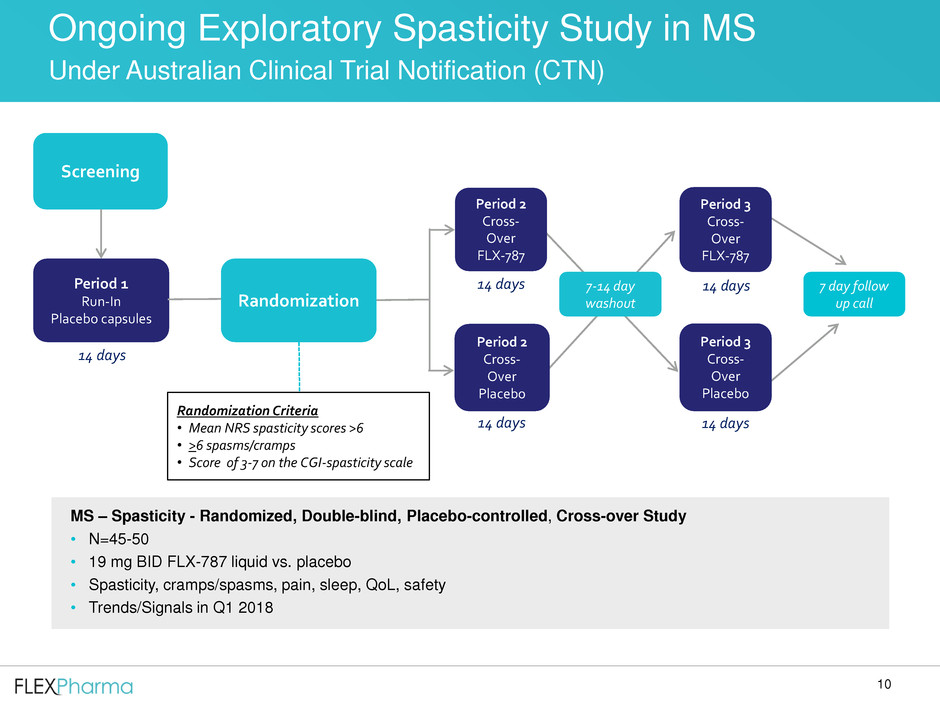
Screening
Period 1
Run-In
Placebo capsules
Period 2
Cross-
Over
FLX-787
Period 2
Cross-
Over
Placebo
7 day follow
up call
14 days
14 days
14 days
Period 3
Cross-
Over
Placebo
14 days
Period 3
Cross-
Over
FLX-787
14 days
MS – Spasticity - Randomized, Double-blind, Placebo-controlled, Cross-over Study
• N=45-50
• 19 mg BID FLX-787 liquid vs. placebo
• Spasticity, cramps/spasms, pain, sleep, QoL, safety
• Trends/Signals in Q1 2018
7-14 day
washout
Ongoing Exploratory Spasticity Study in MS
Under Australian Clinical Trial Notification (CTN)
Randomization
Randomization Criteria
• Mean NRS spasticity scores >6
• >6 spasms/cramps
• Score of 3-7 on the CGI-spasticity scale

11
MOA for Topical Modulation of CNS by FLX-787:
Stimulation-Processing-Motor Output
Spicy!
Alpha-motor neurons
connecting to muscle
Spinal cord
Descending
Inhibitory
Pathway
Nerves
Central processing in
brainstem leads to inhibition
through descending fibers in
spinal cord
Sensory neurons with TRPA1/V1
channels stimulated by FLX-787
Firing of alpha-motor
neurons slows down halting
muscle cramp
1
2
3

12
Muscle Cramping:
ALS, CMT, MS, Renal Dialysis,
Chemotherapy Induced Nausea,
Hereditary Spastic Paraplegia
Autonomic Control:
Migraine/Cluster Headache, Overactive
Bladder, Obstructive Sleep Apnea,
Raynaud’s, Gastroparesis, Emesis,
Menstrual Cramping
Non-autonomic Control
(complex motor):
Cervical Dystonia, Bruxism, Dysphagia
Neuro-Psychiatric:
Epilepsy, Depression, PTSD, Tinnitus,
Panic Attack
Potential Applications of Chemical Neurostimulation
1. United States Renal Data Service 2015 Annual Data Report/ 2. Rocco MV and Burkart JM, J Am Soc Nephrol. 1993; 4:1178-1183/ 3. Muscaritoli M, et al, Nutrition(2012) 28(10):959–66/ 4.
Cook IJ, Kahrillas PJ. Gastroenterology 1999; 116: 455-78/ 5. K Tjaden, Top Geriatr Rehabil. 2008 ; 24(2): 115–126/ 6. Rofes L, et al, J. Gastroenterol(2014) 49: 1517-1523/ 7. Hirata A, et al,
Biol. Pharm. Bull(2016) 39, 1107–1111
Dysphagia (Difficult/Unsafe Swallowing):
• Dysphagia emerges in more than 80% of ALS patients
during the advanced phases of the disease3
Aspiration pneumonia is a common cause of death in
ALS4
• 40% to 95% of persons with Parkinson Disease have
dysphagia5
• TRP channel activators such as piperine6 and ginger
extract7 have been shown to improve swallowing in the
elderly
Renal Dialysis:
• In the US, ~468,000 individuals are on hemodialysis1.
• Cramping was the most common reason (17.9%) for
early terminations from hemodialysis sessions.2
850,000 sessions terminated early (of 70MM sessions)
• FLX-787 has demonstrated anti-cramping activity in an
EIC model and in NLC subjects with intact nervous
systems.

13
Anticipated Upcoming Milestones
H1 2017: File IND (Effective April)
July 2017: Fast Track Designation for ALS
Q3 2017: Initiate ALS/MND Phase 2 parallel-design study (US)
Oct. 2017: Initiate CMT Phase 2 parallel-design study (US)
2018: Clinical Readouts
• Exploratory MS Spasticity Phase 2 study (Australia)
• Phase 2 ALS trial (US)
• Phase 2 CMT trial (US)
• Phase 2a Renal Dialysis Cramping (Observational)
• Phase 2a Dysphagia (swallowing difficulty)/tongue fasciculations in ALS

14
Financial Profile
NASDAQ: FLKS
~$39 M Cash balance as of 9/30/17
Cash into early 2019 based on
current operating plan
~17.9 million shares outstanding
No debt

Novel Treatments for Neuromuscular Conditions
APPENDICES

16
The objectives are:
To assess the effects of FLX-787 on muscle cramps, spasticity and sleep as measured by:
• Number of Cramps, and the Number of Cramp Free Days
• Pain and Intensity of Cramps
• Numerical Rating Scale (NRS) for Spasticity
• Modified Ashworth Scale (MAS)
• Tardieu Scale
• Patient Global Impression of Change (PGI-C) scale
• Clinical Global Impression of Change (CGI-C) scale
• Insomnia Severity Index (ISI) Survey*
• ALS Assessment Questionnaire (ALSAQ)*
• Penn Spasm Frequency Scale (PSFS)*
And, to assess the safety and tolerability of FLX-787 treatment in subjects with ALS, as determined by:
• Adverse Events (AEs)
• Laboratory Evaluations, Vital Signs, and ECGs
* Analysis pending
Study Objectives and Endpoints

17
Baseline
(N=12)
Crossover Period 1
(N=11)
Crossover Period 2
(N=11)
Patient Disposition and Analysis Populations
Per Protocol Population:
• N=8 received and completed both treatments
• All efficacy analyses based upon this population
Washout
Per
Protocol
n = 8n = 12
4 subjects not included in per protocol efficacy analyses:
• 1 subject discontinued due to non-drug related AE
• 3 Treatment Repeated subjects
Control
FLX-787
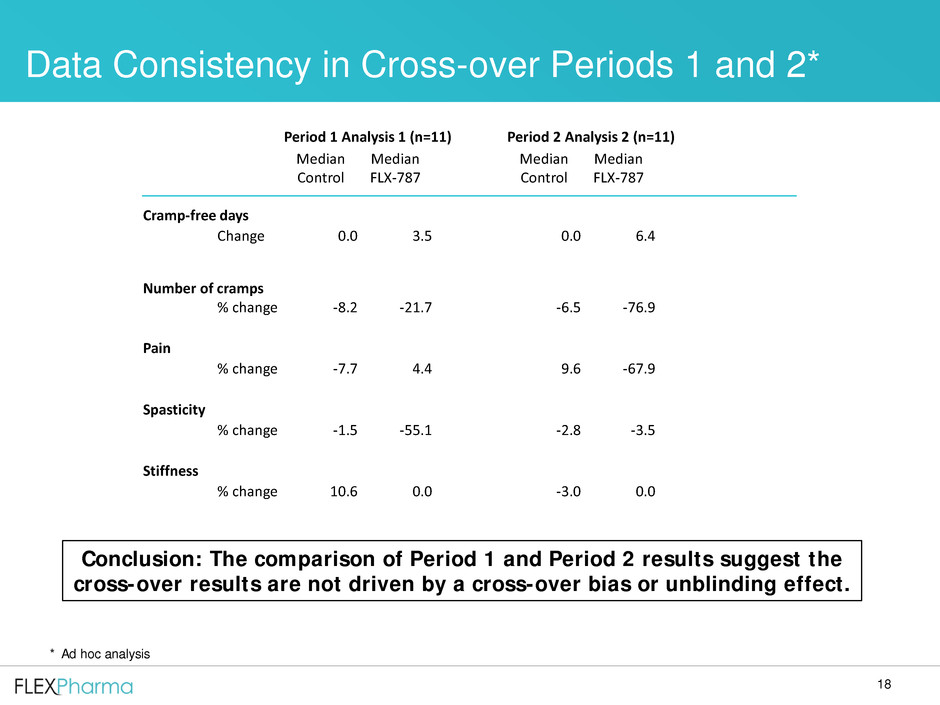
18
Period 1 Analysis 1 (n=11) Period 2 Analysis 2 (n=11)
Median
Control
Median
FLX-787
Median
Control
Median
FLX-787
Cramp-free days
Change 0.0 3.5 0.0 6.4
Number of cramps
% change -8.2 -21.7 -6.5 -76.9
Pain
% change -7.7 4.4 9.6 -67.9
Spasticity
% change -1.5 -55.1 -2.8 -3.5
Stiffness
% change 10.6 0.0 -3.0 0.0
Data Consistency in Cross-over Periods 1 and 2*
Conclusion: The comparison of Period 1 and Period 2 results suggest the
cross-over results are not driven by a cross-over bias or unblinding effect.
* Ad hoc analysis
