Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - PHASERX, INC. | tv478117_ex99-2.htm |
| 8-K - 8-K - PHASERX, INC. | tv478117_8k.htm |
Exhibit 99.1

Unlocking the value of mRNA ® m RNA THERAPEUTICS FOR UREA CYCLE DISORDERS 5 th International mRNA Conference November 1 - 2, 2017, Berlin Robert W. Overell, Ph.D. President and CEO

.. FORWARD - LOOKING STATEMENTS This presentation contains forward - looking statements which are based on current expectations, estimates and projections. Statements that are not historical facts are forward - looking statements and typically are identified by words like “may”, “believe”, “anticipate”, “could”, “should”, “estimate”, “expect”, “intend”, “plan”, “project”, “will”, “forecast”, “budget”, “pro forma”, and similar terms. These statements are not guarantees of future performance, events or results and involve potential risks and uncertainties. Although we believe that such statements are based on reasonable assumptions, these forward - looking statements are subject to numerous factors, risks and uncertainties that could cause actual outcomes and results to be materially different from those projected or assumed in our forward - looking statements. We caution you that any forward - looking statement reflects only our belief at the time the statement is made. Accordingly, the Company’s actual results may differ from our current expectations, estimates and projections. We undertake no obligation to update any forward - looking statements, whether as a result of new information, future events or otherwise. 2

.. UCD s CAUSE CUMULATIVE, PERMANENT BRAIN DAMAGE 3 • Single gene defects resulting in inability to metabolize ammonia • High blood ammonia leads to cumulative and permanent brain damage, coma and death • Liver transplant is the only corrective therapy • The only available drug treatment is the ammonia scavengers, which do not correct the disease • Lead Product is PRX - OTC to treat Ornithine Transcarbamylase (OTC) Deficiency (OTCD) • Goal is to deliver mRNA encoding the missing protein, thereby correcting the disease • Lowering of blood ammonia is an approvable endpoint in the UCDs Urea Cycle Disorders (UCDs) Source: Summar et al., 2008 Acta Paediatr. 97:1420 - 25; Enns et al., 2007 NEJM 356: 2282 - 92

.. Formulation administered by infusion Polymer mediates mRNA delivery into cell HYBRID m RNA TECHNOLOGY™ 4 Hybrid mRNA Technology GalNAc - Targeted Polymer Provides Delivery to Cytoplasm Inert LNP Provides mRNA Protection and Delivery to Liver Hybrid mRNA Technology™: Highly Effective mRNA Delivery Advantages: • Actively targets synthesis to the hepatocytes • High levels of expression and activity • Enables repeat mRNA dosing regimens • Well - tolerated in multiple species

.. LUCIFERASE EXPRESSION AT 6 H POST - DOSE 1 mg/kg 3 mg/kg 10 mg/kg PF0112/PRX847 formulation with luciferase mRNA led to dose responsive protein production up to 10 mg/kg. 5

.. TISSUE - SPECIFIC EXPRESSION WITH G al NA c TARGETING GalNAc targeting results in specific expression in liver • No expression observed in spleen, lymph nodes, kidney, lungs, or other tissues Ex Vivo Luciferase Analysis of CD1 Mouse Tissues Spleen Liver Kidney Lung Heart Pancreas Uterus/Ovaries 6

.. PRX - OTC MAKES ABUNDANT OTC ENZYME 7 • PRX - OTC makes OTC protein out to ≥ day 10 post - dose by Western blot • PRX - OTC treatment results in a ≈ 100% increase in OTC enzyme activity at day 10 post - dose Treatment With PRX - OTC Results in High Levels of OTC Enzyme for >10 days in Normal Mice 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Buffer 48 h Day 4 OTC Ab HSP90 Day 8 Day 10 Days Post 3 mg/kg Dose 1 OTC Enzyme Assay Described in Wang et. al. (2012 Mol. Genetics Metabol . 105: 203) 50 100 150 200 250 Buffer hOTC mRNA % of Wild - Type OTC Enzyme Activity 1 at Day 10 Post - Dose 3 mg/kg

.. PRX - OTC FULLY CORRECTS DISEASE IN OTCD MICE 8 • PRX - OTC normalizes blood ammonia levels in OTC spf - ash OTCD mice • PRX - OTC completely rescues mice from toxic effects of ammonia: 100% survival PRX - OTC Treatment Normalizes Blood Ammonia and Rescues 100% of Mice in Well - Accepted Mouse Model of OTCD 0 50 100 150 200 250 300 350 Buffer OTC mRNA Normal Mice Hyperammonemia Induced spf-ash Mice Plasma Ammonia (µM) Day 14 Day 21 0 5 10 15 20 25 30 35 0 20 40 60 80 100 Buffer OTC mRNA Days Post Induction of Hyperammonemia % S u r v i v a l Control mRNA

.. PRX - OTC REPEAT DOSING IN RATS: OTC EXPRESSION 9 9.0 2.3 4.3 0 2 4 6 8 10 Normal Human Liver 1 dose 3 doses ng hOTC/ug total protein hOTC Protein Level in Rat Liver 48 h post Final Dose • In a weekly repeat dose study in rats, we have seen the predicted accumulation of OTC protein – Week 3 protein level is nearly double week 1 level, and is ≈ 50% amount in normal human liver • This suggests a dosing frequency for PRX - OTC of once every two weeks or once monthly could be fully corrective in humans Western Blotting of Liver Lysates PRX - OTC 3 mg/kg OTC mRNA

.. PRX - OTC REPEAT DOSING IN RATS: LIVER ENZYMES 10 Dose 1 Dose 1 Dose 3 0 100 200 300 P631: Serum ALT at 24 h Post-Dose A L T ( U / L ) 3X ULN 3 mg/kg OTC mRNABuffer Dose 1 Dose 1 Dose 3 0 100 200 300 400 500 P631: Serum AST at 24 h Post-Dose A S T ( U / L ) 3X ULN 3 mg/kg OTC mRNABuffer • After 3 weekly repeat doses, normal ALT and AST levels
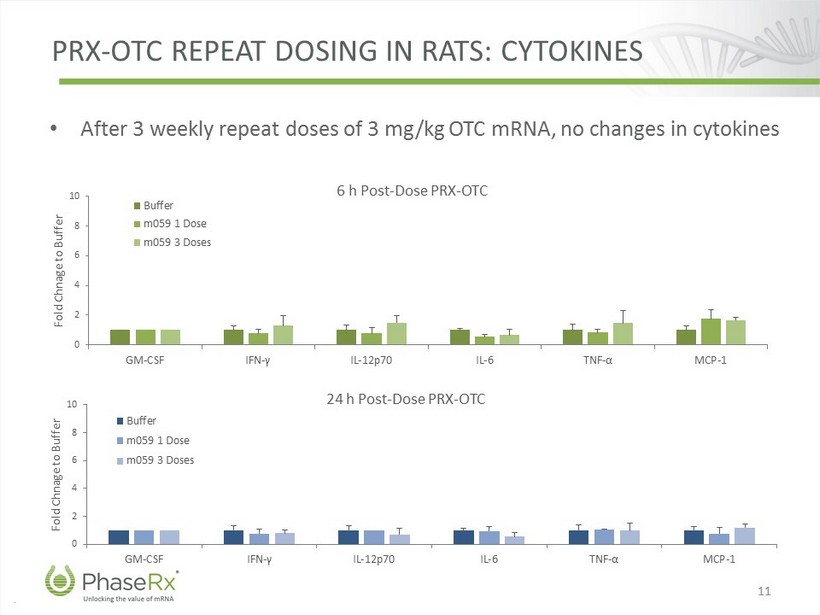
.. PRX - OTC REPEAT DOSING IN RATS: CYTOKINES 11 • After 3 weekly repeat doses of 3 mg/kg OTC mRNA, no changes in cytokines 0 2 4 6 8 10 GM-CSF IFN - γ IL-12p70 IL-6 TNF - α MCP-1 Fold Chnage to Buffer 6 h Post - Dose PRX - OTC Buffer m059 1 Dose m059 3 Doses 0 2 4 6 8 10 GM-CSF IFN - γ IL-12p70 IL-6 TNF - α MCP-1 Fold Chnage to Buffer 24 h Post - Dose PRX - OTC Buffer m059 1 Dose m059 3 Doses

.. PRX - OTC EXPECTED TO SHOW CLINICAL POC IN 2018 12 PROGRAM LEAD OPTIMIZATION PRECLINICAL IND - ENABLING PHASE 2a/2b PHASE 3 PRX - OTC Ornithine Transcarbamylase Deficiency PRX - ASL Argininosuccinate Lyase Deficiency PRX - ASS1 Argininosuccinate Synthase 1 Deficiency IND Filing Expected 2018 Data Expected 2H 2018 Lower Clinical Development Risk Simple mechanism; rare disease drugs have a higher rate of success in clinical trials Expedited Development Phase 1 in healthy volunteers is generally not necessary in rare disease Fast Endpoints 24h ammonia is approvable endpoint; ~12 patients in 2a/2b trial

.. SINGLE 2 a / b TRIAL DESIGN HAS RAPID ENDPOINTS 13 Phase 2a • Single Dose • Adult and Pediatric OTCD Patients • Multi - Center at UCDC sites • ~12 patients • Safety • Blood ammonia (24h) • Ureagenesis (2h) OTCD Patients On Scavenger >2 years old 0.1 mg/kg mRNA 0.3 mg/kg mRNA 1.0 mg/kg mRNA ~12 Patients Receive 4 Repeat Doses at OBD Identify OBD; Select Dosing Interval Phase 2b • Multiple Dose • Same patients OBD, Optimum Biologic Dose • Safety • Blood ammonia (24h) • Ureagenesis (2h)

.. P hase R x TEAM Chemistry: • Sean Monahan, Ph.D. • Debashish Roy, Ph.D. • Anna Galperin, Ph.D. • Jean - Rene Ella - Menye, Ph.D. • Matt Waldheim, MSc. Biology: • Mary Prieve, Ph.D. • Allen Li, M.D., Ph.D. • Pierrot Harvie, Ph.D. • Amber Paschal, MSc. • Teri Blevins, B.S.,LAT • Eric Bell, B.S., LATG 14 • Robert Overell, Ph.D., President and CEO • Michael Houston, Ph.D., CSO • Gordon Brandt, M.D., CMO
