Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Sesen Bio, Inc. | ebio-form8xkoctoberdeck.htm |

© 2017 Eleven Biotherapeutics. All rights reserved.
© 2017 Eleven Biotherapeutics. All rights reserved.
Targeting Cancer with
POWER+PRECISION™

© 2017 Eleven Biotherapeutics. All rights reserved.
This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than
statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, clinical
development of our protein therapies, potential milestone and royalty payments under the Roche license agreement, future financial
position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words
“anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,”
“continue,” and similar expressions are intended to identify forward-looking statements within the meaning of the Private Securities
Litigation Reform Act of 1995, although not all forward-looking statements contain these identifying words.
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place
undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and
expectations disclosed in the forward-looking statements we make as a result of various important factors, including: the occurrence of any
event change or other circumstances that could give rise to the termination of the Roche license agreement, the uncertainties inherent in
receiving future payments pursuant to the Roche license agreement, the uncertainties inherent in the initiation and conduct of clinical trials,
our ability to successfully develop our product candidates and complete our planned clinical programs, our ability to obtain marketing
approvals for our product candidates, expectations regarding our ongoing clinical trials, availability and timing of data from clinical trials,
whether interim results from a clinical trial will be predictive of the final results of the trial or results of early clinical studies will be indicative
of the results of future studies, the adequacy of any clinical models, expectations regarding regulatory approvals, our ability to obtain,
maintain and protect our intellectual property for our technology and products, availability of funding sufficient for the Company’s
foreseeable and unforeseeable operating expenses and capital expenditure requirements, other matters that could affect the financial
performance of the Company, other matters that could affect the availability or commercial potential of the Company’s product candidates
and other factors discussed in the “Risk Factors” section of the Company’s Annual Report on Form 10-K, Quarterly Reports on Form 10-Q
and other reports on file with the Securities and Exchange Commission (SEC). The forward-looking statements contained in this
presentation are made as of the date hereof, and Eleven assumes no obligation to update any forward-looking statements whether as a
result of new information, future events, or otherwise except as required by applicable law.
Forward-Looking Statements
2

© 2017 Eleven Biotherapeutics. All rights reserved.
Eleven Biotherapeutics
• Cambridge, Massachusetts-based company founded in 2010 by Flagship and
Third Rock Ventures to engineer protein therapeutics (NASDAQ:EBIO)
• In August 2016, we granted Roche an exclusive license to EBI-031, a humanized
monoclonal antibody that binds interleukin-6 (IL-6) and inhibits all forms of IL-6
cytokine signaling
‒ Received $30 million in upfront and milestone payments
‒ Eligible for additional $240 million upon achievement of future regulatory,
development and commercialization milestones
• Next milestone $20 million on initiation of Phase 2
‒ Entitled to tiered royalties on net sales
• In September 2016, we acquired Viventia Bio Inc. to create a targeted protein
therapeutics oncology company
‒ Acquisition included our lead product candidates, ViciniumTM, in a Phase 3
registration trial for non-muscle invasive bladder cancer, and ProxiniumTM,
for the treatment of squamous cell carcinoma of the head and neck
3

© 2017 Eleven Biotherapeutics. All rights reserved.
Investment Rationale
Broad pipeline of locally and systemically administered product candidates
for future development
• ProxiniumTM, for squamous cell carcinoma of the head and neck (SCCHN)
- Anti-tumor activity demonstrated in over 100 patients in prior clinical trials
- Next step a Phase 1/2a trial in combination with a checkpoint inhibitor
• VB6-845d, systemically administered TPT, utilizes proprietary deBouganin payload
- Next step is to file IND with FDA
Proprietary Targeted Protein Therapeutics designed to improve upon and
overcome challenges of existing ADCs and SMDCs
• Dual MOA: directly kills cancer cells and promotes anti-tumor immune response
• Utilizes antibody fragments which are associated with better tumor penetration
• Improved payload is effective against quiescent and multidrug resistant cancer cells
• Stable single protein product candidates
Lead drug candidate ViciniumTM in Phase 3 registration trial for high-grade
non-muscle invasive bladder cancer (NMIBC)
• Topline three-month data expected mid-2018; topline 12-month data expected Q2 2019
• In Phase 2 trial, demonstrated complete response rate of 40% at three months
Over $15 million in cash at end of Q2 2017; funds operations into Q1 2018
4
Vicinium entering Phase 1 combination study with checkpoint inhibitor
• Vicinium + durvalumab, AZ’s PD-L1 inhibitor to be studied under CRADA at NCI
• Immune response biomarker data could inform broader combo trials

© 2017 Eleven Biotherapeutics. All rights reserved.
Product Candidate Payload Indication Preclinical Ph 1 Ph 2 Ph 3
Locally administered TPTs
Vicinium ETA
BCG refractory
high-grade NMIBC
Locally administered TPT + Systemic Checkpoint Inhibitor
Vicinium + Durvalumab ETA & IO
BCG refractory
high-grade NMIBC
Proxinium
(combination with
checkpoint inhibitor)
ETA & IO SCCHN
Systemically administered TPTs
VB6-845d deBoug Solid tumors
Partnered Assets
EBI-031 (Roche) n/a
Diabetic Macular
Edema
Product Pipeline
5

© 2017 Eleven Biotherapeutics. All rights reserved.
NCI/ AZ - Vicinium + durvalumab NMIBC Phase 1 first patient
treated
Q4 2017
VISTA - Vicinium NMIBC Phase 3 complete enrollment Q1 2018
VISTA - Vicinium NMIBC Phase 3 topline three-month data Mid 2018
NCI/AZ - Vicinium + durvalumab NMIBC Phase 1 initial
biomarker data
Q3 2018
VISTA - Vicinium NMIBC Phase 3 topline 12-month data Q2 2019
Projected Newsflow
6

© 2017 Eleven Biotherapeutics. All rights reserved.
Targeted Protein Therapeutics (TPTs) are expressed as a single protein
molecule comprised of an anti-tumor antibody fragment, a tether and a
cytotoxic payload
Can be designed either for loco-regional or systemic delivery
Antibody Fragment Tether Protein toxin
Stable
Peptide
sequence
Anti-tumor
Single chain Fv
Pseudomonas Exotoxin A (ETA)
Extremely potent
Requires antibody to enter cell
7
Engineered Fusion Proteins “Smart Missiles”
Vicinium:

© 2017 Eleven Biotherapeutics. All rights reserved.
TPTs Designed to Overcome Limitations and
Improve on Existing ADCs
• TPTs are designed to deliver a greater amount of drug to the tumor with deeper
penetration and faster systemic elimination
‒ Utilize antibody fragments instead of intact Mabs
• Are designed to kill a broader array of cancer cells within a targeted tumor
‒ Utilize powerful protein synthesis inhibitor payloads designed to kill both rapidly proliferating and
slower growing cancer cells including cancer stem cells
‒ Not subject to multidrug resistance (MDR) mechanisms
• May promote therapeutic immune responses
‒ Data suggest TPTs drive immunogenic cell death
‒ Potential synergistic combinations with checkpoint inhibitors and other I/O products
• Potentially improved safety profile due to stable, genetically engineered linkers - product
candidates are single protein molecules
‒ Designed to remain intact until internalized into targeted cancer cell
• One-step manufacturing
‒ More efficient than existing ADC manufacturing
‒ Cost-effective E. Coli expression system
8

© 2017 Eleven Biotherapeutics. All rights reserved. 9
TPT Mechanism of Action
Focused delivery and potent anti-cancer effect

© 2017 Eleven Biotherapeutics. All rights reserved.
TPTs Induce Immunogenic Cell Death
Suggests Second Mechanism of Action, Synergy with I-O Agents
• Preclinical and clinical trial data suggest that VB4 (Vicinium and Proxinium) is promoting
host anti-tumor immune responses
‒ Immune checkpoint therapy requires an ongoing immune response and is believed to be most
effective in tumors with somatic mutations
• Immunogenic Cell Death (ICD)*
‒ Promotes pro-inflammatory environment and drives adaptive cellular immune responses by
releasing tumor neoantigens into this environment
‒ Cell death signals recognized by APCs (M1 phenotype)
‒ Hallmark of ICD is the presence of three Damage Associated Molecular Patterns (DAMPs)
• Calreticulin cell surface expression;
• Active ATP release from the cell; and
• Passive release of high mobility group box 1 protein (HMGB1)
• TPT treatment of tumor cells in vitro results in increase in DAMPs** indicating that TPTs kill
in a manner consistent with ICD
‒ Suggests that TPTs could be employed to drive host anti-tumor immune responses that synergize
with checkpoint inhibitors and other I-O agents
• Reviewed in Vandenabaele, p et al, Adv. Exp Medical Biology 930:133-49 2016
** Presented at AACR, 2017
10
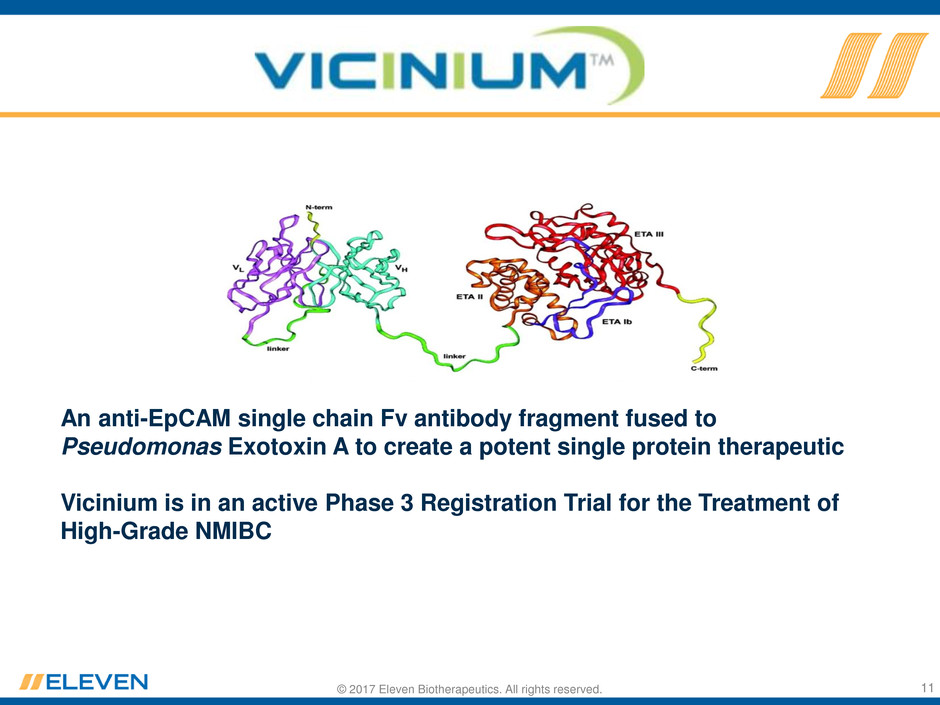
© 2017 Eleven Biotherapeutics. All rights reserved.
An anti-EpCAM single chain Fv antibody fragment fused to
Pseudomonas Exotoxin A to create a potent single protein therapeutic
Vicinium is in an active Phase 3 Registration Trial for the Treatment of
High-Grade NMIBC
11

© 2017 Eleven Biotherapeutics. All rights reserved.
Vicinium Overview
Extremely Potent
• Subpicomolar IC50
Targeting EpCAM in NMIBC
• >98% of high grade NMIBCs express EpCAM in prior studies
• Minimal to no EpCAM expression on normal bladder cells
Phase I and Phase 2 completed
• Demonstrated complete responses in patients with carcinoma-in-situ (CIS)
• Well tolerated
Vicinium VISTA Study In Bladder Cancer
• Ongoing Phase 3 single-arm open label registrational study
• Lead position vs. competition
Recent FDA Draft Guidance on Developing Drugs and Biologics for
Treatment of BCG-Unresponsive NMIBC:
• Supports single arm Phase 3 trial design
• Indicates potential for full approval
• VISTA study aligned with FDA guidance
12

© 2017 Eleven Biotherapeutics. All rights reserved.
Bladder Cancer
• Approx. 75-85% of new bladder cancer cases are NMIBC
‒ Ta: 57% -75%
• Low-grade: 77%
• High-grade: 23%
‒ T1: 20-30% - almost all high grade
‒ CIS: 5-13% - all high grade
Proprietary and Confidential 13

© 2017 Eleven Biotherapeutics. All rights reserved.
Opportunity in Bladder Cancer
In US, bladder cancer is the 6th most common1 but the most expensive cancer
to treat with indirect costs ranging from $184,762 to $461,907 per patient2
• In 2014 the prevalence of bladder cancer was 696,440 in the US3
• In the US the expected incidence in 2017 is 79,000 cases1
‒ The European incident rate is estimated at approx. twice the US rate
• NMIBC comprise 75-85% of bladder cancers4
• Over half of all NMIBC receive treatment with BCG and approx. 40% of patients
fail BCG within a two year span5
• Our initial target market includes these 23,700-26,860 patients diagnosed
annually as well as the prevalence of patients who have previously failed
BCG and have refused cystectomy
• Valstar, the only drug approved (1998) in this space is priced at $37,000
14
1ACS 2017, 2Sievert, K, World J. Urol. 2009, 3US SEER Data base 2014, 4Anastasiadis A. Therapeutic Advances in Urology. 2012, 5Steinberg,
R. Bladder Cancer 2 (2016)
© 2017 Eleven Biotherapeutics. All rights reserved.
Current Approach After BCG Failure
No Major Advances in 30 Years
• Survivors of initial treatment face a life-long risk of recurrence with stage
progression
‒ 30% risk of dying from bladder cancer
• Radical cystectomy is the primary recommendation following BCG failure
‒ 50% lifetime risk of cystectomy
‒ Complex, long surgical procedure associated with significant morbidity (28-45%) and mortality
(8% w/in 6 months of surgery)
‒ Further complicated as most patients with NMIBC are elderly and often have co-morbidities
‒ >25% of patients require readmission for surgery-related complications within 90 days following
surgery, and 34% require emergency room visits.
• Potential for future BCG shortage
‒ Sanofi announced plans to stop producing BCG in mid-2017 due to manufacturing challenges
‒ No anticipated impact on accrual to VISTA study
‒ May represent a market-expansion opportunity
• Valrubicin (Valstar®)
‒ Approved in the United States for BCG refractory CIS in patients for whom immediate
cystectomy would be associated with unacceptable morbidity or mortality.
‒ Approval based upon a single registration study of 90 patients with BCG refractory CIS disease,
18% CR rate at 6 months after starting treatment. Associated with significant toxicity.
15
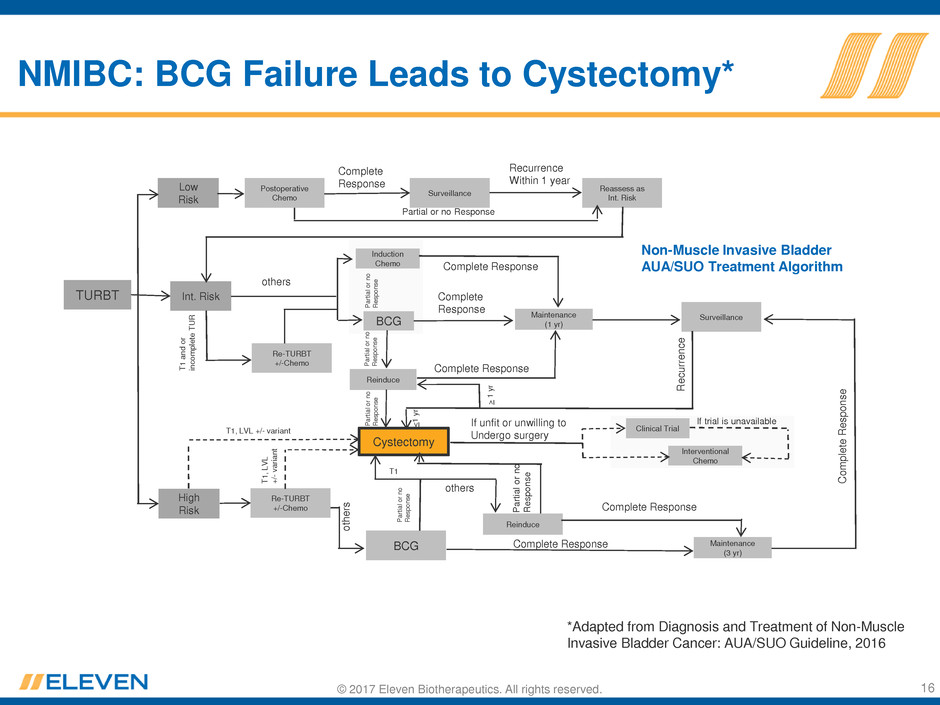
© 2017 Eleven Biotherapeutics. All rights reserved. 16
NMIBC: BCG Failure Leads to Cystectomy*
*Adapted from Diagnosis and Treatment of Non-Muscle
Invasive Bladder Cancer: AUA/SUO Guideline, 2016
Low
Risk
Int. Risk
High
Risk
TURBT
Postoperative
Chemo
Surveillance
Reassess as
Int. Risk
Re-TURBT
+/-Chemo
Re-TURBT
+/-Chemo
BCG
Reinduce
Reinduce
Maintenance
(1 yr)
Surveillance
Maintenance
(3 yr)
Cystectomy
Induction
Chemo
BCG
Interventional
Chemo
Clinical Trial
Complete Response
Complete
Response
Complete Response
Complete Response
Complete
Response
others
Recurrence
Within 1 year
If unfit or unwilling to
Undergo surgery
C
o
m
p
le
te
R
e
s
p
o
n
s
e
Complete Response
others
P
a
rti
a
l
o
r
n
o
Res
p
o
n
s
e
P
a
rt
ia
l o
r
n
o
R
e
sp
o
n
se
P
a
rt
ia
l o
r
n
o
R
e
sp
o
n
se
P
a
rt
ia
l o
r
n
o
R
e
sp
o
n
se
P
a
rt
ia
l o
r
n
o
R
e
sp
o
n
se
T1, LVL +/- variant
T
1
,
L
V
L
+
/-
v
a
ri
a
n
t
T1
o
th
e
rs
>
1
y
r
<
1
y
r
R
e
c
u
rr
e
n
c
e
Partial or no Response
Non-Muscle Invasive Bladder
AUA/SUO Treatment Algorithm
T
1
a
n
d
o
r
in
c
o
m
p
le
te
T
U
R
If trial is unavailable

© 2017 Eleven Biotherapeutics. All rights reserved.
Development of Drugs and Biologics for
NMIBC
In November 2016, the FDA released draft guidance “BCG-Unresponsive Nonmuscle Invasive
Bladder Cancer: Developing Drugs and Biologics for Treatment - Guidance for Industry:
‒ CIS is not completely resected and will persist or progress without treatment
‒ Standard of Care for BCG-unresponsive disease is cystectomy
‒ Effective drugs are not available for this population
‒ Single-arm trials of patients with BCG-unresponsive CIS disease with or without papillary
disease with a response rate endpoint may be appropriate for full approval
17
The Phase 3 VISTA study is consistent with the FDA draft guidance for providing evidence
of effectiveness to support a marketing application
Only recent review in this disease space was Telesta’s MCNA – eliciting a similar inflammatory
response in the bladder as BCG
- Completed a clinical trial in high grade NMIBC. Demonstrated 18.7% CR rate at 1 year in
CIS patients, but only 15.1% in key BCG refractory population
- Missed primary endpoint but still received FDA review and panel in 2015
- Advisory board voted against approval (18 to 6) – we believe transcript suggests decision
not based on sufficient efficacy but issues with protocol design and lack of MOA distinct
from BCG
FDA issued a Complete Response Letter to Telesta

© 2017 Eleven Biotherapeutics. All rights reserved.
Vicinium: Phase 1 Clinical Results
• 64 patients with Grade 2 or 3 BCG refractory or intolerant EpCAM “+” NMIBC*
• Trial design
‒ Treated weekly x 6 weeks
‒ Dose escalation (0.1- 30.16 mg)
‒ Efficacy assessment
‒ Cystoscopy, Biopsy (Random and Directed), Urine Cytology
*Source: Kowalski et. al., Drug Design, Development and Therapy 4: 313, 2010.
Safety and Exploratory Efficacy
• CIS population Complete Response (CR) rate at 3 months:
• Low dose (0.1 mg - <1.0mg): 1/6 (17%)
• High Dose (≥ 10.0mg): 4/11 (36%)
• No dose limiting toxicities (DLTs) reported. Well tolerated at these doses.
• No maximum tolerated dose (MTD) was reached
• Only 2 subjects with detectible systemic levels (very near the LLQ). No clinical
complications.
18
+Refractory was defined as recurrence within 2 years after at least one full cycle of
BGC. 5 of 64 patients were BCG intolerant
*Source: Kowalski et. al., Drug Design, Development and Therapy 4: 313, 2010.
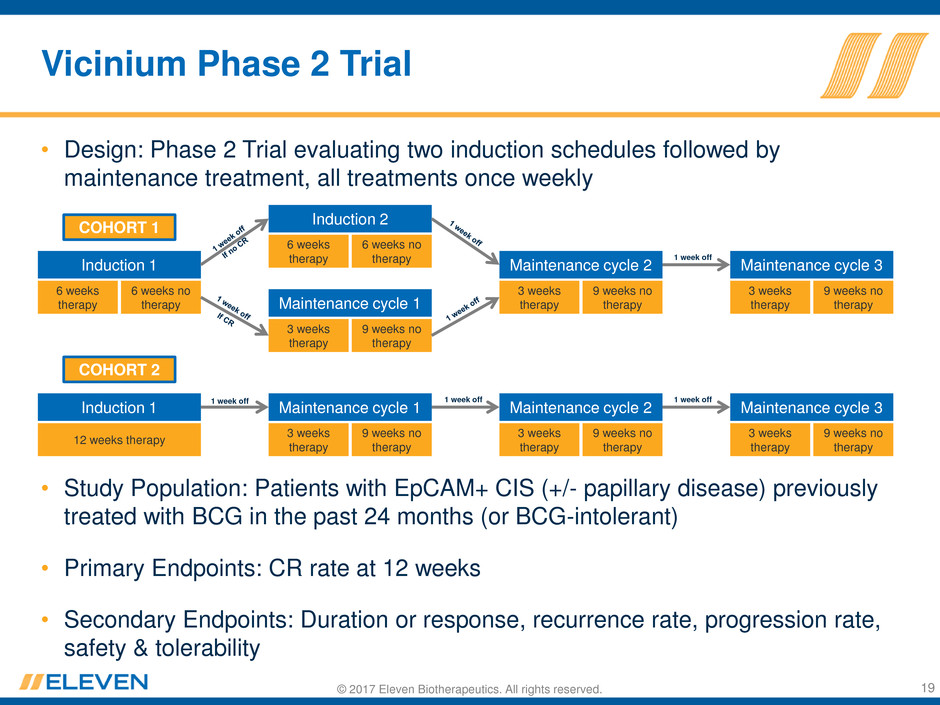
© 2017 Eleven Biotherapeutics. All rights reserved.
Vicinium Phase 2 Trial
• Design: Phase 2 Trial evaluating two induction schedules followed by
maintenance treatment, all treatments once weekly
• Study Population: Patients with EpCAM+ CIS (+/- papillary disease) previously
treated with BCG in the past 24 months (or BCG-intolerant)
• Primary Endpoints: CR rate at 12 weeks
• Secondary Endpoints: Duration or response, recurrence rate, progression rate,
safety & tolerability
Induction 1
6 weeks
therapy
6 weeks no
therapy
Induction 2
6 weeks
therapy
6 weeks no
therapy
Maintenance cycle 1
3 weeks
therapy
9 weeks no
therapy
Maintenance cycle 2
3 weeks
therapy
9 weeks no
therapy
Maintenance cycle 3
3 weeks
therapy
9 weeks no
therapy
Induction 1
12 weeks therapy
Maintenance cycle 1
3 weeks
therapy
9 weeks no
therapy
Maintenance cycle 2
3 weeks
therapy
9 weeks no
therapy
Maintenance cycle 3
3 weeks
therapy
9 weeks no
therapy
COHORT 1
COHORT 2
1 week off
1 week off
1 week off 1 week off
19
© 2017 Eleven Biotherapeutics. All rights reserved.
• No significant toxicity or tolerability issues
‒ Generally well tolerated
‒ No subjects unable to complete treatment
‒ 65% of subjects had an AE related to treatment
• The majority being local bladder
‒ Three subjects reported SAEs. None of the SAEs were
determined to be study drug related
20
Vicinium Phase 2 Trial: Safety Results

© 2017 Eleven Biotherapeutics. All rights reserved.
Vicinium Phase 2 Trial: Efficacy Results
Source: Kowalski et. al., Drug Design, Development and Therapy 4: 313, 2010.
• CR rates
‒ Three months: 40.9% (cohort 1) and 39.1% (cohort 2)
‒ 12 months: 13.6% (cohort 1) and 17.4% (cohort 2)
• All patients who were a CR at one year remained disease-free at least 18 months (18-25
months)
21
Complete Response Rates in Evaluable Population
No. / Total No.
Cohort 1 Cohort 2 Totals
At any point(1) 11/22 (50.0%) 9/23 (39.1%) 20/45 (44.4%)
At 3 mos 9/22 (40.9%) 9/23 (39.1%) 18/45 (40.0%)
At 6 mos 6/22 (27.3%) 6/23 (26.1%) 12/45 (26.7%)
At 9 mos 3/22 (13.6%) 5/23 (21.7%) 8/45 (17.8%)
At 12 mos 3/22 (13.6%) 4/23 (17.4%) 7/45 (15.6%)
1Two subjects in Cohort 1 received additional Vicinium after 3 months and achieved CRs

© 2017 Eleven Biotherapeutics. All rights reserved.
-100
0
100
200
300
Combined Phase 2 data
CIS with or without Ta, T1
Increased
Area Affected
Stable Disease (SD)
Partial Response (PR)
Complete Responders (CR)
• 73% of non CRs had clinical benefit
• Two patients in Phase 2, Arm 1 achieved a CR after receiving a second induction
• Increased induction frequency of treatment in Phase 3 in effort to drive PRs and SDs to CRs
• Increased maintenance frequency in Phase 3 to achieve more durable CRs
Vicinium: Rationale for Increasing
Frequency of Dosing
22
Only subjects (40/46) with baseline and 3 months bladder map data are included

An Open-Label, Multicenter, Phase 3 Study to Evaluate
the Efficacy and Tolerability of Intravesical ViciniumTM
in Subjects with Non Muscle Invasive Carcinoma in
Situ (CIS) and/or High-Grade Papillary Disease of the
Bladder Previously Treated with Bacillus Calmette
Guérin (BCG)
https://clinicaltrials.gov/ct2/show/NCT02449239

© 2017 Eleven Biotherapeutics. All rights reserved. 24
Phase 3 Study Design and Endpoints
Single-arm, open label registrational Phase 3 study
• Sample size: 134 patients with BCG-unresponsive disease
‒ 77 subjects with BGC-unresponsive CIS (Full enrollment expected Q1 2018)
• Primary Endpoint
‒ CR at 12 months and duration of response (DoR) in CIS patients (top line 3 mo data expected
mid 2018)
• Key Secondary Endpoints
‒ Event-free survival (EFS), 18 month EFS
‒ Time to cystectomy
‒ Time to disease recurrence
• Exploratory endpoint for biomarkers
‒ Progression-free survival (PFS)
‒ Overall survival (OS)
Induction Phase
Weeks 1-6
twice weekly
Maintenance Phase
Up to week 104
Every other week
Weeks 7-12
Once weekly

© 2017 Eleven Biotherapeutics. All rights reserved.
Recent Vicinium Advances
• June 1, 2017
“Eleven Biotherapeutics Announced Data and Safety Monitoring Board (DSMB)
Recommendation to Continue Phase 3 Registration Trial with ViciniumTM in Non-
Muscle Invasive Bladder Cancer Based on Review of Safety and Efficacy Data”.
- Phase 3 Trial Enrollment Exceeds 50%
- First time looking at safety and efficacy
• June 6, 2017
“Eleven Biotherapeutics to Collaborate with Astra Zeneca and the National
Cancer Institute on Development of ViciniumTM in Combination with durvalumab
for the Treatment of Non-Muscle Invasive Bladder Cancer”
25

© 2017 Eleven Biotherapeutics. All rights reserved.
Our AstraZeneca & National Cancer Institute Collaboration
26

© 2017 Eleven Biotherapeutics. All rights reserved.
Phase 1 Checkpoint Inhibitor Combination
Vicinium + Durvalumab in NMIBC
• The clinical and preclinical observations that suggest VB4 (Vicinium and Proxinium) may
induce anti-tumor immune responses have lead to a combination clinical study.
• The NCI has entered into CRADAs with EBIO and AstraZeneca to perform a clinical trial
evaluating the combination of Vicinium and durvalumab, AZ’s PD-L1 checkpoint inhibitor, in
patients with NMIBC.
• This study will provide clinical validation of Vicinium’s ability to induce anti-tumor immune
responses that are required for the effective use of checkpoint inhibitors.
• Assessment of critical biomarkers will be performed on patient samples. The results will be
assessed periodically and will be available to EBIO on an ongoing basis.
• We believe this biomarker data could lead to broader combination trials
• Key endpoints of the study include safety, tolerability and preliminary anti-tumor activity of
the combination of the two agents.
Induction
Vicinium weekly for 12
weeks
Maintenance Cycle
Vicinium every other week
Durvalumab q 4 weeks Durvalumab q 4 weeks
Evaluate every three months (Cysto, Cytol, Biopsy)
If CR repeat Maintenance Cycle up to 7 cycles (2 years
including Induction Phase)
27

© 2017 Eleven Biotherapeutics. All rights reserved.
Treatment of Late-Stage SCCHN
28

© 2017 Eleven Biotherapeutics. All rights reserved.
• Single chain anti-EpCAM antibody fragment fused with ETA
• Late-stage SCCHN is dominated by the primary tumor
‒ 40-60% of deaths result from local or regional disease1
‒ 90% of patients with disseminated disease die as a result of uncontrolled disease at the primary site
or in the neck2
• The annual incidence of head and neck cancers worldwide is more than 650,000 cases with
around 350,000 deaths each year3
‒ 2016 estimate of 46,330 new cases of oral cavity, pharyngeal and laryngeal tumors in the U.S. with
~9,570 deaths4
• Surgery highly invasive and associated with significant morbidity
‒ Five year survival rate of only 40-50%, depending on stage of advancement
‒ Up to 70% of patients present with advanced disease
‒ Recurrent disease often not suitable for additional surgery or radiation
• Standalone chemo/biologics have limited benefit
‒ 2nd line chemotherapy median survival is 103 days2; best supportive care median survival is 56 days2
• Opportunity to demonstrate potential complementary MOA with checkpoint inhibitors
1Clayman & Dreiling, Injectable Modalities as Local and Regional Strategies for Head and Neck Cancer
2Leon et. al. Clin Oncol 17: 418, 2005.
3Heroiu et al. Maedica, 2013; 8(1), 80-85
4American Cancer Society (http://www.cancer.org/cancer/oralcavityandoropharyngealcancer/detailedguide/oral-cavity-and-oropharyngeal-cancer-key-statistics
Proxinium: Targeting Late-Stage SCCHN
29
© 2017 Eleven Biotherapeutics. All rights reserved.
• Completed two Phase 1 trials, Russia (daily) and Brazil (weekly)
‒ Proxinium was generally well-tolerated
‒ Anti-tumor activity in 43% Russia trial, 62% Brazil trial
‒ Three out of the four patients with complete responses of their injected tumor had
regression or complete resolution of a non-injected tumor
• Suggests immune driven response
‒ Mean OS for EpCAM “+” subjects was 207 days vs. 125 days for EpCAM “-” subjects
‒ Phase 1 results suggest that Proxinium may be effective in the treatment of EpCAM “+”
SCCHN
• Completed US Phase 2 trial
‒ Proxinium administered weekly at 500 mg or 700 mg via intratumoral injection
‒ Confirmed 700 µg as the recommended dose
‒ Proxinium well-tolerated with pain at the injection site as the most common AE
‒ Reduction in the bidirectional size of the principle targeted tumor observed in 71%
(10/14) of the evaluable subjects
• RECIST criteria not employed
‒ Growth control of the initial treated tumor was achieved in four of five subjects with
multiple tumors, leading to treatment of additional tumors
Proxinium: Targeting Late-Stage SCCHN
30

© 2017 Eleven Biotherapeutics. All rights reserved.
After four weeks of treatment
Targeted tumor cell cytotoxicity may lead to cross-priming and immune
therapy (T cell-mediated killing) of non-targeted tumors
Injected Tumor
Subject A:
Subject B:
Pre-treatment
Injected Tumor
Non-Injected Tumor
Proxinium: Responses in Injected & Non-Injected
Tumors
31

© 2017 Eleven Biotherapeutics. All rights reserved.
Proxinium + BSC vs. BSC. Summarized Phase 2 and Phase 3 data1
RECIST criteria not employed
Progression
Stable Disease
Response
1Only subjects with baseline and post-treatment tumor measurements are included (N = 36 for Proxinium + BSC; N = 26 for BSC only).
32
Proxinium: Phase 2/3 Clinical Trial Data

© 2017 Eleven Biotherapeutics. All rights reserved.
• Observed responses in non-injected tumors during prior clinical trials
and evidence from preclinical in vitro and animal studies suggest that
Proxinium generates a host anti-tumor immune response that may
improve the efficacy of checkpoint inhibitors
• Two checkpoint inhibitors that act by blocking PD-1, Opdivo and
Keytruda, have been approved for treatment of SCCHN
• Next Step: initiate a Phase 1/2a trial evaluating Proxinium in
combination with checkpoint inhibitors in patients with SCCHN
‒ Currently deferring development of Proxinium to focus efforts and resources on
ongoing development of Vicinium
‒ NCI biomarker could unlock partnerships
Proxinium: Clinical Development Plan
33

© 2017 Eleven Biotherapeutics. All rights reserved.
De-Immunized
Systemic
Product Pipeline
Bouganvillea sp.
deBouganin toxin
34

© 2017 Eleven Biotherapeutics. All rights reserved.
• Highly potent plant toxin
‒ Picomolar killing, avoidance of MDR, potentially effective against cancer stem cells
‒ Safety profile provides broad therapeutic window
• Engineered de-immunized variant for systemic delivery
• Type 1 ribosome inactivating protein
‒ RNA N-glycosidase causes deadenylation of the 28S ribosome
‒ Blocks translation inducing apoptotic cell death
• Cell cycle independent
‒ Potentially effective against cancer stem cells
• Pilot Phase 1 trial showed
significant reduction in payload
immunogenicity
• Plan to file IND for
deimmunized TPT, VB6-845d at
appropriate time
• Currently on hold while focused on Phase 3
trial, pursuing collaborations
35
deBouganin: Next Generation Payload

© 2017 Eleven Biotherapeutics. All rights reserved.
Corporate
36

© 2017 Eleven Biotherapeutics. All rights reserved.
Experienced Leadership Team
Stephen Hurly
President, CEO, and Director
David Brooks, MD, PhD
SVP Clinical Development
Rich Fitzgerald
Interim Chief Financial Officer (effective Oct 20, 2017)
Greg Adams, PhD
Chief Scientific Officer
Glen MacDonald, PhD
Chief Technology Officer
Wendy L. Dixon, PhD - Chair
Abbie C. Celniker, PhD
Paul Chaney
Leslie L. Dan, BSc Phm, MBA, CM
Dan Lynch
Jay S. Duker, MD
Barry J. Gertz, MD, PhD
Jane V. Henderson
MANAGEMENT TEAM BOARD OF DIRECTORS
37

© 2017 Eleven Biotherapeutics. All rights reserved.
Vicinium + durvalumab NMIBC Phase 1 first patient treated Q4 2017
Vicinium NMIBC Phase 3 complete enrollment Q1 2018
Vicinium NMIBC Phase 3 topline three-month data Mid 2018
Vicinium + durvalumab NMIBC Phase 1 initial biomarker data Q3 2018
Vicinium NMIBC Phase 3 topline 12-month data Q2 2019
EBIO Corporate / Projected News Flow
38
• $15.8M cash reported at end of last quarter (June 30, 2017)
• 24.7M shares outstanding as of Sept 30, 2017

© 2017 Eleven Biotherapeutics. All rights reserved.
© 2017 Eleven Biotherapeutics. All rights reserved.
Targeting Cancer with
POWER+PRECISION™
