Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Biohaven Pharmaceutical Holding Co Ltd. | a17-24134_18k.htm |
October 2017 1 ©2017 Biohaven DEVELOPING NOVEL THERAPIES TARGETING NEUROLOGICAL DISEASES CHANGING CURRENT TREATMENT PARADIGMS

Disclaimer This presentation contains forward-looking statements, including: statements about our plans to develop and commercialize our product candidates, our planned clinical trials for our rimegepant, BHV-3500, trigriluzole, BHV-0223 and BHV-5000 development programs, the timing of the availability of data from our clinical trials, the timing of our planned regulatory filings, the timing of and our ability to obtain and maintain regulatory approvals for our product candidates and the clinical utility of our product candidates, alone and as compared to other treatment options. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. For further information regarding these risks, uncertainties and other factors you should read the “Risk Factors” section of the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (the ”SEC”) on August 14, 2017 and the Company’s other periodic reports filed with the SEC. This presentation also contains market data and other statistical information that are based on independent industry publications, reports by market research firms or published independent sources. Some market data and statistical information are also based on the Company's good faith estimates, which are derived from management's knowledge of its industry and such independent sources referred to above. While the Company is not aware of any misstatements regarding the market and industry data presented herein, such data involve risks and uncertainties and are subject to change based on various factors. 2 ©2017 Biohaven

Company Overview Driving towards multiple milestones across two platforms Potential for First-in-Class and Best-in-Class Treatments CGRP Receptor Antagonist Platform for Migraine (oral or intranasal therapies) Glutamate Modulator Platform for Orphan and Other Neurologic Indications Nimble, Entrepreneurial Approach to Value Creation 3 ©2017 Biohaven
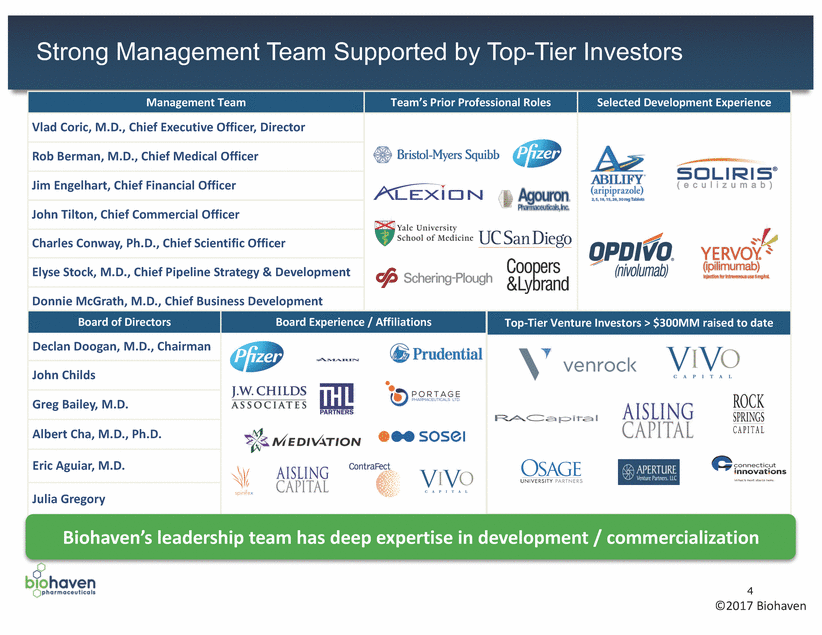
Strong Management Team Supported by Top-Tier Investors Biohaven’s leadership team has deep expertise in development / commercialization 4 ©2017 Biohaven Management Team Team’s Prior Professional Roles Selected Development Experience Vlad Coric, M.D., Chief Executive Officer, Director Rob Berman, M.D., Chief Medical Officer Jim Engelhart, Chief Financial Officer John Tilton, Chief Commercial Officer Charles Conway, Ph.D., Chief Scientific Officer Elyse Stock, M.D., Chief Pipeline Strategy & Development Donnie McGrath, M.D., Chief Business Development Board of Directors Board Experience / Affiliations Top-Tier Venture Investors > $300MM raised to date Declan Doogan, M.D., Chairman John Childs Greg Bailey, M.D. Albert Cha, M.D., Ph.D. Eric Aguiar, M.D. Julia Gregory
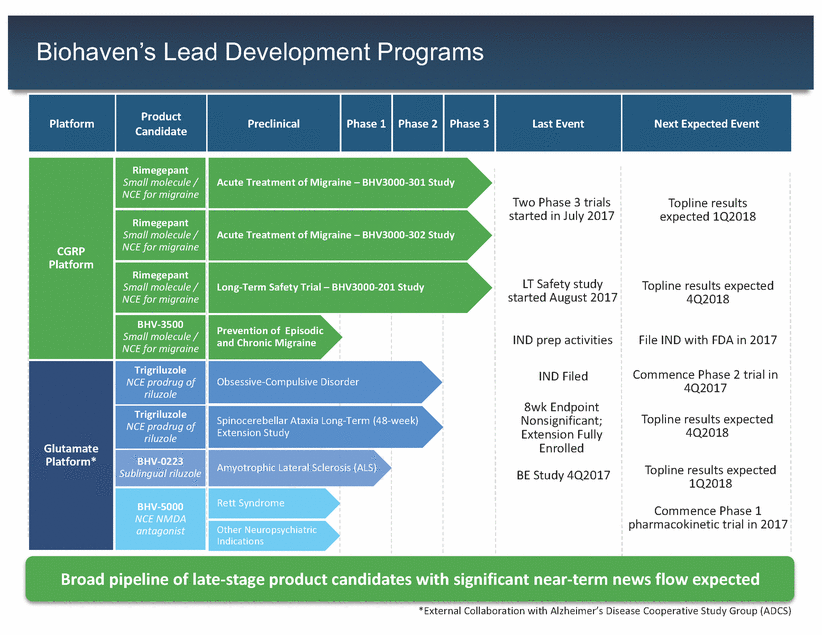
Biohaven’s Lead Development Programs Small molecule / Small molecule / NCE prodrug of Obsessive-Compulsive Disorder 4Q2017 NCE prodrug of Sublingual riluzole 1Q2018 NCE NMDA Other Neuropsychiatric Broad pipeline of late-stage product candidates with significant near-term news flow expected 5 *External Collaboration with Alzheimer’s Disease Cooperative Study Group (ADCS) Platform Product Candidate Preclinical Phase 1 Phase 2 Phase 3 Last Event Next Expected Event Acute Treatment of Migraine – BHV3000-301 Study Two Phase 3 trialsTopline results started in July 2017expected 1Q2018 Acute Treatment of Migraine – BHV3000-302 Study Long-Term Safety Trial – BHV3000-201 Study LT Safety studyTopline results expected started August 20174Q2018 Prevention of Episodic and Chronic Migraine IND prep activitiesFile IND with FDA in 2017 IND FiledCommence Phase 2 trial in 8wk Endpoint Spinocerebellar Ataxia Long-Term (48-week) Nonsignificant;Topline results expected Extension Study Extension Fully4Q2018 Enrolled Amyotrophic Lateral Sclerosis (ALS) BE Study 4Q2017Topline results expected Rett Syndrome Commence Phase 1 pharmacokinetic trial in 2017 Indications CGRP Platform Rimegepant Small molecule / NCE for migraine Rimegepant Small molecule / NCE for migraine Rimegepant NCE for migraine BHV-3500 NCE for migraine Glutamate Platform* Trigriluzole riluzole Trigriluzole riluzole BHV-0223 BHV-5000 antagonist
6 ©2017 Biohaven CGRP Receptor Antagonist Platform Rimegepant BHV-3500
Migraine is a Debilitating Illness – Not Just a ‘Bad Headache’ • World’s third most prevalent illness – Approximately 36 million individuals in the US are affected by migraine – Includes more than 4 million people in the US with chronic migraine • Disease alters ability to perform normal daily activities – Attacks last 4 - 72 hours (or more) with multiple symptoms Pulsating moderate to severe headache pain, typically one-sided Nausea or vomiting Sensitivity to light and sound Visual disturbances or aura – – – – • Migraine attacks associated with severe disability – Seventh highest specific cause of disability worldwide 7 ©2017 Biohaven
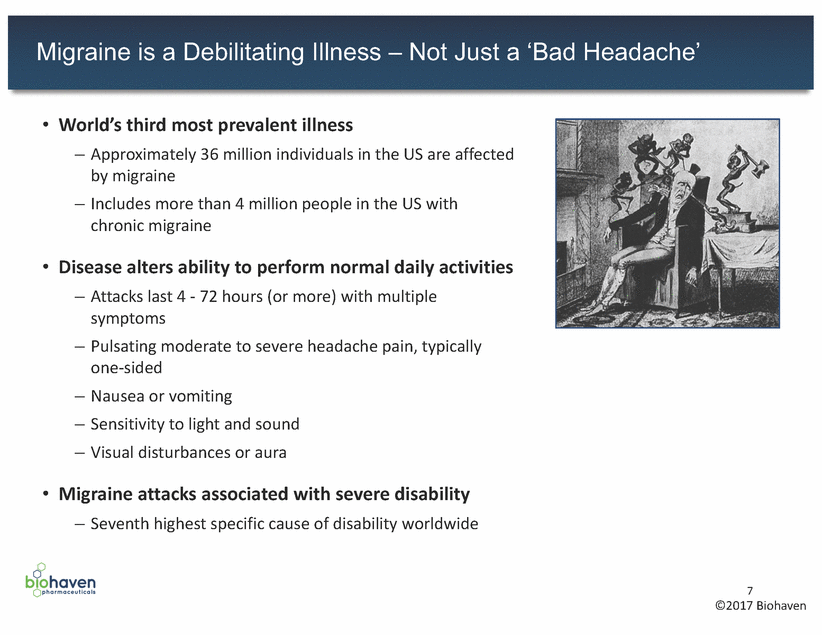
Migraine Treatment Can Be Segmented Into Two Categories ~36M Migraineurs USA(1) Acute Migraineurs Chronic Migraineurs ~32M (90%) ~4M (10%) – Small molecules: Biohaven, Allergan 8 (1) American Migraine Foundation (2) ICHD-3b International Classification of Headache Disorders Acute Treatment(2) • <15 headache days/month – Dx: Migraine w/w-o Aura • Take as-needed to abort attack • CGRP targeting agents in the clinic Chronic Treatment(2) • 15 headache days/month – Dx: Chronic Migraine • Prophylactic – Typically requires additional acute therapy for breakthrough attacks • CGRP agents targeting indication – Antibodies: Alder, Amgen, Lilly, Teva – Small molecules: Biohaven, Allergan
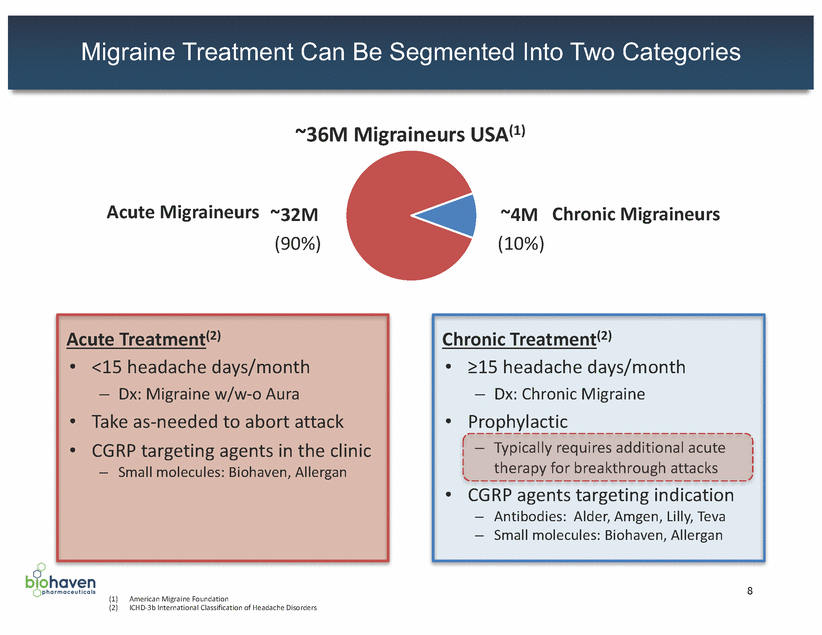
Triptans: Safety & Efficacy Limitations Result in Significant Unmet Need of Action We believe there is a significant unmet need for a novel migraine medication that does not increase the risk of CV liability (1) Estimate according to a recent study published in the journal Headache 9 ©2017 Biohaven Safety Limitations •Display dose-dependent vasoconstriction due to 5-HT1B-mediated effects •Contraindicated in patients with cardiovascular (CV) or cerebrovascular disease (or significant risk factors for either), or uncontrolled hypertension. Serious adverse cardiac events, including acute myocardial infarction, life-threatening disturbances of cardiac rhythm, and death have been reported within a few hours following administration of triptans •Package insert includes warnings and precautions for patients with risk factors for CV disease – Periodic CV evaluation should be considered for long-term triptan users with CV risk factors •Even in patients with a negative CV evaluation, triptan labels recommend consideration of first dose administration in a medically-supervised setting and subsequent performance of an ECG •2.6MM migraine sufferers in the US have a CV event, condition or procedure that limits the potential of triptans as a treatment option (1) Efficacy Limitations •Triptans can be limited by headache recurrence, which are headaches that are relieved and then reoccur within 24 hours after taking migraine medication Usage in Acute Migraine •Current standard of care; >13.9 million annual prescriptions in the US •Represent ~80% of anti-migraine therapies prescribed at office visits by healthcare providers Triptan Mechanism •Serotonin 5-HT1B/1D receptor agonists
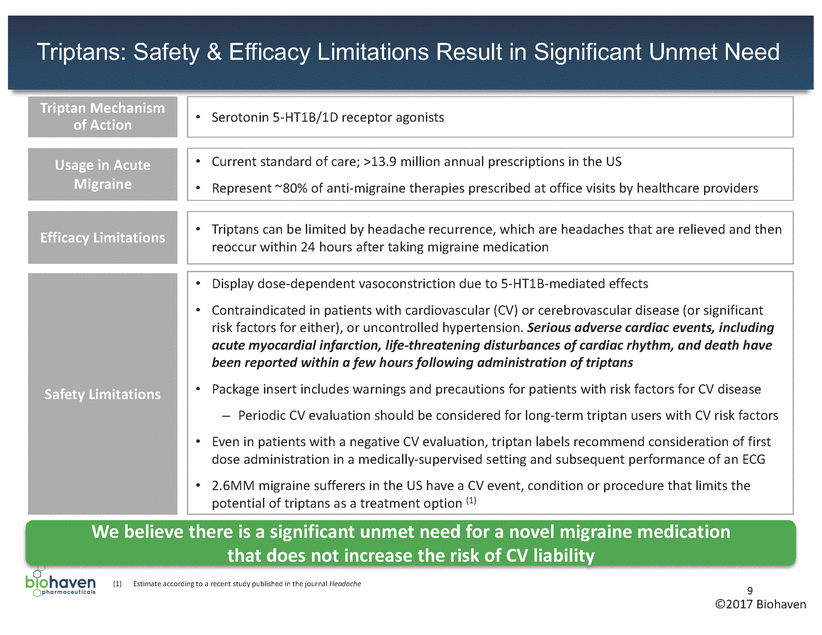
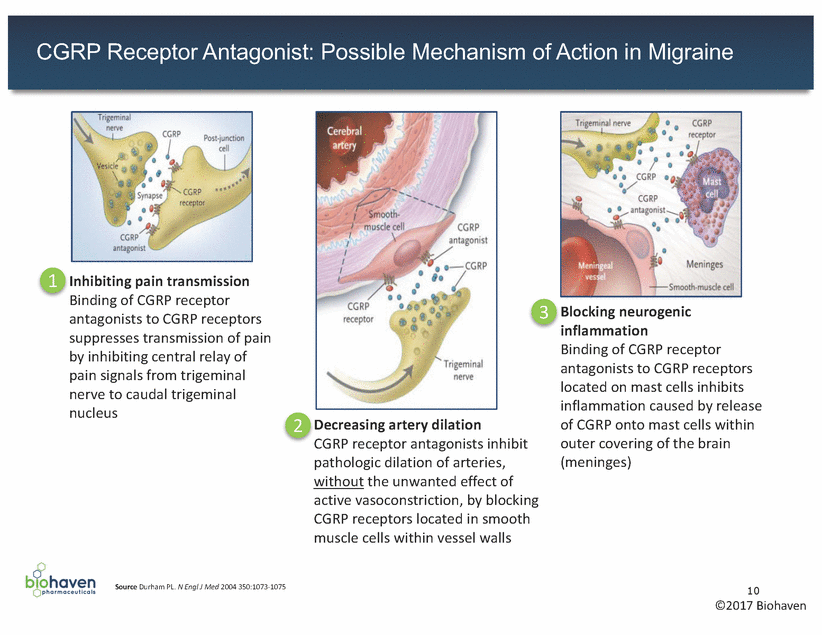
CGRP Receptor Antagonist: Possible Mechanism of Action in Migraine 1 Inhibiting pain transmission Binding of CGRP receptor antagonists to CGRP receptors suppresses transmission of pain by inhibiting central relay of pain signals from trigeminal nerve to caudal trigeminal nucleus 3 Blocking neurogenic inflammation Binding of CGRP receptor antagonists to CGRP receptors located on mast cells inhibits inflammation caused by release of CGRP onto mast cells within outer covering of the brain (meninges) 2 Decreasing artery dilation CGRP receptor antagonists inhibit pathologic dilation of arteries, without the unwanted effect of active vasoconstriction, by blocking CGRP receptors located in smooth muscle cells within vessel walls Source Durham PL. N Engl J Med 2004 350:1073-1075 10 ©2017 Biohaven
Rimegepant: Overview 11 ©2017 Biohaven (1) Marcus R, et al. (2014). Cephalalgia 34(2): 114-125 Clinical Status •Phase 2b data demonstrated that rimegepant may have the ability to address important unmet needs within migraine (1) – Statistically significant improvement relative to placebo observed in all four traditional migraine symptoms – pain, nausea, photophobia and phonophobia – Evidence of durable treatment effect, with statistically significant effects on 2-24 hour and 2-48 hour pain freedom and 2-24 hour pain relief – No chest-pain related symptoms observed in rimegepant patients, unlike observations in patients dosed with sumatriptan •Commenced two Phase 3 trials for the acute treatment of migraine in July 2017 •Commenced Long-term safety study in August 2017 Background •Licensed from Bristol-Myers Squibb (BMS) in July 2016 – BMS selected rimegepant as a lead CGRP receptor antagonist compound for its potential best-in-class chemical profile after 10 years of research on this drug target – To date, six clinical trials have been completed in healthy volunteers and patients with migraine that inform pharmacokinetic, metabolic interactions, safety, tolerability and efficacy of rimegepant – Prior to initiation of the Phase 3 trials, approximately 687 subjects had been dosed with rimegepant, which has been observed to be generally safe and well tolerated – US composition of matter patent protection to October 2030, not including patent term adjustment or any potential patent term extensions Description •Orally available, selective and potent small molecule CGRP receptor antagonist
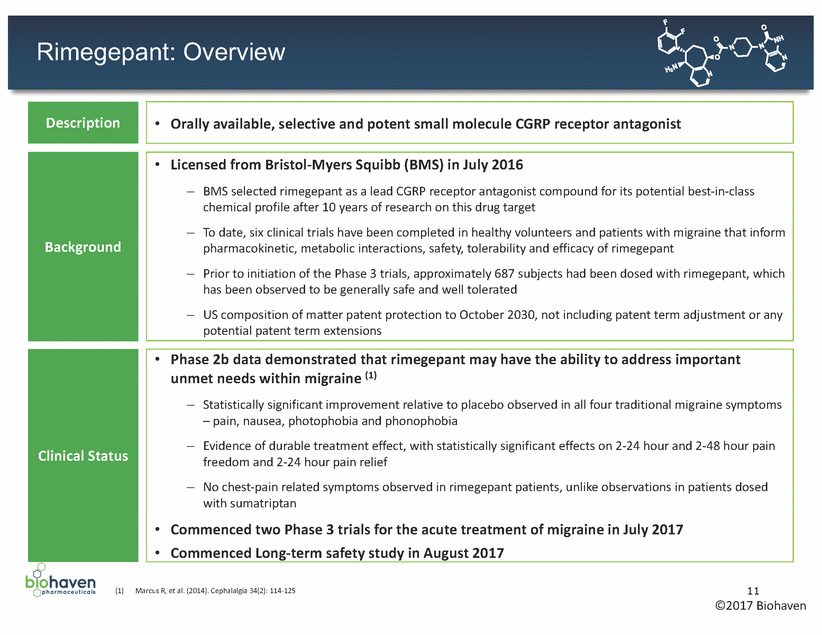
Rimegepant: Comprehensive and Durable Treatment Effect Observed in Phase 2b ** 40 20 Placebo Sumatriptan, 10 25 75 150 300 600 Patients (n) 203 100 71 61 86 85 111 82 ** ** ** ** ** ** Placebo Sumatriptan, 10 25 75 150 300 600 Patients (n) 203 100 71 61 86 85 111 82 Nausea Freedom Phonophobia Freedom Photophobia Freedom 12 ©2017 Biohaven (1) Marcus R, et al. (2014). Cephalalgia 34(2): 114-125 Patients (%) Patients (%) Patients (%) Nausea, Phonophobia and Photophobia Freedom Two Hours Post-Dosing 100 75 ** ** 50 * ** ** 25 0 100mg Rimegepant (mg) * p < 0.05 ** p < 0.01 ** ** ** Sustained Pain Freedom and Pain Relief 2-24 Hours Post-Dosing 100 75 * ** 50 25 0 Placebo Sumatriptan, 10 25 75 150 300 600 100mg Rimegepant (mg) Patients (n) 203 100 71 61 86 85 111 82 * p < 0.05 ** p < 0.01 Pain Freedom Pain Relief ** ** ** * ** ** * ** ** Pain Freedom Two Hours Post-Dosing 50 30 10 0 100mg Rimegepant (mg) ** p < 0.01 ** ** ** Overview of Phase 2b Trial (1) •Double-blind, randomized, placebo-controlled, dose-ranging clinical trial completed by BMS •812 patients suffering from migraine attacks received either placebo, sumatriptan 100mg or rimegepant dosed at 10, 25, 75, 150, 300 or 600mg •Rimegepant dosed at 75mg was observed to have comprehensive and durable treatment effect
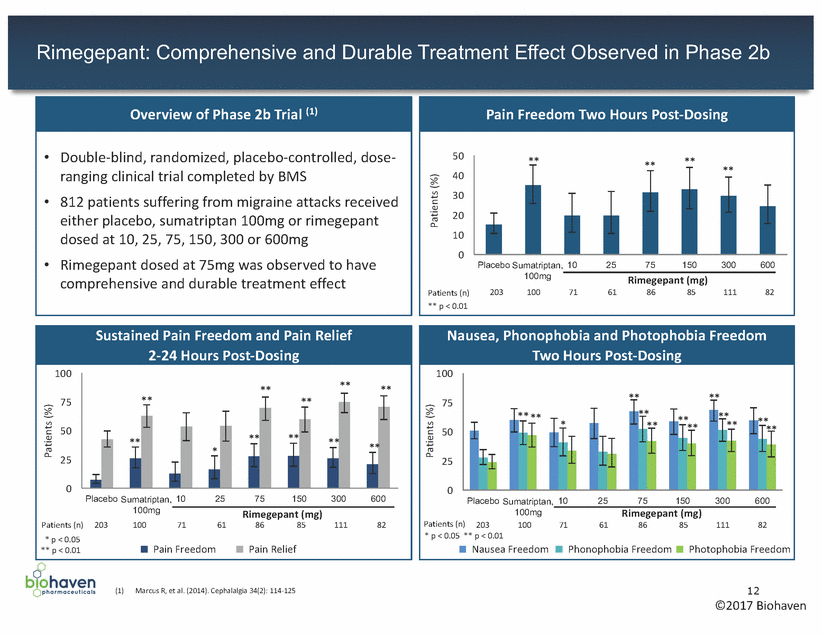
Rimegepant: Well Tolerated in Phase 1 and Phase 2b Testing of very high doses in Phase 1 and 2b provides evidence of acceptable tolerability for 75mg dose (1) (2) (3) (4) Marcus R, et al. (2014). Cephalalgia 34(2): 114-125 Adverse events occurring in ³2% of patients in any treatment group; ordered by frequency in the rimegepant 600mg group. n: number (%) of patients who took at least one tablet of study drug NOEL = no observable effect level 13 NOAEL = no observable adverse effect level ©2017 Biohaven Phase 2b Safety Data Number (%) of Patients Reporting a Commonly Occurring Adverse Event within 48 Hours Post-Dose (1)(2) Placebo Sumatriptan Rimegepant Patients, n (%) n=209 100mg n=100 10mg n=72 25mg n=62 75mg n=86 150mg n=86 300mg n=112 600mg n=84 Nausea 5 (2) 2 (2) 1 (1) - 3 (3) 3 (3) 5 (4) 7 (8) Dizziness 2 (1) 1 (1) 2 (3) 1 (2) 1 (1) 2 (2) - 3 (4) Vomiting 5 (2) 1 (1) - 2 (3) 2 (2) - - 2 (2) Diarrhea - - 4 (6) - - - 1 (1) - Paresthesia 2 (1) 2 (2) - - - - - - Dysgeusia - - 2 (3) - - - - - Chest Discomfort - 2 (2) - - - - - - Myalgia - - 2 (3) - - - - - Summary of Clinical Safety Data •Phase 1: Well tolerated at high doses – Ultra-high exposures up to 1,500mg well tolerated – high doses achieved daily exposures >50-fold therapeutic dose – 600mg administered for up to 14 days •Phase 2b: Well tolerated at all doses – Most AEs of mild or moderate severity – 75mg dose with comparable AEs to placebo – AEs of chest discomfort only reported in sumatriptan group – No clinically important findings on ECG, physical exam, lab assessments or vital signs •Preclinical: Large safety multiples at 75mg (AUC) – 23x below rat NOEL (3) – 56x below monkey NOAEL (4)
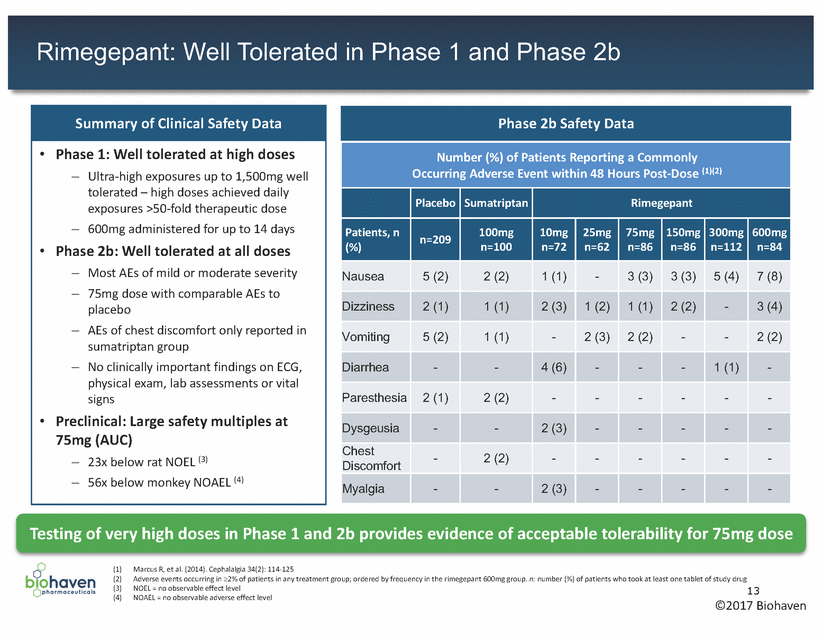
Rimegepant: Clinical Development Plan • FDA approval for acute treatment of migraine historically required demonstrating an effect on all four co-primary endpoints: – Pain, nausea, photophobia and phonophobia Recently, FDA has considered approval based on an effect on two co-primary endpoints: (1) Headache pain freedom, and (2) freedom from patient’s most bothersome symptom (MBS) - nausea, photophobia or phonophobia Two concurrent pivotal Phase 3 clinical trials commenced in 2017 for the acute treatment of migraine: • • – Co-primary endpoints: (1) Pain freedom - headache pain freedom at two hours post-dosing (1) (2) Freedom from MBS at two hours post-dosing Secondary endpoints:Assessment of nausea, photophobia and phonophobia – • In designing the Phase 3 trials, care was taken to minimize any changes in study populations compared to the already completed Phase 2 trial with rimegepant, with no major changes in inclusion and exclusion criteria. 2 Pivotal Trials: 1100 patients per trial, rimegepant 75mg versus placebo (2) - 18 - 65 years old Start: 2017 - Attacks last ~4-72 hours 4Q 2018 - 8 attacks per month, of (1) (2) (3) Headache pain intensity reported as “no pain” using four-point numeric rating scale: no pain, mild pain, moderate pain, severe pain Trials will be double-blinded, randomized, and placebo-controlled A subset of ~600 patients will have frequent migraine attacks (i.e., more than eight migraine attacks per month) and will be able to take up to 30 doses of 75mg rimegepant in one month. Study visits for all patients will be monthly for the first three months and every three months thereafter 14 ©2017 Biohaven Overview of Ongoing Clinical Trials Pivotal Enrollment Criteria:Start: 2017 -Male & femaleTopline Results: 1Q 2018 -1+ year history of migraine -Age of onset: before 50 years -At least 2 attacks per monthLong-term Safety Study: 12-month, open label study in 2000 patients (3)Topline Results: moderate to severe intensity
Rimegepant: Value Proposition • Oral availability – Believed to be one of only two small molecule CGRP antagonists currently in late-stage clinical development Comprehensive treatment effect across most common migraine symptoms – 75mg dose of rimegepant showed statistically significant treatment effect compared to placebo on pain, photophobia, phonophobia and nausea – Other small molecule CGRP receptor antagonist, ubrogepant, failed to show statistically significant treatment effect on nausea and pain relief in Phase 2 trial Durable treatment effect over time – 75mg dose of rimegepant superior to placebo at 2-to-24 hour and 2-to-48 hour sustained pain freedom after dosing – 3.9-fold less likelihood for pain recurrence among responders compared to sumatriptan (data from the Phase 2 study) Well tolerated with favorable adverse event profile • • • – – – – Low rate of AEs Tolerability in 75mg treatment group of Phase 2b trial comparable to placebo Does not cross the blood brain barrier thus conferring low potential for centrally mediated adverse effects Preclinical and clinical evidence suggests that CGRP receptor antagonists have an absence of vasoconstrictor activity and lack other undesirable cardiovascular side effects that are commonly associated with triptans • Potency – Ultra-high potency with subnanomolar affinity for the human CGRP receptor – Allows for a low dose to provide maximal treatment effect Small molecule provides low cost of goods • 15 ©2017 Biohaven
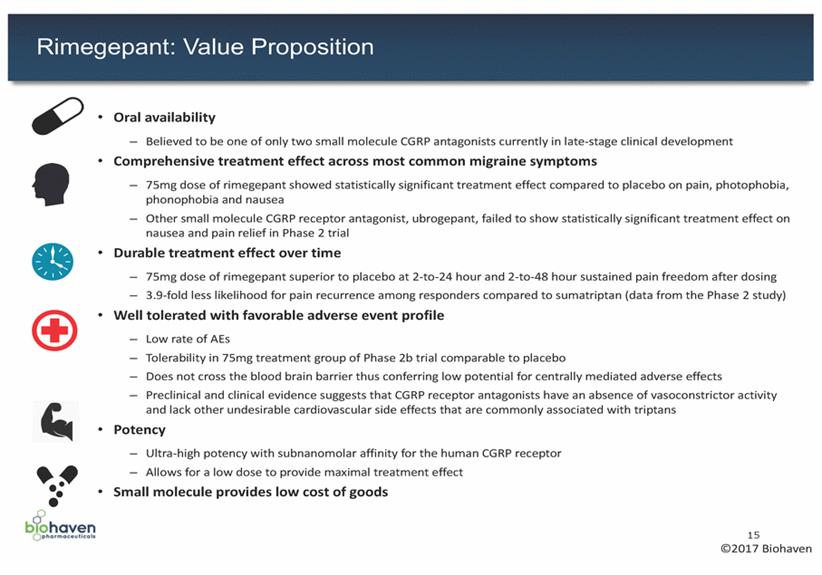
Rimegepant: Potential To Be The Favored Choice for Acute Treatment of Migraine bothersome symptom O Durable treatment effect: hour not different from placebo migraines sustained pain relief O Cumbersome route of triptans directed against circulating non-responders 16 ©2017 Biohaven Compound Rimegepant Ubrogepant Lasmiditan CGRP Antibodies Mechanism of Action • CGRP receptor antagonist • CGRP receptor antagonist • 5-HT1F receptor agonist • CGRP antibodies Stage of Development • Phase 3 • Phase 3 • Phase 3 • Phase 3 and earlier Effectiveness in Acute Treatment of Migraine ü Comprehensive treatment effect: pain, nausea, photophobia and phonophobia at 2 hours post-dose in a Phase 2b trial ü Durable treatment effect: 2 - 24 and 2 - 48 hour sustained pain freedom ü Improvement in pain, photophobia and phonophobia O Comprehensive treatment effect: did not show improvement on nausea or pain relief at 2 hours in Phase 2 O Durable treatment effect: did not show 2 - 48 hour sustained pain freedom at 1 - 50 mg in Phase 2 (present at 100 mg) ü Improvement shown in pain and most O Durable treatment effect: Phase 2 – Headache recurrence at 24 Phase 2/3 – No data showing 2 - 24 hour or 2 - 48 hour sustained pain freedom or O Preventive use only vast majority of patients show residual acute Safety / Tolerability ü Well tolerated ü Well tolerated O Reports of LFT elevations between 3x to 10x ULN in late stage clinical trials O Phase 2 – Higher rates of severe AEs and treatment-emergent AEs compared to placebo (dizziness, fatigue, vertigo, paresthesias, somnolence and sensation of heaviness) O Phase 3 – Higher rates of treatment-emergent AEs compared to placebo (dizziness, paresthesia, somnolence, nausea, fatigue, lethargy and vertigo) ü Well tolerated administration (IV, SC) Benefits / Considerations of Mechanism ü Novel alternative for patients who are triptan intolerant or unresponsive ü No reason to expect headache recurrence phenomena ü Novel alternative for patients who are triptan intolerant or unresponsive ü No reason to expect headache recurrence phenomena ü Mechanism represents an advance on • Uncertainty regarding triptans – e.g., rebound • Uncertain appeal in triptan ü Monoclonal antibody CGRP or CGRP receptors
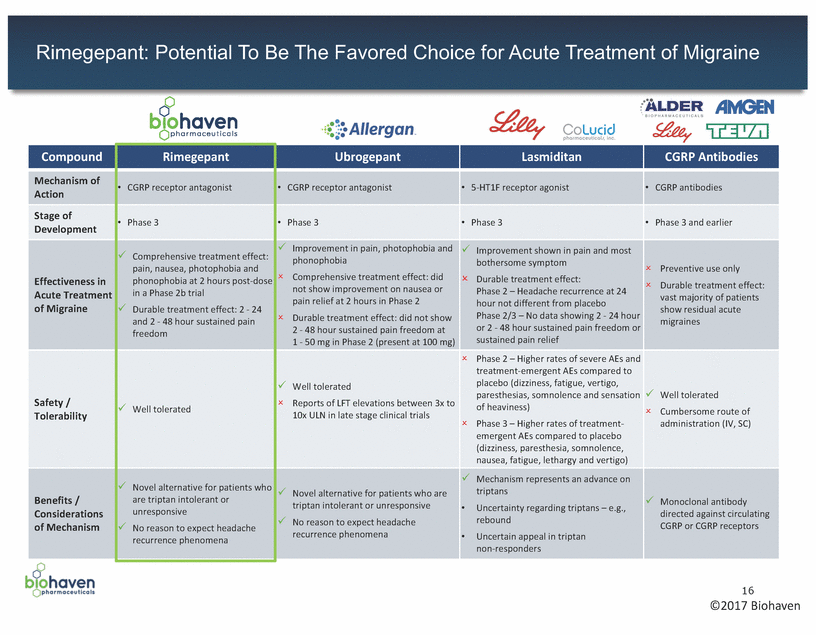
BHV-3500: Third Generation CGRP Receptor Antagonist 17 ©2017 Biohaven Clinical Status •Toxicology program underway to support submission of an IND in 2017 Potential Benefits vs. Standard of Care •Superior chemical attributes – Highly soluble, potent antagonist at the human CGRP receptor •Multiple potential routes of delivery – Potential for nasal, subcutaneous, inhaled or oral routes of administration – May provide rapid onset of treatment effect vs. anti-CGRP monoclonal antibodies which are given in IV or sub-Q form •Enhanced safety profile expected – Expect favorable safety and tolerability profile, similar to rimegepant – Has not demonstrated any propensity for liver abnormalities, even at very high dose levels •Preventative treatments involve chronic daily dosing; any effects on the liver could be problematic •Expected liver safety profile may provide a substantial benefit over other agents with such propensities •Higher value to patients and payors – As a small molecule, expect that BHV-3500 will have a lower cost of goods than monoclonal antibodies •Potential for multiple indications – Expected safety and efficacy profile make BHV-3500 especially attractive for daily administration and development for prevention of episodic and chronic migraine – Also has the potential to be developed in the acute treatment of migraine Description •BHV-3500 is the second compound from the CGRP receptor antagonist platform – In development for prevention of episodic and chronic migraine – Licensed from BMS in July 2016 with US composition of matter patent protection to March 2031, not including patent term adjustment or any potential patent term extensions
18 ©2017 Biohaven Glutamate Modulator Platform Trigriluzole BHV-0223 BHV-5000
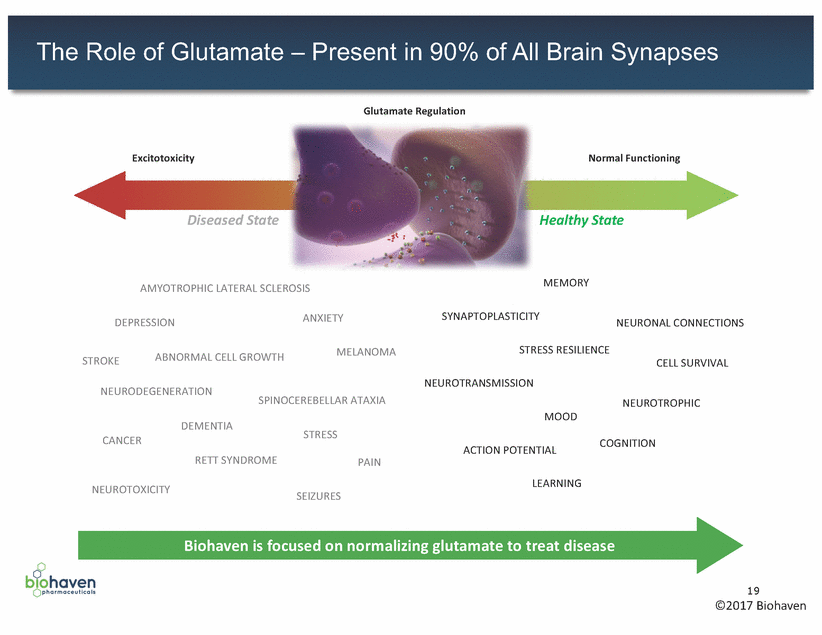
The Role of Glutamate – Present in 90% of All Brain Synapses Glutamate Regulation Excitotoxicity Normal Functioning Level of Glutamate Diseased State Healthy State MEMORY AMYOTROPHIC LATERAL SCLEROSIS SYNAPTOPLASTICITY ANXIETY DEPRESSION NEURONAL CONNECTIONS STRESS RESILIENCE MELANOMA ABNORMAL CELL GROWTH STROKE CELL SURVIVAL NEUROTRANSMISSION NEURODEGENERATION SPINOCEREBELLAR ATAXIA NEUROTROPHIC MOOD DEMENTIA STRESS CANCER COGNITION ACTION POTENTIAL RETT SYNDROME PAIN LEARNING NEUROTOXICITY SEIZURES Biohaven is focused on normalizing glutamate to treat disease 19 ©2017 Biohaven
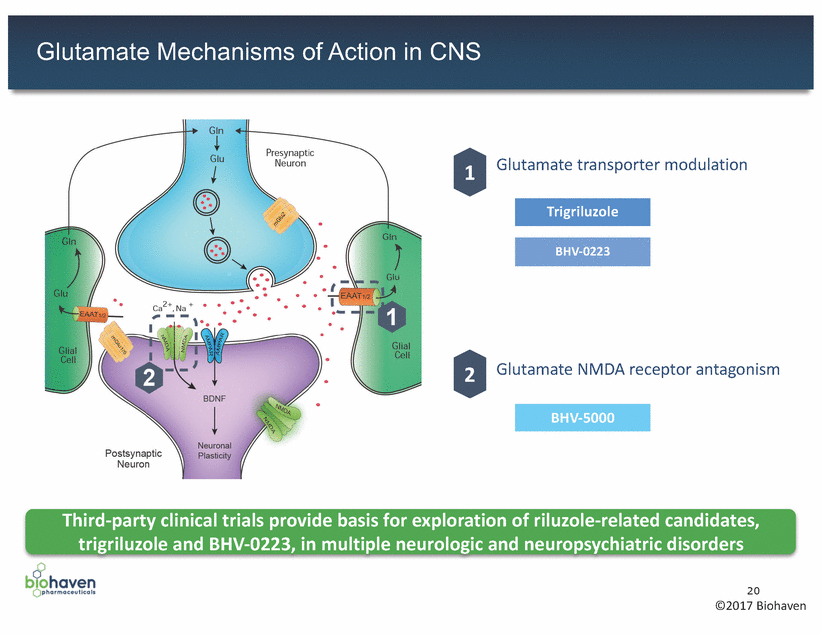
Glutamate Mechanisms of Action in CNS Glutamate transporter modulation 1 Glutamate NMDA receptor antagonism 2 Third-party clinical trials provide basis for exploration of riluzole-related candidates, trigriluzole and BHV-0223, in multiple neurologic and neuropsychiatric disorders 20 ©2017 Biohaven BHV-5000 BHV-0223 Trigriluzole
Riluzole: Use and Limitations Riluzole is the approved for the treatment of patients with amyotrophic lateral sclerosis (ALS) and proven to extend survival at approved dose n Originally marketed by Sanofi, Rilutek® (riluzole) received FDA approval in 1995 In 2013, the FDA approved the first generic versions of riluzole n û¨ û¨ û¨ Fasting required for 6 hours/day, n Doses above 100mg for efficacy not approved due to dose-dependent liver effects Marked PK variability Only one approved indication (ALS) (1) LFT = liver function test ©2017 Biohaven (2) Poor oral bioavailability results in a high liver burden relative to efficacy as ~40% is either not absorbed or is metabolized in the liver LIMITATIONS û¨Twice daily dosing, low bioavailability can’t be taken with meals û¨Dose dependent LFT liability(1) û¨High drug burden relative to efficacy(2) 21 BENEFITS ü¨Mechanism of action well understood ü ALS ¨Neuroprotective, survival benefit in ü¨Well tolerated, safe in clinical settings
( !u nique Properties Third Generation Prodrug Selected from > 300 Synthesized • Prodrug Candidates -•Molecular Weight 419.4 g/mol "'--• Molecular Formula C15H16F3N504S•HCL --G 0 bohaven 22 ©2017 Biohaven Qpharmoceutlcols
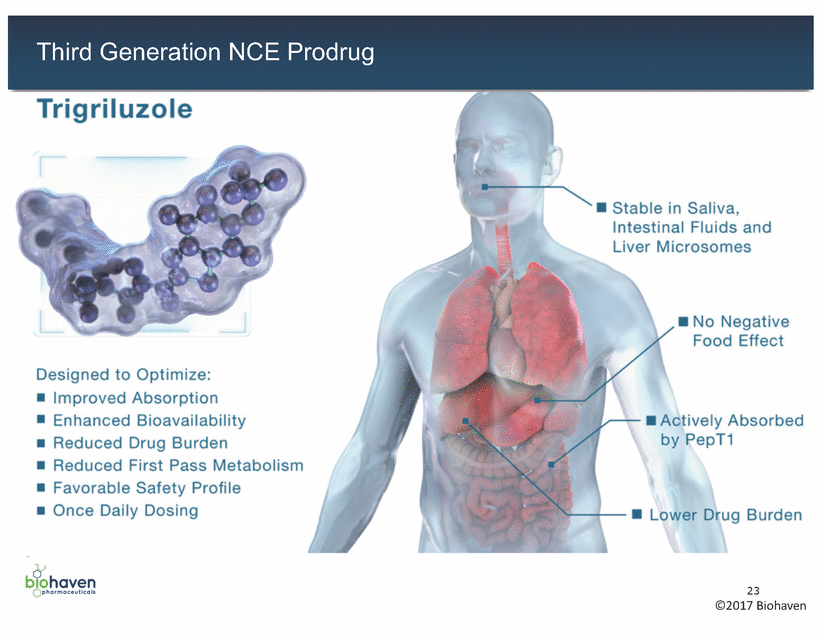
Trigriluzole • Stable in Saliva, Intestinal Fluids and Liver Microsomes No Negative Food Effect Designed to Optimize: • • • • • • Improved Absorption Enhanced Bioavailability Reduced Drug Burden Reduced First Pass Metabolism Favorable Safety Profile Once Daily Dosing Actively Absorbed by PepT1 ":------+--• Lower Drug Burden b1ohaven 23 ©2017 Biohaven Qpharmoceutlcols
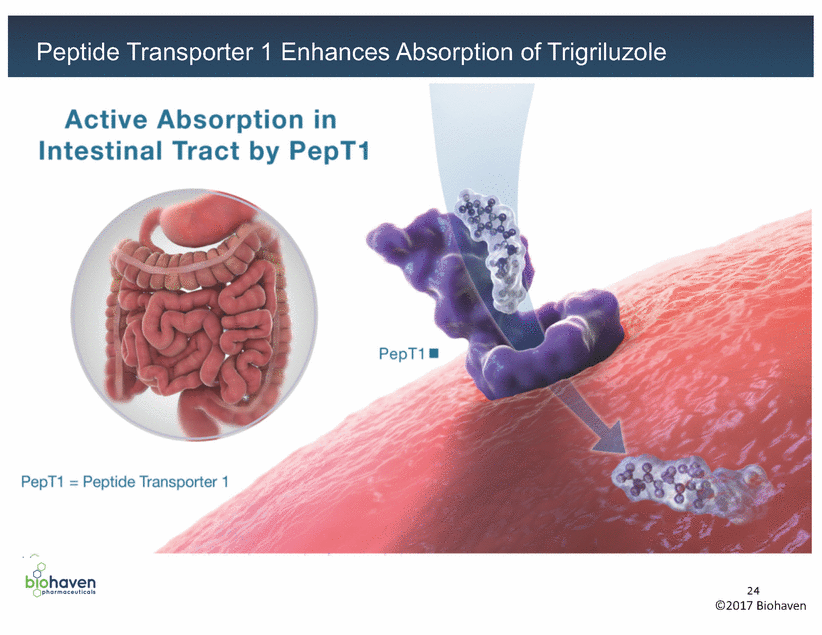
Trigriluzole Active Absorption in Intestinal Tract by PepT1 PepT1 • PepT1 = Peptide Transporter 1 . . 00 b1ohaven 24 ©2017 Biohaven Qpharmoceutlcols Peptide Transporter 1 Enhances Absorption of
Trigriluzole: Advantages vs. Riluzole • Improved bioavailability – Trigriluzole is a substrate for the gut transporters (PepT1), improving bioavailability of the drug • No negative food effect – Trigriluzole will not be associated with special meal restrictions – In contrast to oral riluzole tablets, which require a period of fasting around dosing or levels may not be therapeutic • Lower overall drug burden to the liver – Due to mitigated first-pass liver metabolism and enhanced bioavailability, therapeutic concentrations of the active metabolite riluzole can be achieved with a lower drug dose as compared to riluzole tablets Release of the active metabolite over time will result in a reduced bolus hepatic concentration vs. riluzole tablets These attributes are expected to reduce the potential for adverse liver effects – – • Optimized dosing regimen and compliance – Trigriluzole has been developed as a convenient once-daily dose – Important for compliance and long-term outcomes in chronic disease • Potential for developing multiple formulations – High solubility and negligible affinity for receptors/channels responsible for oral numbness – Potential to be developed in multiple formulations including intranasal, subcutaneous, intravenous, sublingual and other forms Trigriluzole offers potential for significant advantages over riluzole 25 ©2017 Biohaven
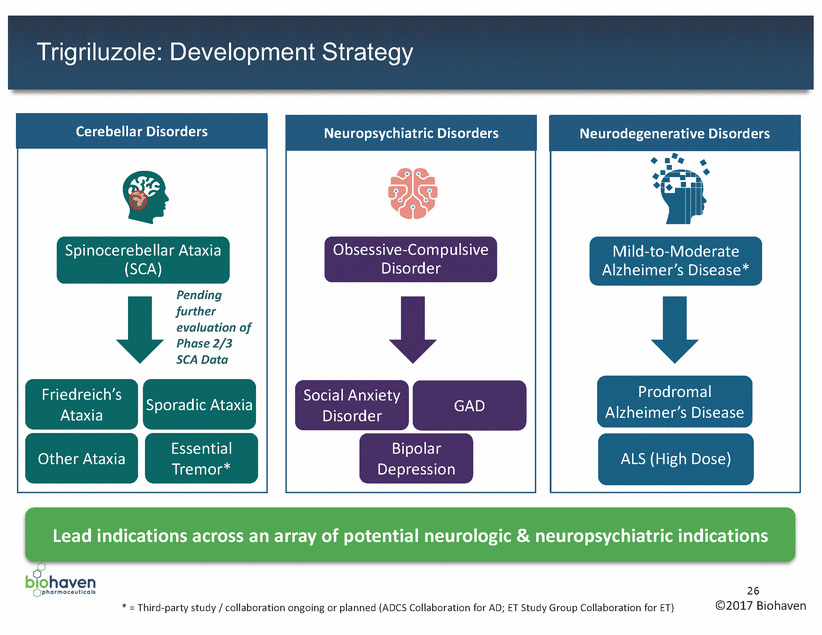
Trigriluzole: Development Strategy Alzheimer’s Disease* Alzheimer’s Disease Lead indications across an array of potential neurologic & neuropsychiatric indications 26 ©2017 Biohaven * = Third-party study / collaboration ongoing or planned (ADCS Collaboration for AD; ET Study Group Collaboration for ET) Neurodegenerative Disorders Mild-to-Moderate Prodromal ALS (High Dose) Neuropsychiatric Disorders Obsessive-Compulsive Disorder Social Anxiety Disorder GAD Bipolar Depression Cerebellar Disorders Spinocerebellar Ataxia (SCA) Pending further evaluation of Phase 2/3 SCA Data Friedreich’s Ataxia Sporadic Ataxia Essential Other Ataxia Tremor*
Trigriluzole in SCA: Signal-to-Noise Challenges at 8 Weeks • • Two double-blind(1-2), placebo-controlled academic trials with riluzole show therapeutic effects (p<0.05) Biohaven Phase 2/3 SCA trial with trigriluzole failed to separate from pbo at 8 week time point; extension phase ongoing over a 12-month period diagnosed with either SCA or Friedreich’s ataxia (enrolled 2:1) placebo or 100mg riluzole (50mg riluzole tablets, BID) improvement on the SARA(3) after 12 months cerebellar ataxia (both SCA and Friedreich’s ataxia) Change in SARA Scores Improved SARA Score 1 pt 40% -1.00 48-Week Extension Phase Ongoing to Assess Efficacy (Topline 4Q2018) (1) Ristori et al., Neurology 2010; 74: 839-845 (2) Romano et al., Lancet Neurol 2015; 14: 985–91 (3) Scale for the Assessment and Rating of Ataxia 27 ©2017 Biohaven % of Patients Change in SARA Score % of Patients Change in SARA Score Romano et al. 2015 (2) • Study on the use of riluzole in patients with hereditary cerebellar ataxias • Multi-center, double-blind, placebo-controlled trial with 60 subjects • Subjects randomized to receive 12 months of treatment with either • Primary endpoint was the proportion of patients with a minimum 1-point • Demonstrated statistically significant efficacy in patients with hereditary % of Patients with 60% 2.00 1.00 0.00 20% 0% -2.00 3 Months 12 months 3 Months 12 months Riluzole (n=28) Placebo (n=27) ** p < 0.01 ** ** Biohaven Multicenter Phase 2/3 (Oct 2017) • This was the first multi-center randomized clinical trial in North America for SCA. High variability observed in primary rating scale (SARA) and unexpected high placebo response rates at 8 weeks. • At 8 wks, trigriluzole with improvement of -0.81 points [95% CI: -1.4 to -0.2] on the SARA vs. -1.05 points [95% CI: -1.6 to -0.4] in placebo, p-value = 0.52. • Trigriluzole demonstrated a favorable safety and tolerability profile, with no drug-related SAEs and low discontinuation rates due to adverse events. • Long-term, open-label, extension phase is ongoing. This extension phase will allow for potential signal detection at later time points. Topline data from the extension phase is expected in 4Q2018. % of Patients with Improved SARA Score 1 pt Change in SARA Scores 60% 2.00 1.00 40% 0.00 20% -1.00 0% -2.00 8 weeks 8 weeks Trigriluzole (n=63) Placebo (n=68) p = 0.52
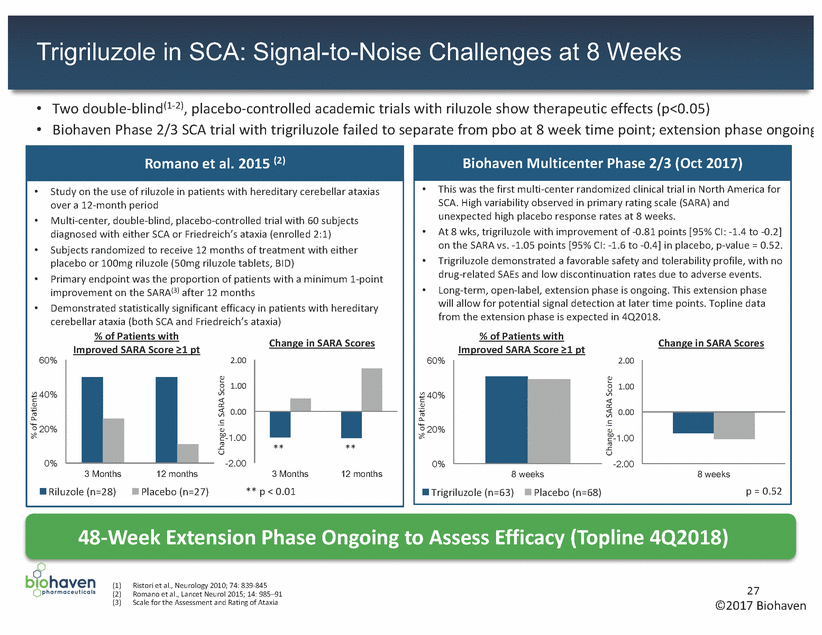
Scale Learnings Inform SCA Analyses and Trial Considerations • Based upon previous literature, a 1pt change in total SARA was believed to be clinically relevant – In this trial, 2 pt change was unexpectedly observed between screening and baseline in over 1/3 patients – Limits ability to detect 1pt efficacy signal at 8 weeks Observations from 8-week randomization phase • – High scale variability between screening and baseline without drug on-board, limits signal detection at 8 weeks Placebo response for progressive neurodegenerative disease unexpectedly high Site rater experience appears critical to reducing variance and placebo response Cannot rule out short term efficacy signal Trigriluzole well tolerated with favorable safety profile 36% of patients with 2pt change in SARA between screening and baseline - 2pt + 2pt – – – – • Full dataset analysis and ongoing extension to be presented at medical conferences – Favorable trends identified in subpopulations defined to reduce variability Additional analyses of data set, interactions with regulatory agency planned 28 ©2017 Biohaven
2nd BHV-0223: Generation Candidate for ALS and Other Disorders ALS 29 ©2017 Biohaven Clinical Status •Phase 1 single dose PK studies show BVH-0223 associated with less PK variability than riluzole •Plan to launch a bioequivalence study in 2017 comparing BHV-0223 to riluzole ALS Therapies •Riluzole was the first FDA approved therapy for ALS – Since approval in 1995, there have been minimal further advances in ALS drug therapeutics •Riluzole extends survival and/or time to tracheostomy – However, riluzole tablets have pharmacokinetic and pharmaceutic limitations that have restricted their broader clinical application Overview of •Progressive neurodegenerative motor neuron disease that affects nerve cells in the brain / spinal cord – Belongs to a group of disorders known as motor neuron diseases, which are characterized by the gradual degeneration and death of motor neurons – Typically presents in patients with painless muscle weakness, trouble swallowing and muscle atrophy that ultimately progresses to paralysis, impaired breathing and death •Affects up to 20,000 individuals in the US Description •Sublingually absorbed and oral disintegrating tablet (ODT) of riluzole – Makes use of proprietary Zydis ODT technology licensed from Catalent •Designed to advance beyond the limitations of riluzole tablets for application in ALS
BHV-0223: Advantages vs. Riluzole • Ease of administration – An early symptom of ALS is difficulty swallowing; makes use of riluzole tablets challenging – BHV-0223 uses fast-dissolving technology that does not require swallowing or administration of liquids More predictable pharmacokinetic performance • – – – Some patients with ALS crush their solid riluzole tablets and take with food to ease administration (1) With BHV-0223, patients will not have to crush or alter the form of administration In Phase 1 trial, observed less pharmacokinetic variability with BHV-0223 compared to 50mg riluzole • No food effect – Prescribing instructions for riluzole tablets state that it should be taken at least an hour before, or two hours after, a meal to avoid food-related decreases in bioavailability Patients who do not strictly adhere to these fasting requirements or administer crushed riluzole in food, may not be obtaining desired therapeutic levels of riluzole BHV-0223 was designed to be absorbed sublingually; since absorption of BHV-0223 occurs in the vasculature under the tongue, fasting requirements are not anticipated This will be particularly beneficial to patients who require a continuous feeding tube for nutrition – – – • Reduced drug load and liver exposure – Riluzole associated with dose-dependent liver issues resulting from high dose loads / extensive liver metabolism – BHV-0223 is sublingually absorbed, bypassing first-pass liver metabolism and reducing the dosage size that needs to be administered, thereby reducing potential risk for hepatic enzyme elevations BHV-0223 offers potential advantages over conventional riluzole tablets for ALS patients (1) Leads to uncertain pharmacokinetic performance as well as oral numbness 30 ©2017 Biohaven
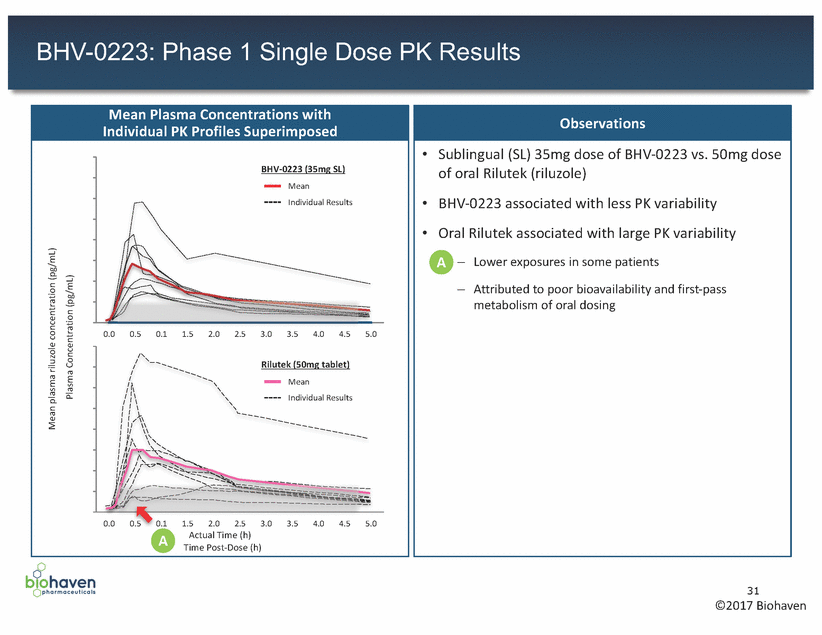
BHV-0223: Phase 1 Single Dose PK Results Time Post-Dose (h) 31 ©2017 Biohaven Mean plasma riluzole concentration (pg/mL) Plasma Concentration (pg/mL) Observations •Sublingual (SL) 35mg dose of BHV-0223 vs. 50mg dose of oral Rilutek (riluzole) •BHV-0223 associated with less PK variability •Oral Rilutek associated with large PK variability A– Lower exposures in some patients – Attributed to poor bioavailability and first-pass metabolism of oral dosing Mean Plasma Concentrations with Individual PK Profiles Superimposed 400000 350000 300000 250000 200000 150000 100000 50000 0 0.0 0.5 0.1 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 400000 350000 300000 250000 200000 150000 100000 50000 0 0.0 0.5 0.1 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 AActual Time (h) Rilutek (50mg tablet) Mean Individual Results BHV-0223 (35mg SL) Mean Individual Results
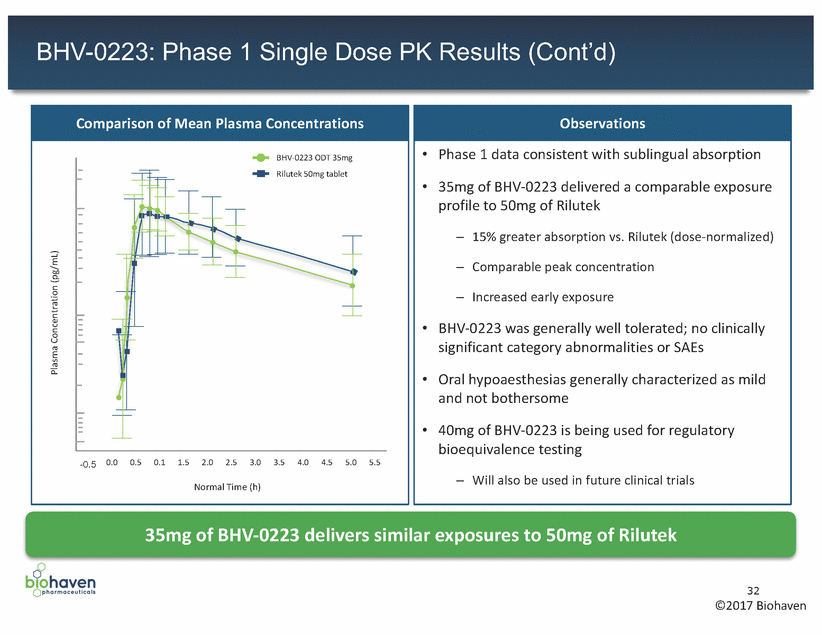
BHV-0223: Phase 1 Single Dose PK Results (Cont’d) 35mg of BHV-0223 delivers similar exposures to 50mg of Rilutek 32 ©2017 Biohaven Plasma Concentration (pg/mL) Observations •Phase 1 data consistent with sublingual absorption •35mg of BHV-0223 delivered a comparable exposure profile to 50mg of Rilutek – 15% greater absorption vs. Rilutek (dose-normalized) – Comparable peak concentration – Increased early exposure •BHV-0223 was generally well tolerated; no clinically significant category abnormalities or SAEs •Oral hypoaesthesias generally characterized as mild and not bothersome •40mg of BHV-0223 is being used for regulatory bioequivalence testing – Will also be used in future clinical trials Comparison of Mean Plasma Concentrations BHV-0223 ODT 35mg 2e+05 1e+05 5e+04 2e+04 1e+04 5e+03 2e+03 1e+03 5e+02 -0.5 0.0 0.5 0.1 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 Normal Time (h) Rilutek 50mg tablet
BHV-0223: Clinical Development Plan • Received orphan drug designation from the FDA in December 2016 for treatment of ALS – Eligible for orphan exclusivity contingent on showing BHV-0223 is clinically superior to Rilutek or other approved riluzole compounds – Clinical superiority may be demonstrated by showing greater effectiveness, greater safety in a substantial portion of the target population, or making a major contribution to patient care • Pivotal bioequivalence study (4Q17) comparing BHV-0223 to riluzole in healthy subjects – Includes a dosing arm to assess the impact of meals on drug absorption in order to potentially support dosing instructions that can avoid the need for the dietary restrictions that accompany Rilutek • Also planning a clinical trial in healthy subjects to assess the effect of BHV-0223 on transaminase levels and other markers of liver function – This trial may also be sufficient to demonstrate the clinical superiority of BHV-0223 to Rilutek • Plan to submit an NDA to the FDA in 2018 under Section 505(b)(2) regulatory pathway 33 ©2017 Biohaven

BHV-5000: A Novel Low-trapping NMDA Antagonist 34 ©2017 Biohaven Clinical Status •Plan to rapidly advance BHV-5000 into trials for the treatment of breathing irregularities associated with Rett syndrome, an orphan disease •NMDA modulation has the potential for applicability across a number of CNS disorders – Subsequently plan to pursue development for other neurological or neuropsychiatric indications with high unmet medical needs, e.g. depression and neuropathic pain •AstraZeneca studied BHV-5000 in a Phase 1 single and multiple ascending dose trial – Doses up to 95mg were studied and were observed to be well tolerated •Active metabolite, lanicemine, has been administered to ~770 subjects in single or multiple doses in 18 clinical trials conducted by AstraZeneca and has been generally well tolerated Clinical Differentiation •NMDA targeting agents are typically associated with psychotomimetic / dissociative effects •However, as a low-trapping antagonist, BHV-5000 is able to uncouple from the NMDA receptor more freely than other agents – Thought to mitigate risk of dissociative effects Description •First-in-class, low-trapping, NMDA receptor antagonist; oral prodrug of the IV drug lanicemine – NMDA receptors may play a role in neurodegenerative diseases – Potential to be best-in-class •BHV-5000 and lanicemine were licensed from AstraZeneca in 2H 2016
BHV-5000: Rationale for Use in Rett Syndrome • Symptoms emerge by 6 months and patients typically diagnosed by 18 months • Anecdotal clinical reports regarding the use of ketamine link treatment effect demonstrated in (reduction of apneic episodes) BHV-5000 has potential to positively impact Rett syndrome patients 35 ©2017 Biohaven Rationale for Use in Rett Syndrome Rett Syndrome (Orphan Disease) •Neurodevelopmental disorder caused by X-linked dominant gene mutation (MECP2) •~15,000 affected-females in US – treated at specialty clinics Progressive Symptoms Eventually Necessitating 24-hour Care •Global deceleration of psychomotor development •Loss of acquired cognitive and motor skills, e.g. speech •Breathing disturbances (apneic periods) •Other: seizures; gait disturbances, tremors, emotional outbursts, sound sensitivity Clinical and Preclinical Rationale •NMDA antagonists improve phenotype (e.g. respiratory symptoms) in transgenic mouse models of Rett syndrome preclinical models with clinical benefit •BHV-5000 / lanicemine show positive treatment effect in preclinical models Regulatory Pathway •FDA interactions with other sponsors, including agreement on primary outcome variable Treatments •None
BHV-5000: Clinical Development Plan • BHV-5000 is rapidly metabolized to lanicemine and, in a Phase 1 trial, concentrations of BHV-5000 were detectable in only a few subjects who received the highest dose – Clinical program for BHV-5000 will build upon AstraZeneca’s previous development efforts for lanicemine – Intend to rely on long-term GLP toxicology, reproductive toxicology and carcinogenicity studies of lanicemine to potentially expedite the safety package for BHV-5000 • In the process of developing a commercial formulation of BHV-5000 – Subsequently intend to conduct a brief Phase 1 trial to confirm BHV-5000’s PK attributes, which we expect to commence by the end of 2017 • Initiate a Phase 2/3 placebo-controlled, randomized, double-blind trial in Rett syndrome in 2018 • Primary outcome measure will be reduction in respiratory abnormalities (number of apneic episodes) – – This endpoint has been advanced with regulatory authorities and used by other sponsors Meaningful benefit for patients and caregivers, improving quality of life and potentially reducing secondary cardio-respiratory complications Will observe impacts on other symptom domains as secondary outcome measures – • NMDA receptor modulation has potential for broad applicability across a number of CNS disorders – Proposed Phase 2/3 trial in Rett, if positive, will serve as proof-of-concept for BHV-5000 across neuroscience indications – Future development may include exploration of BHV-5000 in other conditions such as depression, neuropathic pain and other disorders involving NMDA receptor dysfunction 36 ©2017 Biohaven

37 ©2017 Biohaven Milestones

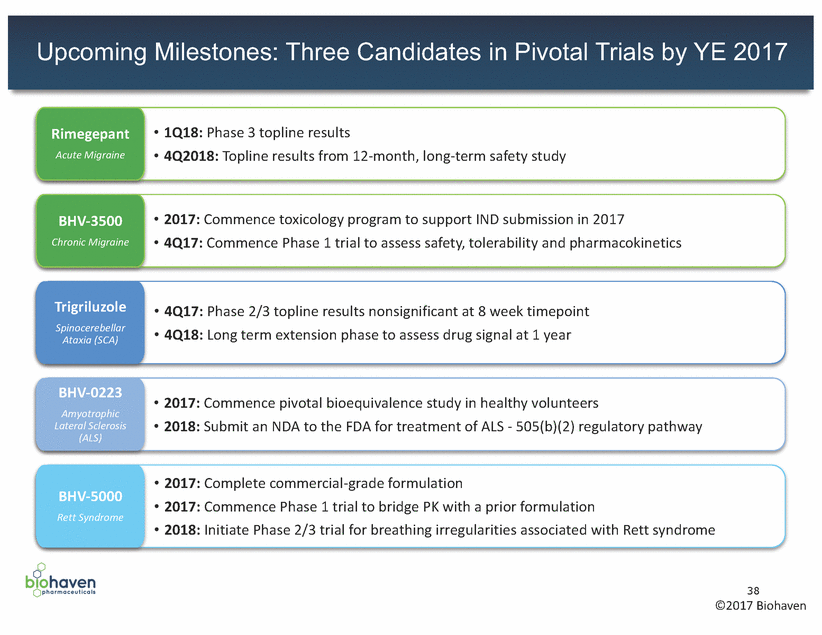
Upcoming Milestones: Three Candidates in Pivotal Trials by YE 2017 • 1Q18: Phase 3 topline results • 4Q2018: Topline results from 12-month, long-term safety study Rimegepant Acute Migraine • 2017: Commence toxicology program to support IND submission in 2017 • 4Q17: Commence Phase 1 trial to assess safety, tolerability and pharmacokinetics BHV-3500 Chronic Migraine Trigriluzole Spinocerebellar Ataxia (SCA) • 4Q17: Phase 2/3 topline results nonsignificant at 8 week timepoint • 4Q18: Long term extension phase to assess drug signal at 1 year BHV-0223 Amyotrophic Lateral Sclerosis (ALS) • 2017: Commence pivotal bioequivalence study in healthy volunteers • 2018: Submit an NDA to the FDA for treatment of ALS - 505(b)(2) regulatory pathway • 2017: Complete commercial-grade formulation • 2017: Commence Phase 1 trial to bridge PK with a prior formulation • 2018: Initiate Phase 2/3 trial for breathing irregularities associated with Rett syndrome BHV-5000 Rett Syndrome 38 ©2017 Biohaven
Biohaven Investment Highlights LATE-STAGE product candidates targeting neurological and rare diseases INNOVATIVE platforms: CGRP receptor and glutamate modulation BEST-IN-CLASS potential for CGRP candidate, rimegepant, in acute migraine FIRST-IN-CLASS potential for glutamate candidate trigriluzole BROAD PIPELINE of additional product candidates 39 ©2017 Biohaven
- - 0 b7ohaven 40 ©2017 Biohaven Qphormaceuticals

