Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ImmunoCellular Therapeutics, Ltd. | d457951d8k.htm |

NYSE American: IMUC.BC October 2017 Corporate Presentation Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding our plans, timelines, prospects, objectives, expectations and intentions with respect to the potential for success of our potential products, cancer immunotherapy research, development efforts and timelines, regulatory status, business, operations, intellectual property, financial condition and other statements that are not historical in nature, are forward-looking statements. You should consider forward-looking statements carefully because they may include statements regarding our future expectations or projections about future events. These statements involve known and unknown risks, uncertainties, assumptions and other factors, including those described in our filings with the Securities and Exchange Commission (SEC). You are cautioned that forward-looking statements are not guarantees of future performance. The forward-looking statements included in this presentation are made only as of the date of this presentation, and, except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future.
ImmunoCellular Therapeutics Developing treatments to stimulate the ability of the immune system to fight cancer Cancer immunotherapy we believe to be the wave of the future – TODAY Development-stage company committed to developing immunotherapeutic solutions for intractable cancers, extending the lives of cancer patients, and providing hope for a potential cure IMUC’s Stem-to-T-Cell program is a novel approach that enables the patient’s immune system to produce targeted killer T cells Multiple value driving milestones next expected in the 12-18 months Need for immunotherapy for cancer: Surgery may not remove every cancer cell Chemotherapy and radiation therapy may damage normal tissue Target all rapidly dividing cells leading to significant toxicity Cancer can recur after apparently successful treatment Cancer stem cells Goal of creating “plug & play” scenario – menu of solutions for patient’s specific tumor

Immunotherapy has several potential advantages over traditional approaches Harnesses the power of each patient’s immune system Tumor-specific Longer-lasting Advantageous safety profile IMUC focus on solid tumors Personalized Cancer Immunotherapy Potential to address severe unmet medical needs
Potential Advantages of Stem-to-T-Cell Approach Unlike CAR-T therapy, T cells will be “produced” by the treated HSCs that are reintroduced to the patient Slower production and subsequent release of targeted CD8+ T cells should be less toxic to the patient More measured killing can be cleaned up by the patient without triggering a cytokine release syndrome Because the treated HSCs will return to the bone marrow, we believe there should be a level of surveillance against recurrence of the tumor Autologous nature of the product we believe will prevent GVHD Careful selection of TCRs will ensure only the tumor is targeted Expect be able to address many tumor types
Three Clinical-Stage Programs ImmunoCellular actively looking to partner, sell or license all three programs Dendritic cell-based research: ICT-107 Phase 3 ready for glioblastoma ICT-121 Phase 1 completed for recurrent glioblastoma (warrants continued testing) ICT-140 Phase 1/2 ready for ovarian cancer ImmunoCellular has the advantage of two personalized immunotherapeutic approaches to drive T cell killing of cancer cells
ImmunoCellular Therapeutics Stem-to-T-Cell Program Early-stage research program, using IP from Dr. David Baltimore’s lab, a Nobel laureate of Caltech, testing effects of transfecting a selected T cell receptor into a patient’s hematopoietic stem cells These transfected HSCs would then be reintroduced to the patient and they would return to the bone marrow Goal is to develop a TCR library to allow a “plug and play” scenario where a patient’s specific tumor can be addressed Improve cancer treatment by stimulating patients’ immune systems to generate a long-term population of cytotoxic T cells directed against the tumor, resulting in a potentially curative therapy for many types of cancers
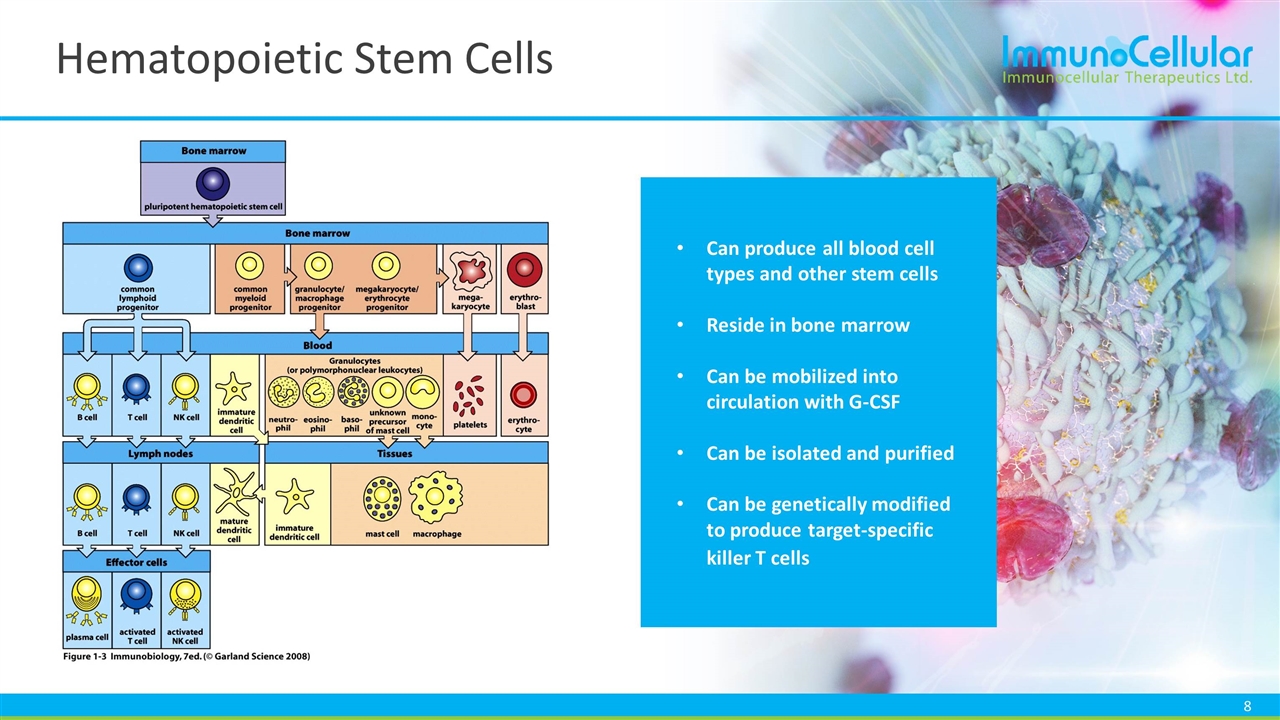
Hematopoietic Stem Cells Can produce all blood cell types and other stem cells Reside in bone marrow Can be mobilized into circulation with G-CSF Can be isolated and purified Can be genetically modified to produce target-specific killer T cells
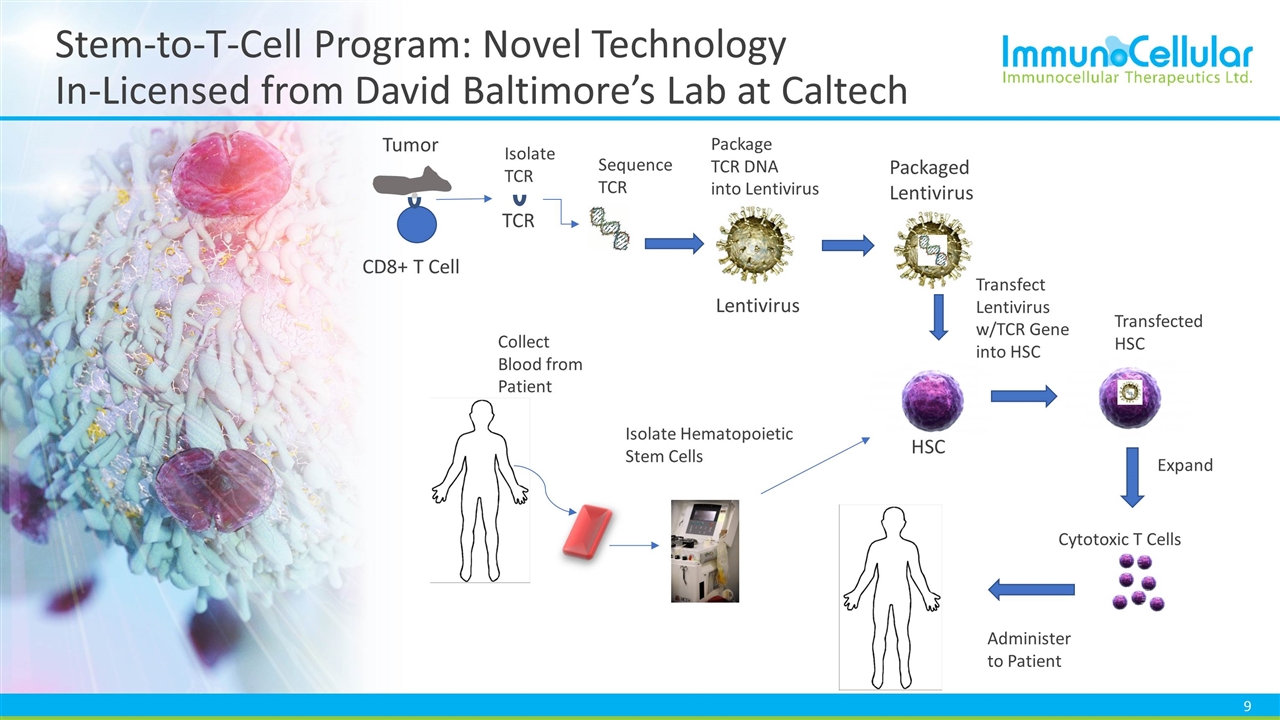
Stem-to-T-Cell Program: Novel Technology In-Licensed from David Baltimore’s Lab at Caltech HSC CD8+ T Cell Tumor TCR Lentivirus Collect Blood from Patient Isolate Hematopoietic Stem Cells Package TCR DNA into Lentivirus Transfect Lentivirus w/TCR Gene into HSC Transfected HSC Isolate TCR Sequence TCR Expand Administer to Patient Packaged Lentivirus Cytotoxic T Cells

Collaborations University of Maryland, Baltimore (“UMB”) Sponsored research partner Conducting three preclinical research programs California Institute of Technology (“Caltech”) Stem-to-T-Cell preclinical technology licensed in 2014 MD Anderson Cancer Center For the discovery of novel T cell receptors
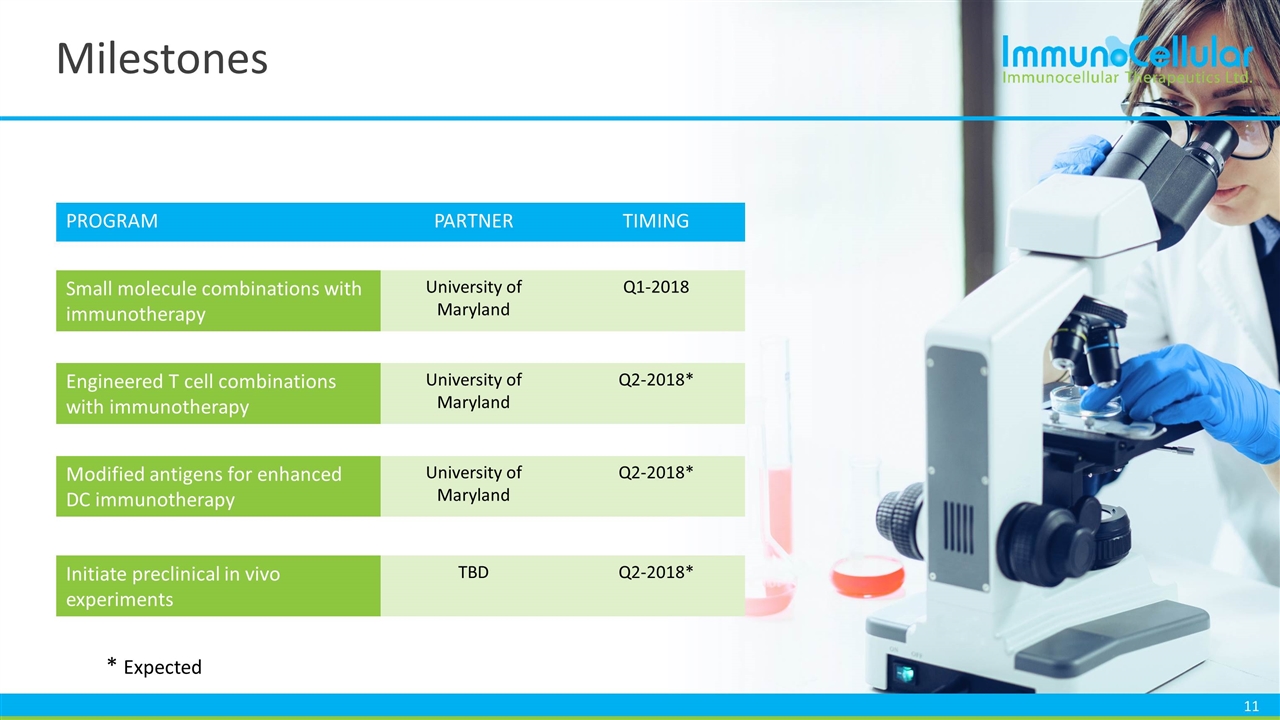
Milestones PROGRAM PARTNER TIMING Small molecule combinations with immunotherapy University of Maryland Q1-2018 Engineered T cell combinations with immunotherapy University of Maryland Q2-2018* Modified antigens for enhanced DC immunotherapy University of Maryland Q2-2018* Initiate preclinical in vivo experiments TBD Q2-2018* * Expected

Recent Financing July 2017 – Initially raised $5.0 million from sale of 5,000 shares of convertible preferred stock Issued warrants to purchase 9,000 shares of convertible preferred stock Potential additional gross proceeds of $9.0 million Warrants mature October 2017 January 2018 July 2018 Underwriter: Maxim Group, LLC Use of proceeds (i) reduce outstanding accounts payable and (ii) restructuring costs to transition to immune-oncology research program Warrants exercise will allow Company to execute its research and development projects

Financial Overview Stock price: $0.40 (as of 10/6/17) Shares outstanding: 15.8 million shares (as of 8/9/17) Market capitalization: $6.35 million (as of 10/6/17) Cash and cash equivalents: $1.8 million (as of 6/30/17) Excludes $5.0 million initial proceeds from July 2017 financing Average daily trading volume (3-months): 2.3 million shares Fiscal year-end: December 31 Financial Recap – six month period, as of June 30, 2017 Research and development expenses: $15.0 million Expect reduced R&D going forward General and administrative expense: $1.8 million Expect reduced G&A going forward “Out of the money” warrants outstanding: 1.6 million “Out of the money” stock options outstanding: 153,000

Management Anthony J. Gringeri, Ph.D. - President and Chief Executive Officer Appointed Chief Executive Officer in December 2016 25+ years of executive experience in the pharmaceutical and biotechnology industry Chief Operating Officer for Amsterdam Molecular Therapeutics Amgen for 15 years in a series of executive leadership roles Ph.D. in Pharmacology from the University of Rochester Steven J. Swanson, Ph.D. - Senior Vice President, Research 15 years at Amgen as Department Head for Clinical Immunology Led the immunoassay laboratory in the Biotechnology department at Schering Plough Research Institute Actively involved in the American Association of Pharmaceutical Scientists (AAPS), Ph.D. Microbiology from the University of Iowa David Fractor, CPA - Chief Financial Officer Appointed Chief Financial Officer April 2017; Previously, Treasurer and Vice President, Finance since April 2011; Since 2003 provided financial consulting and strategic planning services Chief Financial Officer of HemaCare Corporation from 1999 through 2003 Previously served as an audit manager at Deloitte and at a regional accounting firm B.S. in Accounting from the University of Southern California in 1982 Member of AICPA and the California Society of CPAs

Developing immunotherapeutic solutions for intractable cancers, extend the lives of cancer patients, and provide hope for a potential cure IMUC’s Stem-to-T-Cell approach is potentially “game changing” treatment for cancer Harnesses the patient’s immune system in manufacturing specific cytotoxic T cells to destroy cancer cells Provide long-term immuno-surveillance Immuno-oncology is fast evolving field Multiple approaches using immunotherapy being evaluated in clinical trials Potential for combination therapies Immunotherapy has the potential to change cancer treatment Specificity in targeting tumor cells Enhanced patient safety compared to current standard of care Opportunities for partnering with pharma and big biotech seeking novel cancer immunotherapy assets IMUC represents opportunity for value creation for investors today. Summary

NYSE American: IMUC.BC October 2017 Corporate Presentation
