Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Astria Therapeutics, Inc. | a17-22790_1ex99d1.htm |
| 8-K - 8-K - Astria Therapeutics, Inc. | a17-22790_18k.htm |
MoveDMD® Open-Label Extension Edasalonexent is an oral small molecule designed to inhibit NF-kB for the treatment of Duchenne muscular dystrophy October 4, 2017
Forward Looking Statements This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, including statements regarding our expectations and beliefs about our business, future financial and operating performance, clinical trial plans, product development plans and prospects, including statements about future clinical trial plans including, among other things, statements about our plans to commence a single global Phase 3 trial in Duchenne muscular dystrophy, or DMD, in the first half of 2018 to evaluate the efficacy and safety of edasalonexent for registration purposes, our plans to report top-line results from this trial in 2020 and our plans to continue to evaluate data from the open-label extension of our MoveDMD® clinical trial of edasalonexent for the treatment of DMD. The words “believe”, “anticipate”, “plans,” “expect”, “could”, “should”, “will”, “would”, “may”, “intend” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The forward-looking statements contained in this presentation and in remarks made during this presentation and the following Q&A session are subject to important risks and uncertainties that may cause actual events or results to differ materially from our current expectations and beliefs, including: uncertainties inherent in the initiation and completion of preclinical studies and clinical trials and clinical development of the Company’s product candidates, including the final trial design of our planned Phase 3 trial in DMD; availability and timing of results from preclinical studies and clinical trials, including the availability of top-line results from our planned Phase 3 trial in DMD in 2020; whether interim results from a clinical trial will be predictive of the final results of the trial or the results of future trials; expectations for regulatory approvals to conduct trials or to market products, including our expected target product profile for edasalonexent in DMD; our ability to obtain financing on acceptable terms and in a timely manner to fund our planned Phase 3 trial in DMD to evaluate the efficacy and safety of edasalonexent for registration purposes; availability of funding sufficient for the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements; other matters that could affect the availability or commercial potential of the Company’s product candidates; and general economic and market conditions and other factors discussed in the “Risk Factors” section of the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2017, which is on file with the Securities and Exchange Commission, and in other filings that the Company may make with the Securities and Exchange Commission in the future. In addition, the forward-looking statements included in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. 2

Topics of Today’s Call 3 Duchenne Muscular Dystrophy and Edasalonexent Overview MoveDMD Open-Label Extension Results Phase 3 Edasalonexent Phase 3 Clinical Trial Plan Question and Answer Session
Topics of Today’s Call 4 Duchenne Muscular Dystrophy and Edasalonexent Overview MoveDMD Open-Label Extension Results Phase 3 Edasalonexent Phase 3 Clinical Trial Plan Question and Answer Session
Duchenne Muscular Dystrophy Unmet Need Continues Rare disease with well identified patient population – Approximately 15,000 patients in US and 19,000 in EU Initial marketed therapies for small subsets of the patient most patients do not have a targeted therapy available population, Standard of care, corticosteroid treatment, has significant associated adverse events – Including Cushing’s syndrome, obesity, behavioral changes, pubertal delay, osteoporosis and fractures Research shows that effects on muscle function are the most important aspect of a therapy for Duchenne for the parents of affected boys – Parent Project Muscular Dystrophy (PPMD) 5
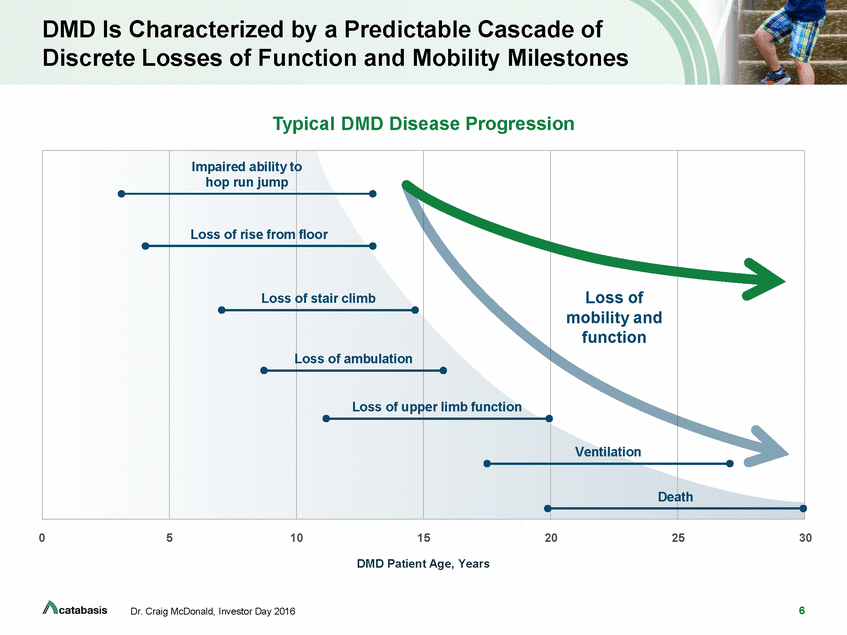
DMD Is Characterized by a Predictable Cascade of Discrete Losses of Function and Mobility Milestones Typical DMD Disease Progression o Loss of ambulation eath 0 5 10 15 20 25 30 DMD Patient Age, Years 6 Dr. Craig McDonald, Investor Day 2016 Impaired ability t hop run jump Loss of rise from Loss floor of stair climb Loss of upp er limb function Loss of mobility and function Ventilation D
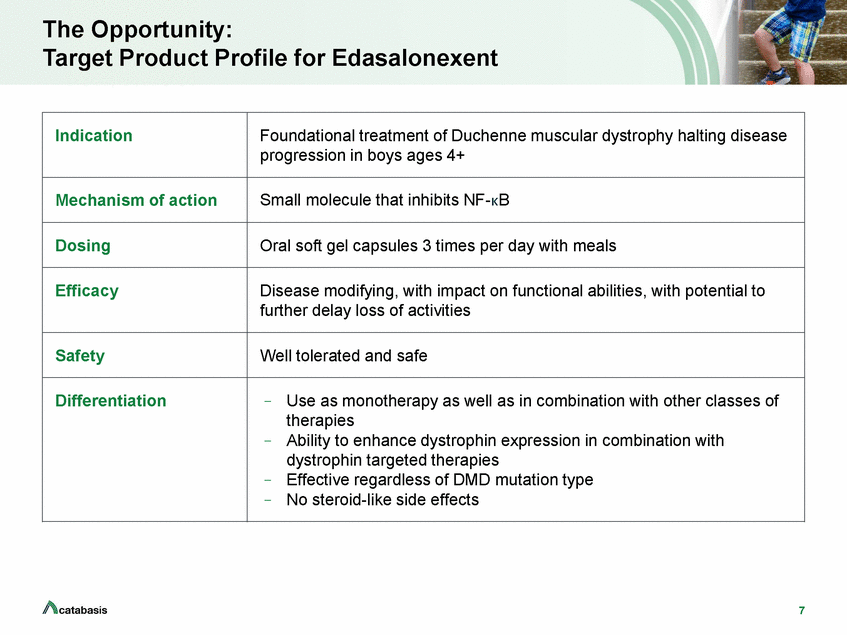
The Opportunity: Target Product Profile for Edasalonexent 7 Indication Foundational treatment of Duchenne muscular dystrophy halting disease progression in boys ages 4+ Mechanism of action Small molecule that inhibits NF-kB Dosing Oral soft gel capsules 3 times per day with meals Efficacy Disease modifying, with impact on functional abilities, with potential to further delay loss of activities Safety Well tolerated and safe Differentiation – Use as monotherapy as well as in combination with other classes of therapies – Ability to enhance dystrophin expression in combination with dystrophin targeted therapies – Effective regardless of DMD mutation type – No steroid-like side effects
Topics of Today’s Call 8 Duchenne Muscular Dystrophy and Edasalonexent Overview MoveDMD Open-Label Extension Results Phase 3 Edasalonexent Phase 3 Clinical Trial Plan Question and Answer Session
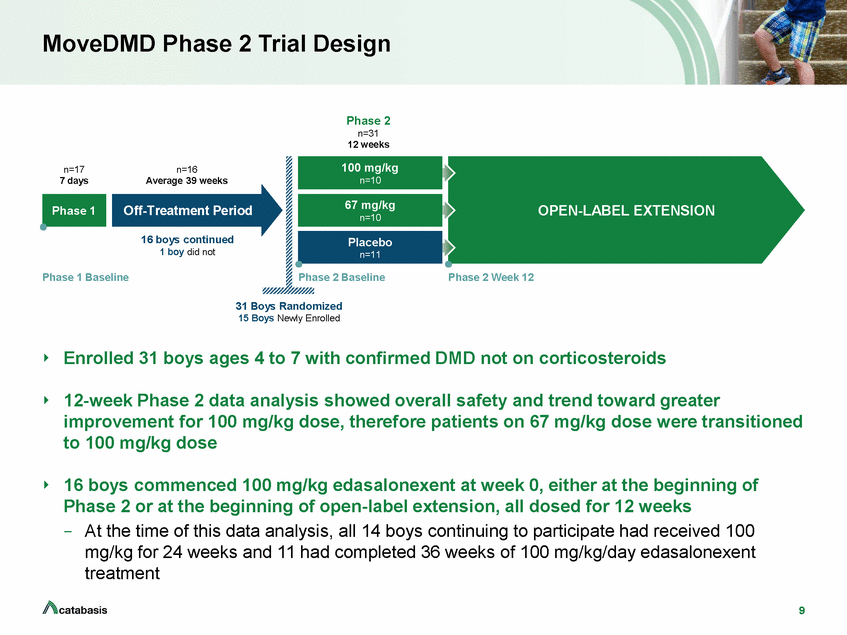
MoveDMD Phase 2 Trial Design Phase 2 n=31 12 weeks n=17 7 days n=16 Average 39 weeks OPEN-LABEL EXTENSION 16 boys continued 1 boy did not Phase 1 Baseline Phase 2 Baseline Phase 2 Week 12 31 Boys Randomized 15 Boys Newly Enrolled Enrolled 31 boys ages 4 to 7 with confirmed DMD not on corticosteroids 12-week Phase 2 data analysis showed overall safety and trend toward greater improvement for 100 mg/kg dose, therefore patients on 67 mg/kg dose were transitioned to 100 mg/kg dose 16 boys commenced 100 mg/kg edasalonexent at week 0, either at the beginning of Phase 2 or at the beginning of open-label extension, all dosed for 12 weeks –At the time of this data analysis, all 14 boys continuing to participate had received 100 mg/kg for 24 weeks and 11 had completed 36 weeks of 100 mg/kg/day edasalonexent treatment 9 Placebo n=11 Off-Treatment Period 67 mg/kg n=10 Phase 1 100 mg/kg n=10
Improvementsin Assessments of Muscle Function 10

Prespecified Analysis Approach to Open-Label Assessments of Muscle Function Changes during the control period were measured prior to commencing edasalonexent, either prior to Phase 2 or for the placebo group at the start of the open-label extension, for time periods averaging 39 weeks Comparison of control period to on-treatment period assumes that the rate of decline remains linear –However, DMD natural history data indicates a more rapid decline as boys get older 0.20 5 Solid line represents average of actual control period observations for boys in the 100 mg/kg treatment group (n=12) ntrol 0.18 0.16 Dashed line represents extension of control period observations 0.14 0.12 10 -36 Weeks -24 -12 Time of edasalonexent 12 24 36 treatment initiation 11 Time (Seconds) Speed (1/Seconds) Co
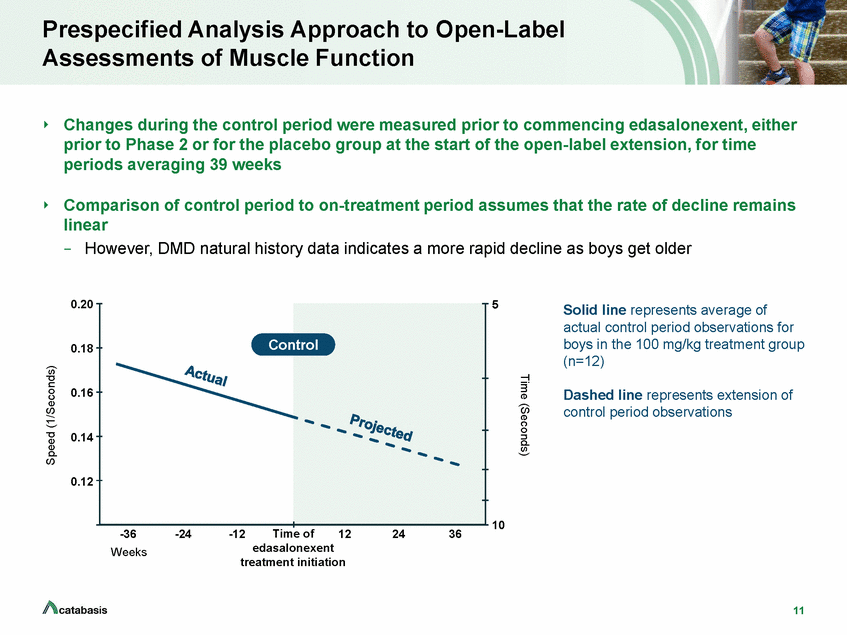
10-Meter Walk/Run Speed Stabilized with Edasalonexent Treatment 10-Meter Walk/Run 0.20 5 0.18 0.16 0.14 0.12 10 -36 -24 -12 0 Weeks 12 24 36 Disease progression on edasalonexent improved compared with rate of change on control 12 Means ± SEM shown Time (Seconds) Speed (1/Seconds) Edasalonexent 100 mg/kg
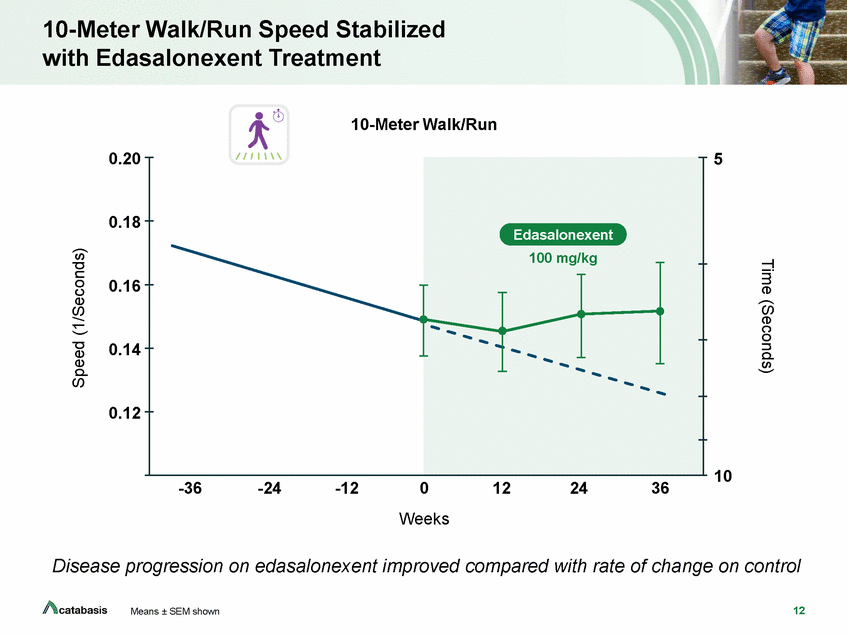
4-Stair Climb Speed Stabilized with Edasalonexent Treatment 4-Step Climb 0.4 0.3 5 0.2 0.1 10 15 -36 -24 -12 0 Weeks 12 24 36 Disease progression on edasalonexent improved compared with rate of change on control 13 Means ± SEM shown Time (Seconds) Speed (1/Seconds) Edasalonexent 100 mg/kg
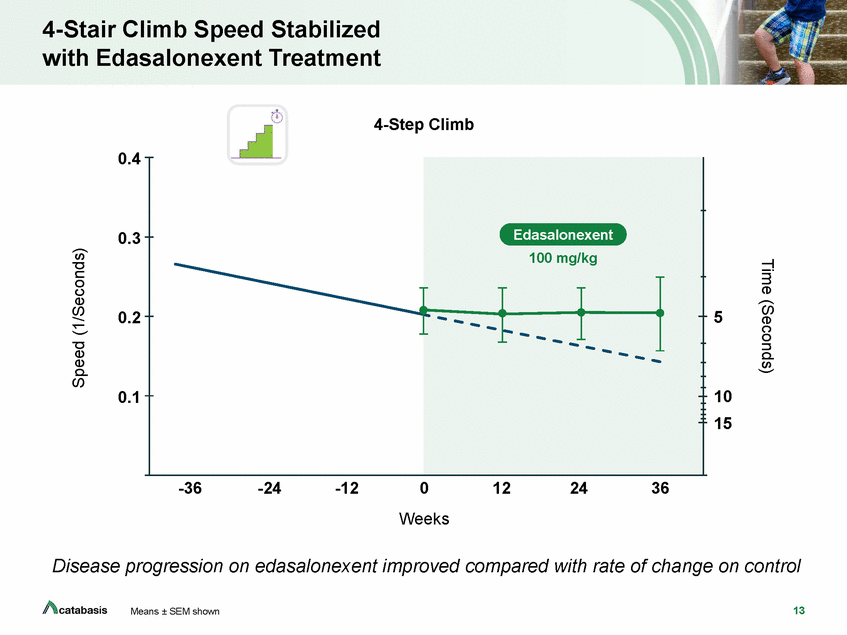
Time to Stand Speed Stabilized with Edasalonexent Treatment Time to Stand 0.3 5 0.2 10 0.1 15 -36 -24 -12 0 Weeks 12 24 36 Disease progression on edasalonexent improved compared with rate of change on control 14 Means ± SEM shown Time (Seconds) Speed (1/Seconds) Edasalonexent 100 mg/kg
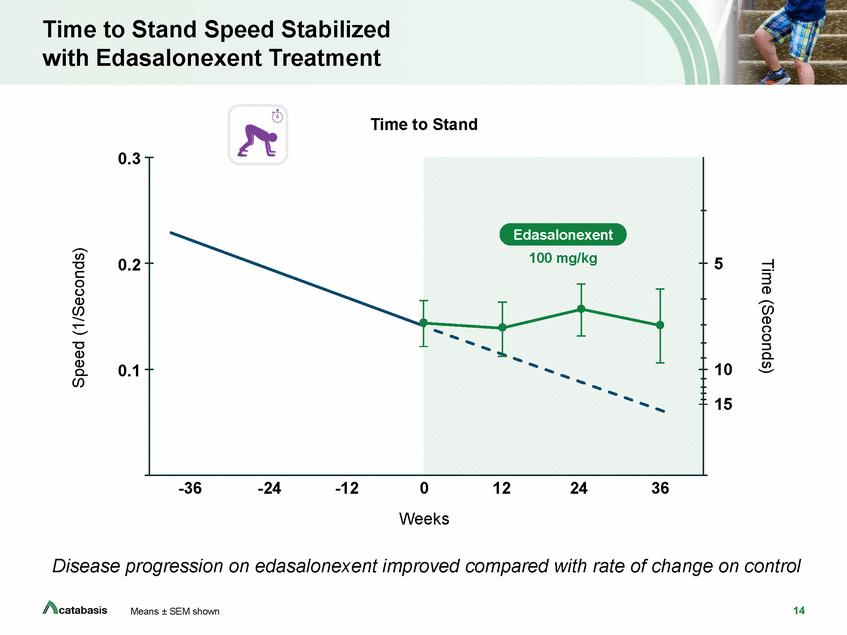
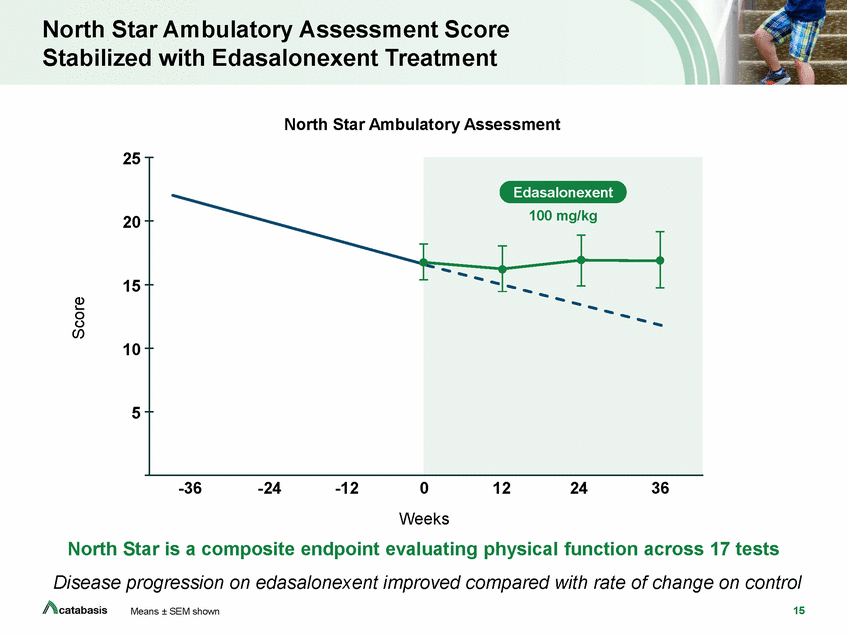
North Star Ambulatory Assessment Score Stabilized with Edasalonexent Treatment North Star Ambulatory Assessment 25 20 15 10 5 -36 -24 -12 0 Weeks 12 24 36 North Star is a composite endpoint evaluating physical function across 17 tests Disease progression on edasalonexent improved compared with rate of change on control 15 Means ± SEM shown Score Edasalonexent 100 mg/kg
OtherSupportiveChangesin Measures of Muscle Health 16

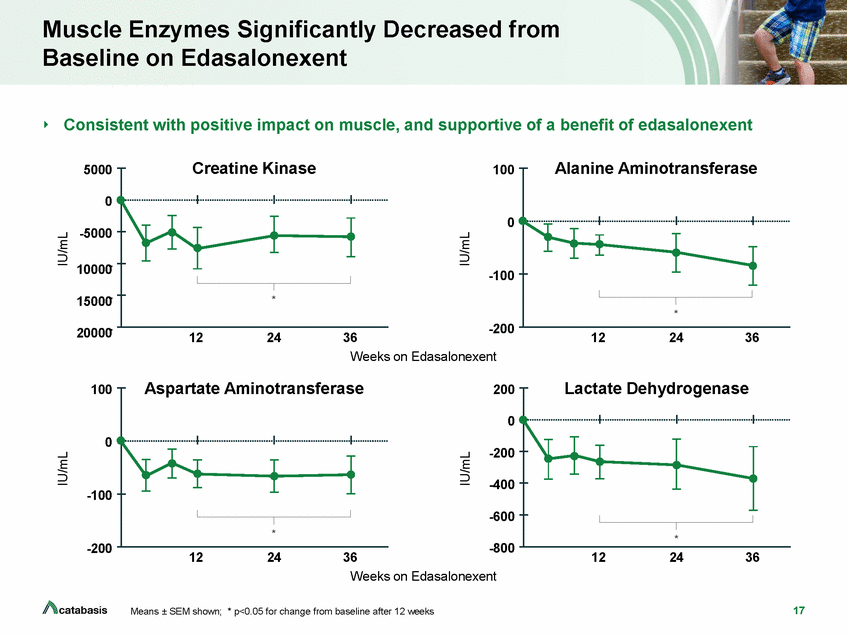
Muscle Enzymes Significantly Decreased from Baseline on Edasalonexent Consistent with positive impact on muscle, and supportive of a benefit of edasalonexent Creatine Kinase Alanine Aminotransferase 5000 100 0 0 -5000 10000-15000-20000--100 * -200 12 24 36 12 24 36 Weeks on Edasalonexent Aspartate Aminotransferase Lactate Dehydrogenase 100 200 0 0 -200 -400 -100 -600 -200 -800 12 24 36 12 24 36 Weeks on Edasalonexent 17 Means ± SEM shown; * p<0.05 for change from baseline after 12 weeks IU/mL IU/mL IU/mL IU/mL * * *
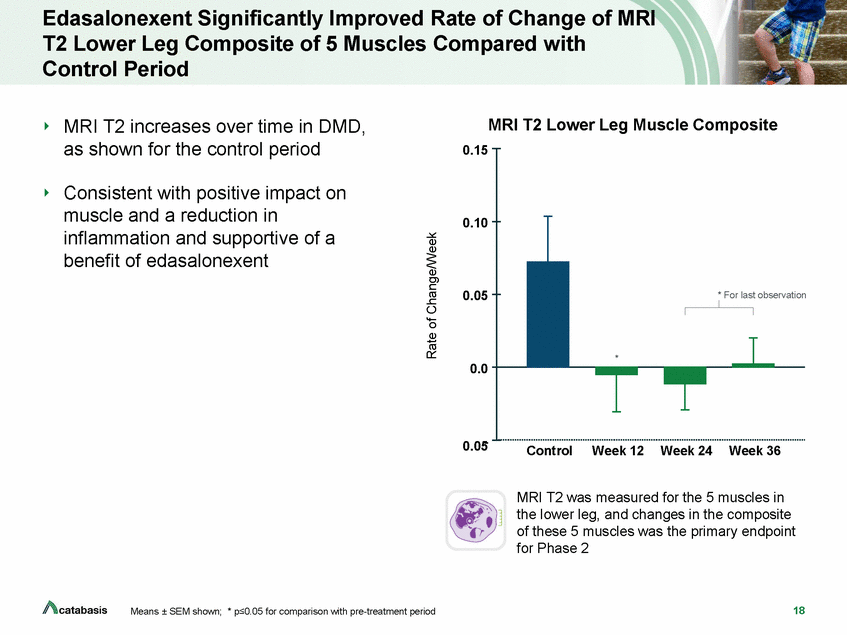
Edasalonexent Significantly Improved Rate of Change of MRI T2 Lower Leg Composite of 5 Muscles Control Period Compared with MRI T2 increases over time in DMD, as shown for the control period MRI T2 Lower Leg Muscle Composite 0.15 Consistent with positive impact on muscle and a reduction in inflammation and supportive of a benefit of edasalonexent 0.10 0.05 ion 0.0 0.05-Control Week 12 Week 24 Week 36 MRI T2 was measured for the 5 muscles in the lower leg, and changes in the composite of these 5 muscles was the primary endpoint for Phase 2 18 Means ± SEM shown; * p<0.05 for comparison with pre-treatment period Rate of Change/Week * For last observat *
WellToleratedwith No Safety Signals 19

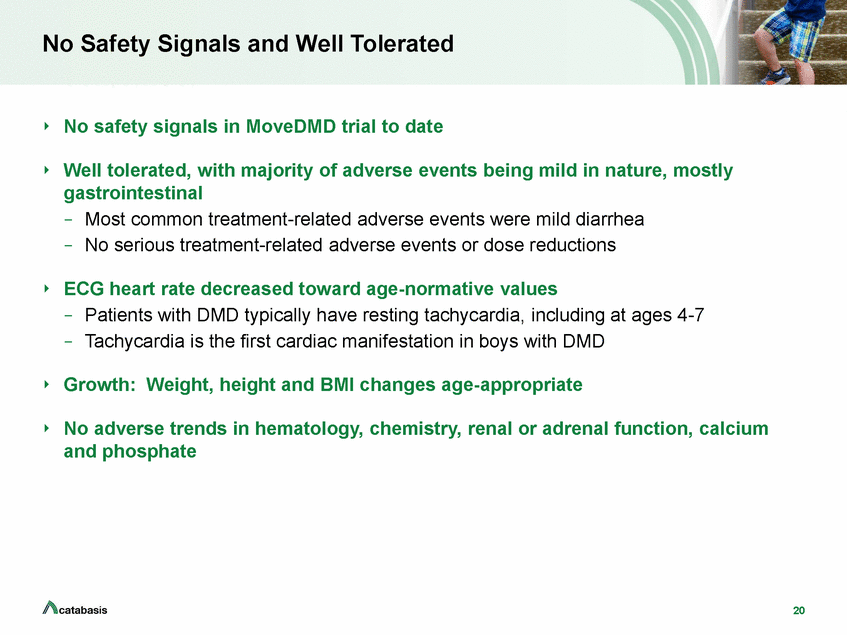
No Safety Signals and Well Tolerated No safety signals in MoveDMD trial to date Well tolerated, with majority of adverse events being mild in nature, mostly gastrointestinal –Most common treatment-related adverse events were mild diarrhea –No serious treatment-related adverse events or dose reductions ECG heart rate decreased toward age-normative values –Patients with DMD typically have resting tachycardia, including at ages 4-7 –Tachycardia is the first cardiac manifestation in boys with DMD Growth: Weight, height and BMI changes age-appropriate No adverse trends in hematology, chemistry, renal or adrenal function, calcium and phosphate 20
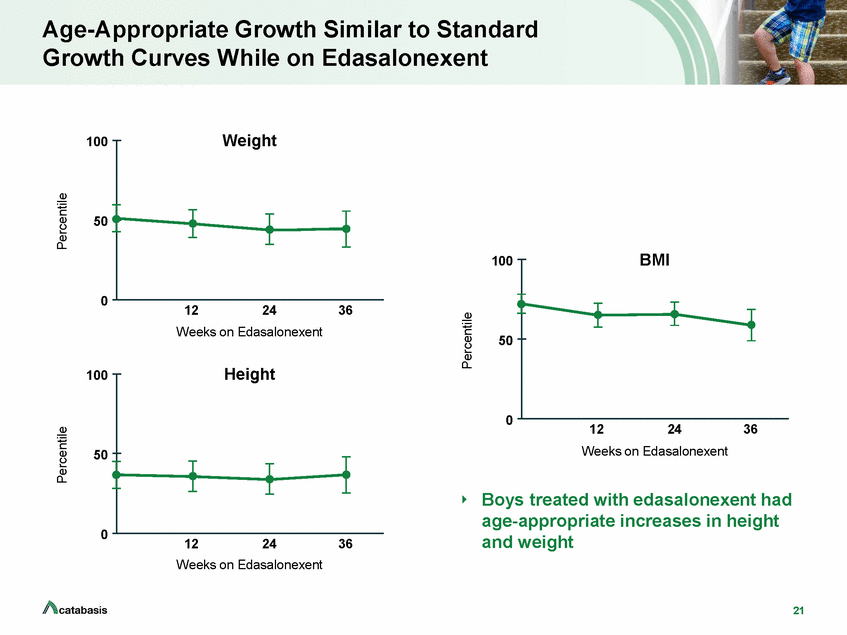
Age-Appropriate Growth Similar to Standard Growth Curves While on Edasalonexent Weight 100 50 BMI 100 0 12 24 36 Weeks on Edasalonexent 50 Height 100 0 12 24 36 Weeks on Edasalonexent 50 Boys treated with edasalonexent had age-appropriate increases in height and weight 0 12 24 36 Weeks on Edasalonexent 21 Percentile Percentile Percentile
Open-Label Extension Results: Edasalonexent Substantially Slowed DMD Disease Progression Disease progression on edasalonexent improved compared to rate of change in control period – North Star Ambulatory Assessment – Timed function tests 10-meter walk/run, 4-stair climb and time to stand Additional measures provide further support for positive edasalonexent treatment effects – Muscle enzymes significantly decreased from baseline at 12 weeks and later time points – Rate of change of lower leg muscle MRI T2 significantly improved compared to control period progression Safety profile – No safety signal and well tolerated – Heart rate decreased toward age-normative values – Height, weight and BMI growth patterns continued to be similar to unaffected boys Endpoints evaluated have regulatory support for ages 4-7 22
Topics of Today’s Call 23 Duchenne Muscular Dystrophy and Edasalonexent Overview MoveDMD Open-Label Extension Results Phase 3 Edasalonexent Phase 3 Clinical Trial Plan Question and Answer Session

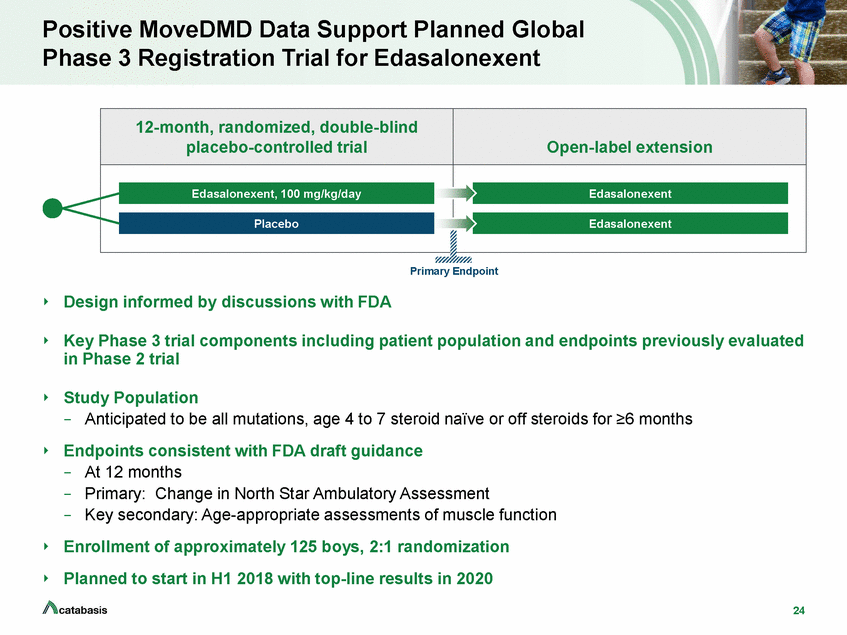
Positive MoveDMD Data Support Planned Global Phase 3 Registration Trial for Edasalonexent Primary Endpoint Design informed by discussions with FDA Key Phase 3 trial components including patient population and endpoints previously evaluated in Phase 2 trial Study Population –Anticipated to be all mutations, age 4 to 7 steroid naïve or off steroids for >6 months Endpoints consistent with FDA draft guidance –At 12 months –Primary: Change in North Star Ambulatory Assessment –Key secondary: Age-appropriate assessments of muscle function Enrollment of approximately 125 boys, 2:1 randomization Planned to start in H1 2018 with top-line results in 2020 24 12-month, randomized, double-blind placebo-controlled trial Open-label extension Placebo Edasalonexent Edasalonexent, 100 mg/kg/day Edasalonexent
Thank You Thank you to the boys and parents involved in the MoveDMD trial, the trial site staff, patient groups and members of the Duchenne community for their support 25

Edasalonexent: Potential to Slow Disease Progression for All Boys with DMD Investigational oral disease-modifying product candidate for all patients with DMD, regardless of mutation type Edasalonexent substantially slowed DMD disease progression through 36 weeks Path to registration defined based on MoveDMD positive data and supportive regulatory input –Phase 3 expected to start in H1 2018, top-line results expected in 2020 Developing as monotherapy and also exploring potential to combine with dystrophin-targeted and other therapies 26
Topics of Today’s Call 27 Duchenne Muscular Dystrophy and Edasalonexent Overview MoveDMD Open-Label Extension Results Phase 3 Edasalonexent Phase 3 Clinical Trial Plan Question and Answer Session
i \catabasis

