Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GENOCEA BIOSCIENCES, INC. | gnca_92717-8k.htm |

Company Update
September 2017
Exhibit 99.1

This presentation contains “forward-looking” statements that are within the meaning of federal securities laws
and are based on our management’s beliefs and assumptions and on information currently available to
management. Forward-looking statements include information concerning our possible or assumed future
results of operations, business strategies, clinical trials and pre-clinical studies, regulatory approval of our
product candidates, liquidity position and capital needs, financing plans, industry environment, potential
growth opportunities, potential market opportunities and the effects of competition.
Forward-looking statements include all statements that are not historical facts and can be identified by terms
such as “anticipates,” “believes,” “could,” “seeks,” “estimates,” “intends,” “may,” “plans,” “potential,”
“predicts,” “projects,” “should,” “will,” “would” or similar expressions and the negatives of those terms. Forward-
looking statements represent our management’s beliefs and assumptions only as of the date of this
presentation. Our operations involve risks and uncertainties, many of which are outside our control, and any
one of which, or combination of which, could materially affect our results of operations and whether the
forward-looking statements ultimately prove to be correct. Factors that may materially affect our results of
operations include, among other things, the timing of results of our ongoing and planned our ability to progress
any product candidates in clinical and clinical trials, the ability of ATLAS to identify promising oncology vaccine
and immunotherapy product candidates, the scope, rate and progress of our preclinical and clinical trials and
other research and development activities, anticipated timing of new clinical trails, our estimates regarding the
amount of funds we require to complete conduct our clinical trials for GEN-003, our plans to commercialize
GEN-003, the timing of, and ability to, obtain and maintain necessary regulatory approvals for our product
candidates, GEN-003 and those listed in our Annual Report on Form 10-K and other filings with the Securities and
Exchange Commission (“SEC”). Except as required by law, we assume no obligation to update these forward-
looking statements publicly, or to update the reasons actual results could differ materially from those
anticipated in the forward-looking statements, even if new information becomes available in the future.
You may get copies of our Annual Report on Form 10-K, Quarterly Report on Form 10-Q and our other SEC filings
for free by visiting EDGAR on the SEC website at http://www.sec.gov.
2
Disclaimer

• Positive 12-month Phase 2b data did not lead to a pathway for
Genocea to independently advance GEN-003 given time and
cost of Phase 3 trials
– Workforce reduction reflects this
• Strategic process underway to seek to maximize value for GEN-
003 through sale, partnership etc.
• Continue to believe that GEN-003 address unmet medical needs
in genital herpes patients
• Opportunity to focus organization and financial resources to
create value through neoantigen cancer vaccine investment
3
Exploring Strategic Alternatives for GEN-003

Leadership in Neoantigen
Cancer Vaccines

• Our mission: to create breakthrough vaccines based on
the right antigens
5
Our vision: Curing cancer with next-generation cancer
vaccines
ATLAS™
Unique, proven platform
for neoantigen selection
Proven team of vaccine experts
Outstanding Board and Scientific Advisors
Efficacious
cancer vaccines

Neoantigens are an exciting new target class in
immuno-oncology
6
• Personalized
tumor mutations
(neoantigens)
are “foreign” to
immune system
Schumacher, Schreiber, 2015 Ott, Sahin, 2017
• Response to
neoantigens
drives checkpoint
inhibitor (CPI)
efficacy
• Possible to
vaccinate
against
neoantigens
Yadav, Gubin, 2015

• Complementary MoA:
“steering wheel” once brakes
are off
• Well tolerated1,2
• Applicable to most cancer
types
7
After CAR-T therapy, neoantigen vaccines represent a
next wave of personalized cancer immunotherapy
Potential synergy with CPI Opportunity to benefit millions
Disease +
Good PS
All Patients
No
Evidence of
Disease
ILLUSTRATIVE
Over
time
Across solid tumors
1Ott et al., 2017 Nature
2Sahin et al., 2017 Nature

Neoantigen Selection
Crucial to Vaccine
Success
8

9
Cancer biology creates significant antigen selection
challenges
Tumor mutational burden by cancer type
Up to thousands of candidate antigens per patient
10
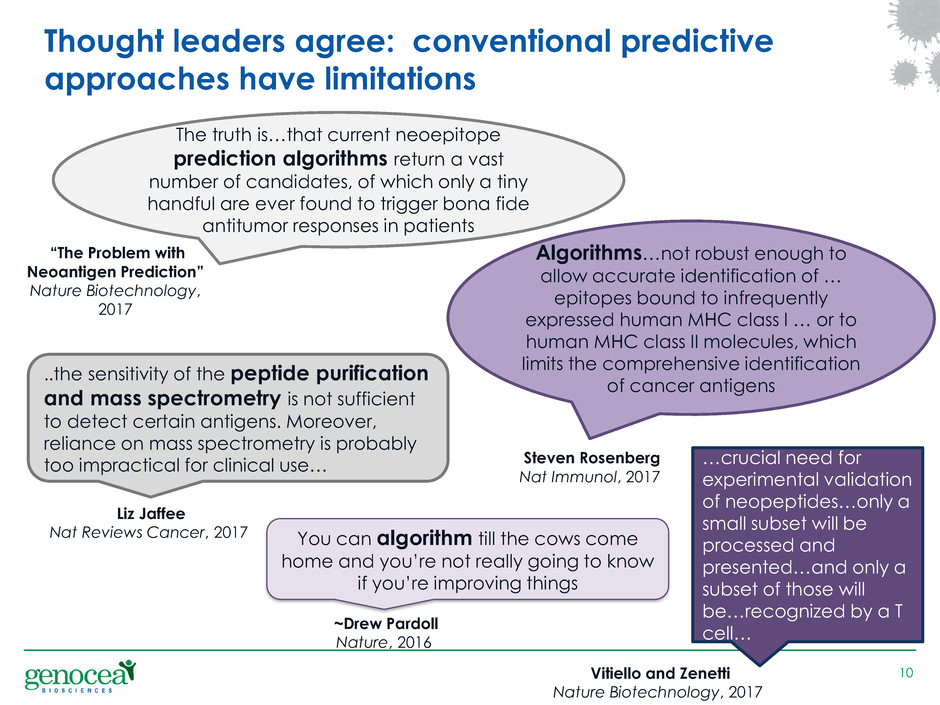
Thought leaders agree: conventional predictive
approaches have limitations
You can algorithm till the cows come
home and you’re not really going to know
if you’re improving things
~Drew Pardoll
Nature, 2016
The truth is…that current neoepitope
prediction algorithms return a vast
number of candidates, of which only a tiny
handful are ever found to trigger bona fide
antitumor responses in patients
“The Problem with
Neoantigen Prediction”
Nature Biotechnology,
2017
…crucial need for
experimental validation
of neopeptides…only a
small subset will be
processed and
presented…and only a
subset of those will
be…recognized by a T
cell…
Vitiello and Zenetti
Nature Biotechnology, 2017
..the sensitivity of the peptide purification
and mass spectrometry is not sufficient
to detect certain antigens. Moreover,
reliance on mass spectrometry is probably
too impractical for clinical use…
Liz Jaffee
Nat Reviews Cancer, 2017
Algorithms…not robust enough to
allow accurate identification of …
epitopes bound to infrequently
expressed human MHC class I … or to
human MHC class II molecules, which
limits the comprehensive identification
of cancer antigens
Steven Rosenberg
Nat Immunol, 2017

*Cohort level; number of SLP with responses in all patients/total SLP immunized across all patients
**Presumed CD4 (total PBMC). Text states majority of responses were CD4 but not disclosed in figure.
11
Conventional approaches yield limited immune
responses regardless of delivery system/modality
% Response to Neoantigens*
ex vivo ELISPOT
Cultured ELISPOTs
(in vitro stim 10-21 days)
CD4+ CD8+ CD4+ CD8+
Neon1
Peptide + adjuvant
20% 0% 40% 16%
BioNTech2
RNA
12%
**
57% 17%
1Ott et al., 2017 Nature
2Sahin et al., 2017 Nature

12
ATLAS platform uses biology – patients’ own T cells – to
identify true neoantigens
Patient-
specific
HLA
agnostic
CD4+ &
CD8+
antigens
Hot and
cold
tumors
*For CD4+ T cell screening the cLLO is not co-
expressed, resulting in conventional endosomal
processing and presentation by MHC cII
Input:
Every candidate
neoantigen
ATLAS cytokine
readout:
- Antigen or not?
- Stimulatory or
inhibitory?
Patient’s
own T cells
*

• Not all neoantigens are
“good”
• CD4+ and CD8 + antigens
are not the same
• No association with key
algorithm inputs:
– Binding affinity
– RNA expression
– Allele frequency
– Frame shifts
13
ATLAS data supports superiority in neoantigen
identification; exposes flaws in predictive approaches
* CD8+ predictions (NetMHC, NetCTLpan, IEDB)
ATLAS identifies true neoantigens ATLAS challenges conventions
Of 202 identified tumor-specific mutations
Algorithms*
Predicted
ATLAS**
Found
Neoantigens
Inhibitory Antigens
False Positives
**ATLAS also identified CD4+ T cell antigens; algorithms do not model CD4+ T cell epitopes well;
Sample from NSCLC patient with long term response to ICB; collaboration with Tim Chan and Jedd Wolchok, MSKCC

14
Prioritizing neoantigens with pre-existing T cell responses
may increase probability of protective vaccination
Tumor-specific mutations
ATLAS output:
IFN-gamma pre-treatment responses
CD4+
CD8+
Actual neoantigens
Pre-existing immunity
associated with
effective
vaccination:
• Malignant glioma1
• HER-2/neu in
prostate cancer2
• Sarcoma NY-ESO-
1 vaccine3
1Yajima et al., 2005 Clin Cancer Res
2Voutsas et al., 2016 JITC
3 Pollack et al., 2017 ASCO poster

15
ATLAS: Don’t Guess. Know.
ATLAST cell antigen
T cell
Tumor Cell

Neoantigen vaccine program
GEN-009
16

Collect tumor and
blood samples,
sequence exome
ATLAS to identify
true neoantigens of
CD4+ and CD8+
T cell responses
Synthesize vaccine
with ATLAS-identified
T cell antigens
Ship personalized
product to clinical
site
ATLAS neoantigen selection drives potential
best-in-class personalized cancer vaccine program
17
GEN-009 composition:
Synthetic long
peptides + adjuvant
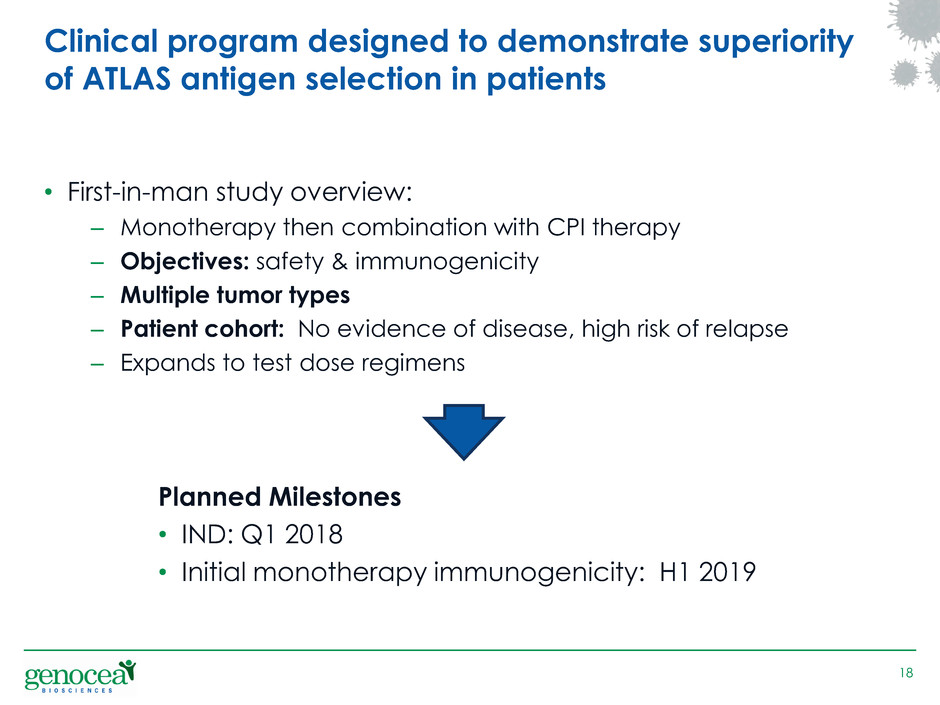
• First-in-man study overview:
– Monotherapy then combination with CPI therapy
– Objectives: safety & immunogenicity
– Multiple tumor types
– Patient cohort: No evidence of disease, high risk of relapse
– Expands to test dose regimens
Planned Milestones
• IND: Q1 2018
• Initial monotherapy immunogenicity: H1 2019
18
Clinical program designed to demonstrate superiority
of ATLAS antigen selection in patients

Investment Opportunity
19
20
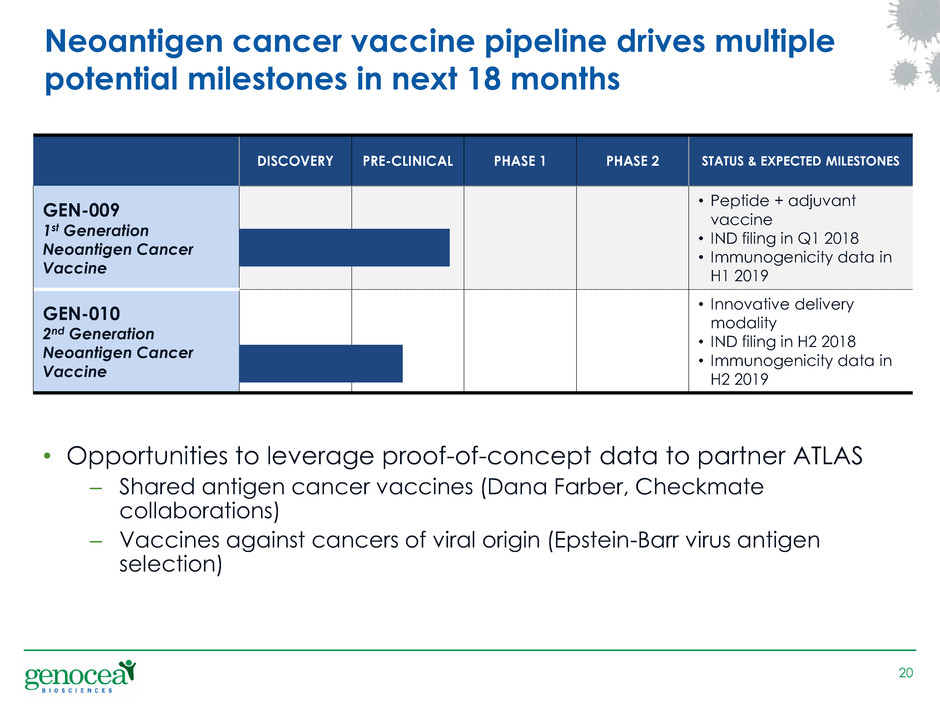
Neoantigen cancer vaccine pipeline drives multiple
potential milestones in next 18 months
DISCOVERY PRE-CLINICAL PHASE 1 PHASE 2 STATUS & EXPECTED MILESTONES
GEN-009
1st Generation
Neoantigen Cancer
Vaccine
• Peptide + adjuvant
vaccine
• IND filing in Q1 2018
• Immunogenicity data in
H1 2019
GEN-010
2nd Generation
Neoantigen Cancer
Vaccine
• Innovative delivery
modality
• IND filing in H2 2018
• Immunogenicity data in
H2 2019
• Opportunities to leverage proof-of-concept data to partner ATLAS
– Shared antigen cancer vaccines (Dana Farber, Checkmate
collaborations)
– Vaccines against cancers of viral origin (Epstein-Barr virus antigen
selection)

• SAB
– Elizabeth Jaffee, MD, Johns Hopkins, Deputy Director Sidney Kimmel
Comprehensive Cancer Center
• President, AACR; Chair, NCI Moonshot
– Chuck Drake, MD, PhD, Columbia, Director of Genitourinary
Oncology and Associate Director for Clinical Research
– Luis Diaz, MD, MSKCC, Head of Division of Solid Tumor Oncology
– Kwok Wong, MD, NYU, Chef of Hematology and Medical Oncology
– George Siber, MD, PhD, Former CSO Wyeth Vaccines
• Scientific founders:
– Darren Higgins, PhD, Harvard
– David Sinclair, PhD, Harvard
21
Strong science

• Compelling role for effective neoantigen vaccines in the I-O
revolution
• Genocea brings differentiated vaccine technology to bear to
create best-in-class vaccines
• Important milestones delivered over next 2 years
• Strong science, proven team
22
We are creating the leading next-generation cancer
vaccine company

• Cash at Q2 2017 – $35.2m
• $49m ATM facility capacity
• Debt facility – $15.8m outstanding
• Funded into middle of 2018
• Shares outstanding (08/07/17)
– Basic – 28.7m
– Fully diluted – 33.5m
• Exploring strategic alternatives for GEN-003
– Phase 3-ready genital herpes immunotherapy
23
Financial summary

Genocea Biosciences, Inc.
NASDAQ: GNCA
Cambridge Discovery Park
100 Acorn Park Drive
5th floor
Cambridge, MA 02140
USA
Phone: +1 617.876.8191
www.genocea.com
24
