Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Verastem, Inc. | a17-21987_28k.htm |
FORWARD-LOOKING STATEMENTS This presentation and other matters discussed today, or answers that may be given today, include forward-looking statements about Verastem’s strategy, future plans and prospects, including statements regarding the development and activity of Verastem’s investigational product candidates, including duvelisib and defactinib, and Verastem’s PI3K and FAK programs generally, the structure of our planned and pending clinical trials and the timeline and indications for clinical development. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Each forward-looking statement is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statement. Applicable risks and uncertainties include the risks that the full data from the DUO study will not be consistent with the top-line results of the study; that the preclinical testing of Verastem’s product candidates and preliminary or interim data from clinical trials may not be predictive of the results or success of ongoing or later clinical trials; that even if data from clinical trials is positive, regulatory authorities may require additional studies for approval and the product may not prove to be safe and effective; that the degree of market acceptance of product candidates, if approved, may be lower than expected; that the timing, scope, and rate of reimbursement for our product candidates is uncertain; that there may be competitive developments affecting our product candidates; that data may not be available when we expect it to be; that enrollment of clinical trials may take longer than expected; that our product candidates will cause unexpected safety events or result in an unmanageable safety profile as compared to their level of efficacy; that duvelisib will be ineffective at treating patients with lymphoid malignancies; that Verastem will be unable to successfully initiate or complete the clinical development of its product candidates; that the development of Verastem’s product candidates will take longer or cost more than planned; that Verastem may not have sufficient cash to fund its contemplated operations; that Verastem or Infinity Pharmaceuticals, Inc. (Infinity) will fail to fully perform under the duvelisib license agreement; that Verastem will not pursue or submit regulatory filings for its product candidates; and that Verastem’s product candidates will not receive regulatory approval, become commercially successful products, or result in new treatment options being offered to patients. Other risks and uncertainties include those identified under the heading “Risk Factors” in Verastem’s Annual Report on Form 10-K for the year ended December 31, 2016, and in any subsequent filings with the U.S. Securities and Exchange Commission. The forward-looking statements contained in this presentation reflect Verastem’s views as of the date of this presentation, and Verastem does not undertake and specifically disclaims any obligation to update any forward-looking statements. 2 Page Verastem, Inc.

VERASTEM AT A GLANCE SCIENTIFIC FOUNDATION Novel drugs targeting malignant cells both directly and through modulation of the tumor microenvironment VALUE DRIVERS Presentation of full DUO data Duvelisib NDA filing targeted 1H 2018 Clinical POC of FAK/I-O combinations in 2018 NASDAQ: VSTM DUVELISIB PI3K-δ,γ inhibitor DEFACTINIB FAK inhibitor Positive Phase 3 readout in R/R CLL/SLL Positive Phase 2 data in iNHL Potential applicability in other lymphoid malignancies Key collaborations exploring combination with leading immuno-oncology agents Page Verastem, Inc. 3
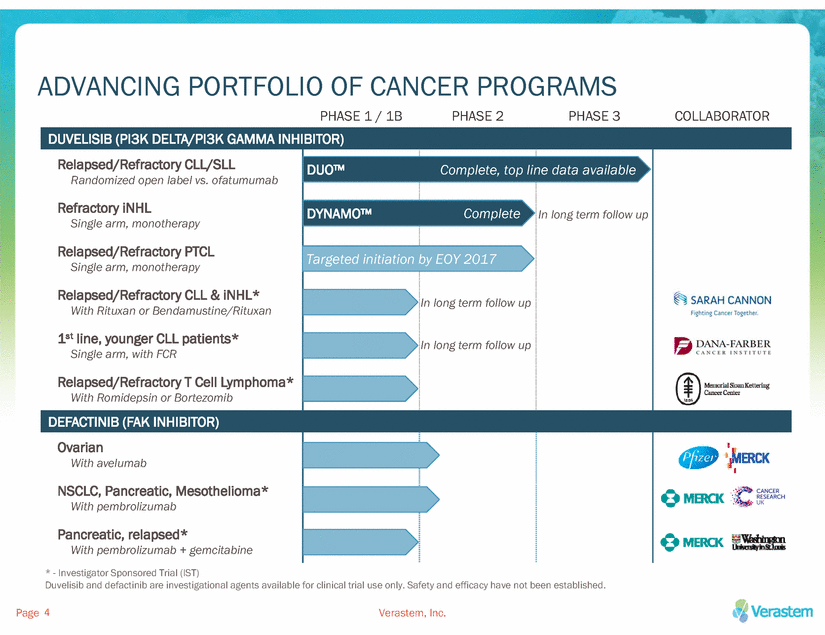
ADVANCING PORTFOLIO OF CANCER PROGRAMS PHASE 1 / 1B PHASE 2 PHASE 3 COLLABORATOR line data available * - Investigator Sponsored Trial (IST) Duvelisib and defactinib are investigational agents available for clinical trial use only. Safety and efficacy have not been established. Page Verastem, Inc. 4 DUVELISIB (PI3K DELTA/PI3K GAMMA INHIBITOR) Relapsed/Refractory CLL/SLL Randomized open label vs. ofatumumab Refractory iNHL Single arm, monotherapy Relapsed/Refractory PTCL Single arm, monotherapy Relapsed/Refractory CLL & iNHL* With Rituxan or Bendamustine/Rituxan 1st line, younger CLL patients* Single arm, with FCR Relapsed/Refractory T Cell Lymphoma* With Romidepsin or Bortezomib DUO™ DYNAMO™ Targeted initiation Complete, top Complete by EOY 2017 In long term follow up In long term follow up In long term follow up DEFACTINIB (FAK INHIBITOR) Ovarian With avelumab NSCLC, Pancreatic, Mesothelioma* With pembrolizumab Pancreatic, relapsed* With pembrolizumab + gemcitabine
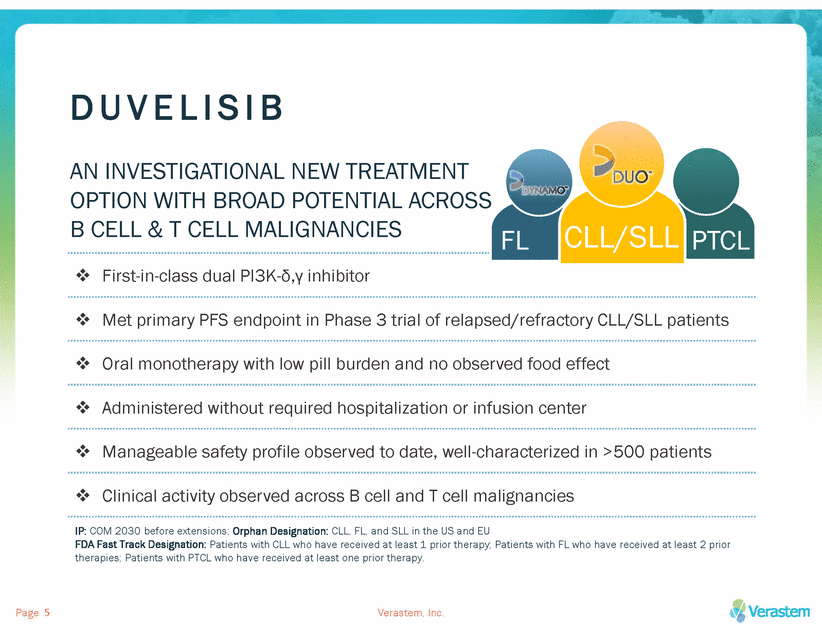
D U V E L I S I B AN INVESTIGATIONAL NEW TREATMENT OPTION WITH BROAD POTENTIAL ACROSS B CELL & T CELL MALIGNANCIES First-in-class dual PI3K-δ,γ inhibitor Met primary PFS endpoint in Phase 3 trial of relapsed/refractory CLL/SLL patients Oral monotherapy with low pill burden and no observed food effect Administered without required hospitalization or infusion center Manageable safety profile observed to date, well-characterized in >500 patients Clinical activity observed across B cell and T cell malignancies IP: COM 2030 before extensions; Orphan Designation: CLL, FL, and SLL in the US and EU FDA Fast Track Designation: Patients with CLL who have received at least 1 prior therapy; Patients with FL who have received at least 2 prior therapies; Patients with PTCL who have received at least one prior therapy. Page Verastem, Inc. 5
DUO™: A POSITIVE PHASE 3 STUDY OF DUVELISIB IN RELAPSED/REFRACTORY CHRONIC LYMPHOCYTIC LEUKEMIA POSITIVE PHASE 3 STUDY, TOP LINE DATA ANNOUNCED SEPTEMBER 2017 Treatment arm 1 Study end points Duvelisib 25 mg BID • Primary: ‒ Progression-free survival (PFS) Secondary: Relapsed or Refractory CLL/SLL patients (N = 319) (Randomized 1:1) • ‒ ‒ ‒ ‒ Overall response rate (ORR) Overall survival (OS) Duration of response (DOR) Safety Treatment arm 2 Ofatumumab Dosage and administration consistent with approved prescribing information* First Phase 3 trial showing PI3K inhibitor monotherapy efficacy in CLL/SLL * 8 weekly infusions, starting with an initial IV dose of 300 mg ofatumumab on Day 1 followed by 7 weekly doses of 2,000 mg. Thereafter, 2,000 mg ofatumumab monthly for 4 months. Page Verastem, Inc. 6
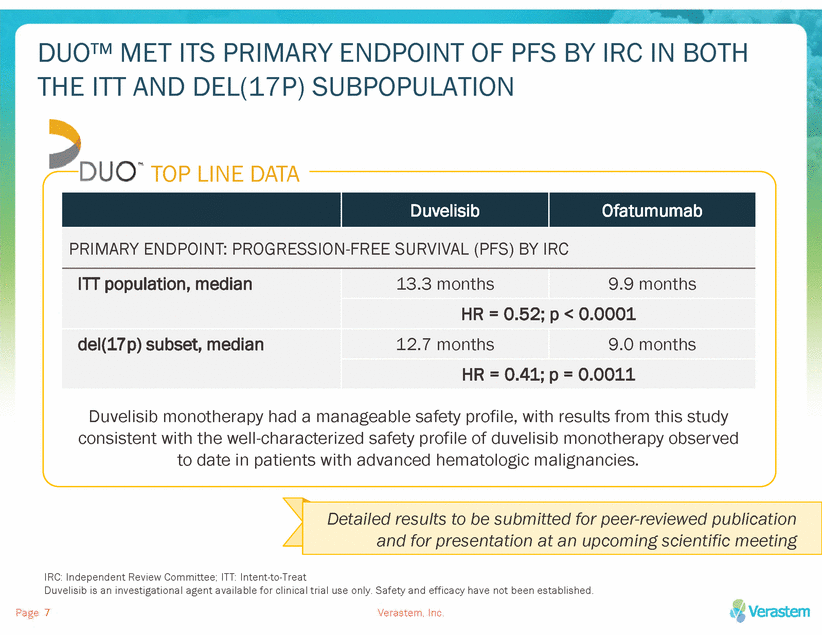
DUO™ MET ITS PRIMARY ENDPOINT OF PFS BY IRC IN BOTH THE ITT AND DEL(17P) SUBPOPULATION TOP LINE DATA Duvelisib monotherapy had a manageable safety profile, with results from this study consistent with the well-characterized safety profile of duvelisib monotherapy observed to date in patients with advanced hematologic malignancies. IRC: Independent Review Committee; ITT: Intent-to-Treat Duvelisib is an investigational agent available for clinical trial use only. Safety and efficacy have not been established. Page Verastem, Inc. 7 Detailed results to be submitted for peer-reviewed publication and for presentation at an upcoming scientific meeting Duvelisib Ofatumumab PRIMARY ENDPOINT: PROGRESSION-FREE SURVIVAL (PFS) BY IRC ITT population, median 13.3 months 9.9 months HR = 0.52; p < 0.0001 del(17p) subset, median 12.7 months 9.0 months HR = 0.41; p = 0.0011
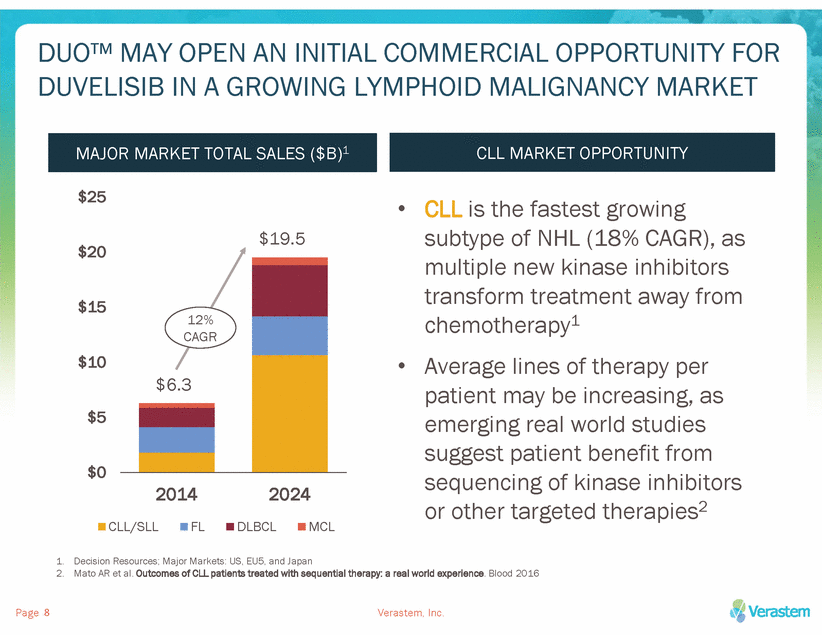
DUO™ MAY OPEN AN INITIAL COMMERCIAL OPPORTUNITY FOR DUVELISIB IN A GROWING LYMPHOID MALIGNANCY MARKET $25 • CLL is the fastest growing subtype of NHL (18% CAGR), as multiple new kinase inhibitors transform treatment away from chemotherapy1 Average lines of therapy per patient may be increasing, as emerging real world studies suggest patient benefit from sequencing of kinase inhibitors or other targeted therapies2 $19.5 $20 $15 $10 • $5 $0 2014 2024 CLL/SLL FL DLBCL MCL 1. 2. Decision Resources; Major Markets: US, EU5, and Japan Mato AR et al. Outcomes of CLL patients treated with sequential therapy: a real world experience. Blood 2016 Page Verastem, Inc. 8 12% CAGR $6.3 MAJOR MARKET TOTAL SALES ($B)1 CLL MARKET OPPORTUNITY
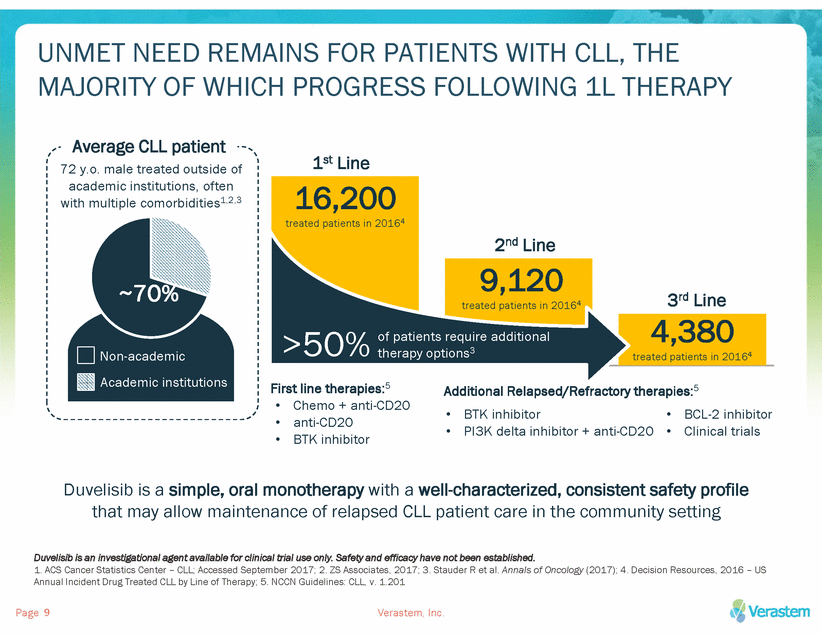
UNMET NEED REMAINS FOR PATIENTS WITH CLL, THE MAJORITY OF WHICH PROGRESS FOLLOWING 1L THERAPY Average CLL patient 1st Line 72 y.o. male treated outside of academic institutions, often with multiple comorbidities1,2,3 First line therapies:5 Additional Relapsed/Refractory therapies:5 • • • Chemo + anti-CD20 anti-CD20 BTK inhibitor • • BTK inhibitor PI3K delta inhibitor + anti-CD20 • • BCL-2 inhibitor Clinical trials Duvelisib is a simple, oral monotherapy with a well-characterized, consistent safety profile that may allow maintenance of relapsed CLL patient care in the community setting Duvelisib is an investigational agent available for clinical trial use only. Safety and efficacy have not been established. 1. ACS Cancer Statistics Center – CLL; Accessed September 2017; 2. ZS Associates, 2017; 3. Stauder R et al. Annals of Oncology (2017); 4. Decision Resources, 2016 – US Annual Incident Drug Treated CLL by Line of Therapy; 5. NCCN Guidelines: CLL, v. 1.201 Page Verastem, Inc. 9 16,200 treated patients in 20164 2nd Line 9,120 treated patients in 20164 3rd Line 4,380 treated patients in 20164
PHYSICIANS ENVISION A CHEMO-FREE FUTURE FOR CLL PATIENTS DRIVEN BY ADDITIONAL TARGETED ORAL MONOTHERAPY OPTIONS US oncologist opinion, academic (n = 16) and non-academic (n = 61-63): Disagree (1-3) Neither Agree nor Disagree (4) Agree (5-7) “In the future, many CLL patients will be treated with targeted agents, and without the need for chemotherapy” “Multiple targeted approaches will be needed to realize a chemo-free future” “There is a need for additional oral treatment options for patients who are not able to tolerate Imbruvica” “Patients prefer an oral treatment regimen to one requiring oral and IV therapy” “Agents targeting PI3K will play an important role in the future treatment of CLL patients” Source: ZS ATU & Chart Audit (W5 - Q1 2017) Page Verastem, Inc. 10 17% 80% 16% 82% 9% 17% 73% 5% 17% 77% 6% 21% 73%
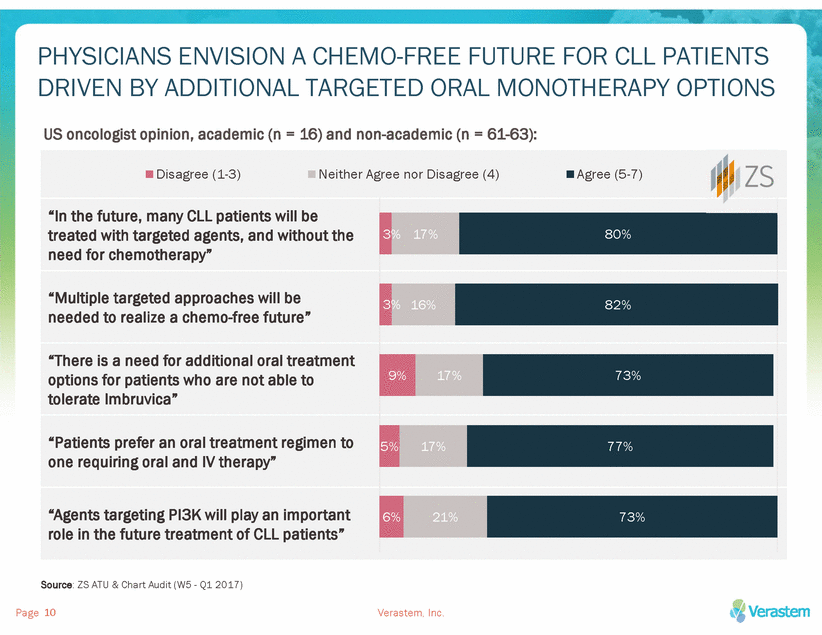
AN INCREASE IN THERAPY OPTIONS OFFERS CLL PATIENTS OPPORTUNITY FOR MORE PERSONALIZED TREATMENT PLANS Imbruvica® (ibrutinib) Zydelig® (idelalisib) + Rituxan (rituximab) Venclexta™ (venetoclax) WEEK 2 DAILY Rituxan® DOSE “IMBRUVICA may increase the risk of hemorrhage in patients receiving antiplatelet or anticoagulant therapies and patients should be monitored for signs of bleeding.” 4 DAILY “Atrial fibrillation and atrial flutter (range, 6 to 9%) have occurred in patients treated with IMBRUVICA, particularly in patients with cardiac risk factors, hypertension, acute infections, and a previous history of atrial fibrillation.” Hospitalization required for IV hydration & blood chemistry monitoring “Relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients for whom rituximab alone would be considered appropriate therapy due to other co-morbidities.” Consider inpatient blood chemistry monitoring during ramp up for patients with reduced renal function and comorbidities; Otherwise, outpatient monitoring required ! Outpatient monitoring required during ramp up Venclexta® induction requires hydration, dose titration, and careful monitoring for TLS, with hospitalization required for the ~1/3 of R/R CLL patients with high tumor burden. Zydelig® is approved only in combination with IV rituximab, increasing the cost and complexity of treatment for patients. A subset of patients are not eligible for or intolerant to BTK inhibitors due to co-morbidities or risk factors. Sources: Ibrutinib product insert; Venclexta dosing and administration guide; ZS ATU & Chart Audit (W5 - Q1 2017); Zydelig product insert Page Verastem, Inc. 11 W A RNINGS & PRECA U TIONS INDICA TION 1.5-2L WATER DAILY 26% Low Tumor Burden 36% Medium Tumor Burden ALLOPURINOL 5+ DAILY 32% High Tumor Burden 3DAILY Zydelig® DAILY Rituxan® INITIAL 375 mg/m2 Rituxan® 500 mg/m2 DOSES 2 – 5: BI-WEEKLY DOSES 6 – 9: MONTHLY 1DAILY DAILY
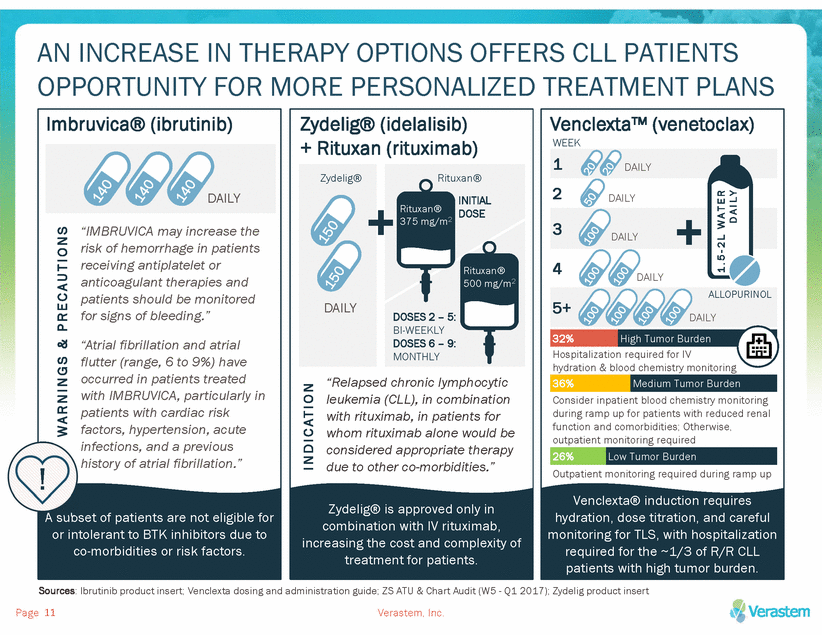
DUVELISIB TARGETED IS WELL-POSITIONED TO BE A DIFFERENTIATED THERAPY OPTION FOR R/R CLL PATIENTS D U V E L I S I B Novel, dual kinase inhibitor with an alternative safety profile to currently approved oral monotherapies Simple, at home, oral monotherapy dosing enables convenient treatment in the community setting May be appropriate for R/R CLL patients regardless of tumor burden or del(17p) status Page Verastem, Inc. 12
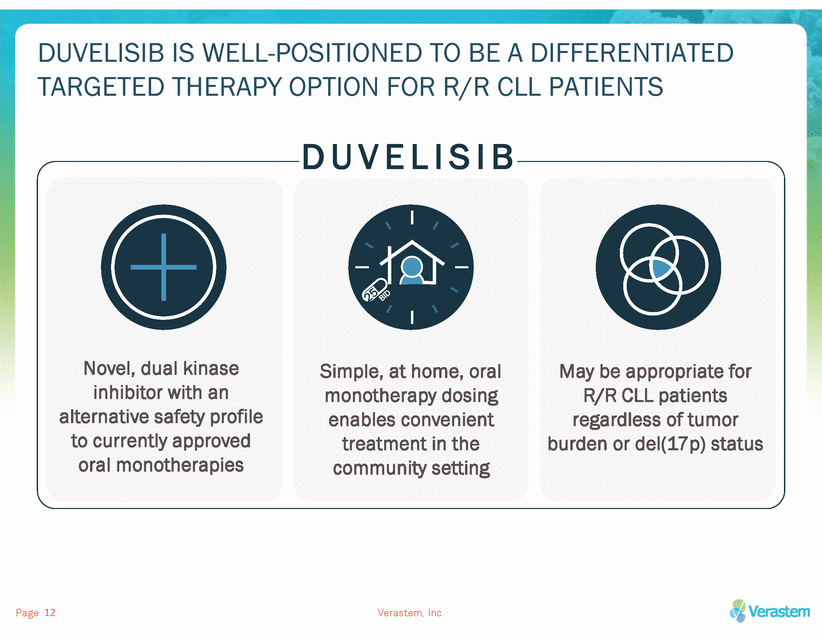
POSITIVE TOP-LINE RESULTS FROM THE PHASE 3 DUO™ STUDY ESTABLISH THE FOUNDATION FOR A POTENTIAL HEMATOLOGICAL FRANCHISE The positive DUO™ study supports: Pursuit of first FDA approval: duvelisib monotherapy in R/R CLL/SLL NDA filing targeted 1H 2018 Establishment of US commercial capability Planned partnership Ex-US Initiation of multiple company-sponsored & investigator-sponsored trials for expansion of duvelisib market potential DUVELISIB MONOTHERAPY FOR R/R CLL/SLL Page Verastem, Inc. 13
DYNAMOTM: A PHASE 2 STUDY OF DUVELISIB MONOTHERAPY IN DOUBLE REFRACTORY iNHL POPULATIONS PHASE 2 STUDY, FINAL ANALYSIS COMPLETED Study end points • Primary: Overall response rate (ORR) by Independent Review Committee (IRC) Double refractory* iNHL patients (N = 129) Duvelisib 25 mg BID • Key secondary: ‒ ‒ Safety Duration of response (DOR) Progression-free survival (PFS) Overall survival (OS) ‒ ‒ * Heavily pretreated patient population: • • Median number of prior treatments = 3 Inclusion criteria: Refractory to both rituximab (R) and a chemotherapy regimen or radioimmunotherapy (RIT) Page Verastem, Inc. 14 Accrual complete November 2015 Final analysis (April 2016) presented at ASH 2016 Mature follow up (March 2017) presented at ICML 2017
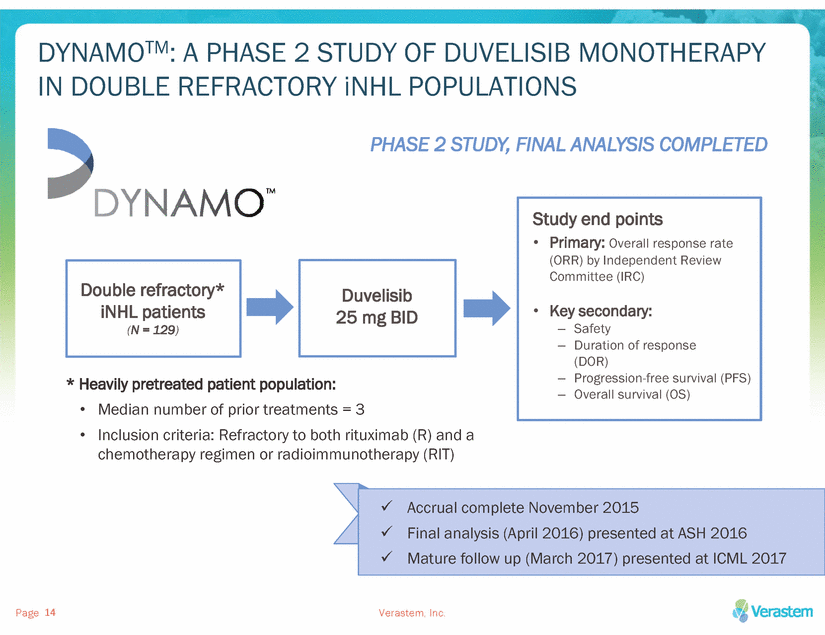
DYNAMO™ MET ITS PRIMARY ENDPOINT OF ORR BY IRC IN DOUBLE REFRACTORY iNHL PATIENTS AT FINAL ANALYSIS Primary endpoint: ORR per IRC at mature follow up 80% 70% 60% 50% 40% 30% 20% 10% 0% •ORR by IRC at per-protocol final analysis: (p=0.0001) 68% 43% Secondary endpoints, at mature follow-up by IRC: •Median PFS on duvelisib: 9.0 months •Median DOR: 10 months ITT (iNHL; n=129) Follicular Lymphoma (FL; n=83) Small Lymphocytic Lymphoma (SLL; n=28) Marginal Zone Lymphoma (MZL; n=18) Zinzani et al., ICML 2017 Duvelisib is an investigational agent available for clinical trial use only. Safety and efficacy have not been established. Verastem, Inc. Page 15 Overall Response Rate (%) 47% 33%
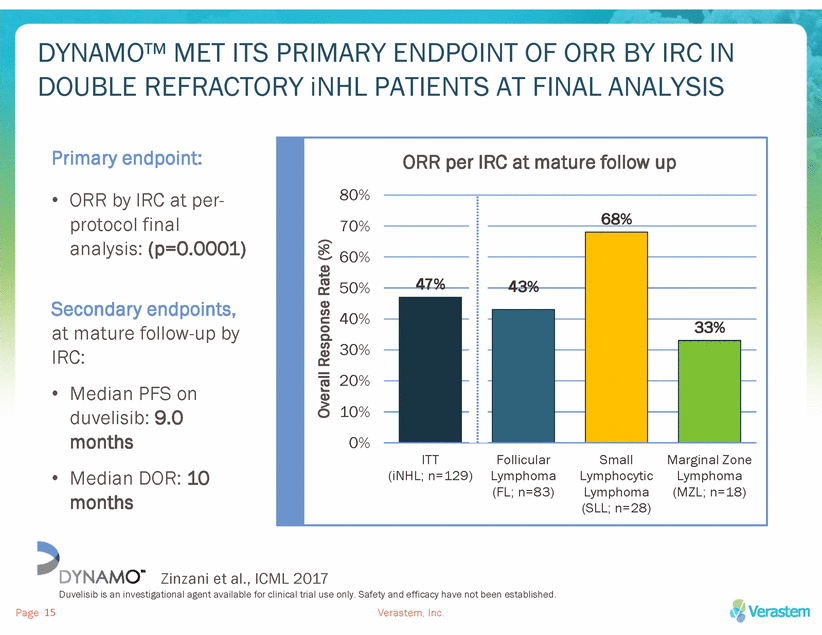
AT MATURE FOLLOW UP, 88% OF PATIENTS ON DYNAMO™ HAD REDUCTION IN TARGET LYMPH NODES BY IRC 88% of patients had reduction in target lymph nodes (IRC data) Zinzani et al., ICML 2017 Duvelisib is an investigational agent available for clinical trial use only. Safety and efficacy have not been established. Verastem, Inc. Page 16
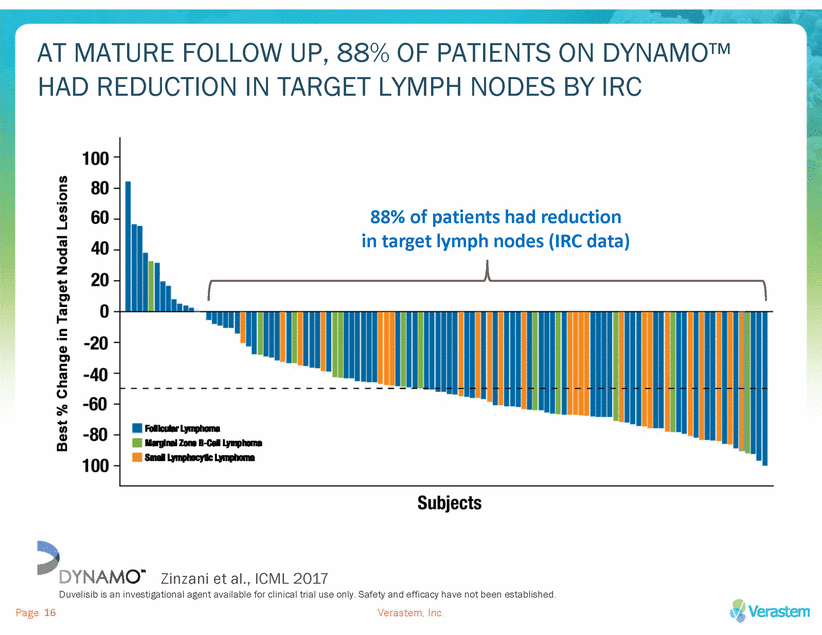
DUVELISIB HAS SHOWN A GENERALLY WELL TOLERATED, MANAGEABLE SAFETY PROFILE WITH APPROPRIATE RISK MITIGATION ADVERSE EVENTS OF INTEREST, IRC data on mature follow up • • • Few discontinuations due to severe AEs of interest Serious opportunistic infections < 4%: PCP (unconfirmed) (n=1); CMV (n=2); fungal pneumonia (n=2) Deaths attributed to treatment (n=6)* *colitis (n=1); toxic epidermal necrolysis/sepsis syndrome (n=1); drug reaction/eosinophilia/systemic symptoms (n=1); pneumonitis/pneumonia (n=1); viral infection (n=1); septic shock (n=1) Zinzani et al., ICML 2017 Duvelisib is an investigational agent available for clinical trial use only. Safety and efficacy have not been established. Verastem, Inc. Page 17
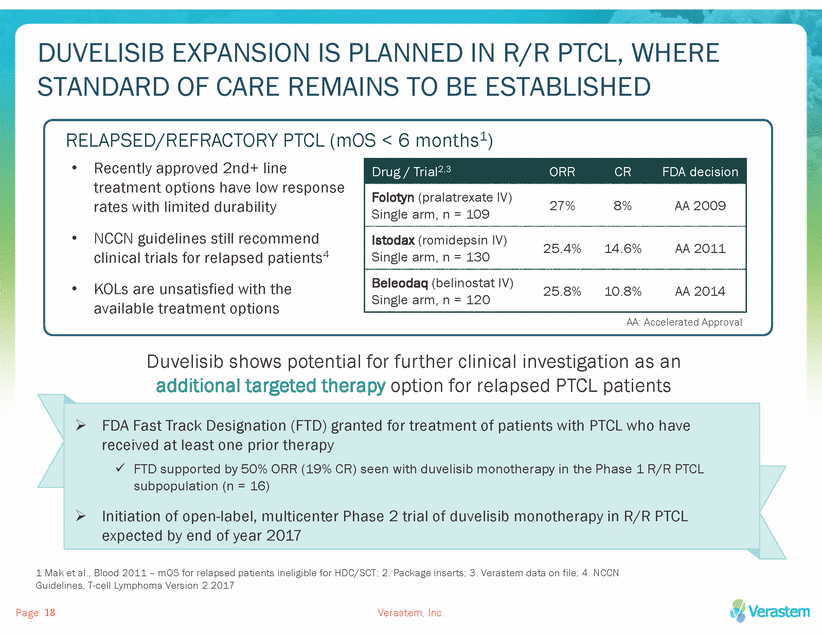
DUVELISIB EXPANSION IS PLANNED IN R/R PTCL, WHERE STANDARD OF CARE REMAINS TO BE ESTABLISHED RELAPSED/REFRACTORY PTCL (mOS < 6 months1) • Recently approved 2nd+ line treatment options have low response rates with limited durability NCCN guidelines still recommend clinical trials for relapsed patients4 KOLs are unsatisfied with the available treatment options 27% 8% AA 2009 Single arm, n = 109 • • AA: Accelerated Approval Duvelisib shows potential for further clinical investigation as an additional targeted therapy option for relapsed PTCL patients 1 Mak et al., Blood 2011 – mOS for relapsed patients ineligible for HDC/SCT; 2. Package inserts; 3. Verastem data on file; 4. NCCN Guidelines, T-cell Lymphoma Version 2.2017 Page Verastem, Inc. 18 FDA Fast Track Designation (FTD) granted for treatment of patients with PTCL who have received at least one prior therapy FTD supported by 50% ORR (19% CR) seen with duvelisib monotherapy in the Phase 1 R/R PTCL subpopulation (n = 16) Initiation of open-label, multicenter Phase 2 trial of duvelisib monotherapy in R/R PTCL expected by end of year 2017 Drug / Trial2,3ORRCRFDA decision Folotyn (pralatrexate IV) Istodax (romidepsin IV) Single arm, n = 13025.4%14.6%AA 2011 Beleodaq (belinostat IV)25.8%10.8%AA 2014 Single arm, n = 120
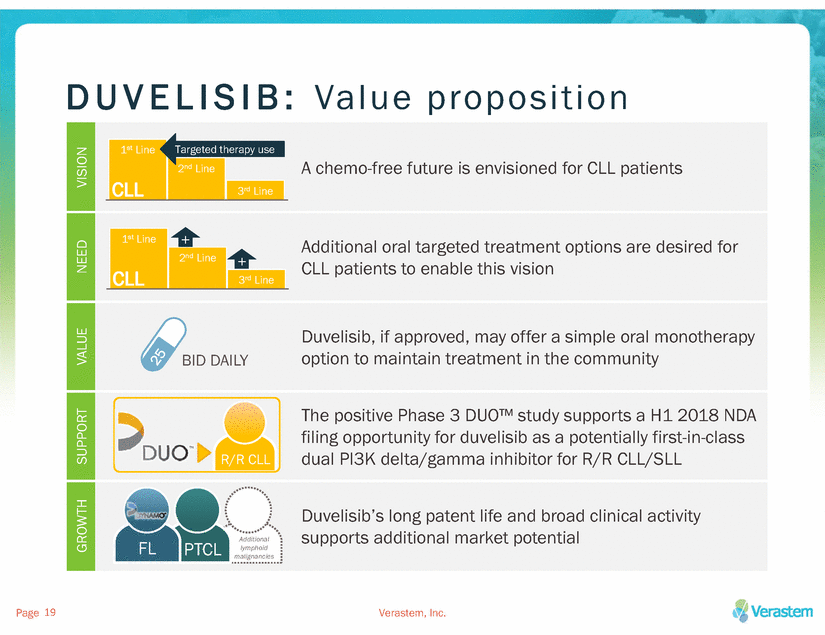
D U V E L I S I B :Va luepr oposition VISION A chemo-free future is envisioned for CLL patients NEED +CLL patients to enable this vision VALUE Duvelisib, if approved, may offer a simple oral monotherapy option to maintain treatment in the community SUPPORT filing opportunity for duvelisib as a potentially first-in-class dual PI3K delta/gamma inhibitor for R/R CLL/SLL GROWTH Duvelisib’s long patent life and broad clinical activity supports additional market potential Page 19 Verastem, Inc.
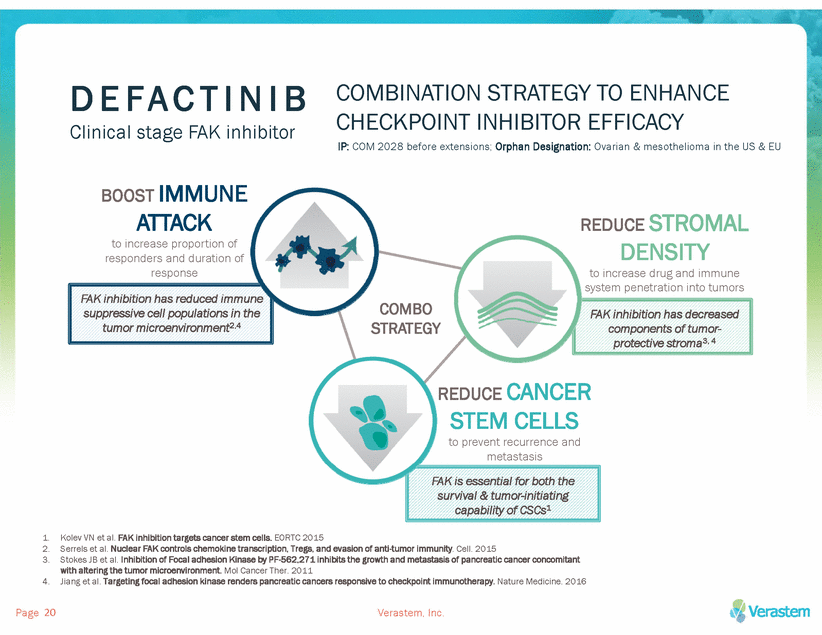
D E F A C T I N I B COMBINATION STRATEGY TO ENHANCE CHECKPOINT INHIBITOR EFFICACY IP: COM 2028 before extensions; Orphan Designation: Ovarian & mesothelioma in the US & EU Clinical stage FAK inhibitor BOOST IMMUNE ATTACK to increase proportion of responders and duration of response REDUCE STROMAL DENSITY to increase drug and immune system penetration into tumors FAK inhibition has reduced immune suppressive cell populations in the tumor microenvironment2,4 COMBO STRATEGY FAK inhibition has decreased components of tumor-protective stroma3, 4 REDUCE CANCER STEM CELLS to prevent recurrence and metastasis FAK is essential for both the survival & tumor-initiating capability of CSCs1 1. 2. 3. Kolev VN et al. FAK inhibition targets cancer stem cells. EORTC 2015 Serrels et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell. 2015 Stokes JB et al. Inhibition of Focal adhesion Kinase by PF-562,271 inhibits the growth and metastasis of pancreatic cancer concomitant with altering the tumor microenvironment. Mol Cancer Ther. 2011 Jiang et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nature Medicine. 2016 4. Page Verastem, Inc. 20
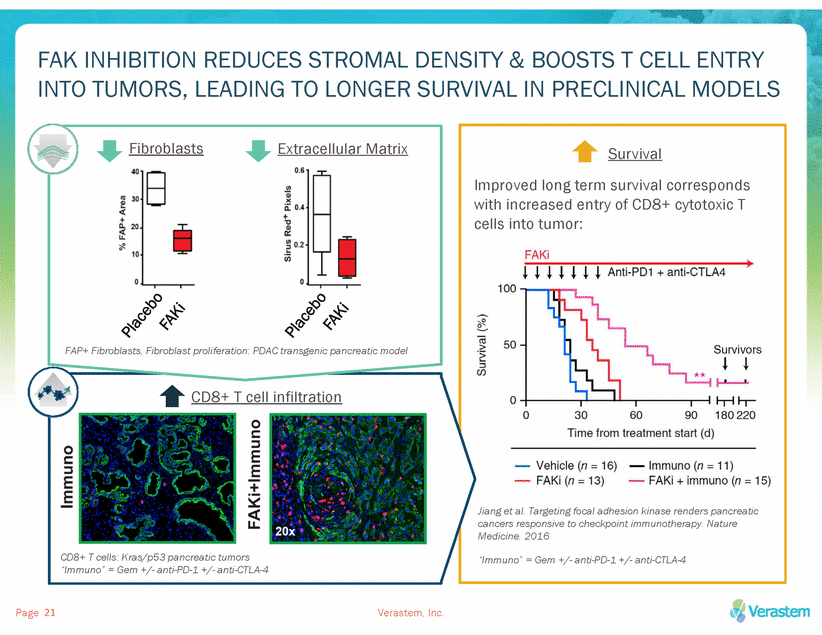
FAK INHIBITION REDUCES STROMAL DENSITY & BOOSTS T CELL ENTRY INTO TUMORS, LEADING TO LONGER SURVIVAL IN PRECLINICAL MODELS Fibroblasts Extracellular Matrix Survival Improved long term survival corresponds with increased entry of CD8+ cytotoxic T cells into tumor: FAP+ Fibroblasts, Fibroblast proliferation: PDAC transgenic pancreatic model CD8+ T cell infiltration Jiang et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nature Medicine. 2016 CD8+ T cells: Kras/p53 pancreatic tumors “Immuno” = Gem +/-anti-PD-1 +/-anti-CTLA-4 “Immuno” = Gem +/-anti-PD-1 +/-anti-CTLA-4 Page Verastem, Inc. 21
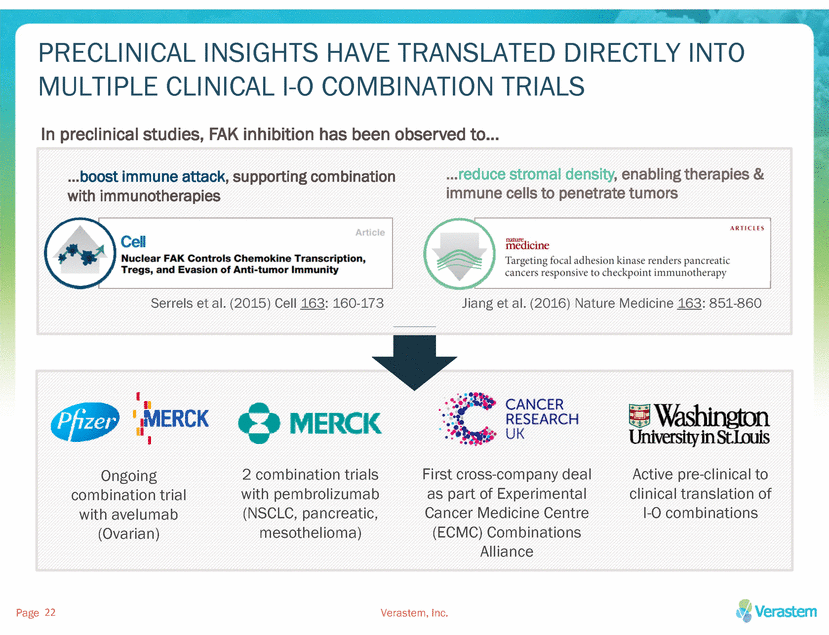
PRECLINICAL INSIGHTS HAVE TRANSLATED DIRECTLY INTO MULTIPLE CLINICAL I-O COMBINATION TRIALS In preclinical studies, FAK inhibition has been observed to… …reduce stromal density, enabling therapies & immune cells to penetrate tumors …boost immune attack, supporting combination with immunotherapies Serrels et al. (2015) Cell 163: 160-173 Jiang et al. (2016) Nature Medicine 163: 851-860 First cross-company deal as part of Experimental Cancer Medicine Centre (ECMC) Combinations Alliance Active pre-clinical to clinical translation of I-O combinations 2 combination trials with pembrolizumab (NSCLC, pancreatic, mesothelioma) Ongoing combination trial with avelumab (Ovarian) Page Verastem, Inc. 22
EXECUTIVE MANAGEMENT Robert Forrester President/CEO, BOD CEO/CFO - CombinatoRx, COLY MeesPierson, Barclays, UBS Daniel Paterson Chief Operating Officer CEO - The DNA Repair Co. (now On-Q-ity) PharMetrics (now IMS), Axion Julie Feder Chief Financial Officer CFO, Clinton Health Access Initiative VP, Finance, Genzyme Steven Bloom Senior Vice President, Corporate Development SVP Commercial Strategy and Business Development - Ziopharm PharMetrics (now IMS) , Eli Lilly and Company Jonathan Pachter, Ph.D. Chief Scientific Officer Head of Cancer Biology - OSI (now Astellas) Schering-Plough (now Merck) Hagop Youssoufian, M.Sc., M.D. Head of Hematology and Oncology Development CMO - BIND Therapeutics Progenics, Ziopharm, ImClone Sprycel®, Taxotere® and Erbitux® Cathy Carew Vice President, Human Resources Principal - HR Collaborative Ironwood, ActiveBiotics, Dynogen, Tufts Health Plan Page Verastem, Inc. 23

BOARD OF DIRECTORS Michael Kauffman, M.D., Ph.D. Robert Forrester President/CEO Verastem (VSTM) Lead Director CEO Karyopharm (KPTI), former CMO Onyx Timothy Barberich Former CEO/Chair Sepracor (SEPR) Alison Lawton COO, Aura Biosciences, former Genzyme (now Sanofi) Louise Phanstiel BOD: Cedars Sinai, MYGN Eric Rowinsky, M.D. Former CMO ImClone Erbitux®, Taxotere®, Tarceva® Brian Stuglik, R.Ph. Former VP and Chief Marketing Officer – Oncology Global Marketing, Eli Lilly & Co. Bruce Wendel CSO Hepalink USA, former CEO Abraxis BioScience CLINICAL AND SCIENTIFIC ADVISORY BOARD Robert Weinberg, Ph.D. Co-founder & Chairman of CSAB Whitehead Institute/MIT Lori Kunkel, M.D. BOD – Loxo Oncology Former Executive/CMO – Pharmacyclics, Proteolix, Xencor Greg I. Berk, M.D. Former Executive/CMO – Verastem, Sideris, BIND Intellikine, Abraxis Biosciences Edmund J. Pezalla, M.D., Ph.D. Former VP – Pharmaceutical Policy and Strategy at Aetna Scholar in Residence – Duke-Margolis Health Policy Center Cheryl Cohen BOD – Tokai, Protein Sciences, Vital Former CCO – Medivation (Xtandi®) Steve Sherwin, M.D. UCSF, San Francisco General Hospital Director – Biogen Idec, Neurocrine Biosciences, Rigel Paul Friedman, M.D. CEO Madrigal (SNTA), Former President/CEO Incyte (INCY) Max Wicha, M.D. Director – University of Michigan Comprehensive Cancer Center Page Verastem, Inc. 24
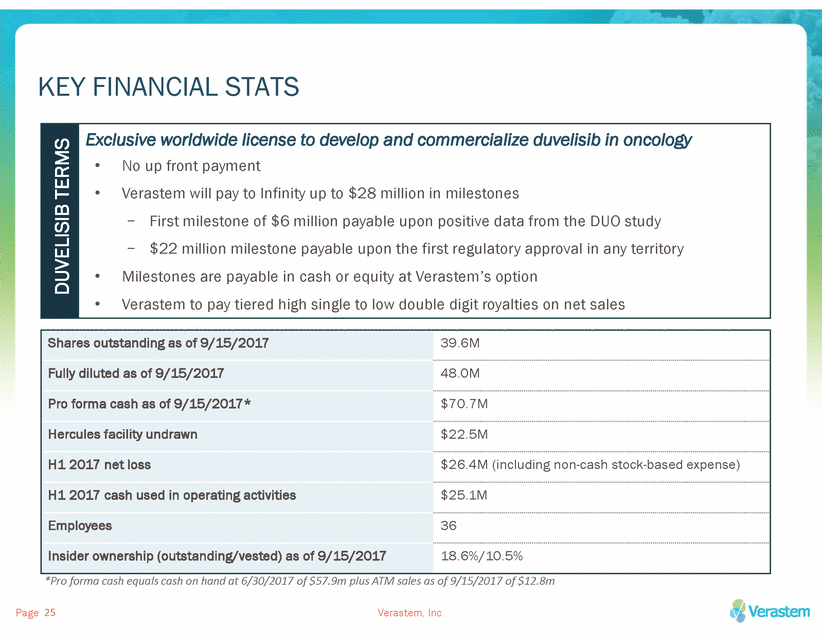
KEY FINANCIAL STATS DUVELISIB TERMS •No up front payment •Verastem will pay to Infinity up to $28 million in milestones -First milestone of $6 million payable upon positive data from the DUO study -$22 million milestone payable upon the first regulatory approval in any territory •Milestones are payable in cash or equity at Verastem’s option •Verastem to pay tiered high single to low double digit royalties on net sales Shares outstanding as of 9/15/201739.6M Fully diluted as of 9/15/201748.0M Pro forma cash as of 9/15/2017*$70.7M Hercules facility undrawn$22.5M H1 2017 net loss$26.4M (including non-cash stock-based expense) H1 2017 cash used in operating activities$25.1M Employees36 Insider ownership (outstanding/vested) as of 9/15/201718.6%/10.5% *Pro forma cash equals cash on hand at 6/30/2017 of $57.9m plus ATM sales as of 9/15/2017 of $12.8m Page 25 Verastem, Inc.
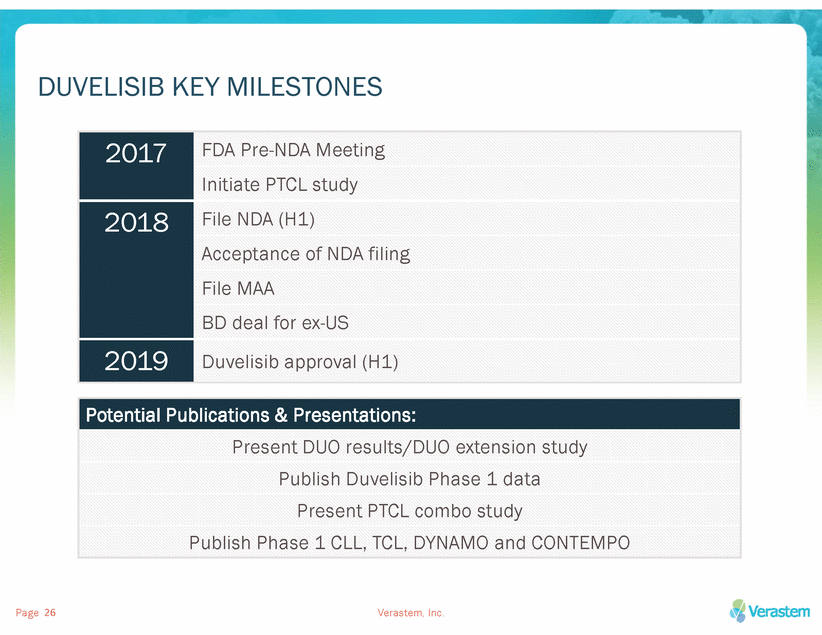
DUVELISIB KEY MILESTONES Page Verastem, Inc. 26 Potential Publications & Presentations: Present DUO results/DUO extension study Publish Duvelisib Phase 1 data Present PTCL combo study Publish Phase 1 CLL, TCL, DYNAMO and CONTEMPO 2017 FDA Pre-NDA Meeting Initiate PTCL study 2018 File NDA (H1) Acceptance of NDA filing File MAA BD deal for ex-US 2019 Duvelisib approval (H1)
VERASTEM AT A GLANCE SCIENTIFIC FOUNDATION Novel drugs targeting malignant cells both directly and through modulation of the tumor microenvironment VALUE DRIVERS Presentation of full DUO data Duvelisib NDA filing targeted 1H 2018 Clinical POC of FAK/I-O combinations in 2018 NASDAQ: VSTM DUVELISIB PI3K-δ,γ inhibitor DEFACTINIB FAK inhibitor Positive Phase 3 readout in R/R CLL/SLL Positive Phase 2 data in iNHL Potential applicability in other lymphoid malignancies Key collaborations exploring combination with leading immuno-oncology agents Page Verastem, Inc. 27