Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - ATOSSA THERAPEUTICS, INC. | s107500_ex99-1.htm |
| 8-K - 8-K - ATOSSA THERAPEUTICS, INC. | s107500_8k.htm |
Exhibit 99.2

Preliminary Topical Endoxifen Phase 1 Results All Objectives Successfully Met September 14, 2017

Forward - Looking Statements Some of the information presented herein may contain projections or other forward - looking statements regarding future events or the future financial performance of the Company which the Company undertakes no obligation to update. These statements are based on management’s current expectations and are subject to risks and uncertainties that may cause actual results to differ materially from the anticipated or estimated future results, including the risks and uncertainties associated with preliminary study results varying from final results, estimates of potential markets for drugs under development, clinical trials, actions by the FDA and other governmental agencies, regulatory clearances, responses to regulatory matters, the market demand for and acceptance of Atossa's products and services, performance of clinical research organizations and other risks detailed from time to time in Atossa's filings with the Securities and Exchange Commission, including without limitation its most recent annual report on form 10 - K, subsequent quarterly reports on Forms 10 - Q and Forms 8 - K, each as amended and supplemented from time to time . 107 Spring St. Seattle WA 98104 2

DISCUSSION TOPICS • Atossa Genetics Overview • Endoxifen • Phase 1 Study • Safety Summary • Tolerability Summary • Pharmacokinetic Summary • Upcoming Milestones 107 Spring St. Seattle WA 98104 3

Atossa Genetics Overview Atossa Genetics, Inc. 1616 Eastlake Ave, Seattle, WA 98102 4

Atossa Genetics: • Clinical - stage • Novel drugs & delivery methods • Breast Cancer & other breast conditions • Headquartered in Seattle, WA 107 Spring St. Seattle WA 98104 5

Endoxifen 107 Spring St. Seattle WA 98104 6

Endoxifen • Most active metabolite of tamoxifen • Tamoxifen has been widely studied • Tamoxifen was first approved in 1977 107 Spring St. Seattle WA 98104 7 Tamoxifen Metabolites, including Endoxifen

Current Paradigm for Tamoxifen Use in US 107 Spring St. Seattle WA 98104 8 1 million breast cancer patients take oral tamoxifen for at least five years 10 million patients with a high risk of breast cancer are indicated for chemoprevention with oral tamoxifen Source: Freedman AN, Graubard BI, Rao SR, McCaskill - Stevens W, Ballard - Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526 – 32

Unmet Medical Needs Are >$1B Markets 107 Spring St. Seattle WA 98104 9 500,000 breast cancer patients don’t benefit from tamoxifen because of metabolism problems 9.9 million high risk patients won’t take oral tamoxifen because of actual or the risk of systemic side effects Source: Freedman AN, Graubard BI, Rao SR, McCaskill - Stevens W, Ballard - Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526 – 32

Emerging Unmet Medical Need • Breast Density – Independent risk for breast cancer – Patients required to be notified in 30 states – No approved treatment • Tamoxifen reduces breast density 107 Spring St. Seattle WA 98104 10

Mandatory Patient Notification of BD 107 Spring St. Seattle WA 98104 11 PINK : Enacted Law — RED: Introduced Bill — BLUE: Working on Bill — WHITE: No Action — BLACK : Insurance Coverage Law From: AreYouDense.org

Why Endoxifen? • Address unmet medical needs for: – Providing a topical alternative to oral tamoxifen for reducing breast density – Enh ancing the chemoprevention properties of tamoxifen in those patients who are refractory • Extensive characterization of tamoxifen, the parent drug 107 Spring St. Seattle WA 98104 12

107 Spring St. Seattle WA 98104 13 Phase 1 Study

Preliminary Phase 1 Topical Results • Study objectives achieved • Demonstrated: - Safety - Tolerability - Verification of transdermal delivery Supports continued development 107 Spring St. Seattle WA 98104 14

107 Spring St. Seattle WA 98104 15 Q2 2106 • Contracted to develop endoxifen API Q3 2016 • First batch of API for clinical use Q4 2016 • Topical formulation development in US • Retained CRO in Australia Q1 2017 • Retained CMO in Australia (for topical and oral presentations) Q2 2017 • Approval to start study • First cohort enrolled • Shipped drug product to CRO Q3 2017 • Last cohort completed

Preliminary Study Conclusions • All study objectives successfully achieved – Safety: There were no clinically significant safety signals and no clinically significant adverse events in participants receiving topical Endoxifen. – Tolerability: Topical Endoxifen was well tolerated at each dose level and for the dosing duration utilized in the study. – Pharmacokinetics: Topical Endoxifen crossed the skin barrier when applied daily to the breast, as demonstrated by low but measurable Endoxifen blood levels detected in a dose - dependent fashion. 107 Spring St. Seattle, WA 98104 16

Phase 1 Study Design PART A (Topical). Cohorts, dose levels and number of participants. Cohort Dose Level Number of Participants (mg per breast) (Total mg) (Z) - Endoxifen Placebo 1 1 2 6 2 2 3 6 6 2 3 5 10 6 2 PART B (Oral). Cohorts, dose levels and number of participants. Dose Level (mg/day) (Z) - Endoxifen Placebo 4 1 6 2 5 2 6 2 6 4 6 2 107 Spring St. Seattle WA 98104 17

Study Design Summary 107 Spring St. Seattle, WA 98104 18 • Two - part double - blinded, placebo controlled, dose escalation trial investigating the safety and pharmacokinetics of (Z) - Endoxifen in healthy female volunteers – Part A: Topical – liquid applied to the breasts – Part B: Capsule – taken orally • Single dose “sachet” (one per breast/day)

Topical Design Summary Cohort Dose Number of Participants mg per Breast Total mg (Z) - Endoxifen Placebo 1 1 2 6 2 2 3 6 6 2 3 5 10 6 2 107 Spring St. Seattle WA 98104 19 x Healthy females, 18 to 65 years of age x Body Mass Index of 18 to 30 x No chronic or acute disease x Daily administration diaries

Safety Summary 107 Spring St. Seattle WA 98104 20

Safety Summary • No safety signals observed in weekly assessments of/in: – Blood chemistry – Coagulation parameters – Hematology parameters – Urinalysis – Vital Signs – Heart – Physical Examinations 107 Spring St. Seattle WA 98104 21

Adverse Events • There were no Serious Adverse Events • Two subjects (one placebo and one active) experienced a Treatment Emergent Adverse Event of moderate severity and possibly drug - related – Headache – Nausea 107 Spring St. Seattle WA 98104 22

Tolerability Summary 107 Spring St. Seattle WA 98104 23
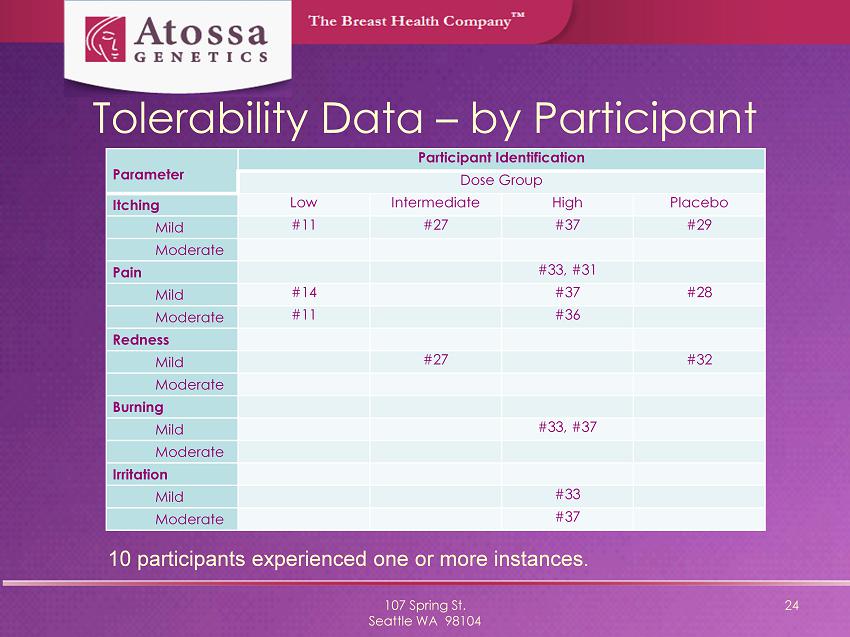
Tolerability Data – by Participant 107 Spring St. Seattle WA 98104 24 Parameter Participant Identification Dose Group Itching Low Intermediate High Placebo Mild #11 #27 #37 #29 Moderate Pain #33, #31 Mild #14 #37 #28 Moderate #11 #36 Redness Mild #27 #32 Moderate Burning Mild #33, #37 Moderate Irritation Mild #33 Moderate #37 10 participants experienced one or more instances.

Local Tolerability By Instances • A daily self - assessment of local tolerance was performed – 24 subjects for 28 days for a total of 672 daily assessments • Redness, Burning, Pain, Itching and Irritation were assessed each day • Scoring was None, Mild, Moderate or Severe for all five parameters 107 Spring St. Seattle , WA 98104 25
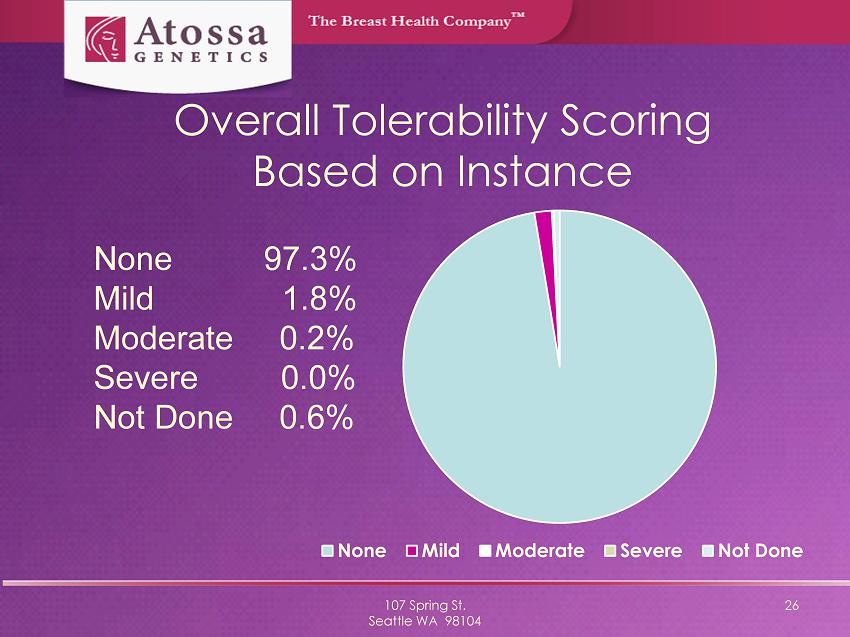
Overall Tolerability Scoring Based on Instance 107 Spring St. Seattle WA 98104 26 None Mild Moderate Severe Not Done None 97.3% Mild 1.8% Moderate 0.2% Severe 0.0% Not Done 0.6%

Parameter Summary by Instance • One subject had mild irritation 17/28 days and moderate irritation on the End of Study assessment 107 Spring St. Seattle , WA 98104 27 Parameter None Redness 99% Burning 99% Pain 98% Itching 97% Irritation 97%

In - person Interview • Each participant was interviewed every seven days for side - effect information • The participant at the highest dose who was bothered by side - effects was the same who reported topical irritation 107 Spring St. Seattle WA 98104 28 Result Cohort Not at All A little Bit Some - what Quite a Bit Very Much Not Done Low 6/6 Intermediate 6/6 High 3/6 2/6 1/6 Placebo 6/6 1/6

107 Spring St. Seattle WA 98104 29 Pharmacokinetic Summary

Pharmacokinetic Objective 107 Spring St. Seattle WA 98104 30 Formulation placed on skin surface Endoxifen absorbed into breast tissue to bind to ER and retard growth Breast blood vessels connect to systemic circulation taking “excess” endoxifen into systemic circulation Goal was to prove absorption with measurable blood levels that are low enough to limit systemic exposure

Endoxifen was detected in blood 107 Spring St. Seattle WA 98104 31 11 7 2 0 0 2 4 6 8 10 12 10 mg 6 mg 2 mg Placebo Blood Level > 2.0 ng/mL N umber of Samples

Preliminary Study Conclusions • All primary and secondary endpoints successfully met – Safety: There were no clinically significant safety signals and no clinically significant adverse events in participants receiving topical Endoxifen . – Tolerability: Topical Endoxifen was well tolerated at each dose level and for the dosing duration utilized in the study . – Pharmacokinetics: Topical Endoxifen crossed the skin barrier when applied daily to the breast, as demonstrated by low but measurable Endoxifen blood levels detected in a dose - dependent fashion. • Therefore, the topical Endoxifen is suitable for continued development 107 Spring St. Seattle, WA 98104 32

Up Coming Milestones • 2017 – Complete analysis of oral endoxifen Phase 1 data – Finalize Phase 2 Study design for topical endoxifen – Finalize Phase 2 Study design for oral endoxifen – Final report of combined topical and oral Phase 1 by CRO – IRB and government submission for Phase 2 – Advance both oral and topical formulations 107 Spring St. Seattle WA 98104 33

NASDAQ: ATOS
