Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Aldeyra Therapeutics, Inc. | d449398dex992.htm |
| 8-K - FORM 8-K - Aldeyra Therapeutics, Inc. | d449398d8k.htm |

Dry Eye Disease Phase 2a Results September 12, 2017 Exhibit 99.1

Disclaimers and Forward-Looking Statements This presentation and various remarks which may be made during this presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations, expenses and financing needs, business strategies and plans, research and development plans or expectations, trends, the structure, timing and success of Aldeyra’s planned or pending clinical trials, expected milestones, market sizing, pricing and reimbursement, competitive position, regulatory matters, industry environment and potential growth opportunities, among other things. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan” or similar expressions and the negatives of those terms. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These statements reflect Aldeyra’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the development and clinical plans for Aldeyra’s product candidates and Aldeyra’s continuing review and quality control analysis of clinical data. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Aldeyra’s Annual Report on Form 10-K for the year ended December 31, 2016 and Quarterly Report on Form 10-Q for the quarter ended June 30, 2017, which are on file with the Securities and Exchange Commission (SEC) and available on the SEC's website at www.sec.gov. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information in this presentation is provided only as of September 12, 2017, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law.

Dry Eye Disease Phase 2a Data Highlights Statistically significant and clinically relevant improvement in multiple dry eye disease signs and symptoms Rapid onset of activity within one week of dosing Improvement increased over time, and a modest dose-response was observed, supporting activity of ADX-102 Pro-inflammatory aldehyde levels reduced, supporting novel mechanism of ADX-102 Primary objective of trial achieved: 0.1% ADX-102, which demonstrated consistent statistically and clinically significant activity and was the best-tolerated formulation, selected to advance to Phase 2b testing No safety concerns observed
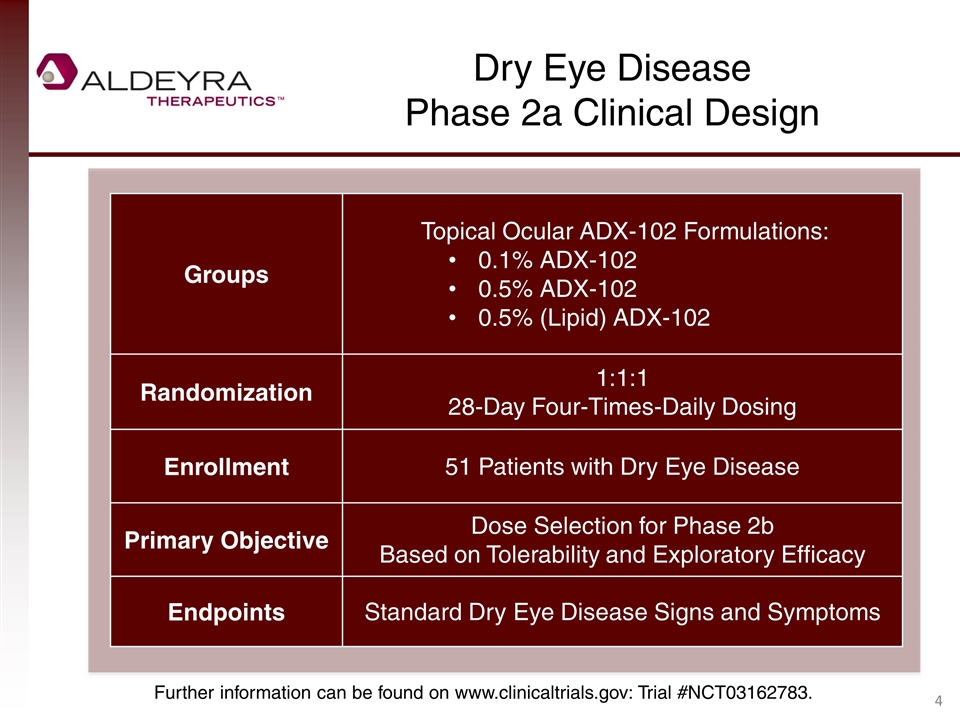
Dry Eye Disease Phase 2a Clinical Design Groups Topical Ocular ADX-102 Formulations: 0.1% ADX-102 0.5% ADX-102 0.5% (Lipid) ADX-102 Randomization 1:1:1 28-Day Four-Times-Daily Dosing Enrollment 51 Patients with Dry Eye Disease Primary Objective Dose Selection for Phase 2b Based on Tolerability and Exploratory Efficacy Endpoints Standard Dry Eye Disease Signs and Symptoms Further information can be found on www.clinicaltrials.gov: Trial #NCT03162783.
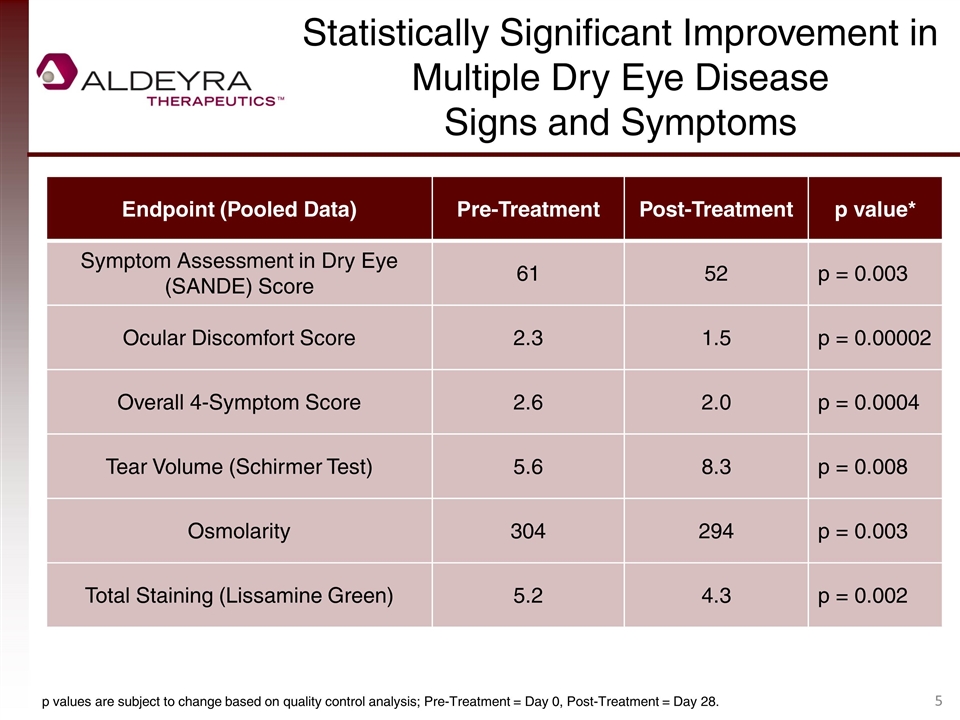
Statistically Significant Improvement in Multiple Dry Eye Disease Signs and Symptoms Endpoint (Pooled Data) Pre-Treatment Post-Treatment p value* Symptom Assessment in Dry Eye (SANDE) Score 61 52 p = 0.003 Ocular Discomfort Score 2.3 1.5 p = 0.00002 Overall 4-Symptom Score 2.6 2.0 p = 0.0004 Tear Volume (Schirmer Test) 5.6 8.3 p = 0.008 Osmolarity 304 294 p = 0.003 Total Staining (Lissamine Green) 5.2 4.3 p = 0.002 p values are subject to change based on quality control analysis; Pre-Treatment = Day 0, Post-Treatment = Day 28.
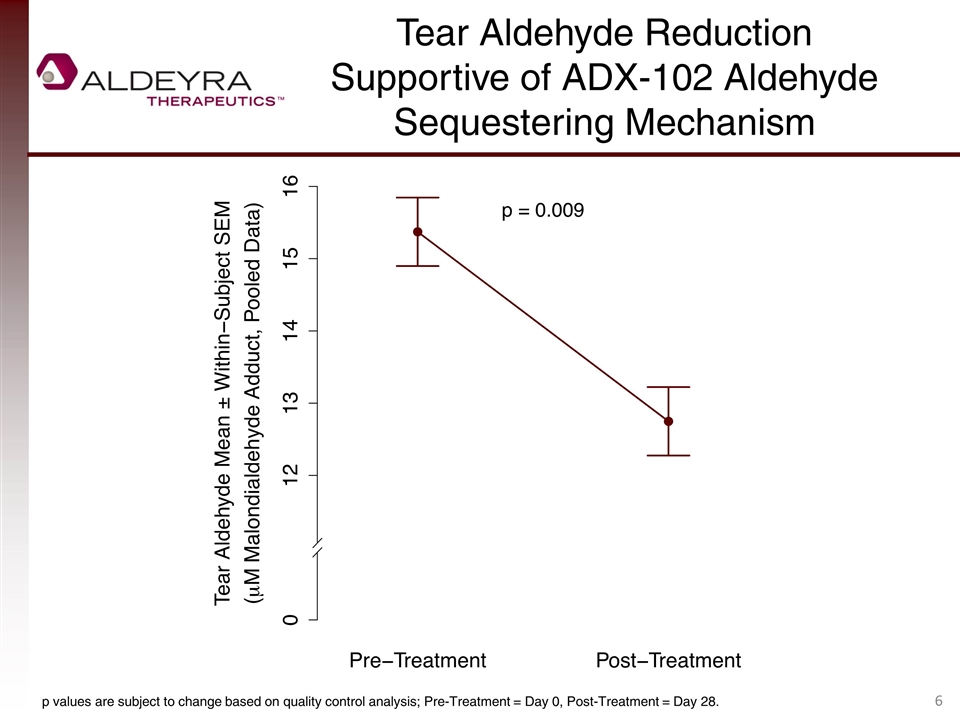
Tear Aldehyde Reduction Supportive of ADX-102 Aldehyde Sequestering Mechanism p values are subject to change based on quality control analysis; Pre-Treatment = Day 0, Post-Treatment = Day 28.
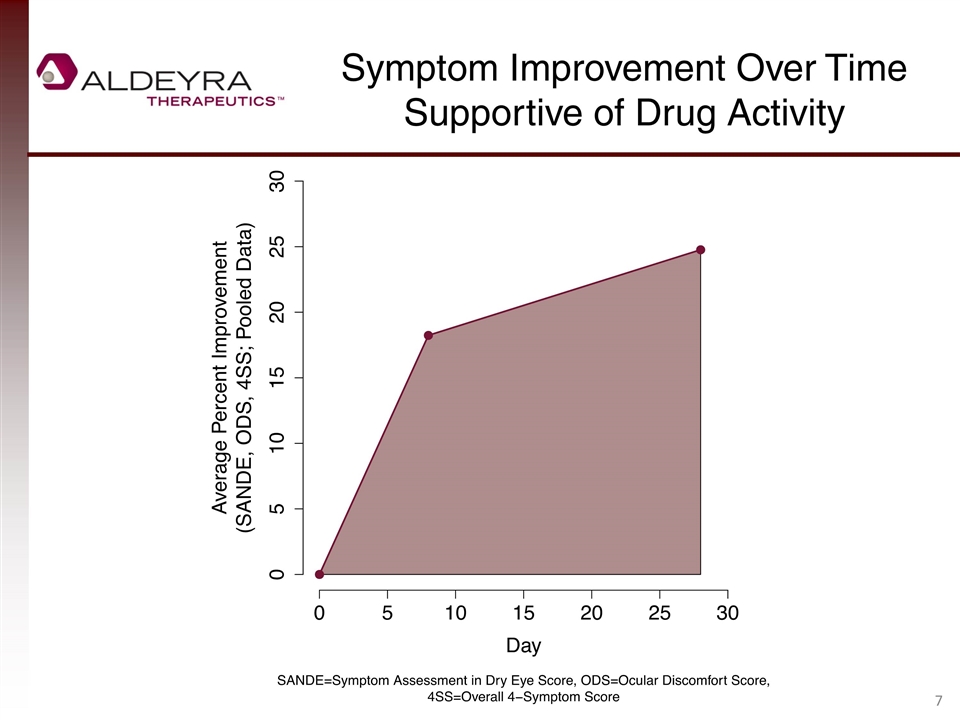
Symptom Improvement Over Time Supportive of Drug Activity
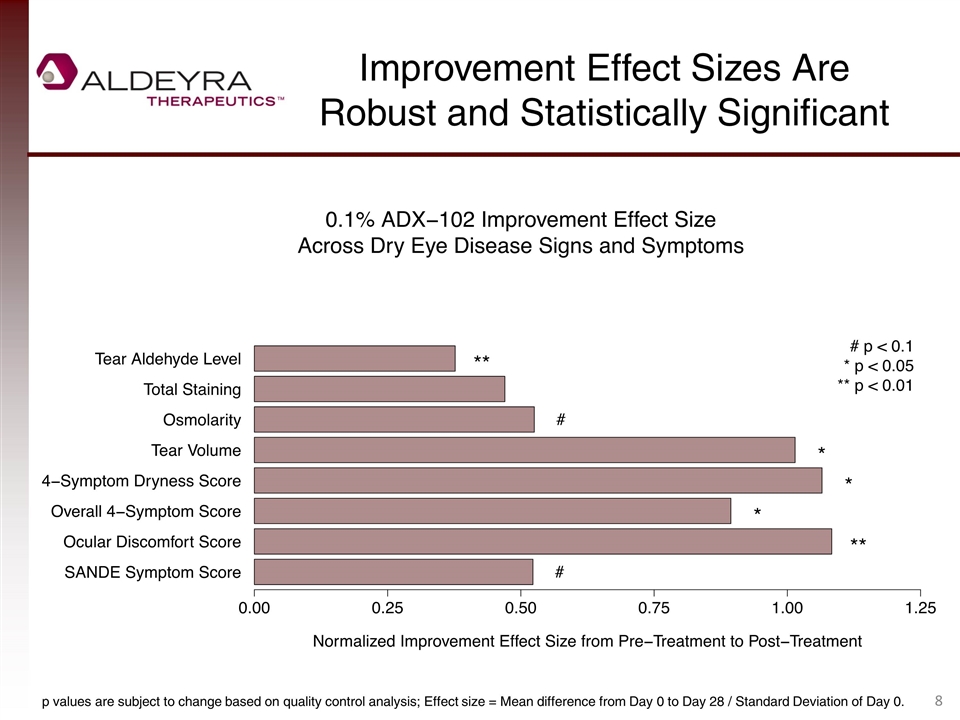
Improvement Effect Sizes Are Robust and Statistically Significant p values are subject to change based on quality control analysis; Effect size = Mean difference from Day 0 to Day 28 / Standard Deviation of Day 0.

Dose Selection for Phase 2b Clinical Testing No observed safety concerns Stinging consistent with other eye drops and prior ADX-102 clinical experience, generally resolving within minutes Tolerability of 0.1% ADX-102 consistent with standard of care, and was better than that of 0.5% ADX-102 formulations in the dry eye disease patient population 0.1% ADX-102, which demonstrated consistent statistically and clinically significant activity and was the best-tolerated formulation, selected to advance to Phase 2b clinical testing in dry eye disease
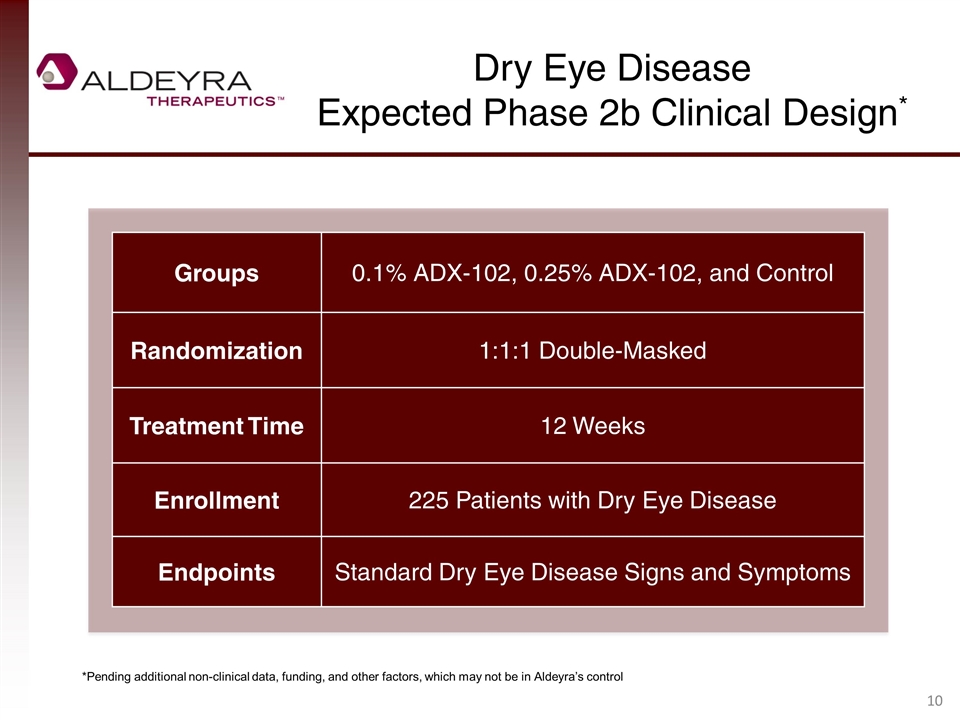
Dry Eye Disease Expected Phase 2b Clinical Design* Groups 0.1% ADX-102, 0.25% ADX-102, and Control Randomization 1:1:1 Double-Masked Treatment Time 12 Weeks Enrollment 225 Patients with Dry Eye Disease Endpoints Standard Dry Eye Disease Signs and Symptoms *Pending additional non-clinical data, funding, and other factors, which may not be in Aldeyra’s control

Dry Eye Disease Results Strategic Implications Aldeyra R&D Day October 10, 2017 A novel mechanism of action with confirmed clinically relevant activity suggests that ADX-102 offers a differentiated approach for treatment of dry dye disease patients, which accounted for approximately $1.8B in 2016 US prescription sales*. Positive dry eye disease efficacy results, combined with clinically relevant results in allergic conjunctivitis and noninfectious anterior uveitis, reinforce the anti-inflammatory potential of Aldeyra’s novel aldehyde trap platform in ophthalmology. Clinical results to date suggest the potential to position ADX-102 as the only non-corticosteroid eye drop with efficacy in dry eye disease and allergic conjunctivitis, a substantial unmet medical need for large numbers of patients that suffer from both conditions. Based on the positive results, Aldeyra plans to advance to Phase 2b clinical testing in dry eye disease. *IMS data
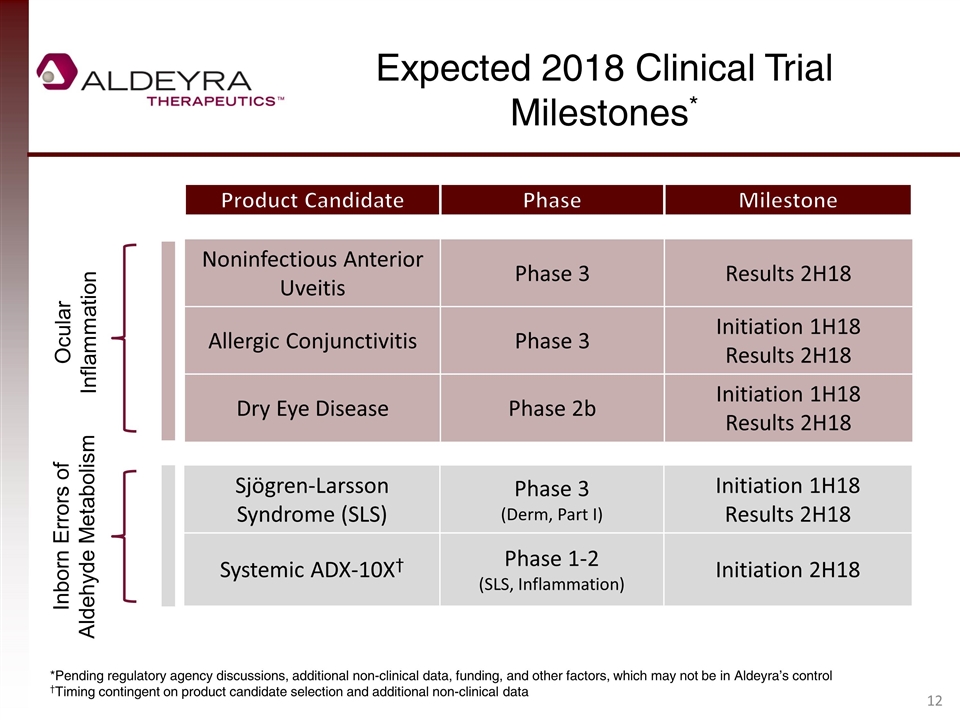
Expected 2018 Clinical Trial Milestones* *Pending regulatory agency discussions, additional non-clinical data, funding, and other factors, which may not be in Aldeyra’s control †Timing contingent on product candidate selection and additional non-clinical data Product Candidate Phase Noninfectious Anterior Uveitis Phase 3 Results 2H18 Allergic Conjunctivitis Phase 3 Initiation 1H18 Results 2H18 Dry Eye Disease Phase 2b Initiation 1H18 Results 2H18 Milestone Sjögren-Larsson Syndrome (SLS) Phase 3 (Derm, Part I) Initiation 1H18 Results 2H18 Systemic ADX-10X† Phase 1-2 (SLS, Inflammation) Initiation 2H18 Ocular Inflammation Inborn Errors of Aldehyde Metabolism
