Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Ignyta, Inc. | d453232dex991.htm |
| 8-K - FORM 8-K - Ignyta, Inc. | d453232d8k.htm |

Entrectinib ROS1 Lung Cancer Update September 6, 2017 Exhibit 99.2

Safe Harbor Statement This document contains forward-looking statements, as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, about Ignyta, Inc. (“us” or the “Company”). Statements that are not purely historical are forward-looking statements. These include statements regarding, among other things: references to the development of entrectinib and our other product candidates, including CNS activity as a potential differentiating factor; the clinical and/or non-clinical data or plans underlying entrectinib or any of our other development programs, and the timelines associated with such programs; our ability to design and conduct development activities for entrectinib and our other development programs; our ability to obtain regulatory approvals in order to market any of our product candidates; references to the potential market size for our products; and our ability to successfully commercialize any approved products. Forward-looking statements involve known and unknown risks that relate to future events or the Company’s future financial performance, some of which may be beyond our control, and the actual results could differ materially from those discussed in this document. Accordingly, the Company cautions investors not to place undue reliance on the forward-looking statements contained in, or made in connection with, this document. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements, include, among others, the potential for results of past or ongoing clinical or non-clinical studies to differ from expectations or previous results; the interpretation of data from our clinical and non-clinical studies; our ability to initiate and complete clinical trials and non-clinical studies; regulatory developments; our dependence on third party manufacturers for supply of our product candidates and any approved products; the potential advantages of our product candidates; the markets any approved products are intended to serve; and our capital needs; as well as those set forth under the headings “Special Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in the Company’s Form 10-K filed with the Securities and Exchange Commission (“SEC”) on March 14, 2017, and similar disclosures made in the Company’s Form 10-Q filings and other SEC filings and press releases. The forward-looking statements contained in this document represent our estimates and assumptions only as of the date of this document, and we undertake no duty or obligation to update or revise publicly any forward-looking statements contained in this document as a result of new information, future events or changes in our expectations. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company.
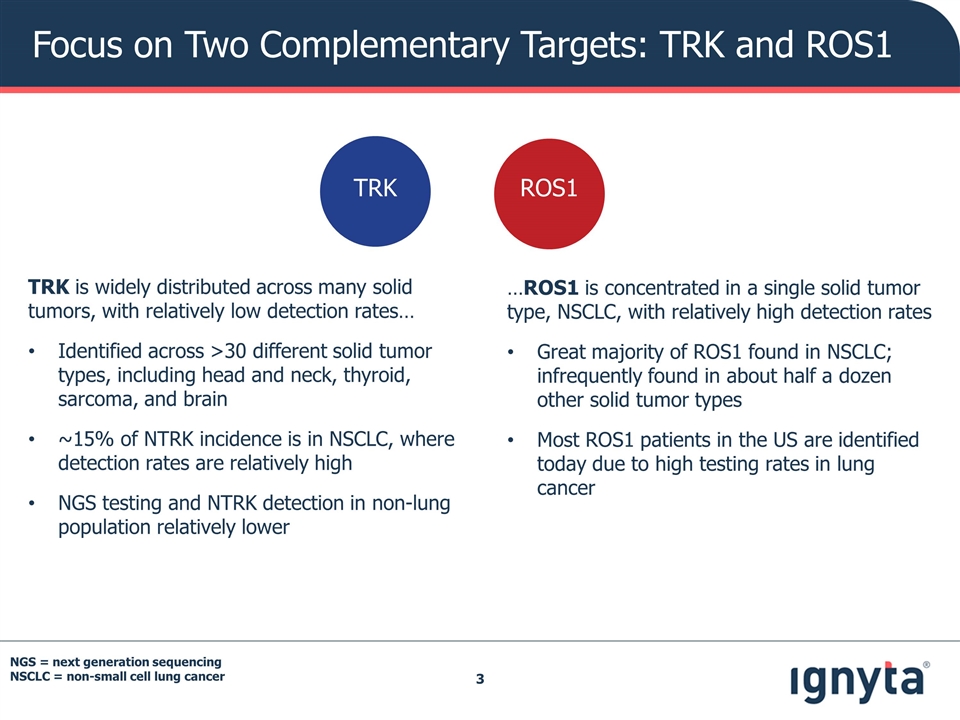
Focus on Two Complementary Targets: TRK and ROS1 TRK is widely distributed across many solid tumors, with relatively low detection rates… Identified across >30 different solid tumor types, including head and neck, thyroid, sarcoma, and brain ~15% of NTRK incidence is in NSCLC, where detection rates are relatively high NGS testing and NTRK detection in non-lung population relatively lower …ROS1 is concentrated in a single solid tumor type, NSCLC, with relatively high detection rates Great majority of ROS1 found in NSCLC; infrequently found in about half a dozen other solid tumor types Most ROS1 patients in the US are identified today due to high testing rates in lung cancer ROS1 TRK NGS = next generation sequencing NSCLC = non-small cell lung cancer
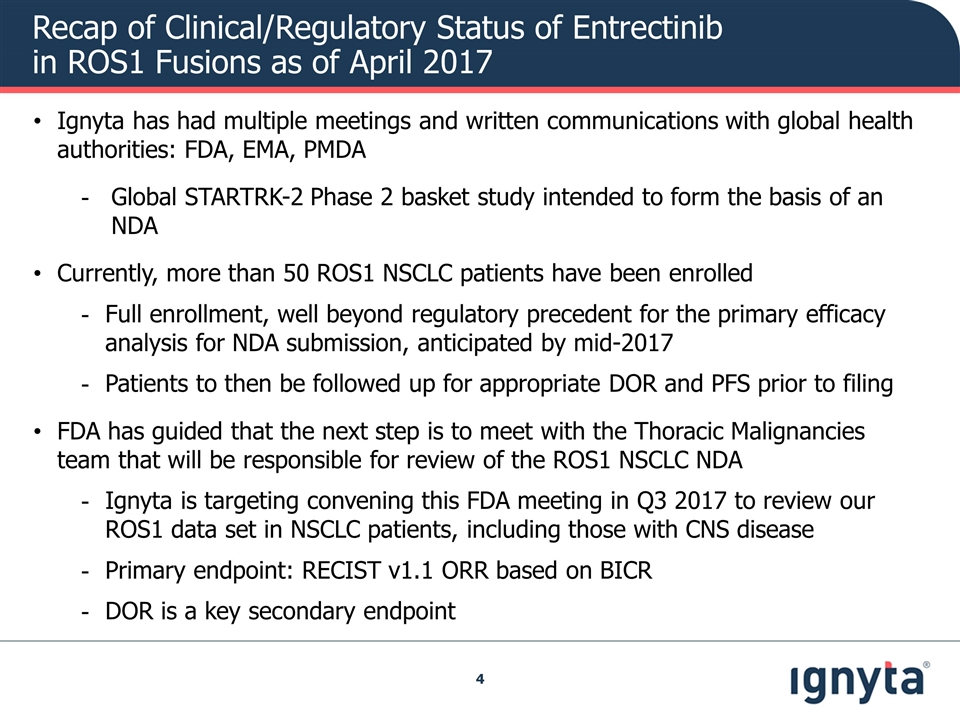
Recap of Clinical/Regulatory Status of Entrectinib in ROS1 Fusions as of April 2017 Ignyta has had multiple meetings and written communications with global health authorities: FDA, EMA, PMDA Global STARTRK-2 Phase 2 basket study intended to form the basis of an NDA Currently, more than 50 ROS1 NSCLC patients have been enrolled Full enrollment, well beyond regulatory precedent for the primary efficacy analysis for NDA submission, anticipated by mid-2017 Patients to then be followed up for appropriate DOR and PFS prior to filing FDA has guided that the next step is to meet with the Thoracic Malignancies team that will be responsible for review of the ROS1 NSCLC NDA Ignyta is targeting convening this FDA meeting in Q3 2017 to review our ROS1 data set in NSCLC patients, including those with CNS disease Primary endpoint: RECIST v1.1 ORR based on BICR DOR is a key secondary endpoint

FDA Feedback on Proposed Registration Plan for Entrectinib in “ROS1 Fusion-Positive, Locally Advanced or Metastatic, NSCLC” Based on written feedback from Thoracic Malignancies team that will be responsible for review of the ROS1 NSCLC NDA: Registrational dataset will derive from STARTRK-2, STARTRK-1 and ALKA studies No additional studies were requested Primary endpoint: RECIST v1.1 ORR, based on blinded independent central review, in all patients receiving >1 dose of entrectinib Follow-up >12 months required for all responders to assess durability of response Guidance provided for inclusion of CNS efficacy data in entrectinib prescribing information Guidance provided regarding proposed safety database size Data from current studies sufficient for potential ROS1 NSCLC approval
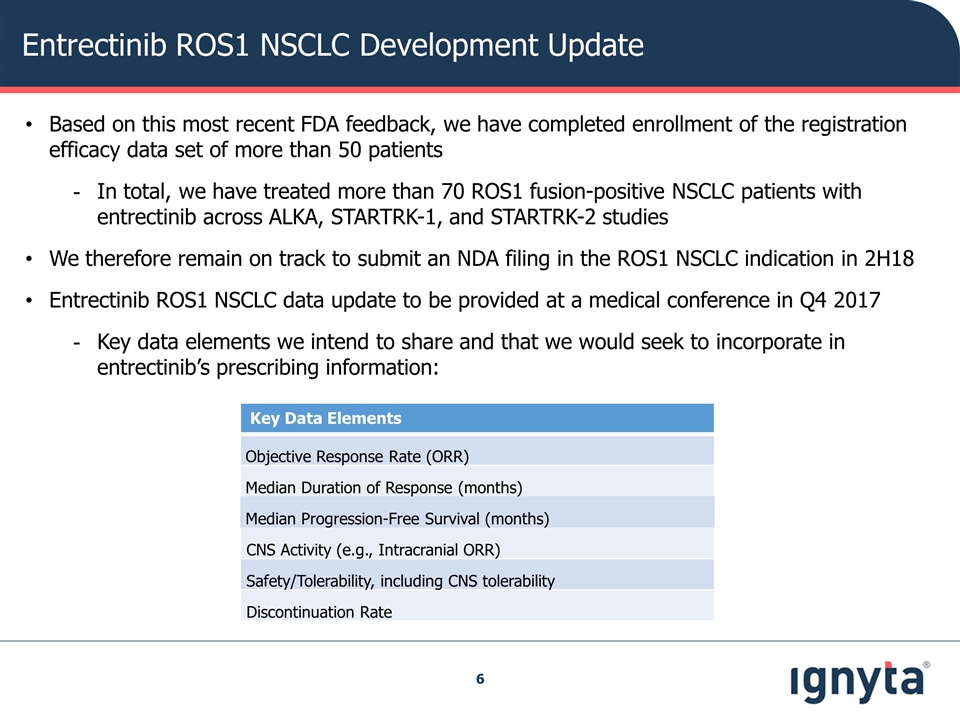
Entrectinib ROS1 NSCLC Development Update Key Data Elements Objective Response Rate (ORR) Median Duration of Response (months) Median Progression-Free Survival (months) CNS Activity (e.g., Intracranial ORR) Safety/Tolerability, including CNS tolerability Discontinuation Rate Based on this most recent FDA feedback, we have completed enrollment of the registration efficacy data set of more than 50 patients In total, we have treated more than 70 ROS1 fusion-positive NSCLC patients with entrectinib across ALKA, STARTRK-1, and STARTRK-2 studies We therefore remain on track to submit an NDA filing in the ROS1 NSCLC indication in 2H18 Entrectinib ROS1 NSCLC data update to be provided at a medical conference in Q4 2017 Key data elements we intend to share and that we would seek to incorporate in entrectinib’s prescribing information:
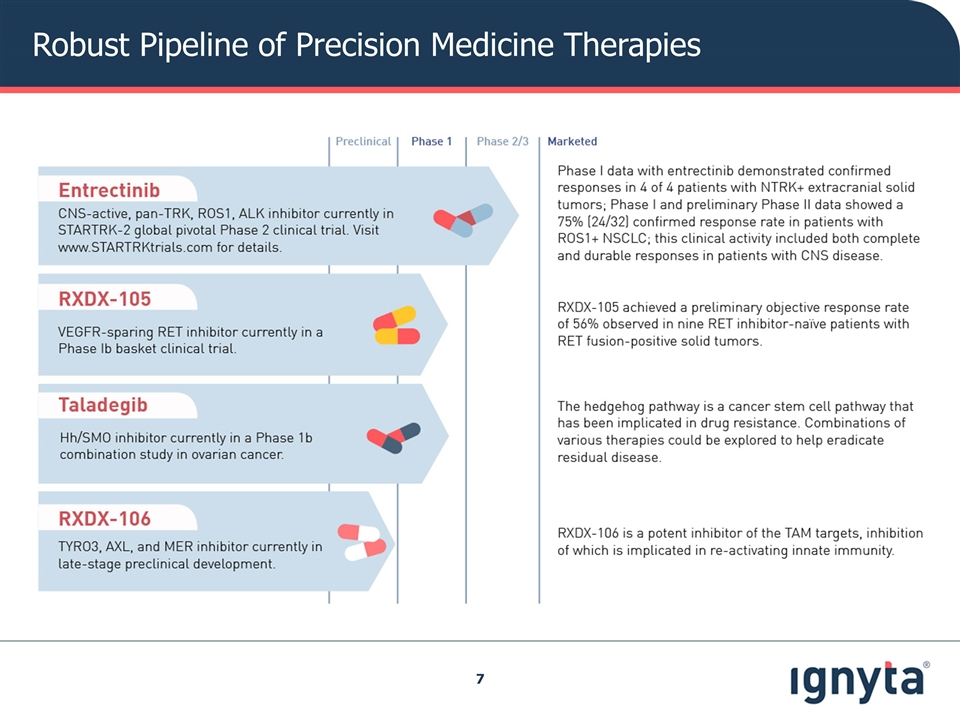
Robust Pipeline of Precision Medicine Therapies
