Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - CHIASMA, INC | d438713dex992.htm |
| EX-99.1 - EX-99.1 - CHIASMA, INC | d438713dex991.htm |
| 8-K - FORM 8-K - CHIASMA, INC | d438713d8k.htm |

Chiasma, Inc. Regulatory update call August 10, 2017 NASDAQ: CHMA Exhibit 99.3

Forward-Looking Statements These slides contain forward-looking statements and information. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward-looking statements. All forward-looking statements are based on estimates and assumptions by our management that, although we believe them to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These statements are also subject to a number of material risks and uncertainties that are described under the heading “Risk Factors” in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2017 filed with the Securities and Exchange Commission on August 10, 2017, as well as discussions of potential risks, uncertainties and other important factors in Chiasma’s subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.
Announced SPA Agreement Reached agreement with FDA on design of new Phase 3 trial (OPTIMAL) for octreotide capsules, conditionally trade-named Mycapssa® Agreed-upon study is designed to address FDA’s concerns raised in the Complete Response Letter from April 2016 Provides regulatory clarity of clinical path forward for octreotide capsules in the United States Important step in advancing octreotide capsules as potential maintenance treatment for adult acromegaly patients who are currently treated with injections SPA Agreement indicates concurrence by FDA with the adequacy and acceptability of specific critical elements of overall protocol design (e.g., study population, study duration, entry criteria, endpoints and planned analyses) Based on current projections, current Company resources sufficient to reach top-line data for the study, which is expected by the end of 2019
Company Overview Focus: Improving the lives of patients with rare and serious chronic diseases Approximately $80.1M in cash and investments as of June 30, 2017; believed sufficient to reach anticipated release of Phase 3 OPTIMAL study top-line data by the end of 2019, while conducting MPOWERED in parallel If approved, Chiasma’s patent-protected octreotide capsules may be the first oral somatostatin analog in an injectable-only market Initial target indication is adult patients with the rare disease acromegaly Special Protocol Assessment (SPA) Agreement reached with FDA for OPTIMAL Phase 3 study in August 2017 Phase 3 MPOWEREDTM trial accepted by EMA and enrolling since March 2016 Clinically tested, patent-protected technology platform developed to enable oral delivery of certain peptides and small molecules
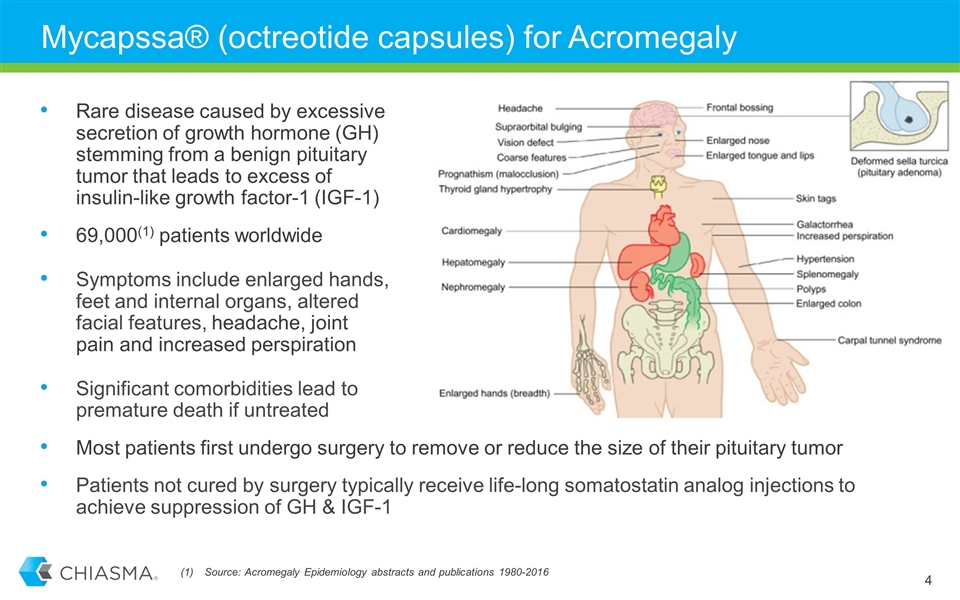
Mycapssa® (octreotide capsules) for Acromegaly Rare disease caused by excessive secretion of growth hormone (GH) stemming from a benign pituitary tumor that leads to excess of insulin-like growth factor-1 (IGF-1) 69,000(1) patients worldwide Symptoms include enlarged hands, feet and internal organs, altered facial features, headache, joint pain and increased perspiration Significant comorbidities lead to premature death if untreated Most patients first undergo surgery to remove or reduce the size of their pituitary tumor Patients not cured by surgery typically receive life-long somatostatin analog injections to achieve suppression of GH & IGF-1 Source: Acromegaly Epidemiology abstracts and publications 1980-2016
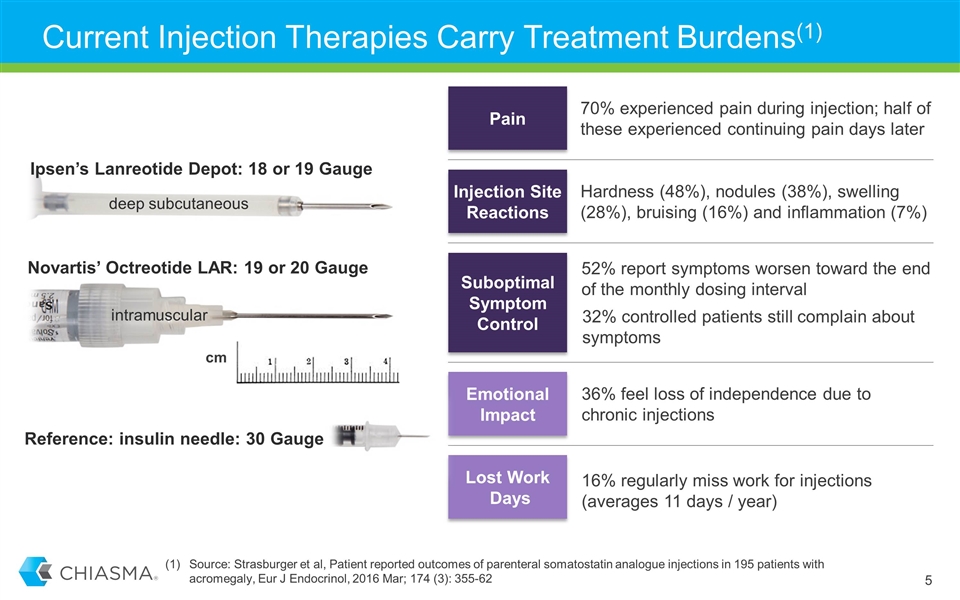
Current Injection Therapies Carry Treatment Burdens(1) intramuscular Novartis’ Octreotide LAR: 19 or 20 Gauge Ipsen’s Lanreotide Depot: 18 or 19 Gauge deep subcutaneous Emotional Impact 36% feel loss of independence due to chronic injections Injection Site Reactions Hardness (48%), nodules (38%), swelling (28%), bruising (16%) and inflammation (7%) Lost Work Days 16% regularly miss work for injections (averages 11 days / year) Suboptimal Symptom Control 32% controlled patients still complain about symptoms 52% report symptoms worsen toward the end of the monthly dosing interval Pain 70% experienced pain during injection; half of these experienced continuing pain days later cm Reference: insulin needle: 30 Gauge Source: Strasburger et al, Patient reported outcomes of parenteral somatostatin analogue injections in 195 patients with acromegaly, Eur J Endocrinol, 2016 Mar; 174 (3): 355-62

OPTIMAL - Octreotide capsules versus Placebo Treatment In MultinationAL centers; New Phase 3 SPA Agreement with FDA New Phase 3 trial will be a randomized, double-blind, placebo-controlled study and is expected to enroll 50 adult acromegaly patients Treatment duration of nine months At least 20% of patients to be recruited in the U.S. Primary endpoint: The proportion of patients who maintain biochemical response (IGF-1< 1.0xULN) at the end of the double-blind placebo controlled period Adequately powered to assess maintenance of biochemical control with octreotide capsules, compared to placebo Chiasma remains committed to MPOWERED trial while we initiate and conduct OPTIMAL
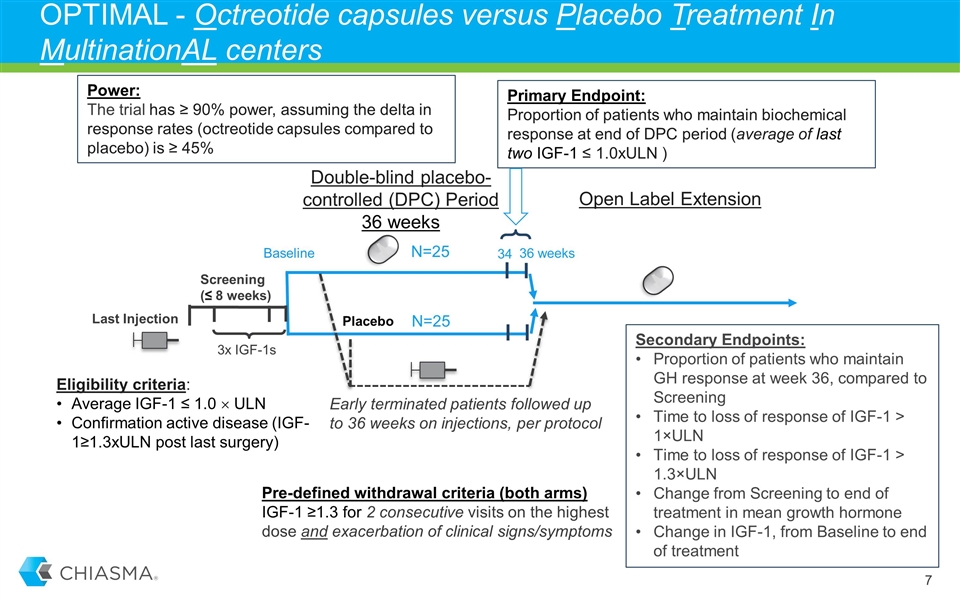
OPTIMAL - Octreotide capsules versus Placebo Treatment In MultinationAL centers Last Injection Open Label Extension Double-blind placebo-controlled (DPC) Period 36 weeks Eligibility criteria: Average IGF-1 ≤ 1.0 ´ ULN Confirmation active disease (IGF-1≥1.3xULN post last surgery) Screening (≤ 8 weeks) Baseline Placebo Primary Endpoint: Proportion of patients who maintain biochemical response at end of DPC period (average of last two IGF-1 ≤ 1.0xULN ) N=25 N=25 Pre-defined withdrawal criteria (both arms) IGF-1 ≥1.3 for 2 consecutive visits on the highest dose and exacerbation of clinical signs/symptoms Early terminated patients followed up to 36 weeks on injections, per protocol 36 weeks 34 Power: The trial has ≥ 90% power, assuming the delta in response rates (octreotide capsules compared to placebo) is ≥ 45% 3x IGF-1s Secondary Endpoints: Proportion of patients who maintain GH response at week 36, compared to Screening Time to loss of response of IGF-1 > 1×ULN Time to loss of response of IGF-1 > 1.3×ULN Change from Screening to end of treatment in mean growth hormone Change in IGF-1, from Baseline to end of treatment
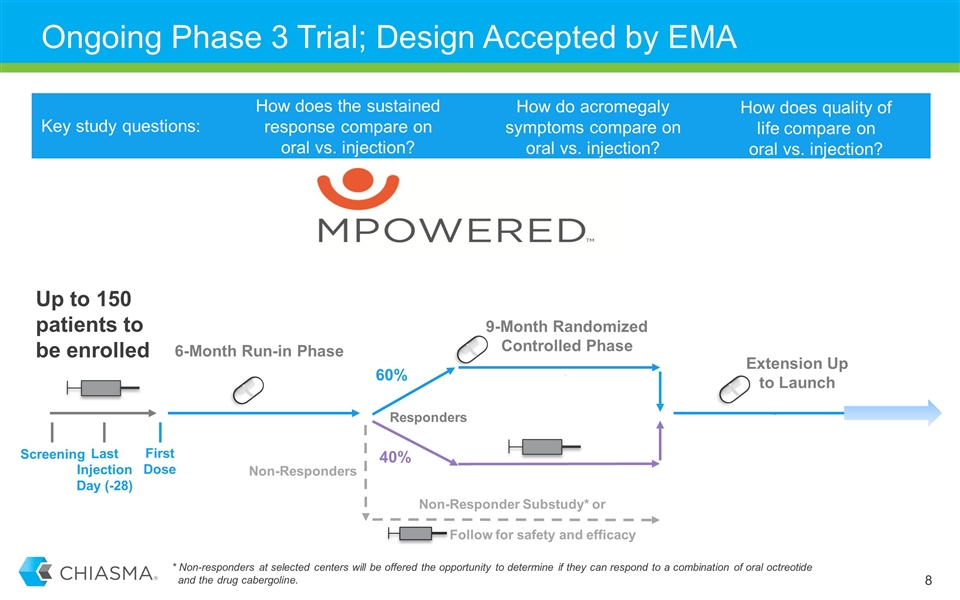
Ongoing Phase 3 Trial; Design Accepted by EMA How does the sustained response compare on oral vs. injection? Key study questions: How do acromegaly symptoms compare on oral vs. injection? How does quality of life compare on oral vs. injection? Last Injection Day (-28) Screening First Dose 6-Month Run-in Phase Responders 9-Month Randomized Controlled Phase Extension Up to Launch Follow for safety and efficacy Up to 150 patients to be enrolled 60% 40% Non-Responder Substudy* or Non-Responders * Non-responders at selected centers will be offered the opportunity to determine if they can respond to a combination of oral octreotide and the drug cabergoline.

Chiasma Financial Update $80.1M in cash and investments as of June 30, 2017 Cash burn of $13M in H1:2017 Total direct cost of OPTIMAL projected at between $18M and $20M Remaining direct cost of MPOWERED projected at between $15M and $17M Expect cash balance of >$60M at December 31, 2017 Based on current plans, believe existing cash and investments sufficient to fund operations through release of OPTIMAL study top-line data by the end of 2019 and support in parallel the MPOWERED trial
Mycapssa – Regulatory Clarity in U.S. Optimistic about the path forward for Mycapssa® SPA agreement with FDA a significant milestone for Chiasma Clears way for new Phase 3 study; expected to result in resubmission of NDA OPTIMAL clinical trial material (Placebo and Mycapssa) is ready for initiation Plan to secure sufficient number of sites to recruit requisite number of patients in OPTIMAL study Forecasted that existing cash and investments are sufficient to fund operations through expected release of top-line OPTIMAL data by the end of 2019

Thank You August 10, 2017 NASDAQ: CHMA
