Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Theravance Biopharma, Inc. | a17-19129_2ex99d1.htm |
| 8-K - 8-K - Theravance Biopharma, Inc. | a17-19129_28k.htm |
Exhibit 99.2
Q2 2017 Financial Results and Business Update August 8, 2017 Theravance Biopharma, Inc. (NASDAQ: TBPH)

Cautionary Statement Regarding Forward-Looking Statements Under the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, the company cautions investors that any forward-looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward-looking statements or projections. Examples of forward-looking statements in this presentation include statements relating to the company’s business plans and objectives, including financial and operating results, potential partnering transactions and sales targets, the company’s regulatory strategies and timing and results of clinical studies, the potential benefits and mechanisms of action of the company’s product and product candidates (including their potential as components of combination therapies). The company’s forward-looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective (including when our product candidates are studied in combination with other compounds), delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with third parties to discover, develop and commercialize products, risks associated with establishing and maintaining sales, marketing and distribution capabilities. Other risks affecting the company are described under the heading “Risk Factors” and elsewhere in the company’s Form 10-Q filed with the Securities and Exchange Commission (SEC) on May 9, 2017, and other periodic reports filed with the SEC.
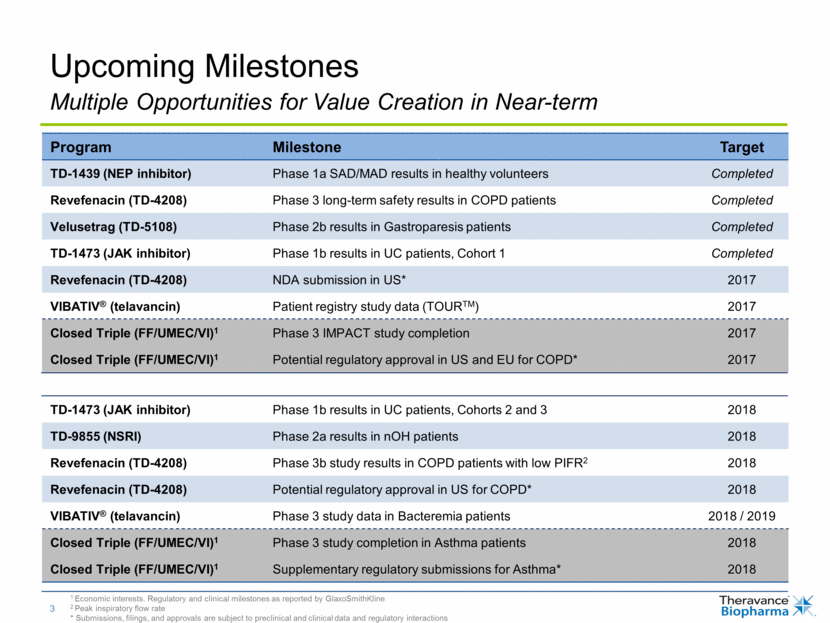
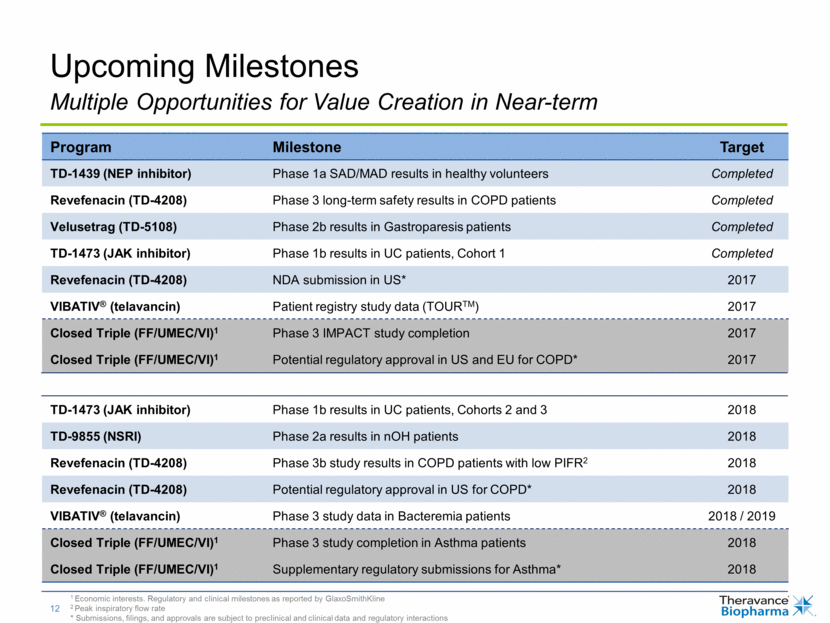
Upcoming Milestones Program Milestone Target TD-1439 (NEP inhibitor) Phase 1a SAD/MAD results in healthy volunteers Completed Revefenacin (TD-4208) Phase 3 long-term safety results in COPD patients Completed Velusetrag (TD-5108) Phase 2b results in Gastroparesis patients Completed TD-1473 (JAK inhibitor) Phase 1b results in UC patients, Cohort 1 Completed Revefenacin (TD-4208) NDA submission in US* 2017 VIBATIV® (telavancin) Patient registry study data (TOURTM) 2017 Closed Triple (FF/UMEC/VI)1 Phase 3 IMPACT study completion 2017 Closed Triple (FF/UMEC/VI)1 Potential regulatory approval in US and EU for COPD* 2017 TD-1473 (JAK inhibitor) Phase 1b results in UC patients, Cohorts 2 and 3 2018 TD-9855 (NSRI) Phase 2a results in nOH patients 2018 Revefenacin (TD-4208) Phase 3b study results in COPD patients with low PIFR2 2018 Revefenacin (TD-4208) Potential regulatory approval in US for COPD* 2018 VIBATIV® (telavancin) Phase 3 study data in Bacteremia patients 2018 / 2019 Closed Triple (FF/UMEC/VI)1 Phase 3 study completion in Asthma patients 2018 Closed Triple (FF/UMEC/VI)1 Supplementary regulatory submissions for Asthma* 2018 Multiple Opportunities for Value Creation in Near-term 1 Economic interests. Regulatory and clinical milestones as reported by GlaxoSmithKline 2 Peak inspiratory flow rate * Submissions, filings, and approvals are subject to preclinical and clinical data and regulatory interactions
JAK Inhibitor Program Oral intestinally-restricted pan-Janus kinase (JAK) inhibitors for ulcerative colitis and other inflammatory intestinal diseases

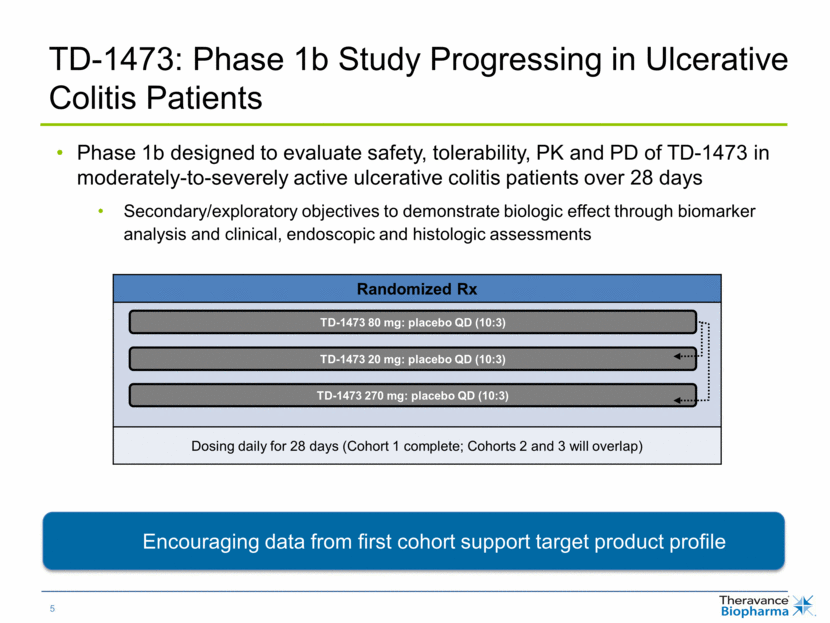
Phase 1b designed to evaluate safety, tolerability, PK and PD of TD-1473 in moderately-to-severely active ulcerative colitis patients over 28 days Secondary/exploratory objectives to demonstrate biologic effect through biomarker analysis and clinical, endoscopic and histologic assessments TD-1473: Phase 1b Study Progressing in Ulcerative Colitis Patients Randomized Rx Dosing daily for 28 days (Cohort 1 complete; Cohorts 2 and 3 will overlap) TD-1473 20 mg: placebo QD (10:3) TD-1473 80 mg: placebo QD (10:3) TD-1473 270 mg: placebo QD (10:3) Encouraging data from first cohort support target product profile
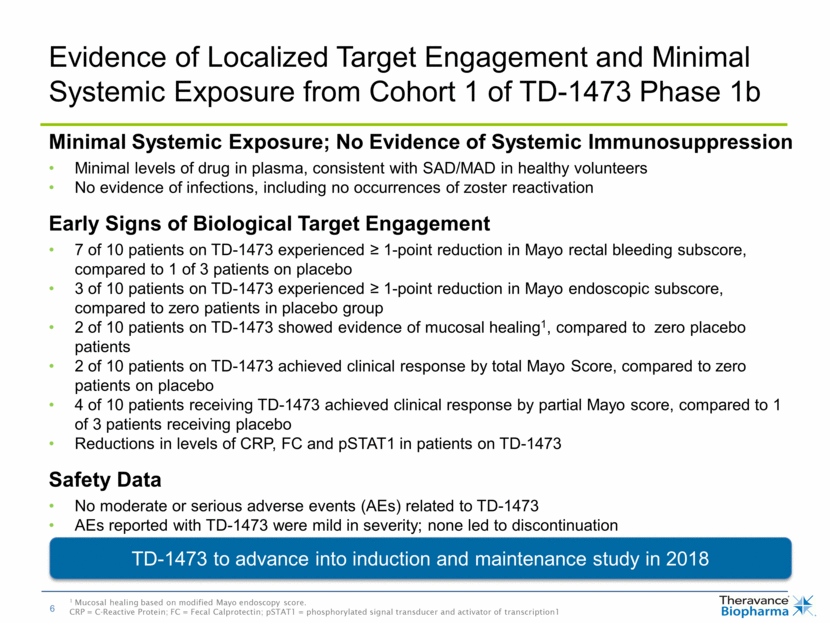
Evidence of Localized Target Engagement and Minimal Systemic Exposure from Cohort 1 of TD-1473 Phase 1b TD-1473 to advance into induction and maintenance study in 2018 Minimal Systemic Exposure; No Evidence of Systemic Immunosuppression Minimal levels of drug in plasma, consistent with SAD/MAD in healthy volunteers No evidence of infections, including no occurrences of zoster reactivation Early Signs of Biological Target Engagement 7 of 10 patients on TD-1473 experienced > 1-point reduction in Mayo rectal bleeding subscore, compared to 1 of 3 patients on placebo 3 of 10 patients on TD-1473 experienced > 1-point reduction in Mayo endoscopic subscore, compared to zero patients in placebo group 2 of 10 patients on TD-1473 showed evidence of mucosal healing1, compared to zero placebo patients 2 of 10 patients on TD-1473 achieved clinical response by total Mayo Score, compared to zero patients on placebo 4 of 10 patients receiving TD-1473 achieved clinical response by partial Mayo score, compared to 1 of 3 patients receiving placebo Reductions in levels of CRP, FC and pSTAT1 in patients on TD-1473 Safety Data No moderate or serious adverse events (AEs) related to TD-1473 AEs reported with TD-1473 were mild in severity; none led to discontinuation 1 Mucosal healing based on modified Mayo endoscopy score. CRP = C-Reactive Protein; FC = Fecal Calprotectin; pSTAT1 = phosphorylated signal transducer and activator of transcription1
Velusetrag (TD-5108) Highly selective 5-HT4 agonist for gastroparesis

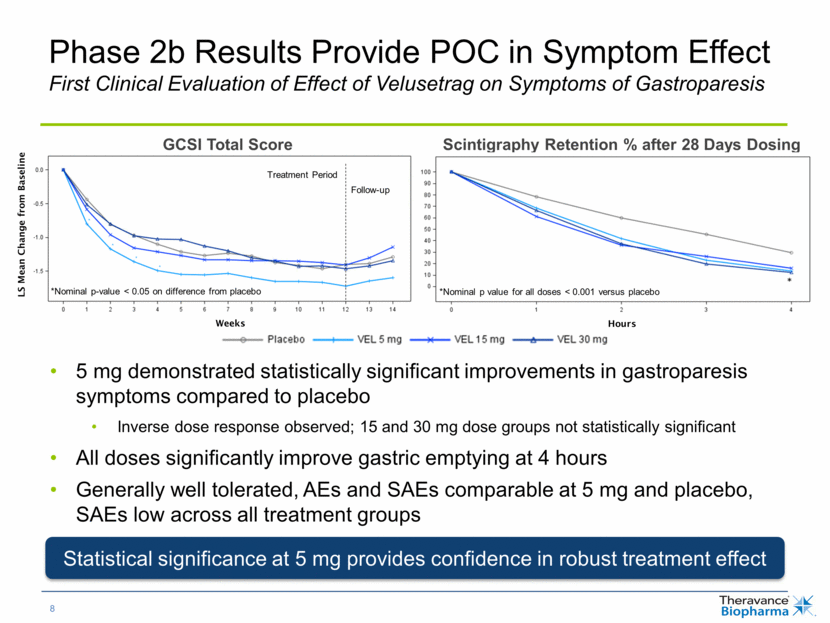
Phase 2b Results Provide POC in Symptom Effect First Clinical Evaluation of Effect of Velusetrag on Symptoms of Gastroparesis Scintigraphy Retention % after 28 Days Dosing LS Mean Change from Baseline GCSI Total Score Weeks Hours *Nominal p value for all doses < 0.001 versus placebo *Nominal p-value < 0.05 on difference from placebo Treatment Period Follow-up 5 mg demonstrated statistically significant improvements in gastroparesis symptoms compared to placebo Inverse dose response observed; 15 and 30 mg dose groups not statistically significant All doses significantly improve gastric emptying at 4 hours Generally well tolerated, AEs and SAEs comparable at 5 mg and placebo, SAEs low across all treatment groups Statistical significance at 5 mg provides confidence in robust treatment effect
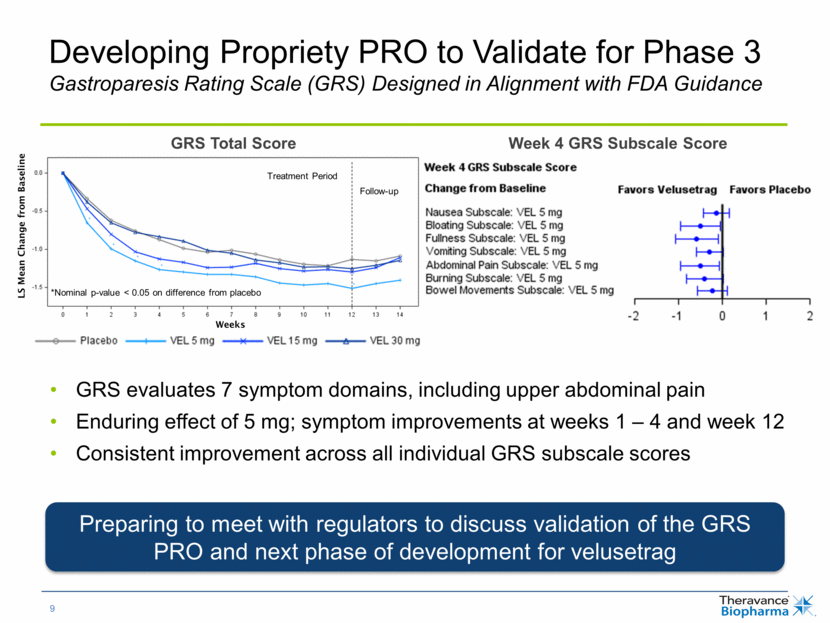
Developing Propriety PRO to Validate for Phase 3 Gastroparesis Rating Scale (GRS) Designed in Alignment with FDA Guidance Week 4 GRS Subscale Score LS Mean Change from Baseline GRS Total Score Weeks *Nominal p-value < 0.05 on difference from placebo Treatment Period Follow-up GRS evaluates 7 symptom domains, including upper abdominal pain Enduring effect of 5 mg; symptom improvements at weeks 1 – 4 and week 12 Consistent improvement across all individual GRS subscale scores Preparing to meet with regulators to discuss validation of the GRS PRO and next phase of development for velusetrag
Revefenacin (TD-4208) Nebulized Long-Acting Muscarinic Antagonist (LAMA) for COPD
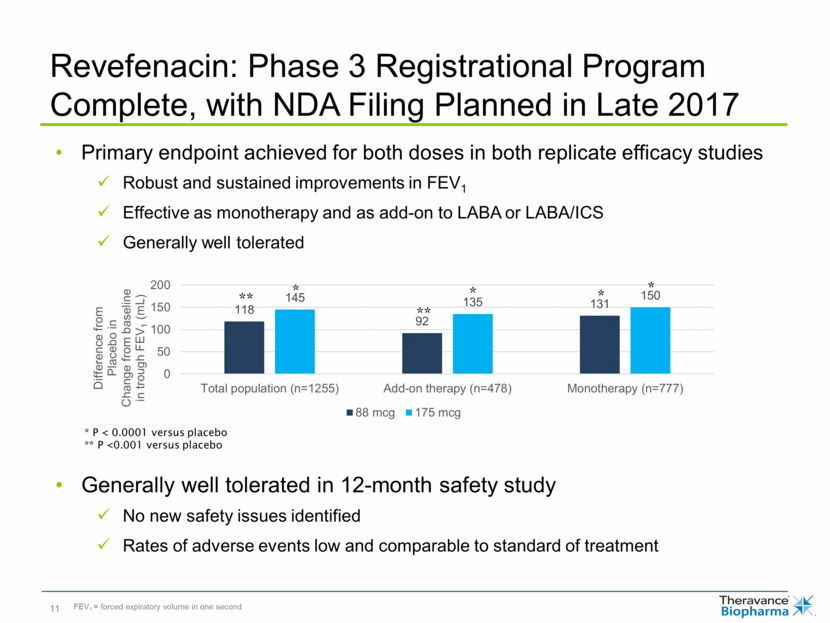
* P < 0.0001 versus placebo ** P <0.001 versus placebo * ** * * * ** Revefenacin: Phase 3 Registrational Program Complete, with NDA Filing Planned in Late 2017 Primary endpoint achieved for both doses in both replicate efficacy studies Robust and sustained improvements in FEV1 Effective as monotherapy and as add-on to LABA or LABA/ICS Generally well tolerated Generally well tolerated in 12-month safety study No new safety issues identified Rates of adverse events low and comparable to standard of treatment FEV1 = forced expiratory volume in one second
Upcoming Milestones Program Milestone Target TD-1439 (NEP inhibitor) Phase 1a SAD/MAD results in healthy volunteers Completed Revefenacin (TD-4208) Phase 3 long-term safety results in COPD patients Completed Velusetrag (TD-5108) Phase 2b results in Gastroparesis patients Completed TD-1473 (JAK inhibitor) Phase 1b results in UC patients, Cohort 1 Completed Revefenacin (TD-4208) NDA submission in US* 2017 VIBATIV® (telavancin) Patient registry study data (TOURTM) 2017 Closed Triple (FF/UMEC/VI)1 Phase 3 IMPACT study completion 2017 Closed Triple (FF/UMEC/VI)1 Potential regulatory approval in US and EU for COPD* 2017 TD-1473 (JAK inhibitor) Phase 1b results in UC patients, Cohorts 2 and 3 2018 TD-9855 (NSRI) Phase 2a results in nOH patients 2018 Revefenacin (TD-4208) Phase 3b study results in COPD patients with low PIFR2 2018 Revefenacin (TD-4208) Potential regulatory approval in US for COPD* 2018 VIBATIV® (telavancin) Phase 3 study data in Bacteremia patients 2018 / 2019 Closed Triple (FF/UMEC/VI)1 Phase 3 study completion in Asthma patients 2018 Closed Triple (FF/UMEC/VI)1 Supplementary regulatory submissions for Asthma* 2018 Multiple Opportunities for Value Creation in Near-term 1 Economic interests. Regulatory and clinical milestones as reported by GlaxoSmithKline 2 Peak inspiratory flow rate * Submissions, filings, and approvals are subject to preclinical and clinical data and regulatory interactions
Q&A