Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Natera, Inc. | a17-19124_18k.htm |
| EX-99.1 - EX-99.1 - Natera, Inc. | a17-19124_1ex99d1.htm |
Exhibit 99.2
August 2017 Natera, Inc. Q2 2017 Earnings Call

Safe Harbor This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding the market opportunity, products, commercial partners, user experience, clinical trials, financial performance, strategies, anticipated future performance and general business conditions of Natera, Inc. (“Natera”, the “Company”, “we” or “us”), are forward-looking statements. These forward-looking statements are subject to known and unknown risks and uncertainties that may cause actual results to differ materially, including: we face numerous uncertainties and challenges in achieving the financial guidance provided; we may be unable to further increase the use and adoption of Panorama, through our direct sales efforts or through our laboratory partners, or to develop and successfully commercialize new products, including our cancer products; we have incurred losses since our inception and we anticipate that we will continue to incur losses for the foreseeable future; our quarterly results may fluctuate significantly; our estimates of market opportunity and forecasts of market growth may prove to be inaccurate, and even if the market in which we compete achieves the forecasted growth, our business could fail to grow at similar rates; we may be unable to compete successfully with either existing or future prenatal testing products or other test methods; we may not be successful in commercializing our cloud-based distribution model; our products may not perform as expected; the results of our clinical studies may not support the use of our tests, particularly in the average-risk pregnancy population or for microdeletions screening, or may not be able to be replicated in later studies required for regulatory approvals or clearances; if our sole CLIA-certified laboratory facility becomes inoperable, we will be unable to perform our tests and our business will be harmed; we rely on a limited number of suppliers or, in some cases, single suppliers, for some of our laboratory instruments and materials and may not be able to find replacements or immediately transition to alternative suppliers; our cord blood and tissue banking activities are subject to regulations that may impose significant costs and restrictions on us; the marketing, sale, and use of Panorama and our other products could result in substantial damages arising from product liability or professional liability claims that exceed our resources; we may be unable to expand third-party payer coverage and reimbursement for Panorama and our other tests, and we may be required to refund reimbursements already received; third-party payers may withdraw coverage or provide lower levels of reimbursement due to changing policies, billing complexities or other factors; if the FDA were to begin actively regulating our tests, we could incur substantial costs and delays associated with trying to obtain premarket clearance or approval and incur costs associated with complying with post-market controls; we could be subject to third party claims of intellectual property infringement, which could result in litigation or other proceedings and could limit our ability to commercialize our products or services; and any failure to obtain, maintain, and enforce our intellectual property rights could impair our ability to protect our proprietary technology and our brand. We discuss these and other risks and uncertainties in greater detail in the sections entitled “Risk Factors” and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our Form 10-Q for the quarter ended June 30, 2017. Further information on potential risks that could affect actual results will be included in other filings we make with the SEC from time to time. Given these uncertainties, you should not place undue reliance on the forward-looking statements. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on its business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statement. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied. Except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations. We file reports, proxy statements, and other information with the SEC. Such reports, proxy statements, and other information concerning us can be read and copied at the SEC’s Public Reference Room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549 or on the Internet at http://www.sec.gov.Please call the SEC at 1-800-SEC-0330 for further information on the Public Reference Room. Our common stock is listed on the NASDAQ Global Select Market, and these reports, proxy statements and other information are also available for inspection at the offices of the NASDAQ Stock Market, Inc. located at 1735 K Street, NW, Washington, D.C. 20006. We will provide without charge upon written or oral request a copy of any or all of the documents that are incorporated by reference into this prospectus, other than exhibits which are specifically incorporated by reference into such documents. Requests should be directed to our Investor Relations department at Natera, Inc., 201 Industrial Road, Suite 410, San Carlos, California 94070. Our telephone number is (650) 249-9090. 2

Recent Highlights Total revenues of $53.6M in Q2 2017, up 14% from Q1 2017 Revenues grew 3% YoY despite adoption of in-network pricing Revenues grew 14% sequentially vs. Q1 2017 Broader insurance coverage, stable in-network contracts driving ASP increases Blended Average Selling Price of $452 vs. $408 in Q1 2017 Gross margins of 36% in Q2 2017, up ~800 basis points from Q1 2017 Panorama V3 improvements are on track for further COGS reductions Processed 125,700 tests in Q2 2017, 17% growth vs. Q2 2016 Panorama®: approximately 89,400 tests processed, 8% growth YoY HorizonTM: approximately 30,700 tests accessioned, 63% growth YoY Closed $100 million debt facility with Orbimed Advisors 3

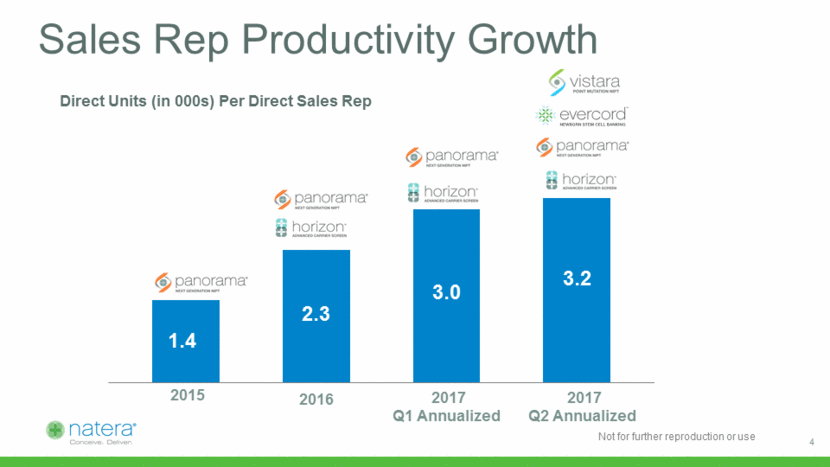
2015 2016 2017 Q1 Annualized 2017 Q2 Annualized Direct Units (in 000s) Per Direct Sales Rep Sales Rep Productivity Growth 4
Millions of Covered Lives NIPT for All Women – Expanding Coverage 79.6M 29 plans 97.1M 33 plans 108.1 M 49 plans UHC Aetna Other 56.7M 5 0 50 100 150 200 250 2Q2015 4Q2015 2Q2016 4Q2016 2Q2017 Expected Future NIPT Average Risk Covered Lives by Quarter
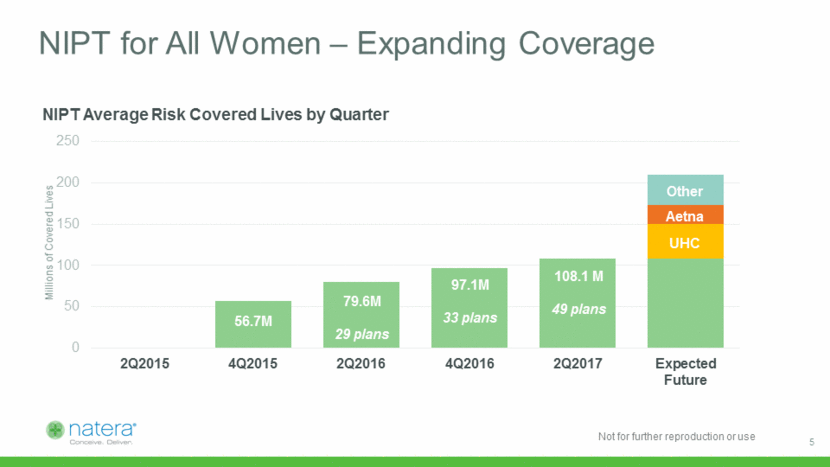
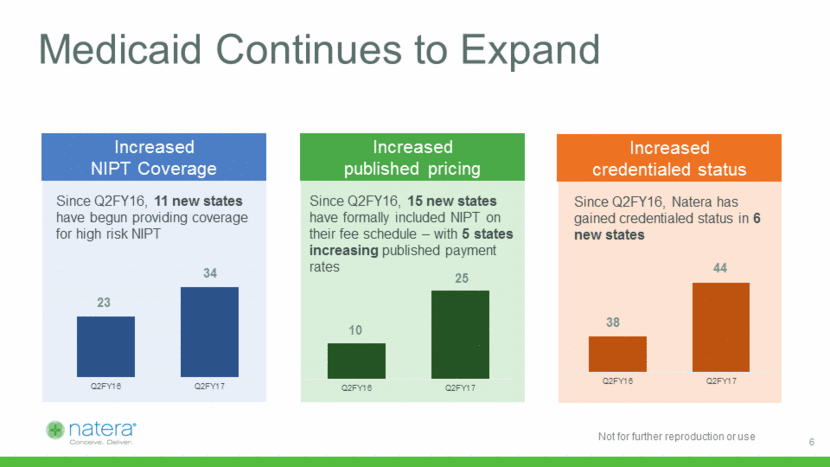
Medicaid Continues to Expand Increased NIPT Coverage Since Q2FY16, 11 new states have begun providing coverage for high risk NIPT 34 Increased published pricing Since Q2FY16, 15 new states have formally included NIPT on their fee schedule – with 5 states increasing published payment rates 25 Increased credentialed status Since Q2FY16, Natera has gained credentialed status in 6 new states 44 23 10 38 6
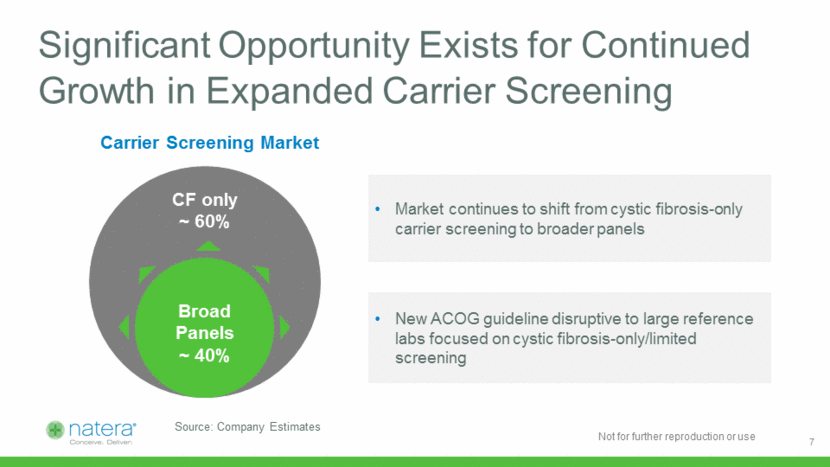
Significant Opportunity Exists for Continued Growth in Expanded Carrier Screening Source: Company Estimates Market continues to shift from cystic fibrosis-only carrier screening to broader panels CF only ~ 60% Broad Panels ~ 40% New ACOG guideline disruptive to large reference labs focused on cystic fibrosis-only/limited screening Carrier Screening Market 7
Focus on Direct Channel Yielding Higher Growth 63% 14% 8 18,800 30,700 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 2Q 2016 2Q 2017 Year Over Year 26,900 30,700 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 1Q 2017 2Q 2017 Quarter Over Quarter
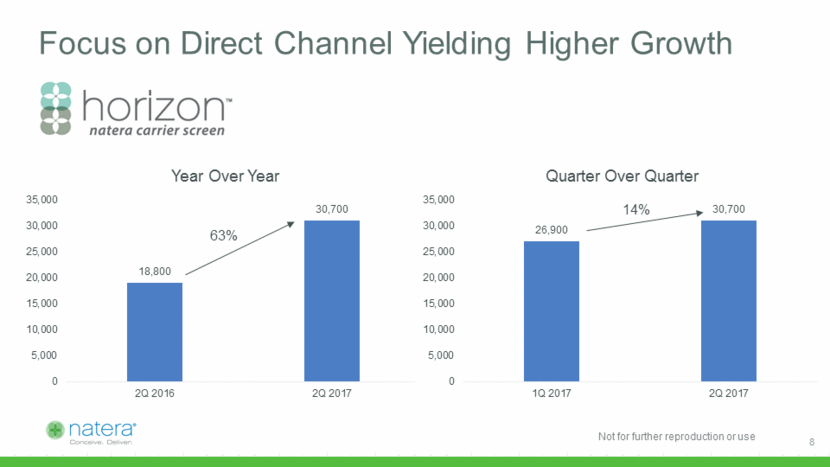
Vistara Beta Successful 1. Snijders, et al. Ultrasound Obstet Gynecol 1999;13:167–170 2 Company estimates based on publically available epidemiology of individual diseases in the panel Two confirmed positives so far, reimbursement and turnaround times consistent with expectations Screens for 30 genes tied to life-altering genetic disorders Complement to Panorama Conditions typically undetected with conventional screening Vistara conditions2 Down syndrome1 Maternal Age 9 1/2000 1/1000 1/500 1/250 20 22 24 26 28 30 32 34
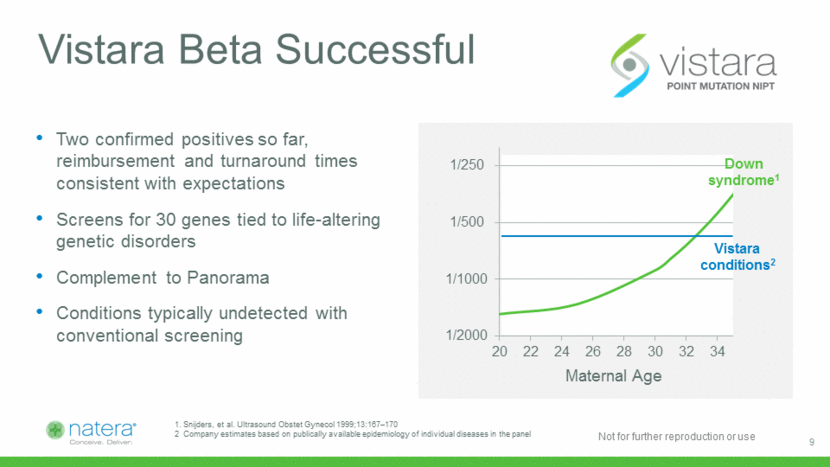
Evercord Launch Underway 35 Horizon identifies 35 conditions that have been treated with cord blood stem cells 80 Treats nearly 80 diseases 30,000 transplants performed since 1988 80% of medical professionals prefer a company that provides products from conception through pregnancy and beyond.* 80% Yes 20% No *TechValidate survey of medical professionals Proven Quality & Experience Expanded Product Offering Bloodworks collects nearly 50 units per month for the national registry 10
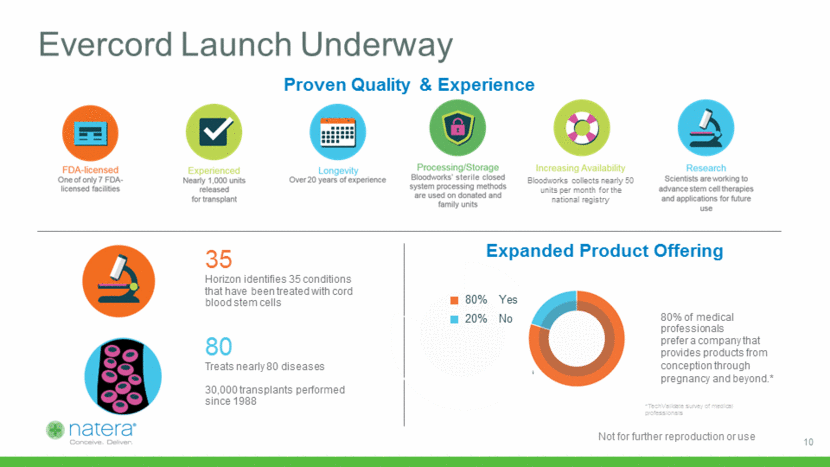
Reach 500k Patients With Quality Message Natera patients become Evercord customers Panorama order and engagment with Patient Portal Build trust in Natera as a leader in women’s health and resource for patients Evercord awareness and education via email, SMS, and Patient Portal Evercord purchase and deposit Ongoing engagement with Natera 11
RUO Launch of Signatera *Based on prevalent population which has received a treatment with the intent to cure (~87% of total prevalent), 2 tests a year Assumes incidence of ~1.7M new cancer cases annually (SEER data), 50% eligible for treatment monitoring with 5 tests per diagnosis at $500 / test Sources: SEER Data, L.E.K. Interviews and Analysis Company Estimates Not for further reproduction or use Total CLIA & RUO U.S. TAM: $12,645M Collection efforts underway for 3,000 samples on >500 patients for 6 indications Indications with sample collection efforts underway 12
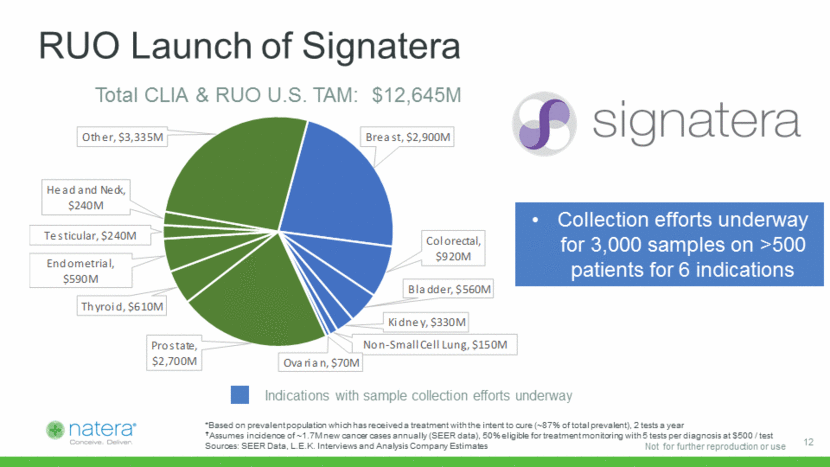
x Launching Pan-Cancer Technology Platform Single molecule sensitivity in a tube of blood Personalized assay designed for each individual Fast turnaround time Expected low cost of goods sold <$200 Predicted relapse with 93% sensitivity, zero false positives Lead time up to 11 months Cumulative relapse ctDNA Positive ctDNA Negative Source: Phylogenetic ctDNA analysis depicts early stage lung cancer evolution. Abbosh C, et al. Nature. 2017 Apr 26. doi: 10.1038/nature22364 Natera Advantages Proven Capability Days to relapse 13
Natera’s Path to Cash Flow Breakeven Reimbursement for existing test volumes COGS Improvements New product launches in women’s health channel Stable operating expenses as revenues grow 14
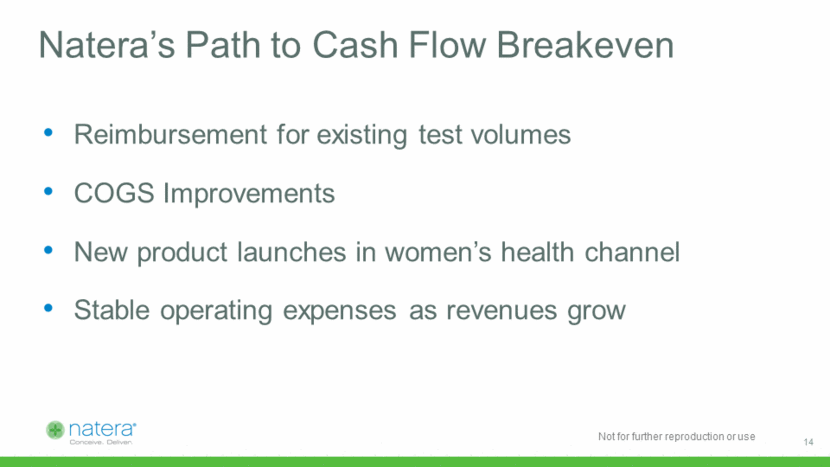
Average Selling Prices Stable and Improving Multi-year, fixed price payer contracts Increasing average risk NIPT Coverage Increasing coverage for microdeletions Shift from procedure code to NIPT Code In-Network shift Shift from procedure code to Microdeletions code Total revenues / tests accessioned Three Distinct Pricing Headwinds Pricing Drivers Going Forward 15 $0 $1,000 2014 2015 2016 2017E 2018E 2019E
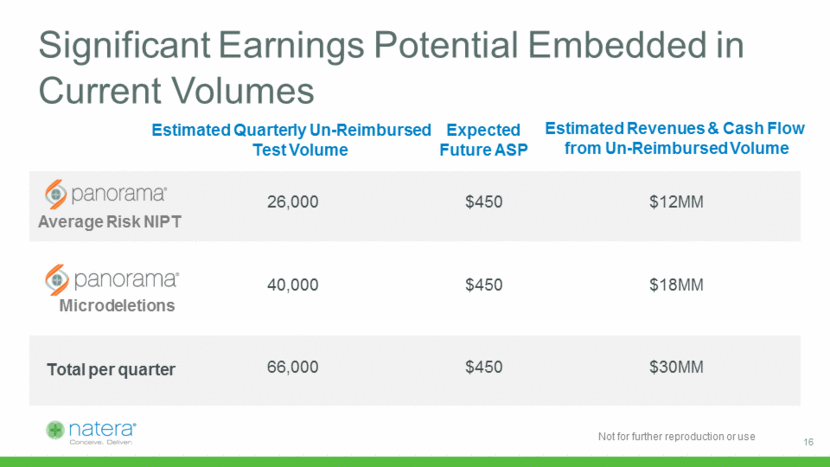
Significant Earnings Potential Embedded in Current Volumes Estimated Quarterly Un-Reimbursed Test Volume Expected Future ASP Estimated Revenues & Cash Flow from Un-Reimbursed Volume Average Risk NIPT 26,000 $450 $12MM Microdeletions 40,000 $450 $18MM Total per quarter 66,000 $450 $30MM 16
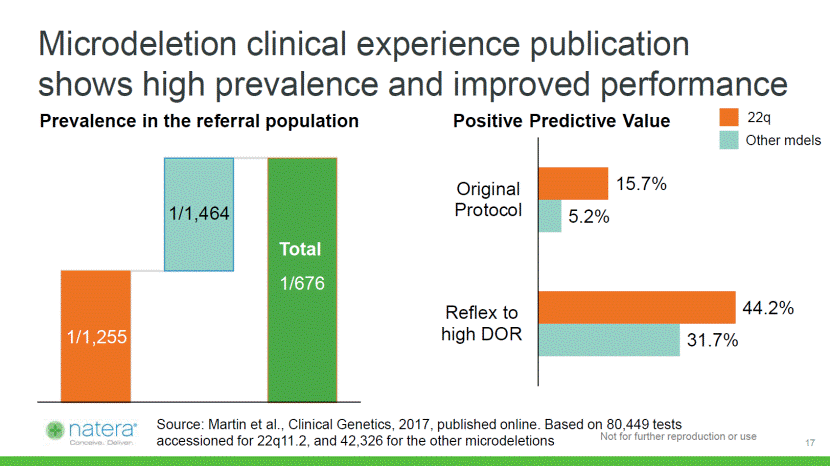
Microdeletion clinical experience publication shows high prevalence and improved performance 31.7% 5.2% 44.2% 15.7% Reflex tohigh DOR OriginalProtocol Positive Predictive Value 1/1,255 1/1,464 1/676 Prevalence in the referral population Total 22q Other mdels Source: Martin et al., Clinical Genetics, 2017, published online. Based on 80,449 tests accessioned for 22q11.2, and 42,326 for the other microdeletions
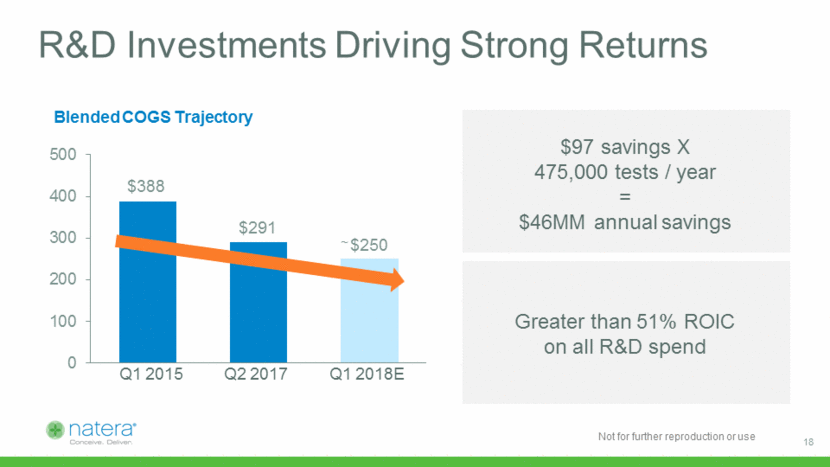
Q1 2015 Q2 2017 R&D Investments Driving Strong Returns Q1 2018E ~ Blended COGS Trajectory $97 savings X 475,000 tests / year = $46MM annual savings Greater than 51% ROIC on all R&D spend 18 $388 $291 $250 0 100 200 300 400 500
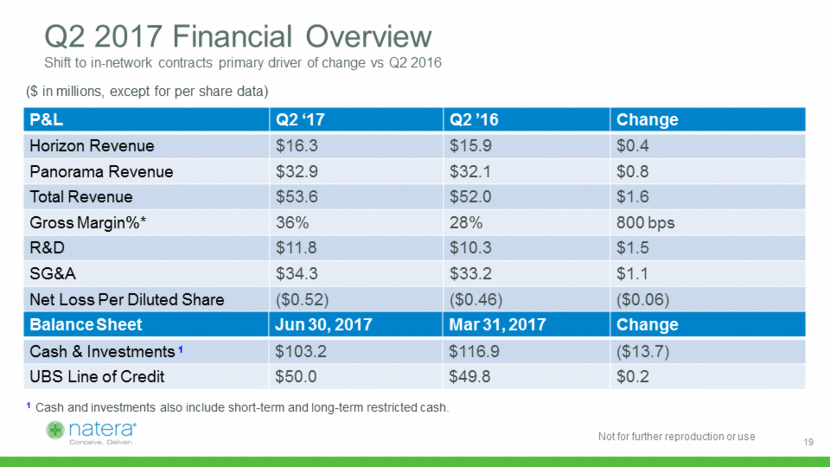
Q2 2017 Financial Overview Shift to in-network contracts primary driver of change vs Q2 2016 P&L Q2 ‘17 Q2 ’16 Change Horizon Revenue $16.3 $15.9 $0.4 Panorama Revenue $32.9 $32.1 $0.8 Total Revenue $53.6 $52.0 $1.6 Gross Margin%* 36% 28% 800 bps R&D $11.8 $10.3 $1.5 SG&A $34.3 $33.2 $1.1 Net Loss Per Diluted Share ($0.52) ($0.46) ($0.06) Balance Sheet Jun 30, 2017 Mar 31, 2017 Change Cash & Investments 1 $103.2 $116.9 ($13.7) UBS Line of Credit $50.0 $49.8 $0.2 ($ in millions, except for per share data) 1 Cash and investments also include short-term and long-term restricted cash. 19
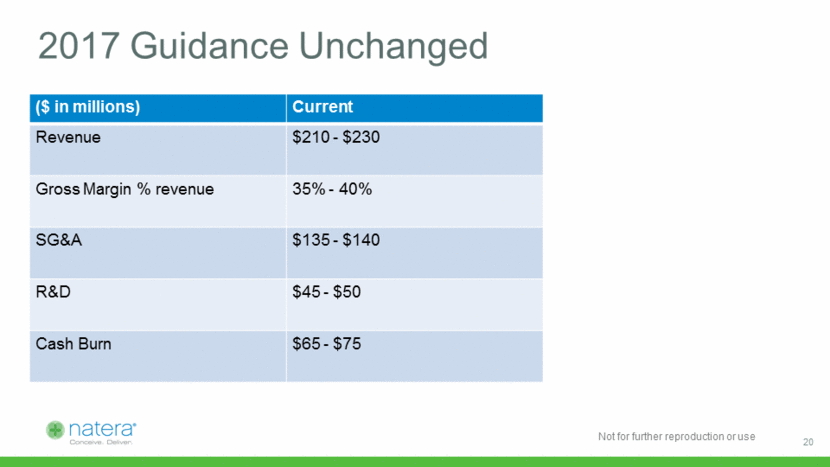
2017 Guidance Unchanged ($ in millions) Current Revenue $210 - $230 Gross Margin % revenue 35% - 40% SG&A $135 - $140 R&D $45 - $50 Cash Burn $65 - $75 20
Orbimed Debt Financing $100 million commitment $75 million funded at close; additional $25 million available at Natera’s option through 2018 7 year loan Interest only until maturity LIBOR + 8.75%, subject to a 1% LIBOR Floor May be repaid prior to maturity date 21
[LOGO]

