Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Lipocine Inc. | v472588_8k.htm |

Enabling oral drug delivery to improve patient compliance CORPORATE PRESENTATION August 2017 Exhibit 99.1

Forward - Looking Statements This presentation contains forward - looking statements about Lipocine Inc. (the “Company”). These forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward - looking statement s relate to the Company’s product candidates, clinical and regulatory processes and objectives, potential benefits of the Company’s product c and idates, intellectual property and related matters, all of which involve known and unknown risks and uncertainties. Actual results may d iffer materially from the forward - looking statements discussed in this presentation . Accordingly, the Company cautions investors not to place undue reliance on the forward - looking statements contained in, or made in connection with, this presentation . Several factors may affect the initiation and completion of clinical trials, the potential advantages of the Company’s product candidates and the Company’s capital needs. Among other things, the projected commencement and completion of the Company’s clinical trials may be affected by difficulties or delays. In addition, the Company’s results may be affected by i ts ability to manage its financial resources, difficulties or delays in developing manufacturing processes for its product candidates, preclinical an d toxicology testing and regulatory developments. Delays in clinical programs, whether caused by competitive developments, adverse events , p atient enrollment rates, regulatory issues or other factors, could adversely affect the Company’s financial position and prospects. Pr ior clinical trial program designs and results are not necessarily predictive of future clinical trial designs or results. If the Company’s pro duc t candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will no t be able to market them. The Company may not be able to enter into any strategic partnership agreements. The Company’s commercial success de pends on its ability to manufacture, market and sell products without infringing the proprietary rights of third parties. Operating ex pense and cash flow projections involve a high degree of uncertainty, including variances in future spending rates due to changes in corpora te priorities, the timing and outcomes of clinical trials, competitive developments and the impact on expenditures and available capital from li cen sing and strategic collaboration opportunities. If the Company is unable to raise additional capital when required or on acceptable t erm s, it may have to significantly delay, scale back or discontinue one or more of its drug development or discovery research programs. The Co mpa ny is at an early stage of development and may not ever have any products that generate significant revenue. The forward - looking statements contained in this presentation are further qualified by the detailed discussion of risks and uncertainties set forth in the documents f ile d by the Company with the Securities and Exchange Commission, all of which can be obtained on the Company’s website at www.lipocine.com or on the SEC website at www.sec.gov . The forward - looking statements contained in this document represent the Company’s estimates and assumptions only as of the date of this presentation and the Company undertakes no duty or obligation to update or revise publicly any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations.

Lipocine Investment Highlights 3 Innovative Oral Products for Men’s and Women’s Health Proprietary oral drug delivery technology to improve patient compliance TLANDO™ (LPCN 1021): Potential first oral Testosterone Replacement Therapy option • Differentiated product targeting ~$2.0 Billion established US TRT market • Validated "No Titration" Dosing Regimen With Positive Topline Efficacy Results • New Drug Application resubmission in August 2017 Additional pipeline assets advancing towards “Phase 3 ready” status • LPCN 1111: Next generation potential once - daily oral TRT option - Positive top - line Phase 2b study results - End of Phase 2 meeting with FDA anticipated in 4Q 2017 • LPCN 1107: Orphan designated oral alternative to current injectable for the prevention of preterm birth - Phase 3 protocol submitted to FDA via Special Protocol Assessment

4 Late - Stage Pipeline First Oral Products Targeting Significant Opportunities PRODUCT (Indication) RESEARCH / PRECLINICAL PHASE 1 PHASE 2 PHASE 3 NDA MEN’S HEALTH TLANDO (Oral Testosterone Replacement Therapy) NDA Resubmission August 2017 LPCN 1111 (Next Generation Oral T) End of P2 Meeting 4Q 2017 WOMEN’S HEALTH LPCN 1107 (Prevention of Preterm Birth) CMC: process characterization & scale - up complete 4Q 2017

Hypogonadism Affects Up to 20 M American Men 1,2 5 Significant Number of Untreated Hypogonadal Males ~6M Men with diagnosed hypogonadism 3 2.2M Men currently being treated 4 700,000 New naïve patients each year 5 1. US Census data. http://www.infoplease.com/us/census/data/demographic.html. 2. Mulligan T, et al. Int J Clin Pract . 2006 Jul;60(7):762 - 9. 3. Araujo, et al. J Clin Endo Metabol 2007. 92(11):4241 - 7. 4. Symphony Healthcare 2014 for FDA Advisory Meeting. 5. IMS Health Sept 2015. Asymptomatic and/or Undiagnosed Hypogonadism 70% Diagnosed Untreated 19% 68% 32% Treated 11% Previously Treated Treatment Naïve

2017 2016 2014 2015 350 375 400 425 450 475 500 525 550 575 600 625 Sep 14 Oct 14 Nov 14 Dec 14 Jan 15 Feb 15 Mar 15 Apr 15 May 15 Jun 15 Jul 15 Aug 15 Sep 15 Oct 15 Nov 15 Dec 15 Jan 16 Feb 16 Mar 16 Apr 16 May 16 Jun 16 Jul 16 Aug 16 Sep 16 Oct 16 Nov 16 Dec 16 Jan 17 Feb 17 Mar 17 Apr 17 May 17 Jun 17 Monthly TRx (000s) FDA Label Guidance TRT Market: Monthly TRx Trend Stable Following FDA Label Guidance ▪ Annual estimates of 6.8 million TRx 6 Source: Company reports, IMS database and UBS estimates TRx = Total prescriptions
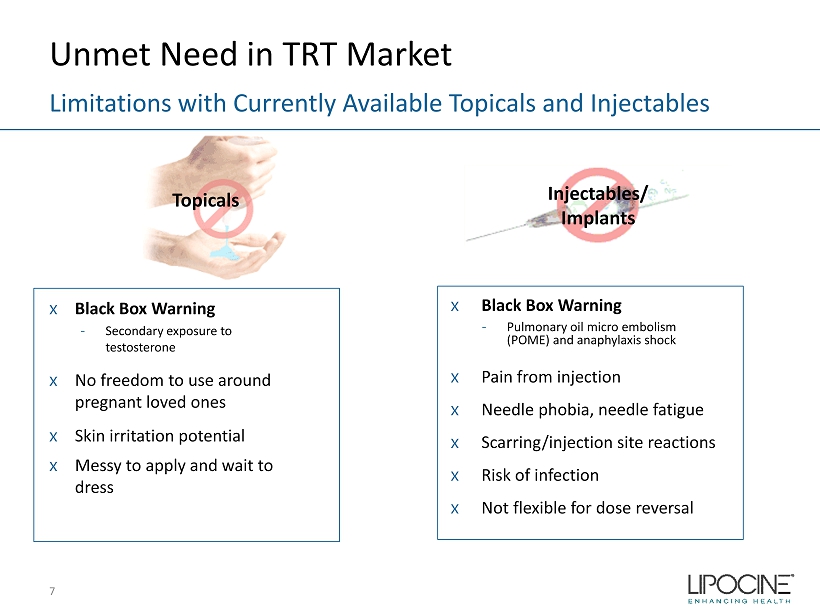
Unmet Need in TRT Market x Black Box Warning - Secondary exposure to testosterone x No freedom to use around pregnant loved ones x Skin irritation potential x Messy to apply and wait to dress Limitations with Currently Available Topicals and Injectables x Black Box Warning - Pulmonary oil micro embolism (POME) and anaphylaxis shock x Pain from injection x Needle phobia, needle fatigue x Scarring/injection site reactions x Risk of infection x Not flexible for dose reversal 7 Topicals Injectables / Implants

8 10.90% 23.40% 16.10% 19.20% 36.70% 16.30% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% M+1 M+2 M+3 M+4 M+5 M+6 M+7 M+8 M+9 M+10 M+11 M+12 Percentage of Patients Remaining “On Therapy” Androderm AndroGel 1.62% Axiron Fortesta Injectables Testim TRT Market: “On Therapy” Persistency Based on New - to - Market Cohorts (July 2013) 1 1. MABI’s TRT Patient Metrics (Powered by Source Healthcare Analytics Patient Data). All trademarks acknowledged. TRx leader $$ leader Poor Persistence Reflects Need for Oral Therapy Average Days on Therapy is 100 Days

Most Patients Need Dosage Adjustment with Current TRT Options A Significant Burden with Numerous Doctor Visits 9 40% Other 0 X 47% 1 X 33% 2 X 15% 3 X 2% >3 X 3% 0 X 37% 1 X 26% 2 X 20% 3 X 12% >3 X 5% Number of Current TRT Dose Adjustments by Form* Gel (n=200) Injectable (n=137) * Current TRT n=412 Q16. Since you started using your current testosterone medication, how many times was the dose adjusted up or down until you reached your current dose level?

TLANDO 10

• Met primary endpoint: 80% response rate with fixed twice daily (“BID”) dosing regimen vs. FDA requirement of 75% * using baseline carried forward approach with missing data/discontinuances (“worst - case analysis”) • Secondary endpoints generally consistent with approved products • No eligible subjects * with T levels >2500 ng/ dL • T levels not affected by food fat content EFFICACY ▪ 52 week long term exposure dataLPCN 1021 met Primary end • 525 unique hypogonadal men exposure - Well tolerated in 52 week exposure - AE profile comparable to active control, including GI * - No cardiac, hepatic or drug related SAEs • No apparent correlation of the observed Cmax excursions with ADRs, AEs or meaningful changes in critical lab parameters * SAFETY ▪ 52 week long term exposure dataLPCN 1021 met Primary end • Preferred Oral option - Non - invasive - Less cumbersome • Fixed dose/no dose titration - Simpler to prescribe - Fewer doctor visits - Easier for patients to properly use • No risk of accidental T transference BENEFITS 11 * See Appendix ✔ TLANDO: Potential First Oral Option Profile Demonstrated Clinically with Target Label Regimen

TLANDO: Patient Market Research 12 Patient Enthusiasm Evident About Oral TRT 12 80% of interviewed TRT patients 30% 30% “Oral Form ” 30% “It’s not Gel”, “ Not Injection” “ Ease of Use” Likely or Highly Likely to Ask Physician to Prescribe TLANDO What Most Like About TLANDO? Other • TLANDO generates strong patient enthusiasm among current and prior TRT users – Oral administration and lack of transference viewed as key benefits • For transdermal users, most common concern is transference risk – “Always worry with the kids.”; “Right now we have to plan sex.” – Gels and roll - on are messy to apply and often cause skin irritation • Injectable users complain about swings in testosterone levels resulting in “crash” before next dose – “I keep crashing two weeks after the injection” – Needle phobia/needle fatigue are common • Both transdermal and injection users also want better symptomatic efficacy 40% Other

TLANDO: Physician Market Research 13 Oral Agent is Most Important Attribute for Prescribers QUESTION: What is the most important advantage of TLANDO? 66% 10% 8% 8% 5% 3% No/Low Risk of… Good Efficacy Easy Dosing/ Administration Easy/Less Titration Few Side Effects/ Well-tolerated N=212 (All Respondents; URO=54, ENDO=53, PCP=105), TVG conducted market research. Q35a. In your opinion, what is the most important advantage of TLANDO? Oral Agent No Competing TRT Agent Can Offer

TLANDO: Development Plan 14 Next Steps to Bring TLANDO to Patients • Dosing Validation (“DV”) clinical study (pivotal efficacy study) results confirm efficacy with no titration – Addresses label titration related deficiency cited in CRL • Study of Oral Androgen Replacement (“SOAR”) Phase 3 clinical study (pivotal safety study) results support long - term safety NDA Resubmission Next steps: • NDA resubmission in August 2017 • Fully re - engage in partnering/licensing discussions • Targeted NDA acceptance by FDA in 3Q 2017 • Expected PDUFA date of 1Q 2018

TLANDO: DV Study (Pivotal Efficacy) – Efficacy Results Primary Endpoints – Demonstrated Robust Efficacy Measure FDA Targets Safety Set* “Worst Case” Analysis # Number of subjects 95 95 % subjects with C avg w ithin normal range (300 - 1080 ng/ dL ) ≥75% 81% 80% 95% CI lower bound ≥ 65% 72% 72% * Subjects randomized into the study and who took at least one dose of the study drug. Missing data imputed by multiple imput ati on. # Subjects randomized into the study and who took at least one dose of the study drug. Missing data imputed by baseline carri ed forward (i.e., considered treatment failures). 15

TLANDO: DV Study – Efficacy Results Secondary Endpoints Generally Met FDA Targets ▪ Proportion of subjects with T Cmax in predefined ranges against FDA targets (permissible excursions) that originally developed for transdermals - Key secondary endpoint (> 2500 ng/ dL ) • No trial eligible patient exceeded the predefined target* - Other secondary endpoints • Cmax per dose (highest peak in 12 hrs , each dose) o ≤ 1500 ng/ dL : 85% vs. target of ≥ 85% o Between 1800 and 2500 ng/ dL : 7% vs. target of ≤ 5% • Cmax per day (highest peak a day, two doses a day) o Deviations observed for ≤ 1500 , and between 1800 - 2500 thresholds vs FDA targets 16 * One single measurement of Cmax of 2720 ng/ dL was observed in the subject who was a major protocol violator as had a gastric surgery of cholecystectomy (i.e., gall bladder removal) and ineligible for the study

TLANDO: Long Term Safety Demonstrated Over 52 Weeks in SOAR (Pivotal Safety) Study AE’s greater than 5% TLANDO Active Control Upper Respiratory Tract Infection 5.2% 5.8% Fatigue 2.4% 6.7% ADRs greater than 2% TLANDO Active Control Headache 0.5% 2.9% Acne 2.4% 0.0% Safety Population: Subjects who received at least one dose of study drug, comprised of 314 subjects; 210 who received TLANDO and 104 who received the active control 17

TLANDO: Safety Summary Well Tolerated with No Drug Related SAE’s • Total TLANDO exposure in 525 unique patients – Mild to moderate Adverse Drug Reactions (ADRs) • Safety relevance of Cmax excursions * – No apparent correlation between observed Cmax excursions >1500 ng/ dL and observed safety parameters (ADRs, AEs of special interest, or changes in key lab. parameters) • Possibly due to transiency of excursions unlike QD transdermal products • Cmax excursions are generally non repeatable * See Appendix 18

LPCN 1111 19

LPCN 1111: Next - Generation Oral TRT • Novel bio - reversible prodrug of testosterone for oral delivery • Once - daily potential expected to sustain and improve market share of oral T franchise • Once - daily feasibility established in Phase 2a and 2b clinical trials - Single - daily oral dose provides T levels in eugonadal range • Development status - Next steps: • Preclinical toxicity study ongoing • End of Phase 2 meeting with FDA in 4Q 2017 post preclinical toxicity study 20 Potential Once - Daily Dosing

LPCN 1107 21

22 1 Pediatric Research (2006) 60, 775 – 776 LPCN 1107: Prevention of Preterm Birth (PTB) An Unmet Medical Need Preterm Birth O week 20 weeks 34 weeks 37 weeks 40 weeks O NE P RETERM B IRTH E VERY M INUTE 1

High PTB Medical Costs 23 ≥ $26 Billion Economic Impact 3 • 12% of all US pregnancies 1 (475 - 500K) result in PTB (< 37 weeks) - a leading cause of neonatal mortality and morbidity • First year medical costs for PTB infants are ~ 10x higher than for full term infants 2 • 28% of preterm births are to women with histories of early delivery 1. CDC (2010) 2. J. Maternal - Fetal and Neonatal Medicine, Dec. 2006, 19(12), 773 – 782 3 . Institute of Medicine of the National Academies. Jul.200

LPCN 1107: First Oral PTB Candidate 24 Addresses Unmet Need IM HPC, Makena ® : Current preterm birth standard of care • $334 M in sales in 2016 • Total of 18 - 22 injections – Viscous oily weekly injection – Injection takes up to 1 min – Weekly visit to/by health care provider – ~35% of patients experienced injection site pain during clinical trial – ~17% of patients reported site swelling - much greater than placebo during clinical trial Makena 21 gauge needle

LPCN 1107: First Oral PTB Candidate 25 Addresses Unmet Need LPCN 1107 - Oral HPC • Twice daily dose • Higher HPC levels with Phase 3 target dose vs. IM Injection, Makena® • No patient discomfort upon administration • Steady state achieved in 7 days • Orphan drug designation – Major contribution to patient care

LPCN 1107: HPC PK - PD Correlation 26 HPC Concentration and PTB Rate with IM HPC, Makena 1 46.3 % 27.0 % 29.6 % 31.3 % Quartile 1: 3.7 - 8.1 ng/mL Quartile 2: 8.2 - 9.8 ng/mL Quartile 3: 9.9 - 12.4 ng/mL Quartile 4: 12.5 - 56 ng/mL 15 25 35 45 55 6 8 10 12 14 16 PTB rate (%, N=315) HPC trough plasma concentration (ng/mL) • Lower % PTB rate can be expected with daily Cavg 2 HPC levels ≥ 8.2 ng/mL 1. Caritis et al., Am J Obstet Gynecol. 2014 (N=315 subjects) 2. Ctrough Cavg for IM HPC, Makena N=315

LPCN 1107: Dose Finding Study Design • Open - label, four - period, four - treatment study • 12 healthy pregnant women - Ages 18 - 35 years; 16 - 18 weeks gestation • All subjects received all four treatments 27 PK Study: Oral LPCN 1107 vs IM HPC, Makena Treatment A 400 mg BID Treatment B 600 mg BID Treatment C 800 mg BID Treatment D 250 mg Weekly LPCN 1107, Oral HPC IM HPC, Makena Multiple doses for 8 days Multiple dose: 5 weeks

LPCN 1107: Dose - Finding PK Study Results 1 28 Oral LPCN 1107 vs IM HPC, Makena 1. PK results obtained post 8 days of BID dosing for LPCN 1107 and post 5 weeks for weekly IM HPC, Makena Lower reported PTB rate threshold 0 10 20 30 40 50 60 0 200 400 600 800 1000 Cavg(0 - 24/ 0 - 168) (ng/mL) Dose (mg) IM 250 mg Oral 400 mg Oral 600 mg Oral 800 mg Target Phase 3 dose • HPC levels below 8.2 ng/mL: – Target LPCN 1107 Phase 3 dose was 0% vs 20% subjects using IM HPC Makena per label • Average HPC levels at target LPCN 1107 Phase 3 dose – ~ 3x greater than the comparator, IM HPC, Makena

LPCN 1107: Development Status 29 Key Design Elements for Phase 3 Study Proposed indication • To reduce the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth Design Elements • Open label, RCT, non - inferiority (“NI”) study with two treatment arms (LPCN 1107 and IM, Makena®) with 1:1 randomization • The primary efficacy analysis to demonstrate NI to Makena using a pre - specified NI margin of 7% • A standard statistical NI design of 90% power leads to ~ 1,100 subjects per arm • Phase 3 protocol includes an adaptive design • Interim analysis with pre - specified actions Next steps • Conduct CMC ongoing activities in preparation of Phase 3 study
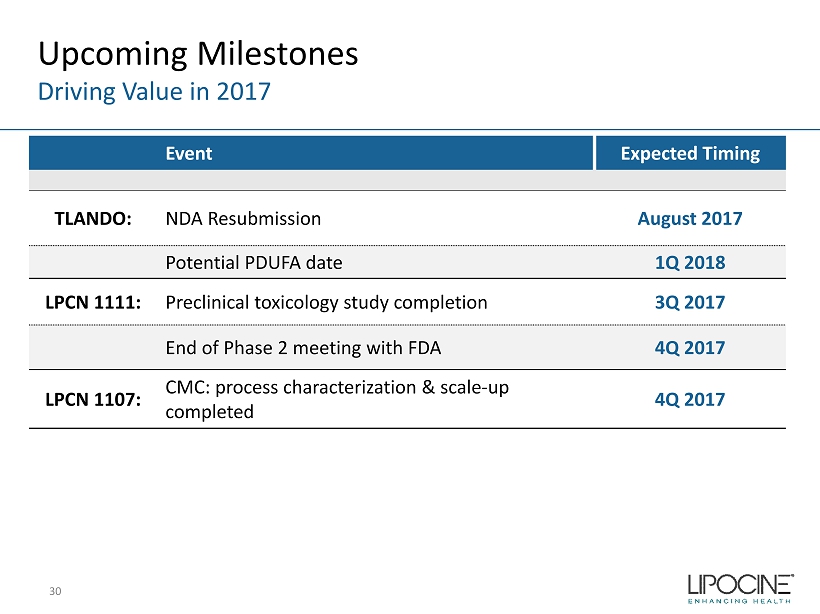
Upcoming Milestones Driving Value in 2017 30 Event Expected Timing TLANDO: NDA Resubmission August 2017 Potential PDUFA date 1Q 2018 LPCN 1111: Preclinical toxicology study completion 3Q 2017 End of Phase 2 meeting with FDA 4Q 2017 LPCN 1107: CMC: process characterization & scale - up completed 4Q 2017

Key Financial Metrics 31 Stock Price, Market Cap, Cash Balance Ticker Symbol LPCN (Nasdaq Capital Market) Closing Stock Price (8/4/17) $4.42/share Market Capitalization (8/4/17) $91.4 million Fully Diluted Shares Outstanding (6/30/17) 23.0 million Cash Balance (6/30/17) $27.8 million Bank Debt (6/30/17) None

Lipocine Investment Highlights 32 Innovative Oral Products for Men’s and Women’s Health Proprietary oral drug delivery technology to improve patient compliance TLANDO: Potential first oral Testosterone Replacement Therapy option • Differentiated product targeting ~$2.0 billion established US TRT market • Validated "No Titration" dosing regimen with positive topline efficacy results • New Drug Application resubmission in August 2017 Additional pipeline assets advancing towards “Phase 3 ready” status • LPCN 1111: Next generation potential once - daily oral TRT option - Positive top - line Phase 2b study results - End of Phase 2 meeting with FDA anticipated in 4Q 2017 • LPCN 1107: Orphan designated oral alternative to current injectable for the prevention of preterm birth - Phase 3 protocol submitted to FDA via Special Protocol Assessment

Appendix 33

TLANDO: Phase 3 Design Study of Androgen Replacement (SOAR) 34 Open - label, randomized, active - controlled study of LPCN 1021 in hypogonadal men Screening N=315 0 Week 4 Week 8 Randomization LPCN 1021 225 mg, TU, BID with Meal (n=210) Active Control (n=105) PK/Dose Titration PK/Dose Titration PK/Efficacy Assessment Safety Assessment Week 13 Week 52 Safety Extension (up to Week 52)

TLANDO: DV Study vs SOAR Trial Key Design & Efficacy Result Differences DV Study – 225 mg BID without titration – Taken with meal – No BMI restriction – Mean Cavg (CV): 476 ng/ dL (37%)* SOAR Trial – 225 mg BID with titration – Taken with standard (20% – 35% fat) meal – Exclude BMI > 38 kg/m2 – Mean Cavg (CV): 471 ng/ dL (41%)** 35 * Safety set **Full Analysis Set (FAS) ▪ Cmax excursions in DV study (fixed dose) are comparable or better than in SOAR Trial (titration) ▪ Much lower unacceptably high (> 2500 ng/ dL ) T level excursions in DV study than in SOAR trial ▪ AE profile and changes in key lab parameters were generally consistent between studies

Safety Relevance of TLANDO Cmax Excursions 36

TLANDO: DV Study vs SOAR Trial Cmax * Outside the Pre - defined Ranges are Transient 37 22.3 23.0 21.9 22.0 1.7 1.0 2.1 2.0 0 4 8 12 16 20 24 T Cmax > 1500 ng/dL T Cmax > 1800 ng/dL T Cmax > 1500 ng/dL T Cmax > 1800 ng/dL Time ( hr ) Time Spent in a day below (hr) Time Spent in a day above (hr) *All Cmax including permissible excursions DV Study SOAR Trial
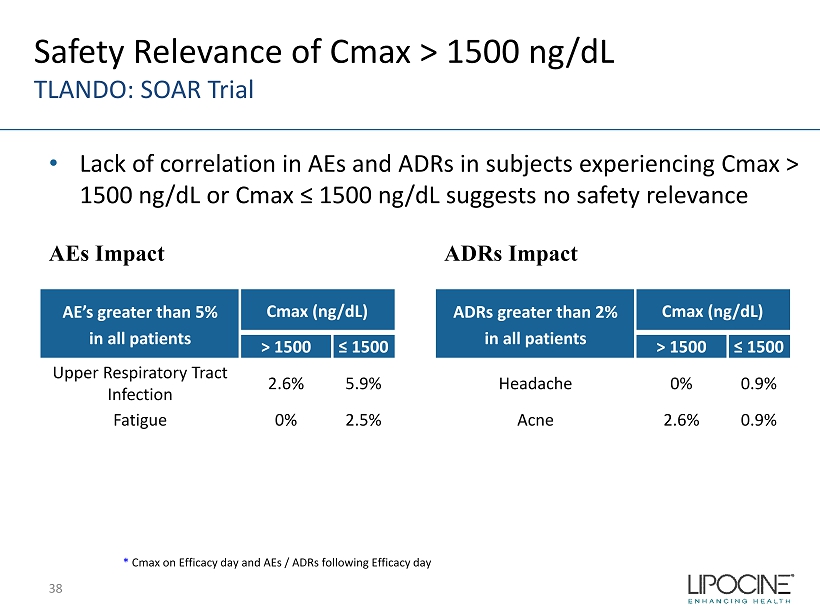
AE’s greater than 5% in all patients Cmax (ng/dL) > 1500 ≤ 1500 Upper Respiratory Tract Infection 2.6% 5.9% Fatigue 0% 2.5% ADRs greater than 2% in all patients Cmax (ng/dL) > 1500 ≤ 1500 Headache 0% 0.9% Acne 2.6% 0.9% AEs Impact * Cmax on Efficacy day and AEs / ADRs following Efficacy day Safety Relevance of Cmax > 1500 ng/ dL TLANDO: SOAR Trial ADRs Impact • Lack of correlation in AEs and ADRs in subjects experiencing Cmax > 1500 ng/ dL or Cmax ≤ 1500 ng/ dL suggests no safety relevance 38
