Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Aclaris Therapeutics, Inc. | ex-99d1.htm |
| 8-K - 8-K - Aclaris Therapeutics, Inc. | f8-k.htm |
Exhibit 99.2
|
|
© Copyright 2017 Aclaris Therapeutics. All rights reserved. A-1 Dr. Neal Walker President and CEO August 2017 CONFLUENCE LIFE SCIENCES ACQUISITION |
|
|
Any statements contained in this presentation that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as "believe", "expect", "may", "plan," "potential," "will," and similar expressions, and are based on Aclaris' current beliefs and expectations. These forward-looking statements include expectations regarding the potential benefits of the Confluence acquisition and the clinical development of the combined companies’ drug candidates. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the conduct of clinical trials, Aclaris' reliance on third parties over which it may not always have full control, and other risks and uncertainties that are described in the Risk Factors section of Aclaris' Annual Report on Form 10-K for the year ended December 31, 2016, Aclaris’ Quarterly Report in Form 10-Q for the quarter ended June 30, 2017, and other filings Aclaris makes with the U.S. Securities and Exchange Commission from time to time. These documents are available under the "Financial Information" section of the Investors page of Aclaris' website at http://www.aclaristx.com. Any forward-looking statements speak only as of the date of this presentation and are based on information available to Aclaris as of the date of this release, and Aclaris assumes no obligation to, and does not intend to, update any forward-looking statements, whether as a result of new information, future events or otherwise This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Disclaimer |
|
|
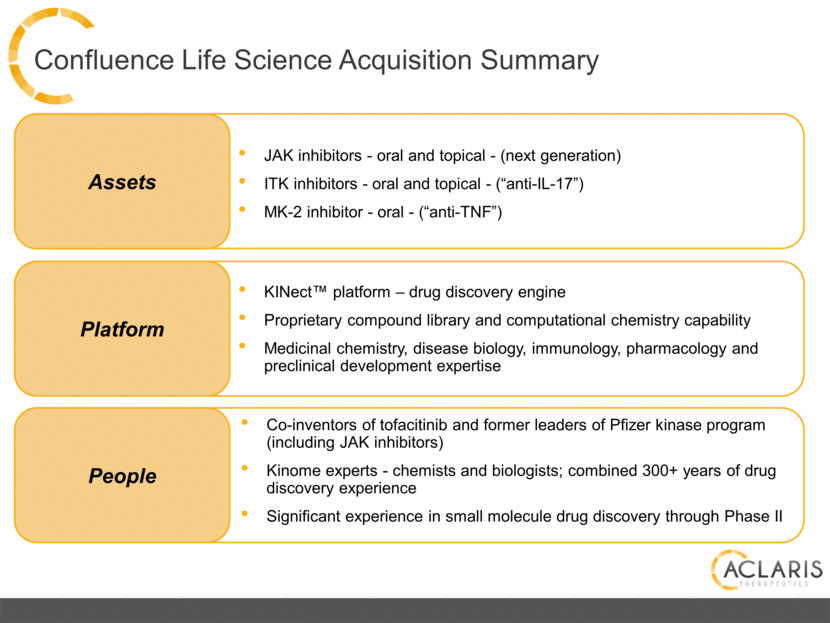
Confluence Life Science Acquisition Summary Co-inventors of tofacitinib and former leaders of Pfizer kinase program (including JAK inhibitors) Kinome experts - chemists and biologists; combined 300+ years of drug discovery experience Significant experience in small molecule drug discovery through Phase II People Platform Assets JAK inhibitors - oral and topical - (next generation) ITK inhibitors - oral and topical - (“anti-IL-17”) MK-2 inhibitor - oral - (“anti-TNF”) KINect™ platform – drug discovery engine Proprietary compound library and computational chemistry capability Medicinal chemistry, disease biology, immunology, pharmacology and preclinical development expertise |
|
|
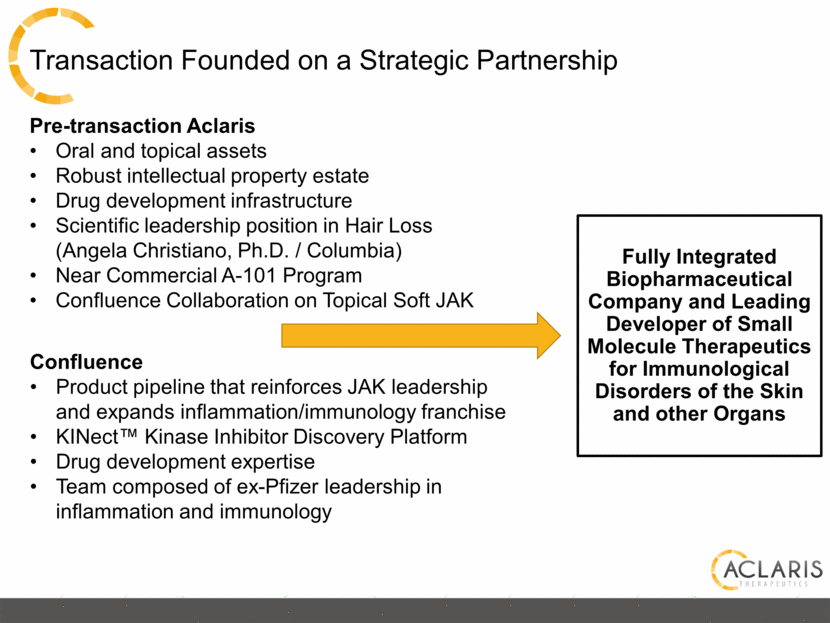
Transaction Founded on a Strategic Partnership Fully Integrated Biopharmaceutical Company and Leading Developer of Small Molecule Therapeutics for Immunological Disorders of the Skin and other Organs Pre-transaction Aclaris Oral and topical assets Robust intellectual property estate Drug development infrastructure Scientific leadership position in Hair Loss (Angela Christiano, Ph.D. / Columbia) Near Commercial A-101 Program Confluence Collaboration on Topical Soft JAK Confluence Product pipeline that reinforces JAK leadership and expands inflammation/immunology franchise KINect™ Kinase Inhibitor Discovery Platform Drug development expertise Team composed of ex-Pfizer leadership in inflammation and immunology |
|
|
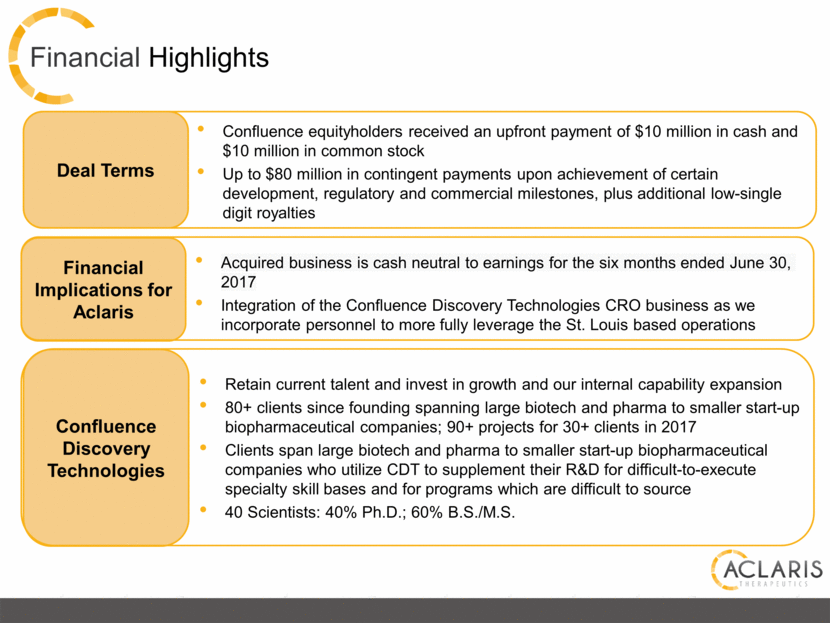
Financial Highlights Confluence equityholders received an upfront payment of $10 million in cash and $10 million in common stock Up to $80 million in contingent payments upon achievement of certain development, regulatory and commercial milestones, plus additional low-single digit royalties Deal Terms Financial Implications for Aclaris Acquired business is cash neutral to earnings for the six months ended June 30, 2017 Integration of the Confluence Discovery Technologies CRO business as we incorporate personnel to more fully leverage the St. Louis based operations Retain current talent and invest in growth and our internal capability expansion 80+ clients since founding spanning large biotech and pharma to smaller start-up biopharmaceutical companies; 90+ projects for 30+ clients in 2017 Clients span large biotech and pharma to smaller start-up biopharmaceutical companies who utilize CDT to supplement their R&D for difficult-to-execute specialty skill bases and for programs which are difficult to source 40 Scientists: 40% Ph.D.; 60% B.S./M.S. Confluence Discovery Technologies |
|
|
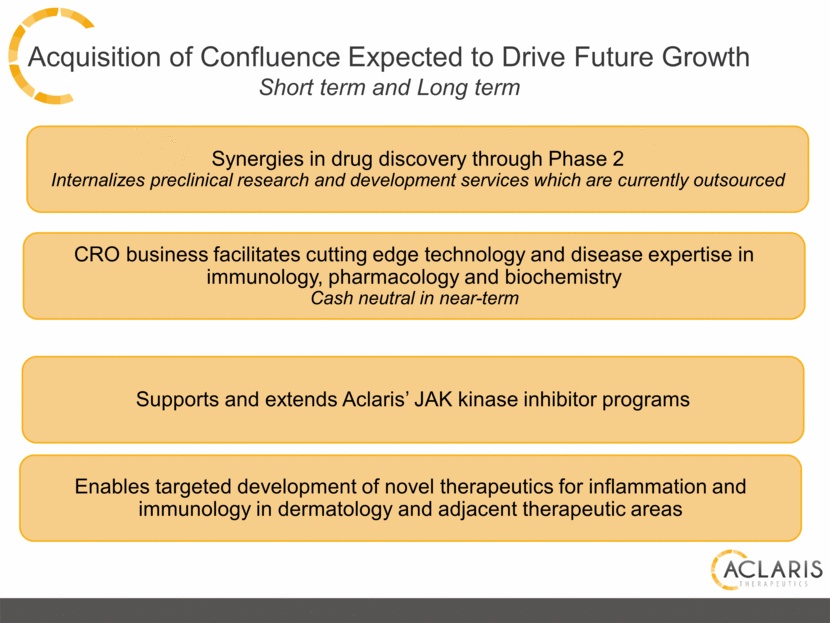
Acquisition of Confluence Expected to Drive Future Growth Short term and Long term Supports and extends Aclaris’ JAK kinase inhibitor programs Enables targeted development of novel therapeutics for inflammation and immunology in dermatology and adjacent therapeutic areas Synergies in drug discovery through Phase 2 Internalizes preclinical research and development services which are currently outsourced CRO business facilitates cutting edge technology and disease expertise in immunology, pharmacology and biochemistry Cash neutral in near-term |
|
|
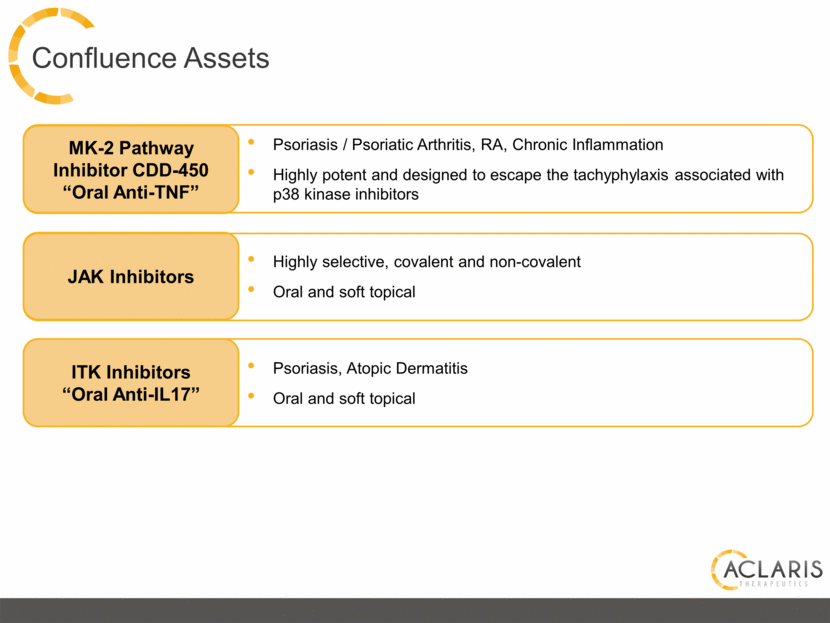
Confluence Assets ITK Inhibitors “Oral Anti-IL17” Psoriasis, Atopic Dermatitis Oral and soft topical Highly selective, covalent and non-covalent Oral and soft topical JAK Inhibitors Psoriasis / Psoriatic Arthritis, RA, Chronic Inflammation Highly potent and designed to escape the tachyphylaxis associated with p38 kinase inhibitors MK-2 Pathway Inhibitor CDD-450 “Oral Anti-TNF” |
|
|
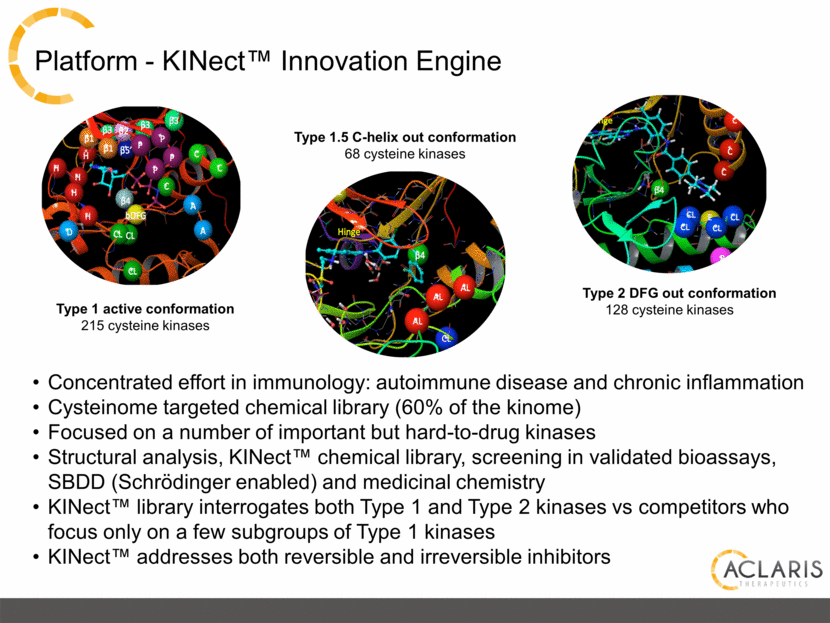
Platform - KINect™ Innovation Engine Type 1 active conformation 215 cysteine kinases Type 2 DFG out conformation 128 cysteine kinases Type 1.5 C-helix out conformation 68 cysteine kinases Concentrated effort in immunology: autoimmune disease and chronic inflammation Cysteinome targeted chemical library (60% of the kinome) Focused on a number of important but hard-to-drug kinases Structural analysis, KINect™ chemical library, screening in validated bioassays, SBDD (Schrödinger enabled) and medicinal chemistry KINect™ library interrogates both Type 1 and Type 2 kinases vs competitors who focus only on a few subgroups of Type 1 kinases KINect™ addresses both reversible and irreversible inhibitors |
|
|
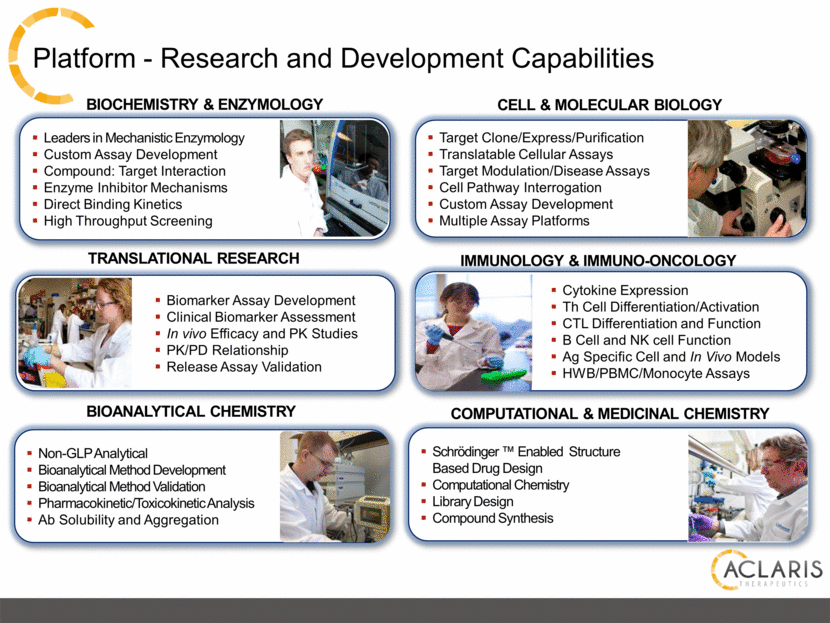
Platform - Research and Development Capabilities Biomarker Assay Development Clinical Biomarker Assessment In vivo Efficacy and PK Studies PK/PD Relationship Release Assay Validation TRANSLATIONAL RESEARCH COMPUTATIONAL & MEDICINAL CHEMISTRY Leaders in Mechanistic Enzymology Custom Assay Development Compound: Target Interaction Enzyme Inhibitor Mechanisms Direct Binding Kinetics High Throughput Screening CELL & MOLECULAR BIOLOGY BIOCHEMISTRY & ENZYMOLOGY BIOANALYTICAL CHEMISTRY IMMUNOLOGY & IMMUNO-ONCOLOGY Cytokine Expression Th Cell Differentiation/Activation CTL Differentiation and Function B Cell and NK cell Function Ag Specific Cell and In Vivo Models HWB/PBMC/Monocyte Assays Non-GLP Analytical Bioanalytical Method Development Bioanalytical Method Validation Pharmacokinetic/Toxicokinetic Analysis Ab Solubility and Aggregation Schrödinger ™ Enabled Structure Based Drug Design Computational Chemistry Library Design Compound Synthesis Target Clone/Express/Purification Translatable Cellular Assays Target Modulation/Disease Assays Cell Pathway Interrogation Custom Assay Development Multiple Assay Platforms |
|
|
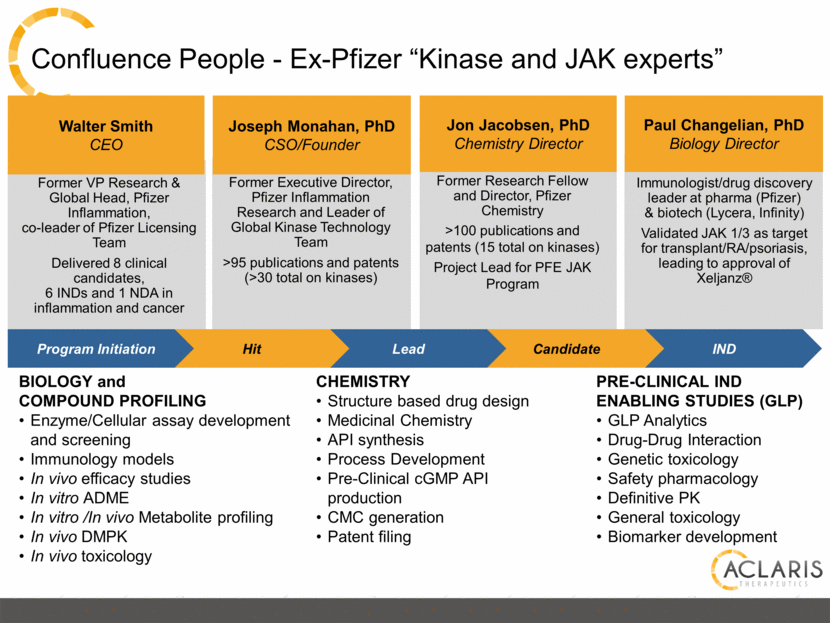
Confluence People - Ex-Pfizer “Kinase and JAK experts” Former Executive Director, Pfizer Inflammation Research and Leader of Global Kinase Technology Team >95 publications and patents (>30 total on kinases) Joseph Monahan, PhD CSO/Founder Former VP Research & Global Head, Pfizer Inflammation, co-leader of Pfizer Licensing Team Delivered 8 clinical candidates, 6 INDs and 1 NDA in inflammation and cancer Immunologist/drug discovery leader at pharma (Pfizer) & biotech (Lycera, Infinity) Validated JAK 1/3 as target for transplant/RA/psoriasis, leading to approval of Xeljanz® Paul Changelian, PhD Biology Director Walter Smith CEO Jon Jacobsen, PhD Chemistry Director Former Research Fellow and Director, Pfizer Chemistry >100 publications and patents (15 total on kinases) Project Lead for PFE JAK Program IND Candidate Lead Hit Program Initiation BIOLOGY and COMPOUND PROFILING Enzyme/Cellular assay development and screening Immunology models In vivo efficacy studies In vitro ADME In vitro /In vivo Metabolite profiling In vivo DMPK In vivo toxicology CHEMISTRY Structure based drug design Medicinal Chemistry API synthesis Process Development Pre-Clinical cGMP API production CMC generation Patent filing PRE-CLINICAL IND ENABLING STUDIES (GLP) GLP Analytics Drug-Drug Interaction Genetic toxicology Safety pharmacology Definitive PK General toxicology Biomarker development |
|
|
The Kinase Opportunity – Rational Targeted Drug Discovery Creating New Medicines Targeting Previously Inaccessible Parts of the Kinome Kinase Drugs Represented $240B in Aggregate Global Sales from 2011-2015 Proprietary chemical library and integrated capabilities for interrogating the Kinome Solves challenges encountered in the class Selectivity Biochemical efficiency Validity of targeting kinases is commercially established Plethora of validated kinase targets are inadequately drugged Kinect™ platform allows rational targeting of validated kinase targets KINect™ Technology Platform 500 member class, representing 2% of the human genome |
|
|
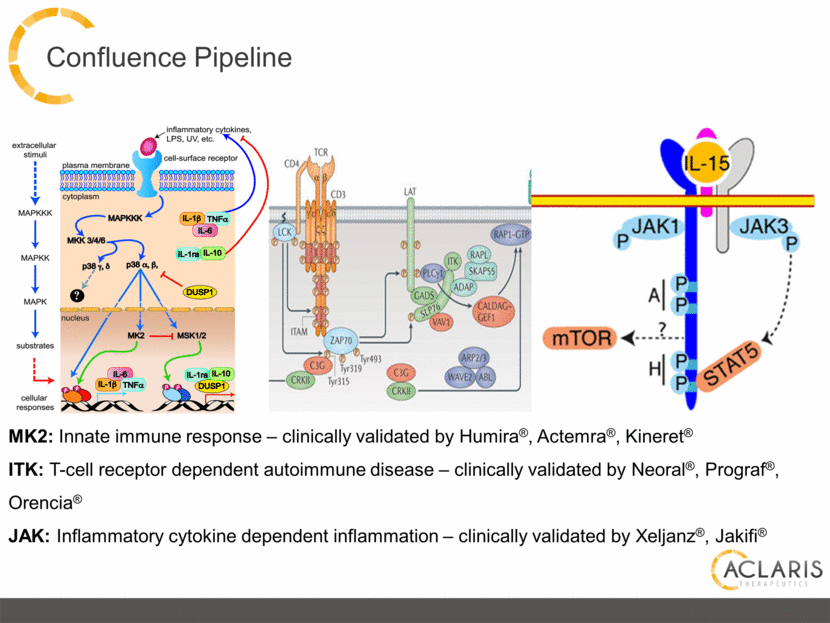
Confluence Pipeline MK2: Innate immune response – clinically validated by Humira®, Actemra®, Kineret® ITK: T-cell receptor dependent autoimmune disease – clinically validated by Neoral®, Prograf®, Orencia® JAK: Inflammatory cytokine dependent inflammation – clinically validated by Xeljanz®, Jakifi® |
|
|
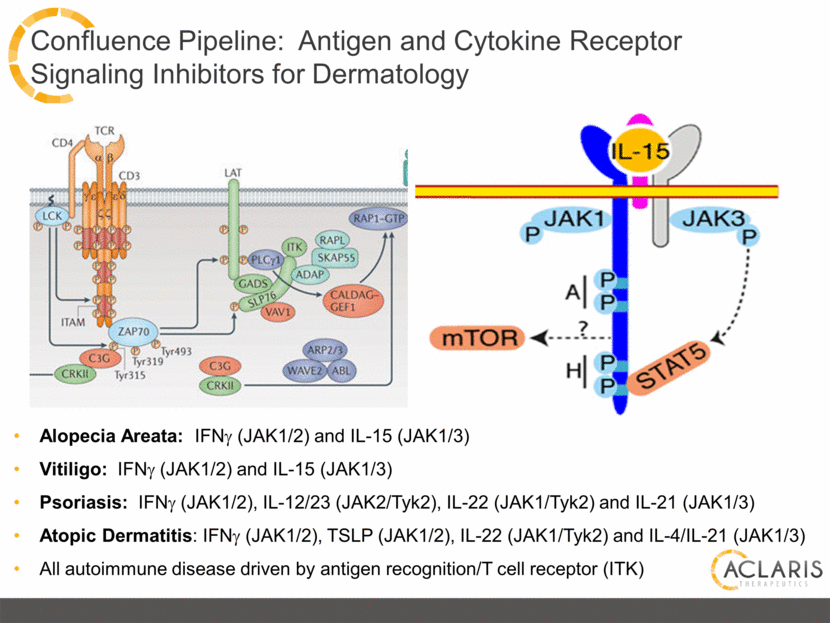
Confluence Pipeline: Antigen and Cytokine Receptor Signaling Inhibitors for Dermatology Alopecia Areata: IFNg (JAK1/2) and IL-15 (JAK1/3) Vitiligo: IFNg (JAK1/2) and IL-15 (JAK1/3) Psoriasis: IFNg (JAK1/2), IL-12/23 (JAK2/Tyk2), IL-22 (JAK1/Tyk2) and IL-21 (JAK1/3) Atopic Dermatitis: IFNg (JAK1/2), TSLP (JAK1/2), IL-22 (JAK1/Tyk2) and IL-4/IL-21 (JAK1/3) All autoimmune disease driven by antigen recognition/T cell receptor (ITK) |
|
|
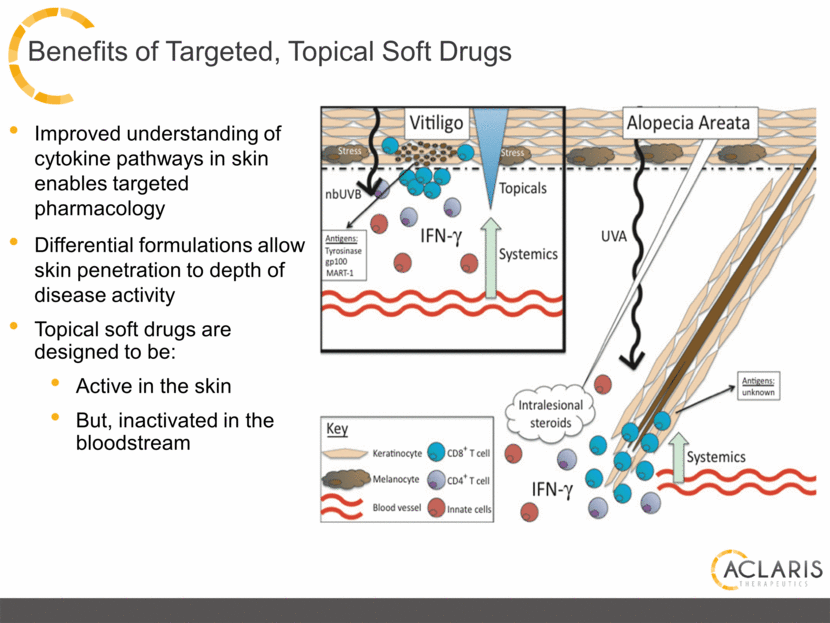
Benefits of Targeted, Topical Soft Drugs Improved understanding of cytokine pathways in skin enables targeted pharmacology Differential formulations allow skin penetration to depth of disease activity Topical soft drugs are designed to be: Active in the skin But, inactivated in the bloodstream |
|
|
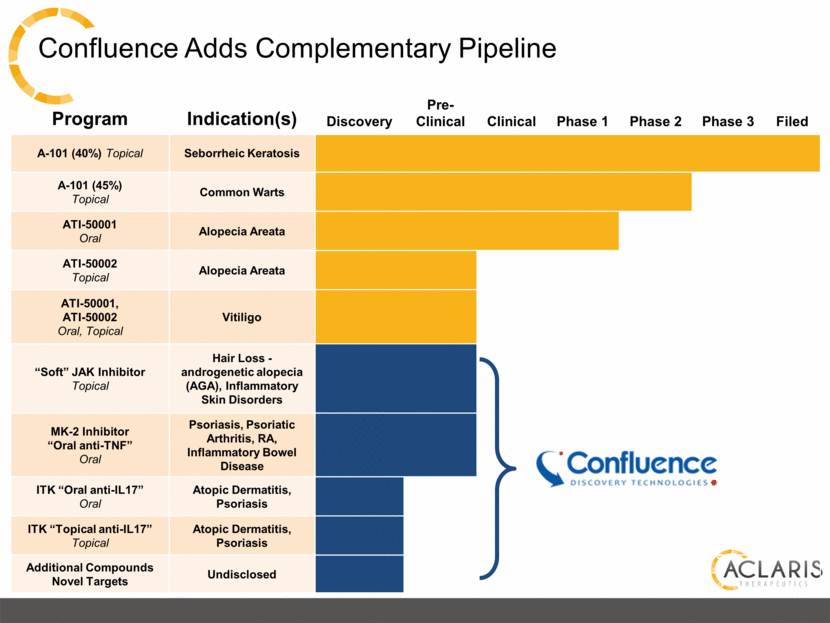
Program Indication(s) Discovery Pre-Clinical Clinical Phase 1 Phase 2 Phase 3 Filed A-101 (40%) Topical Seborrheic Keratosis A-101 (45%) Topical Common Warts ATI-50001 Oral Alopecia Areata ATI-50002 Topical Alopecia Areata ATI-50001, ATI-50002 Oral, Topical Vitiligo “Soft” JAK Inhibitor Topical Hair Loss - androgenetic alopecia (AGA), Inflammatory Skin Disorders MK-2 Inhibitor “Oral anti-TNF” Oral Psoriasis, Psoriatic Arthritis, RA, Inflammatory Bowel Disease ITK “Oral anti-IL17” Oral Atopic Dermatitis, Psoriasis ITK “Topical anti-IL17” Topical Atopic Dermatitis, Psoriasis Additional Compounds Novel Targets Undisclosed Confluence Adds Complementary Pipeline |
|
|
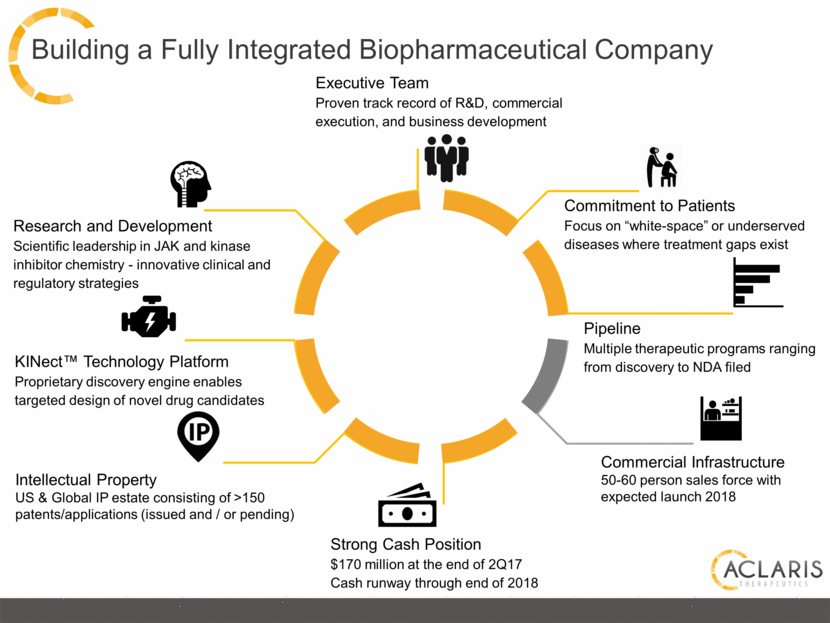
Building a Fully Integrated Biopharmaceutical Company Commitment to Patients Focus on “white-space” or underserved diseases where treatment gaps exist Research and Development Scientific leadership in JAK and kinase inhibitor chemistry - innovative clinical and regulatory strategies Innovative and differentiated clinical and regulatory strategies Executive Team Proven track record of R&D, commercial execution, and business development Pipeline Multiple therapeutic programs ranging from discovery to NDA filed Strong Cash Position $170 million at the end of 2Q17 Cash runway through end of 2018 KINect™ Technology Platform Proprietary discovery engine enables targeted design of novel drug candidates Commercial Infrastructure 50-60 person sales force with expected launch 2018 Intellectual Property US & Global IP estate consisting of >150 patents/applications (issued and / or pending) |