Attached files
| file | filename |
|---|---|
| 8-K - 8-K - CURIS INC | d436163d8k.htm |
| EX-99.1 - EX-99.1 - CURIS INC | d436163dex991.htm |

Corporate Presentation August 2017 Exhibit 99.2

This presentation contains certain forward-looking statements about Curis, Inc. (“we,” “us,” or the “Company”) within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as “expect(s),” “feel(s),” “believe(s),” “will,” “may,” “anticipate(s)” and similar expressions are intended to identify forward-looking statements. Forward-looking statements are statements that are not historical facts, reflect management’s expectations as of the date of this presentation, and involve risks and uncertainties. Forward-looking statements herein include, but are not limited to, statements with respect to the timing and results of future clinical and pre-clinical milestones; the timing of future preclinical studies and clinical trials and results of these studies and trials; the clinical and therapeutic potential of our drug candidates; and management’s ability to successfully achieve its goals. These forward-looking statements are based on our current expectations and may differ materially from actual results due to a variety of important factors including, without limitation, risks relating to: whether any of our drug candidates will advance further in the clinical development process and whether and when, if at all, they will receive approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies; whether historical preclinical results will be predictive of future clinical trial results; whether historical clinical trial results will be predictive of future trial results; whether any of our drug candidate discovery and development efforts will be successful; whether any of our drug candidates will be successfully marketed if approved; our ability to achieve the benefits contemplated by our collaboration agreements; management’s ability to successfully achieve its goals; our ability to raise additional capital to fund our operations on terms acceptable to us; general economic conditions; competition; and the other risk factors contained in our periodic and interim reports filed with the Securities and Exchange Commission which are available on the SEC website at www.sec.gov. You are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events, except as required by law. Forward Looking Statements
Mission: Innovative Medicines to Treat Cancer Effectively Precision Oncology Oncology-Focused Biotech Pipeline of differentiated small molecule cancer drug candidates Developing and intend to commercialize oncology drugs Partnerships: Aurigene, Genentech/Roche Immuno-oncology
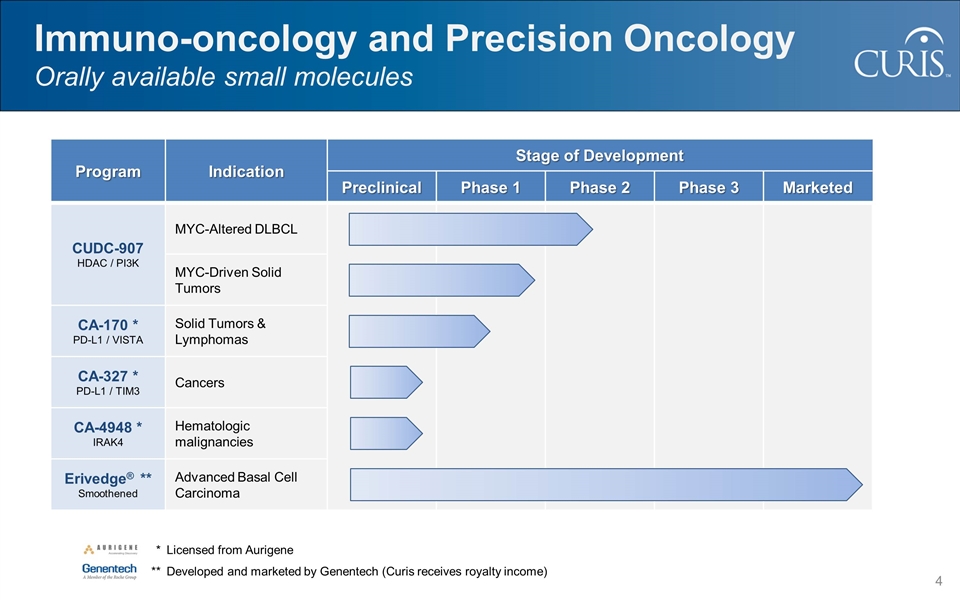
Program Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Marketed CUDC-907 HDAC / PI3K MYC-Altered DLBCL MYC-Driven Solid Tumors CA-170 * PD-L1 / VISTA Solid Tumors & Lymphomas CA-327 * PD-L1 / TIM3 Cancers CA-4948 * IRAK4 Hematologic malignancies Erivedge® ** Smoothened Advanced Basal Cell Carcinoma Immuno-oncology and Precision Oncology Orally available small molecules * Licensed from Aurigene **Developed and marketed by Genentech (Curis receives royalty income)

CUDC-907 has the potential to become a treatment for MYC-altered cancers CUDC-907 Phase 2 drug candidate to treat MYC-driven cancers Program Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Marketed CUDC-907 HDAC / PI3K MYC-Altered DLBCL MYC-Driven Solid Tumors
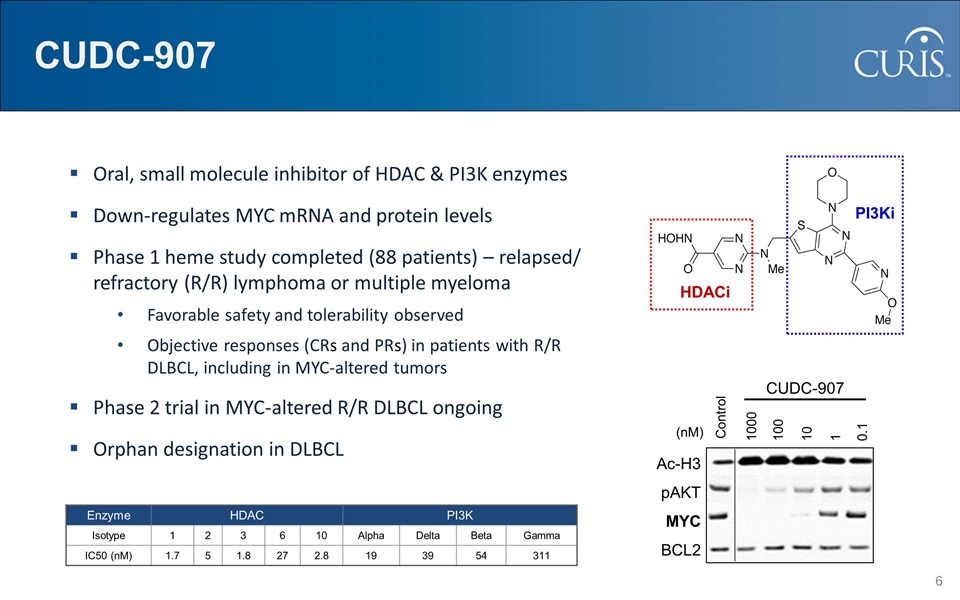
HDACi PI3Ki CUDC-907 Oral, small molecule inhibitor of HDAC & PI3K enzymes Down-regulates MYC mRNA and protein levels Phase 1 heme study completed (88 patients) – relapsed/ refractory (R/R) lymphoma or multiple myeloma Favorable safety and tolerability observed Objective responses (CRs and PRs) in patients with R/R DLBCL, including in MYC-altered tumors Phase 2 trial in MYC-altered R/R DLBCL ongoing Orphan designation in DLBCL Enzyme HDAC PI3K Isotype 1 2 3 6 10 Alpha Delta Beta Gamma IC50 (nM) 1.7 5 1.8 27 2.8 19 39 54 311 CUDC-907 Control 1000 100 10 1 0.1 Ac-H3 pAKT MYC BCL2 (nM)
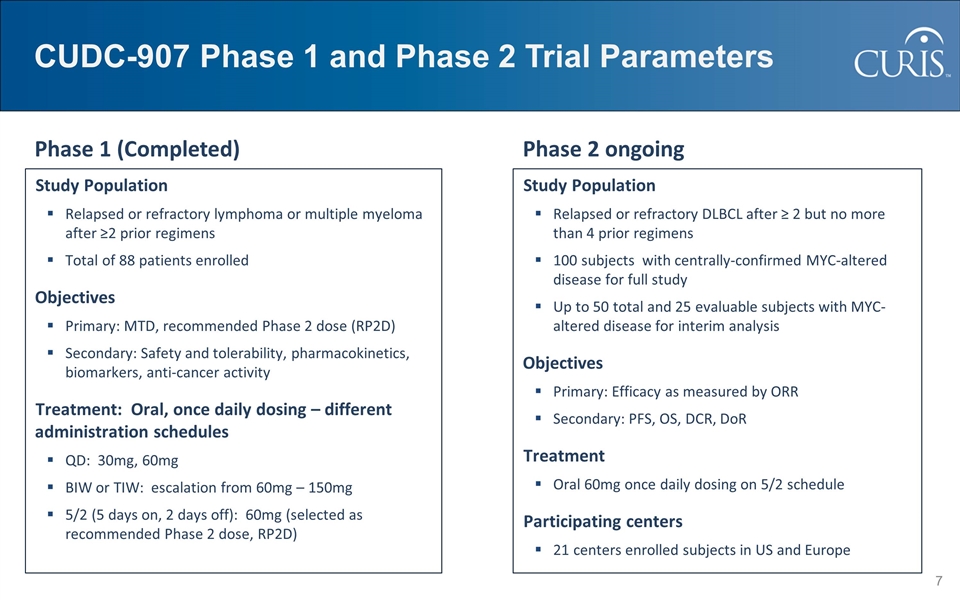
CUDC-907 Phase 1 and Phase 2 Trial Parameters Study Population Relapsed or refractory lymphoma or multiple myeloma after ≥2 prior regimens Total of 88 patients enrolled Objectives Primary: MTD, recommended Phase 2 dose (RP2D) Secondary: Safety and tolerability, pharmacokinetics, biomarkers, anti-cancer activity Treatment: Oral, once daily dosing – different administration schedules QD: 30mg, 60mg BIW or TIW: escalation from 60mg – 150mg 5/2 (5 days on, 2 days off): 60mg (selected as recommended Phase 2 dose, RP2D) Study Population Relapsed or refractory DLBCL after ≥ 2 but no more than 4 prior regimens 100 subjects with centrally-confirmed MYC-altered disease for full study Up to 50 total and 25 evaluable subjects with MYC-altered disease for interim analysis Objectives Primary: Efficacy as measured by ORR Secondary: PFS, OS, DCR, DoR Treatment Oral 60mg once daily dosing on 5/2 schedule Participating centers 21 centers enrolled subjects in US and Europe Phase 1 (Completed) Phase 2 ongoing
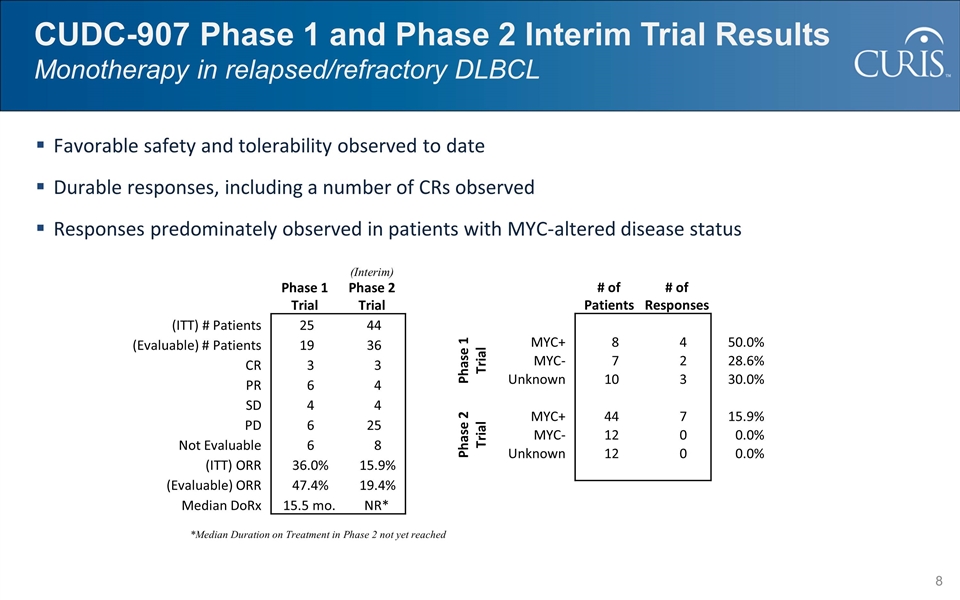
CUDC-907 Phase 1 and Phase 2 Interim Trial Results Monotherapy in relapsed/refractory DLBCL Favorable safety and tolerability observed to date Durable responses, including a number of CRs observed Responses predominately observed in patients with MYC-altered disease status Phase 1 Trial (Interim) Phase 2 Trial (ITT) # Patients 25 44 (Evaluable) # Patients 19 36 CR 3 3 PR 6 4 SD 4 4 PD 6 25 Not Evaluable 6 8 (ITT) ORR 36.0% 15.9% (Evaluable) ORR 47.4% 19.4% Median DoRx 15.5 mo. NR* *Median Duration on Treatment in Phase 2 not yet reached # of Patients # of Responses Phase 1 Trial MYC+ 8 4 50.0% MYC- 7 2 28.6% Unknown 10 3 30.0% Phase 2 Trial MYC+ 44 7 15.9% MYC- 12 0 0.0% Unknown 12 0 0.0%
CUDC-907 Phase 2 Trial Status Monotherapy in MYC-altered relapsed/refractory DLBCL Open label study to evaluate the efficacy and safety of CUDC-907 monotherapy in patients with relapsed/refractory (R/R) DLBCL – after 2 prior treatments Study Status Interim analysis conducted following enrollment of 68 patients No new safety findings Durable CRs and PRs observed selectively in patients with MYC-altered DLBCL ORR of 19.4% in evaluable patients is below internally-set threshold of 30% Evaluating alternative designs for a separate registration-enabling trial to better capture and demonstrate CUDC-907 benefit in patients with MYC-altered DLBCL
CA-170 is a first-in-class oral small molecule immune checkpoint inhibitor In Phase 1 clinical development CA-170 Oral, small molecule checkpoint inhibitor – PDL1 and VISTA Program Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Marketed CA-170 PD-L1 / VISTA Solid Tumors & Lymphomas
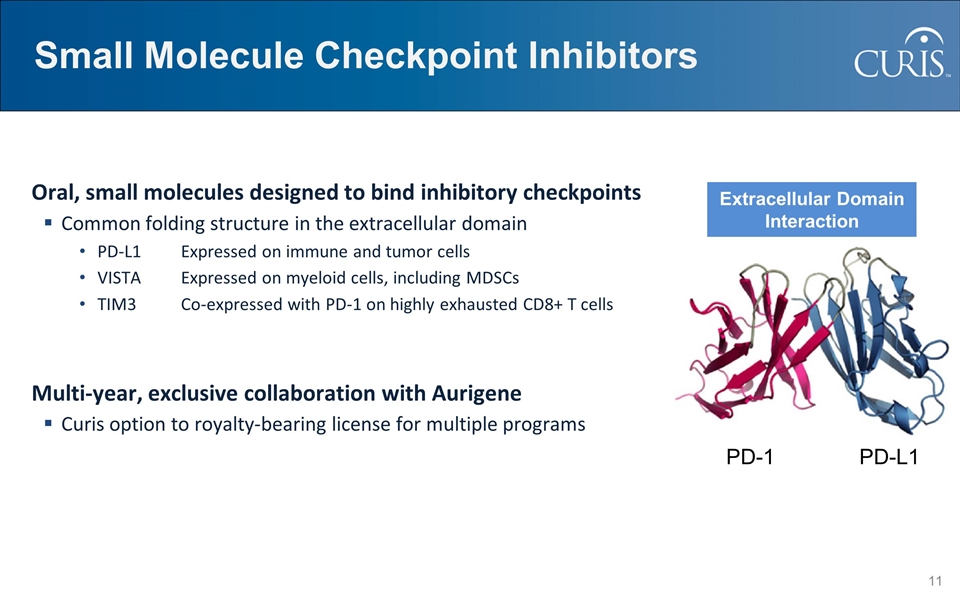
Small Molecule Checkpoint Inhibitors Oral, small molecules designed to bind inhibitory checkpoints Common folding structure in the extracellular domain PD-L1Expressed on immune and tumor cells VISTAExpressed on myeloid cells, including MDSCs TIM3Co-expressed with PD-1 on highly exhausted CD8+ T cells Multi-year, exclusive collaboration with Aurigene Curis option to royalty-bearing license for multiple programs PD-1 PD-L1 Extracellular Domain Interaction
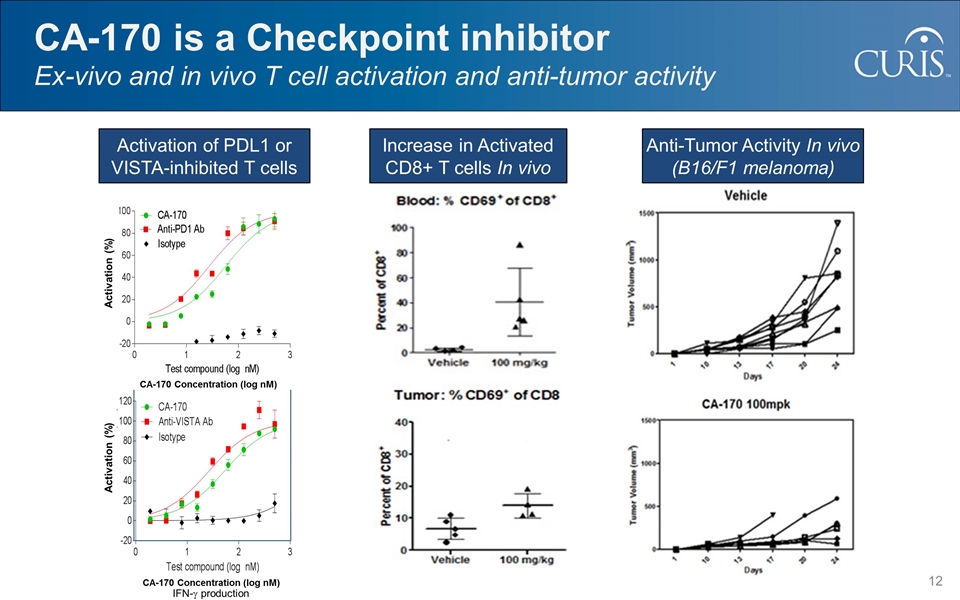
CA-170 Concentration (log nM) Activation (%) CA-170 is a Checkpoint inhibitor Ex-vivo and in vivo T cell activation and anti-tumor activity Increase in Activated CD8+ T cells In vivo Activation of PDL1 or VISTA-inhibited T cells Activation (%) CA-170 Concentration (log nM) Anti-Tumor Activity In vivo (B16/F1 melanoma) IFN-g production
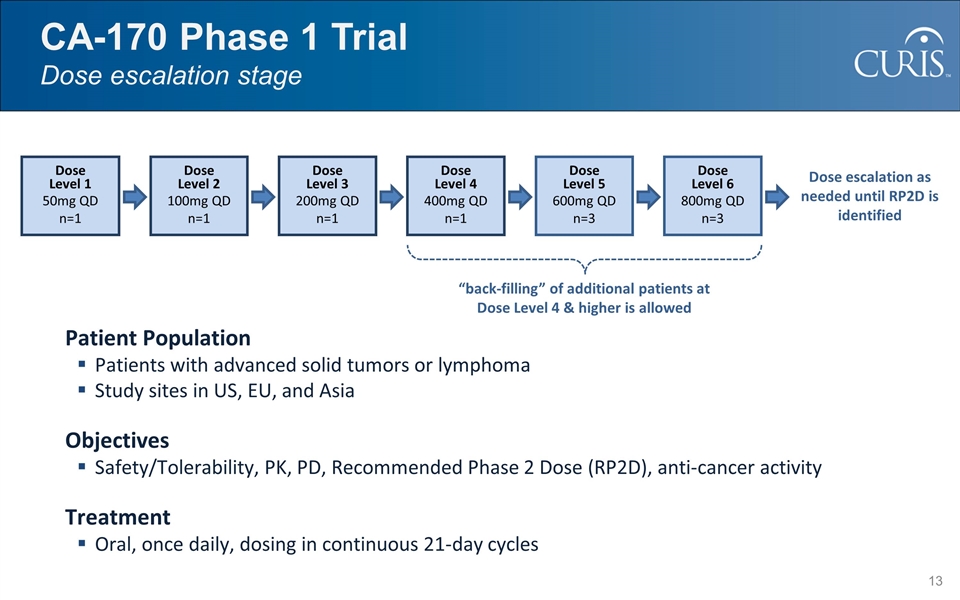
CA-170 Phase 1 Trial Dose escalation stage Patient Population Patients with advanced solid tumors or lymphoma Study sites in US, EU, and Asia Objectives Safety/Tolerability, PK, PD, Recommended Phase 2 Dose (RP2D), anti-cancer activity Treatment Oral, once daily, dosing in continuous 21-day cycles Dose escalation as needed until RP2D is identified “back-filling” of additional patients at Dose Level 4 & higher is allowed Dose Level 1 50mg QD n=1 Dose Level 2 100mg QD n=1 Dose Level 3 200mg QD n=1 Dose Level 4 400mg QD n=1 Dose Level 5 600mg QD n=3 Dose Level 6 800mg QD n=3
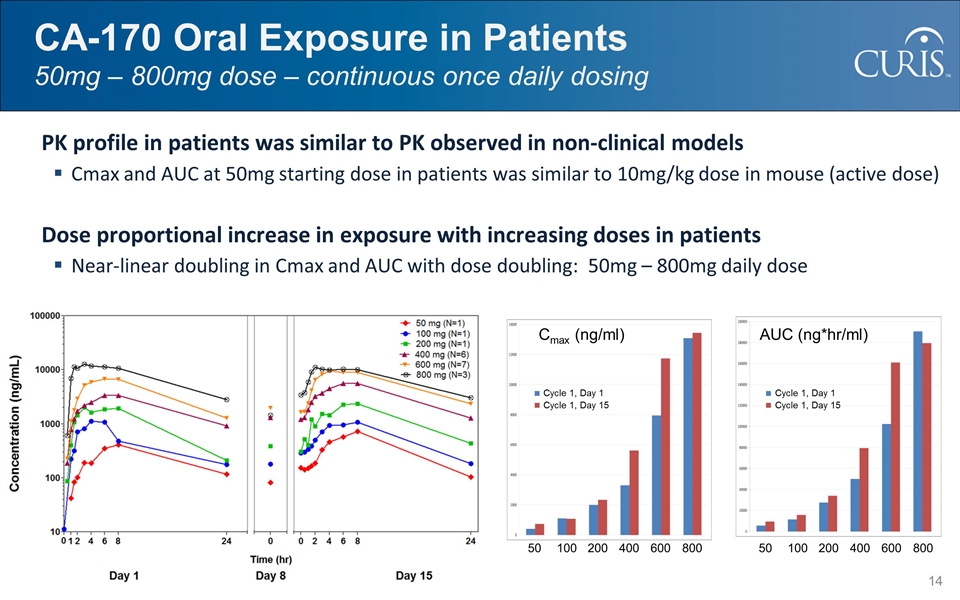
CA-170 Oral Exposure in Patients 50mg – 800mg dose – continuous once daily dosing PK profile in patients was similar to PK observed in non-clinical models Cmax and AUC at 50mg starting dose in patients was similar to 10mg/kg dose in mouse (active dose) Dose proportional increase in exposure with increasing doses in patients Near-linear doubling in Cmax and AUC with dose doubling: 50mg – 800mg daily dose Human PK Profile 50 100 200 400 600 800 50 100 200 400 600 800 Cycle 1, Day 1 Cycle 1, Day 15 Cmax (ng/ml) Cycle 1, Day 1 Cycle 1, Day 15 AUC (ng*hr/ml)
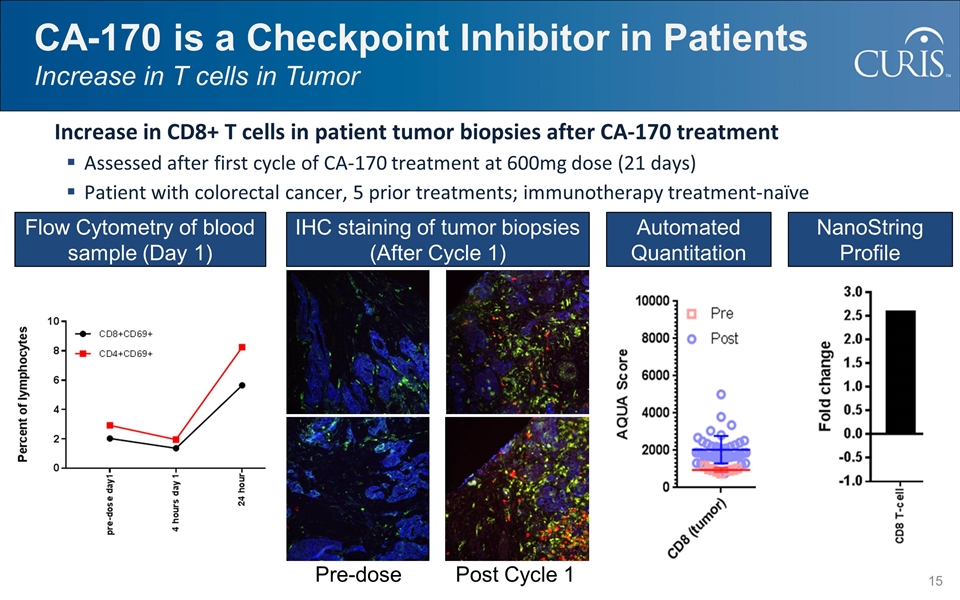
CA-170 is a Checkpoint Inhibitor in Patients Increase in T cells in Tumor Increase in CD8+ T cells in patient tumor biopsies after CA-170 treatment Assessed after first cycle of CA-170 treatment at 600mg dose (21 days) Patient with colorectal cancer, 5 prior treatments; immunotherapy treatment-naïve Flow Cytometry of blood sample (Day 1) IHC staining of tumor biopsies (After Cycle 1) Pre-dose Post Cycle 1 Automated Quantitation NanoString Profile
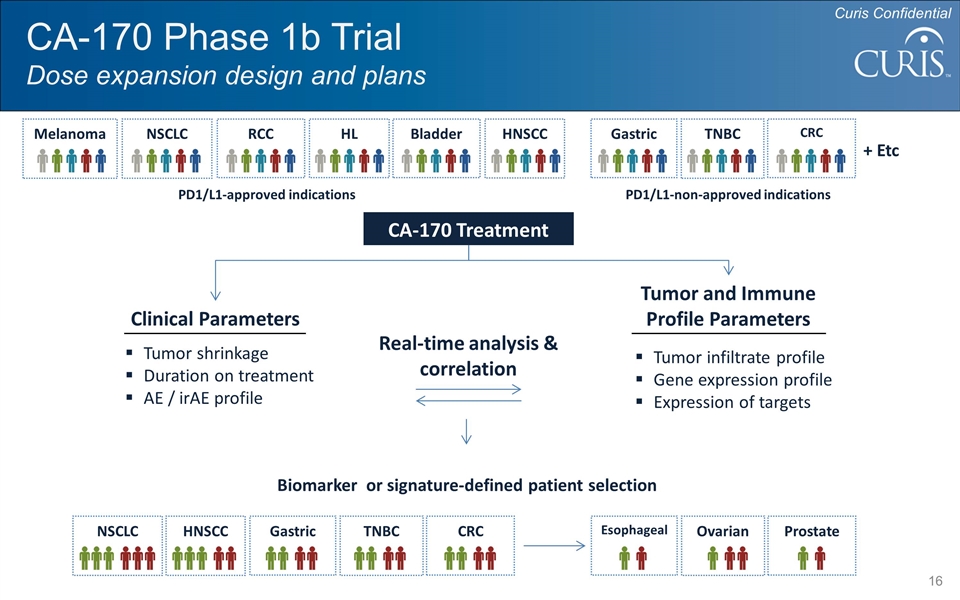
CA-170 Phase 1b Trial Dose expansion design and plans Melanoma NSCLC RCC HL TNBC HNSCC CRC Bladder Gastric + Etc CA-170 Treatment Clinical Parameters Tumor and Immune Profile Parameters Tumor shrinkage Duration on treatment AE / irAE profile Tumor infiltrate profile Gene expression profile Expression of targets Real-time analysis & correlation PD1/L1-approved indications PD1/L1-non-approved indications Curis Confidential Biomarker or signature-defined patient selection NSCLC TNBC HNSCC CRC Gastric Ovarian Prostate Esophageal
CA-327 is an oral, small molecule immune checkpoint inhibitor IND filing expected in Q1 2018 CA-327 Oral, small molecule checkpoint inhibitor – PDL1 and TIM3 Program Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Marketed CA-327 PD-L1 / TIM3 Cancers
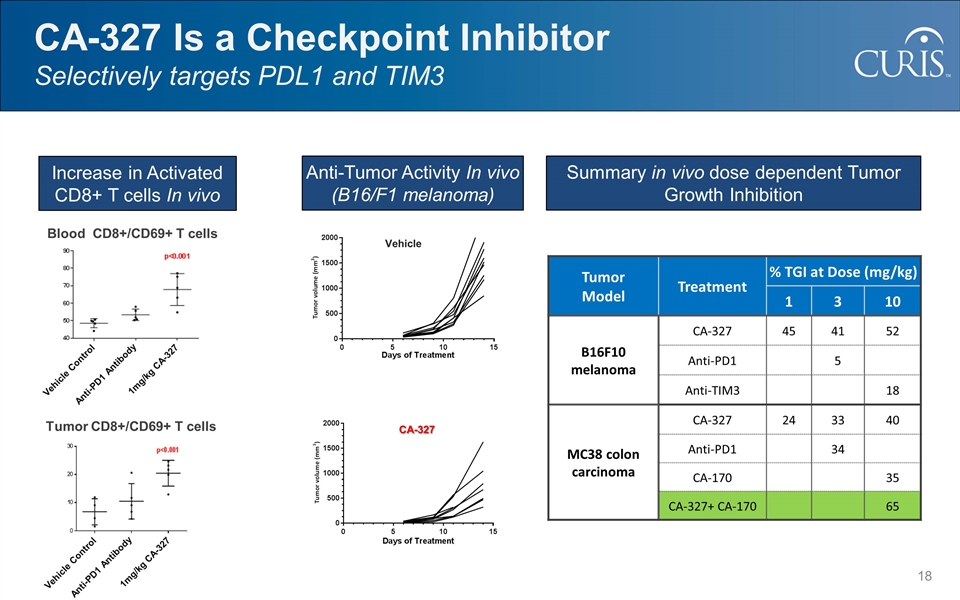
CA-327 Is a Checkpoint Inhibitor Selectively targets PDL1 and TIM3 CA-327 Vehicle Blood CD8+/CD69+ T cells Vehicle Control Anti-PD1 Antibody 1mg/kg CA-327 Tumor CD8+/CD69+ T cells Vehicle Control Anti-PD1 Antibody 1mg/kg CA-327 Tumor Model Treatment % TGI at Dose (mg/kg) 1 3 10 B16F10 melanoma CA-327 45 41 52 Anti-PD1 5 Anti-TIM3 18 MC38 colon carcinoma CA-327 24 33 40 Anti-PD1 34 CA-170 35 CA-327+ CA-170 65 Increase in Activated CD8+ T cells In vivo Anti-Tumor Activity In vivo (B16/F1 melanoma) Summary in vivo dose dependent Tumor Growth Inhibition
CA-4948 is an oral, small molecule inhibitor of IRAK4 IND filing expected in 3Q 2017 (Phase 1 trial in Non-Hodgkin’s Lymphoma) CA-4948 Oral, small molecule inhibitor of IRAK4 Program Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Marketed CA-4948 IRAK4 Heme malignancies
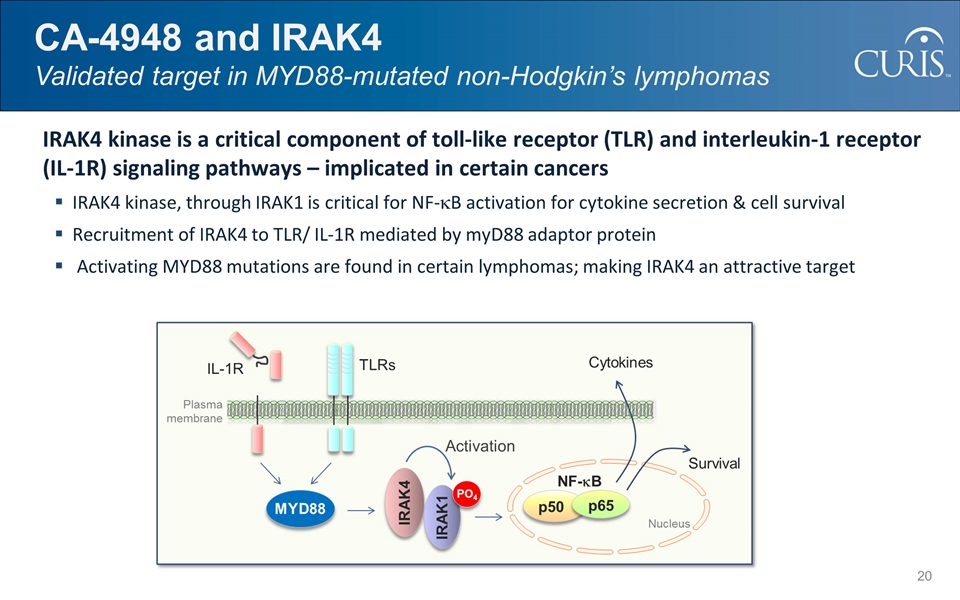
IRAK4 kinase is a critical component of toll-like receptor (TLR) and interleukin-1 receptor (IL-1R) signaling pathways – implicated in certain cancers IRAK4 kinase, through IRAK1 is critical for NF-kB activation for cytokine secretion & cell survival Recruitment of IRAK4 to TLR/ IL-1R mediated by myD88 adaptor protein Activating MYD88 mutations are found in certain lymphomas; making IRAK4 an attractive target CA-4948 and IRAK4 Validated target in MYD88-mutated non-Hodgkin’s lymphomas Activation
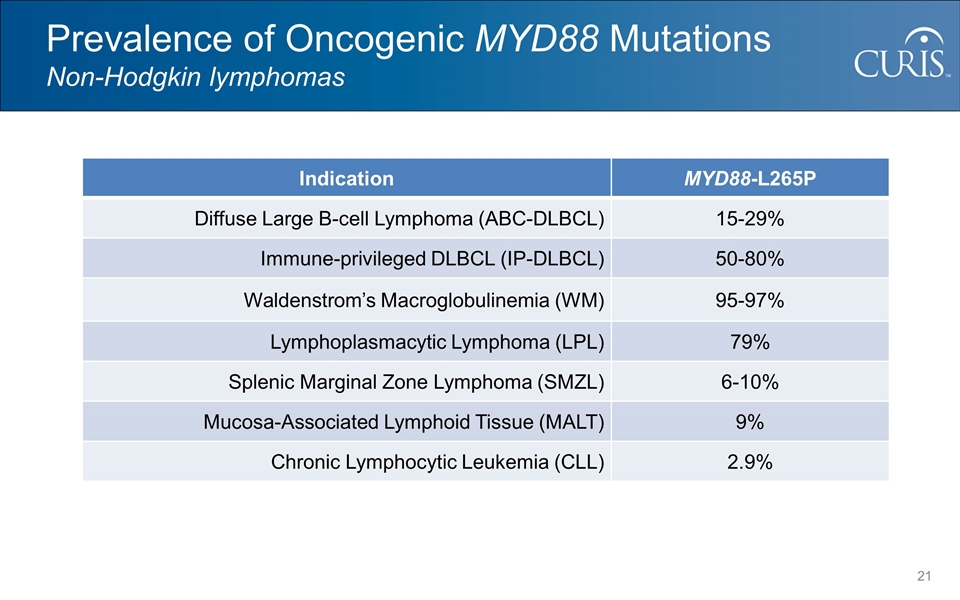
Prevalence of Oncogenic MYD88 Mutations Non-Hodgkin lymphomas Indication MYD88-L265P Diffuse Large B-cell Lymphoma (ABC-DLBCL) 15-29% Immune-privileged DLBCL (IP-DLBCL) 50-80% Waldenstrom’s Macroglobulinemia (WM) 95-97% Lymphoplasmacytic Lymphoma (LPL) 79% Splenic Marginal Zone Lymphoma (SMZL) 6-10% Mucosa-Associated Lymphoid Tissue (MALT) 9% Chronic Lymphocytic Leukemia (CLL) 2.9%
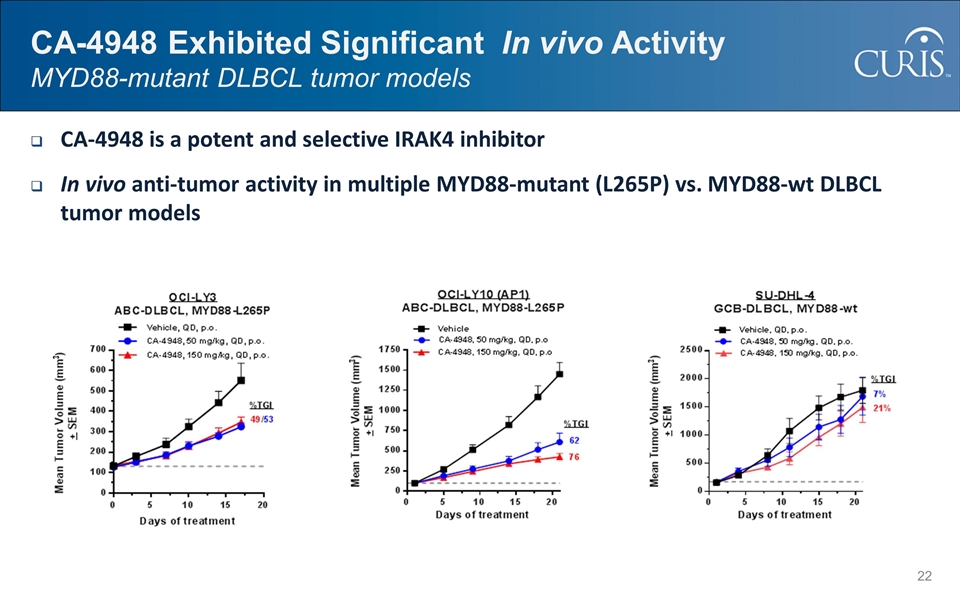
CA-4948 Exhibited Significant In vivo Activity MYD88-mutant DLBCL tumor models CA-4948 is a potent and selective IRAK4 inhibitor In vivo anti-tumor activity in multiple MYD88-mutant (L265P) vs. MYD88-wt DLBCL tumor models
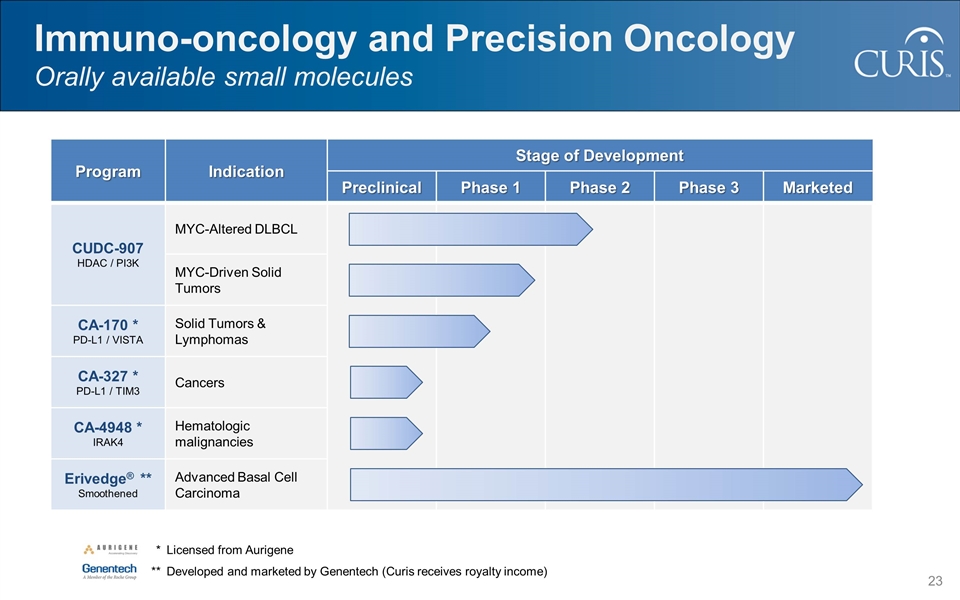
Program Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Marketed CUDC-907 HDAC / PI3K MYC-Altered DLBCL MYC-Driven Solid Tumors CA-170 * PD-L1 / VISTA Solid Tumors & Lymphomas CA-327 * PD-L1 / TIM3 Cancers CA-4948 * IRAK4 Hematologic malignancies Erivedge® ** Smoothened Advanced Basal Cell Carcinoma Immuno-oncology and Precision Oncology Orally available small molecules * Licensed from Aurigene **Developed and marketed by Genentech (Curis receives royalty income)

CA-170 Phase 1 initial data, Clinical profilen Q3 2017ESMO n Q4 2017SITC CA-4948 IND filing n Q3 2017 CA-327 IND filing n Q1 2018 2017 Projected Milestones
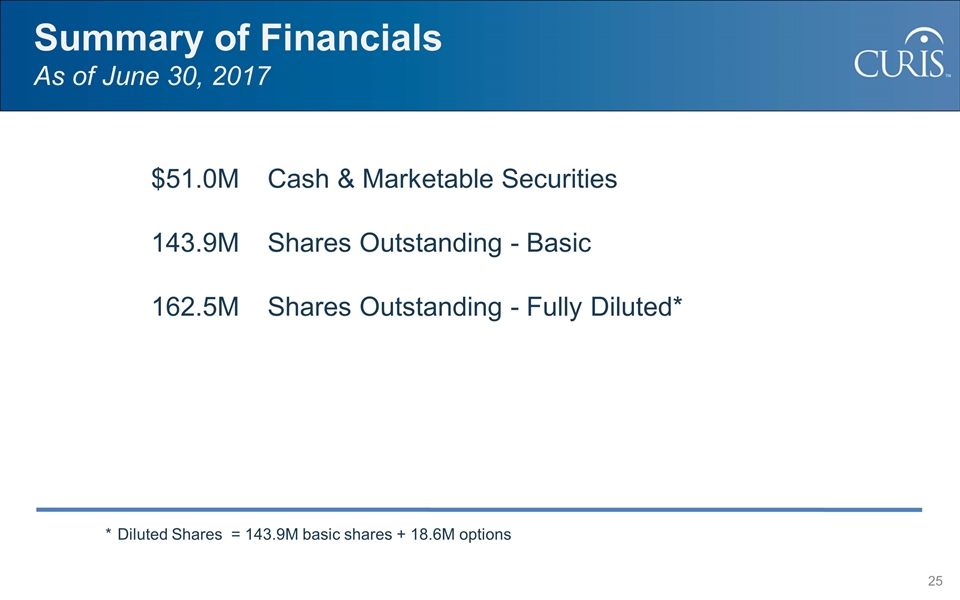
$51.0MCash & Marketable Securities 143.9MShares Outstanding - Basic 162.5MShares Outstanding - Fully Diluted* *Diluted Shares = 143.9M basic shares + 18.6M options Summary of Financials As of June 30, 2017

END
