Attached files
| file | filename |
|---|---|
| EX-31.2 - EXHIBIT 31.2 - Arbutus Biopharma Corp | exhibit312q22017.htm |
| EX-32.2 - EXHIBIT 32.2 - Arbutus Biopharma Corp | exhibit322q22017.htm |
| EX-32.1 - EXHIBIT 32.1 - Arbutus Biopharma Corp | exhibit321q22017.htm |
| EX-31.1 - EXHIBIT 31.1 - Arbutus Biopharma Corp | exhibit311q22017.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
[X] QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended June 30, 2017
OR
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Transition Period from to
Commission File Number: 001-34949
ARBUTUS BIOPHARMA CORPORATION
(Exact Name of Registrant as Specified in Its Charter)
British Columbia, Canada | 98-0597776 | |
(State or Other Jurisdiction of | (I.R.S. Employer | |
Incorporation or Organization) | Identification No.) | |
100-8900 Glenlyon Parkway, Burnaby, BC, Canada V5J 5J8
(Address of Principal Executive Offices)
604-419-3200
(Registrant’s Telephone Number, Including Area Code)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes [X] No [ ]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes [X] No [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer [ ] | Accelerated filer [X] | Non-accelerated filer [ ] | Smaller reporting company [ ] |
(Do not check if a smaller | |||
reporting company) | |||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
[ ]
1
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes [ ] No [X]
As of July 31, 2017, the registrant had 55,026,995 common shares, no par value, outstanding.
2
ARBUTUS BIOPHARMA CORP.
TABLE OF CONTENTS
Page | ||
3
PART I. FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS (UNAUDITED)
ARBUTUS BIOPHARMA CORPORATION
Condensed Consolidated Balance Sheets
(Unaudited)
(Expressed in thousands of U.S. dollars, except share and per share amounts)
(Prepared in accordance with US GAAP)
June 30, | December 31, | ||||||
2017 | 2016 | ||||||
Assets | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 24,212 | $ | 23,413 | |||
Short-term investments (note 2) | 78,797 | 107,146 | |||||
Accounts receivable | 1,042 | 273 | |||||
Accrued revenue | 128 | 128 | |||||
Investment tax credits receivable | 160 | 293 | |||||
Prepaid expenses and other assets | 1,583 | 1,311 | |||||
Total current assets | 105,922 | 132,564 | |||||
Restricted investment (note 2) | 12,601 | 12,601 | |||||
Property and equipment | 24,284 | 17,683 | |||||
Less accumulated depreciation | (11,541 | ) | (10,738 | ) | |||
Property and equipment, net of accumulated depreciation | 12,743 | 6,945 | |||||
Intangible assets (note 3) | 99,445 | 99,445 | |||||
Goodwill (note 3) | 24,364 | 24,364 | |||||
Total assets | $ | 255,075 | $ | 275,919 | |||
Liabilities and stockholders' equity | |||||||
Current liabilities: | |||||||
Accounts payable and accrued liabilities (note 5) | $ | 8,274 | $ | 9,910 | |||
Deferred revenue (note 4) | 2,624 | 15 | |||||
Liability-classified options (note 2) | 983 | 553 | |||||
Warrants | — | 107 | |||||
Total current liabilities | 11,881 | 10,585 | |||||
Deferred revenue, net of current portion (note 4) | 4,130 | — | |||||
Loan payable | 12,001 | 12,001 | |||||
Contingent consideration (notes 2 and 6) | 10,014 | 9,065 | |||||
Deferred tax liability | 41,263 | 41,263 | |||||
Total liabilities | 79,289 | 72,914 | |||||
Stockholders’ equity: | |||||||
Common shares | |||||||
Authorized - unlimited number with no par value | |||||||
Issued and outstanding: 55,026,983 (December 31, 2016 - 54,841,494) | 873,841 | 867,393 | |||||
Additional paid-in capital | 39,758 | 36,543 | |||||
Deficit | (688,031 | ) | (651,149 | ) | |||
Accumulated other comprehensive loss | (49,782 | ) | (49,782 | ) | |||
Total stockholders' equity | 175,786 | 203,005 | |||||
Total liabilities and stockholders' equity | $ | 255,075 | $ | 275,919 | |||
Nature of business and future operations (note 1)
Contingencies and commitments (note 6)
Subsequent event (note 8)
See accompanying notes to the condensed consolidated financial statements.
4
ARBUTUS BIOPHARMA CORPORATION
Condensed Consolidated Statements of Operations and Comprehensive Loss
(Unaudited)
(Expressed in thousands of U.S. dollars, except share and per share amounts)
(Prepared in accordance with US GAAP)
Three months ended | Six months ended | |||||||||||||
June 30, | June 30, | |||||||||||||
2017 | 2016 | 2017 | 2016 | |||||||||||
Revenue (note 4) | ||||||||||||||
Collaborations and contracts | $ | 318 | $ | 32 | $ | 336 | $ | 139 | ||||||
Licensing fees, milestone and royalty payments | 721 | 277 | 938 | 773 | ||||||||||
Total revenue | 1,039 | 309 | 1,274 | 912 | ||||||||||
Expenses | ||||||||||||||
Research, development, collaborations and contracts | 15,445 | 15,215 | 29,317 | 28,359 | ||||||||||
General and administrative | 4,599 | 23,766 | 8,927 | 30,985 | ||||||||||
Depreciation of property and equipment | 480 | 252 | 814 | 469 | ||||||||||
Impairment of intangible assets (note 3) | — | 156,324 | — | 156,324 | ||||||||||
Total expenses | 20,524 | 195,557 | 39,058 | 216,137 | ||||||||||
Loss from operations | (19,485 | ) | (195,248 | ) | (37,784 | ) | (215,225 | ) | ||||||
Other income (losses) | ||||||||||||||
Interest income | 390 | 435 | 758 | 679 | ||||||||||
Interest expense | (68 | ) | — | (110 | ) | — | ||||||||
Foreign exchange gains | 798 | 33 | 1,225 | 2,975 | ||||||||||
Gain on disposition of financial instrument | — | — | — | 1,000 | ||||||||||
Decrease (increase) in fair value of warrant liability (note 2) | — | 168 | (22 | ) | 329 | |||||||||
Decrease (increase) in fair value of contingent consideration (note 7) | 110 | (252 | ) | (949 | ) | (496 | ) | |||||||
Total other income (losses) | 1,230 | 384 | 902 | 4,487 | ||||||||||
Loss before income taxes | (18,255 | ) | (194,864 | ) | (36,882 | ) | (210,738 | ) | ||||||
Income tax benefit | — | 64,864 | — | 64,864 | ||||||||||
Net and comprehensive loss | $ | (18,255 | ) | $ | (130,000 | ) | $ | (36,882 | ) | $ | (145,874 | ) | ||
Loss per common share | ||||||||||||||
Basic and diluted | $ | (0.33 | ) | $ | (2.47 | ) | $ | (0.68 | ) | $ | (2.80 | ) | ||
Weighted average number of common shares | ||||||||||||||
Basic and diluted | 54,647,944 | 52,716,059 | 54,478,221 | 52,052,165 | ||||||||||
See accompanying notes to the condensed consolidated financial statements.
5
ARBUTUS BIOPHARMA CORPORATION
Condensed Consolidated Statement of Stockholders’ Equity
(Unaudited)
(Expressed in thousands of U.S. dollars, except share and per share amounts)
(Prepared in accordance with US GAAP)
Number of shares | Share capital | Additional paid-in capital | Deficit | Accumulated other comprehensive loss | Total stockholders' equity | |||||||||||||||||
December 31, 2016 | 54,841,494 | $ | 867,393 | $ | 36,543 | $ | (651,149 | ) | $ | (49,782 | ) | $ | 203,005 | |||||||||
Stock-based compensation | — | 5,938 | 3,554 | — | — | 9,492 | ||||||||||||||||
Certain fair value adjustments to liability stock option awards | — | — | (315 | ) | — | — | (315 | ) | ||||||||||||||
Issuance of common shares pursuant to exercise of options | 6,489 | 29 | (24 | ) | — | — | 5 | |||||||||||||||
Issuance of common shares pursuant to exercise of warrants | 179,000 | 481 | — | — | — | 481 | ||||||||||||||||
Net loss | — | — | — | (36,882 | ) | — | (36,882 | ) | ||||||||||||||
Balance, June 30, 2017 | 55,026,983 | $ | 873,841 | $ | 39,758 | $ | (688,031 | ) | $ | (49,782 | ) | $ | 175,786 | |||||||||
See accompanying notes to the condensed consolidated financial statements.
6
ARBUTUS BIOPHARMA CORPORATION
Condensed Consolidated Statements of Cash Flow
(Unaudited)
(Expressed in thousands of U.S. dollars)
(Prepared in accordance with US GAAP)
Three months ended | Six months ended | |||||||||||||
June 30, | June 30, | |||||||||||||
2017 | 2016 | 2017 | 2016 | |||||||||||
OPERATING ACTIVITIES | ||||||||||||||
Net loss for the period | $ | (18,255 | ) | $ | (130,000 | ) | $ | (36,882 | ) | $ | (145,874 | ) | ||
Items not involving cash: | ||||||||||||||
Deferred income taxes | — | (64,864 | ) | — | (64,864 | ) | ||||||||
Depreciation of property and equipment | 480 | 252 | 814 | 469 | ||||||||||
Stock-based compensation - research, development, collaborations and contract expenses | 3,055 | 2,519 | 5,678 | 5,466 | ||||||||||
Stock-based compensation - general and administrative expenses | 2,048 | 19,592 | 3,929 | 24,558 | ||||||||||
Unrealized foreign exchange (gains) losses | (824 | ) | 51 | (1,250 | ) | (2,956 | ) | |||||||
Change in fair value of warrant liability | — | (168 | ) | 22 | (329 | ) | ||||||||
Change in fair value of contingent consideration | (110 | ) | 252 | 949 | 496 | |||||||||
Impairment of intangible assets (note 3) | — | 156,324 | — | 156,324 | ||||||||||
Net change in non-cash operating items: | ||||||||||||||
Accounts receivable | 6,691 | (116 | ) | (914 | ) | 552 | ||||||||
Investment tax credits receivable | 294 | 98 | 279 | 98 | ||||||||||
Prepaid expenses and other assets | (9 | ) | (573 | ) | (273 | ) | (847 | ) | ||||||
Accounts payable and accrued liabilities | 2,205 | 42 | (1,708 | ) | (1,074 | ) | ||||||||
Deferred revenue | (653 | ) | (213 | ) | 6,739 | (370 | ) | |||||||
Net cash used in operating activities | (5,078 | ) | (16,804 | ) | (22,617 | ) | (28,351 | ) | ||||||
INVESTING ACTIVITIES | ||||||||||||||
Disposition (acquisition) of short and long-term investments, net | 1,731 | (84,439 | ) | 28,349 | (97,745 | ) | ||||||||
Acquisition of property and equipment | (3,121 | ) | (954 | ) | (6,539 | ) | (1,230 | ) | ||||||
Net cash provided by (used) in investing activities | (1,390 | ) | (85,393 | ) | 21,810 | (98,975 | ) | |||||||
FINANCING ACTIVITIES | ||||||||||||||
Proceeds from issuance of common shares, net of issuance costs | — | — | — | — | ||||||||||
Issuance of common shares pursuant to exercise of options | 4 | 1 | 5 | 116 | ||||||||||
Issuance of common shares pursuant to exercise of warrants | — | 445 | 353 | 445 | ||||||||||
Net cash provided by financing activities | 4 | 446 | 358 | 561 | ||||||||||
Effect of foreign exchange rate changes on cash and cash equivalents | 825 | (51 | ) | 1,248 | 2,956 | |||||||||
(Decrease) Increase in cash and cash equivalents | (5,639 | ) | (101,802 | ) | 799 | (123,809 | ) | |||||||
Cash and cash equivalents, beginning of period | 29,851 | 144,772 | 23,413 | 166,779 | ||||||||||
Cash and cash equivalents, end of period | $ | 24,212 | $ | 42,970 | $ | 24,212 | $ | 42,970 | ||||||
Supplemental cash flow information | ||||||||||||||
Non-cash transactions: | ||||||||||||||
Investment tax credit received | 108 | $ | — | 108 | — | |||||||||
Acquired property and equipment in trade payables | 72 | — | 72 | — | ||||||||||
See accompanying notes to the condensed consolidated financial statements.
7
ARBUTUS BIOPHARMA CORPORATION
Notes to Condensed Consolidated Financial Statements
(Tabular amounts in thousands of US Dollars, except share and per share amounts)
1. Nature of business and future operations
Arbutus Biopharma Corporation (the “Company” or “Arbutus”) is a biopharmaceutical business dedicated to discovering, developing, and commercializing a cure for patients suffering from chronic hepatitis B infection, a disease of the liver caused by the hepatitis B virus (“HBV”). The Company's portfolio of assets includes a broad pipeline of drug candidates to develop a cure for HBV and leverages the Company’s expertise in Lipid Nanoparticle ("LNP") technology.
The success of the Company is dependent on obtaining the necessary regulatory approvals to bring its products to market and achieve profitable operations. The continuation of the research and development activities and the commercialization of its products are dependent on the Company’s ability to successfully complete these activities and to obtain adequate financing through a combination of financing activities and operations. It is not possible to predict either the outcome of future research and development programs or the Company’s ability to continue to fund these programs in the future.
2. Significant accounting policies
Basis of presentation
These unaudited condensed consolidated financial statements have been prepared in accordance with generally accepted accounting principles of the United States of America (“U.S. GAAP”) for interim financial statements and accordingly, do not include all disclosures required for annual financial statements. These statements should be read in conjunction with the Company’s audited consolidated financial statements and notes thereto for the year ended December 31, 2016 and included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2016. The unaudited condensed consolidated financial statements reflect, in the opinion of management, all adjustments and reclassifications necessary to present fairly the financial position, results of operations and cash flows at June 30, 2017 and for all periods presented. The results of operations for the three and six months ended June 30, 2017 and June 30, 2016 are not necessarily indicative of the results for the full year. These unaudited condensed consolidated financial statements follow the same significant accounting policies as those described in the notes to the audited consolidated financial statements of the Company for the year ended December 31, 2016, except as described below.
Principles of Consolidation
These unaudited condensed consolidated financial statements include the accounts of the Company and its two wholly-owned subsidiaries, Arbutus Biopharma Inc. ("Arbutus Inc.") and Protiva Biotherapeutics Inc. ("Protiva"). All intercompany transactions and balances have been eliminated on consolidation.
Income or loss per share
Income or loss per share is calculated based on the weighted average number of common shares outstanding. Diluted loss per share does not differ from basic loss per share since the effect of the Company’s stock options, liability-classified stock option awards, and warrants are anti-dilutive. During the six months ended June 30, 2017, potential common shares of 5,848,138 (June 30, 2016 – 5,488,162) were excluded from the calculation of loss per common share because their inclusion would be anti-dilutive, of which 226,588 (June 30, 2016 - 1,258,824) relates to shares issued subject to repurchase provisions as part of consideration paid for the acquisition of Arbutus Inc.
Fair value of financial instruments
The Company measures certain financial instruments and other items at fair value.
8
To determine the fair value, the Company uses the fair value hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use to value an asset or liability and are developed based on market data obtained from independent sources. Unobservable inputs are inputs based on assumptions about the factors market participants would use to value an asset or liability. The three levels of inputs that may be used to measure fair value are as follows:
• | Level 1 inputs are quoted market prices for identical instruments available in active markets. |
• | Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability either directly or indirectly. If the asset or liability has a contractual term, the input must be observable for substantially the full term. An example includes quoted market prices for similar assets or liabilities in active markets. |
• | Level 3 inputs are unobservable inputs for the asset or liability and will reflect management’s assumptions about market assumptions that would be used to price the asset or liability. |
The following table presents information about the Company’s assets and liabilities that are measured at fair value on a recurring basis, in thousands, and indicates the fair value hierarchy of the valuation techniques used to determine such fair value:
Level 1 | Level 2 | Level 3 | June 30, 2017 | |||||||||||
Assets | ||||||||||||||
Cash and cash equivalents | $ | 24,212 | — | — | $ | 24,212 | ||||||||
Short-term investments | 78,797 | — | — | 78,797 | ||||||||||
Restricted investment | 12,601 | — | — | 12,601 | ||||||||||
Total | $ | 115,610 | — | — | $ | 115,610 | ||||||||
Liabilities | ||||||||||||||
Liability-classified options | — | — | $ | 983 | $ | 983 | ||||||||
Contingent consideration | — | — | 10,014 | 10,014 | ||||||||||
Total | — | — | $ | 10,997 | $ | 10,997 | ||||||||
Level 1 | Level 2 | Level 3 | December 31, 2016 | |||||||||||
Assets | ||||||||||||||
Cash and cash equivalents | $ | 23,413 | — | — | $ | 23,413 | ||||||||
Short-term investments | 107,146 | — | — | 107,146 | ||||||||||
Restricted investment | 12,601 | — | — | 12,601 | ||||||||||
Total | $ | 143,160 | — | — | $ | 143,160 | ||||||||
Liabilities | ||||||||||||||
Warrants | — | — | $ | 107 | $ | 107 | ||||||||
Liability-classified options | 553 | 553 | ||||||||||||
Contingent consideration | — | — | 9,065 | 9,065 | ||||||||||
Total | — | — | $ | 9,725 | $ | 9,725 | ||||||||
9
The following table presents the changes in fair value of the Company’s warrants:
Liability at beginning of the period | Fair value of warrants exercised in the period | Increase (decrease) in fair value of warrants | Liability at end of the period | ||||||||||||
Six months ended June 30, 2016 | $ | 883 | $ | (246 | ) | $ | (329 | ) | $ | 308 | |||||
Six months ended June 30, 2017 | $ | 107 | $ | (129 | ) | $ | 22 | $ | — | ||||||
During the six months ended June 30, 2017, there were 179,000 warrants exercised for $353,000 in cash (June 30, 2016 - 170,500) and no warrants were exercised using the cashless exercise provision (June 30, 2016 - nil). On March 1, 2017, 22,000 of the Company's warrants expired, resulting in a nil liability balance for the period-ended June 30, 2017. The change in fair value of warrant liability for the six months ended June 30, 2017 is recorded in the statement of operations and comprehensive loss.
The following table presents the changes in fair value of the Company’s liability-classified stock option awards:
Liability at beginning of the period | Increase (decrease) in fair value of liability | Liability at end of the period | |||||||||
Six months ended June 30, 2016 | $ | 1,909 | $ | (597 | ) | $ | 1,312 | ||||
Six months ended June 30, 2017 | $ | 553 | $ | 430 | $ | 983 | |||||
The following table presents the changes in fair value of the Company’s contingent consideration:
Liability at beginning of the period | Increase in fair value of Contingent Consideration | Liability at end of the period | |||||||||
Six months ended June 30, 2016 | $ | 7,497 | $ | 496 | $ | 7,993 | |||||
Six months ended June 30, 2017 | $ | 9,065 | $ | 949 | $ | 10,014 | |||||
Recent accounting pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (FASB) or other standard setting bodies that are adopted by the Company as of the specified effective date.
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers (ASC 606). The standard, as subsequently amended, is intended to clarify the principles for recognizing revenue and to develop a common revenue standard for U.S. GAAP and IFRS by creating a new Topic 606, Revenue from Contracts with Customers. This guidance supersedes the revenue recognition requirements in ASC 605, Revenue Recognition, and supersedes some cost guidance included in Subtopic 605-35, Revenue Recognition – Construction-Type and Production-Type Contracts. The core principle of the accounting standard is that an entity recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those good or services. The amendments should be applied by either (1) retrospectively to each prior reporting period presented; or (2) retrospectively with the cumulative effect of initially applying this ASU recognized at the date of initial application ("modified retrospective method"). The new guidance would be effective for fiscal years beginning after December 15, 2017, which for the Company means January 1, 2018. The Company anticipates applying the modified retrospective method for its implementation, and continues to evaluate the expected impact that the standard could have on its consolidated financial statements and related disclosures, which the Company believes most materially relates to its licensing and collaboration contracts that are described in note 4.
10
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842): Recognition and Measurement of Financial Assets and Financial Liabilities. The update supersedes Topic 840, Leases and requires the recognition of lease assets and lease liabilities by lessees for those leases classified as operating leases under previous GAAP. Topic 842 retains a distinction between finance leases and operating leases, with cash payments from operating leases classified within operating activities in the statement of cash flows. The amendments in this update are effective for fiscal years beginning after December 15, 2018 for public business entities, which for the Company means January 1, 2019. The Company does not plan to early adopt this update. The extent of the impact of this adoption has not yet been determined.
In August 2016, the FASB issued ASU 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments. The update addresses eight specific cash flow issues with the objective of reducing the existing diversity in practice. Under this update, the classification of cash receipts and payments that have aspects of more than one class of cash flows should be determined first by applying specific guidance in GAAP. In the absence of specific guidance, an entity should determine each separately identifiable source or use within the cash receipts and cash payments on the basis of the nature of the underlying cash flows. An entity should then classify each separately identifiable source or use within the cash receipts and payments on the basis of their nature in financing, investing, or operating activities. In situations in which cash receipts and payments have aspects of more than one class of cash flows and cannot be separated by source or use, the appropriate classification should depend on the activity that is likely to be the predominant source or use of cash flows for the item. The amendments in this update are effective for public business entities for fiscal years beginning after December 31, 2017, which for the Company means January 1, 2018, and interim periods within those fiscal years. Early adoption is permitted. The amendments in this update should be applied using a retrospective transition method to each period presented. If it is impracticable to apply the amendments retrospectively for some of the issues, the amendments for those issues would be applied prospectively as of the earliest date practicable. The Company is currently evaluating the extent of the impact of this adoption.
In November 2016, the FASB issued ASU 2016-18, Statement of Cash Flows (Topic 230): Statement of Cash Flows: Restricted Cash. The update requires the statement of cash flows to explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash or restricted cash equivalents. Therefore, amounts generally described as restricted cash and restricted cash equivalents should be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. The amendments in this update are effective for public business entities for fiscal years beginning after December 15, 2017, which for the Company means January 1, 2018. Early adoption is permitted, including adoption in an interim period. If an entity early adopts the amendments in an interim period, any adjustments should be reflected as of the beginning of the fiscal year that included that interim period. The amendments in this update should be applied using a retrospective transition method to each period presented. The Company is currently evaluating the extent of the impact of this adoption.
In January 2017, the FASB issued ASU 2017-04, Intangibles - Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment. The update simplifies the subsequent measurement of goodwill by eliminating Step 2 from the goodwill impairment test. In computing the implied fair value of goodwill under Step 2, an entity had to perform procedures to determine the fair value at impairment testing date of its assets and liabilities following the procedure that would be required in determining the fair value of assets required and liabilities assumed in a business combination. Instead, under the amendments in this update, an entity should perform its annual, or interim, goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount. The amendments in this update are effective for public business entities should be adopted for its annual or any interim goodwill impairment tests in fiscal years beginning after December 15, 2019, which for the Company means January 1, 2020. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. The Company is currently evaluating the extent of the impact of this adoption.
11
In May 2017, the FASB issued ASU 2017-09, Compensation - Stock Compensation (Topic 718): Scope of Modification Accounting. The amendments in this Update provide guidance about which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting under Topic 718. An entity should account for effects of a modification unless all of the following are met: (1) the fair value of the modified award is the same as the fair value of the original award immediately before the original award is modified; (2) the vesting conditions of the modified award are the same as the vesting conditions of the original award immediately before the original award is modified; (3) the classification of the modified award as an equity instrument or a liability instrument is the same as the classification of the original award immediately before the original award is modified. The amendments in this Update are effective for all entities for annual periods and interim periods within those annual periods, beginning after December 15, 2017, which for the Company means January 1, 2018. Early adoption is permitted, including adoption in any interim period for public business entities for reporting periods for which financial statements have not yet been issued. The amendments in this Update should be applied prospectively to an award modified on or after the adoption date. The Company has early adopted the amendments in this Update effective for its interim financial statements for the period ended June 30, 2017. The impact of this adoption did not have an effect in the Company's statement of operations and comprehensive loss for the period ended June 30, 2017 as no award modifications occurred.
3. Intangible assets and goodwill
All in-process research and development (IPR&D) acquired is currently classified as indefinite-lived and is not currently being amortized. IPR&D becomes definite-lived upon the completion or abandonment of the associated research and development efforts, and will be amortized from that time over an estimated useful life based on respective patent terms. The Company evaluates the recoverable amount of intangible assets on an annual basis and performs an annual evaluation of goodwill as of December 31 each year, unless there is an event or change in the business that could indicate impairment, in which case earlier testing is performed.
The following table summarizes the carrying values of the intangible assets as at June 30, 2017:
June 30, 2017 | December 31, 2016 | |||||
IPR&D – Immune Modulators | $ | 40,798 | $ | 40,798 | ||
IPR&D – Antigen Inhibitors | 14,811 | 14,811 | ||||
IPR&D – cccDNA Sterilizers | 43,836 | 43,836 | ||||
Total Intangible Assets | $ | 99,445 | $ | 99,445 | ||
Impairment evaluation of goodwill
At June 30, 2017, the Company did not identify any new indicators of impairment. No impairment charge on intangible assets or goodwill was recorded for the period ended June 30, 2017 (three and six months ended June 30, 2016 - $156,324,000).
4. Collaborations, contracts and licensing agreements
The following tables set forth revenue recognized under collaborations, contracts and licensing agreements, in thousands:
12
Three months ended June 30, | Six months ended June 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | ||||||||||||
Collaborations and contracts | |||||||||||||||
Alexion (a) | $ | 318 | $ | — | 336 | — | |||||||||
Dicerna (b) | — | 32 | — | 139 | |||||||||||
Total research and development collaborations and contracts | 318 | 32 | 336 | 139 | |||||||||||
Licensing fees, milestone and royalty payments | |||||||||||||||
Alexion licensing fee (a) | 652 | — | 761 | — | |||||||||||
Dicerna licensing fee (b) | — | 214 | — | 427 | |||||||||||
Other milestone and royalty payments (c) | 69 | 63 | 177 | 346 | |||||||||||
Total licensing fees, milestone and royalty payments | 721 | 277 | 938 | 773 | |||||||||||
Total revenue | $ | 1,039 | $ | 309 | $ | 1,274 | $ | 912 | |||||||
The following table sets forth deferred collaborations and contracts revenue:
June 30, 2017 | December 31, 2016 | ||||||
Alexion current portion (a) | $ | 2,624 | $ | — | |||
DoD | — | 15 | |||||
Deferred revenue, current portion | 2,624 | 15 | |||||
Alexion long-term portion (a) | 4,130 | — | |||||
Total deferred revenue | $ | 6,754 | $ | 15 | |||
(a) License Agreement with Alexion Pharmaceuticals, Inc. ("Alexion")
On March 16, 2017, the Company signed a license agreement with Alexion that entitles Alexion to research, develop, manufacture, and commercialize products with the Company's lipid nanoparticle ("LNP") technology in their single orphan disease target. In consideration for the rights granted under the agreement, the Company received a $7,500,000 non-refundable upfront cash payment. The Company is also entitled to receive payments from Alexion for services provided, as well as further subsequent payments up to $75,000,000 for the achievement of development, regulatory and commercial milestones, as well as single digit royalties. Further, under the agreement, a joint steering committee has been established to provide guidance and direction on the progression of the collaboration.
The Company determined the deliverables under the agreement included the rights granted, participation in the joint steering committee, and other services provided, as directed under the joint steering committee. The license and participation in the joint steering committee have been determined by the Company to not have standalone value due to the uniqueness of the subject matter under the agreement. Therefore, these deliverables are treated as one unit of accounting and recognized as revenue over the performance period.
The Company has determined that other services to be provided have standalone value. The relative fair values are estimated upon the execution of each activity, with actual expenditures charged at rates comparable to market with embedded margins on each service activity.
The Company believes the development and regulatory milestones are substantive, due to the existence of substantive uncertainty upon the execution of the arrangement, and that the achievement of the development and regulatory events are based, in part, on the Company’s performance and the occurrence of a specific outcome resulting from performance. The Company has not received any milestone payments to date.
13
In July 2017, the Company received notice of termination from Alexion for the Company's LNP license agreement - refer to note 8 for further details related to the subsequent event.
(b) License and Development and Supply Agreement with Dicerna Pharmaceuticals, Inc. (“Dicerna”)
On November 16, 2014, the Company signed a License Agreement and a Development and Supply Agreement (together, the “Agreements”) with Dicerna to develop, manufacture, and commercialize products directed to the treatment of Primary Hyperoxaluria 1 (“PH1”). In consideration for the rights granted under the Agreements, Dicerna paid the Company an upfront cash payment of $2,500,000. The Company is also entitled to receive payments from Dicerna on manufacturing and services provided, as well as further payments with the achievement of development and regulatory milestones of up to $22,000,000, in aggregate, and potential commercial royalties. Further, under the Agreements, a joint development committee has been established to provide guidance and direction on the progression of the collaboration.
The Company determined the deliverables under the Agreements included the rights granted, participation in the joint development committee, materials manufactured and other services provided, as directed under the joint development committee. The license and participation in the joint development committee have been determined by the Company to not have standalone value due to the uniqueness of the subject matter under the Agreements. Therefore, these deliverables are treated as one unit of accounting and recognized as revenue over the performance period. In September 2016, Dicerna announced the discontinuation of their DCR-PH1 program using the Company's technology. As such, the Company revised the estimated completion date of performance period from March 2017 to September 30, 2016, at which time the Company had no further remaining performance obligations. This resulted in the recognition of $1,066,000 in Dicerna license fee revenue for year ended December 31, 2016 and no revenue thereafter.
(c) Agreements with Spectrum Pharmaceuticals, Inc. (“Spectrum”)
On May 6, 2006, the Company signed a number of agreements with Talon Therapeutics, Inc. (“Talon”, formerly Hana Biosciences, Inc.) including the grant of worldwide licenses (the “Talon License Agreement”) for three of the Company’s chemotherapy products, Marqibo®, Alocrest ™ (Optisomal Vinorelbine) and Brakiva ™ (Optisomal Topotecan).
On August 9, 2012, the Company announced that Talon had received accelerated approval for Marqibo from the FDA for the treatment of adult patients with Philadelphia chromosome negative acute lymphoblastic leukemia in second or greater relapse or whose disease has progressed following two or more anti-leukemia therapies. Marqibo is a liposomal formulation of the chemotherapy drug vincristine. In the year ended December 31, 2012, the Company received a milestone of $1,000,000 based on the FDA's approval of Marqibo and will receive royalty payments based on Marqibo's commercial sales. There are no further milestones related to Marqibo but the Company is eligible to receive total milestone payments of up to $18,000,000 on Alocrest and Brakiva.
Talon was acquired by Spectrum in July 2013. The acquisition does not affect the terms of the license between Talon and the Company. On September 3, 2013, Spectrum announced that they had shipped the first commercial orders of Marqibo. For the three and six months ended June 30, 2017, the Company recorded $65,000 and $123,000 in Marqibo royalty revenue (three and six months ended June 30, 2016 - $60,000 and $91,000 respectively). For the six months ended June 30, 2017, the Company accrued 2.5% in royalties due to TPC in respect of the Marqibo royalty earned by the Company – see note 7, contingencies and commitments.
5. Accounts payable and accrued liabilities
Accounts payable and accrued liabilities are comprised of the following, in thousands:
14
June 30, 2017 | December 31, 2016 | ||||||
Trade accounts payable | $ | 3,368 | $ | 3,215 | |||
Research and development accruals | 3,215 | 3,131 | |||||
Professional fee accruals | 305 | 498 | |||||
Deferred lease inducements | 825 | 350 | |||||
Payroll accruals | 214 | 2,178 | |||||
Other accrued liabilities | 347 | 538 | |||||
$ | 8,274 | $ | 9,910 | ||||
6. Contingencies and commitments
Product development partnership with the Canadian Government
The Company entered into a Technology Partnerships Canada ("TPC") agreement with the Canadian Federal Government on November 12, 1999. Under this agreement, TPC agreed to fund 27% of the costs incurred by the Company, prior to March 31, 2004, in the development of certain oligonucleotide product candidates up to a maximum contribution from TPC of $7,179,000 (C$9,323,000). As at June 30, 2017, a cumulative contribution of $2,853,000 (C$3,702,000) has been received and the Company does not expect any further funding under this agreement. In return for the funding provided by TPC, the Company agreed to pay royalties on the share of future licensing and product revenue, if any, that is received by the Company on certain non-siRNA oligonucleotide product candidates covered by the funding under the agreement. These royalties are payable until a certain cumulative payment amount is achieved or until a pre-specified date. In addition, until a cumulative amount equal to the funding actually received under the agreement has been paid to TPC, the Company agreed to pay 2.5% royalties on any royalties the Company receives for Marqibo. For the three and six months ended June 30, 2017, the Company earned royalties on Marqibo sales in the amount of $65,000 and $123,000 respectively (three and six months ended June 30, 2016 – $60,000 and $91,000 respectively) (see note 4(c)), resulting in $3,000 being recorded by the Company as royalty payable to TPC (June 30, 2016 -$2,000). The cumulative amount paid or accrued up to June 30, 2017 was $20,000, therefore the remaining contingent amount due to TPC is $2,833,000 (C$3,676,000).
Arbitration with the University of British Columbia (“UBC”)
Certain early work on lipid nanoparticle delivery systems and related inventions was undertaken at the Company and assigned to the University of British Columbia (UBC). These inventions are licensed to the Company by UBC under a license agreement, initially entered in 1998 as amended in 2001, 2006 and 2007. The Company has granted sublicenses under the UBC license to Alnylam. Alnylam has in turn sublicensed back to the Company under the licensed UBC patents for discovery, development and commercialization of siRNA products.
On November 10, 2014, UBC filed a notice of arbitration against the Company and on January 16, 2015, filed a Statement of Claim, which alleges entitlement to $3,500,000 in allegedly unpaid royalties based on publicly available information, and an unspecified amount based on non-public information. UBC also seeks interest and costs, including legal fees. The Company filed its Statement of Defense to UBC's Statement of Claims, as well as filed a Counterclaim involving a patent application that Arbutus alleges UBC wrongly licensed to a third party rather than to Arbutus. The Company continues to dispute UBC’s allegations, and seeks any license payments for said application, and an exclusive worldwide license to said application. However, the Company notes that arbitration is subject to inherent uncertainty and an arbitrator could rule against the Company. The Company has not recorded an estimate of the possible loss associated with this arbitration, due to the uncertainties related to both the likelihood and amount of any possible loss or range of loss. The proceedings have been bifurcated into three phases, with a first hearing that took place in June 2017. Arbitration and related matters are costly and may divert the attention of the Company’s management and other resources that would otherwise be engaged in other activities. Costs related to the arbitration are recorded by the Company as incurred.
15
Contingent consideration from Arbutus Inc. acquisition of Enantigen and License Agreements between Enantigen and the Baruch S. Blumberg Institute (“Blumberg”) and Drexel University (“Drexel”)
In October 2014, Arbutus Inc. acquired all of the outstanding shares of Enantigen pursuant to a stock purchase agreement. Through this transaction, Arbutus Inc. acquired a HBV surface antigen secretion inhibitor program and a capsid assembly inhibitor program, each of which are now assets of the Company, following the Company’s merger with Arbutus Inc.
Under the stock purchase agreement, Arbutus Inc. agreed to pay up to a total of $21,000,000 to Enantigen’s selling stockholders upon the achievement of certain triggering events related to Enantigen’s two programs in pre-clinical development related to HBV therapies. The first triggering event is the enrollment of the first patient in a Phase 1b clinical trial in HBV patients, which the Company believes is likely to occur in the next twelve-month period.
The regulatory, development and sales milestone payments had an initial estimated fair value of approximately $6,727,000 as at the date of acquisition of Arbutus Inc., and have been treated as contingent consideration payable in the purchase price allocation, based on information available at the date of acquisition, using a probability weighted assessment of the likelihood the milestones would be met and the estimated timing of such payments, and then the potential contingent payments were discounted to their present value using a probability adjusted discount rate that reflects the early stage nature of the development program, time to complete the program development, and market comparative data.
Contingent consideration is recorded as a financial liability, and measured at its fair value at each reporting date, based on an updated consideration of the probability-weighted assessment of expected milestone timing, with any changes in fair value from the previous reporting date recorded in the statement of operations and comprehensive loss (see note 2).
Drexel and Blumberg
In February 2014, Arbutus Inc. entered into a license agreement with Blumberg and Drexel that granted an exclusive, worldwide, sub-licensable license to three different compound series: cccDNA inhibitors, capsid assembly inhibitors and HCC inhibitors.
In partial consideration for this license, Arbutus Inc. paid a license initiation fee of $150,000 and issued warrants to Blumberg and Drexel. The warrants were exercised in 2014. Under this license agreement, Arbutus Inc. also agreed to pay up to $3,500,000 in development and regulatory milestones per licensed compound series, up to $92,500,000 in sales performance milestones per licensed product, and royalties in the mid-single digits based upon the proportionate net sales of licensed products in any commercialized combination. The Company is obligated to pay Blumberg and Drexel a double digit percentage of all amounts received from the sub-licensees, subject to customary exclusions.
In November 2014, Arbutus Inc. entered into an additional license agreement with Blumberg and Drexel pursuant to which it received an exclusive, worldwide, sub-licensable license under specified patents and know-how controlled by Blumberg and Drexel covering epigenetic modifiers of cccDNA and STING agonists. In consideration for these exclusive licenses, Arbutus Inc. made an upfront payment of $50,000. Under this agreement, the Company is required to pay up to $1,200,000 for each licensed product upon the achievement of a specified regulatory milestone and a low single digit royalty, based upon the proportionate net sales of compounds covered by this intellectual property in any commercialized combination. The Company is also obligated to pay Blumberg and Drexel a double digit percentage of all amounts received from its sub-licensees, subject to exclusions.
16
Research Collaboration and Funding Agreement with Blumberg
In October 2014, Arbutus Inc. entered into a research collaboration and funding agreement with Blumberg under which the Company will provide $1,000,000 per year of research funding for three years, renewable at the Company’s option for an additional three years, for Blumberg to conduct research projects in HBV and liver cancer pursuant to a research plan to be agreed upon by the parties. Blumberg has exclusivity obligations to Arbutus with respect to HBV research funded under the agreement. In addition, the Company has the right to match any third party offer to fund HBV research that falls outside the scope of the research being funded under the agreement. Blumberg has granted the Company the right to obtain an exclusive, royalty bearing, worldwide license to any intellectual property generated by any funded research project. If the Company elects to exercise its right to obtain such a license, the Company will have a specified period of time to negotiate and enter into a mutually agreeable license agreement with Blumberg. This license agreement will include the following pre-negotiated upfront, milestone and royalty payments: an upfront payment in the amount of $100,000; up to $8,100,000 upon the achievement of specified development and regulatory milestones; up to $92,500,000 upon the achievement of specified commercialization milestones; and royalties at a low single to mid-single digit rates based upon the proportionate net sales of licensed products from any commercialized combination.
On June 5, 2016, the Company and Blumberg entered into an amended and restated research collaboration and funding agreement, primarily to: (i) increase the annual funding amount to Blumberg from $1,000,000 to $1,100,000; (ii) extend the initial term through to October 29, 2018; (iii) provide an option for the Company to extend the term past October 29, 2018 for two additional one year terms; and (iv) expand our exclusive license under the Agreement to include the sole and exclusive right to obtain and exclusive, royalty-bearing, worldwide and all-fields license under Blumberg's rights in certain other inventions described in the agreement.
7. Concentrations of credit risk
Credit risk is defined by the Company as an unexpected loss in cash and earnings if a collaborative partner is unable to pay its obligations on a timely basis. The Company’s main source of credit risk is related to its accounts receivable balance which principally represents temporary financing provided to collaborative partners in the normal course of operations.
The Company does not currently maintain a provision for bad debts as the majority of accounts receivable are from collaborative partners or government agencies and are considered low risk.
The carrying amount of financial assets represents the maximum credit exposure. The maximum exposure to credit risk at June 30, 2017 was the accounts receivable balance of $1,042,000 (December 31, 2016 - $273,000).
All accounts receivable balances were current at June 30, 2017 and at December 31, 2016.
8. Subsequent event
Termination of License Agreement with Alexion
On July 27, 2017, the Company announced that it received notice of termination from Alexion for the Company's LNP license agreement. The termination was driven by a strategic review of Alexion's business and research and development portfolio, which included a decision to discontinue development of mRNA therapeutics. The $7,500,000 upfront payment received in March 2017 is non-refundable, and the Company anticipates recording the remaining deferred revenue balance, as well as any revenue and costs related to closeout procedures in the statement of comprehensive loss for the period ended September 30, 2017.
17
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis by our management of our financial position and results of operations in conjunction with our audited consolidated financial statements and related notes thereto included as part of our Annual Report on Form 10-K for the year ended December 31, 2016 and our unaudited condensed consolidated financial statements for the three and six month periods ended June 30, 2017. Our consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles and are presented in U.S. dollars.
FORWARD-LOOKING STATEMENTS
The information in this report contains forward-looking statements within the meaning of the Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and forward looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). Forward-looking statements in this report include statements about our strategy, future operations, clinical trials, prospects and the plans of management; the discovery, development and commercialization of a cure for HBV; our beliefs and development path and strategy to achieve a cure for HBV; obtaining necessary regulatory approvals; obtaining adequate financing through a combination of financing activities and operations; receiving payments from Dicerna on manufacturing and services provided, as well as further payments with the achievement of development and regulatory milestones of up to $22,000,000, in aggregate, and potential commercial royalties; the possibility of receiving total milestone payments of up to $18,000,000 on Alocrest and Brakiva; enrollment of the first patient in Enantigen’s Phase 1b clinical trial in HBV patients in the next twelve-month period; evaluating different treatment durations to determine the optimal finite duration of therapy; selecting combination therapy regimens and treatment durations to conduct Phase III clinical trials intended to ultimately support regulatory filings for marketing approval; approval for a single product from our pipeline by combining with available agents to improve upon the cure rate with the current standard of care; expanding our HBV drug candidate pipeline through internal development, acquisitions and in-licenses; initial results from Cohort 4 of the Phase II trial of ARB-1467 from three months of bi-weekly dosing expected in the third quarter of 2017; initiating longer bi-weekly dosing combination studies with ARB-1467 with results expected in 2019; beginning a AB-423 multi-dosing study in HBV patients in the second half of 2017; evaluating AB-423’s utility in combination with our RNAi candidate ARB-1467, NAs and possibly interferon; an IND (or equivalent) filing for AB-506 and AB-452 in 2018; exploring partnership opportunities to enable further study of TKM-PLK-1 in HCC; results from Alnylam's patisiran (ALN-TTR02) Phase 3 cohort and a subsequent New Drug Application filing in 2017, with possible low to mid-single-digit royalty payments escalating based on sales performance as Alnylam’s LNP-enabled products are commercialized; recording the remaining deferred revenue from Alexion for the non-refundable upfront payment, as well as revenue for any work done related to closeout procedures in Q3 2017; the belief that current legal proceedings will not have a material adverse effect on our consolidated results of operations, cash flows, or financial condition; the expected return from strategic alliances, licensing agreements, and research collaborations; statements with respect to revenue and expense fluctuation and guidance; having sufficient cash resources to fund our operations for at least the next 12 months; and the quantum and timing of potential funding.
With respect to the forward-looking statements contained in this report, we have made numerous assumptions regarding, among other things: LNP’s status as a leading RNAi delivery technology; our research and development capabilities and resources; the effectiveness of our products as a treatment for chronic Hepatitis B infection or other diseases; continued positive results from pre-clinical and clinical trials; the timing and quantum of payments to be received under contracts with our partners; assumptions related to our share price volatility, expected lives of warrants and warrant issuances and/or exercises; and our financial position and its ability to execute our business strategy. While we consider these assumptions to be reasonable, these assumptions are inherently subject to significant business, economic, competitive, market and social uncertainties and contingencies.
Our actual results could differ materially from those discussed in the forward-looking statements as a result of a number of important factors, including the risk factors discussed in this report and the risk factors discussed in our Annual Report on Form 10-K under the heading “Risk Factors,” and the risks discussed in our other filings with the Securities and Exchange Commission and Canadian Securities Regulators. Readers are cautioned not to place undue reliance on these forward-looking statements, which reflect management’s analysis, judgment, belief or expectation only as of the date hereof. All forward-looking statements herein are qualified in their entirety by this cautionary statement, and we explicitly disclaim any obligation to revise or update any such forward-looking statements or to publicly announce the result of any revisions to any of the forward-looking statements contained herein to reflect future results, events or developments, except as required by law.
3
OVERVIEW
Arbutus Biopharma Corporation ("Arbutus", the "Company", "we", "us", and "our") is a publicly traded industry-leading Hepatitis B Virus (HBV) therapeutic solutions company. HBV represents a significant unmet medical need, and given the complex biology of the disease, we believe combination therapies are the key to HBV treatment and a potential cure, and development can be accelerated when multiple components of a combination therapy regimen are controlled by the same company. We have assembled an HBV pipeline consisting of multiple drug candidates with complementary mechanisms of action, and plan to continue to broaden our pipeline.
HBV Product Pipeline
Our product pipeline, like our business, is focused on finding a cure for chronic HBV infection, with the objective of developing a combination of products that intervene at different points in the viral life cycle, and reactivating the host immune system. Initially, these combinations will include a single product from our pipeline combined with approved agents. Given our strong scientific and research capabilities in-house, we are able to conduct preclinical combination studies to evaluate combinations of our proprietary pipeline candidates with approved agents and with each other. Once compounds within the portfolio with sufficient activity have been identified, we intend, subject to discussions with regulatory authorities, to evaluate combinations of two or more drug candidates in cohorts of patients with chronic HBV infection. We expect to use these results to adaptively design additional treatment regimens for the next cohorts. We also plan to evaluate different treatment durations to determine the optimal finite duration of therapy. We plan to continue this iterative process until we select combination therapy regimens and treatment durations to conduct Phase III clinical trials intended to ultimately support regulatory filings for marketing approval. In the meantime, we believe that there is a potential path to approval for a single product from our pipeline by combining with available agents to improve upon the cure rate with the current standard of care.
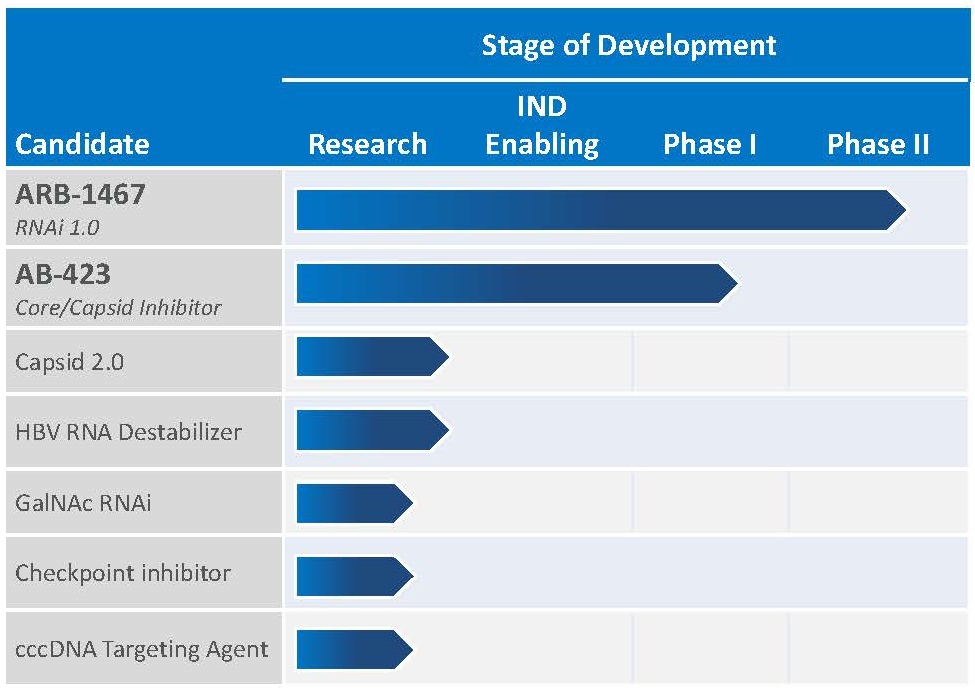
We intend to continue to expand our HBV pipeline through internal development, acquisitions and in-licenses. We also have a research collaboration agreement with the Baruch S. Blumberg Institute that provides exclusive rights to in-license any intellectual property generated through the collaboration.
4
RNAi (ARB-1467 & ARB-1740)
Our lead RNAi HBV candidate, ARB-1467 (formerly TKM-HBV), is designed to reduce Hepatitis B surface antigen (HBsAg) expression in patients chronically infected with HBV. Reducing HBsAg is thought to be a key prerequisite to enable a patient’s immune system to raise an adequate immune response against the virus. The ability of ARB-1467 to inhibit numerous viral elements in addition to HBsAg increases the likelihood of affecting the viral infection.
ARB-1467 is a multi-component RNAi therapeutic that simultaneously targets three sites on the HBV genome, including the HBsAg coding region. Targeting three distinct and highly conserved sites on the HBV genome is intended to facilitate potent knockdown of all viral mRNA transcripts and viral antigens across a broad range of HBV genotypes and reduce the risk of developing antiviral resistance. In preclinical models, ARB-1467 treatment results in reductions in intrahepatic and serum HBsAg, HBV DNA, cccDNA, Hepatitis B e antigen (HBeAg) and Hepatitis B c antigen (HBcAg). ARB-1467 was evaluated in a Phase I Single Ascending Dose (SAD) trial designed to assess the safety, tolerability and pharmacokinetics of intravenous administration of the product in healthy adult subjects. In the Phase I SAD study, healthy volunteer subjects were dosed up to a dose of 0.4 mg/kg but a defined maximum tolerated dose was not reached.
The Phase II trial is a multi-dose study in chronic HBV patients who are also receiving nucleot(s)ide analog therapy. The trial consists of four cohorts. The first three cohorts each enrolled eight subjects; six receiving three monthly doses of ARB-1467, and two receiving placebo. Cohort 4 enrolled twelve patients, all receiving five bi-weekly doses of ARB-1467. Cohorts 1, 2, and 4 enrolled HBeAg- patients and Cohort 3 enrolled HBeAg+ patients. ARB-1467 is administered at 0.2 mg/kg in Cohort 1 and 0.4 mg/kg in Cohorts 2, 3, and 4. Results from this trial based on multiple dose administration of ARB-1467 in Cohorts 1, 2 and 3 demonstrated significant reductions in serum HBsAg levels and showed a step-wise, additive reduction in serum HBsAg with each subsequent dose. The HBsAg reduction achieved after three monthly doses of 0.4mg/kg in Cohort 2 was greater than that seen at 0.2 mg/kg in Cohort 1, demonstrating a dose-response seen with repeat dosing. There were no significant differences in serum HBsAg reductions between HBeAg-negative and HBeAg-positive patients. Overall treatment was well tolerated in all three cohorts (Cohorts 1, 2 and 3). Cohort 4 began bi-weekly dosing in the first quarter of 2017 and is fully enrolled. Initial results from the first three months are expected in the third quarter of 2017. Cohort 4 also has an extension phase where patients who meet predefined response criteria after the first three months of dosing will go on to receive monthly dosing of ARB-1467 for up to an additional nine months (enabling up to one year of dosing in total).
Our follow-on RNAi HBV candidate, ARB-1740, is chemically distinct from ARB-1467 (comprising variations to the trigger sequences) and employs the same Lipid Nanoparticle (LNP) formulation as ARB-1467. In preclinical studies, ARB-1740 demonstrated greater potency over ARB-1467, offering the potential to be effective at lower clinical doses than ARB-1467. In early 2017 we started an ARB-1740 Phase II Multi Ascending Dose (MAD) study that dosed HBV patients in Cohorts 1 and 2 to enable a clinical potency comparison between ARB-1467 and ARB-1740. While ARB-1740 posed no safety concerns, the lack of a significant potency advantage led us to discontinue any further development of ARB-1740. We are continuing to invest in the development of our more clinically advanced RNAi agent, ARB-1467.
To further develop our HBV candidates, we are initiating longer bi-weekly dosing combination studies with our RNAi agent, ARB-1467, with current standard of care nucleoside/nucleotide analogues (NAs) and interferon (IFN) therapies, which offer the potential to improve current cure rates with a finite dosing period. Results from this trial are expected in 2019 and could inform the trial design of Phase 2b and Phase 3 combination studies to follow. Combination treatment has the potential to result in sustained HBV DNA and HBsAg loss in patients. Achieving this in a significant proportion of patients would put this Arbutus therapeutic on a potential approval pathway.
Core Protein/ Capsid Assembly Inhibitor (AB-423)
HBV core protein, or capsid, is required for viral replication and core protein may have additional roles in cccDNA function. Current nucleot(s)ide analog therapy significantly reduces HBV DNA levels in the serum but HBV replication continues in the liver, thereby enabling HBV infection to persist. Effective therapy for patients requires new agents which will effectively block viral replication. We are developing core protein inhibitors (also known as capsid assembly inhibitors) as oral therapeutics for the treatment of chronic HBV infection. By inhibiting assembly of the viral capsid, the ability of hepatitis B virus to replicate is impaired, resulting in reduced cccDNA. We initiated a healthy volunteer clinical study of AB-423 in March 2017 with single ascending dose cohorts followed by multiple ascending dose cohorts, and expect to begin a multi-dosing study in HBV patients by the end of 2017. Following initial studies of AB-423 in patients we will consider inclusion in a combination study, with our RNAi candidate ARB-1467, NAs and possibly interferon. In addition to AB-423, our core protein/capsid assembly inhibitor discovery effort is ongoing and has already generated promising back-up compounds.
5
We recently nominated AB-506, a second-generation capsid inhibitor, for Investigational New Drug (IND)-enabling studies. In preclinical studies, this new capsid inhibitor has shown to have improved potency and pharmacokinetics when compared to AB-423. Pending successful IND-enabling studies, this product candidate could be the subject of an IND (or equivalent) filing in 2018.
Research Programs
In addition to our clinical candidates, we have a number of research programs aimed at the discovery and development of proprietary HBV candidates with different and complementary mechanisms of action. One of our most advanced preclinical programs, AB-452, an HBV RNA Destabilizer (formerly known as our oral surface antigen (HBsAg) inhibitor program), which has broad activity against HBV RNAs and reduces surface antigen, has yielded a product candidate that has been nominated for IND enabling studies based on its novel activity in destabilizing HBV RNA. This molecule has the potential for once daily oral dosing. Pending successful IND-enabling studies, this product candidate could be the subject of an IND (or equivalent) filing in 2018.
We have designed a number of highly potent HBV-targeting RNAi payloads for use with our proprietary GalNAc conjugate platform to enable subcutaneous delivery. In preclinical models, our molecules display acute knockdown of viral proteins and a duration of effect that is highly competitive in the field. We observe a significant dose response, and a stepwise reduction in viral proteins when multi-dosing. We expect to nominate a clinical development candidate in early 2018.
Our Proprietary Delivery Technology
Development of RNAi therapeutic products is currently limited by the instability of the RNAi trigger molecules in the bloodstream and the inability of these molecules to access target cells or tissues following administration. Delivery technology is necessary to protect these drugs in the bloodstream to allow efficient delivery and cellular uptake by the target cells. Arbutus has developed a proprietary delivery LNP platform. The broad applicability of this platform to RNAi development has established Arbutus as a leader in this new area of innovative medicine.
Our proprietary LNP delivery technology allows for the successful encapsulation of RNAi trigger molecules in LNP administered intravenously, which travel through the bloodstream to target tissues or disease sites. LNPs are designed to protect the triggers, and stay in the circulation long enough to accumulate at disease sites, such as the liver or cancerous tumors. LNPs are then taken up into the target cells by a process called endocytosis. Subsequent activation by the changing environment inside the cell causes the LNP to release the trigger molecules, which can then successfully mediate RNAi.
Ongoing Advancements in LNP Technology
Our LNP technology represents the most widely adopted delivery technology in RNAi, which has enabled several clinical trials and has been administered to hundreds of human subjects. We continue to explore opportunities to generate value from our LNP platform technology, which is well suited to deliver therapies based on RNAi, mRNA, and gene editing constructs. We have also made progress in developing a proprietary GalNAc conjugate technology to enable subcutaneous delivery of an RNAi therapeutic targeting hepatitis B surface antigen and/or other HBV targets.
6
Partner Programs
Patisiran (ALN-TTR02)
Alnylam has a license to use our intellectual property to develop and commercialize products and may only grant access to our LNP technology to its partners if it is part of a product sublicense. Alnylam’s license rights are limited to patents that we have filed, or that claim priority to a patent that was filed, before April 15, 2010. Alnylam's patisiran (ALN-TTR02) program represents the most clinically advanced application of our LNP delivery technology, and results demonstrate that multi-dosing with our LNP has been well-tolerated with treatments out to 25 months. Results from this study are expected in the third quarter of 2017. We are entitled to low to mid-single-digit royalty payments escalating based on sales performance as Alnylam’s LNP-enabled products are commercialized.
Marqibo®
Marqibo, originally developed by Arbutus, is a novel, sphingomyelin/cholesterol liposome-encapsulated formulation of the FDA-approved anticancer drug vincristine. Marqibo’s approved indication is for the treatment of adult patients with Philadelphia chromosome-negative acute lymphoblastic leukemia (Ph-ALL) in second or greater relapse or whose disease has progressed following two or more lines of anti-leukemia therapy. Our licensee, Spectrum Pharmaceuticals, Inc. (Spectrum), launched Marqibo through its existing hematology sales force in the United States. Spectrum has ongoing trials evaluating Marqibo in three additional indications, which are: first line use in patients with Philadelphia Negative Acute Lymphoblastic Leukemia (Ph-ALL), Pediatric ALL and Non-Hodgkin’s lymphoma. We are receiving mid-single digit royalty payments on sales of Marqibo.
Alexion Pharmaceuticals Inc.
In March 2017, we entered into a license agreement with Alexion that granted them exclusive use of our proprietary LNP technology in one of Alexion's rare disease programs. Under the terms of the license agreement, Alexion paid us $7.5 million upfront, and was set to pay $75 million for achievement of development, regulatory, and commercial milestones, as well as single digit royalties. Arbutus conducted technology development and provided manufacturing and regulatory support. In July 2017, Alexion terminated its LNP licensing agreement with Arbutus driven by a strategic review of Alexion’s business and research and development portfolio, which included a decision to discontinue development of mRNA therapeutics. The preclinical work completed during this period enabled refinement of the LNP formulation process for mRNA development at a larger scale.
Recent Developments
Acuitas Therapeutics Inc.
In December 2013, we entered into a cross-license agreement with Acuitas Therapeutics Inc., or Acuitas. The terms of the cross-license agreement provided Acuitas with access to certain of our earlier intellectual property (IP) generated prior to April 2010 for a specific field. On August 29, 2016, we provided Acuitas with notice that Arbutus considered Acuitas to be in material breach of the cross-license agreement. On October 25, 2016, Acuitas filed a Notice of Civil Claim in the Supreme Court of British Columbia seeking an order that we perform our obligations under the cross-license agreement , for damages ancillary to specific performance, injunctive relief, interest and costs. We disputed Acuitas' position and filed a counterclaim seeking, among other relief, a declaration that the cross-license agreement had been terminated. On January 10, 2017, we filed an application seeking an order to enjoin Acuitas from entering into any further agreements purporting to sublicense Arbutus' technology from the date of the order to the date of trial or further order from the court. Acuitas filed a response to Arbutus' application and the matter was the subject of a hearing on January 26, 2017, which resulted in the Supreme Court of British Columbia granting a pre-trial injunction against Acuitas. On March 7, 2017, Acuitas appealed the injunction decision and on April 3, 2017, the appeal was denied. The case regarding the termination of Acuitas' license from Arbutus will be tried in court subject to scheduling.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
There are no changes to our critical accounting policies and estimates from those disclosed in our annual MD&A contained in our Annual Report Form 10-K for the year ended December 31, 2016.
7
RECENT ACCOUNTING PRONOUNCEMENTS
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board or other standard setting bodies that are adopted by us as of the specified effective date. Unless otherwise discussed, we believe that the impact of recently issued standards that are not yet effective will not have a material impact on our financial position or results of operations upon adoption.
Please refer to Note 2 to our consolidated financial statements included in Part I, Item 1, "Financial Statements (Unaudited)" of this Quarterly Report on Form 10-Q for a description of recent accounting pronouncements applicable to our business.
SUMMARY OF QUARTERLY RESULTS
The following table presents our unaudited quarterly results of operations for each of our last eight quarters. These data have been derived from our unaudited condensed consolidated financial statements, which were prepared on the same basis as our annual audited financial statements and, in our opinion, include all adjustments necessary, consisting solely of normal recurring adjustments, for the fair presentation of such information.
(in millions $ except per share data) – unaudited
Q2 | Q1 | Q4 | Q3 | Q2 | Q1 | Q4 | Q3 | ||||||||||||||||||||||||
2017 | 2017 | 2016 | 2016 | 2016 | 2016 | 2015 | 2015 | ||||||||||||||||||||||||
Revenue | |||||||||||||||||||||||||||||||
Collaborations and contracts: | |||||||||||||||||||||||||||||||
DoD | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | (0.1 | ) | $ | 2.0 | ||||||||||||||
Monsanto | — | — | — | — | — | — | 3.9 | 0.3 | |||||||||||||||||||||||
Dicerna | — | — | — | 0.1 | — | 0.1 | 0.7 | 0.7 | |||||||||||||||||||||||
Alexion | 0.3 | 0.02 | — | — | — | — | — | — | |||||||||||||||||||||||
0.3 | 0.02 | — | 0.1 | — | 0.1 | 4.5 | 3.0 | ||||||||||||||||||||||||
Monsanto licensing fees and milestone payments | — | — | — | — | — | 7.9 | 0.7 | ||||||||||||||||||||||||
Dicerna licensing fee | — | — | — | 0.6 | 0.2 | 0.2 | 0.3 | 0.3 | |||||||||||||||||||||||
Alexion licensing fee | 0.7 | 0.1 | — | — | — | — | — | — | |||||||||||||||||||||||
Other milestone and royalty payments | 0.1 | 0.1 | (0.2 | ) | — | 0.1 | 0.3 | 0.1 | 0.1 | ||||||||||||||||||||||
Total revenue | 1.0 | 0.2 | (0.2 | ) | 0.7 | 0.3 | 0.6 | 12.7 | 4.1 | ||||||||||||||||||||||
Expenses | (20.5 | ) | (18.5 | ) | (257.2 | ) | (19.7 | ) | (195.6 | ) | (20.6 | ) | (24.4 | ) | (62.2 | ) | |||||||||||||||
Other income (losses) | 1.2 | (0.3 | ) | (1.4 | ) | (0.6 | ) | 0.4 | 4.1 | 5.5 | 14.0 | ||||||||||||||||||||
Loss before income taxes | (18.3 | ) | (18.6 | ) | (258.8 | ) | (19.6 | ) | (194.9 | ) | (15.9 | ) | (6.2 | ) | (44.2 | ) | |||||||||||||||
Income tax benefit | — | — | 40.1 | — | 64.9 | — | 1.0 | 15.2 | |||||||||||||||||||||||
Net loss | (18.3 | ) | (18.6 | ) | (218.7 | ) | (19.6 | ) | (130.0 | ) | (15.9 | ) | (5.2 | ) | (29.0 | ) | |||||||||||||||
Basic and diluted net loss per share | $ | (0.33 | ) | $ | (0.34 | ) | $ | (4.05 | ) | $ | (0.37 | ) | $ | (2.47 | ) | $ | (0.31 | ) | $ | (0.10 | ) | $ | (0.57 | ) | |||||||
Quarterly Trends
Revenue / Our revenue is derived from research and development collaborations and contracts, licensing fees, milestone and royalty payments.
In Q3 2010 we signed a contract with the DoD to develop TKM-Ebola. In July 2015, we announced that activities had been suspended and in Q4 2015 the DoD contract was terminated. We are currently conducting contract close out procedures with the DoD.
8
In January 2014, we signed an Option Agreement and a Services Agreement with Monsanto for the use of our proprietary delivery technology and related intellectual property in agriculture. Over the option period, which was expected to be approximately four years, Monsanto made payments to us to maintain their option rights. In Q1 2014, we received $14.5 million of the $17.5 million near term payments, of which $4.5 million related to research services and $10.0 million for the use of our technology. In Q4 2015, we did not receive further payments from Monsanto for the continuance of research activities under the arrangement. As such, we revised our estimated option period end date to December 31, 2015, resulting in the full release of Monsanto deferred revenue and recognition of $11.8 million in Monsanto revenue in Q4 2015. In March 2016, Monsanto exercised its option to acquire 100% of the outstanding shares of Protiva Agricultural Development Company Inc. (PADCo), for which Monsanto paid us an exercise fee of $1.0 million in Q1 2016. We recorded this receipt in Q1 2016 as Other income (losses).
In November 2014, we signed a License Agreement and a Development and Supply Agreement with Dicerna for the use of our proprietary delivery technology and related technology intended to develop, manufacture, and commercialize products related to the treatment of PH1. In Q4 2014, we received an upfront payment of $2.5 million, which was being recognized over the period over which we expected to provide services to Dicerna. In September 2016, Dicerna announced the discontinuation of their DCR-PH1 program using our technology. As such, in Q3 2016, we recognized the remaining balance of Dicerna license fee revenue of $0.6 million, as well as other Dicerna collaboration revenue for the provision of development services.
In March 2017, we signed a License Agreement with Alexion that granted them exclusive use of our proprietary lipid nanoparticle (LNP) technology in one of Alexion's rare disease programs. Under the terms of the license agreement, we received a $7.5 million non-refundable upfront payment in April 2017, which is being recognized over the expected period we provide services to Alexion. In addition, we began recognizing revenue on the services provided to Alexion related to technology development, manufacturing and regulatory support for the advancement of Alexion's mRNA product candidate. In July 2017, we received notice of termination from Alexion for our LNP license agreement. The termination was driven by a strategic review of Alexion's business and research and development portfolio, which included a decision to discontinue development of mRNA therapeutics. We anticipate recording the remaining deferred revenue for the non-refundable upfront payment, as well as revenue for any work done related to closeout procedures in Q3 2017.
Under our licensing and collaboration arrangements with Alnylam and Acuitas, we earn licensing fee revenue from Acuitas as well as further potential development and commercial milestones and royalties from Alnylam for the use of our LNP technology.
In 2013, we began to earn royalties from Spectrum with respect to the commercial sales of Marqibo.
Expenses / Expenses consist primarily of clinical and pre-clinical trial expenses, personnel expenses, consulting and third party expenses, reimbursable collaboration expenses, consumables and materials, patent filing expenses, facilities, stock-based compensation and general corporate costs. Impairment of intangible assets and goodwill is also included in operating expenses.
We continue to incur research and development expense as we continue to move more products into the clinic while continuing to advance our HBV research pipeline. In Q3 2015, we incurred $5.5 million in incremental R&D expenses primarily related to an increase in HBV and HCC clinical trial expenses due to an increase in patient enrollment and a ramp up in spending on other HBV programs.
In Q3 2015, we recorded an estimated impairment charge of $38.0 million (before deferred tax) as we discontinued our cyclophilin inhibitor program based on our conclusion that cyclophilins do not play a meaningful role in HBV biology. From Q4 2015 to Q2 2017, we continued to incur significant R&D expense related to our HBV programs, including initiation of our ARB-1467 and ARB-1740 in Phase 2 clinical trials, and incurred costs in Q4 2016 to Q2 2017 to advance our AB-423 to Phase 1 clinical trials. As well as ongoing clinical trials costs, in Q2 2017, we also incurred costs related to two recently nominated product candidates, a second generation capsid inhibitor and a HBV RNA Destabilizer (formerly known as our oral surface antigen (HBsAg) inhibitor program). In Q2, 2016, we recorded an impairment charge of $156.3 million (before deferred tax) for the discontinuance of the ARB-1598 program in the Immune Modulators drug class after extensive research and analysis, as well as a delay for additional exploration of the biology of the cccDNA Sterilizer drug class. In Q4 2016, we recorded an impairment charge of $96.9 million for our intangible assets (before deferred tax) and impairment charge of $138.1 million for our goodwill which resulted from a change in estimated cost of capital and resulting discount rate used in our annual impairment assessment. This change in the discount rate was made to address the sustained discrepancy between our market capitalization and the carrying value of intangible assets and goodwill. Following the merger with Arbutus Inc., we have recorded, to date, non-cash compensation expense of $55.0 million related to the expiry of repurchase rights on shares issued as part of the consideration paid for the merger with Arbutus Inc. - see "Results of Operations".
9
Other income (losses) / Other income (losses) consist primarily of changes in the fair value of our contingent consideration, warrant liability and foreign exchange gains and losses. During Q1 2017, our remaining warrants were exercised or expired, resulting in a decrease of $0.1 million in fair value of warrant liability recorded in our statement of operations, and an ending warrant liability balance of nil. In Q1 and Q2 2017, we recorded a net increase in the fair value of contingent consideration of $0.9 million.
We have recorded large foreign exchange gains and losses over the past eight quarters including a gain of $11.8 million in Q3 2015. Up until December 31, 2015, our foreign exchange gains and losses largely related to U.S. dollar cash and investment holdings and fluctuations in the U.S./Canadian dollar exchange rate. We expect to record future foreign exchange gains and losses, on conversion from the Canadian dollar, to the U.S. dollar, as the functional currency for the company changed to the U.S. dollar effective January 1, 2016. This change in functional currency results in a smaller proportion of our cash and investments being held in a foreign currency and therefore reduces the level of gains and losses we expect to record in this respect compared to periods prior to January 1, 2016.
Income tax benefit / Income tax benefit relates to the decrease in deferred tax liability associated with impairment charges recorded on acquired intangible assets as discussed above.
Net loss / Fluctuations in our net loss are explained by changes in revenue, expenses, other income (losses) and taxes as discussed above.
RESULTS OF OPERATIONS
The following summarizes the results of our operations for the periods shown, in thousands (except for per share figures):
Three Months Ended | Six Months Ended | ||||||||||||||
June 30, | June 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | ||||||||||||
Total revenue | $ | 1,039 | $ | 309 | $ | 1,274 | $ | 912 | |||||||
Operating expenses | 20,524 | 195,557 | 39,058 | 216,137 | |||||||||||
Loss from operations | (19,485 | ) | (195,248 | ) | (37,784 | ) | (215,225 | ) | |||||||
Net loss | $ | (18,255 | ) | $ | (130,000 | ) | $ | (36,882 | ) | $ | (145,874 | ) | |||
Basic and diluted loss per share | (0.33 | ) | (2.47 | ) | (0.68 | ) | (2.80 | ) | |||||||
Revenue / Revenue is summarized in the following table, in thousands:
Three months ended June 30, | |||||||||||||
2017 | % of Total | 2016 | % of Total | ||||||||||
Dicerna | — | — | % | 32 | 10 | % | |||||||
Alexion | 318 | 31 | % | — | — | % | |||||||
Total collaborations and contracts revenue | 318 | 31 | % | 32 | 10 | % | |||||||
Dicerna licensing fee | — | — | % | 214 | 69 | % | |||||||
Alexion licensing fee | 652 | 63 | % | — | — | % | |||||||
Other milestone and royalty payments | 69 | 7 | % | 63 | 20 | % | |||||||
Total revenue | $ | 1,039 | $ | 309 | |||||||||
10
Six months ended June 30, | |||||||||||||
2017 | % of Total | 2016 | % of Total | ||||||||||
Dicerna | — | — | % | 139 | 15 | % | |||||||
Alexion | 336 | 26 | % | — | — | % | |||||||
Total collaborations and contracts revenue | 336 | 26 | % | 139 | 15 | % | |||||||
Dicerna licensing fee | — | — | % | 427 | 47 | % | |||||||
Alexion licensing fee | 761 | 60 | % | — | — | % | |||||||
Other milestone and royalty payments | 177 | 14 | % | 346 | 38 | % | |||||||
Total revenue | $ | 1,274 | $ | 912 | |||||||||
Revenue contracts are addressed in detail in the Overview section of Item 2 above.
Dicerna revenue
In November 2014, we signed a License Agreement and a Development and Supply Agreement with Dicerna for the use of our proprietary delivery technology and related technology intended to develop, manufacture, and commercialize products related to the treatment of PH1. Licensing fee revenue recognized in the three and six months ended June 30, 2016 relates to the earned portion of the upfront payment of $2.5 million for the use of our technology, which was being recognized over the period we provided services to Dicerna. In September 2016, Dicerna announced the discontinuation of their DCR-PH1 program using the Company's technology. As such, we revised the estimated completion date of performance period from March 2017 to September 30, 2016, at which time we had no further remaining performance obligations.
Alexion revenue
In March 2017, we signed a License Agreement with Alexion that granted them exclusive use of our proprietary lipid nanoparticle (LNP) technology in one of Alexion's rare disease programs. Licensing fee revenue recognized in the three and six months ended June 30, 2017 relates to the earned portion of the non-refundable upfront payment of $7.5 million for the use of our technology, which is being recognized over the expected period we provide services to Alexion. In addition, we began recognizing revenue on the services provided to Alexion related to technology development, manufacturing and regulatory support for the advancement of Alexion's mRNA product candidate.
In July 2017, we received notice of termination from Alexion for our LNP license agreement. The termination was driven by a strategic review of Alexion's business and research and development portfolio, which included a decision to discontinue development of mRNA therapeutics. We anticipate recording the remaining deferred revenue for the non-refundable upfront payment, as well as revenue for any work done related to closeout procedures in Q3 2017.
Other milestone and royalty payments
Under our licensing and collaboration arrangements with Alnylam and Acuitas, we earn licensing fee revenue from Acuitas as well as further potential development and commercial milestones from Alnylam for the use of our LNP technology.
In September 2013, Spectrum announced that they had shipped the first commercial orders of Marqibo. We continue to earn royalties on the sales of Marqibo, which uses a license to our technology.
Expenses / Expenses are summarized in the following table, in thousands:
Three months ended June 30, | |||||||||||||
2017 | % of Total | 2016 | % of Total | ||||||||||
Research, development, collaborations and contracts | $ | 15,445 | 75 | % | $ | 15,215 | 8 | % | |||||
General and administrative | 4,599 | 22 | % | 23,766 | 12 | % | |||||||
Depreciation | 480 | 2 | % | 252 | — | % | |||||||
Impairment of intangible assets | — | — | % | $ | 156,324 | 80 | % | ||||||
Total operating expenses | $ | 20,524 | $ | 195,557 | |||||||||
11
Six months ended June 30, | |||||||||||||
2017 | % of Total | 2016 | % of Total | ||||||||||
Research, development, collaborations and contracts | $ | 29,317 | 75 | % | $ | 28,359 | 13 | % | |||||
General and administrative | 8,927 | 23 | % | 30,985 | 14 | % | |||||||
Depreciation | 814 | 2 | % | 469 | — | % | |||||||
Impairment of intangible assets | — | — | % | $ | 156,324 | 72 | % | ||||||
Total operating expenses | $ | 39,058 | $ | 216,137 | |||||||||
Research, development, collaborations and contracts
Research, development, collaborations and contracts expenses consist primarily of clinical and pre-clinical trial expenses, personnel expenses, consulting and third party expenses, consumables and materials, as well as a portion of stock-based compensation and general overhead costs.
R&D expenses increased in the three and six months ended June 30, 2017 as compared to the three and six months ended June 30, 2016. During the first half of 2017, we initiated a Phase 1 clinical trial for AB-423 and incurred increased clinical costs as we continued our trials for ARB-1467 and ARB-1740. In addition, during Q2 2017, we incurred costs to prepare for our recent candidate nominations, a second generation capsid inhibitor and a HBV RNA Destabilizer (formerly known as our oral surface antigen (HBsAg) inhibitor program). We also continue to incur costs related to research and preclinical studies for our other HBV programs.
A significant portion of our research, development, collaborations and contracts expenses are not tracked by project as they benefit multiple projects or our technology platform and because our most-advanced programs are not yet in late-stage clinical development. However, our collaboration agreements contain cost-sharing arrangements pursuant to which certain costs incurred under the project are reimbursed. Costs reimbursed under collaborations typically include certain direct external costs and hourly or full-time equivalent labor rates for the actual time worked on the project. As a result, although a significant portion of our research, development, collaborations and contracts expenses are not tracked on a project-by-project basis, we do, however, track direct external costs attributable to, and the actual time our employees worked on and our collaborations.
General and administrative
General and administrative expenses decreased in the three and six months ended June 30, 2017 compared to the three and six months ended June 30, 2016 due to a decrease in non-cash compensation expense related to the expiry of repurchase rights effective Q2 2016 related to the departure of two of the four former Arbutus Inc. shareholders in June 2016. As a result of this change, our quarterly non-cash compensation general and administrative expense decreased to $1.5 million and $3.0 million in Q2 and 1H2017, respectively, as compared to $18.5 and $23.0 million in Q2 and 1H2016, respectively. The following table summarizes the non-cash compensation expense recorded related to the expiry of repurchase rights since the Arbutus Inc. acquisition in March 2015:
Q2 | Q1 | Q4 | Q3 | Q2 | Q1 | Q4 | Q3 | Q2 | Q1 | ||||||||||||||||||||||||||||||
2017 | 2017 | 2016 | 2016 | 2016 | 2016 | 2015 | 2015 | 2015 | 2015 | ||||||||||||||||||||||||||||||
Research and development | $ | 1.5 | $ | 1.5 | $ | 1.5 | $ | 1.5 | $ | 1.5 | $ | 1.5 | $ | 1.5 | $ | 1.4 | $ | 1.0 | $ | 0.3 | |||||||||||||||||||
General and administrative | 1.5 | 1.5 | 1.5 | 1.5 | 18.5 | 4.5 | 4.5 | 4.3 | 3.1 | 0.9 | |||||||||||||||||||||||||||||
Total non-cash compensation for repurchase rights expiration | $ | 3.0 | $ | 3.0 | $ | 3.0 | $ | 3.0 | $ | 20.0 | $ | 6.0 | $ | 6.0 | $ | 5.7 | $ | 4.1 | $ | 1.2 | |||||||||||||||||||
Impairment of intangible assets
For the three and six months ended June 30, 2016, we recorded an impairment charge of $156.3 million for the discontinuance of the ARB-1598 program in the Immune Modulator drug class after extensive research and analysis, as well as a delay for additional exploration of the biology of the cccDNA Sterilizer drug class. No impairment has been required to be recognized in 2017.
12
Other income (losses) / Other income (losses) are summarized in the following table, in thousands:
Three Months Ended | Six Months Ended | ||||||||||||||
June 30, | June 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | ||||||||||||
Interest income | $ | 390 | $ | 435 | $ | 758 | $ | 679 | |||||||
Interest expense | (68 | ) | — | (110 | ) | — | |||||||||
Foreign exchange gains | 798 | 33 | 1,225 | 2,975 | |||||||||||
Gain on disposition of financial instrument | — | — | — | 1,000 | |||||||||||
Decrease (increase) in fair value of warrant liability | — | 168 | (22 | ) | 329 | ||||||||||
Decrease (increase) in fair value of contingent consideration | 110 | (252 | ) | (949 | ) | (496 | ) | ||||||||
Total other income (losses) | $ | 1,230 | $ | 384 | $ | 902 | $ | 4,487 | |||||||
Foreign exchange gains (losses)
We continue to incur substantial expenses and hold cash and investment balances in Canadian dollars, and as such, will remain subject to risks associated with foreign currency fluctuations. For the three and six months ended June 30, 2017, we recorded a foreign exchange gain of $0.8 million and $1.2 million, respectively, which is primarily an unrealized gain related to an appreciation in the value of our Canadian dollar funds, from the previous period, when converted to our functional currency of U.S. dollars.
Gain on disposition of financial instrument
On March 4, 2016, Monsanto exercised its option to acquire 100% of the outstanding shares of our wholly-owned subsidiary, PADCo, as described above, and paid us an exercise fee of $1.0 million.
Decrease in fair value of warrant liability
On March 1, 2017, any remaining outstanding warrants expired. The increase in the fair value of warrants in the first half of 2017 relates to warrants exercised offset by reducing our warrant liability balance to nil as at June 30, 2017.
Increase in fair value of contingent consideration
Contingent consideration is a liability assumed by the Company from our acquisition of Arbutus Inc. in March 2015. In general, increases in the fair value of the contingent consideration are related to the progress of our programs as they get closer to triggering contingent payments.
Income tax benefit
In the three and six months ended June 30, 2016, we recorded an income tax benefit of $64.9 million due to the decrease in deferred tax liability resulting from the impairment charge we recorded, as discussed above.
13
LIQUIDITY AND CAPITAL RESOURCES
The following table summarizes our cash flow activities for the periods indicated, in thousands:
Three Months Ended | Six Months Ended | ||||||||||||||
June 30, | June 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | ||||||||||||
Net loss for the period | $ | (18,255 | ) | $ | (130,000 | ) | $ | (36,882 | ) | $ | (145,874 | ) | |||
Adjustments to reconcile net loss to net cash provided by operating activities | 4,649 | 113,958 | 10,142 | 119,164 | |||||||||||
Changes in operating assets and liabilities | 8,528 | (762 | ) | 4,123 | (1,641 | ) | |||||||||
Net cash used in operating activities | (5,078 | ) | (16,804 | ) | (22,617 | ) | (28,351 | ) | |||||||
Net cash provided by (used in) investing activities | (1,390 | ) | (85,393 | ) | 21,810 | (98,975 | ) | ||||||||
Net cash provided by financing activities | 4 | 446 | 358 | 561 | |||||||||||
Effect of foreign exchange rate changes on cash & cash equivalents | 825 | (51 | ) | 1,248 | 2,956 | ||||||||||
Net increase (decrease) in cash and cash equivalents | (5,639 | ) | (101,802 | ) | 799 | (123,809 | ) | ||||||||
Cash and cash equivalents, beginning of period | 29,851 | 144,772 | 23,413 | 166,779 | |||||||||||
Cash and cash equivalents, end of period | 24,212 | 42,970 | 24,212 | 42,970 | |||||||||||
Since our incorporation, we have financed our operations through the sales of shares, units, debt, revenues from research and development collaborations and licenses with corporate partners, interest income on funds available for investment, and government contracts, grants and tax credits.
At June 30, 2017, we had an aggregate of $115.6 million in cash and cash equivalents, short-term investments, and restricted investments as compared to an aggregate of $143.2 million in cash and cash equivalents, short-term investments, and restricted investments at December 31, 2016.
For the six months ended June 30, 2017, operating activities used $22.6 million in cash as compared to $28.4 million of cash used in the six months ended June 30, 2016. The decrease in net cash used from operating activities is largely related to the $7.5 million license payment we received from Alexion during the six months ended June 30, 2017.
For the six months ended June 30, 2017, investing activities increased cash by $21.8 million primarily due to the maturity and monetization of our short-term investments, offset by acquisition of capital assets for our new U.S. facility in Warminster.
For the six months ended June 30, 2017, financing activities increased cash by $0.4 million due to options and warrants exercised during the period. All unexercised outstanding warrants expired on March 1, 2017.
Cash requirements / At June 30, 2017 we held $24.2 million in cash and cash equivalents, $78.8 million in short-term investments, and $12.6 million in restricted investments. We believe we have sufficient cash resources to fund our operations for at least the next 12 months. In the future, substantial additional funds will be required to continue with the active development of our pipeline products and technologies. In particular, our funding needs may vary depending on a number of factors including:
14
•the need for additional capital to fund future business development programs;
• | revenue earned from our legacy collaborative partnerships and licensing agreements, including milestone and royalty payments from Alnylam and royalties from sales of Marqibo from Spectrum; |
• | the extent to which we continue the development of our product candidates, add new product candidates to our pipeline, or form collaborative relationships to advance our products; |
• | our decisions to in-license or acquire additional products or technology for development, in particular for our HBV therapeutics programs; |
• | our ability to attract and retain corporate partners, and their effectiveness in carrying out the development and ultimate commercialization of our product candidates; |
• | whether batches of drugs that we manufacture fail to meet specifications resulting in delays and investigational and remanufacturing costs; |
• | the decisions, and the timing of decisions, made by health regulatory agencies regarding our technology and products; |
• | competing technological and market developments; and |
• | costs associated with prosecuting and enforcing our patent claims and other intellectual property rights, including litigation and arbitration arising in the course of our business activities. |
We intend to seek to obtain funding to maintain and advance our business from a variety of sources including public or private equity or debt financing, collaborative arrangements with pharmaceutical companies and government grants and contracts. There can be no assurance that funding will be available at all or on acceptable terms to permit further development of our products.
If adequate funding is not available, we may be required to delay, reduce or eliminate one or more of our research or development programs or reduce expenses associated with non-core activities. We may need to obtain funds through arrangements with collaborators or others that may require us to relinquish most or all of our rights to product candidates at an earlier stage of development or on less favorable terms than we would otherwise seek if we were better funded. Insufficient financing may also mean failing to prosecute our patents or relinquishing rights to some of our technologies that we would otherwise develop or commercialize.
OFF-BALANCE SHEET ARRANGEMENTS
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
CONTRACTUAL OBLIGATIONS
The following table summarizes our contractual obligations as at June 30, 2017:
(in millions) | Payments Due by Period | ||||||||||||||||||
Total | Less than 1 year | 1 – 3 years | 3 – 5 years | More than 5 years | |||||||||||||||
Contractual Obligations | |||||||||||||||||||
Facility leases | $ | 8.8 | $ | 1.6 | $ | 2.4 | $ | 1.3 | $ | 3.5 | |||||||||
Loan payable | $ | 12.0 | $ | — | $ | 12.0 | $ | — | $ | — | |||||||||
Total | $ | 20.8 | $ | 1.6 | $ | 14.4 | $ | 1.3 | $ | 3.5 | |||||||||
IMPACT OF INFLATION
Inflation has not had a material impact on our operations.
RELATED PARTY TRANSACTIONS
We have not entered into any related party transactions in the periods covered by this discussion.
15
OUTSTANDING SHARE DATA
At July 31, 2017, we had 55,026,995 common shares issued and outstanding and outstanding options to purchase an additional 5,621,550 common shares.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
There have been no material changes in our quantitative and qualitative disclosures about market risk from those disclosed in our Annual Report on Form 10-K for the year ended December 31, 2016.
ITEM 4. CONTROLS AND PROCEDURES
As of June 30, 2017, an evaluation of the effectiveness of our “disclosure controls and procedures” (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934) was carried out by our management, with the participation of our Chief Executive Officer (CEO) and Chief Financial Officer (CFO). Based upon this evaluation, the CEO and CFO have concluded that as of June 30, 2017, our disclosure controls and procedures are effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in Securities and Exchange Commission (the “Commission”) rules and forms and (ii) accumulated and communicated to the management of the registrant, including the CEO and CFO, to allow timely decisions regarding required disclosure.
It should be noted that while the CEO and CFO believe that our disclosure controls and procedures provide a reasonable level of assurance that they are effective, they do not expect that our disclosure controls and procedures or internal control over financial reporting will prevent all errors and fraud. A control system, no matter how well conceived or operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
During management's year-end assessment process for the period-ended December 31, 2016, a material weakness was identified over management's review of the annual impairment evaluation of intangible assets and goodwill; specifically the judgments made with respect to the estimated discount rate and the mathematical accuracy of the impairment calculation. Given that management did not perform a quantitative calculation of impairment at June 30, 2017 (refer to Note 3 of the condensed consolidated interim financial statements), this matter did not have any impact on the period ended June 30, 2017. Management intends to continue to evaluate and, to the extent necessary, remediate this material weakness throughout 2017.
There have been no changes in our internal control over financial reporting (as defined in Rules 13a–15(f) and 15d–15(f) under the Exchange Act) during the three months ended June 30, 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
16
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
We are involved with various legal matters arising in the ordinary course of business. We make provisions for liabilities when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated. Such provisions are reviewed at least quarterly and adjusted to reflect the impact of any settlement negotiations, judicial and administrative rulings, advice of legal counsel, and other information and events pertaining to a particular case. Litigation is inherently unpredictable. Although the ultimate resolution of these various matters cannot be determined at this time, we do not believe that such matters, individually or in the aggregate, will have a material adverse effect on our consolidated results of operations, cash flows, or financial condition.
UBC Arbitration
Certain early work on liposomal delivery systems and related inventions was undertakenby us and assigned to the University of British Columbia (UBC). These inventions are licensed to us by UBC under a license agreement initially entered into in 1998 and subsequently amended in 2001, 2006 and 2007. We have granted sublicenses to these inventions to Alnylam. Alnylam has in turn sublicensed these inventions back to us for discovery, development and commercialization of siRNA products.
On November 10, 2014, UBC filed a demand for arbitration against us, BCICAC File No.: DCA-1623. We received UBC’s Statement of Claims on January 16, 2015. In its Statement of Claims, UBC alleges that it is entitled to C$3.5 million in allegedly unpaid royalties based on publicly available information, and an unspecified amount based on non-public information. UBC also seeks interest and costs, including legal fees. Arbutus filed its Statement of Defense to UBC’s Statement of Claims on April 27, 2015, denying that UBC is entitled to any unpaid royalties. Arbutus also filed a Counterclaim involving a patent application that Arbutus alleges UBC wrongly licensed to a third party rather than to Arbutus. Arbutus seeks any license payments for said application, and an exclusive worldwide license to said application. The proceeding has been bifurcated into three phases, beginning with a first liability phase, addressing UBC’s Claims and Arbutus’ Counterclaim that was the subject of a hearing that took place June 19-30, 2017.
Acuitas Therapeutics
On August 29, 2016, Arbutus provided Acuitas with notice that Arbutus considered Acuitas to be in material breach of the cross-license agreement. The cross-license agreement provides that it may be terminated upon any material breach by the other party 60 days after receipt of written notice of termination describing the material breach in reasonable detail. On October 25, 2016, Acuitas filed a Notice of Civil Claim in the Supreme Court of British Columbia seeking an order that Arbutus perform its obligations under the Cross License Agreement, for damages ancillary to specific performance, injunctive relief, interest and costs. We dispute Acuitas’ position; and filed our response within the time frame prescribed by the Court. On January 10, 2017, we filed an application seeking an order to enjoin Acuitas from, among other things, entering into any further agreements purporting to sublicense Arbutus’ technology from the date of the order to the date of trial or further order from the Court. On February 8, 2017, we announced that the Supreme Court of British Columbia granted Arbutus’ request for a pre-trial injunction against Acuitas, preventing Acuitas from further sublicensing of Arbutus’ lipid nanoparticle (LNP) technology until the end of October, or further order of the Court. Under the terms of the pre-trial injunction, Acuitas is prevented from entering into any new agreements which include sublicensing of Arbutus’ LNP. On March 7, 2017, Acuitas appealed the injunction decision and on April 3, 2017, the appeal was denied. The case regarding the termination of Acuitas’ license from Arbutus will be tried in court subject to scheduling.
ITEM 1A. RISK FACTORS
There have been no material changes in our risk factors from those disclosed in our Annual Report on Form 10-K for the fiscal year ended December 31, 2016.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
None.
17
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. OTHER INFORMATION
None.
ITEM 6. EXHIBITS
See the Exhibit Index hereto.
18
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on August 3, 2017.
ARBUTUS BIOPHARMA CORPORATION | ||
By: | /s/ Mark Murray | |
Mark Murray | ||
President and Chief Executive Officer | ||
19
EXHIBIT INDEX
Number | Description |
31.1* | Certification of Chief Executive Officer pursuant to Rule 13a14 or 15d14 of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the SarbanesOxley Act of 2002 |
31.2* | Certification of Chief Financial Officer pursuant to Rule 13a14 or 15d14 of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the SarbanesOxley Act of 2002 |
32.1* | Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the SarbanesOxley Act of 2002 |
32.2* | Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the SarbanesOxley Act of 2002 |
101 | Interactive Data Files |
* Filed herewith.
20
