Attached files
| file | filename |
|---|---|
| 8-K - 8-K CARDINAL PART 1 DATA AND PART 2 INITIATION - REATA PHARMACEUTICALS INC | reta-8k_20170724.htm |
| EX-99.1 - EX-99.1 PRESS RELEASE CARDINAL - REATA PHARMACEUTICALS INC | reta-ex991_194.htm |

CARDINAL PHASE 2 RESULTS AND UPDATE Exhibit 99.2

Forward-Looking Statements This presentation contains certain “forward-looking” statements that are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical or present facts, are forward-looking statements, including statements regarding our future financial condition, business strategy, and plans and objectives of management for future operations. In some cases, you can identify forward-looking statements by terminology such as “believe,” “will,” “may,” “estimate,” “continue,” “aim,” “assume,” “anticipate,” “contemplate,” “intend,” “target,” “project,” “should,” “plan,” “expect,” “predict,” “could,” “possible,” “seek,” “goal”, “potential,” “hypothesize,” “likely,” “modeled” or the negative of these terms or other similar terms or expressions that concern our expectations, strategy, plans, or intentions. These statements are based on our intentions, beliefs, projections, outlook, analyses, or current expectations using currently available information, are not guarantees of future performance, and involve certain risks and uncertainties. Although we believe that the expectations reflected in these forward-looking statements are reasonable, we cannot assure you that our expectations will prove to be correct. Therefore, actual outcomes and results could materially differ from what is expressed, implied, or forecast in these statements. Any differences could be caused by a number of factors including but not limited to: the success, cost, and timing of our product development activities and clinical trials; our ability to advance our NRF2 activators and other technologies; our ability to obtain and maintain regulatory approval of our product candidates, and limitations and warnings in the label of an approved product candidate; our ability to obtain funding for our operations, including funding necessary to complete further development and commercialization of our product candidates; our plans to research, develop, and commercialize our product candidates; the commercialization of our product candidates, if approved; the rate and degree of market acceptance of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to identify target patient populations and serve those markets, especially for diseases with small patient populations; the success of competing therapies that are or may become available; our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates; the ability to license additional intellectual property relating to our product candidates and to comply with our existing license agreements; our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately; our ability to attract collaborators with development, regulatory, and commercialization expertise; our ability to attract and retain key scientific or management personnel; our ability to grow our organization and increase the size of our facilities to meet our anticipated growth; the accuracy of our estimates regarding expenses, future revenue, capital requirements, and needs for additional financing; and regulatory developments in the United States and foreign countries. Additional factors that could cause actual results to differ materially from our expectations can be found in our Securities and Exchange Commission filings. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors nor can we assess the effects of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. All forward-looking statements included in this presentation are expressly qualified in their entirety by these cautionary statements. The forward-looking statements speak only as of the date made and, other than as required by law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise. 2

Overview of Alport Syndrome Alport Syndrome Pathophysiology Type IV collagen mutations cause defects in the glomerular basement membrane (GBM) Patients present with hematuria and then develop proteinuria and deterioration of kidney function, leading to end-stage renal disease (ESRD) Deafness and ocular abnormalities are also common Like diabetic CKD, oxidative stress, inflammation, and fibrosis are known to promote disease and drive progressive loss of kidney function Patient Statistics Prevalence of approximately 12,000 patients in the U.S. and 40,000 globally Median age at initiation of ESRD for males with most common form is 25 Median survival of approximately 55 years Current Disease Management No approved therapies ACE inhibitors and ARBs are commonly used off-label Dialysis or renal transplant is needed in most patients 3

CARDINAL Phase 2 Trial Design Open-label trial designed with input from international key opinion leaders and the Alport Syndrome Foundation Key eligibility criteria include: Genetically-confirmed diagnosis Age range from 12 to 60 years old Broad range of kidney function (eGFR between 30-90 mL/min/1.73 m2) Patients with stage 4 CKD, cardiovascular disease, or fluid retention are excluded Titration is used to reach goal dose of 20 or 30 mg given orally, once daily Week 12 treatment effect from ongoing Phase 2 trial used to guide decision making 4 Week 12 eGFR Change (ml/min/1.73 m2) Decision < 3 Do not proceed to Phase 3 3 to < 5.5 Proceed to Phase 3 and increase sample size ≥ 5.5 Proceed to Phase 3 without increasing sample size
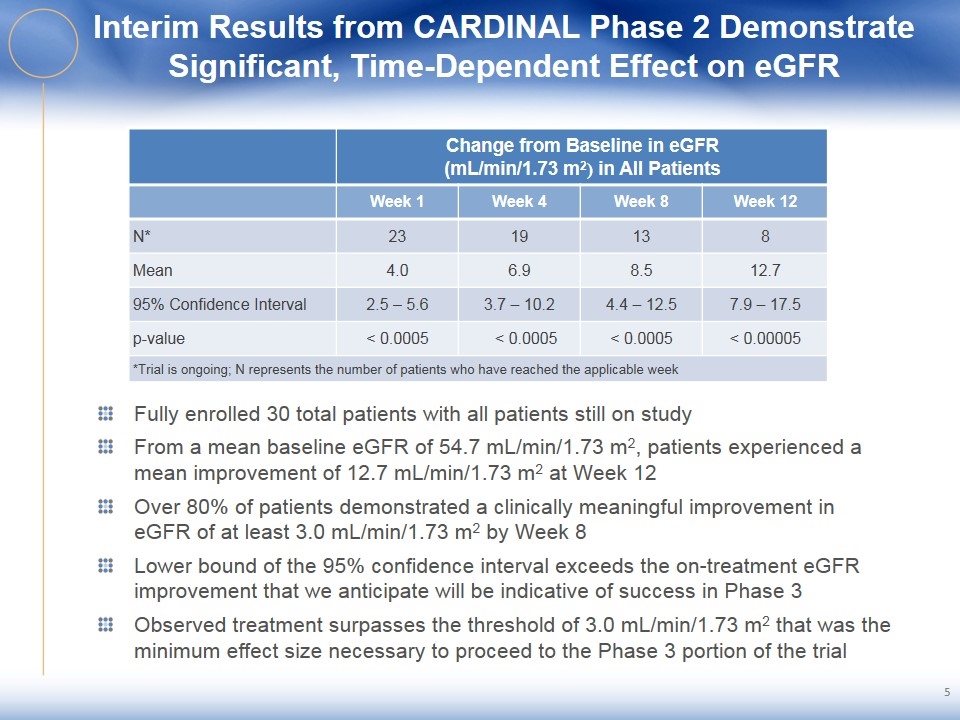
Interim Results from CARDINAL Phase 2 Demonstrate Significant, Time-Dependent Effect on eGFR Change from Baseline in eGFR (mL/min/1.73 m2) in All Patients Week 1 Week 4 Week 8 Week 12 N* 23 19 13 8 Mean 4.0 6.9 8.5 12.7 95% Confidence Interval 2.5 – 5.6 3.7 – 10.2 4.4 – 12.5 7.9 – 17.5 p-value < 0.0005 < 0.0005 < 0.0005 < 0.00005 *Trial is ongoing; N represents the number of patients who have reached the applicable week Fully enrolled 30 total patients with all patients still on study From a mean baseline eGFR of 54.7 mL/min/1.73 m2, patients experienced a mean improvement of 12.7 mL/min/1.73 m2 at Week 12 Over 80% of patients demonstrated a clinically meaningful improvement in eGFR of at least 3.0 mL/min/1.73 m2 by Week 8 Lower bound of the 95% confidence interval exceeds the on-treatment eGFR improvement that we anticipate will be indicative of success in Phase 3 Observed treatment surpasses the threshold of 3.0 mL/min/1.73 m2 that was the minimum effect size necessary to proceed to the Phase 3 portion of the trial
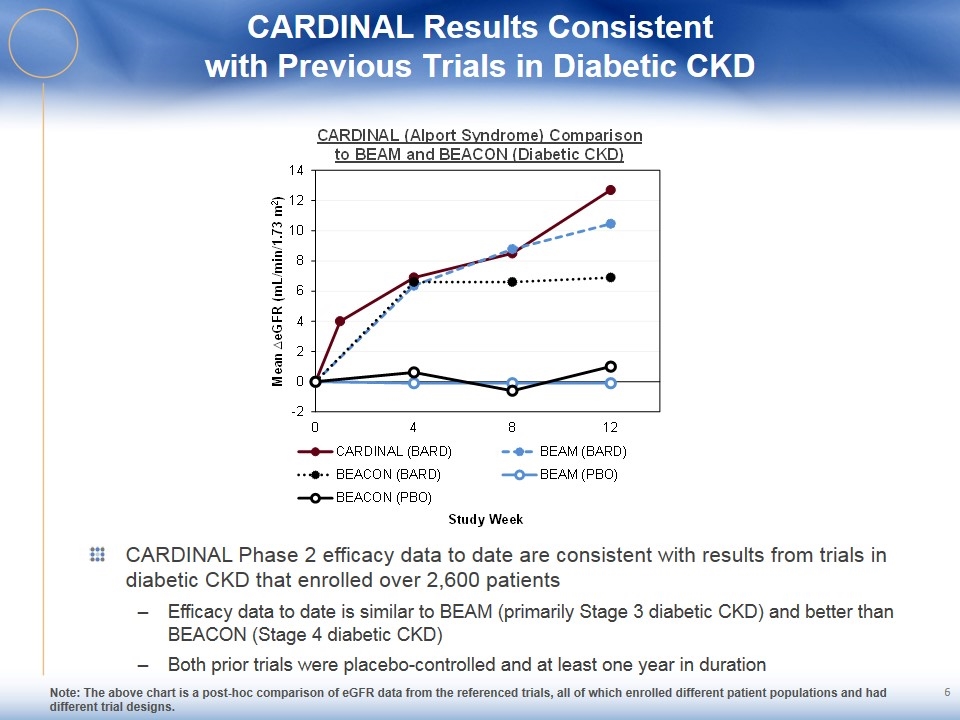
CARDINAL Results Consistent with Previous Trials in Diabetic CKD CARDINAL Phase 2 efficacy data to date are consistent with results from trials in diabetic CKD that enrolled over 2,600 patients Efficacy data to date is similar to BEAM (primarily Stage 3 diabetic CKD) and better than BEACON (Stage 4 diabetic CKD) Both prior trials were placebo-controlled and at least one year in duration Note: The above chart is a post-hoc comparison of eGFR data from the referenced trials, all of which enrolled different patient populations and had different trial designs. 6
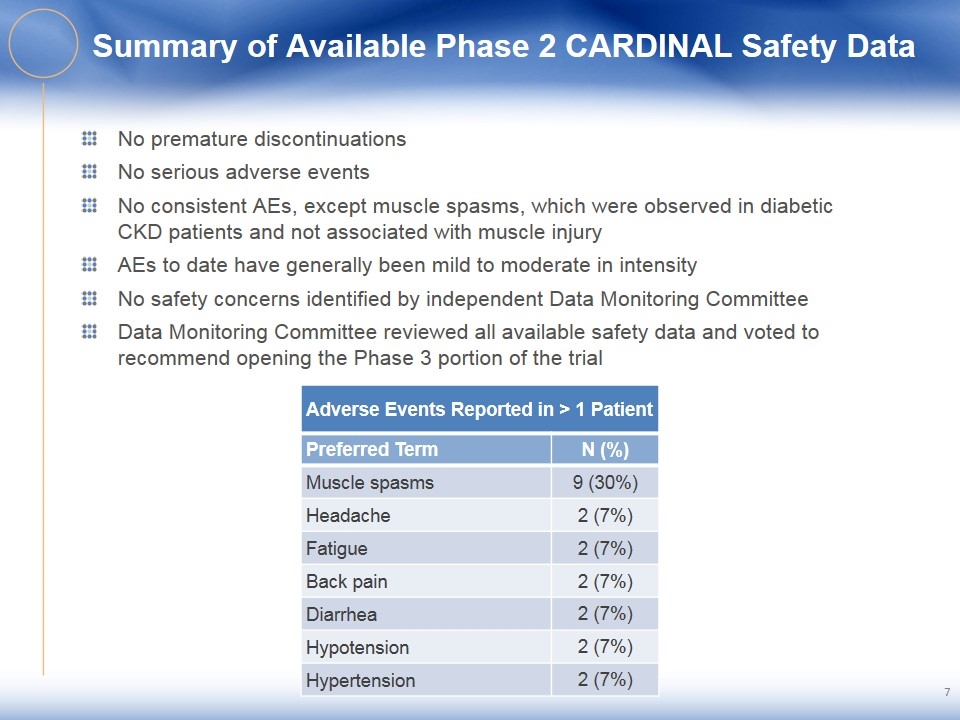
Summary of Available Phase 2 CARDINAL Safety Data No premature discontinuations No serious adverse events No consistent AEs, except muscle spasms, which were observed in diabetic CKD patients and not associated with muscle injury AEs to date have generally been mild to moderate in intensity No safety concerns identified by independent Data Monitoring Committee Data Monitoring Committee reviewed all available safety data and voted to recommend opening the Phase 3 portion of the trial Adverse Events Reported in > 1 Patient Preferred Term N (%) Muscle spasms 9 (30%) Headache 2 (7%) Fatigue 2 (7%) Back pain 2 (7%) Diarrhea 2 (7%) Hypotension 2 (7%) Hypertension 2 (7%)

CARDINAL Phase 3 Trial Phase 3 portion of the trial is designed to support regulatory approval for Alport syndrome Design incorporates insights from all prior CKD studies including dose-response, predictors of response, and other design elements Double-blind, placebo-controlled with 1:1 randomization at up to 100 sites worldwide Enrolling up to 150 patients with two years of follow-up All key endpoints are eGFR-based and objective Primary endpoint: Change in on-treatment eGFR relative to placebo at Week 48 Key secondary endpoint: Retained eGFR relative to placebo at Week 52 following a 4-week withdrawal period Patients will be re-started on study drug and treated for additional year to measure on treatment and retained eGFR at year two FDA guidance: Retained eGFR after one year may support accelerated approval and retained eGFR after two years may support full approval
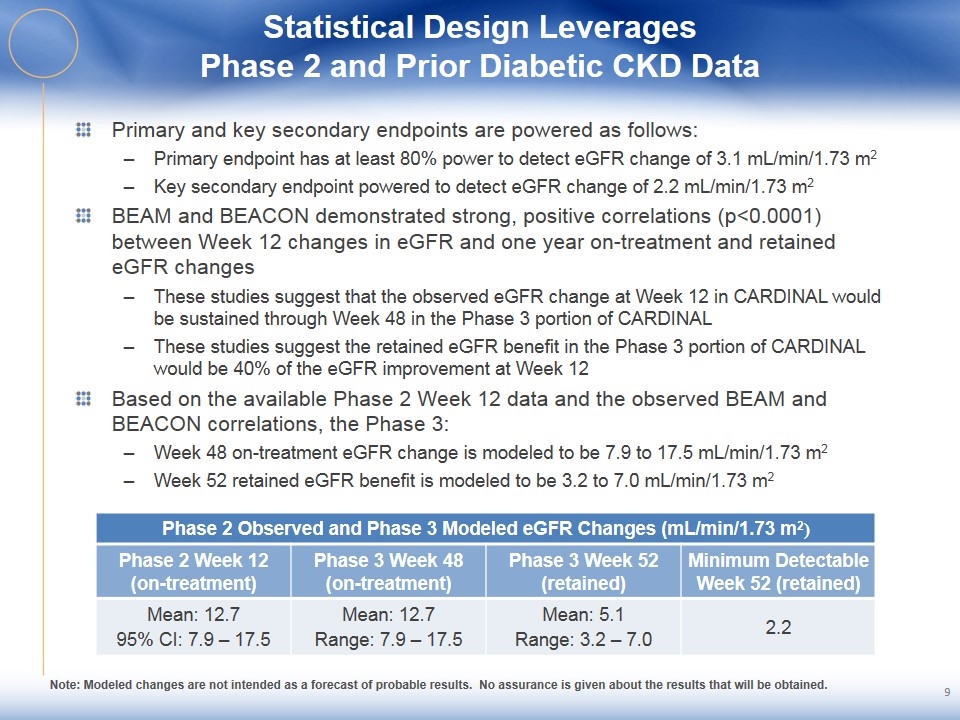
Statistical Design Leverages Phase 2 and Prior Diabetic CKD Data Primary and key secondary endpoints are powered as follows: Primary endpoint has at least 80% power to detect eGFR change of 3.1 mL/min/1.73 m2 Key secondary endpoint powered to detect eGFR change of 2.2 mL/min/1.73 m2 BEAM and BEACON demonstrated strong, positive correlations (p<0.0001) between Week 12 changes in eGFR and one year on-treatment and retained eGFR changes These studies suggest that the observed eGFR change at Week 12 in CARDINAL would be sustained through Week 48 in the Phase 3 portion of CARDINAL These studies suggest the retained eGFR benefit in the Phase 3 portion of CARDINAL would be 40% of the eGFR improvement at Week 12 Based on the available Phase 2 Week 12 data and the observed BEAM and BEACON correlations, the Phase 3: Week 48 on-treatment eGFR change is modeled to be 7.9 to 17.5 mL/min/1.73 m2 Week 52 retained eGFR benefit is modeled to be 3.2 to 7.0 mL/min/1.73 m2 Phase 2 Observed and Phase 3 Modeled eGFR Changes (mL/min/1.73 m2) Phase 2 Week 12 (on-treatment) Phase 3 Week 48 (on-treatment) Phase 3 Week 52 (retained) Minimum Detectable Week 52 (retained) Mean: 12.7 95% CI: 7.9 – 17.5 Mean: 12.7 Range: 7.9 – 17.5 Mean: 5.1 Range: 3.2 – 7.0 2.2 Note: Modeled changes are not intended as a forecast of probable results. No assurance is given about the results that will be obtained.
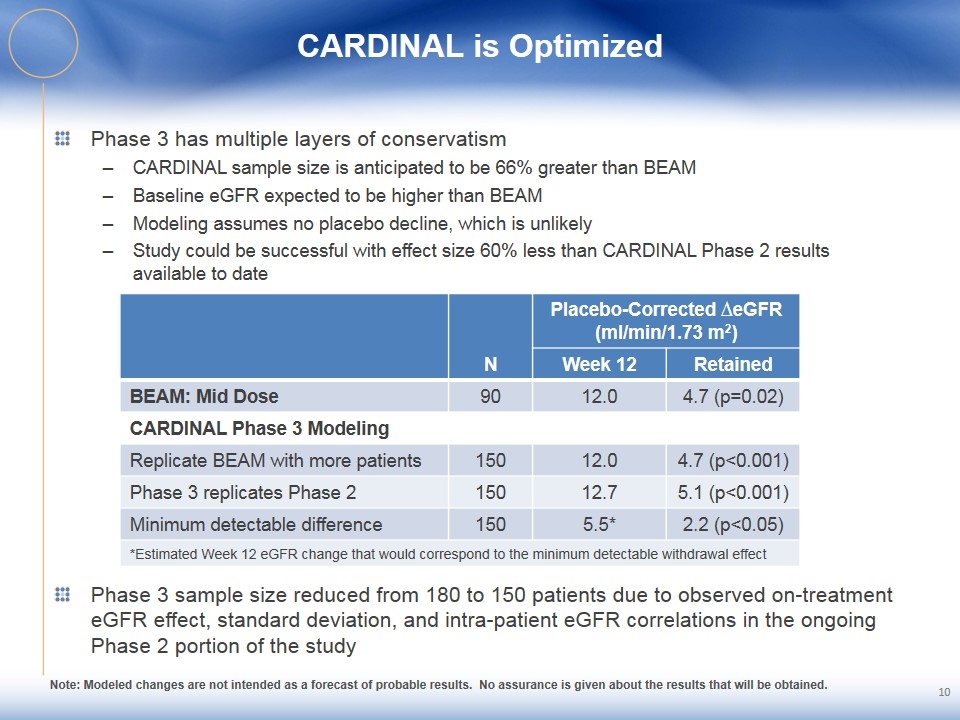
CARDINAL is Optimized Phase 3 has multiple layers of conservatism CARDINAL sample size is anticipated to be 66% greater than BEAM Baseline eGFR expected to be higher than BEAM Modeling assumes no placebo decline, which is unlikely Study could be successful with effect size 60% less than CARDINAL Phase 2 results available to date N Placebo-Corrected ∆eGFR (ml/min/1.73 m2) Week 12 Retained BEAM: Mid Dose 90 12.0 4.7 (p=0.02) CARDINAL Phase 3 Modeling Replicate BEAM with more patients 150 12.0 4.7 (p<0.001) Phase 3 replicates Phase 2 150 12.7 5.1 (p<0.001) Minimum detectable difference 150 5.5* 2.2 (p<0.05) *Estimated Week 12 eGFR change that would correspond to the minimum detectable withdrawal effect Phase 3 sample size reduced from 180 to 150 patients due to observed on-treatment eGFR effect, standard deviation, and intra-patient eGFR correlations in the ongoing Phase 2 portion of the study Note: Modeled changes are not intended as a forecast of probable results. No assurance is given about the results that will be obtained.

Potential Bard Alport Syndrome Target Product Profile Bard has potential to become the first treatment approved for Alport syndrome No therapies are approved for this deadly, chronic, orphan disease Off-label use of RAAS agents has minimal impact of slowing disease progression Bard has a novel, potent mechanism that affects inflammation, fibrosis, and GFR Bard targets the underlying renal pathophysiology driving disease Bard inhibits the acute and chronic inflammation-mediated pathways, and exerts anti-proliferative and anti-fibrotic effects that affect tissue remodeling and GFR loss Bard is an easy-to-administer, oral, once-daily capsule Bard has the potential to be combined with any of class of RAAS compounds We believe that meeting approval endpoint of retained benefit is strong evidence of disease-modifying activity and can drive adoption and reimbursement Approximately 12,000 patients in the U.S. and 40,000 globally Bard pricing expected to be consistent with other disease modifying therapies for orphan indications 11
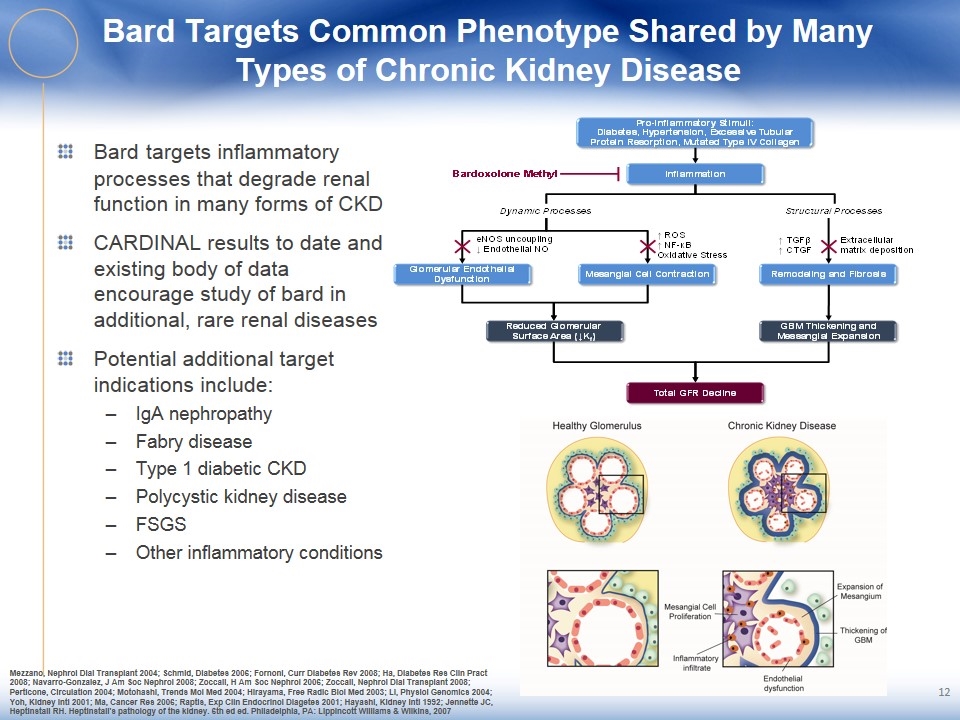
Bard Targets Common Phenotype Shared by Many Types of Chronic Kidney Disease Bard targets inflammatory processes that degrade renal function in many forms of CKD CARDINAL results to date and existing body of data encourage study of bard in additional, rare renal diseases Potential additional target indications include: IgA nephropathy Fabry disease Type 1 diabetic CKD Polycystic kidney disease FSGS Other inflammatory conditions Mezzano, Nephrol Dial Transplant 2004; Schmid, Diabetes 2006; Fornoni, Curr Diabetes Rev 2008; Ha, Diabetes Res Clin Pract 2008; Navarro-Gonzalez, J Am Soc Nephrol 2008; Zoccali, H Am Soc Nephrol 2006; Zoccali, Nephrol Dial Transplant 2008; Perticone, Circulation 2004; Motohashi, Trends Mol Med 2004; Hirayama, Free Radic Biol Med 2003; Li, Physiol Genomics 2004; Yoh, Kidney Intl 2001; Ma, Cancer Res 2006; Raptis, Exp Clin Endocrinol Diagetes 2001; Hayashi, Kidney Intl 1992; Jennette JC, Heptinstall RH. Heptinstall's pathology of the kidney. 6th ed ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007 12

