Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HELIUS MEDICAL TECHNOLOGIES, INC. | d423913d8k.htm |
Exhibit 99.1
Fact Sheet: July 10, 2017

Developer of the Portable Neuromodulation Stimulator (PoNS™) Therapy
Helius Medical Technologies is a Newtown, PA-based medical device company developing and commercializing the PoNS Therapy platform, a powerful wearable direct current stimulation technology to treat movement and gait disorders in patients with traumatic brain injury (TBI) and chronic neurological disease. The first targeted application of the PoNS is for balance disorder tied to mild to moderate TBI. The US neurostimulation device market is forecast to grow to $4 billion in 2018.
Features of the PoNS Therapy platform:
| • | Proprietary non-invasive wearable platform for treating symptoms of neurological disease or trauma; |
| • | Direct impulses delivered to the brain and brainstem via disposable arrays placed on the tongue (razor blade), powered by wearable pulse generator (razor); |
| • | The mild to moderate TBI trial with PoNS Therapy was deemed a non-significant risk trial by the FDA; we expect to report on results of this trial in early 2H2017; |
| • | Class II regulatory process (120-day timeline from submission to FDA decision), with expected regulatory clearance in 1H2018; |
| • | Pilot studies support motor and gait disturbance correction, across a range of neurological conditions, including Traumatic Brain Injury (TBI), Multiple Sclerosis (MS), and Cerebral Palsy (CP); |
| • | Reimbursement framework for physical and cognitive therapy portion of treatment in place with commercial and government payers; |
| • | Protected by over 40 patents including proprietary medical methods (5), as well as design and utility of therapeutic delivery mechanisms (35); |
| • | Potential opportunity for human performance improvement applications post medical therapy development. |
Company Highlights:
Helius is led by an experienced management team. CEO Phil Deschamps, CFO & COO Joyce LaViscount and CMO Jonathan Sackier each bring over 30+ years of experience in Health Sciences industry with a focus on development and commercialization of healthcare products.
The company is nearing completion of a 120 patient double-blind, randomized, sham -controlled study of the safety and effectiveness of the PoNS device for cranial nerve noninvasive neuromodulation in subjects with a chronic balance deficit due to mild to moderate TBI. The Company plans to submit for FDA, Health Canada and CE Mark approval and TGA regulatory approval of the PoNS device in 2H 2017.
In addition to over $7 million in grants raised by the inventors, the Company has raised over $40 million in equity capital since June 2014, and has received $1.8 million in funding from the U.S. Army in support of PoNS clinical development, with a further $1 million expected later in 2017.
The Company filed a registration statement on Form S-3 in January 2017 establishing a $100 million shelf; $9.5 million was taken down in the February 2017 registered offering. Helius also completed a private placement financing in June 2017, raising an additional $5.275 million.
Helius shares are currently traded on the Toronto Stock Exchange (symbol: HSM) & Over-The-Counter Bulletin Board in the U.S. (symbol: HSDT) with a goal to be listed on a US national securities exchange.
The Company has received ISO 13485 Certification, which permits submission to Health Canada, CE Mark in Europe and TGA in Australia.
Helius Medical’s PoNS™ Therapy
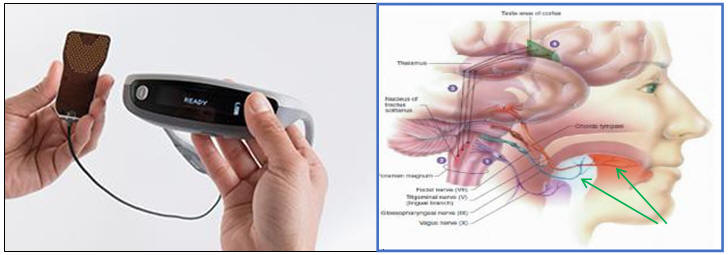
Targeted Nerves
The PoNS device delivers specially-patterned electrical stimulation developed to mirror nerve impulses to the brain through 143-gold-plated electrodes on the mouthpiece which is placed on the tongue;
Stimulation of the brain and brain-stem via cranial nerve innervation of the tongue is hypothesized to encourage neuroplasticity, or a “re-routing”, enabling the damaged brain to compensate for lost function due to trauma or disease, by recruiting existing working neurons;
The PoNS device, when used in combination with specialized physical or cognitive therapy, has been shown to improve symptoms of patients with chronic neurological symptoms caused by disease or trauma;
Relative to most neurological drug and device treatments, shorter clinical timelines, fewer study subjects and a shortened regulatory pathway offer relatively lower-cost opportunities to bring new indications to market;
Key near-term focus is the ongoing FDA registrational trial for PoNS Therapy for the treatment of balance deficit associated with mild to moderate TBI.
Investment Highlights for Helius Medical Technologies
One of the few pure-play medical device company in the large and growing neuromodulation market. The neuromodulation market is estimated at nearly $6 billion worldwide by 2020 and is expected to grow in the double digits on a percentage basis annually. Significant industry consolidation by large, diversified medical device companies has left few pure-play companies developing and commercializing novel therapies. Differentiated technologies like the PoNS™ device capture market share relatively quickly post-launch.
Helius has a number of compelling milestones. The company has a goal to have its shares listed on a US national securities exchange, a critical component in potentially broadening the US institutional investor base. Data from the 120 patient double-blind, randomized, sham-controlled trial in TBI is expected to read out in early 2H2017. Assuming FDA clearance, the 510(k)-de Novo to Class II device classification could allow for commercial launch as early as 1H2018.
PoNS Therapy could play a unique role in the treatment paradigm for multiple neurological deficits. The unique and non-invasive neuromodulation approach, in conjunction with physical or cognitive therapy, as well as pharmacotherapies, could allow for potential broad applicability across a range of indications beyond TBI. Positive outcomes with PoNS Therapy could reduce the cost of pharmaceutical and rehabilitation treatments for patients that are incurred with today’s care plans.
Flexible business model. If the PoNS Therapy is approved for commercialization, the combination of the wearable pulse generator and disposable mouthpiece could result in a recurring revenue stream. The service model is expected to launch in a clinic setting initially, with potential expansion to a home-based therapy care plan support model, with existing reimbursement for treatment. The de Novo device classification affords for relatively short clinical trials with a small number of subjects, translating to relatively low cost development to expand into additional indications.
Risk Factors
Our business is subject to a number of risks of which you should be aware before making a decision to invest in our common stock. These risks are discussed more fully in the “Risk Factors” section of our Transition Report on Form 10-K for the period ended December 31, 2016, which we filed with the SEC on April 3, 2017. These risks include the following:
| • | risks related to the Company’s limited operating history; |
| • | the Company being dependent on the ability and expertise of its Chief Executive Officer, Chief Medical Officer and a very limited number of employees; |
| • | the Company having incurred losses since its inception and its anticipation that it will continue to incur substantial net losses for the foreseeable future and may never achieve or sustain profitability; |
| • | risks relating to the Company requiring additional financing to carry out its plan of operations; |
| • | the Company’s independent registered public accounting firm having included an explanatory paragraph relating to the Company’s ability to continue as a going concern in its report on the Company’s audited financial statements for the year ended December 31, 2016; |
| • | the Company’s failure to maintain effective internal controls over financial reporting; |
| • | risks related to the Company raising additional capital by issuing securities or through debt financing or licensing arrangements that may cause dilution to existing shareholders, restrict its operations or require the Company to relinquish proprietary rights; |
| • | risks concerning the Company only having one product candidate, which is still in development, and the Company not having obtained clearance from the United States Food and Drug Administration (the “FDA”), CE Mark, TGA (Australia) or Health Canada with respect thereto; |
| • | the Company’s dependence on outside scientists and third-party research institutions for its research and development in order to be able to commercialize its product candidates; |
| • | the risk that if the Company fails to obtain FDA clearance for commercialization of or otherwise fails to ensure that the PoNS™ device is available for purchase by the U.S. Government by December 31, 2017, the Company will be subject to significant risk of loss of data and proprietary rights and a US$2,000,000 contract penalty payable to A&B pursuant to the Strategic Agreement with A&B; |
| • | the risk that the Strategic Agreement with A&B may be terminated; |
| • | risks related to the limited market awareness of the Company and its product; |
| • | risks related to the neuromodulation market being new and growing but undefined; |
| • | the Company’s PoNS™ technology being a new “untested” form of neurostimulation therapy and the medical community tending to be very conservative in adopting new therapies; |
| • | risks related to the Company needing to expand its products beyond its single product by commercializing new product candidates, and the Company not being able to do so in a timely fashion and at expected costs, or at all; and |
| • | development by others of new or improved devices or products that may result in the Company’s present and future products from becoming obsolete. |
Forward-Looking Statements
This fact sheet contains forward-looking statements and forward-looking information as such terms are defined under applicable U.S. federal securities and Canadian securities legislation. All statements other than statements of historical fact contained in this fact sheet constitute forward-looking statements and forward-looking information, including, without limitation, statements containing the words “believe”, “may”, “plan”, “should”, “predict”, “potential”, “will”, “estimate”, “continue”, “anticipate”, “intend”, “expect”, “seek”, “mission”, “goal” and similar words, variations, expressions or the negative thereof. Forward-looking statements are necessarily based on estimates and assumptions made by management of the Helius Medical Technologies, Inc. (“Helius”, “we” or the “Company”) in light of experience and perception of historical trends, current conditions and expected future developments, as well as the factors management of the Company believes are appropriate. Forward-looking statements and information in this fact sheet include but are not limited to statements relating to:
| • | the potential results of the Company’s ongoing and planned clinical trials; |
| • | the Company’s estimate of the size of the potential markets for its products; |
| • | the estimated clinical, regulatory and commercial milestones and the timelines for achieving such milestones; |
| • | the therapeutic benefits, effectiveness and safety of the Company’s product candidates; |
| • | whether the Company will receive, and the timing and costs of obtaining, regulatory clearances in the U.S., Canada, EU and Australia; |
| • | regulatory developments and the regulatory environments in which the Company operates; and |
| • | anticipated trends and challenges in the Company’s business and the markets in which it operates. |
Such statements reflect management of the Company’s current views with respect to future events and are subject to risks and uncertainties and are necessarily based upon a number of estimates and assumptions that, while considered reasonable by the Company, are inherently subject to significant business, economic, competitive, political and social uncertainties and contingencies. Many factors could cause our actual results, performance or achievements to be materially different from any future results, performance, or achievements that may be expressed or implied by such forward-looking statements, including, among others, those risks described in “Risk Factors” above and the risks described in our SEC filings, including our Transition Report on Form 10-K for the period ended December 31, 2016 and our other SEC filings from time to time.
This fact sheet speaks as of its date and we, our advisors, and our and their affiliates and representatives undertake no obligation to update, revise or correct the information contained herein, except as required by law.
Industry and Market Data
This fact sheet contains industry and market data that we obtained from independent industry publications. We have not independently confirmed this data and, although we believe it is generally reliable, it involves a number of assumptions and limitations, it is inherently imprecise, and you are cautioned not to place undue reliance on it.
