Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - Calyxt, Inc. | d370817dex231.htm |
| EX-5.1 - EX-5.1 - Calyxt, Inc. | d370817dex51.htm |
Table of Contents
As filed with the Securities and Exchange Commission on July 10, 2017
Registration No. 333-218924
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 2
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
CALYXT, INC.
(Exact name of Registrant as specified in its charter)
Not Applicable
(Translation of Registrant’s name into English)
| Delaware | 2870 | 27-1967997 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
600 County Road D West
Suite 8
New Brighton, MN 55112
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Calyxt, Inc.
600 County Road D West
Suite 8
New Brighton, MN 55112
(Name, address, including zip code, and telephone number, including area code, of agent for service)
| Copies to: | ||||
| Richard D. Truesdell, Jr. Derek J. Dostal Davis Polk & Wardwell LLP 450 Lexington Avenue New York, New York 10017 (212) 450-4000 |
Shayne Kennedy Brian Cuneo Brett Urig Latham & Watkins LLP 650 Town Center Drive, 20th Floor Costa Mesa, California 92626 (714) 540-1235 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ (Do not check if a smaller reporting company) | Smaller reporting company | ☐ | |||
| Emerging growth company | ☒ | |||||
If an emerging growth company, indicate by check mark if the registrant has not elected to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
|
| ||||||||
| Title Of Each Class Of Securities To Be Registered |
Amount To Be Registered(1) |
Proposed Maximum Offering Price Per Share(2) |
Proposed Maximum Aggregate |
Amount Of Registration Fee(3) | ||||
| Common stock, par value $0.0001 per share |
6,969,697 | $18.00 | $125,454,546 | $8,746 | ||||
|
| ||||||||
|
| ||||||||
| (1) | Includes the offering price of shares of common stock that may be sold if the option to purchase additional shares of common stock granted to the underwriters is exercised. |
| (2) | Estimated solely for the purpose of computing the amount of the registration fee pursuant to Rule 457(a) under the Securities Act of 1933. |
| (3) | Filing fees in the amount of $5,795 were previously paid. |
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. We may not offer these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JULY 10, 2017
PRELIMINARY PROSPECTUS

6,060,606 Shares
Calyxt, Inc.
Common Stock
This is our initial public offering. We are offering 6,060,606 shares of our common stock.
Prior to this offering, there has been no public market for our common stock. We have applied to list the common stock on the NASDAQ Global Market (the “NASDAQ”) under the symbol “CLXT.” We anticipate that the initial public offering price will be between $15.00 and $18.00 per share of common stock.
We are an “emerging growth company” as that term is defined in the Jumpstart Our Business Startups Act of 2012 and, as such, will be subject to certain reduced public company reporting requirements. See “Summary—Implications of Being an Emerging Growth Company.”
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 13.
| Per Share | Total | |||||||
| Public offering price |
$ | $ | ||||||
| Underwriting discounts(1) |
$ | $ | ||||||
| Proceeds to us before expenses(1) |
$ | $ | ||||||
| (1) | We have agreed to reimburse the underwriters for certain FINRA-related expenses. See “Underwriting.” |
Our parent company, Cellectis S.A., has reserved the option to potentially purchase up to $20.0 million of our common stock in the offering at a price per share equal to the initial public offering price. The shares would be purchased directly from Calyxt and not through the underwriters and there would be no payment of any underwriting discount.
The underwriters have the option to purchase up to 909,091 additional shares of common stock from us at the initial public offering price less the underwriting discount.
The underwriters expect to deliver the shares of common stock to purchasers on or about , 2017 through the book-entry facilities of The Depository Trust Company.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| Citigroup | Jefferies | Wells Fargo Securities |
| BMO Capital Markets | Ladenburg Thalmann |
The date of this prospectus is , 2017
Table of Contents

Table of Contents
| PAGE | ||||
| 1 | ||||
| 9 | ||||
| 11 | ||||
| 13 | ||||
| Special Note Regarding Forward-Looking Statements and Industry Data |
47 | |||
| 48 | ||||
| 49 | ||||
| 50 | ||||
| 51 | ||||
| 53 | ||||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
55 | |||
| 69 | ||||
| 100 | ||||
| 106 | ||||
| 119 | ||||
| 121 | ||||
| 126 | ||||
| 132 | ||||
| Material U.S. Federal Tax Considerations for Non-U.S. Holders |
134 | |||
| 137 | ||||
| 146 | ||||
| 146 | ||||
| 146 | ||||
| F-1 | ||||
We, Cellectis S.A. and the underwriters have not authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses prepared by or on behalf of us or to which we have referred you. We, Cellectis S.A. and the underwriters take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may provide you. We are offering to sell, and seeking offers to buy, shares of common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of the shares of common stock.
For investors outside the United States: Neither we nor any of the underwriters have taken any action that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
Unless the context requires otherwise: (a) references to “Calyxt,” the “Company,” our “company,” “we,” “us” or “our” refer to Calyxt, Inc., a Delaware corporation (formerly known as Cellectis Plant Sciences, Inc.) and (b) references to “Cellectis” refer to Cellectis S.A., a French corporation, and its subsidiaries other than Calyxt and its subsidiaries. Unless the context requires otherwise, statements relating to our history in this prospectus describe the history of Cellectis’ plant products business. See “Certain Relationships and Related Party Transactions—Relationship with Cellectis.”
i
Table of Contents
We have made rounding adjustments to some of the figures included in this prospectus. Accordingly, numerical figures shown as totals in some tables may not be an arithmetic aggregation of the figures that precede them.
Currency amounts in this prospectus are stated in U.S. dollars, unless otherwise indicated.
This prospectus includes industry and market data that we obtained from periodic industry publications, third-party studies and surveys, filings of public companies in our industry and internal company surveys. These sources include government and industry sources. Industry publications and surveys generally state that the information contained therein has been obtained from sources believed to be reliable. Although we believe the industry and market data to be reliable as of the date of this prospectus, this information could prove to be inaccurate. Industry and market data could be wrong because of the method by which sources obtained their data and because information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties. We do not know all of the assumptions regarding general economic conditions or growth that were used in preparing the forecasts from the sources relied upon or cited herein. Assumptions and estimates of our and our industry’s future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause future performance to differ materially from our assumptions and estimates. See “Special Note Regarding Forward-Looking Statements.”
The name and trademark, Cellectis, and other trademarks, trade names and service marks of Cellectis appearing in this prospectus are the property of Cellectis. Prior to the completion of this offering, Calyxt and other trademarks, trade names and service marks of Calyxt appearing in this prospectus are the property of Cellectis, and after the completion of this offering, they will be the property of, or licensed to, Calyxt. This prospectus also contains additional trade names, trademarks and service marks belonging to Cellectis and to other companies. We do not intend our use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties.
ii
Table of Contents
This summary highlights information included elsewhere in this prospectus and does not contain all of the information you should consider in making an investment decision. You should read this entire prospectus carefully, including the sections entitled “Risk Factors,” “Special Note Regarding Forward-Looking Statements,” “Selected Historical Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the notes thereto before making an investment decision regarding our common stock.
Our Company
Overview
We are a consumer-centric, food- and agriculture-focused company. By combining our leading gene-editing technology and technical expertise with our innovative commercial strategy, we are pioneering a paradigm shift to deliver healthier specialty food ingredients, such as healthier oils and high fiber wheat, for consumers and agriculturally advantageous crop traits, such as herbicide tolerance, to farmers. While the traits that enable these characteristics may occur naturally and randomly through evolution—or under a controlled environment through traditional agricultural technologies—those processes are imprecise and take many years, if not decades. Our technology enables us to precisely and specifically edit a plant genome to elicit the desired traits and characteristics, resulting in a final product that has no foreign DNA. We believe the precision, specificity, cost effectiveness and development speed of our gene-editing technologies will enable us to provide meaningful disruption to the food and agriculture industries.
Food-related issues including obesity and diabetes are some of the most prevalent health issues today and continue to grow rapidly. As awareness of these diet-related health issues grows, consumers are emphasizing a healthier lifestyle and a desire for nutritionally rich foods that are more nutritious, better tasting, less processed and more convenient. This trend is leading to an increase in the demand for higher valued, premium segments of the food industry, such as higher fiber, reduced gluten and reduced fat products. As a result of these trends, food companies are looking for specialty ingredients and solutions that can help them satisfy their customers’ evolving needs and drive growth in market share and new value-added products.
While food companies are focused on these trends, we believe the legacy agriculture companies have overlooked society’s food-related issues and are not properly equipped to address health-driven consumer food trends. These legacy agriculture companies have historically focused on increasing yields, profit margins and market share. They have been burdened by high research and development costs and a high degree of commoditization in their deep, farmer-focused supply chains.
We have developed a robust product pipeline with our proprietary technology. Our first product candidate, which we expect to be commercialized by the end of 2018, is a high oleic soybean designed to produce a healthier oil that has zero trans fats and reduced saturated fats. We are also developing a high fiber wheat to create flour with up to three times more dietary fiber than standard white flour while maintaining the same flavor and convenience of use. Another product candidate we are developing is a herbicide tolerant wheat designed to provide farmers with better weed control options to increase yields and profitability. We believe each of these product candidates addresses a potential multi-billion dollar market opportunity.
We believe that our proprietary gene-editing technologies and innovative commercial strategy will allow us to bridge the divide between evolving consumer preferences and the historical approach by the large legacy companies in the agriculture supply chain.
Using our proprietary technologies and expertise, we edit the genome of food crops by using our “molecular scissors” to precisely cut DNA in a single plant cell, use the plant’s natural repair machinery to make our desired
1
Table of Contents
edit and finally regenerate the single cell into a full plant. We believe we are able to develop targeted traits—some of which would be nearly impossible to develop using traditional trait-development methods—quicker, more efficiently and more cost effectively than traditional trait-development methods. Our technology positions us to assess the probability of success early on in the research and development process, potentially eliminating expensive late stage failures and allowing for a larger breadth of products to be developed. We have a strong track record with respect to our technologies and expertise as we have successfully edited more than 20 unique genes in 6 plant species since our inception in 2010.
Our commercial strategy is centered on two core elements: developing healthier specialty food ingredients, such as healthier oils and high fiber wheat, to enable the food industry to address evolving consumer trends and developing agriculturally advantageous traits, such as herbicide tolerance, for farmers. This will involve developing and leveraging our supply chain to effectively bring our consumer- and farmer-centric products to the marketplace. For our consumer-centric products we intend to repurpose and leverage existing supply chain capacity by contracting, tolling or partnering with players in the existing supply chain, such as seed production companies, farmers, crushers, refiners or millers, which we expect will allow us to apply our resources to maximizing innovation and product development while minimizing our capital expenditures and overhead. For our farmer-centric products, we intend to broadly out-license our products to the seed industry.
Our Competitive Strengths
We believe that we are strategically well-positioned to develop high-value and innovative products. Our competitive strengths include:
| • | Proprietary technologies creating a powerful platform to design and develop new products. Since our founding, we have been at the forefront of the research, development and application of plant-based gene-editing technologies. Our capabilities enable us to precisely edit specific genes from a target food crop to improve the nutritional composition or provide agricultural benefits to farmers. Three examples of our technological innovation include: |
| • | High Oleic Soybean: We deactivated key genes associated with fatty acid biosynthesis to achieve a healthier soybean oil. |
| • | High Fiber Wheat: We simultaneously deactivated all six copies of a gene within a single wheat plant with the purpose of increasing fiber content. |
| • | Herbicide Tolerance: We believe we can develop crop varieties that will be tolerant to certain herbicides by identifying and making a subtle base substitution that we believe will be sufficient to confer herbicide tolerance. We expect to be able to replicate this process in various crops. |
| • | Innovative portfolio of product candidates with an accelerated path to market. We are currently developing a diversified portfolio spanning across five core crops—soybean, wheat, canola, potato and alfalfa—and a multitude of product candidates. These include innovative consumer-centric product candidates like our high-fiber wheat that is designed to produce flour with up to three times the fiber content of standard white flour, as well as innovative, farmer-centric solutions like herbicide tolerant wheat and products with valuable supply chain benefits like cold storable potatoes that are designed to store longer and produce much less acrylamide in the frying process, a human health concern that has been linked to cancer. We believe our portfolio of product candidates, coupled with our ability to quickly develop future product candidates, affords us the opportunity to disrupt the food industry. |
| • | Significant barriers to entry through our first-mover advantage and strong intellectual property. We command a first-mover advantage in the editing of genes in plants. As a pioneer in gene-editing technologies, we are building on more than two decades of hands-on experience and process |
2
Table of Contents
| optimization which we believe cannot be easily replicated by competitors. Our proprietary technologies and product candidates benefit from the licensing of a portfolio of 81 issued patents and 170 pending patent applications. |
| • | Faster, cheaper and innovative product development process focused on end user needs. Genetic modification has traditionally taken an average of 13 years and over $130 million to develop a commercially viable product. By contrast, a key advantage of our gene-editing technology platform is that we believe we can develop products from concept to commercialization in three to six years and at a fraction of the cost. For example, we created our high oleic soybean product candidate by generating fewer than 20 independent plants that were edited with TALEN. This contrasts with traditional genetic modification methods which we believe require thousands of plants to achieve the same result. We developed our high oleic soybean from concept to field in under four years and expect to commercialize this product by the end of 2018. We believe we will continue to be able to react quickly to consumer and farmer needs that we identify. |
| • | Supply chain flexibility enabling us to capture significant downstream value. We plan to develop consumer traits and leverage existing supply chain capacity and our existing relationships to provide differentiated specialty ingredients to food companies. By doing so, we believe that we will be able to capture significant value as our innovative products move from “field to fork.” Our supply chain is flexible, enabling us to layer on new products to our existing ones and capture additional value. By stacking additional characteristics on the same seeds, we believe we can create additional value without necessarily increasing acreage, volumes of grain produced or the amount of oil commercialized, but rather by aiming to increase the value of our products on a per unit basis. For example, we believe this value creation could be implemented in our high oleic soybean product candidate if we were to stack a trait that improves the protein composition of the meal, a trait that further improves the soybean oil fatty acid profile or a farmer-focused trait such as herbicide tolerance. |
| • | A world-class management team with deep industry expertise. Our executive team has more than 120 years of collective experience in the agriculture and gene-editing fields. This includes over 95 years of collective experience in agricultural supply chain, product development and commercial operations, during which several members of our executive team have managed billions of dollars of revenue and cost at large multinational corporations. Several members of our executive team previously worked at well-known technology and agri-business companies, such as Monsanto, Syngenta, Stine Seed Company and Cargill. As pioneers in plant-based gene editing, members of our management team invented TALEN, one of the premier gene-editing tools. Dr. Daniel Voytas, our Chief Science Officer, is a Professor of Genetics, Cell Biology and Development at the University of Minnesota and Director of the Beckman Center for Genome Engineering. He is best known for his pioneering work to develop methods for precisely editing DNA sequences in living plant cells. |
Our Growth Strategy
We believe that there are significant opportunities to grow our business both domestically and internationally by executing on the following key elements of our strategy:
| • | Commercialize our current product candidates in North America. Our near-term focus is to launch our product candidates in North America where we believe the markets for healthier specialty food ingredients for consumers and unique, plant-trait solutions for farmers are significant and the existing supply chain enables us to accelerate growth. |
| • | Identify new opportunities through our consumer- and farmer-centric approaches. We intend to continue to elicit desired traits and to include additional crops in our product pipeline. We continuously evaluate the evolving needs of the consumer and the farmer and seek to apply our technologies and |
3
Table of Contents
| expertise to provide better solutions to meet their demands. We also expect to be able create additional opportunities from our existing product candidates once they are commercialized through combining traits, which may allow us to create products with additional benefits without adding significant cost. |
| • | Accelerate our R&D productivity with enhanced automation and high throughput capabilities. We consistently strive to optimize our product development processes. Through our planned facility expansion, we are combining gene-editing automation with food science capabilities to enable us to rapidly identify new growth opportunities. We believe that this automation and our high-throughput platform will allow for greater standardization in our processes. We expect this standardization to increase our research and development productivity significantly and add a greater number of projects to our product pipeline. |
| • | Expand through R&D agreements and acquisitions. We plan to selectively partner, in-license or acquire key enabling technologies and businesses across the value chain. This may include acquiring complementary technologies and intellectual property or fully developed products. In each case, we plan to look for R&D partners and acquisitions that give us a significant presence in the health focused specialty food ingredient markets where we will be able to accelerate the launch and commercialization of our innovative products. |
| • | Leverage our North American market presence to globalize our products. While we believe the North American market opportunity remains attractive and extensive, in the future we plan to explore the possibility of expanding our business globally. This may involve exporting our products to international markets or establishing new supply chains in other attractive markets. |
4
Table of Contents
Our Product Pipeline
We have several products under development including: high oleic soybeans, powdery mildew resistant wheat, cold storable potato, high fiber wheat, reduced browning potato and herbicide tolerant wheat. Our current product candidates are depicted in the figure below:
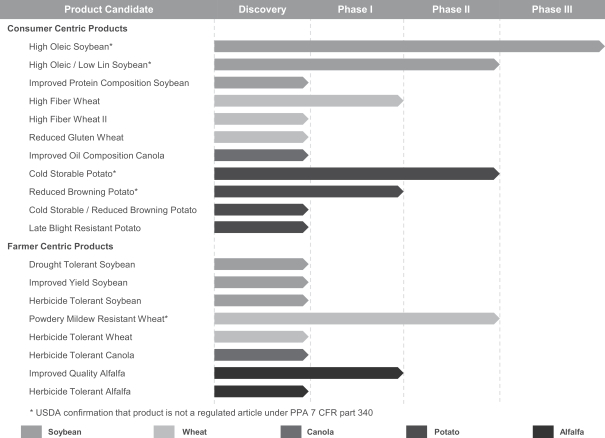
We categorize our stages of pre-commercial development from Phase I to Phase III. Prior to entering Phase I, in Discovery, we identify genes of interest. In Phase I, we edit the identified genes of interest, target edits we desire to make, and produce an initial seed that contains the desired edit. Phase II is trait validation, where we perform small-scale and large-scale tests to confirm phenotype and ingredient functionality. In this phase we also perform replicated, multi-location field testing, after confirming that the product is not a regulated article by the USDA. In Phase III, we develop the first commercial-scale pilot production, begin to build out the supply chain and inventory and perform customer testing prior to commercialization.
High Oleic Soybean (Consumer Trait)
Soybean oil has historically been partially hydrogenated to enhance its oxidative stability in order to increase shelf life and improve frying characteristics. This process, however, creates trans-unsaturated fatty acids, or trans fats, which have been demonstrated to raise low-density lipoprotein (LDL) cholesterol levels and lower high-density lipoprotein (HDL), both of which contribute to cardiovascular disease.
We developed a soybean trait that has produced oils with a fatty acid profile that contains 80% oleic acid, 20% less saturated fatty acids compared to commodity soybean oil and zero trans fats. Our high oleic soybean oil
5
Table of Contents
enhances oxidative stability more than fivefold when compared to commodity oil and also offers a threefold increase in fry-life. Our high oleic soybean was created using our TALEN gene-editing technology. We designed TALEN to specifically target two fatty acid desaturase genes (designated FAD2A and FAD2B). By key measures, including yield, our high oleic soybean variety performs comparably to its unedited counterpart. Our soybean product candidate is in Phase III of our development process. We are currently completing our commercialization plan and anticipate commercialization by the end of 2018.
High Fiber Wheat (Consumer Trait)
Research has shown that fiber may play a large role in maintaining bowel health, lowering cholesterol, stabilizing blood glucose levels and controlling weight gain. In recent years, the awareness of the health benefits of high fiber diets has increased. This has translated to a strong growth in demand for high fiber food products, with 35% of grocery shoppers now seeking high fiber foods. By 2018, the global market for high-fiber bread is expected to be $36 billion, a 25% jump from 2013.
We are developing high fiber wheat traits that could be used to produce white flour with up to three times more dietary fiber than standard white flour. We anticipate that by altering the proportion of certain slower digested carbohydrates in the wheat grain, we will increase dietary fiber. We believe our high fiber wheat flour will be incorporated into many food products—from pasta to bread. The product candidate is currently in Phase I of our development process.
Herbicide Tolerant Wheat (Farmer Trait)
With the constant need to increase yields, herbicides are an important component of commercial food production. Herbicide tolerance traits in crops can provide additional crop protection chemistry alternatives to control weeds and increase crop yields.
We are pioneering the development of herbicide tolerant traits in wheat without the use of foreign DNA. Herbicides act by inhibiting the activity of certain plant-encoded proteins that promote growth. We aim to achieve herbicide tolerance by specifically making a subtle repair to prevent herbicides from being able to recognize and block functions of these proteins, such that the edited plant survives the application of the herbicide. Our product will contain no foreign DNA. We believe this solution, if successfully developed and commercialized, will have the potential to increase the farmer’s yield and revenue. The product candidate is currently in the Discovery phase of our development process.
Other Products in Our Development Pipeline
Our extensive product pipeline includes a variety of consumer centric and farmer centric traits for soybean, wheat, canola, alfalfa and potato. We will conduct further development programs to build upon our current pipeline which currently includes improved oil composition canola, herbicide tolerant canola, improved quality alfalfa and herbicide tolerant alfalfa, late blight resistant potato, cold storable / reduced browning potato, improved protein composition soybean, drought tolerant soybean, herbicide tolerant soybean and improved yield soybean. In the future, we anticipate expanding our product pipeline to include other food crops.
We plan to develop gene-editing automation processes that will enable us to implement a high throughput discovery platform to identify new growth opportunities. This high-throughput platform is intended to allow us to discover more gene traits and make more complex edits, enabling us to drive innovation at a significantly faster rate. We believe all of these steps will enable us to remain at the forefront of food and agriculture innovation.
6
Table of Contents
Relationship with Cellectis
Prior to the completion of this offering, we were a wholly owned subsidiary of Cellectis, and all of our outstanding shares of common stock were owned by Cellectis. Immediately prior to the completion of this offering, we and Cellectis intend to enter into, or will have entered into, certain agreements that will provide a framework for our ongoing relationship with Cellectis. For a description of these agreements, see “Certain Relationships and Related Party Transactions—Relationship with Cellectis.”
Risk Factors
There are a number of risks that you should understand before making an investment decision regarding this offering. These risks are discussed more fully in the section entitled “Risk Factors” following this prospectus summary. These risks include, but are not limited to:
| • | Our limited operating history; |
| • | Our incurrence of significant losses since our inception and likelihood that we will continue to incur significant losses for the foreseeable future; |
| • | Our product development efforts use complex integrated technology platforms and require substantial time and resources; |
| • | Our crops are new, and producers may require instruction to successfully establish, grow and harvest our crops; |
| • | Our ability to produce high-quality plants and seeds cost-effectively on a large scale and to accurately forecast demand for our products; |
| • | Significant competition in plant biotechnology and the substantially greater financial, technical and other resources of our competitors; |
| • | Challenges from public perceptions of genetically engineered products and ethical, legal, environmental and social concerns and the potential future government regulation of our products; |
| • | Our ability to adequately protect our proprietary rights; |
| • | Our success in obtaining or maintaining necessary rights to product components and processes for our development pipeline through acquisitions and in-licenses; |
| • | Our ability to retain and attract senior management and key employees; |
| • | Our independent registered public accounting firm has identified a material weakness relating to our lack of a control in place to review forward purchase derivative contracts entered into by us, and this will require remediation; |
| • | The influence of Cellectis over us after this offering, including its contractual right to nominate a majority of our directors and other contractual rights; and |
| • | Our being a “controlled company” and, as a result, qualifying for, and intending to rely on, exemptions from certain corporate governance requirements. |
Corporate Information
We were incorporated in Delaware on January 8, 2010. The address of our principal executive offices is currently 600 County Road D West, Suite 8, New Brighton, MN 55112. Our website is currently www.calyxt.com. Information on, or accessible through, our website is not part of this prospectus.
7
Table of Contents
Implications of Being an Emerging Growth Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage of certain reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
| • | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act; |
| • | only two years of audited financial statements are required in addition to any required interim financial statements, and correspondingly reduced disclosure in management’s discussion and analysis of financial condition and results of operations; and |
| • | (i) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and (ii) exemptions from the requirements of holding a non-binding advisory vote on executive compensation, including golden parachute compensation. |
We may take advantage of these provisions for up to five years or until such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company upon the earliest to occur of (1) the last day of the fiscal year in which we have more than $1.07 billion in annual revenue; (2) the date we qualify as a “large accelerated filer,” with at least $700 million of equity securities; (3) the issuance, in any three-year period, by us of more than $1.07 billion in non-convertible debt securities held by non-affiliates; and (4) the last day of the fiscal year ending after the fifth anniversary of our initial public offering.
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can use the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended (the “Securities Act”) for complying with new or revised accounting standards. This permits an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We intend to take advantage of the exemptions discussed above. Accordingly, the information contained herein may be different than the information you receive from other public companies.
8
Table of Contents
| Share of common stock offered by us |
6,060,606 shares |
| Total shares of common stock to be outstanding after this offering |
25,660,606 shares (26,569,697 shares if the underwriters exercise their option in full) |
| Underwriters’ option to purchase additional shares |
The underwriters have a 30-day option to purchase up to 909,091 additional shares of common stock from us as described under the heading “Underwriting.” |
| Use of proceeds |
We estimate that the net proceeds to us from this offering will be approximately $90.9 million, or approximately $104.8 million if the underwriters exercise in full their option to purchase additional shares of our common stock, assuming an initial public offering price of $16.50 per share (the midpoint of the range set forth on the cover page of this prospectus), after deducting estimated underwriting discounts and commissions and estimated offering expenses. |
| We intend to use the net proceeds of this offering to fund research and development, to build out commercial capabilities and for working capital and general corporate purposes, as described in greater detail under “Use of Proceeds.” |
| Voting rights |
Holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholder. |
| See “Description of Capital Stock—Common Stock” for a description of the material terms of our common stock. |
| Directed share program |
At our request, the underwriters have reserved for sale, at the initial public offering price, up to 3.0% of the shares offered by this prospectus for sale to some of our or Cellectis’ directors, consultants and employees and their families. Any purchases of reserved shares by these persons would reduce the number of shares available for sale to the general public. Any reserved shares that are not so purchased will be offered by the underwriters to the general public on the same terms as the other shares offered by this prospectus. |
| NASDAQ symbol |
We have applied to list the common stock on the NASDAQ under the symbol “CLXT.” |
The number of shares outstanding after this offering is based on 19,600,000 shares outstanding as of June 30, 2017 and excludes:
| • | 1,909,775 shares of common stock issuable upon the exercise of outstanding stock options under the Calyxt, Inc. Equity Incentive Plan (the “Existing Plan”) and 2,119,699 shares of common stock issuable upon the exercise of outstanding stock options under the new equity compensation plan that we adopted in connection with this offering (the “Omnibus Plan”) at a blended weighted-average exercise price of $9.09 per share; |
9
Table of Contents
| • | 1,452,333 shares of common stock deliverable upon the vesting and settlement of outstanding restricted stock units under the Omnibus Plan; |
| • | 127,400 shares of common stock reserved for future grants or for sale under the Existing Plan; and |
| • | 1,327,969 shares of common stock reserved for future grants or for sale under the Omnibus Plan. |
Unless we specifically state otherwise or the context otherwise requires, the information in this prospectus (i) reflects the 100-for-1 stock split completed on June 14, 2017 as described in note 15 to our unaudited condensed financial statements included elsewhere in this prospectus and (ii) assumes:
| • | except in our historical financial statements included in this prospectus, the consummation of another stock split immediately prior to and contingent on closing of this offering pursuant to which each share of common stock held of record by the holder thereof will be reclassified into 2.45 shares; |
| • | no exercise of the outstanding options described above; |
| • | no purchases by our directors, officers or their affiliates pursuant to the directed share program or by Cellectis; and |
| • | the underwriters’ option to purchase up to an additional 909,091 shares of common stock from us is not exercised. |
10
Table of Contents
SUMMARY HISTORICAL FINANCIAL DATA
The summary financial information appearing below for the years ended December 31, 2016 and 2015 has been derived from our audited financial statements, included elsewhere in this prospectus with the exception of the basic and diluted net loss per share and the weighted average shares outstanding—basic and diluted, which reflect the consummation of a stock split immediately prior to and contingent on closing of this offering pursuant to which each share held of record by the holder thereof will be reclassified into 2.45 shares. The summary financial information appearing below as of March 31, 2017 and for the three months ended March 31, 2017 and 2016 has been derived from our unaudited condensed financial statements, included elsewhere in this prospectus. The unaudited condensed financial statements have been prepared on the same basis as our audited financial statements and include all normal recurring adjustments that we consider necessary for a fair statement of our financial position and operating results as of the dates and for the periods presented. The summary financial data below should be read together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and in conjunction with the financial statements, related notes and other information included elsewhere in this prospectus.
Our historical results are not necessarily indicative of our future results and our interim results are not necessarily reflective of the results to be expected for the full year. The summary financial information below does not contain all the information included in our financial statements.
Statements of Operations
| (unaudited) | ||||||||||||||||
| Three Months Ended March 31, | Year Ended December 31, | |||||||||||||||
| 2017 | 2016 | 2016 | 2015 | |||||||||||||
| (in thousands, except per share data) | ||||||||||||||||
| Revenue |
$ | 55 | $ | 106 | $ | 399 | $ | 1,272 | ||||||||
| Operating expenses: |
||||||||||||||||
| Cost of revenue |
— | 100 | 200 | 751 | ||||||||||||
| Research and development |
1,266 | 1,370 | 5,638 | 2,766 | ||||||||||||
| Sales, general, and administrative |
1,578 | 1,197 | 6,670 | 3,569 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
2,844 | 2,667 | 12,508 | 7,086 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(2,789 | ) | (2,561 | ) | (12,109 | ) | (5,814 | ) | ||||||||
| Interest expense |
(14 | ) | (4 | ) | (5 | ) | (261 | ) | ||||||||
| Foreign currency transaction gains (losses) |
(29 | ) | (4 | ) | 28 | 186 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before income taxes |
(2,832 | ) | (2,569 | ) | (12,086 | ) | (5,889 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income tax benefit |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (2,832 | ) | $ | (2,569 | ) | $ | (12,086 | ) | $ | (5,889 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted net loss per share(1) |
$ | (0.14 | ) | $ | (0.13 | ) | $ | (0.62 | ) | $ | (0.88 | ) | ||||
| Weighted average shares outstanding—basic and diluted |
19,600,000 | 19,600,000 | 19,600,000 | 6,725,740 | ||||||||||||
11
Table of Contents
Balance Sheet Data:
| (unaudited) | ||||||||
| As at March 31, 2017 | ||||||||
| Actual | As Adjusted(2) | |||||||
| (in thousands) | ||||||||
| Cash and cash equivalents |
$ | 3,007 | $ | 93,873 | ||||
| Total assets |
15,092 | 105,958 | ||||||
| Accumulated deficit |
(31,400 | ) | (31,400 | ) | ||||
| Total stockholder’s equity |
10,421 | 101,287 | ||||||
| Total liabilities and stockholder’s equity |
15,092 | 105,958 | ||||||
| (1) | See note 8 to our audited financial statements and note 7 to our unaudited condensed financial statements, included elsewhere in this prospectus, for an explanation of the method used to calculate basic and diluted net loss per share. |
| (2) | As adjusted to give effect to the issuance and sale of 6,060,606 shares of common stock in this offering, assuming an initial public offering price of $16.50 per share (the midpoint of the range set forth on the cover page of this prospectus), after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
A $1.00 increase (decrease) in the assumed initial public offering price of $16.50 per share would increase (decrease) each of as adjusted cash and cash equivalents, total assets total stockholder’s equity and total liabilities and stockholder’s equity by $5.6 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriting discount and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 1,000,000 in the number of shares offered by us would increase (decrease) as adjusted cash and cash equivalents, total stockholder’s equity and liabilities and stockholder’s equity by $15.3 million, assuming an initial public offering price of $16.50 per share, after deducting the underwriting discount and estimated offering expenses payable by us.
12
Table of Contents
Investing in our common stock involves a high degree of risk. You should consider carefully the following risks, together with all the other information in this prospectus, including our financial statements and notes thereto, before you invest in our common stock. If any of the following risks actually materializes, our operating results, financial condition and liquidity could be materially adversely affected. As a result, the trading price of our common stock could decline and you could lose all or part of your investment.
Risks Related to Our Business and Industry
We have a limited operating history, which makes it difficult to evaluate our current business and future prospects and may increase the risk of your investment.
We are an early-stage gene-editing company with a limited operating history that to date has been focused primarily on research and development, conducting field trials and building our management team. Investment in agricultural biotechnology product development is a highly speculative endeavor. It entails substantial upfront research and development investment and there is significant risk that we will not be able to edit the genes in a particular plant to express a desired trait, or, once edited, we will not be able to replicate that trait across entire crops in order to commercialize the product candidate. Moreover, the regulatory pathway for some of our product candidates can be uncertain and could add significant additional cost and time to development. We have not yet generated any revenue from sales of these products.
Our limited operating history may make it difficult to evaluate our current business and our future prospects. We have encountered, and will continue to encounter, risks and difficulties frequently experienced by growing companies in rapidly developing and changing industries, such as the agricultural biotechnology industry, including challenges in forecasting accuracy, determining appropriate investments of our limited resources, gaining market acceptance of the products created using our gene-editing platform, managing a complex regulatory landscape and developing new product candidates. We may also face challenges in scaling our supply chain in a cost-effective manner, as we will rely on contracting with seed production companies, farmers, crushers, refiners, logistics and transportation providers and/or millers, in order to get our various products to market. Our current operating model may require changes in order for us to scale our operations efficiently. We may not be able to fully implement or execute on our business strategy or realize, in whole or in part within our expected time frames, the anticipated benefits of our growth strategies. You should consider our business and prospects in light of the risks and difficulties we face as an early-stage company focused on developing products in the field of agricultural biotechnology.
We have incurred significant losses since our inception, have no commercial products and anticipate that we will continue to incur significant losses for the foreseeable future.
Our net loss for the three months ended March 31, 2017 and for the years ended December 31, 2016 and 2015 was $2.8 million, $12.1 million and $5.9 million, respectively. As of March 31, 2017, we had an accumulated deficit of $31.4 million. The amount of our future net losses will depend, in part, on the pace and amount of our future expenditures and our ability to obtain funding through equity or debt financings, funding provided by Cellectis, and on additional grants or tax credits. We currently have no commercial products and do not expect to have sales until the end of the 2018 at the earliest for our high oleic soybean product candidate, and we do not expect potential commercial launch of our high fiber wheat product candidate until 2020 at the earliest. Even then, our sales will be limited to a single product. As a result, we expect to continue to incur significant expenses and operating losses for the foreseeable future. We anticipate that such expenses will increase substantially if and as we:
| • | establish a sales, marketing and distribution infrastructure, including relationships across our supply chain, to commercialize any products that have completed the development process; |
| • | conduct additional field trials of our current and future product candidates; |
13
Table of Contents
| • | secure manufacturing arrangements for commercial production; |
| • | continue to advance the research and development of our current and future product candidates; |
| • | seek to identify and validate additional product candidates; |
| • | acquire or in-license other product candidates, technologies, germplasm or other biological material; |
| • | are required to seek regulatory and marketing approvals for our product candidates; |
| • | make royalty and other payments under any in-license agreements; |
| • | maintain, protect, expand and defend our intellectual property portfolio; |
| • | seek to attract and retain new and existing skilled personnel; and |
| • | experience any delays or encounter issues with any of the above. |
The net losses we incur may fluctuate significantly from year-to-year and quarter-to-quarter, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. In any particular period or periods, our operating results could be below the expectations of securities analysts or investors, which could cause the price of our common stock to decline.
We have never commercialized a product candidate and we may lack the necessary expertise, personnel and resources to successfully commercialize any of our product candidates.
We have never commercialized a product candidate. Our products are still in development, and there is no established market for them. Completion of product development could be protracted, and any products may not be ready for commercial launch for several years, if ever. If we are not able to commercialize our existing or future product candidates on a significant scale, then we may not be successful in building a sustainable or profitable business. Moreover, we expect to price our products based on our assessment of the value that we believe they will provide to food manufacturers or farmers, rather than on the cost of production. If food manufacturers or farmers attribute a lower value to our products than we do, they may not be willing to pay the premium prices that we expect to charge. Pricing levels may also be negatively affected if our products are unsuccessful in producing the yields or traits we expect. Food manufacturers or farmers may also be cautious in their adoption of new products and technologies, with conservative initial purchases and proof of product required prior to widespread deployment. It may take several growing seasons for food manufacturers or farmers to adopt our products on a large scale.
To achieve commercial success of our product candidates, we will have to develop our own sales, marketing and supply capabilities by outsourcing these activities to third parties. Factors that may affect our ability to commercialize our product candidates on our own include recruiting and retaining adequate numbers of effective sales and marketing personnel, obtaining access to or persuading adequate numbers of food manufacturers or farmers to purchase and use our product candidates and other unforeseen costs associated with creating an independent sales and marketing organization. Developing and maintaining a sales and marketing organization requires significant investment, is time-consuming and could delay the launch of our product candidates. We may not be able to build or maintain an effective sales and marketing organization in North America or other key global markets. If we are unable to find suitable partners for the commercialization of our product candidates, we may have difficulties generating revenue from them.
We will rely on contractual counterparties and they may fail to perform adequately.
Our commercial strategy depends on our ability to contract with counterparties that provide, and in the future may provide, a variety of seed production companies, farmers, crushers, refiners, millers, transportation and logistics companies and lab equipment service providers. We plan to rely on these third parties to provide services along our supply chain and in our research and development functions. The failure of these
14
Table of Contents
counterparties to fulfill the terms of our agreements could cause disruptions in our supply chains, research efforts, commercialization efforts, and otherwise inhibit our ability to bring our products to market at the times and in the quantities as planned. For example, if our crushers and refiners fail to process our crops at the times and at the quantities as agreed, we may be unable to meet the demands of food manufacturers who we have contracted with to purchase our products, leading to lower sales and potential reputational damage and contractual liabilities. While we may have certain indemnification rights in our contracts with such counterparties, there is no assurance that such indemnification rights will be sufficient to cover any damage to us that would result from a failure of such a counterparty in their contractual arrangements with us.
We face significant competition and many of our competitors have substantially greater financial, technical and other resources than we do.
The market for agricultural biotechnology products is highly competitive, and we face significant direct and indirect competition in several aspects of our business. Competition for improving plant genetics comes from conventional and advanced plant breeding techniques, as well as from the development of advanced biotechnology traits. Other potentially competitive sources of improvement in crop yields include improvements in crop protection chemicals, fertilizer formulations, farm mechanization, other biotechnology, and information management. Programs to improve genetics and crop protection chemicals are generally concentrated within a relatively small number of large companies, while non-genetic approaches are underway with broader set of companies. Mergers and acquisitions in the plant science, specialty food ingredient and agricultural biotechnology, seed and chemical industries may result in even more resources being concentrated among a smaller number of our competitors. Additionally, competition for providing more nutritious ingredients for food companies come from chemical-based ingredients, additives and substitutes, which are developed by various companies. The majority of these competitors have substantially greater financial, technical, marketing, sales, distribution and other resources than we do, such as larger research and development staff, more experienced marketing and manufacturing organizations and more well-established sales forces. As a result, we may be unable to compete successfully against our current or future competitors, which may result in price reductions, reduced margins and the inability to achieve market acceptance for our products. We expect to continue to face significant competition in the markets in which we intend to commercialize our products.
Many of our competitors engage in ongoing research and development, and technological developments by our competitors could render our products less competitive, resulting in reduced sales compared to our expectations. Our ability to compete effectively and to achieve commercial success depends, in part, on our ability to: control manufacturing and marketing costs; effectively price and market our products; successfully develop an effective marketing program and an efficient supply chain; develop new products with properties attractive to food manufacturers or farmers; and commercialize our products quickly without incurring major regulatory costs. We may not be successful in achieving these factors and any such failure may adversely affect our business, results of operations and financial condition.
We also anticipate increased competition in the future as new companies enter the market and new technologies become available, particularly in the area of gene editing. Our technology may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors, which will prevent or limit our ability to generate revenue from the commercialization of our products. At the same time, the expiration of patents covering existing products reduces the barriers to entry for competitors.
The successful commercialization of our products may face challenges from public perceptions of genetically engineered products and ethical, legal, environmental, health and social concerns.
The successful commercialization of our product candidates depends, in part, on public acceptance of genetically engineered agricultural products. Any increase in negative perceptions of gene editing or more restrictive government regulations in response thereto, would have a negative effect on our business and may delay or impair the development and commercialization of our products.
15
Table of Contents
The commercial success of our products may be adversely affected by claims that biotechnology plant products are unsafe for consumption or use, pose risks of damage to the environment, or create legal, social and ethical dilemmas.
If we are not able to overcome these concerns, our products may not achieve market acceptance. Any of the risks discussed below could result in expenses, delays or other impediments to our development programs or the market acceptance and commercialization of our products:
| • | public attitudes about the safety and environmental hazards of, and ethical concerns over, genetic research and biotechnology plant products, which could influence public acceptance of our technologies and products; |
| • | public attitudes regarding, and potential changes to laws governing, ownership of genetic material, which could weaken our intellectual property rights with respect to our genetic material and discourage R&D partners from supporting, developing or commercializing our products and technologies; and |
| • | failure to maintain or secure consumer confidence in, or to maintain or receive governmental approvals for, our products. |
Any future labeling requirements could heighten these concerns and make consumers less likely to purchase food products containing gene-edited ingredients.
Regulatory requirements for genetically engineered products are uncertain and evolving. Changes in the current application of these laws would have a significant adverse impact on our ability to develop and commercialize our products.
Changes in applicable regulatory requirements could result in a substantial increase in the time and costs associated with developing our products and negatively impact our operating results. In the United States, the United States Department of Agriculture, or USDA, regulates, among other things, the introduction (including the importation, interstate movement, or release into the environment such as field testing) of organisms and products altered or produced through genetic engineering that are plant pests or that there is reason to believe are plant pests. Such organisms and products are considered “regulated articles.” However, a petitioner may submit a request for a determination by the USDA of “nonregulated status” for a particular article. A petition for determination of nonregulated status must include detailed information, including relevant experimental data and publications, and a description of the genotypic differences between the regulated article and the nonmodified recipient organism, among other things. We previously submitted a request for a determination of “nonregulated status” to the USDA for our potato product candidates, our high oleic and low linolineic soybean product candidates and our powdery mildew-resistant wheat product candidate. The USDA confirmed in writing that each of these product candidates is not deemed to be a “regulated article” under the Plant Protection Act because it does not contain genetic material from plant pests. In the event any of our product candidates are found to contain inserted genetic material or otherwise differ from the descriptions we have provided to the USDA, the USDA could determine that such product candidates are regulated articles, which would require us to comply with the permit and notification requirements of the Plant Protection Act. While we believe that the USDA’s reasoning will continue to extend to our other product candidates, we have not obtained a determination from the USDA that any of our other product candidates are not “regulated articles” under these regulations. USDA’s regulations also require that companies obtain a permit or file a notification before engaging in the introduction (including the importation, interstate movement, or release into the environment such as field testing) of “regulated articles.” We cannot predict whether the USDA or advocacy groups will challenge our interpretation, or whether the USDA will alter the manner in which it interprets its own regulations or institutes new regulations, or otherwise modifies regulations in a way that will subject our products to more burdensome standards, thereby substantially increasing the time and costs associated with developing our product candidates. Moreover, we cannot assure you that the USDA will apply this same analysis to any of our other product candidates in development. Complying with USDA’s plant pest regulations, including permitting requirements, is a costly, time-consuming process and could substantially delay or prevent the commercialization of our products.
16
Table of Contents
Our products may also be subject to extensive FDA food product regulations. Under sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act, or FDCA, any substance that is reasonably expected to become a component of food added to food is a food additive, and is therefore subject to FDA premarket review and approval, unless the substance is generally recognized, among qualified experts, as having been adequately shown to be safe under the conditions of its intended use (generally recognized as safe, or GRAS), or unless the use of the substance is otherwise excluded from the definition of a food additive, and any food that contains an unsafe food additive is considered adulterated under section 402(a)(2)(C) of the FDCA. The FDA may classify some or all of our product candidates as containing a food additive that is not GRAS or otherwise determine that our products contain significant compositional differences from existing plant products that require further review. Such classification would cause these product candidates to require pre-market approval, which could delay the commercialization of these products. In addition, the FDA is currently evaluating its approach to the regulation of gene-edited plants. For example, on January 18, 2017, the FDA announced a Request for Comments, or RFC, seeking public input to help inform its regulatory approach to human and animal foods derived from plants produced using gene editing. Among other things, the RFC asks for data and information in response to questions about the safety of foods from gene-edited plants, such as whether categories of gene-edited plants present food safety risks different from other plants produced through traditional plant breeding. If the FDA enacts new regulations or policies with respect to gene-edited plants, such policies could result in additional compliance costs and/or delay the commercialization of our product candidates, which could negatively affect our profitability. Any delay in the regulatory consultation process, or a determination that our products do not meet regulatory approval, by the FDA could cause a delay in the commercialization of our products, which may lead to reduced acceptance by food manufacturers, farmers or the public and an increase in competitor products that may directly compete with ours.
The regulatory environment outside the United States varies greatly from region to region and is less developed than in the United States.
The regulatory environment around gene editing in plants for food ingredients is greatly uncertain outside of the United States and varies greatly from jurisdiction to jurisdiction. Each jurisdiction may have its own regulatory framework regarding genetically modified foods, which may include restrictions and regulations on planting and growing genetically modified plants and in the consumption and labeling of genetically modified foods, and which may encapsulate our products. The two leading jurisdictions, the United States and the European Union, or the EU, do, and may continue to in the future, have distinctly different regulatory regimes with different rules and requirements. We cannot predict how the global regulatory landscape regarding gene editing in plants for food ingredients will evolve and may incur increased regulatory costs as regulations in the jurisdictions in which we operate change.
In the EU, genetically modified foods can only be allowed on the market once they have been authorized subject to rigorous safety assessments. The procedures for evaluation and authorization of genetically modified foods are governed by Regulation (EC) 1829/2003 on genetically modified food and feed and Directive 2001/18/EC on the release of genetically modified organisms, or GMOs, into the environment. If the GMO is not to be used in food or feed, then an application must be made under Directive 2001/18/EC. If the GMO is to be used in food or feed (but it is not grown in the EU) then a single application for both food and feed purposes under Regulation 1829/2003 should be made. If the GMO is used in feed or food and it is also grown in the EU, an application for both cultivation and food/feed purposes needs to be carried out under Regulation (EC) 1829/2003. A different EU regulation, Regulation (EC) 1830/2003, regulates the labeling of products that contain GMOs that are placed on the EU market. There are currently legislative proposals in the EU that would allow EU Member States to restrict or prohibit growing GMOs in their territory, on a range of environmental grounds, even if such crops were previously authorized at EU level. Should these proposals become law, growing GMOs may become more difficult in individual EU Member States.
We cannot predict whether or when any jurisdiction will change its regulations with respect to our products. Advocacy groups have engaged in publicity campaigns and filed lawsuits in various countries against companies
17
Table of Contents
and regulatory authorities, seeking to halt regulatory approval activities or influence public opinion against genetically engineered products. In addition, governmental reaction to negative publicity concerning our products could result in greater regulation of genetic research and derivative products or regulatory costs that render our products cost prohibitive.
The scale of the commodity food industry may make it difficult to monitor and control the distribution of our products. As a result, our products may be sold inadvertently within jurisdictions where they are not approved for distribution. Such sales may lead to regulatory challenges or lawsuits against us, which could result in significant expenses and management attention.
Government policies and regulations, particularly those affecting the agricultural sector and related industries, could adversely affect our operations and profitability.
Agricultural production and trade flows are subject to government policies and regulations. Governmental policies and approvals of technologies affecting the agricultural industry, such as taxes, tariffs, duties, subsidies, incentives and import and export restrictions on agricultural commodities and commodity products can influence the planting of certain crops, the location and size of crop production, and the volume and types of imports and exports. Future government policies in the United States or in other countries may discourage food manufacturers or farmers from using our products or encourage the use of products more advantageous to our competitors, which would put us at a commercial disadvantage and could negatively impact our future revenues and results of operations.
The overall agricultural industry is susceptible to commodity price changes and we, along with our food manufacturing customers and farmer customers, are exposed to market risks from changes in commodity prices.
Changes in the prices of certain commodity products could result in higher overall cost along the agricultural supply chain, which may negatively affect our ability to commercialize our products. We will be susceptible to changes in costs in the agricultural industry as a result of factors beyond our control, such as general economic conditions, seasonal fluctuations, weather conditions, demand, food safety concerns, product recalls and government regulations. As a result, we may not be able to anticipate or react to changing costs by adjusting our practices, which could cause our operating results to deteriorate. We do not engage in hedging or speculative financial transactions nor do we hold or issue financial instruments for trading purposes.
Our product development efforts use complex integrated technology platforms and require substantial time and resources; these efforts may not be successful, or the rate of product improvement may be slower than expected.
Development of successful agricultural products using complex technology platforms such as gene-editing technologies requires significant levels of investment in research and development, including laboratory, greenhouse and field testing, to demonstrate their effectiveness and can take several years or more. For the three months ended March 31, 2017 and for the years ended December 31, 2016 and 2015, we spent $1.3 million, $5.6 million and $2.8 million, respectively, on research and development expenses. We intend to continue to invest in research and development, including additional and expanded field testing, to validate our product candidates in real world conditions. Our investment in research and development may not result in significant product revenue over the next several years, if ever. Moreover, the successful application of gene-editing technologies can be unpredictable, and may prove to be unsuccessful when attempting to achieve desired traits in different crops and plants. For example, our gene-editing techniques may prove to be unsuccessful very early on during the discovery phase of new crop development based on technology limitations. Alternatively, even though we successfully implemented gene edits during the discovery phase, that trait may not ultimately appear in crops during field testing or crops may also exhibit other undesirable traits that adversely affect their commercial value.
18
Table of Contents
Development of new or improved agricultural products involves risks of failure inherent in the development of products based on innovative and complex technologies. These risks include the possibility that:
| • | our products will fail to perform as expected in the field; |
| • | our products will not receive necessary regulatory permits and governmental clearances in the markets in which we intend to sell them; |
| • | our products may have adverse effects on consumers; |
| • | consumer preferences, which are unpredictable and can vary greatly, may change quickly, making our products no longer desirable; |
| • | our competitors develop new products that taste better or have other more appealing characteristics than our products; |
| • | our products will be viewed as too expensive by food companies or farmers as compared to competitive products; |
| • | our products will be difficult to produce on a large scale or will not be economical to grow; |
| • | intellectual property and other proprietary rights of third parties will prevent us, our R&D partners, or our licensees from marketing and selling our products; |
| • | we may be unable to patent or otherwise obtain intellectual property protection for our discoveries in the necessary jurisdictions; |
| • | we or the food manufacturers that we sell our ingredients to may be unable to fully develop or commercialize products containing our products in a timely manner or at all; and |
| • | third parties may develop superior or equivalent products. |
Lastly, the field of gene editing, particularly in the area of plants, is still in its infancy, and no products using this technology have reached the market. Negative developments in the field of gene editing, including with respect to adverse side effects, could harm the reputation of the industry and negatively impact our business.
We are currently reliant on certain gene-editing technologies that may become obsolete in the future.
We currently rely on our proprietary TALEN technology in the development of our product candidates. There are several other gene-editing technologies currently available, including CRISPR/Cas9, meganucleases and zinc finger nucleases. If our competitors are able to refine existing gene-editing technologies—or develop new ones—that allow them to develop products faster, with lower research costs or with more desirable traits than we can, we may face a decline in the demand for our products. Our technologies may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors, which will prevent or limit our ability to generate revenues from the commercialization of our products.
We may need to raise additional funding, which may not be available on acceptable terms or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
The process of developing and commercializing product candidates is expensive, lengthy and risky. We expect our research and development expenses to increase substantially as we continue to develop our existing product candidates and identify new product candidates for development. We are beginning to prepare for the commercialization of our first product candidate, high-oleic soybean oil, which we expect to occur by the end of 2018. As a result, our selling, general and administrative expense will also increase significantly.
19
Table of Contents
As of March 31, 2017, we had cash and cash equivalents of approximately $3.0 million. We believe our cash and cash equivalents, together with the net proceeds from this offering, will be sufficient to fund our operations through at least 2020. However, in order to complete the development process, obtain regulatory approval for, if necessary, and commercialize our products, we may require additional funding. Also, our operating plan, including our product development plans, may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements and other strategic alliances and licensing arrangements, or a combination of these approaches. To commercialize our products, we will require significant working capital to operate our business and maintain our supply chain. To the extent that we raise additional capital through the sale of additional equity or convertible securities, your ownership interest may be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, would result in increased fixed payment obligations and a portion of our operating cash flows, if any, being dedicated to the payment of principal and interest on such indebtedness. In addition, debt financing may involve agreements that include restrictive covenants that impose operating restrictions, such as restrictions on the incurrence of additional debt, the making of certain capital expenditures or the declaration of dividends. To the extent we raise additional funds through arrangements with R&D partners or otherwise, we may be required to relinquish some of our technologies, product candidates or revenue streams, license our technologies or product candidates on unfavorable terms, or otherwise agree to terms unfavorable to us. Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all, or that we will be able to complete the sale leaseback transaction to finance the building for the planned facility expansion described under “Business—Facilities.” Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or in light of specific strategic considerations. If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research or product candidate development programs or the commercialization of any product candidate or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, operating results and prospects and cause the price of the common stock to decline.
We rely on third parties to conduct, monitor, support, and oversee field trials and other research services for product candidates in development, and any performance issues by third parties, or our inability to engage third parties on acceptable terms, may impact our ability to successfully commercialize such product candidates.
Prior to commercializing any product candidate we must conduct large scale field trials to validate the desired product trait. These field trials can take one to two years to complete. We currently conduct field trials, and plan to conduct further field trials, of our product candidates in various geographies. We currently rely on third parties to conduct, monitor, support and oversee these field trials. In some cases, these field trials are conducted outside of the United States, making it difficult for us to monitor the daily activity of the work being conducted by the third parties that we engage. Although we provide our third-party contractors with extensive protocols regarding the establishment, management, harvest, transportation and storage of our product candidates, we have limited control over the execution of field trials. Consequently, the success of these field trials depends upon the ability of these third parties to correctly follow our suggested protocols. However, there is no guarantee that third parties will devote adequate time and resources to our field trials or conduct the field trials in accordance with our protocols, including maintenance of all required field trial information. Any such failures may result in delays in the development of our product candidates or the incurrence of additional costs. Even if our third-party contractors adhere to our suggested protocols, field trials may fail to succeed for a variety of other reasons, including weather, disease or pests, improper timing of planting our seeds, or incorrect fertilizer use. Ultimately, we remain responsible for ensuring that each of our field trials is conducted in accordance with
20
Table of Contents
the applicable protocol, legal, regulatory and scientific standards, and our reliance on third parties does not relieve us of our responsibilities.
Additionally, if we are unable to maintain or enter into agreements with third-party contractors on acceptable terms, or if any such engagement is terminated prematurely, we may be unable to conduct or complete our field trials in the manner we anticipate. If our relationship with any of these third-party contractors is terminated, we may be unable to enter into arrangements with alternative contractors on commercially reasonable terms, or at all. Switching or adding third-party contractors can involve substantial cost and require extensive management time and focus. In addition, there is a natural transition period when any new third party commences field trial work. As a result, delays may occur, which could materially impact our ability to meet our desired development timelines.
Our crops are new, and if farmers and food processors are unable to work effectively with our crops, our various relationships, our reputation and our results of operations will be harmed.
We plan to provide farmers with information and protocols regarding the establishment, management, harvest, transportation and storage of our crops. These crop management recommendations may include equipment selection, planting and harvest timing, application of crop protection chemicals or herbicides and storage systems and protocols. Our general or specific protocols may not apply in all circumstances, may be improperly implemented, may not be sufficient, or may be incorrect, leading to reduced yields, crop failures or other production problems or losses. If farmers that are producing crops for our food ingredients experience these failures, we may be unable to provide product ingredients to food manufacturers on a timely basis or at all. If we are unable to deliver products in a timely basis or at all, or if farmers that are purchasing our seed in an a effort to meet their yields experience these failures, or if our food processors are unable to process our crops effectively and efficiently, we will experience damage to our relationships, reputation and ability to successfully market our products. Further, the use of our seeds may require a change in current planting, rotation or agronomic practices, which may be difficult to implement or may discourage the use of our products by agricultural producers.
There are various reasons why our crops, once available, may fail to succeed, including weather, disease or pests, improper timing of planting our seeds, or incorrect fertilizer use. In addition, cross contamination of our products can happen in any step of the supply chain. Statements by potential customers about negative experiences with our products could harm our reputation, and the decision by these parties not to proceed with large-scale seed purchases could harm our business, revenue and ability to achieve profitability.
The successful commercialization of our products depends on our ability to produce high-quality plants and seeds cost-effectively on a large scale and to accurately forecast demand for our products, and we may be unable to do so.
The production of commercial-scale quantities of seeds requires the multiplication of the plants or seeds through a succession of plantings and seed harvests. The cost-effective production of high-quality, high-volume quantities of any product candidates we successfully develop depends on our ability to scale our production processes to produce plants and seeds in sufficient quantity to meet demand. For example, food ingredients such as soybean oil and wheat flour will require optimized production and commercialization of the underlying plant and seed harvests. We cannot assure that our existing or future seed production techniques will enable us to meet our large-scale production goals cost-effectively for the products in our pipeline. Even if we are successful in developing ways to increase yields and enhance quality, we may not be able to do so cost-effectively or on a timely basis, which could adversely affect our ability to achieve profitability. If we are unable to maintain or enhance the quality of our plants and seeds as we increase our production capacity, including through the expected use of third parties, we may experience reductions in food manufacturer or farmer demand, higher costs and increased inventory write-offs.
In addition, because of the length of time it takes to produce commercial quantities of marketable plants and seeds, we will need to make seed production decisions well in advance of product sales. Our ability to accurately
21
Table of Contents
forecast demand can be adversely affected by a number of factors outside of our control, including changes in market conditions, environmental factors, such as pests and diseases, and adverse weather conditions. A shortfall in the supply of our products may reduce product revenue, damage our reputation in the market and adversely affect relationships. Any surplus in the amount of products we have on hand may negatively impact cash flows, reduce the quality of our inventory and ultimately result in write-offs of inventory. Additionally, we will take financial risk in our inventory given that we will have to keep the inventory marked to market on our balance sheet. Fluctuations in the spot price our crops in inventory could have negative impacts on our financial statements. Any failure on our part to produce sufficient inventory, or overproduction of a particular product, could harm our business, results of operations and financial condition. In addition, food manufacturers or farmers may cancel orders or request a decrease in quantity at any time prior to delivery of the plants or seeds, which may lead to a surplus of our products.
In addition, while we estimate that the potential size of our target markets for our products is significant, that estimate has not been independently verified and is based on certain assumptions that may not prove to be accurate. As a result, these estimates could differ materially from actual market sizes, which could result in decreased demand for our products and therefore adversely impact our future business prospects, results of operation and financial condition.
The commercial success of our consumer-centric products is reliant on the needs of food manufacturers and the recognition of shifting consumer preferences.
The commercial success of our consumer-centric products will depend in part on the success of the food manufacturer’s products that our products are included in. We will not control the marketing, distribution, labeling or any other aspects of the sale and commercialization of the food manufacturers’ food products in which our products are an ingredient. Consumer preferences may be a significant driver in the success of our food manufacturer customers in their efforts to sell foods products including our products. While current trends indicate that consumer preferences may be moving towards “healthier” options, we cannot predict whether such trends will continue or which types of food products will be demanded by consumers in the future. Additionally, as health and nutritional science continues to progress, consumer perception of what foods, nutrients and ingredients are considered “healthy” may shift. We and our food manufacturer customers may not be dynamic enough in responding to consumer trends and creating products that will be demanded by consumers in the future. Failure by our food manufacturer customers to successfully recognize consumer trends and commercialize and sell their products which contain our ingredients could lower demand for our products and harm our business, results of operations and financial condition.
Farmers may not recognize the value in our farmer-centric products.
The commercial success of our farmer-centric products will rely on convincing farmers of the benefits to yield and natural resource usage. Farmers may not recognize the value of our farmer-centric products and may opt to use other seed products in the market with different varieties. The margins in the farmer-centric seed industry have historically been very narrow, so we may not be able to produce farmer-centric seed products at costs that would be competitive for our farmer customers, which may lead to a reduction in demand for our products.
Adverse weather conditions, natural disasters, crop disease, pests and other natural conditions can impose significant costs and losses on our business.
The ability to grow our products is vulnerable to adverse weather conditions, including windstorms, floods, drought and temperature extremes, which are quite common but difficult to predict, the effects of which may be influenced and intensified by ongoing global climate change. Unfavorable growing conditions can reduce both crop size and crop quality. This risk is particularly acute with respect to regions or countries in which we plan to source a significant percentage of our products. In extreme cases, entire harvests may be lost in some geographic
22
Table of Contents
areas. Such adverse conditions can increase costs, decrease revenues and lead to additional charges to earnings, which may have a material adverse effect on our business, financial position and results of operations.
The ability to grow our products is also vulnerable to crop disease and to pests, which may vary in severity and effect, depending on the stage of production at the time of infection or infestation, the type of treatment applied, climatic conditions and the risks associated with ongoing global climate change. The costs to control disease and other infestations vary depending on the severity of the damage and the extent of the plantings affected. Moreover, there can be no assurance that available technologies to control such infestations will continue to be effective. These infestations can also increase costs, decrease revenues and lead to additional charges to earnings, which may have a material adverse effect on our business, financial position and results of operations.
We expect our business will be highly seasonal and subject to weather conditions and other factors beyond our control, which may cause our sales and operating results to fluctuate significantly.
The sale of plant products is dependent upon planting and growing seasons, which vary from year to year, and are expected to result in both highly seasonal patterns and substantial fluctuations in quarterly sales and profitability. As we have not yet made any sales of our products, we have not yet experienced the full nature or extent to which this business may be seasonal. Furthermore, significant fluctuations in market prices for agricultural inputs and crops could also have an adverse effect on the value of our products. Weather conditions and natural disasters, such as heavy rains, hurricanes, hail, floods, tornadoes, freezing conditions, drought or fire, also affect decisions by food manufacturers or farmers about the types and amounts of seeds to plant and the timing of harvesting and planting such seeds, as well as adversely impact the agricultural industry as a whole in various regions. Disruptions that cause delays by food manufacturers or farmers in harvesting or planting can result in the movement of orders to a future quarter. Disruptions that cause delays by our farmers in harvesting could create us to be delayed, or to fail entirely in delivering food ingredients to food manufacturers. Any of those delays or failures would negatively affect the quarter in which they occur and cause fluctuations in our operating results.
We may expend our limited resources to pursue a particular product candidate and fail to capitalize on product candidates that may be more profitable or for which there is a greater likelihood of success.
We have limited financial and managerial resources. As a result, we may forego or delay pursuit of opportunities with other product candidates that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates may not yield any commercially viable products.
If we are sued for defective products and if such lawsuits were determined adversely, we could be subject to substantial damages, for which insurance coverage is not available.
We may be held liable if any product we develop, or any product that uses or incorporates, any of our technologies, causes injury or is found otherwise unsuitable during marketing, sale or consumption. For example, the detection of unintended trait in a commercial seed variety or the crops and products produced may result in physical injury to consumers resulting in potential liability for us as the seed producer or technology provider. If this were to occur, we could be subject to claims by multiple parties based not only on the cost of our plant products but also on their lost profits and business opportunities, including but not limited to trade disruption. Courts could levy substantial damages against us in connection with claims for injuries allegedly caused by use of our products. We do not currently have insurance coverage for such claims. In addition, the detection of unintended traits in our seeds could result in governmental actions such as mandated crop destruction, product recalls or environmental cleanup or monitoring. Concerns about seed quality could also lead to additional regulations being imposed on our business, such as regulations related to testing procedures, mandatory
23
Table of Contents
governmental reviews of biotechnology advances, or the integrity of the food supply chain from the farm to the finished product.
Our business activities are currently conducted at a limited number of locations, which makes us susceptible to damage or business disruptions caused by natural disasters or acts of vandalism.
Our current headquarters and certain research and development operations are located in New Brighton, Minnesota and our new headquarters and research facilities are located in Roseville, Minnesota. The greenhouse for the new headquarters is operational and the remainder of the new facility which includes an office, labs and demonstration kitchen are expected to be operational in the first half of 2018. Our seed production takes place primarily in the United States and Argentina. Warehousing for seed storage, which is conducted by a third-party contractor, is located primarily in Minnesota and Wisconsin. We may use a limited number of processing partners which may be located in concentrated areas. We take precautions to safeguard our facilities, including insurance, health and safety protocols, and off-site storage of critical research results and computer data. However, a natural disaster, such as a hurricane, drought, fire, flood, tornado, earthquake, or acts of vandalism, could cause substantial delays in our operations, damage or destroy our equipment, inventory or development projects, and cause us to incur additional expenses.
Our ability to use our net operating losses to offset future taxable income may be subject to certain limitations.
As of December 31, 2016, we had approximately $24.7 million of net operating losses, or NOLs, for federal and state income tax purposes, which may be available to offset federal income tax liabilities in the future. In addition, we may generate additional NOLs in future years. Under Section 382 of the Internal Revenue Code of 1986 (as amended, the “Code”), a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change NOLs to offset future taxable income. For this purpose, an ownership change generally means a more than 50 percentage point change in the ownership of a corporation by one or more stockholders or specified groups of stockholders, each of which owns 5% or more of the corporation (determined after the application of certain attribution and grouping rules) over a three-year period. Although we do not believe that any of our NOLs are currently subject to limitation under Section 382 of the Code, future changes in our stock ownership, some of which may be outside of our control, could result in an ownership change under Section 382 of the Code, which could limit our ability to use our existing or future NOLs to offset future taxable income. In addition, under current law, we are permitted to carry forward an NOL from any taxable year to only the succeeding twenty taxable years, and, if we do not generate sufficient taxable income to utilize an NOL carryforward within this period, it may expire unused. There is also a risk that future legal or regulatory changes my limit our ability to use our current or future NOLs to offset our future federal income tax liabilities, or otherwise diminish the value of our NOLs to us.
Risks Related to Intellectual Property
We will license a significant portion of our intellectual property from Cellectis S.A., our controlling stockholder, and principally rely upon it to prosecute and defend such intellectual property.
Our business relies heavily on the intellectual property we will license from Cellectis S.A., or Cellectis. Our license from Cellectis will be exclusive in the field of researching, developing and commercializing agricultural and food products, including traits, seeds, and feed and food ingredients (excluding any application in connection with animals and animal cells), except that such license will be non-exclusive in such field for any activities relating to researching, developing or commercializing certain modified or mutated I-CreI homing endonucleases. In addition, Cellectis has previously granted other third parties non-exclusive rights to use the same intellectual property for research purposes and therefore our exclusive license will be subject to such previously granted rights. Pursuant to our license agreement with Cellectis, we will be required to pay Cellectis certain royalties and other consideration based upon our commercialization and exploitation of the licensed
24
Table of Contents
intellectual property. If we do not comply with our obligations under the license agreement, including the foregoing payment obligations, we may be subject to damages, which may be significant, and in some cases Cellectis may have the right to terminate the license agreement. Any termination of our license agreement with Cellectis would have a material adverse effect on our business and results of operations.
Under our license agreement with Cellectis, and as between the parties, Cellectis will have the first right to control the prosecution, maintenance, defense and enforcement of the licensed intellectual property and we will have the right to step in and assume such control with respect to the patents owned by Cellectis and exclusively licensed to us under the agreement if Cellectis elects to not prosecute, maintain, defend or enforce such patents. In addition, in certain circumstances, if Cellectis elects to abandon any patents owned by Cellectis and exclusively licensed to us under the agreement, we will have the right to assume ownership of such patents. However, there can be no assurance that Cellectis will prosecute, maintain, defend and enforce such intellectual property, either in the best interests of our business or at all. Moreover, any enforcement of the licensed intellectual property could subject it to challenge by third parties and if any such challenge is successful, such intellectual property could be narrowed in scope or held to be invalid or unenforceable, which would materially impair any competitive advantage afforded to us by such intellectual property.
In addition, some of the intellectual property that will be licensed to us by Cellectis consists of a sublicense of intellectual property originally licensed to Cellectis by the Regents of the University of Minnesota, which we refer to as the University of Minnesota to exploit such intellectual property in our exclusive agricultural field of use. Therefore, as to such sublicensed intellectual property, our license from Cellectis will be subject to the terms and conditions of the license agreement between the University of Minnesota and Cellectis, and to the extent our activities under such sublicense violate any terms and conditions of the license agreement between Cellectis and the University of Minnesota, we will be responsible for any damages that Cellectis may incur. In addition, under the license agreement between Cellectis and the University of Minnesota, the University of Minnesota has the first right to control the prosecution and maintenance of the licensed intellectual property. There can be no assurance that the University of Minnesota will prosecute and maintain such intellectual property in the best interests of our business or at all, and, if the University of Minnesota fails to properly prosecute and maintain such intellectual property, we could lose our rights to such intellectual property, which would materially impair any competitive advantage afforded to us by such intellectual property. For more information regarding our license agreement with Cellectis or the license agreement between Cellectis and the University of Minnesota, please see “Business—Intellectual Property.”
Our ability to compete may decline if we do not, or Cellectis does not, adequately protect our proprietary rights.
Our commercial success depends, in part, on obtaining and maintaining proprietary rights to our and our licensors’ intellectual property estate, including with respect to our product candidates, as well as successfully defending these rights against third-party challenges. Cellectis will only be able to protect our product candidates from unauthorized use by third parties to the extent that valid and enforceable patents, or effectively protected trade secrets, cover them. Our ability to obtain patent protection for our product candidates is uncertain due to a number of factors, including:
| • | we or our licensors may not have been the first to invent the technology covered by our or their pending patent applications or issued patents; |
| • | we cannot be certain that we or our licensors were the first to file patent applications covering our product candidates, including their compositions or methods of use, as patent applications in the United States and most other countries are confidential for a period of time after filing; |
| • | others may independently develop identical, similar or alternative products or compositions or methods of use thereof; |
| • | the disclosures in our or our licensors’ patent applications may not be sufficient to meet the statutory requirements for patentability; |
25
Table of Contents
| • | any or all of our or our licensors’ pending patent applications may not result in issued patents; |
| • | we or our licensors may not seek or obtain patent protection in countries or jurisdictions that may eventually provide us a significant business opportunity; |
| • | any patents issued to us or our licensors may not provide a basis for commercially viable products, may not provide any competitive advantages, or may be successfully challenged by third parties, which may result in our or our licensors’ patent claims being narrowed, invalidated or held unenforceable; |
| • | our compositions and methods may not be patentable; |
| • | others may design around our or our licensors’ patent claims to produce competitive products that fall outside of the scope of our or our licensors’ patents; and |
| • | others may identify prior art or other bases upon which to challenge and ultimately invalidate our or our licensors’ patents or otherwise render them unenforceable. |
Even if we own, obtain or in-license patents covering our product candidates or compositions, we may still be barred from making, using and selling our product candidates or technologies because of the patent rights or other intellectual property rights of others. Others may have filed, and in the future may file, patent applications covering compositions, products or methods that are similar or identical to ours, which could materially affect our ability to successfully develop and commercialize our product candidates. In addition, because patent applications can take many years to issue, there may be currently pending applications unknown to us that may later result in issued patents that our product candidates or compositions may infringe. These patent applications may have priority over patent applications filed by us or our licensors.
Obtaining and maintaining a patent portfolio entails significant expense of resources. Part of such expense includes periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications due over the course of several stages of prosecuting patent applications, and over the lifetime of maintaining and enforcing issued patents. We or our licensors may or may not choose to pursue or maintain protection for particular intellectual property in our or our licensors’ portfolio. If we or our licensors choose to forgo patent protection or to allow a patent application or patent to lapse purposefully or inadvertently, our competitive position could suffer. In some cases, the prosecution and maintenance of our licensed patents is controlled by the applicable licensor. If such licensor fails to properly prosecute and maintain such patents, we could lose our rights to them which could materially impair any competitive advantage afforded by such patents. Furthermore, we and our licensors employ reputable law firms and other professionals to help comply with the various procedural, documentary, fee payment and other similar provisions we and they are subject to and, in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. There are situations, however, in which failure to make certain payments or noncompliance with certain requirements in the patent prosecution and maintenance process can result in abandonment or lapse of a patent or patent application, resulting in a partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market, which would have a material adverse effect on our business.
Legal action that may be required to enforce our patent rights can be expensive and may involve the diversion of significant management time. In addition, these legal actions could be unsuccessful and could also result in the invalidation of our or our licensors’ patents or a finding that they are unenforceable. We or our licensors may or may not choose to pursue litigation or other actions against those that have infringed on our or their patents, or have used them without authorization, due to the associated expense and time commitment of monitoring these activities. In some cases, the enforcement and defense of patents we in-license is controlled by the applicable licensor. If such licensor fails to actively enforce and defend such patents, any competitive advantage afforded by such patents could be materially impaired. In addition, some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we or our licensors can because of their greater financial resources and more mature and developed intellectual property portfolios. Accordingly,
26
Table of Contents
despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating or from successfully challenging our intellectual property rights. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could harm our results of operations.
In addition to patent protection, because we operate in the highly technical field of biosciences, we rely in part on trade secret protection in order to protect our proprietary technology and processes. However, trade secrets are difficult to protect. Monitoring unauthorized uses and disclosures is difficult, and we do not know whether the steps we have taken to protect our proprietary technologies will be effective. We cannot guarantee that our trade secrets and other proprietary and confidential information will not be disclosed or that competitors will not otherwise gain access to our trade secrets. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. We enter into confidentiality and intellectual property assignment agreements with our employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors. These agreements generally require that the other party keep confidential and not disclose to third parties all confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. These agreements also generally provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, these agreements may be breached or held unenforceable and may not effectively assign intellectual property rights to us.
In addition to contractual measures, we try to protect the confidential nature of our proprietary information using physical and technological security measures. Such measures may not provide adequate protection for our proprietary information. For example, our security measures may not prevent an employee or consultant with authorized access from misappropriating our trade secrets and providing them to a competitor, and the recourse we have available against such misconduct may not provide an adequate remedy to protect our interests fully. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. Furthermore, our proprietary information may be independently developed by others in a manner that could prevent legal recourse by us. If any of our confidential or proprietary information, including our trade secrets, were to be disclosed or misappropriated, or if any such information was independently developed by a competitor, our competitive position could be harmed and our business could be materially and adversely affected.
Patents and patent applications involve highly complex legal and factual questions, which, if determined adversely to us, could negatively impact our competitive position.
The patent positions of biotechnology companies and other actors in our fields of business can be highly uncertain and typically involve complex scientific, legal and factual analyses. In particular, the interpretation and breadth of claims allowed in some patents covering biological compositions may be uncertain and difficult to determine, and are often affected materially by the facts and circumstances that pertain to the patented compositions and the related patent claims. The standards of the United States Patent and Trademark Office, or USPTO, and foreign patent offices are sometimes uncertain and could change in the future. Consequently, the issuance and scope of patents cannot be predicted with certainty. Patents, if issued, may be challenged, invalidated, narrowed or circumvented. U.S. patents and patent applications may also be subject to interference proceedings, and U.S. patents may be subject to reexamination proceedings, post-grant review, inter partes review, or other administrative proceedings in the USPTO. Foreign patents as well may be subject to opposition or comparable proceedings in corresponding foreign patent offices. Challenges to our or our licensors’ patents and patent applications, if successful, may result in the denial of our or our licensors’ patent applications or the loss or reduction in their scope. In addition, such interference, reexamination, post-grant review, inter partes review, opposition proceedings and other administrative proceedings may be costly and involve the diversion of significant management time. Accordingly, rights under any of our or our licensors’ patents may not provide us with sufficient protection against competitive products or processes and any loss, denial or reduction in scope of any of such patents and patent applications may have a material adverse effect on our business.
27
Table of Contents
Furthermore, even if not challenged, our or our licensors’ patents and patent applications may not adequately protect our product candidates or technology or prevent others from designing their products or technology to avoid being covered by our or our licensors’ patent claims. If the breadth or strength of protection provided by the patents we own or license with respect to our product candidates is threatened, it could dissuade companies from partnering with us to develop, and could threaten our ability to successfully commercialize, our product candidates. Furthermore, for U.S. patent applications in which claims are entitled to a priority date before March 16, 2013, an interference proceeding can be provoked by a third party or instituted by the USPTO in order to determine who was the first to invent any of the subject matter covered by such patent claims.
In addition, changes in, or different interpretations of, patent laws in the United States and other countries may permit others to use our discoveries or to develop and commercialize our technology and products without providing any notice or compensation to us, or may limit the scope of patent protection that we or our licensors are able to obtain. The laws of some countries do not protect intellectual property rights to the same extent as U.S. laws and those countries may lack adequate rules and procedures for defending our intellectual property rights.
If we or our licensors fail to obtain and maintain patent protection and trade secret protection of our product candidates and technology, we could lose our competitive advantage and competition we face would increase, reducing any potential revenues and have a material adverse effect on our business.
The lives of our patents may not be sufficient to effectively protect our products and business.
Patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after its first effective filing date. Although various extensions may be available, the life of a patent, and the protection it affords, is limited. Our or our licensors’ issued patents will expire on dates ranging from 2020 to 2033, subject to any patent extensions that may be available for such patents. If patents are issued on our or our licensors’ pending patent applications, the resulting patents are projected to expire on dates ranging from 2023 to 2037. In addition, although upon issuance in the United States a patent’s life can be increased based on certain delays caused by the USPTO, this increase can be reduced or eliminated based on certain delays caused by the patent applicant during patent prosecution. If we or our licensors do not have sufficient patent life to protect our products, our business and results of operations will be adversely affected.
Developments in patent law could have a negative impact on our business.
From time to time, the United States Supreme Court, or the Supreme Court, other federal courts, the United States Congress, the USPTO and similar foreign authorities may change the standards of patentability and any such changes could have a negative impact on our business.
The Leahy-Smith America Invents Act, or the America Invents Act, which was signed into law in 2011, includes a number of significant changes to U.S. patent law. These changes include a transition from a “first-to-invent” system to a “first-to-file” system, changes to the way issued patents are challenged, and changes to the way patent applications are disputed during the examination process. As a result of these changes, the patent law in the United States may favor larger and more established companies that have greater resources to devote to patent application filing and prosecution. The USPTO has developed new and untested regulations and procedures to govern the full implementation of the America Invents Act, and many of the substantive changes to patent law associated with the America Invents Act, and, in particular, the first-to-file provisions became effective on March 16, 2013. Substantive changes to patent law associated with the America Invents Act may affect our ability to obtain patents, and if obtained, to enforce or defend them. Accordingly, it is not clear what, if any, impact the America Invents Act will have on the cost of prosecuting our or our licensors’ patent applications and the ability for us and our licensors to obtain patents and enforce or defend any patents that may issue from such patent applications, all of which could have a material adverse effect on our business.
28
Table of Contents
In addition, recent Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the Supreme Court, the United States Congress, the federal courts, the USPTO and similar foreign authorities, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future.
We will not seek to protect our intellectual property rights in all jurisdictions throughout the world and we may not be able to adequately enforce our intellectual property rights even in the jurisdictions where we seek protection.
Filing, prosecuting and defending patents on our product candidates in all countries and jurisdictions throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States could be less extensive than those in the United States, assuming that rights are obtained in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions.
Competitors may use our technologies in jurisdictions where we or our licensors do not pursue and obtain patent protection to develop their own products and further, may export otherwise infringing products to territories where we or our licensors have patent protection, but where the ability to enforce our or our licensors’ patent rights is not as strong as in the United States. These products may compete with our products and our intellectual property rights and such rights may not be effective or sufficient to prevent such competition.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Patent protection must be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we and our licensors may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries. In addition, the legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to biotechnologies, and the requirements for patentability differ, in varying degrees, from country to country, and the laws of some foreign countries do not protect intellectual property rights, including trade secrets, to the same extent as federal and state laws of the United States. As a result, many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. Such issues may make it difficult for us to stop the infringement, misappropriation or other violation of our intellectual property rights. For example, many foreign countries, including the EU countries, have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. In those countries, we and our licensors may have limited remedies if patents are infringed or if we or our licensors are compelled to grant a license to a third party, which could materially diminish the value of those patents. This could limit our potential revenue opportunities. Accordingly, our and our licensors’ efforts to enforce intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we own or license. Similarly, if our trade secrets are disclosed in a foreign jurisdiction, competitors worldwide could have access to our proprietary information and we may be without satisfactory recourse. Such disclosure could have a material adverse effect on our business. Moreover, our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in foreign intellectual property laws.
Furthermore, proceedings to enforce our licensors’ and our patent rights and other intellectual property rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other
29
Table of Contents
aspects of our business, could put our or our licensors’ patents at risk of being invalidated or interpreted narrowly, could put our or our licensors’ patent applications at risk of not issuing and could provoke third parties to assert claims against us or our licensors. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded to us, if any, may not be commercially meaningful, while the damages and other remedies we may be ordered to pay such third parties may be significant. Accordingly, our licensors’ and our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Third parties may assert rights to inventions we develop or otherwise regard as our own.
Third parties may in the future make claims challenging the inventorship or ownership of our or our licensors’ intellectual property. We have written agreements with R&D partners that provide for the ownership of intellectual property arising from our strategic alliances. These agreements provide that we must negotiate certain commercial rights with such partners with respect to joint inventions or inventions made by our partners that arise from the results of the strategic alliance. In some instances, there may not be adequate written provisions to address clearly the allocation of intellectual property rights that may arise from the respective alliance. If we cannot successfully negotiate sufficient ownership and commercial rights to the inventions that result from our use of a third-party partner’s materials when required, or if disputes otherwise arise with respect to the intellectual property developed through the use of a partner’s samples, we may be limited in our ability to capitalize on the full market potential of these inventions. In addition, we may face claims by third parties that our agreements with employees, contractors, or consultants obligating them to assign intellectual property to us are ineffective, or are in conflict with prior or competing contractual obligations of assignment, which could result in ownership disputes regarding intellectual property we have developed or will develop and could interfere with our ability to capture the full commercial value of such inventions. Litigation may be necessary to resolve an ownership dispute, and if we are not successful, we may be precluded from using certain intellectual property and associated products and technology, or may lose our rights in that intellectual property. Either outcome could have a material adverse effect on our business.
In addition, the research resulting in certain of our in-licensed patent rights and technology was funded in part by the United States government. As a result, the United States government has certain rights to such patent rights and technology, which include march-in rights. When new technologies are developed with government funding, the government generally obtains certain rights in any resulting patents, including a non-exclusive license authorizing the government to use the invention or to have others use the invention on its behalf. The government can exercise its march-in rights if it determines that action is necessary because we fail to achieve practical application of the government-funded technology, or because action is necessary to alleviate health or safety needs, to meet requirements of federal regulations, or to give preference to the United States industry. Any exercise by the government of any of the foregoing rights could have a material adverse effect on our business.
We may not identify relevant third party patents or may incorrectly interpret the relevance, scope or expiration of a third party patent which might adversely affect our ability to develop and market our products.
We cannot guarantee that any of our patent searches or analyses, including but not limited to the identification of relevant patents, the scope of patent claims or the expiration of relevant patents, are complete or thorough, nor can we be certain that we have identified each and every third party patent and pending application in the United States and abroad that is relevant to or necessary for the commercialization of our product candidates in any jurisdiction.
The scope of a patent claim is determined by an interpretation of the law, the written disclosure in a patent and the patent’s prosecution history. Our interpretation of the relevance or the scope of a patent or a pending application may be incorrect, which may negatively impact our ability to market our products. We may incorrectly determine that our products are not covered by a third party patent or may incorrectly predict whether a third party’s pending application will issue with claims of relevant scope. Our determination of the expiration
30
Table of Contents
date of any patent in the United States or abroad that we consider relevant may be incorrect, which may negatively impact our ability to develop and market our product candidates. Our failure to identify and correctly interpret relevant patents may negatively impact our ability to develop and market our products.
Third parties may assert that our employees or consultants have wrongfully used or disclosed confidential information or misappropriated trade secrets.
We currently employ, and in the future may employ, individuals who were previously employed at universities or other biotechnology companies, including our competitors or potential competitors. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Any infringement, misappropriation or other violation by us of intellectual property rights of others may prevent or delay our product development efforts and may prevent or increase the costs of our successfully commercializing our product candidates, if approved.
Our success will depend in part on our ability to operate without infringing, misappropriating or otherwise violating the intellectual property and proprietary rights of third parties. We cannot assure you that our business operations, products, product candidates and methods and the business operations, products, product candidates and methods of our partners do not or will not infringe, misappropriate or otherwise violate the patents or other intellectual property rights of third parties.
The biotechnology industry is characterized by extensive litigation regarding patents and other intellectual property rights. Other parties may allege that our products, product candidates or the use of our technologies infringe, misappropriate or otherwise violate patent claims or other intellectual property rights held by them or that we are employing their proprietary technology without authorization. Patent and other types of intellectual property litigation can involve complex factual and legal questions, and their outcome is uncertain. Any claim relating to intellectual property infringement that is successfully asserted against us may require us to pay substantial damages, including treble damages and attorneys’ fees if we or our partners are found to be willfully infringing another party’s patents, for past use of the asserted intellectual property and royalties and other consideration going forward if we are forced to take a license. Such a license may not be available on commercially reasonable terms, or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same intellectual property rights or technologies licensed to us. In addition, if any such claim were successfully asserted against us and we could not obtain a license, we or our partners may be forced to stop or delay developing, manufacturing, selling or otherwise commercializing our products, product candidates or other infringing technology, or those we develop with our R&D partners.
Even if we are successful in these proceedings, we may incur substantial costs and divert management time and attention pursuing these proceedings, which could have a material adverse effect on us. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. If we are unable to avoid infringing the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court, or
31
Table of Contents
redesign our products. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion. In addition, intellectual property litigation or claims could force us to do one or more of the following:
| • | cease developing, selling or otherwise commercializing our product candidates; |
| • | pay substantial damages for past use of the asserted intellectual property; |
| • | obtain a license from the holder of the asserted intellectual property, which license may not be available on reasonable terms, if at all; and |
| • | in the case of trademark claims, redesign, or rename trademarks we may own, to avoid infringing the intellectual property rights of third parties, which may not be possible and, even if possible, could be costly and time-consuming. |
Any of these risks coming to fruition could have a material adverse effect on our business, results of operations, financial condition and prospects.
We may be unsuccessful in licensing or acquiring intellectual property from third parties that may be required to develop and commercialize our product candidates.
Because our programs may involve additional product candidates that may require the use of intellectual property or proprietary rights held by third parties, the growth of our business will likely depend in part on our ability to acquire, in-license or use these intellectual property and proprietary rights. For example, if we determined to use a technology other than TALEN to perform our gene editing, such as CRISPR, we would likely need one or more licenses to use that technology. However, we may be unable to acquire or in-license any third party intellectual property or proprietary rights. Even if we are able to acquire or in-license such rights, we may be unable to do so on commercially reasonable terms. The licensing and acquisition of third party intellectual property and proprietary rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third party intellectual property and proprietary rights that we may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, capital resources and agricultural development and commercialization capabilities.
For example, we sometimes partner with academic institutions to accelerate our research or development under written agreements with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution’s rights in technology resulting from the strategic alliance. Regardless of such option, we may be unable to negotiate a license within the specified time frame or under terms that are acceptable to us, and the institution may license such intellectual property rights to third parties, potentially blocking our ability to pursue our development and commercialization plans.
Further, our consulting agreement with Dr. Voytas generally obligates Dr. Voytas to assign to us any intellectual property solely or jointly conceived, developed or reduced to practice by him in the course of the performance of his services to us. However, we do not have any rights, including any assignment or right of first refusal rights, to intellectual property conceived, developed or reduced to practice by Dr. Voytas outside the course of the performance of his services to us, including in connection with his employment at the University of Minnesota.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license intellectual property and proprietary rights to us. We also may be unable to license or acquire third party intellectual property and proprietary rights on terms that would allow us to make an appropriate return on our investment or at all. If we are unable to successfully acquire or in-license rights to required third party intellectual property and proprietary rights or maintain the existing intellectual property and proprietary rights we have, we may have to cease development of the relevant program, product or product candidate, which could have a material adverse effect on our business.
32
Table of Contents
Loss of or damage to our germplasm libraries would significantly slow our product development efforts.
We have access to a comprehensive collection of germplasm for our product candidates, in part, through licensing agreements with leading institutions. Germplasm comprises genetic material covering the diversity of a crop, the attributes of which are inherited from generation to generation. Germplasm is a key strategic asset since it forms the basis of plant breeding programs. To the extent that we lose access to the germplasm because of the termination or breach of our licensing agreements or as a result of insufficient quantities of germplasm for testing, breeding and commercial use in relevant geographies, our product development capabilities could be negatively impacted. In addition, loss of or damage to our germplasm would significantly impair our research and development activities. Although we restrict access to our germplasm at our facilities to protect this valuable resource, we cannot guarantee that our efforts to protect our germplasm will be successful. The destruction or theft of a significant portion of our germplasm collection would adversely affect our business and results of operations.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose license rights that are important to our business.
We are a party to a number of intellectual property license agreements that are important to our business and expect to enter into additional license agreements in the future. Our existing license agreements impose, and we expect that future license agreements will impose, various diligence, royalty and other obligations on us. If we fail to comply with our obligations under these agreements, or we are subject to a bankruptcy, our licensors may have the right to terminate the license, in which event we would not be able to market products or product candidates covered by the license.
In addition, disputes may arise regarding the payment of the royalties or other consideration due to licensors in connection with our exploitation of the rights we license from them. Licensors may contest the basis of payments we retained and claim that we are obligated to make payments under a broader basis. In addition to the costs of any litigation we may face as a result, any legal action against us could increase our payment obligations under the respective agreement and require us to pay interest and potentially damages to such licensors.
In some cases, patent prosecution of our licensed technology is controlled solely by the licensor. If such licensor fails to obtain and maintain patent or other protection for the proprietary intellectual property we license from such licensor, we could lose our rights to such intellectual property or the exclusivity of such rights, and our competitors could market competing products using such intellectual property. In addition, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. In that event, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected products and product candidates, which could harm our business significantly. In other cases, we control the prosecution of patents resulting from licensed technology. In the event we breach any of our obligations related to such prosecution, we may incur significant liability to our licensing partners. We may also require the cooperation of our licensors to enforce any licensed patent rights, and such cooperation may not be provided. Moreover, we have obligations under these license agreements, and any failure to satisfy those obligations could give our licensor the right to terminate the agreement. Termination of a necessary license agreement could have a material adverse impact on our business.
Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues and is complicated by the rapid pace of scientific discovery in our industry. Disputes may arise regarding intellectual property subject to a licensing agreement (including the intellectual property licensed to us by Cellectis), including:
| • | the scope of rights granted under the license agreement and other interpretation-related issues; |
| • | the basis of royalties and other consideration due to our licensors; |
33
Table of Contents
| • | the extent to which our products, product candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| • | the sublicensing of patent and other rights under our development relationships; |
| • | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| • | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
| • | the priority of invention of patented technology. |
If disputes over intellectual property that we have licensed from third parties prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates, which could have a material adverse effect on our business.
Any partnerships that we may enter into in the future may not be successful, which could adversely affect our ability to develop and commercialize our product candidates.
We may seek R&D partnerships or joint venture arrangements with third parties for the development or commercialization of our product candidates depending on the merits of retaining commercialization rights for ourselves as compared to entering into partnerships or joint venture arrangements. We will face, to the extent that we decide to enter into partnerships or joint venture agreements, significant competition in seeking appropriate partners. Moreover, partnerships or joint venture arrangements are complex and time-consuming to negotiate, document, implement and maintain. We may not be successful in our efforts to establish and implement partnerships, joint ventures, or other alternative arrangements should we so chose to enter into such arrangements. The terms of any partnerships, joint ventures, or other arrangements that we may establish may not be favorable to us.
Any future partnerships or joint ventures that we enter into may not be successful. The success of our R&D partnerships or joint venture arrangements will depend heavily on the efforts and activities of our partners. R&D partnerships and joint ventures are subject to numerous risks, which may include that:
| • | partners have significant discretion in determining the efforts and resources that they will apply to R&D partnerships or joint ventures; |
| • | partners may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on trial results, changes in their strategic focus due to the acquisition of competitive products, availability of funding or other external factors, such as a business combination that diverts resources or creates competing priorities; |
| • | partners may delay trials, provide insufficient funding for a trial program, stop a trial, abandon a product candidate, repeat or conduct new trials or require a new formulation of a product candidate for testing; |
| • | partners could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates; |
| • | a partner with marketing, manufacturing and distribution rights to one or more products may not commit sufficient resources to or otherwise not perform satisfactorily in carrying out these activities; |
| • | we could grant exclusive rights to our partners that would prevent us from collaborating with others; |
| • | partners may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability; |
34
Table of Contents
| • | disputes may arise between us and a partner that causes the delay or termination of the research, development or commercialization of our current or future products or that results in costly litigation or arbitration that diverts management attention and resources; |
| • | partnerships may be terminated, and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable current or future products; |
| • | partners may own or co-own intellectual property covering our products that results from our partnering with them, and in such cases, we would not have the exclusive right to develop or commercialize such intellectual property; and |
| • | a partner’s sales and marketing activities or other operations may not be in compliance with applicable laws resulting in civil or criminal proceedings. |
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names and trademarks, which we need for name recognition by potential partners or food manufacturers or farmers in our markets of interest. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively and our business may be adversely affected.
Risks Related to Our Organization, Structure and Operation
We will need to develop and expand our company, and we may encounter difficulties in managing this development and expansion, which could disrupt our operations.
As of May 31, 2017, we had 27 employees and we expect to increase our number of employees and the scope and location of our operations. To manage our anticipated development and expansion, including the development and the commercialization of our product candidates, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Members of our management team may need to divert a disproportionate amount of their attention away from their day-to-day activities and devote a substantial amount of time to managing these development activities. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. This may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. The physical expansion of our operations may lead to significant costs and may divert financial resources from other projects, such as the development of our product candidates. If our management is unable to effectively manage our expected development and expansion, our expenses may increase more than expected, our ability to generate or increase our revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize our product candidates, if approved, and compete effectively will depend, in part, on our ability to effectively manage the future development and expansion of our company.
We depend on key management personnel and attracting and retaining other qualified personnel, and our business could be harmed if we lose key management personnel or cannot attract and retain other qualified personnel.
Our success depends to a significant degree upon the technical skills and continued service of certain members of our management team: Federico A. Tripodi, our CEO, and Daniel Voytas, Ph.D., our CSO. Dr. Voytas works for us as a consultant pursuant to a consulting agreement under which he is required to work 10 days per month with us. The loss of the services of one or both of these key executive officers could have a material adverse effect on us. We
35
Table of Contents
do not maintain “key man” insurance policies on the lives of any of our employees. Our success also will depend upon our ability to attract and retain additional qualified management, regulatory, technical, and sales and marketing executives and personnel. The failure to attract, integrate, motivate, and retain additional skilled and qualified personnel could have a material adverse effect on our business.
We compete for such personnel against numerous companies, including larger, more established companies with significantly greater financial resources than we possess. In addition, failure to succeed in our product candidates’ development may make it more challenging to recruit and retain qualified personnel. There can be no assurance that we will be successful in attracting or retaining such personnel and the failure to do so could have a material adverse effect on our business, financial condition, and results of operations.
We may not be able to fully enforce covenants not to compete with our key management personnel, and therefore we may be unable to prevent our competitors from benefiting from the expertise of such employees.
Our offer letters with key management personnel, which include executive officers, and our consulting agreement with Dr. Voytas, contain non-compete provisions. These provisions prohibit our key management personnel, if they cease working for us, from competing directly with us or working for our competitors for a period of time. Under applicable laws, we may be unable to enforce these provisions. If we cannot enforce the non-compete provisions with our key management personnel, we may be unable to prevent our competitors from benefiting from the expertise of such management personnel. Even if these provisions are enforceable, they may not adequately protect our interests. The defection of one or more of our management personnel to a competitor could materially adversely affect our business, results of operations and ability to capitalize on our proprietary information.
We must maintain effective internal control over financial reporting, and if we are unable to do so, the accuracy and timeliness of our financial reporting may be adversely affected, which could have a material adverse effect on our business, investor confidence and market price.
We must maintain effective internal control over financial reporting in order to accurately and timely report our results of operations and financial condition. In addition, as a public company, the Sarbanes-Oxley Act requires, among other things, that we assess the effectiveness of our controls over financial reporting at the end of each fiscal year. We anticipate being first required to issue management’s annual report on internal control over financial reporting, pursuant to Section 404 of the Sarbanes-Oxley Act, in respect of our annual report for the fiscal year ended December 31, 2018.
The rules governing the standards that must be met for our management to assess our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act are complex and require significant documentation, testing and possible remediation. These stringent standards require that our audit and finance committee be advised and regularly updated on management’s assessment of internal control over financial reporting. In addition, our independent registered public accounting firm will be required to attest to the effectiveness of our internal controls over financial reporting beginning with our annual report following the date on which we are no longer an “emerging growth company.” If we fail to staff our accounting and finance function adequately or maintain internal control over financial reporting adequate to meet the requirements of the Sarbanes-Oxley Act, our business and reputation may be harmed.
Our independent registered public accounting firm has identified a material weakness in our internal control over financial reporting which requires remediation.
Our independent registered public accounting firm issued a letter to our audit committee and management in which they identified certain matters that they consider to constitute a material weakness in the design and operation of our internal control over financial reporting as of December 31, 2016. A deficiency in internal control over financial reporting exists when the design or operation of a control does not allow management or employees, in the
36
Table of Contents
normal course of performing their assigned functions, to prevent or detect misstatements on a timely basis. A significant deficiency is a deficiency, or a combination of deficiencies, in internal control over financial reporting that is less severe than a material weakness, yet important enough to merit attention by those responsible for the oversight of the company’s financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis.
The material weakness identified by our auditors relates to our lack of a control in place to review forward purchase derivative contracts entered into by us for potential accounting implications or to determine the proper accounting treatment as a result of entering into the contract. We entered into soybean seed and grain production contracts with third parties. The price we paid for the seed and grain was indexed to the soybean commodity price. Payment terms were specified in the contract and often paid before the title for the grain and seed was transferred.
Management is taking steps to remediate this material weakness including standardizing our seed and grain contracts and developing internal accounting policies for recognizing expenses for seed and grain contracts. We cannot assure you that our plans will sufficiently address the identified material weakness, nor can we assure you that there will not be additional material weaknesses or significant deficiencies in our internal controls in the future. If we fail to remediate the material weakness, or if additional material weaknesses arise in the future, we may fail to meet our future reporting obligations, our financial statements may contain material misstatements and our operational results may be harmed. Any such failure could also adversely affect the results of the periodic management evaluations and, to the extent we are no longer an emerging growth company, the annual auditor attestation reports regarding the effectiveness of our internal control over financial reporting that will be required under Section 404 of the Sarbanes-Oxley Act of 2002. Internal control deficiencies could also cause investors to lose confidence in our reported financial information.
We may use hazardous chemicals and biological materials in our business and are subject to numerous environmental, health and safety laws and regulations. Compliance with such laws and regulations and any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
We are subject to numerous federal, state, local and foreign environmental, health and safety laws and regulations, including those governing laboratory procedures, the handling, use, storage, treatment, manufacture and disposal of hazardous materials and wastes, discharge of pollutants into the environment and human health and safety matters. Our research and development processes may involve the controlled use of hazardous materials, including chemicals and biological materials. We cannot eliminate the risk of contamination or discharge and any resultant injury from these materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials, or may otherwise be required to remediate such contamination, and our liability may exceed any insurance coverage and our total assets. Compliance with environmental, health and safety laws and regulations may be expensive and may impair our research and development efforts. If we fail to comply with these requirements, we could incur substantial costs and liabilities, including civil or criminal fines and penalties, clean-up costs or capital expenditures for control equipment or operational changes necessary to achieve and maintain compliance. In addition, we cannot predict the impact on our business of new or amended environmental, health and safety laws or regulations or any changes in the way existing and future laws and regulations are interpreted and enforced. These current or future laws and regulations may impair our research, development or production efforts.
Our internal computer systems, or those of our third-party contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs.
Despite the implementation of security measures, our internal computer systems and those of our third-party contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While we do not believe that we have experienced any such system failure, accident, or security breach to date, if such an event were to occur and cause interruptions in
37
Table of Contents
our operations, it could result in a material disruption of our programs. For example, the loss of field trial data for our product candidates could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Additionally, there have been reported cases in the industry where product candidates have been stolen from the field during field trials. To the extent that any disruption or security breach results in a loss of or damage to our data or applications or other data or applications relating to our technology or product candidates, or inappropriate disclosure of confidential or proprietary information, we could incur liabilities and the further development of our product candidates could be delayed.
Our business and operations would suffer in the event of computer system failures, cyber-attacks or a deficiency in our cyber-security.
Despite the implementation of security measures, our internal computer systems, and those of third parties on which we rely, are vulnerable to damage from computer viruses, malware, natural disasters, terrorism, war, telecommunication and electrical failures, cyber-attacks or cyber-intrusions over the Internet, attachments to emails, persons inside our organization, or persons with access to systems inside our organization. The risk of a security breach or disruption, particularly through cyber-attacks or cyber-intrusion, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our product development programs. For example, the loss of field trial data from completed or ongoing or planned field trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach was to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur material legal claims and liability, damage to our reputation, and the further development of our product candidates could be delayed.
We may acquire businesses or products, or form strategic alliances, in the future, and we may not realize the benefits of such acquisitions.
We plan to selectively partner, in-license or acquired key enabling technologies and businesses across our value chain that we believe will keep us on the cutting edge of our industry. We may not be able to identify appropriate targets or make acquisitions under satisfactory conditions, in particular, satisfactory price conditions. In addition, we may be unable to obtain the financing for these acquisitions under favorable conditions, and could be led to finance these acquisitions using cash that could be allocated to other purposes in the context of existing operations. If we acquire businesses with promising markets or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to successfully integrate them with our existing operations and company culture. We may encounter numerous difficulties in developing, manufacturing and marketing any new products resulting from a strategic alliance or acquisition that delay or prevent us from realizing their expected benefits or enhancing our business. We cannot assure you that, following any such acquisition, we will achieve the expected synergies to justify the transaction, which could have a material adverse effect on our business, financial conditions, earnings and prospects.
Risks Related to Our Relationship with Cellectis
Cellectis controls the direction of our business, and the concentrated ownership of our common stock and certain contractual rights Cellectis has will prevent you and other stockholders from influencing significant decisions.
Immediately following the completion of this offering (and assuming Cellectis does not purchase any shares in this offering), Cellectis will own 76.4% of our outstanding shares of common stock (or 73.8% if the underwriters exercise their option to purchase additional shares in full). Pursuant to our stockholders agreement with Cellectis, Cellectis will have certain contractual rights for so long as it beneficially owns at least 50% of the
38
Table of Contents
then outstanding shares of our common stock, as described under “Certain Relationships and Related Party Transactions—Relationship with Cellectis,” including:
| • | to approve any modification to our or any future subsidiary’s share capital (e.g., share capital increase or decrease), the creation of any subsidiary, any grant of stock-based compensation, any distributions or initial public offering, merger, spin-off, liquidation, winding up or carve-out transactions; |
| • | to approve the annual business plan and annual budget and any modification thereof; |
| • | to approve any external growth transactions exceeding $500,000 and not included in the approved annual business plan and annual budget; |
| • | to approve any investment and disposition decisions exceeding $500,000 and not included in the approved annual business plan and annual budget (it being understood that this clause excludes the purchase and sale of inventory as a part of the normal course of business); |
| • | to approve any related-party agreement and any agreement or transaction between the executives or shareholders of Calyxt, on the one hand, and Calyxt or any of its subsidiaries, on the other hand; |
| • | to approve any decision pertaining to the recruitment, dismissal/removal, or increase of the compensation of executives and corporate officers; |
| • | to approve any material decision relating to a material litigation; |
| • | to approve any decision relating to the opening of a social or restructuring plan or pre-insolvency proceedings; |
| • | to approve any buyback by us of our own shares; |
| • | to approve any new borrowings or debts exceeding $500,000 and early repayment of loans, if any (it being understood that Cellectis will approve the entering into of contracts for revolving loans and other short-term loans and the repayment of such for financing general operating activities, such as revolving loans for inventory or factoring of receivables); |
| • | to approve grants of any pledges on securities; |
| • | to develop new activities and businesses not described in the annual business and annual budget; |
| • | to approve entry into any material agreement or partnership; and |
| • | to approve any offshore and relocation activities. |
In addition, Cellectis will have the following rights for so long as it beneficially owns at least 15% of the then outstanding shares of our common stock, as described under “Certain Relationships and Related Party Transactions—Relationship with Cellectis,” including:
| • | to nominate the greater of three members of our Board of Directors or a majority of the directors; |
| • | to designate the Chairman of our Board of Directors and one member to each of the audit committee of the Board of Directors, the compensation committee of the Board of Directors and the nominating and corporation governance committee of the Board of Directors; |
| • | to approve any amendments to our amended and restated certificate of incorporation or our amended and restated by-laws that would change the name of our company, its jurisdiction of incorporation, the location of its principal executive offices, the purpose or purposes for which our company is incorporated or the Cellectis approval items set forth in the stockholders agreement; |
| • | to approve the payment of any regular or special dividends; |
| • | to approve the commencement of any voluntary proceeding for the dissolution, winding up or bankruptcy of Calyxt or a material subsidiary; |
39
Table of Contents
| • | to approve any public or private offering, merger, amalgamation or consolidation of us or the spinoff of a business of ours or any sale, conveyance, transfer or other disposition of our assets; and |
| • | to approve any appointment to our Board of Directors contrary to the stockholders agreement or our certificate of incorporation or our by-laws. |
As a result, Cellectis controls the direction of our business, and the concentrated ownership of our common stock and the contractual rights described above will prevent you and other stockholders from influencing significant decisions.
If Cellectis sells a controlling interest in our company to a third party in a private transaction, you may not realize any change-of-control premium on shares of our common stock and we may become subject to the control of a presently unknown third party.
Following the completion of this offering, Cellectis will continue to own a significant equity interest in our company. Cellectis will have the ability, should it choose to do so, to sell some or all of its shares of our common stock in a privately negotiated transaction, which, if sufficient in size, could result in a change of control of our company.
The ability of Cellectis to privately sell its shares of our common stock, with no requirement for a concurrent offer to be made to acquire all of the shares of our common stock that will be publicly traded hereafter, could prevent you from realizing any change-of-control premium on your shares of our common stock that may otherwise accrue to Cellectis on its private sale of our common stock. Additionally, if Cellectis privately sells its significant equity interest in our company, we may become subject to the control of a presently unknown third party. Such third party may have conflicts of interest with those of other stockholders. In addition, if Cellectis sells a controlling interest in our company to a third party, any future indebtedness we have may be subject to acceleration, Cellectis may terminate the license agreement, and other transitional arrangements, and our other commercial agreements and relationships could be impacted, all of which may adversely affect our ability to run our business as described herein and may have a material adverse effect on our operating results and financial condition.
We will be a “controlled company” within the meaning of the rules of the NASDAQ and, as a result, will qualify for, and intend to rely on, exemptions from certain corporate governance requirements. You will not have the same protections afforded to stockholders of companies that are subject to such requirements.
Upon completion of this offering, Cellectis will continue to control a majority of the voting power of our outstanding common stock. As a result, we will be a “controlled company” within the meaning of the corporate governance standards of the NASDAQ. Under these rules, a listed company of which more than 50% of the voting power is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance requirements, including:
| • | the requirement that a majority of the board of directors consist of independent directors; |
| • | the requirement that our nominating and corporate governance committee be composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; |
| • | the requirement that our compensation committee be composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; and |
| • | the requirement for an annual performance evaluation of our corporate governance and compensation committees. |
While Cellectis controls a majority of the voting power of our outstanding common stock, we may not have a majority of independent directors or corporate governance and compensation committees consisting entirely of
40
Table of Contents
independent directors and we will not be required to have written charters addressing these committees’ purposes and responsibilities or have annual performance evaluations of these committees. Accordingly, you will not have the same protections afforded to stockholders of companies that are subject to all of the corporate governance requirements of the NASDAQ.
Cellectis may compete with us.
Cellectis will not be restricted from competing with us in the plant sciences industry business, including as a result of acquiring a company that operates an agricultural biotechnology business. Due to the significant resources of Cellectis, including financial resources, name recognition and know-how resulting from the previous management of our business, Cellectis could have a significant competitive advantage over us should it decide to engage in the type of business we conduct, which may cause our operating results and financial condition to be materially adversely affected.
Certain of our directors may have actual or potential conflicts of interest because of their positions with Cellectis.
Following this offering, Dr. André Choulika, Mr. Alain Godard and Mr. Laurent Arthaud will serve on our Board of Directors and retain their positions with Cellectis. In addition, such directors may own Cellectis ordinary shares, options to purchase Cellectis ordinary shares or other Cellectis equity awards. These individuals’ holdings of Cellectis ordinary shares, options to purchase ordinary shares of Cellectis or other equity awards may be significant for some of these persons compared to these persons’ total assets. Their position at Cellectis and the ownership of any Cellectis equity or equity awards creates, or may create the appearance of, conflicts of interest when these directors are faced with decisions that could have different implications for Cellectis than the decisions have for us.
Cellectis and its directors and officers will have limited liability to us or you for breach of fiduciary duty.
Our Certificate of Incorporation provides that, subject to any contractual provision to the contrary, Cellectis will have no obligation to refrain from:
| • | engaging in the same or similar business activities or lines of business as we do; |
| • | doing business with any of our clients or consumers; or |
| • | employing or otherwise engaging any of our officers or employees. |
Under our Certificate of Incorporation, neither Cellectis nor any officer or director of Cellectis, except as provided in our Certificate of Incorporation, is liable to us or to our stockholders for breach of any fiduciary duty by reason of any of these activities.
Cellectis currently performs or supports many of our important corporate functions. We will incur significant charges in connection with this offering and incremental costs as a stand-alone public company.
We will need to replicate or replace certain functions, systems and infrastructure to which we will no longer have the same access after this offering. We may also need to make investments or hire additional employees to operate without the same access to Cellectis’ existing operational and administrative infrastructure. These initiatives may be costly to implement. Due to the scope and complexity of the underlying projects relative to these efforts, the amount of total costs could be materially higher than our estimate, and the timing of the incurrence of these costs is subject to change.
Cellectis currently performs or supports many important corporate functions for our company. Our financial statements reflect charges for these services on an allocation basis. Following this offering, many of these services will be governed by our management services agreement with Cellectis. Under the management services
41
Table of Contents
agreement we will be able to use these Cellectis services for one year terms, which are automatically renewed. However, either party will have the right to terminate the agreement at the anniversary of the agreement by giving three months’ prior notice. In addition, either party will be able to terminate the agreement due to a material breach of the other party, upon prior written notice, subject to limited cure periods and certain change of control, sale and bankruptcy events. See “Certain Relationships and Related Party Transactions—Relationship with Cellectis—Management Services Agreement.”
We will pay Cellectis mutually agreed-upon fees for these services, which will be based on Cellectis’ costs of providing the services.
We may not be able to replace these services or enter into appropriate third-party agreements on terms and conditions, including cost, comparable to those that we will receive from Cellectis under our management services agreement. Additionally, after the agreement terminates, we may be unable to sustain the services at the same levels or obtain the same benefits as when we were receiving such services and benefits from Cellectis. When we begin to operate these functions separately, if we do not have our own adequate systems and business functions in place, or are unable to obtain them from other providers, we may not be able to operate our business effectively or at comparable costs, and our profitability may decline. In addition, we have historically received informal support from Cellectis, which may not be addressed in our management services agreement. The level of this informal support will diminish or be eliminated following this offering.
Risks Related to Ownership of Our Common Stock
The requirements of being a U.S. public company require significant resources and management attention and affect our ability to attract and retain executive management and qualified board members.
As a U.S. public company following this offering, we will incur legal, accounting, and other expenses that we did not previously incur. We will be subject to the Exchange Act, including the reporting requirements thereunder, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the NASDAQ listing requirements and other applicable securities rules and regulations. Compliance with these rules and regulations will increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources, particularly after we are no longer an “emerging growth company.”
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, we will be required to furnish a report by our management on our internal control over financial reporting, including an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. However, while we remain an emerging growth company, we will not be required to include this attestation report on internal control over financial reporting issued by our independent registered public accounting firm. When our independent registered public accounting firm is required to undertake an assessment of our internal control over financial reporting, the cost of complying with Section 404 will significantly increase and management’s attention may be diverted from other business concerns, which could adversely affect our business and results of operations. We may need to hire more employees in the future or engage outside consultants to comply with these requirements, which will further increase our cost and expense. If we fail to implement the requirements of Section 404 in the required timeframe, we may be subject to sanctions or investigations by regulatory authorities, including the SEC and the NASDAQ. Furthermore, if we are unable to conclude that our internal control over financial reporting is effective, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of shares of our common stock could decline, and we could be subject to sanctions or investigations by regulatory authorities. Failure to implement or maintain effective internal control systems required of public companies could also restrict our future access to the capital markets. In addition, enhanced legal and regulatory regimes and heightened standards relating to corporate governance and disclosure for public companies result in increased legal and financial compliance costs and make some activities more time consuming.
42
Table of Contents
An active trading market for our common stock may not develop, and the market price for our common stock may be volatile or may decline regardless of our operating performance.
Prior to the completion of this offering, there has been no public market for our common stock. An active trading market for shares of our common stock may never develop or be sustained following this offering. If an active trading market does not develop, you may have difficulty selling your shares of common stock at an attractive price, or at all. The price for our common stock in this offering will be determined by negotiations among Cellectis, us and representatives of the underwriters, and it may not be indicative of prices that will prevail in the open market following this offering. Consequently, you may not be able to sell your common stock at or above the initial public offering price or at any other price or at the time that you would like to sell. An inactive market may also impair our ability to raise capital by selling our common stock, and it may impair our ability to attract and motivate our employees through equity incentive awards and our ability to acquire other companies, products or technologies by using our common stock as consideration.
The price of our common stock may fluctuate substantially.
You should consider an investment in our common stock to be risky, and you should invest in our common stock only if you can withstand a significant loss and wide fluctuations in the market value of your investment. Some factors that may cause the market price of our common stock to fluctuate, in addition to the other risks mentioned in this section of the prospectus, are:
| • | actual or anticipated fluctuations in our financial condition and operating results; |
| • | our failure to develop and commercialize our product candidates; |
| • | actual or anticipated changes in our growth rate relative to our competitors; |
| • | competition from existing products or new products that may emerge; |
| • | announcements by us, Cellectis, our R&D partners or our competitors of significant acquisitions, strategic partnerships, joint ventures, strategic alliances, or capital commitments; |
| • | the imposition of regulatory requirements on any of our product candidates to be sold in North America; |
| • | the inability to establish additional strategic alliances; |
| • | unanticipated serious safety concerns related to the use of any of our products once commercialized; |
| • | failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public; |
| • | issuance of new or updated research or reports by securities analysts; |
| • | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| • | common stock price and volume fluctuations attributable to inconsistent trading volume levels of our common stock; |
| • | additions or departures of key management or scientific personnel; |
| • | disputes or other developments related to proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our technologies; |
| • | announcement or expectation of additional equity or debt financing efforts; |
| • | sales of common stock by us, Cellectis, our insiders or our other stockholders; |
| • | announcements or actions taken by Cellectis as our principal stockholder; and |
| • | general economic and market conditions. |
These and other market and industry factors may cause the market price and demand for our common stock to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors
43
Table of Contents
from readily selling their common stock and may otherwise negatively affect the liquidity of our common stock. In addition, the stock market in general, and agricultural biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies.
If you purchase common stock in this offering, you will experience substantial and immediate dilution.
If you purchase common stock in this offering, you will experience substantial and immediate dilution of $12.55 per share in the net tangible book value after giving effect to the offering at an assumed initial public offering price of $16.50 per share (the midpoint of the price range on the cover of this prospectus) because the price that you pay will be substantially greater than the net tangible book value per share that you acquire. For a further description of the dilution that you will experience immediately after this offering, see the section of this prospectus titled “Dilution.”
Our historical financial data is not necessarily representative of the results we would have achieved as a stand-alone company and may not be a reliable indicator of our future results.
Our historical financial data included in this prospectus does not reflect the financial condition, results of operations or cash flows we would have achieved as a stand-alone company during the periods presented or those we will achieve in the future. This is primarily the result of the following factors:
| • | our historical financial data reflects expense allocations for certain support functions that are provided on a centralized basis within Cellectis, such as expenses for business technology, facilities, legal, finance, human resources and business development that may be higher or lower than the comparable expenses we would have actually incurred, or will incur in the future, as a stand-alone company; and |
| • | significant increases will occur in our cost structure as a result of this offering, including costs related to public company reporting, investor relations and compliance with the Sarbanes-Oxley Act. |
As a result of the separation, it may be difficult for investors to compare our future results to historical results or to evaluate our relative performance or trends in our business.
Future sales of common stock by Cellectis or others of our common stock, or the perception that such sales may occur, could depress the market price of our common stock.
Immediately following the completion of this offering (and assuming Cellectis does not purchase any shares in this offering), Cellectis will own 76.4% of our outstanding shares of common stock (or 73.8% if the underwriters exercise their option to purchase additional shares in full). Subject to the restrictions described in the paragraph below, future sales of these shares in the public market will be subject to the volume and other restrictions of Rule 144 under the Securities Act of 1933, or the Securities Act, for so long as Cellectis is deemed to be our affiliate, unless the shares to be sold are registered with the Securities and Exchange Commission, or SEC. We are unable to predict with certainty whether or when Cellectis will sell a substantial number of shares of our common stock. The sale by Cellectis of a substantial number of shares after this offering, or a perception that such sales could occur, could significantly reduce the market price of our common stock. Upon completion of this offering, except as otherwise described herein, all shares that are being offered hereby will be freely tradable without restriction, assuming they are not held by our affiliates.
We, our officers and directors and Cellectis have agreed with the underwriters that, without the prior written consent of Citigroup Global Markets Inc. and Jefferies LLC, we and they will not, subject to certain exceptions and extensions, during the period ending 180 days after the date of this prospectus, offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, or enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any shares of common
44
Table of Contents
stock or any securities convertible into or exercisable or exchangeable for shares of common stock. Citigroup Global Markets Inc. and Jefferies LLC may, in their sole discretion and at any time without notice, release all or any portion of the shares of our common stock subject to the lock-up.
Following this offering, we intend to file one or more registration statements with the SEC covering shares of our common stock available for future issuance under our equity incentive plans. Upon effectiveness of such registration statements, any shares subsequently issued under such plans will be eligible for sale in the public market, except to the extent that they are restricted by the lock-up agreements referred to above and subject to compliance with Rule 144 in the case of our affiliates. Sales of a large number of the shares issued under these plans in the public market could have an adverse effect on the market price of our common stock.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, the price of our common stock and trading volume could decline.
The trading market for our common stock depends in part on the research and reports that securities or industry analysts publish about us or our business. If few or no securities or industry analysts cover us, the trading price for our common stock would be negatively impacted. If one or more of the analysts who covers us downgrades our common stock or publishes incorrect or unfavorable research about our business, the price of our common stock would likely decline. If one or more of these analysts ceases coverage of our company or fails to publish reports on us regularly, or downgrades our common stock, demand for our common stock could decrease, which could cause the price of our common stock or trading volume to decline.
We do not currently intend to pay dividends on our common stock.
We do not intend to pay any dividends to holders of our common stock for the foreseeable future. We currently intend to invest our future earnings, if any, to fund our growth. Therefore, you are not likely to receive any dividends on your common stock for the foreseeable future, and the success of an investment in our common stock will depend upon any future appreciation in its value. Consequently, investors may need to sell all or part of their holdings of our common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investment. There is no guarantee that our common stock will appreciate in value or even maintain the price at which our stockholders have purchased our common stock. Investors seeking cash dividends should not purchase our common stock.
Provisions in our Certificate of Incorporation, By-laws and Delaware law may prevent or delay an acquisition of us, which could decrease the trading price of our common stock.
Our Certificate of Incorporation and By-laws contain provisions that are intended to deter coercive takeover practices and inadequate takeover bids and to encourage prospective acquirers to negotiate with our Board of Directors rather than to attempt a hostile takeover. These include the following provisions that become effective once Cellectis’ no longer holds at least 50% of our outstanding shares of common stock:
| • | a Board of Directors that is divided into three classes with staggered terms; |
| • | rules regarding how our stockholders may present proposals or nominate directors for election at stockholder meetings; |
| • | the right of our Board of Directors to issue preferred stock without stockholder approval; and |
| • | limitations on the right of stockholders to remove directors. |
These provisions could make it more difficult for a third party to acquire us, even if the third party’s offer may be considered beneficial by many stockholders. As a result, stockholders may be limited in their ability to obtain a premium for their shares. See “Description of Capital Stock” for a discussion of these provisions.
45
Table of Contents
We are an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We cannot predict if investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
46
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS AND INDUSTRY DATA
We have made statements under the captions “Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business” and in other sections of this prospectus that are forward-looking statements. In some cases, you can identify these statements by forward-looking words such as “may,” “might,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential” or “continue,” the negative of these terms and other comparable terminology. These forward-looking statements, which are subject to risks, uncertainties and assumptions about us, may include projections of our future financial performance, our anticipated growth strategies and anticipated trends in our business. These statements are only predictions based on our current expectations and projections about future events. There are important factors that could cause our actual results, level of activity, performance or achievements to differ materially from the results, level of activity, performance or achievements expressed or implied by the forward-looking statements, including those factors discussed under the caption entitled “Risk Factors.” You should specifically consider the numerous risks outlined under “Risk Factors.”
Although we believe the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, level of activity, performance or achievements. Moreover, neither we nor any other person assumes responsibility for the accuracy and completeness of any of these forward-looking statements. We are under no duty to update any of these forward-looking statements after the date of this prospectus to conform our prior statements to actual results or revised expectations.
Unless otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate is based on information from various sources, including independent industry publications. In presenting this information, we have also made assumptions based on such data and other similar sources, and on our knowledge of, and our experience to date in, the potential markets for our product. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the section entitled “Risk factors.” These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
47
Table of Contents
We estimate that the net proceeds to us from this offering will be approximately $90.9 million, or approximately $104.8 million if the underwriters exercise in full their option to purchase additional shares of common stock, assuming an initial public offering price of $16.50 per share (the midpoint of the range set forth on the cover page of this prospectus), after deducting estimated underwriting discounts and commissions and estimated offering expenses.
Each $1.00 increase (decrease) in the public offering price per share would increase (decrease) our net proceeds, after deducting estimated underwriting discounts and commissions, by $5.6 million (assuming no exercise of the underwriters’ option to purchase additional shares of common stock). An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) the net proceeds to us from this offering, after deducting the underwriting discount and estimated offering expenses payable by us, by approximately $15.3 million, assuming the assumed initial public offering price stays the same.
We intend to use the net proceeds of this offering (including any net proceeds from the underwriters’ exercise of their option to purchase additional shares of common stock) as follows:
| • | approximately $45.0 million to fund research and for development costs to advance our existing product candidates including high fiber wheat and herbicide tolerant wheat and to add additional product candidates to our portfolio; |
| • | approximately $10.0 million to build out commercial capabilities, which includes incremental expansion of our sales force to build commercial relationships with supply chain and food companies, furnishing a demonstration kitchen, and producing demonstration and promotional products, such as high oleic oil and meal samples, to help drive demand creation; |
| • | approximately $20.0 million to be used as working capital related to seed production, grain purchases and meal and oil production; and |
| • | the remainder for general corporate purposes. |
We may also use a portion of the net proceeds to acquire or invest in complementary products, technologies or businesses, although we currently have no agreements or binding commitments to complete any such transaction.
The foregoing intended uses do not include our planned facility expansion, described under “Business—Facilities,” as we intend to finance the building for the planned facility expansion in whole or in part through a sale leaseback transaction. On June 20, 2017, we signed an agreement with a third party buyer for the sale of the land and warehouse facility. The completion of the sale is conditioned on us and the buyer entering into a new facility construction agreement and a lease in respect of the property in the forms contemplated by the sale agreement. We expect to close on the proposed transaction and construction to begin on the new facilities in the second half of 2017.
However, due to the uncertainties inherent in the product development process, it is difficult to estimate with certainty the exact amounts of the net proceeds from this offering that may be used for the above purposes. The amount and timing of our actual expenditures will depend upon numerous factors, including the results of our research and development efforts, the timing and success of our ongoing field tests or field tests we may commence in the future and the timing of regulatory submissions. As a result, our management will have broad discretion over the use of the net proceeds from this offering, and investors will be relying on our judgment regarding the application of the net proceeds. In addition, we might decide to postpone or not pursue certain activities or field tests if the net proceeds from this offering and our other sources of cash are less than expected.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments.
48
Table of Contents
We do not anticipate declaring or paying any cash dividends to holders of our common stock in the foreseeable future. We currently intend to retain future earnings, if any, to finance the growth of our business. If we decide to pay cash dividends in the future, the declaration and payment of such dividends will be at the sole discretion of our Board of Directors and may be discontinued at any time. In determining the amount of any future dividends, our Board of Directors will take into account any legal or contractual limitations, our actual and anticipated future earnings, cash flow, debt service and capital requirements and other factors that our Board of Directors may deem relevant.
49
Table of Contents
The following table sets forth our cash and cash equivalents and capitalization as of March 31, 2017:
| • | on an actual basis; and |
| • | on an as adjusted basis to give effect to the sale and issuance by us of 6,060,606 shares in this offering at an assumed initial public offering price of $16.50 per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses, and assuming that the net proceeds of this offering are held as cash. |
You should read this table in conjunction with our audited financial statements and the notes thereto, included in this prospectus as well as “Use of Proceeds,” “Selected Historical Financial Data,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
| (unaudited) | ||||||||
| As of March 31, 2017 | ||||||||
| Actual | As Adjusted(1) | |||||||
| (in thousands) | ||||||||
| Cash and cash equivalents |
$ | 3,007 | $ | 93,873 | ||||
|
|
|
|
|
|||||
| Long-term debt |
$ | — | $ | — | ||||
| Stockholder’s equity: |
||||||||
| Preferred stock, $0.0001 par value, no shares authorized, actual; 50,000,000 shares authorized and no shares issued and outstanding on an as adjusted basis |
— | — | ||||||
| Common stock, $0.0001 par value, 73,500,000 shares authorized and 19,600,000 shares issued and outstanding; 275,000,000 shares authorized and 25,660,606 shares issued and outstanding on an as adjusted basis |
1 | 3 | ||||||
| Additional paid-in capital |
41,820 | 132,684 | ||||||
| Accumulated (deficit) |
(31,400 | ) | (31,400 | ) | ||||
|
|
|
|
|
|||||
| Total stockholder’s equity |
$ | 10,421 | $ | 101,287 | ||||
|
|
|
|
|
|||||
| Total capitalization |
$ | 10,421 | $ | 101,287 | ||||
|
|
|
|
|
|||||
| (1) | A $1.00 increase (decrease) in the assumed initial public offering price of $16.50 per share would increase (decrease) each of as adjusted cash and cash equivalents, additional paid-in capital, total stockholder’s equity and total capitalization by $5.6 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriting discount and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 1,000,000 in the number of shares offered by us would increase (decrease) as adjusted cash and cash equivalents, additional paid-in capital, total stockholder’s equity and total capitalization by $15.3 million, assuming an initial public offering price of $16.50 per share, after deducting the underwriting discount and estimated offering expenses payable by us. |
50
Table of Contents
Our net tangible book value as of March 31, 2017 was $10.4 million, or $0.53 per share of common stock. Net tangible book value per share represents tangible assets, less liabilities, divided by the aggregate number of shares outstanding. After giving effect to the sale by us of 6,060,606 shares of common stock in this offering, at an assumed initial public offering price of $16.50 per share (the midpoint of the range set forth on the cover page of this prospectus) and the receipt and application of the net proceeds, our adjusted net tangible book value as of March 31, 2017 would have been $101.3 million or $3.95 per share. This represents an immediate increase in adjusted net tangible book value to our existing stockholder of $3.42 per share and immediate dilution to new investors of $12.55 per share. Dilution per share represents the difference between the price per share to be paid by new investors for the shares of common stock sold in this offering and the adjusted net tangible book value per share immediately after this offering. The following table illustrates this per share dilution:
| Assumed initial public offering price per share |
$ | 16.50 | ||||||
| Net tangible book value per share at March 31, 2017 |
$ | 0.53 | ||||||
| Increase in net tangible book value per share attributable to new investors |
$ | 3.42 | ||||||
|
|
|
|||||||
| Adjusted net tangible book value per share as of March 31, 2017 after this offering |
$ | 3.95 | ||||||
|
|
|
|||||||
| Dilution per share to new investors in this offering |
$ | 12.55 | ||||||
|
|
|
Each $1.00 increase (decrease) in the assumed initial public offering price of $16.50 per share (the midpoint of the price range set forth on the cover page of this prospectus) would increase (decrease) our adjusted net tangible book value by approximately $5.6 million, or approximately $0.22 per share, and increase (decrease) the dilution per share to investors participating in this offering by approximately $0.78 per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase of 1,000,000 in the number of shares offered by us would increase our adjusted net tangible book value by approximately $15.3 million, or $0.43 per share, and decrease the dilution per share to investors participating in this offering by $0.43 per share, assuming that the assumed initial public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, a decrease of 1,000,000 shares in the number of shares offered by us would decrease our adjusted net tangible book value by approximately $15.3 million, or $0.46 per share, and increase the dilution per share to investors participating in this offering by $0.46 per share, assuming that the assumed initial public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The adjusted information discussed above is illustrative only and will adjust based on the actual initial price to public and other terms of this offering determined at pricing.
If the underwriters exercise their option in full to purchase 909,091 additional shares of common stock in this offering, the adjusted net tangible book value per share after the offering would be $4.34 per share, the incremental increase in the adjusted net tangible book value per share to our existing stockholder would be $3.81 per share and the adjusted dilution to new investors participating in this offering would be $12.16 per share.
51
Table of Contents
The following table sets forth, on an adjusted basis as described above, as of March 31, 2017, the number of shares of common stock purchased from us, the total consideration paid, or to be paid, and the average price per share paid, or to be paid, by our existing stockholder (assuming our existing stockholder does not purchase any shares in this offering) and by the new investors, at an assumed initial public offering price of $16.50 per share (the midpoint of the range set forth on the cover page of this prospectus) before deducting estimated underwriting discounts and commissions and offering expenses payable by us:
| Shares Purchased | Total Consideration | Average Price Per Share |
||||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||||
| Existing stockholder |
19,600,000 | 76 | % | $ | 41,820,725 | 32 | % | $ | 2.13 | |||||||||||
| New investors |
6,060,606 | 24 | % | $ | 90,865,640 | 68 | % | $ | 14.99 | |||||||||||
| Total |
25,660,606 | 100 | % | $ | 132,686,365 | 100 | % | $ | 5.17 | |||||||||||
Each $1.00 increase (decrease) in the assumed initial public offering price of $16.50 per share would increase (decrease) total consideration paid by new investors by approximately $5.6 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares offered by us would increase (decrease) total consideration paid by new investors by approximately $15.3 million, assuming that the assumed initial public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
52
Table of Contents
SELECTED HISTORICAL FINANCIAL DATA
The selected financial information as of December 31, 2016 and for the years ended December 31, 2016 and 2015 appearing below has been derived from our audited financial statements and the notes thereto, included elsewhere in this prospectus with the exception of the basic and diluted net loss per share and the weighted average shares outstanding—basic and diluted, which reflect the consummation of a stock split immediately prior to and contingent on closing of this offering pursuant to which each share held of record by the holder thereof will be reclassified into 2.45 shares. The selected financial information as of March 31, 2017 and for the three months ended March 31, 2017 and 2016 appearing below has been derived from our unaudited condensed financial statements and the notes thereto, included elsewhere in this prospectus. The unaudited condensed financial statements have been prepared on the same basis as our audited financial statements and include all normal recurring adjustments that we consider necessary for a fair statement of our financial position and operating results as of the dates and for the periods presented. The selected financial information below should be read together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and in conjunction with the financial statements, related notes and other financial information included elsewhere in this prospectus.
Our historical results are not necessarily indicative of our future results and our interim results are not necessarily reflective of the results to be expected for the full year. The summary financial information below does not contain all the information included in our financial statements.
Statements of Operations
| (unaudited) | ||||||||||||||||
| Three Months Ended March 31, | Year Ended December 31, | |||||||||||||||
| 2017 | 2016 | 2016 | 2015 | |||||||||||||
| (in thousands, except per share data) | ||||||||||||||||
| Revenue |
$ | 55 | $ | 106 | $ | 399 | $ | 1,272 | ||||||||
| Operating expenses: |
||||||||||||||||
| Cost of revenue |
— | 100 | 200 | 751 | ||||||||||||
| Research and development |
1,266 | 1,370 | 5,638 | 2,766 | ||||||||||||
| Sales, general, and administrative |
1,578 | 1,197 | 6,670 | 3,569 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
2,844 | 2,667 | 12,508 | 7,086 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(2,789 | ) | (2,561 | ) | (12,109 | ) | (5,814 | ) | ||||||||
| Interest expense |
(14 | ) | (4 | ) | (5 | ) | (261 | ) | ||||||||
| Foreign currency transaction gains (losses) |
(29 | ) | (4 | ) | 28 | 186 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before income taxes |
(2,832 | ) | (2,569 | ) | (12,086 | ) | (5,889 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income tax benefit |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (2,832 | ) | $ | (2,569 | ) | $ | (12,086 | ) | $ | (5,889 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted net loss per share(1) |
$ | (0.14 | ) | $ | (0.13 | ) | $ | (0.62 | ) | $ | (0.88 | ) | ||||
| Weighted average shares outstanding—basic and diluted |
19,600,000 | 19,600,000 | 19,600,000 | 6,725,740 | ||||||||||||
Balance Sheet Data:
| As of |
||||||||
| March 31, 2017 (unaudited) |
December 31, 2016 | |||||||
| (in thousands) |
||||||||
| Cash and cash equivalents |
$ | 3,007 | $ | 5,026 | ||||
| Total assets |
15,092 | 16,623 | ||||||
| Accumulated deficit |
(31,400 | ) | (28,568 | ) | ||||
| Total stockholder’s equity |
10,421 | 13,119 | ||||||
| Total liabilities and stockholder’s equity |
15,092 | 16,623 | ||||||
53
Table of Contents
| (1) | See note 8 to our audited financial statements and note 7 to our unaudited condensed financial statements, included elsewhere in this prospectus for an explanation of the method used to calculate basic and diluted net loss per share. |
54
Table of Contents
ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read together with our financial statements and related notes which are included elsewhere in this prospectus. Our actual results could differ materially from those anticipated in the forward-looking statements included in this discussion as a result of certain factors, including, but not limited to, those discussed in “Risk Factors” included elsewhere in this prospectus.
Overview
We are a consumer-centric, food- and agriculture-focused company. By combining our leading gene-editing technology and technical expertise with our innovative commercial strategy, we are pioneering a paradigm shift to deliver healthier specialty food ingredients, such as healthier oils and high fiber wheat, for consumers and agriculturally advantageous crop traits, such as herbicide tolerance, to farmers. While the traits that enable these characteristics may occur naturally and randomly through evolution—or under a controlled environment through traditional agricultural technologies—those processes are imprecise and take many years, if not decades. Our technology enables us to precisely and specifically edit a plant genome to elicit the desired traits and characteristics, resulting in a final product that has no foreign DNA. We believe the precision, specificity, cost effectiveness and development speed of our gene-editing technologies will enable us to provide meaningful disruption to the food and agriculture industries.
Food-related issues, including obesity and diabetes, are some of the most prevalent health issues today and continue to grow rapidly. As awareness of these diet-related health issues grows, consumers are emphasizing a healthier lifestyle and a desire for nutritionally rich foods that are more nutritious, better tasting, less processed and more convenient. This trend is leading to an increase in the demand for higher valued, premium segments of the food industry, such as higher fiber, reduced gluten and reduced fat products. As a result of these trends, food companies are looking for specialty ingredients and solutions that can help them satisfy their customers’ evolving needs and drive growth in market share and new value-added products.
We have developed a robust product pipeline with our proprietary technology. Our first product candidate, which we expect to be commercialized by the end of 2018, is a high oleic soybean designed to produce a healthier oil that has zero trans fats and reduced saturated fats. We are also developing a high fiber wheat to create flour with up to three times more dietary fiber than standard white flour while maintaining the same flavor and convenience of use. Another product candidate we are developing is a herbicide tolerant wheat designed to provide farmers with better weed control options to increase yields and profitability. We believe each of these product candidates addresses a potential multi-billion dollar market opportunity.
We are an early-stage company and have incurred net losses since our inception. As of March 31, 2017 we had an accumulated deficit of $31.4 million. Our net losses for the three months ended March 31, 2017 and for the years ended December 31, 2016 and 2015 were $2.8 million, $12.1 million and $5.9 million, respectively. Substantially all of our net losses resulted from costs incurred in connection with our research and development (R&D) programs and from selling, general and administrative expenses associated with our operations. As we continue to develop our product pipeline, we expect to continue to incur significant expenses and increasing operating losses for the foreseeable future and those expenses and losses may fluctuate significantly from quarter-to-quarter and year-to-year. We expect that our expenses will increase substantially as we:
| • | establish a sales, marketing and distribution infrastructure, including relationships across our supply chain, to commercialize any products that have completed the development process; |
| • | conduct additional field trials of our current and future product candidates; |
| • | secure manufacturing arrangements for commercial production; |
55
Table of Contents
| • | continue to advance the research and development of our current and future product candidates; |
| • | seek to identify and validate additional product candidates; |
| • | acquire or in-license other product candidates, technologies, germplasm or other biological material; |
| • | are required to seek regulatory and marketing approvals for our product candidates; |
| • | make royalty and other payments under any in-license agreements; |
| • | maintain, protect, expand and defend our intellectual property portfolio; |
| • | seek to attract and retain new and existing skilled personnel; and |
| • | experience any delays or encounter issues with any of the above. |
We do not expect to generate material revenue unless and until we successfully complete development of one or more of our product candidates, which may take a number of years and is subject to significant uncertainty. Until such time that we can generate substantial revenues from sales of our product candidates, if ever, we expect to finance our operating activities through a combination of equity offerings, debt financings, government or other third-party funding, and licensing arrangements. However, we may be unable to raise additional funds or enter into such arrangements when needed on favorable terms, or at all, which would have a negative impact on our financial condition and could force us to delay, limit, reduce or terminate our development programs or commercialization efforts or grant to others rights to develop or market product candidates that we would otherwise prefer to develop and market ourselves. Failure to receive additional funding could cause us to cease operations, in part or in full.
Our audited financial statements for the years ended December 31, 2015 and 2016 and our unaudited condensed financial statements for the three months ended March 31, 2016 and 2017 included elsewhere in this prospectus were prepared in accordance with U.S. Generally Accepted Accounting Principles, or U.S. GAAP, as issued by Financial Accounting Standards Board, or FASB.
Comparability of Our Results and Our Relationship with Cellectis
We currently operate as a wholly owned subsidiary of Cellectis. As a result, our historical financial statements may not be reflective of what our results of operations would have been had we been a stand-alone public company and no longer a subsidiary of Cellectis. In particular, certain legal, finance, human resources and other functions have historically been provided to us by Cellectis at cost plus an agreed-upon markup. Following this offering, pursuant to agreements with Cellectis, we expect that Cellectis will continue to provide us with some of the services related to these functions on a transitional basis in exchange for agreed-upon fees, and we expect to incur other costs to replace the services and resources that will not be provided by Cellectis. We will also incur additional costs as a stand-alone public company. As a stand-alone public company, our total costs related to certain support functions may differ from the costs that were historically allocated to us from Cellectis. In addition, in the future, we expect to incur internal costs to implement certain new systems, including infrastructure and an enterprise resource planning system, while our legacy systems are currently being fully supported by Cellectis. See “Certain Relationships and Related Party Transactions—Relationship with Cellectis” for a description of certain agreements that we have entered into or intend to enter into with Cellectis in connection with this offering that will provide a framework for our ongoing relationship upon and after completion of this offering.
Financial Operations Overview
Revenue
We recognized approximately $0.1 million, $0.4 million and $1.3 million of revenue for the three months ended March 31, 2017 and for the years ended December 31, 2016 and 2015, respectively, from payments we
56
Table of Contents
received pursuant to our R&D agreements under which we conduct research activities for a number of companies. Our R&D agreements provide for non-refundable upfront payments that we receive upon execution of the relevant agreement; milestone payments upon the achievement of certain scientific, regulatory or commercial milestones; license payments from licenses that we grant to third parties; and R&D cost reimbursements that are recognized over the period of these services and royalty payments. Our reliance on revenue from our R&D agreements has been systematically diminishing as we purposely reduce the number of R&D contracts we enter into with other companies and focus on in-house product development.
To date, we have not generated any product revenue. Our ability to generate future product revenue depends upon our R&D partners’ ability to assist us in successfully developing and commercializing our products. If we fail to complete the development of our product candidates in a timely manner or fail to obtain their regulatory approval if necessary, our ability to generate future revenue would be compromised.
Research and Development Expenses
R&D expenses consist of expenses incurred while performing R&D activities to discover and develop potential product candidates. We recognize R&D expenses as they are incurred.
Our R&D expenses consist primarily of:
| • | personnel costs, including salaries and related benefits, for our employees engaged in scientific R&D functions; |
| • | cost of third-party contractors, such as contract research organizations, or CROs, and third-party contractors who support our product candidate development; |
| • | seed increases (small-scale and large-scale testing) for trait validation; |
| • | purchases and manufacturing of biological materials, real-estate leasing costs, as well as conferences and travel costs; |
| • | certain other expenses, such as expenses for use of laboratories and facilities for our R&D activities; and |
| • | costs of in-licensing or acquiring technology from third parties. |
Our R&D efforts are focused on our existing product candidates and in broadening our pipeline with new product candidates. We use our employee and infrastructure resources across multiple R&D programs directed toward identifying and developing product candidates. We manage certain activities such as field trials and the manufacture of product candidates through third-party vendors. Due to the number of ongoing projects and our ability to use resources across several projects, we do not record or maintain information regarding the costs incurred for our R&D programs on a program-specific basis.
Our R&D efforts are central to our business and account for a significant portion of our operating expenses. We expect that our R&D expenses will increase for the foreseeable future as we expand our R&D and process development efforts, access and develop additional technologies and hire additional personnel to support our R&D efforts. Product candidates in later stages of product development generally have higher development costs than those in earlier stages of development, primarily due to the increased size of field trials and commercial scale product testing.
R&D expenses, including licensing fees, are expensed as incurred, due to the uncertainty of future commercial value. At this time, we cannot reasonably estimate or know the nature, timing and estimated costs of the efforts that will be necessary to complete the development of our current product candidates or any new product candidates we may identify and develop.
57
Table of Contents
Selling, General and Administrative Expenses
Selling, general and administrative expenses consist primarily of employee-related expenses for executive, business development, intellectual property, finance and human resource functions. Other selling, general and administrative expenses include facility-related costs not otherwise allocated to R&D expense, professional fees for auditing, tax and legal services, expenses associated with obtaining and maintaining patents, consulting costs, management fees and costs of our information systems.
Cellectis provides us services, which include general sales and administration functions, accounting and finance functions, legal advice, human resources, and information technology. We have a management agreement in which they charge us in euros at cost plus a markup ranging from zero to 10%.
We expect that our selling, general and administrative expense will increase as we continue to operate as a public reporting company and continue to develop and commercialize our product candidates. We also expect to incur increased costs to comply with corporate governance, internal controls, investor relations, disclosure and similar requirements applicable to public reporting companies.
Critical Accounting Policies and Estimates
Some of the accounting methods and policies used in preparing our financial statements under U.S. GAAP are based on complex and subjective assessments by our management or on estimates based on past experience and assumptions deemed realistic and reasonable based on the circumstances concerned. The actual value of our assets, liabilities and shareholders’ equity and of our losses could differ from the value derived from these estimates if conditions changed and these changes had an impact on the assumptions adopted. We believe that the most significant management judgments and assumptions in the preparation of our financial statements and the notes thereto are named below. For further details, see our financial statements and the notes thereto, included elsewhere in this prospectus.
Going Concern
During the three months ended March 31, 2017 and the years ended December 31, 2016 and 2015, we incurred losses from operations and net cash outflows from operating activities as disclosed in the statements of operation and cash flows, respectively. At March 31, 2017, we had an accumulated deficit of $31.4 million and expect to incur losses for the foreseeable future. To date, we have been funded primarily by various cash and equity infusions by Cellectis. Although we believe that we will be able to successfully fund our operations through funding from Cellectis, the proceeds of this offering and cash on hand of $3.0 million at March 31, 2017, there can be no assurance we will be able to do so or that we will ever operate profitably. Cellectis has guaranteed funding for our operations through August 2018, which guarantee will not be released, modified or otherwise affected by the timing of, or amount raised in, this offering. Our ability to continue as a going concern prior to this offering is subject to this guaranteed funding from Cellectis and our ability to develop and successfully commercialize our product candidates.
Revenue
We enter into R&D agreements that may consist of nonrefundable up-front payments, milestone payments, royalties and R&D services. In addition, we may license our technology to third parties, which may be part of the R&D agreements.
For agreements that contain multiple elements, each element within a multiple-element arrangement is accounted for as a separate unit of accounting provided the following criteria are met: the delivered products or services have value to the customer on a stand-alone basis; and for an arrangement that includes a general right of return relative to the delivered products or services, delivery or performance of the undelivered product or service is considered probable and is substantially controlled by us. We consider a deliverable to have stand-
58
Table of Contents
alone value if the product or service is sold separately by us or another vendor or could be resold by the customer. Further, our revenue arrangements do not include a general right of return relative to the delivered products.
Nonrefundable up-front payments are deferred and recognized as revenue over the period of the R&D agreement.
Milestone payments represent amounts received from our R&D partners, the receipt of which is dependent upon the achievement of certain scientific, regulatory or commercial milestones. We recognize milestone payments when the triggering event has occurred, there are no further contingencies or services to be provided with respect to that event, and the counterparty has no right to refund of the payment. The triggering event may be scientific results achieved by us or another party to the arrangement, regulatory approvals, or the marketing of products developed under the arrangement.
Royalty revenue arises from our contractual entitlement to receive a percentage of product sales revenues achieved by counterparties. Royalty revenue is recognized on an accrual basis in accordance with the terms of the agreement when sales can be determined reliably and there is reasonable assurance that the receivables from outstanding royalties will be collected.
License revenue from licenses that we grant to third parties are recognized ratably over the period of the license agreements.
Revenue from R&D services is recognized over the duration of the service period.
Research and Development
R&D expenses represent costs incurred for the development of various products using licensed gene editing technology. R&D expenses consist primarily of salaries and related costs of our scientists, in-licensing of technology, consumables, property and equipment depreciation, and fees paid to third-party consultants. All R&D costs are expensed as incurred.
In the normal course of business, we enter into R&D arrangements with third parties where the arrangements are contractual agreements whereby we perform R&D of certain gene traits for the outside party. We have entered into various multiyear arrangements where we perform the R&D of the gene technology and the third parties generally have primary responsibility for any commercialization of the technology. These arrangements are performed with no guarantee of either technological or commercial success.
We in-license certain technology from third-parties that is a component of ongoing research and product development. We expense up-front license fees upon contracting due to the uncertainty of future commercial value, as well as expensing any ongoing annual fees when incurred.
Forward Purchase Contracts and Derivatives
We enter into supply agreements for grain and seed production with settlement values based on commodity market futures pricing. We account for these derivative financial instruments utilizing the authoritative guidance in ASC 815, Derivatives and Hedging. We recognize the realized gains and losses from derivative contracts and record them as a component of R&D expenses as a result of breeding contract activity. We also recognize the unrealized derivative asset and unrealized derivative liability in other current assets and other current liabilities, respectively.
59
Table of Contents
Stock-Based Compensation
Calyxt, Inc. Equity Incentive Plans
We adopted the Calyxt, Inc. Equity Incentive Plan, or the Existing Plan, which allows for the grant of stock options to attract and retain highly qualified employees. We also adopted in June 2017 an omnibus incentive plan, or the Omnibus Plan, under which we have granted stock options with an exercise price equal to the estimated fair value of the stock at the grant date and restricted stock unit awards. See “Executive Compensation—Equity-Based Awards—Calyxt, Inc. Equity Incentive Plan” and “—2017 Omnibus Incentive Plan” for further information.
The awards granted under the Existing Plan and the Omnibus Plan are only exercisable upon a triggering event or initial public offering as defined by the relevant plan. All stock awards from 2011 to 2016 are accounted for as liability awards and are remeasured each reporting period. Because there were no triggering events that occurred during the three months ended March 31, 2017 and the years ended December 31, 2016 and 2015, no compensation expense was recognized in these respective periods. At March 31, 2017, we had 2,022,475 stock options outstanding, of which 494,410 were fully vested, resulting in total unrecognized stock-based compensation expense estimated to be $4.54 million as of that date.
At June 30, 2017, we had 1,909,775 shares of common stock issuable upon the exercise of outstanding stock options under the Existing Plan, 2,119,699 shares of common stock issuable upon the exercise of stock options under the Omnibus Plan and restricted stock units with respect to 1,452,333 shares of common stock outstanding under the Omnibus Plan. Of that total, 1,198,467 stock options and restricted stock units were fully vested at June 30, 2017 or will vest as a result of this offering. The stock-based compensation expense related to these awards will be recorded upon the consummation of this offering and is estimated to be approximately $16.4 million, based on the assumed initial public offering price of $16.50 per share, the midpoint of the range set forth on the cover of this prospectus. Each $1.00 increase or decrease in the assumed initial public offering price of $16.50 per share would increase or decrease this additional stock compensation expense by approximately $1.2 million.
The fair value of each stock option is estimated using the Black-Scholes option pricing model at each measurement date. The fair value of stock options under the Black-Scholes model requires management to make assumptions regarding projected employee stock option exercise behaviors, risk-free interest rates, volatility of the stock price, and expected dividends. The awards currently outstanding were granted with vesting terms between two and four years. Certain awards contain a 25% acceleration vesting clause upon a triggering event or Initial Public Offering as defined in the Existing Plan.
We have not historically paid cash dividends to our stockholders, and we currently do not anticipate paying any cash dividends in the foreseeable future. As a result we assumed a dividend yield of 0%. The risk free interest rate is based upon the rates of U.S. Treasury bills with a term that approximates the expected life of the option. We use the simplified method to reasonably estimate the expected life of its option awards. Expected volatility is based upon the volatility of comparable public companies.
Parent Awards
Cellectis granted stock options to our employees. Compensation costs related to the grant of the Cellectis awards to our employees have been recognized in our statements of operations with a corresponding credit to stockholder’s equity, representing Cellectis’s capital contribution. The fair value of each stock option is estimated at the grant date using the Black-Scholes option pricing model. The fair value of stock options under the Black-Scholes model requires management to make assumptions regarding projected employee stock option exercise behaviors, risk-free interest rates, volatility of the stock price, and expected dividends.
Cellectis granted certain of our consultants non-employee warrants to purchase Cellectis stock in exchange for services provided. We recorded the fair value of the warrants as a dividend paid to Cellectis in exchange for the warrants issued to them.
We recognized share-based compensation expense related to Cellectis’s grants of stock options and warrants to our employees and consultants of $134,000, $948,000 and $692,000 for the three months ended March 31, 2017 and for the years ended December 31, 2016 and 2015, respectively.
60
Table of Contents
Recent Accounting Pronouncements
In May 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers,” which creates Accounting Standards Codification (“ASC”) 606 “Revenue from Contracts with Customers” and supersedes the revenue recognition requirements in ASC 605 “Revenue Recognition.” The guidance in ASU 2014-09 and subsequently issued amendments ASU 2016-08, “Revenue from Contracts with Customers: Principal versus Agent Considerations (Reporting Revenue Gross versus Net),” ASU 2016-10, “Revenue from Contracts with Customers: Identifying Performance Obligations and Licensing,” and ASU 2016-12, “Revenue from Contracts with Customers: Narrow-Scope Improvements and Practical Expedients” outlines a comprehensive model for all entities to use in accounting for revenue arising from contracts with customers as well as required disclosures. Entities have the option of using either a full retrospective or modified approach to adopt the new guidance. For public entities, certain not-for-profit entities, and certain employee benefit plans, the new revenue standard is effective for annual periods beginning after December 15, 2017, including interim periods within that reporting period. For all other entities, the new revenue standard is effective for annual periods beginning after December 15, 2018, and interim periods within annual periods beginning after December 15, 2019. Early adoption is permitted. We are evaluating the impact of adopting this pronouncement.
In August 2014, the FASB issued ASU 2014-15, “Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern.” The guidance in ASU 2014-15 sets forth management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern as well as required disclosures. ASU 2014-15 indicates that, when preparing financial statements for interim and annual periods, management should evaluate whether conditions or events, in the aggregate, raise substantial doubt about the entity’s ability to continue as a going concern one year from the date the financial statements are issued or are available to be issued. This evaluation should include consideration of conditions and events that are either known or are reasonably knowable at the date the financial statements are issued or are available to be issued, as well as, whether it is probable that management’s plans to address the substantial doubt will be implemented and, if so, whether it is probable that the plans will alleviate the substantial doubt. ASU 2014-15 is effective for annual periods ending after December 15, 2016 for both public and nonpublic entities, and interim periods and annual periods thereafter. We adopted this guidance in the current fiscal year; accordingly, this is disclosed in Note 1 to the financial statements included elsewhere in this prospectus.
In November 2015, the FASB issued ASU 2015-17, “Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes.” The amendment simplifies the presentation of deferred income taxes. Instead of separating deferred income tax liabilities and assets into current and noncurrent amounts in a classified statement of financial position, the amendments in this update require that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. For public entities, ASU 2015-17 is effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods. For nonpublic entities, ASU 2015-17 is effective for annual periods beginning after December 15, 2017, and interim periods within annual periods beginning after December 15, 2018. Early adoption is permitted. We do not expect the adoption of this standard to have a material impact on our financial statements.
In February 2016, the FASB issued ASU 2016-02, “Leases (Topic 842).” The guidance requires that lessees will be required to recognize assets and liabilities on the balance sheet for the rights and obligations created by all leases with terms of more than 12 months. The amendment also will require disclosures designed to give financial statement users information on the amount, timing, and uncertainty of cash flows arising from leases. These disclosures include qualitative and quantitative information. For public entities, not-for-profit entities, or employee benefit plans, ASU 2016-02 is effective for annual periods beginning after December 15, 2018, and interim periods within those annual periods. For all other entities, ASU 2016-02 is effective for annual periods beginning after December 15, 2019, and interim periods within annual periods beginning after December 15, 2020. Entities are required to use a modified retrospective approach for leases that exist or are entered into after the beginning of the earliest comparative period in the financial statements. Early adoption is permitted. We are evaluating the impact of adopting this pronouncement.
61
Table of Contents
In March 2016, the FASB issued ASU No. 2016-09, “Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting.” This ASU eliminates the APIC pool concept and requires that excess tax benefits and tax deficiencies be recorded in the statement of operations when awards are settled. The ASU also addresses simplifications related to statement of cash flows classification, accounting for forfeitures, and minimum statutory tax withholding requirements. For public entities, ASU 2016-09 is effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods. For all other entities, ASU 2016-09 is effective for annual periods beginning after December 15, 2017, and interim periods within annual periods beginning after December 15, 2018. Early adoption is permitted. We are evaluating the impact of adopting this pronouncement.
Results of Operations
Three Months Ended March 31, 2017 Compared to Three Months Ended March 31, 2016 (unaudited)
The following table summarizes key components of our results of operations for the periods indicated:
| Three Months Ended March 31, | % change | |||||||||||
| 2017 | 2016 | March 31, 2017 vs 2016 |
||||||||||
| ($ in thousands) | ||||||||||||
| Revenue |
55 | 106 | (48 | )% | ||||||||
| Operating expenses: |
||||||||||||
| Cost of revenue |
— | 100 | (100 | )% | ||||||||
| Research and development |
1,266 | 1,370 | (8 | )% | ||||||||
| Sales, general, and administrative |
1,578 | 1,197 | 32 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
2,844 | 2,667 | 7 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(2,789 | ) | (2,561 | ) | 9 | % | ||||||
| Interest expense |
(14 | ) | (4 | ) | 250 | % | ||||||
| Foreign currency transaction (losses) |
(29 | ) | (4 | ) | 625 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
(2,832 | ) | (2,569 | ) | 10 | % | ||||||
| Income tax expense |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(2,832 | ) | (2,569 | ) | 10 | % | ||||||
|
|
|
|
|
|
|
|||||||
Revenue
Revenue decreased $51,000, or (48)%, from $106,000 for the three months ended March 31, 2016 to $55,000 for the three months ended March 31, 2017. The decrease was primarily attributable to a strategic decision to focus on in-house development of product candidates and to reduce the amount of R&D we were performing for third parties.
Cost of Revenue
Cost of revenue decreased $100,000, or (100)%, from $100,000 for the three months ended March 31, 2016 to $0 for the three months ended March 31, 2017. The decrease was a result of not having contractual obligations to fulfill in 2017 for our R&D contracts.
Research and development expenses
R&D expenses decreased $104,000, or (8)%, from $1.37 million for the three months ended March 31, 2016 to $1.27 million for the three months ended March 31, 2017. The decrease was primarily attributable to reduced license expenses partially offset by an increase in sub-contracted R&D such as third-party germplasm breeding, third-party germplasm trials and meal and oil product testing.
62
Table of Contents
Selling, general, and administrative expenses
Selling, general, and administrative expenses increased $381,000, or 32%, from $1.20 million for the three months ended March 31, 2016 to $1.58 million for the three months ended March 31, 2017. The increase was primarily attributable to increased personnel expenses, depreciation, facilities costs and recruiting costs and partially offset by a reduction in legal and professional fees.
Interest expense
Interest expense increased $10,000, or 250%, from $4,000 for the three months ended March 31, 2016 to $14,000 for the three months ended March 31, 2017. The increase was primarily attributable to an increase in our accounts payable balance owed to Cellectis.
Foreign currency transaction loss
Foreign currency transaction loss increased $25,000, or 625%, from $4,000 for the three months ended March 31, 2016 to $29,000 for the three months ended March 31, 2017. The increase was primarily attributable to changes in accounts payable balances and foreign exchange rates.
Year Ended December 31, 2016 Compared to Year Ended December 31, 2015
The following table summarizes key components of our results of operations for the periods indicated:
| Year Ended December 31, | % change | |||||||||||
| 2016 | 2015 | 2016 vs 2015 | ||||||||||
| ($ in thousands) | ||||||||||||
| Revenue |
399 | 1,272 | (68.6 | )% | ||||||||
| Operating expenses: |
||||||||||||
| Cost of revenue |
200 | 751 | (73.4 | )% | ||||||||
| Research and development |
5,638 | 2,766 | 103.8 | % | ||||||||
| Sales, general, and administrative |
6,670 | 3,569 | 86.9 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
12,508 | 7,086 | 76.5 | % | ||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(12,109 | ) | (5,814 | ) | 108.3 | % | ||||||
| Interest expense |
(5 | ) | (261 | ) | (98.1 | )% | ||||||
| Foreign currency transaction gains |
28 | 186 | (84.9 | )% | ||||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
(12,086 | ) | (5,889 | ) | 105.2 | % | ||||||
| Income tax expense |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(12,086 | ) | (5,889 | ) | 105.2 | % | ||||||
|
|
|
|
|
|
|
|||||||
Revenue
Revenue decreased $873,000, or (68.6)%, from $1.3 million for the year ended December 31, 2015 to $0.4 million for the year ended December 31, 2016. The decrease was primarily attributable to a strategic decision to focus on in-house development of product candidates and to reduce the amount of R&D we were performing for third parties.
Cost of Revenue
Cost of revenue decreased $551,000, or (73.4)%, from $751,000 for the year ended December 31, 2015 to $200,000 for the year ended December 31, 2016. The decrease was a result of lower R&D expense associated with fulfilling the contractual obligations of our R&D contracts.
63
Table of Contents
Research and development expenses
R&D expenses increased by $2.9 million, or 103.8%, from $2.8 million for the year ended December 31, 2015 to $5.6 million for the year ended December 31, 2016. The increase was primarily attributable to a $1.9 million increase in purchased and external expenses due to increased costs for outsourcing of breeding and product development. We also experienced a $1.0 million increase in personnel expense due to an increase in headcount to support product development activities.
Selling, general, and administrative expenses
Selling, general, and administrative expenses increased $3.1 million, or 86.9%, from $3.6 million for the year ended December 31, 2015 to $6.7 million for the year ended December 31, 2016, primarily due to a $2.7 million increase in purchase and external expenses resulting from increased support from Cellectis and increased costs of professional services firms, in each case to support our growth, and an increase of $400,000 in personnel expenses as we expanded our executive management team.
Interest expense
Interest expense decreased $256,000, or (98.1)%, from $261,000 for the year ended December 31, 2015 to $5,000 for the year ended December 31, 2016, due to a reduction in our accounts payable balance owed to Cellectis throughout the year.
Foreign currency transaction gains
Foreign currency transaction gains decreased $158,000, or (84.9)%, from $186,000 for the year ended December 31, 2015 to $28,000 for the year ended December 31, 2016, due to the reduction in our accounts payable balance owed to Cellectis throughout the year.
Liquidity and Capital Resources
As of March 31, 2017, we had cash and cash equivalents of $3.0 million.
Sources of Liquidity
We have funded our operations primarily through cash infusions provided by Cellectis.
During the three months ended March 31, 2017 and the years ended December 31, 2016 and 2015, we incurred losses from operations of $2.8 million, $12.1 million and $5.9 million, respectively, and net cash used in operating activities of $1.7 million, $9.2 million and $6.7 million, respectively. At March 31, 2017, we had an accumulated deficit of $31.4 million and expect to incur losses for the foreseeable future. Although we believe that we will be able to successfully fund our operations with funding from Cellectis, the proceeds of this offering and our cash and cash equivalents at March 31, 2017, there can be no assurance we will be able to do so or that we will ever operate profitably. Cellectis has guaranteed funding for our operations through August 2018, which guarantee will not be released, modified or otherwise affected by the timing of, or amount raised in, this offering. Our ability to continue as a going concern prior to this offering is subject to this guaranteed funding from Cellectis and our ability to develop and successfully commercialize our product candidates.
64
Table of Contents
Historical Changes in Cash Flows
Three Months Ended March 31, 2017 Compared to Three Months Ended March 31, 2016 (unaudited)
The table below summarizes our sources and uses of cash for the three months ended March 31, 2017 and 2016:
| Three Months Ended March 31, | ||||||||
| 2017 | 2016 | |||||||
| (in thousands) | ||||||||
| Net cash used in operating activities |
$ | (1,707 | ) | $ | (1,627 | ) | ||
| Net cash used in investing activities |
(312 | ) | (6,780 | ) | ||||
| Net cash provided by financing activities |
— | — | ||||||
|
|
|
|
|
|||||
| Total |
$ | (2,019 | ) | $ | (8,407 | ) | ||
|
|
|
|
|
|||||
Net cash used in operating activities was $1.7 million and $1.6 million in the three months ended March 31, 2017 and 2016, respectively. The net cash used in each of these periods primarily reflects the net loss for those periods, offset in part by depreciation, stock-based compensation and the effects of changes in operating assets and liabilities.
Net cash used in investing activities was $0.3 million and $6.8 million in the three months ended March 31, 2017 and 2016, respectively. The majority of cash used in investing activities in the three months ended March 31, 2017 was related to site improvements and architect fees for the design of our headquarters and greenhouse facility in Roseville, MN, and equipment purchases. The majority of the cash used in investing in the three months ended March 31, 2016 was related to a land purchase in Roseville, MN for our future greenhouse and headquarters.
Year Ended December 31, 2016 Compared to Year Ended December 31, 2015
The table below summarizes our sources and uses of cash for the years ended December 31, 2016 and 2015:
| Year Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| ($ in thousands) | ||||||||
| Net cash used in operating activities |
$ | (9,237 | ) | $ | (6,691 | ) | ||
| Net cash used in investing activities |
(10,424 | ) | (665 | ) | ||||
| Net cash provided by financing activities |
— | 31,740 | ||||||
|
|
|
|
|
|||||
| Total |
$ | (19,661 | ) | $ | 24,384 | |||
|
|
|
|
|
|||||
Net cash used in operating activities was $9.2 million and $6.7 million in the years ended December 31, 2016 and 2015, respectively. The net cash used in each of these periods primarily reflects the net loss for those periods, offset in part by depreciation, stock-based compensation and the effects of changes in operating assets and liabilities.
Net cash used in investing activities was $10.4 million and $665,000 in the years ended December 31, 2016 and 2015, respectively. The majority of cash used in investing activities in 2016 was related to the purchase of the land for our headquarters and the construction of our R&D greenhouses. The majority of the cash used in investing in 2015 was for office furniture and equipment associated with our current R&D laboratories and office space.
Net cash provided by financing activities was zero and $31.7 million in the years ended December 31, 2016 and 2015, respectively. Net cash provided by financing activities in 2015 was attributable to a capital contribution from Cellectis.
65
Table of Contents
Contractual Obligations, Commitments and Contingencies
The following table discloses aggregate information about material contractual obligations and periods in which payments were due as of December 31, 2016. Future events could cause actual payments to differ from these estimates.
| As of December 31, 2016 |
Total | Less than 1 year |
1-3 years | More than 3 years |
||||||||||||
| (in thousands) | ||||||||||||||||
| Operating lease agreements |
$ | 121 | 121 | — | — | |||||||||||
| Capital lease obligations |
— | — | — | — | ||||||||||||
| Forward purchase contracts |
$ | 383 | 383 | — | — | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total contractual obligations |
$ | 504 | 504 | — | — | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The commitment amounts in the table above are associated with contracts that are enforceable and legally binding and that specify all significant terms, including fixed or minimum services to be used, fixed, minimum or variable price provisions, and the approximate timing of the actions under the contracts. Purchase contracts consist of purchase commitments with growers to purchase seed and grain at a future date.
The table does not include obligations under agreements that we can cancel without a significant penalty. We have R&D agreements whereby we are obligated to pay royalties and other payments based on future events that are uncertain and therefore, they are not included in the table above.
The above table also does not include the amount of funds necessary to complete our planned facility expansion, described under “Business—Facilities,” as we intend to finance the building for the planned facility expansion in whole or in part through a sale leaseback transaction. On June 20, 2017, we signed an agreement with a third party buyer for the sale of the land and warehouse facility. The completion of the sale is conditioned on us and the buyer entering into a new facility construction agreement and a lease in respect of the property in the forms contemplated by the sale agreement. We expect to close on the proposed transaction and construction to begin on the new facilities in the second half of 2017.
Operating capital requirements
To date, we have not generated any revenues from product sales. We do not know when, or if, we will generate any revenue from product sales. We do not expect to generate significant revenue from product sales unless and until we obtain regulatory approval of and commercialize one of our current or future product candidates. We anticipate that we will continue to generate losses for the foreseeable future, and we expect the losses to increase as we continue the development of our product candidates and begin to commercialize our product candidates that complete the development process. We are subject to all risks incident in the development of new agricultural products, and we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. We also anticipate substantial expenses related to audit, legal, regulatory and tax-related services associated with our public company obligations in the United States and our compliance with applicable U.S. exchange listing and SEC requirements. We anticipate that we will need additional funding in connection with our continuing operations, including for the further development of our existing product candidates and to pursue other development activities related to additional product candidates.
We believe our cash and cash equivalents on hand and our cash flow from operations, after giving effect to our receipt of the net proceeds of this offering, will be sufficient to fund our operations through 2020. However, we may require additional capital for the further development of our existing product candidates and may also need to raise additional funds sooner to pursue other development activities related to additional product candidates.
66
Table of Contents
Until we can generate a sufficient amount of revenue from our products, if ever, we expect to finance a portion of future cash needs through public or private equity or debt financings. Additional capital may not be available on reasonable terms, if at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our product candidates. If we raise additional funds through the issuance of additional debt or equity securities, it could result in dilution to our existing stockholders, increased fixed payment obligations and these securities may have rights senior to those of our ordinary shares. If we incur indebtedness, we could become subject to covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Any of these events could significantly harm our business, financial condition and prospects.
Our assessment of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors. We have based this estimate on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect. Our future funding requirements, both near and long-term, will depend on many factors, including, but not limited to:
| • | the initiation, progress, timing, costs and results of field trials for our product candidates; |
| • | the outcome, timing and cost of regulatory approvals by U.S. and non-U.S. regulatory authorities, including the possibility that regulatory authorities will require that we perform studies; |
| • | the ability of our product candidates to progress through late stage development successfully, including through field trials; |
| • | the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
| • | our need to expand our research and development activities; |
| • | our need and ability to hire additional personnel; |
| • | our need to implement additional infrastructure and internal systems; |
| • | the effect of competing technological and market developments; and |
| • | the cost of establishing sales, marketing and distribution capabilities for any products we commercialize. |
If we cannot expand our operations or otherwise capitalize on our business opportunities because we lack sufficient capital, our business, financial condition and results of operations could be materially adversely affected.
Off Balance Sheet Obligations
We enter into seed and grain production agreements with settlement value based on commodity market future pricing. Otherwise, we do not have any off-balance sheet arrangements as defined under SEC rules.
Internal Control over Financial Reporting
In preparing our financial statements for the year ended December 31, 2016, a material weakness in our internal control over financial reporting was identified, as defined by the SEC guidelines for public companies. The material weakness relates to our lack of a control in place to review forward purchase derivative contracts entered into by us. The derivative contracts were to produce high oleic soybean seed and grain and the purchase price was indexed to the soybean commodity price.
67
Table of Contents
We implemented improvements and remedial measures in response to these assessments, including:
| • | standardizing our production contracts, |
| • | developing a database to track production contracts, and |
| • | implementing written policies for the accounting treatment of the production contracts. |
Although our management is implementing these measures, they may not fully address this material weakness in our internal control over financial reporting, and we, therefore, may not be able to conclude that it has been fully remedied.
Quantitative and Qualitative Disclosure About Market Risk
We are exposed to a limited amount of foreign currency exchange risk, principally in euros, primarily as a result of certain services and infrastructure costs charged to us by Cellectis.
JOBS Act
We are an emerging growth company under the JOBS Act. The JOBS Act provides that an emerging growth company can delay adopting new or revised accounting standards until such time as those standards apply to private companies.
Subject to certain conditions set forth in the JOBS Act, if, as an “emerging growth company”, we choose to rely on such exemptions we may not be required to, among other things, (i) provide an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404, (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) comply with any requirement that may be adopted by the PCAOB regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis), and (iv) disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the CEO’s compensation to median employee compensation. These exemptions will apply for a period of five years following the completion of our initial public offering or until we are no longer an “emerging growth company,” whichever is earlier.
68
Table of Contents
Our mission is to make the food you love a healthier choice.
Overview
We are a consumer-centric, food- and agriculture-focused company. By combining our leading gene-editing technology and technical expertise with our innovative commercial strategy, we are pioneering a paradigm shift to deliver healthier food ingredients for consumers and agriculturally advantageous traits for farmers. We are developing and creating specialty food ingredients, such as healthier oils and high fiber wheat, and food crops with desirable traits, such as herbicide tolerance. While the traits that enable these characteristics may occur naturally and randomly through evolution—or under a controlled environment through traditional agricultural technologies—those processes are imprecise and take many years, if not decades. Our technology enables us to precisely and specifically edit a plant genome to elicit the desired traits and characteristics, resulting in a final product that has no foreign DNA. We believe the precision, specificity, cost effectiveness and development speed of our gene-editing technologies will enable us to provide meaningful disruption to the food and agriculture industries.
Our first product candidate, which we expect to be commercialized by the end of 2018, is a high oleic soybean designed to produce a healthier oil that has increased heat stability with zero trans fats. Among our other product candidates are high fiber wheat and herbicide tolerant wheat. We are developing a high fiber wheat to create flour with up to three times more dietary fiber than standard white flour while maintaining the same flavor and convenience of use. Our high fiber wheat may provide benefits associated with a high fiber diet, including reduced risk of coronary heart disease. Our herbicide tolerant wheat is designed to provide farmers with better weed control options to increase yields. We believe each of these three product candidates addresses a multi-billion dollar market opportunity.
Food-related issues including obesity and diabetes are some of the most prevalent health issues today and continue to grow rapidly. In the last 30 years, obesity rates have doubled in adults, tripled in children and quadrupled in adolescents in the United States, according to data from the Centers for Disease Control and Prevention and other industry sources. The medical care costs—including doctors’ appointments, hospital stays, prescription drugs and home health care—for the obese population in the United States was approximately $315 billion in 2010. This figure represents approximately 20% of all U.S. healthcare costs and a 48% inflation-adjusted increase from 2005. According to data provided by the National Center for Health Statistics, approximately 43% of deaths in 2012 were caused by diseases linked to poor diet, including heart diseases and diabetes. Food allergies, which can be life threatening, have increased by approximately 50% from 1997 to 2011 in children, and the economic costs of children’s food allergies are approximately $25 billion per year.
As awareness of these diet-related health issues grows, consumers have emphasized a healthier lifestyle and a desire for nutritionally rich foods that are more nutritious, better tasting, less processed and more convenient. This trend is leading to an increase in the demand for higher valued, premium segments of the food industry, such as higher fiber, reduced gluten and reduced fat products. As a result of these trends, food companies are looking for specialty ingredients and solutions that can help them satisfy their customers’ evolving needs and drive growth in market share and new value added products.
While food companies are focused on these trends, we believe the legacy agriculture companies have overlooked society’s food-related issues and are not properly equipped with a business model to address health-driven consumer food trends. These legacy agriculture companies have historically focused on increasing yields and volume—to address population growth—while increasing profit margins and market share by reducing input costs. They have been burdened by high research and development costs and a high degree of commoditization in their deep, farmer-focused supply chains. Industry sources indicate it can take approximately 13 years or more and require an investment of over $130 million to generate a desired trait through traditional trait-development
69
Table of Contents
methods. In contrast, the food industry has seen new entrants growing aggressively and taking market share through premium segment product offerings from existing players, who are facing stagnating growth and divestitures in their core businesses. These factors have resulted in a disconnect between the legacy agriculture industry players’ current products and the consumer’s food-related health demands.
We believe that our proprietary gene-editing technologies and innovative commercial strategy will allow us to bridge the divide between evolving consumer preferences and the historical approach by the large legacy companies in the agriculture supply chain.
Using our proprietary technologies and expertise, we edit the genome of food crops by using our “molecular scissors” to precisely cut DNA in a single plant cell, use the plant’s natural repair machinery to make our desired edit and finally regenerate the single cell into a full plant. We believe we are able to develop targeted traits—some of which would be nearly impossible to develop using traditional trait-development methods—quicker, more efficiently and more cost effectively than traditional trait-development methods. Our technology positions us to assess the probability of success early on in the research and development process, potentially eliminating expensive late stage failures and allowing for a larger breadth of products to be developed. We have a strong track record with respect to our technologies and expertise as we have successfully edited more than 20 unique genes in 6 plant species since our inception in 2010.
Our commercial strategy is centered on two core elements: developing healthier specialty food ingredients to enable the food industry to address evolving consumer trends and developing agriculturally advantageous traits, such as herbicide tolerance, for farmers. This will involve developing and leveraging our supply chain to effectively bring our consumer- and farmer-centric products to the marketplace. For our consumer-centric products, we intend to repurpose and leverage existing supply chain capacity by contracting, tolling or partnering with players in the existing supply chain, such as seed production companies, farmers, crushers, refiners or millers, which we expect will allow us to apply our resources to maximizing innovation and product development while minimizing our capital expenditures and overhead. For our farmer-centric products, we intend to broadly out-license our products to the seed industry.
70
Table of Contents
We believe that we are able to identify a consumer or farmer need and develop a product from “concept to fork” or “concept to field” in approximately three to six years by utilizing our proprietary technologies and expertise and leveraging our innovative supply chain. We have an extensive product pipeline, as set forth in the table below, that is intended to address the potential market opportunities we have identified to date.

We categorize our stages of pre-commercial development from Phase I to Phase III. Prior to entering Phase I, in Discovery, we identify genes of interest. In Phase I, we edit the identified genes of interest, target edits we desire to make, and produce an initial seed that contains the desired edit. Phase II is trait validation, where we perform small-scale and large-scale tests to confirm phenotype and ingredient functionality. In this phase we also perform replicated, multi-location field testing, after confirming that the product is not a regulated article by the USDA. In Phase III, we develop the first commercial-scale pilot production, begin to build out the supply chain and inventory and perform customer testing prior to commercialization.
While we intend to initially deploy our commercial strategy in North America with respect to our current product candidates, we also see many avenues of potential future growth beyond North America. In particular, over time we may explore opportunities to apply our commercial strategy elsewhere around the world and leverage our North American products and footprint to target geographies where there are unmet consumer or farmer needs. We also intend to explore the ability to add value through our existing product candidates once they are commercialized by combining traits in the same crop, which may allow us to create products with additional benefits without adding significant cost. In the near term, we are planning an expansion of our campus to enhance our gene-editing automation processes and develop a high-throughput discovery platform to identify new growth opportunities. We believe this high-throughput platform will allow us to discover more products, make more complex edits and enable us to drive product innovation at a significantly faster rate. We believe our expanded campus will be the only “concept to fork” facility of its kind, containing gene-editing labs,
71
Table of Contents
greenhouses, fields and a commercial kitchen to develop, test and showcase products. We believe all of these steps will enable us to remain at the forefront of food and agriculture innovation.
As of May 31, 2017, we employed 27 employees, of whom approximately 74% are involved in research and development and 8 hold a Ph.D. degree. Our multidisciplinary team includes experts in biology, chemistry, plant genetics, agronomics and other related fields. Several members of our executive team previously worked at industry-leading technology and agricultural companies, such as Monsanto Company, Syngenta AG and Cargill, Inc. As pioneers in the field of gene editing for plant sciences, members of our management team have invented TALEN, one of the premier gene-editing tools.
Calyxt was established in 2010 as a wholly owned subsidiary of Cellectis, a leading gene-editing company with a focus on the development of immuno-oncology therapeutics.
Our Competitive Strengths
We believe that we are strategically well-positioned to develop high-value and innovative products. Our competitive strengths include:
| • | Proprietary technologies creating a powerful platform to design and develop new products. Since our founding, we have been at the forefront of the research, development and application of plant-based gene-editing technologies. Our capabilities enable us to precisely edit specific genes from a target food crop to improve the nutritional composition or provide agricultural benefits to farmers. Three examples of our technological innovation include: |
| • | High Oleic Soybean: We deactivated key genes associated with fatty acid biosynthesis to achieve a healthier soybean oil. |
| • | High Fiber Wheat: We simultaneously deactivated all six copies of a gene within a single wheat plant with the purpose of increasing fiber content. |
| • | Herbicide Tolerance: We believe we can develop crop varieties that will be tolerant to certain herbicides by identifying and making a subtle base substitution that we believe will be sufficient to confer herbicide tolerance. We expect to be able to replicate this process in various crops. |
| • | Innovative portfolio of product candidates with an accelerated path to market. We are currently developing a diversified portfolio spanning across five core crops—soybean, wheat, canola, potato and alfalfa—and a multitude of product candidates. These include innovative consumer-centric product candidates like our high-fiber wheat that is designed to produce flour with up to three times the fiber content of standard white flour, as well as innovative, farmer-centric solutions like herbicide tolerant wheat and products with valuable supply chain benefits like cold storable potatoes that are designed to store longer and produce much less acrylamide in the frying process, a human health concern that has been linked to cancer. We believe our portfolio of product candidates, coupled with our ability to quickly develop future product candidates, affords us the opportunity to disrupt the food industry. |
| • | Significant barriers to entry through our first-mover advantage and strong intellectual property. We command a first-mover advantage in the editing of genes in plants. As a pioneer in gene-editing technologies, we are building on more than two decades of hands-on experience and process optimization which we believe cannot be easily replicated by competitors. Our proprietary technologies and product candidates benefit from the licensing of a portfolio of 81 issued patents and 170 pending patent applications. |
| • | Faster, cheaper and innovative product development process focused on end user needs. Genetic modification has traditionally taken an average of 13 years and over $130 million to develop a commercially viable product. By contrast, a key advantage of our gene-editing technology platform is that we believe we can develop products from concept to commercialization in three to six years and at a fraction of the cost. For example, we created our high oleic soybean product candidate by generating |
72
Table of Contents
| fewer than 20 independent plants that were edited with TALEN. This contrasts with traditional genetic modification methods which we believe require thousands of plants to achieve the same result. We developed our high oleic soybean from concept to field in under four years and expect to commercialize this product by the end of 2018. We believe we will continue to be able to react quickly to consumer and farmer needs that we identify. |
| • | Supply chain flexibility enabling us to capture significant downstream value. We plan to develop consumer traits and leverage existing supply chain capacity and our existing relationships to provide differentiated specialty ingredients to food companies. By doing so, we believe that we will be able to capture significant value as our innovative products move from “field to fork.” Our supply chain is flexible, enabling us to layer on new products to our existing ones and capture additional value. By stacking additional characteristics on the same seeds, we believe we can create additional value without necessarily increasing acreage, volumes of grain produced or the amount of oil commercialized, but rather by increasing the value of our products on a per unit basis. For example, we believe this value creation could be implemented in our high oleic soybean product candidate if we were to stack a trait that improves the protein composition of the meal, a trait that further improves the soybean oil fatty acid profile or a farmer-focused trait such as herbicide tolerance. |
| • | A world-class management team with deep industry expertise. Our executive team has more than 120 years of collective experience in the agriculture and gene-editing fields. This includes over 95 years of collective experience in agricultural supply chain, product development and commercial operations, during which several members of our executive team have managed billions of dollars of revenue and cost at large multinational corporations. Several members of our executive team previously worked at well-known technology and agri-business companies, such as Monsanto, Syngenta, Stine Seed Company and Cargill. As pioneers in plant-based gene editing, members of our management team invented TALEN, one of the premier gene-editing tools. Dr. Daniel Voytas, our Chief Science Officer, is a Professor of Genetics, Cell Biology and Development at the University of Minnesota and Director of the Beckman Center for Genome Engineering. He is best known for his pioneering work to develop methods for precisely editing DNA sequences in living plant cells. |
Our Growth Strategy
We believe that there are significant opportunities to grow our business both domestically and internationally by executing on the following key elements of our strategy:
| • | Commercialize our current product candidates in North America. Our near-term focus is to launch our product candidates in North America where we believe the markets for healthier specialty food ingredients for consumers and unique, plant-trait solutions for farmers are significant and the existing supply chain enables us to accelerate growth. The regulatory environment in North America is well established, which we believe provides a stable, predictable and otherwise attractive backdrop as we focus our near term efforts in these markets. |
| • | Identify new opportunities through our consumer- and farmer-centric approaches. We intend to continue to elicit desired traits and to include additional crops in our product pipeline. We continuously evaluate the evolving needs of the consumer and the farmer and seek to apply our technologies and expertise to provide better solutions to meet their demands. We also expect to be able create additional opportunities from our existing product candidates once they are commercialized through combining traits, which may allow us to create products with additional benefits without adding significant cost. |
| • | Accelerate our R&D productivity with enhanced automation and high throughput capabilities. We consistently strive to optimize our product development processes. Through our planned facility expansion, we are combining gene-editing automation with food science capabilities to enable us to rapidly identify new growth opportunities. We believe that this automation and our high-throughput platform will allow for greater standardization in our processes. We expect this standardization to |
73
Table of Contents
| increase our research and development productivity significantly and add a greater number of projects to our product pipeline. |
| • | Expand through R&D agreements and acquisitions. We plan to selectively partner, in-license or acquire key enabling technologies and businesses across the value chain. This may include acquiring complementary technologies and intellectual property or fully developed products. In each case, we plan to look for R&D partners and acquisitions that give us a significant presence in the health focused specialty food ingredient markets where we will be able to accelerate the launch and commercialization of our innovative products. |
| • | Leverage our North American market presence to globalize our products. While we believe the North American market opportunity remains attractive and extensive, in the future we plan to explore the possibility of expanding our business globally. This may involve exporting our products to international markets or establishing new supply chains in other attractive markets. |
The Evolution of the Food and Agriculture Industries
The Food Industry is Struggling to Find Solutions to Address Many of Society’s Food-Related Issues
The United States is experiencing a dramatic increase in the prevalence of food-related health issues. In addition to being an underlying cause of some of the most prevalent diseases in western society, an unhealthy diet is a contributor to three of the ten leading causes of death: heart disease, diabetes and stroke. As shown in the chart below, according to data provided by the National Center for Health Statistics, approximately 43% of deaths in 2012 were caused by these diseases. Furthermore, a 2010 study suggested that poor diet and diet-related risk factors, such as high blood pressure and high cholesterol, were linked to 63% of deaths.
As a result of the increase in food-related health issues, consumers have developed an increasingly heightened awareness of the role that dietary habits play in long-term wellness. This trend is especially prevalent in wealthier, developed nations where consumers have greater access to information that is helping to shift their consumption habits. According to a 2016 survey of U.S. grocery shoppers, 74% of the surveyed shoppers said that the food they eat at home could be healthier. Consumers have also demonstrated a willingness to spend more than in the past on specialty or high quality foods. These high-end items now account for approximately 25% of the average American’s food budget.
74
Table of Contents
The chart below represents the responses of over 1,000 respondents when asked what packaging health claims they looked for when purchasing a food product:
Regulatory agencies are playing a larger role in monitoring which food ingredients reach consumers. Beginning in 2018, the FDA’s will ban partially hydrogenated oils in foods, the primary artificial source of trans fat in processed foods. A recent study showed that New York State counties that implemented a ban on trans fats experienced an approximately 6% decline in hospitalizations for heart attacks and strokes as compared to New York State counties that did not. Additionally, following the passage of the Healthy, Hunger-Free Kids Act of 2010, the USDA gained significant oversight of the federal school lunch program and holds the authority to set new, healthier standards for food sold in U.S. schools. These healthier food mandates include minimum serving requirements for fiber, fruits and vegetables and maximum allowable content standards for fat, sugar and sodium.
With today’s consumers increasingly conscious and interested in the food they eat, buying habits have been creating dynamic shifts in the grocery aisle. The market has shifted from a focus on diet foods to a focus on “real” food as a way to maintain health. Yet it is sometimes difficult for customers to find healthy alternatives without compromising taste or convenience. Consumers now view food—particularly food with health benefits—as the key to good health. More foods are being launched that go beyond basic nutrition to support health, digestive health, and higher energy levels. Locally sourced foods with a direct-to-consumer model are becoming more attractive, the demand for transparency in food sourcing, production and labeling is gaining traction, and consumers are discovering novel, foreign ingredients. We believe that as consumers continue the shift from a traditional production-driven food culture to a modern demand-driven food culture, they will continue to press companies and retailers for more information and accountability about how ingredients are sourced and processed, how “real” their food products are, and how responsive they are to consumers’ desire for choice and customization.
As consumers seek healthier and more nutritious food options, the health and wellness food sector has benefitted. Healthier food options are capturing an increasing share of the consumer wallet. Although this segment currently represents only 6% of total sales for conventional multi-outlet channels (defined as retail outlets spanning grocery, drug and convenience, such as Target, Kroger, Walgreens, Wegmans and Walmart), natural products drove 45% of the dollar growth for these multi-outlet channels. As conventional multi-outlet channels and food processors try to capitalize on the fact that a significant percentage of North American consumers are willing to pay a premium for healthy and nutritious foods, their ability to quickly adapt to changing consumer demands is hampered by commoditized business models and supply chains.
The impact of changing consumer preferences is increasingly evident as market share and sales shifts towards smaller consumer packaged goods companies. Industry sources estimate that consumer packaged goods
75
Table of Contents
companies with annual revenue greater than $5 billion have lost approximately $18 billion in sales and approximately 3% in market share to smaller companies in the last five years. These sources also note that the sales growth in these larger companies has grown at 1.3% as compared to sales growth of upwards of 4% or 5% for smaller companies. These changes in consumer preferences benefit small and medium-sized companies with above-average growth and slowing the growth of the largest food and beverages companies.
As a result of the consumer’s rising demand for healthier food and the inability of traditional outlets and food processors to satisfy this demand, we believe that an opportunity exists for us to provide our innovative solutions for our customers and food industry.
The Agricultural Industry has Overlooked Society’s Food-Related Issues
The agriculture industry has historically been burdened by high infrastructure costs in a market that has focused on price and market share resulting in commoditization. A highly segmented supply chain has also resulted in the legacy agriculture companies focusing on increasing margins and market share through increased yields and consolidation, and on passing along maximum value to the growers, thereby keeping pace with the growing demand for food globally. Over the past few decades the agriculture industry has seen a consolidation of over 200 seed companies, leaving the industry with only a handful of large, dominant players such as Syngenta AG, Bayer AG, Monsanto Company, BASF SE, Dow Chemical Co. and Dupont Pioneer, which together accounted for approximately 76% of the U.S. seed sales for soybeans and 82% for corn in 2014–2015. In addition, development at these legacy agriculture companies has been significantly limited by time and cost constraints. According to industry estimates, genetic modification, a primary method of these companies to improve crops, requires an average of approximately 13 years and more than $130 million to progress a new crop from the discovery stage through commercialization. These innovations have primarily achieved increases in yields and food production volumes through the creation of herbicide tolerance and insect resistance, using genetically modified traits that in many cases contain bacterial DNA. We believe these industry dynamics explain the inability for the agricultural industry to evolve to a consumer- and farmer-focused approach, and thereby effectively meet their demands as societal trends shift and provide new market opportunities.
Traditional Trait-Development Techniques
In addition to these market dynamics, innovation within the industry has been slow given the limitations of traditional trait-development techniques. While plant breeders have been crossbreeding varieties and selecting advantageous traits for thousands of years, the modern agriculture industry has relied primarily on two methods of crop improvement:
| • | Genetic Modification—this involves the use of genetic technologies to randomly insert foreign genetic material, such as bacterial derived genes, into a plant’s genome for the development of seeds in which the inserted genes express specific traits. Historically, genetic modification techniques have been focused on mitigating negative yield impacts related to biotic causes, such as insect protection and herbicide tolerance traits. |
The development process for genetically modified seeds involves:
| • | identifying genes, many times outside the plant kingdom, that may include desirable traits; |
| • | randomly inserting the extracted genetic material into seeds, and delivering transgenic products that contain foreign DNA; |
| • | spending years testing, growing and confirming these random genetic modifications did not cause unintended consequences; |
| • | working with global regulatory bodies to approve the above; and |
| • | working with an agricultural supply chain to ensure broad adoption of the new trait in varieties across multiple countries, justifying the high development costs this process requires. |
76
Table of Contents
The use of genetically modified crops has continued to face challenges due to various factors, including:
| • | high development costs and a lengthy development process; |
| • | a significant regulatory burden associated with obtaining marketing approval; and |
| • | prevalence of durability issues such as weed resistance to chemistries used in herbicide tolerant crops and the evolution of insect resistance to toxins produced by genetically engineered crops with insecticidal traits. |
| • | Chemical Mutagenesis—this process involves the induction of mutagenesis in plants using agents and chemicals. Chemical mutagenesis creates non-specific mutations throughout the whole plant genome. As a result, mutagenesis techniques have proven to be slow, random and have not yielded disruptive agricultural improvements. The major limitations of mutagenesis include: |
| • | off-target effects that can induce unwanted mutations; |
| • | lengthy development process of up to ten years or longer necessary to identify the desired mutations, remove the unwanted mutations and breed the new mutations into commercial seeds; and |
| • | applicability is limited to certain types of mutations that do not require precision or complexity. |
While these primary methods for addressing the agricultural challenges will continue to be applied, we believe these approaches can no longer effectively meet societal demands for innovative solutions demanded by the consumer and the farmer.
Calyxt’s Solution: Bridging the Divide Between Evolving Consumer Preferences and the Agriculture Industry
We believe that our proprietary technologies and commercial strategy will allow us to bridge the divide between evolving consumer preferences and the historical approach by the legacy companies in the food and agriculture industries.
Our Technologies
Using our proprietary technologies and expertise, we edit the genome of food crops by using our “molecular scissors” to precisely cut DNA in a single plant cell, use the plant’s natural repair machinery to make our desired edit and finally regenerate the single cell into a full plant. We are able to develop targeted traits—some of which would be nearly impossible to develop using traditional trait-development methods—quicker, more efficiently and more cost effectively than would be possible using traditional trait-development methods. Our technology also puts us in a position to assess the probability of success early on in the research and development process, potentially eliminating excess cost associated with traditional trait-development methods and further reducing the risk of our product development process.
We believe we are disrupting the agriculture and food industry by utilizing our proprietary gene-editing expertise to prototype and develop traits at a fraction of the cost while simultaneously reducing the time to market. We believe our trait development process makes it possible to commercialize a product in three to six years, which would make it possible to effectively respond to evolving consumer preferences and farmer needs.
We are poised to become a leader in agriculture innovation through the use of gene editing. Our scientists have developed certain critical elements enabling us to make precise edits to DNA in living plant cells, including the use of reagents referred to as sequence-specific nucleases and the delivery methods of those reagents to plant cells. Our proprietary gene-editing platform relies on our capacity to custom design DNA-sequence specific
77
Table of Contents
cutting enzymes, or nucleases, for any chosen gene we need to edit and our capability to introduce such custom-made nucleases into the living plant cells we want to edit. Our platform relies on precisely chosen protein families that can specifically recognize unique DNA sequences and can be tailored to target such sequences in any chosen gene or genetic region.
Our proprietary technologies and intellectual property portfolio enable us to edit the plant genome by knocking out genes or creating precise gene edits. We take advantage of our knowledge about plant gene function to create novel genetic variation that results in traits of value. In all applications, a feature that distinguishes our products from those created through genetic modification is that our crop varieties lack foreign DNA. As such, for each of the five product candidates we have submitted to date, the USDA has confirmed that the products are not regulated articles, which represents regulatory cost savings for the development of these products.
We believe a vast number of favorable traits can be created by editing the genes of the plants or by inserting genes that occur within the same plant species. To date, we have not targeted the development of any products that would require foreign DNA in the final product, and we prefer to focus on finding natural traits existing within a plant species that can be elicited in our products. Where no naturally occurring trait exists within the plant species, we may not be able to create the desired trait without inserting foreign DNA. We believe there are a sufficient number of naturally occurring plant traits to allow us to continue to grow and expand our product pipeline for the foreseeable future.
Our Commercial Strategy
Our commercial strategy is centered on two core elements:
| • | Developing healthier specialty food ingredients for the food industry to benefit consumers—We intend to disrupt the food industry by continuing to identify market opportunities to help food manufacturers provide consumers with healthier and better tasting foods without sacrificing convenience or quality. Given trends in consumer preferences, we envision selling specialty ingredients such as healthier oils, high fiber flours and ingredients with reduced allergen and carcinogen content to the food industry. |
| • | Developing herbicide tolerance and other agriculturally advantageous traits for farmers—We intend to disrupt the agriculture industry by developing agriculturally advantageous traits that have a broad appeal to farmers and the seed companies that sell to farmers. For example, we are developing a herbicide tolerant wheat product that is designed to be resistant to multiple herbicide chemistries currently in the market. Because these traits have broad applicability, we believe it is important to get these traits into as many seed varieties and acres as possible, similar to what has occurred in other major row crops such as corn, soybean and cotton. Our strategy is to invent and develop these agriculturally advantageous traits for farmers and broadly out-license them to the seed industry, with the potential to collect license fees and royalties in return. |
We believe our two-pronged strategy will disrupt the traditional food and agriculture industries by aligning the needs of the consumer, farmer and the food and agriculture value chains. We intend to repurpose and leverage existing supply chain capacity by contracting, tolling or partnering with players in the existing supply chain, which will allow us to apply our resources to maximizing innovation and product development while minimizing our capital expenditures and overhead. As we continue to streamline our supply chain, we expect to enter into additional contracts and strategic partnerships within the various participants and members of the value chain, in an effort to maintain control of the process and help ensure product quality, manage cyclical demand changes and capture value.
We believe our product candidates will benefit from being grown, processed and stored utilizing the same existing third-party infrastructure and standard industry practices that are currently used in the non-GMO products in the industry. Our intent is to outsource to third parties, such as seed production companies, farmers
78
Table of Contents
under identity preservation contracts and soybean crushers or wheat millers, for the production and processing of our consumer-focused products. Given that our strategy is to outsource these processes, we do not anticipate using proceeds to build or purchase processing or farming assets. Once we establish a supply chain for our first consumer-focused product in soybeans and wheat, we anticipate that future products within the same crop will benefit from utilizing the same supply chain.
Farmers are an essential component of our business model as they produce and prepare crops for processing. Within the traditional agriculture industry supply chain, farmers sell to elevators or processors who operate a high volume/low margin business to store crops and convert them into consumer and industrial products. Our strategy is to partner with farmers to grow premium products and have them allocate some of their land to grow our crops and, in turn, we will then buy back the crops produced by the farmers. We can then contract with processors to create our specialty food ingredients, which we will market directly to food manufacturers. We can also take our product and negotiate prices with food manufacturers and capture the incremental value without substantial capital outlays using these “closed-loop” contracts. Due to our partnership-oriented, vertically- integrated supply chain, we believe we can create and retain more value for ourselves that we can share with our farmers and processers, creating a supply chain that is a favorable for all participants involved.
Soybean crushers that manage existing crushing and refining infrastructure in the United States have small margins and compete largely in commoditized low margin markets. We intend to sign tolling agreements, which are common in our industry, to hire the crusher and refiner to transform our grain into meal for animal feed and oil for human consumption. We believe this will enable us to focus our investment on building inventory and commercial activity while leveraging the existing third-party infrastructure and knowledge regarding processing and crushing in the soybean industry. We intend to replicate this model for our consumer-oriented wheat products.
The following chart shows our plans for commercializing our consumer-focused product candidates:
We have made substantial progress in our supply chain execution. We currently have established relationships with multiple seed production partners, crop farmers and crushers, in addition to our internal capabilities. We believe that we can extract meaningful value from each of our relevant supply chains in a similar manner and we expect to establish similar relationships across the value chain with our wheat and canola product candidates.
Our Product Pipeline and Commercial Opportunity
High Oleic Soybean (Consumer Trait)—a Premium Oil Market Commercial Opportunity
Soybean oil has historically been partially hydrogenated to enhance its oxidative stability in order to increase shelf life and improve frying characteristics. This process, however, creates trans-unsaturated fatty acids, or trans fats, which have been demonstrated to raise low-density lipoprotein (LDL) cholesterol levels and lower high-density lipoprotein (HDL), both of which contribute to cardiovascular disease. The discovery that dietary trans fats increase the risk of several health issues led the FDA to rule in 2003 that manufacturers be required to include trans fat content information on the “Nutrition Facts” label of foods. In 2015, the FDA took a further step and banned the use of partially hydrogenated oils, the primary dietary source of artificial trans fat in processed foods, by all food manufacturers beginning in 2018. After the FDA’s 2003 ruling, commodity soybean
79
Table of Contents
oil—which leads to high trans fats in foods—lost approximately 20% market share to other vegetable oils, such as palm oil and canola oil.
Monounsaturated fats, such as oleic acid, have been linked to reducing LDL cholesterol and triglycerides and raising HDL cholesterols. Diets rich in monounsaturated acids are associated with lower fat mass and decreased blood pressure. High levels of oleic acids can be found in olive, canola, sunflower and safflower oils. The following chart shows the oil composition in our soybean oil compared to these and certain other oils:
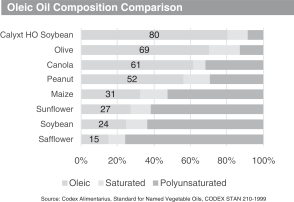
We developed a soybean trait that has produced oils with a fatty acid profile that contains 80% oleic acid, 20% less saturated fatty acids compared to commodity soybean oil and zero transfats, as shown in the chart below.
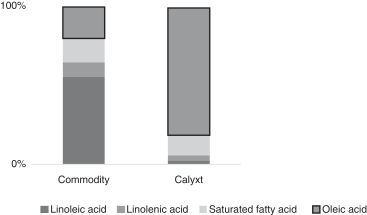
Oil created from our high oleic soybean product candidate has multiple desirable characteristics as an ingredient for the food industry. The high level of oleic acid in our soybean oil enhances oxidative stability more than fivefold when compared to commodity oil. This eliminates the need for partial hydrogenation, and thus no trans fats are produced during oil production. Furthermore, our high oleic soybean oil offers additional potential benefits, including reduced saturated fats, a threefold increase in fry-life, and reduced polymerization upon frying at high temperatures. Soybean oil is also neutral in flavor, odorless and colorless, and is therefore highly desired as a food ingredient because it has limited impact on the sensory characteristics of the final food product.
Our high oleic soybean product candidate was created using our TALEN gene-editing technology. We designed TALEN to specifically target two fatty acid desaturase genes (designated FAD2A and FAD2B). These genes convert oleic acid (a mono-unsaturated fatty acid) to linoleic acid (a polyunsaturated fat). By specifically inactivating both the FAD2A and FAD2B genes by removing DNA with TALEN, oleic acid accumulates in the
80
Table of Contents
seed—increasing from about 20% to 80%. By key measures, including yield, our high oleic soybean variety performs comparably to its unedited counterpart. Further, our improved soybean variety does not contain any foreign DNA. Because our technology is so precise and we target genes with well known functions in the plant, we have not, to date, detected any other changes as a result of the gene-editing process or undesired effects in our product.
In mid-2015, we received a letter from the USDA indicating that our high oleic soybean variety is not a regulated article under the Plant Protection Act. This allowed us to test the performance of our soybean variety in the field. In November 2015, we announced the completion of the second year of multi-location field trials in Minnesota and South Dakota. The agronomic and yield performance of our high oleic soybeans is on par with the non-GMO variety used to create this product. In 2016, 45,000 bushels of soybean seeds were produced by our farmers and we established supply chain partnerships.
Our soybean product candidate is in Phase III of our development process, and we are making preparations to create the capacity to crush on a commercial scale, which will enable product testing by potential food company customers. We are currently completing our commercialization plan and anticipate commercialization by the end of 2018. The interim critical milestones to complete in the remainder of 2017 and in 2018 to achieve that goal include the following:
| • | building up our commercial supply chain for seed, grain and oil; |
| • | creating relationships with customers, and encouraging them to start testing oil created from our soybean product candidate; |
| • | planting additional seed acres in 2017 to have sufficient seed supply in 2018; |
| • | signing tolling agreements with one or more crushers; and |
| • | building up the commercial grain inventory toward the end of the 2018 crop year in order to generate 2019 oil and meal sales. |
In addition, our high oleic soybean product candidate can be stacked with other soybean traits. For example, we intend to combine our high oleic acid trait with a trait that reduces linoleic acid, which would further improve the quality of our oil for frying. We are also developing an improved protein composition trait, which is in the Discovery phase of our development process. We believe this trait will increase the value of the soybean meal. Soybean meal is an important source of dietary protein for animals, and our product may have a better balance of amino acids for animal nutrition. These output traits that affect oil and protein quality will be combined with traits of value to the farmer. Among these are herbicide and drought tolerance and traits that confer improved yield. Thus, we have established a pipeline in which additional traits can be stacked with our initial products to increase their commercial value over time.
Premium Oil Market Opportunity
According to industry sources, the vegetable oil market in North America is estimated at 40.7 billion pounds per year. Certain premium priced oils such as canola, sunflower, corn, cotton and others represent approximately 34% of this market, or approximately 14 billion pounds per year. We believe this market has grown by a 6.7% compound annualized growth rate over the trailing fifteen years. Data from the USDA suggests that the average price of various premium oils over the 10-year period ended 2016 was approximately $0.56 per pound versus soybean oil that we estimate currently sells in a range of approximately $0.33-$0.39 per pound. Based on these estimates, the premium oil market segment could potentially generate approximately $8 billion in annual revenue. Assuming the market grows at a 5% CAGR, which assumes 2% growth for inflation and 3% population growth, we believe this market opportunity could grow to approximately $13 billion in the next ten years. In addition, in the United States, according to a report by Qualisoy, high oleic soybean acreage and oil production is expected to more than double year upon year, resulting in 9.3 billion pounds of available soybean oil within the next decade. Accordingly, we believe the oil generated from our soybean product candidate targets a potential multi-billion dollar market.
81
Table of Contents
Vertically Integrated Soybean Franchise, Supply Chain and Business Model
We intend to pursue a vertically integrated business model for our soybean product candidate that we believe will provide three potential revenue opportunities:
| • | seed sales to farmers that are part of what is called the identity-preserved supply chain, which entails entering into a contract with the farmer, who, in return for a premium, produces and stores our soybeans while maintaining the quality and purity of our product; |
| • | oil sales to food companies; and |
| • | meal sales to animal feed companies. |
The following chart shows a high level indicative approach to our business model within the supply chain:
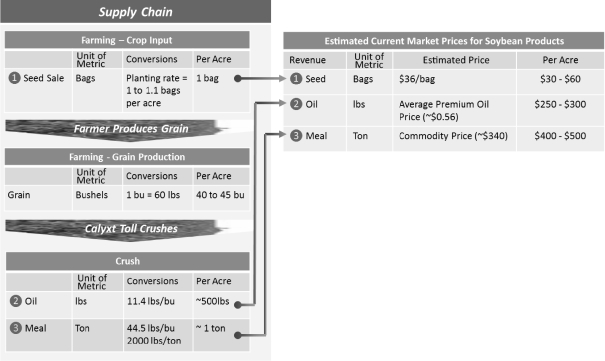
Soybean Germplasm (Seeds) – Soybeans are a crop that is photoperiod sensitive, meaning the crop is sensitive to the length of the day. Plant breeders have developed soybean varieties that are adapted to distinct latitudes and agroclimatic conditions – which are referred to as maturity groups. Different growing regions in the United States require different maturity groups. For example, the northern United States requires soybeans in the 1.5- 2.2 maturity group ranges, with farmers typically planting a few varieties within this range to diversify their risk.
We have in-licensed over thirty non-genetically modified soybean varieties with commercial rights. We intend to initially introduce our high oleic soybean product candidate in the northern United States on one variety within a particular maturity group range and over time expand to additional varieties to farmers in the same region to provide farmers with the ability to diversify their risk. We expect to continue this expansion over time by adding regions in other maturity groups and varieties. Over the next five to 10 years we envision expanding our germplasm portfolio to early, middle and late maturity groups. We believe this strategy will enable us to expand our supply chain through additional crushing plants within target growing regions and at the same time lower potential premiums and production costs as we introduce into the supply chain additional varieties that meet the diversity of seed variety that farmers need to mitigate their risk.
82
Table of Contents
Crusher operators are generally divided into two categories—crushers owned and operated by large companies in the industry and crushers that are independently operated. Each group generally follows a different business strategy. Large crushers typically focus on maximizing utilization and production, whereas the independently operated crushers typically focus on maximizing profitability by specialization and production focus. Within our target regions, we believe there are many independent crushers that process soybean and that are under utilized. We expect this to provide us with a meaningful opportunity to leverage their services in our business model.
Farmers
We plan to initially focus our supply chain strategy on contracting with farmers within a 50-100 mile area from certain predetermined crushing plants with which we are forming commercial tolling relationships. This will allow these farmers and growers to deliver their grain to these crushing plants in a cost-effective manner to avoid high transportation costs. Over time, we foresee supplying farmers with seeds in multiple states near multiple crushing plants with which we have contracted. We believe that we can generate a successful supply chain by contracting with fewer than 2,000 farmers.
There are a number of key metrics by which we will measure our success in the established supply chain, including the number of farmers using our seeds to grow high oleic soybeans, the number of acres planted with our seeds, the number of farmers that we need to contract with in order to add an incremental crushing plant to the supply chain and the retention rate of how many farmers choose to grow our soybeans year after year. We also anticipate farmers continuing to grow multiple varieties from multiple seed companies, which means we may initially represent a small percentage of a particular grower’s soybean acreage. Over time, we plan to seek to increase that percentage and, as our product offering expands, become one of the partners of choice for that farmer’s soybean seed needs.
Soybean Meal Sales to Animal Food Companies – Another potential opportunity for our high oleic soybean is for consumption in soybean meal. Approximately 97% of the soybean meal consumption is in the animal nutrition industry, with the industry experiencing increased demand for differentiated and non-GMO soybean meal. As a result, we believe the soybean meal industry provides us another competitive opportunity as we establish our commercial strategy.
Soybean Oil Customers – Given the characteristics of our soybean oil—neutral taste, heat stability and fatty acid profile similar to olive oil—we believe our oil presents a competitive alternative to existing premium oils in multiple food application categories.
Additional Soybean Growth Opportunities – We intend to initially focus commercialization of our soybean product candidate in North America. However, over time, we foresee a number of expansion opportunities given the global nature of soybean uses, including extensive import and exports of soybean co-products such as meal and oils. Some of these expansion opportunities may include:
| • | Oil Exports: As global regulatory frameworks continue to evolve for gene-edited products, we believe we will have the opportunity to export our soybean oil to other markets, which in turn we expect will create the opportunity for additional market expansion and increased premiums. |
| • | Global Launches of High Oleic Soybeans: Soybeans are grown in other countries such as Brazil and Argentina. We therefore believe that we have the potential to expand our supply chain and business relationships to other countries, providing additional global opportunities. |
| • | Expand Product Offering in Existing Supply Chain: Once a supply chain is established, we believe we will have the potential to leverage the same acres, meal and oil sales to create additional value through increased quality, such as improved protein contents, or farmer-focused traits such as herbicide tolerance, improved yield and drought stress tolerance. |
83
Table of Contents
| • | Expansion of Seed Sales: We believe that once we establish a supply chain in soybeans through commercial relationships with farmers, we may be able to expand our product offerings to these farmers. |
High Fiber Wheat (Consumer Trait)
Fiber is the indigestible portion of food that is essential for healthy digestion. Research has shown that fiber may play a large role in maintaining bowel health, lowering cholesterol, stabilizing blood glucose levels and controlling weight gain. A high fiber diet has the potential to lower the rate of glucose entry into circulation, thus decreasing the risk of food-related chronic diseases, such as coronary artery disease and diabetes. The average American adult consumes approximately 15-18 grams of fiber daily, only half of the amount recommended by the U.S. Department of Health’s dietary guidelines based on the average caloric intake. In recent years, the awareness of the health benefits of high fiber diets has increased. This has translated to a strong growth in demand for high fiber food products, with 38% of grocery shoppers now seeking high fiber foods.
We are developing high fiber wheat traits that could be used to produce white flour with up to three times more dietary fiber than standard white flour. We anticipate that by altering the proportion of certain slower digested carbohydrates in the wheat grain, we will increase dietary fiber. This would allow consumers to reach their daily value of fiber without changing their existing food preferences. These high fiber wheat product candidates will not contain any foreign DNA.
We believe our high fiber wheat flour will be incorporated into many food products—from pasta to bread. Whereas a single serving of whole wheat flour can provide 49% of an individual’s daily fiber needs, a single serving of our high fiber flour may provide up to 100% of the recommended daily requirement thereby allowing food manufacturers to make high fiber products sought after by many consumers.
This product candidate is currently in Phase I of our development process. The gene-edited wheat line has been identified and grown and the fiber levels are under examination in grain derived from greenhouse grown plants.
In addition to our high fiber wheat product candidate, we are also developing other consumer traits in our wheat pipeline, including a reduced gluten product candidate.
High Fiber Wheat Market Opportunity
According to industry sources, the wheat market in North America is estimated at 108 million tons per year with approximately 43% produced for human consumption in the United States. Humans consume approximately 650 million hundredweight, or cwt, of wheat flour in the United States, including an estimated 91 million cwt of premium wheat flour. Assuming an average price of approximately $15 per cwt we believe the premium wheat market could represent a potential multi-billion dollar market opportunity.
High Fiber Wheat Germplasm (Seeds)
The wheat flour market in the United States consists of six classes, which are designated by color, hardness and growing season: hard red winter, hard red spring, soft red winter, soft white, hard white and durum.
The wheat classes grown by a particular farmer depend on geographic location and the germplasm offerings in the different classes of wheat. The classes pose different quality and functionality characteristics, and growing wheat of multiple classes diversifies harvest risk.
We plan to expand our product offerings into potentially all classes of wheat with an emphasis on those that provide superior functionality. We will then selectively in-license and acquire third-party genetics to build germplasm platform in potentially all classes of wheat. These additional product offerings will impact our regional expansion of production as well as market opportunities.
84
Table of Contents
Flour
Miller operators fall into one of two categories—millers owned and operated by large companies in the industry and millers that are independently operated. Each group generally follows a different business strategy. Large millers typically focus on maximizing utilization, blending and economies of scale, whereas independently operated millers typically focus on maximizing profitability by specialization and production focus. We believe there are many independent millers that process flour that are underutilized and eager to work with specialty products. We expect this to provide us with a meaningful opportunity to leverage their services in our business model. We expect to commence commercialization of our wheat product, once developed, using two to four millers, and to expand that number as commercialization progresses.
High Fiber Wheat Customers
The market for wheat-based products is diverse and, in the United States, includes fresh bread and rolls, snack bars and granola bars, pastries and doughnuts, frozen pizza, bakery snacks and Mexican foods. As a result, we believe there are multiple opportunities to supply our high fiber wheat to food producers.
Additional High Fiber Wheat Opportunities
We intend to initially focus commercialization of our high fiber wheat product candidate in North America. However, over time, we foresee a number of expansion opportunities. Some of these expansion opportunities may include:
| • | Global expansion: We believe there would be potential in being able to export our high fiber wheat to other countries with conducive regulatory frameworks with respect to our products. We also believe that we will be able to grow high fiber wheat in other countries given the extensive amount of wheat grown outside of the North American market. We believe both of these opportunities create the potential for increased premiums and market penetration. |
| • | Cross selling: Once we have established an initial relationship with a food industry customer, we believe there is potential opportunity to cross sell products to the customer. For example, the customer may also have a demand for our high oleic soybean or another product as the customer’s needs evolve. |
| • | Trait stacking: Another potential opportunity for us is using trait stacking. For example, we may be able to add characteristics to our high fiber wheat, such as reduced gluten or a more favorable carbohydrate profile. Alternatively, we may be able to add herbicide tolerance to our high fiber wheat. In each case, we would expect the additional trait to enhance the value of our products on a per unit basis and further drive demand. |
Herbicide Tolerant Wheat (Farmer Trait)
Weed control is one of the greatest challenges farmers face in producing crops. Weeds compete not only with crops for water, nutrients, sunlight and space, but also harbor insect and disease pests, clog irrigation and drainage systems, undermine crop quality, and deposit weed seeds into crop harvests. Poorly controlled weeds significantly increase farmers’ cost while reducing crop yield and quality. With the constant need to increase yields, herbicides are an important component of commercial food production and account for 70% of all agricultural chemical use.
Herbicide tolerance is a plant’s ability to withstand a particular chemical herbicide. Herbicide tolerance traits in crops can provide additional crop protection chemistry alternatives to control weeds and increase crop yields. Weed resistance is a growing problem in the United States and other countries in the world. As weeds develop resistance to herbicides they become more difficult to control which can reduce yields and the quality of crops. The use of different chemistries has been an important tool to control herbicide resistant weeds. For example, Glyphosate tolerant weeds are controlled with other herbicide chemistries effective in grasses. Certain crops—such as wheat and soybeans—may not be tolerant to such chemistries, rendering this option non-viable for over-the-top applications.
85
Table of Contents
Herbicide tolerant traits may offer farmers a vital tool in managing weeds effectively during crop production. The deployment of herbicide tolerant traits in wheat significantly lags other major crops and wheat production is constantly faced with yield-robbing weeds that can result in lower yield and higher dockage costs at the elevator. In the United States, nearly 90% of major row crops, including corn and soybeans, contain at least one herbicide tolerant trait. In contrast, no broad acre GMO herbicide tolerant trait has been developed for wheat, largely due to the complexity of the wheat genome. Without effective control, weeds can lower winter wheat yield by up to 23% on average worldwide, and in turn significantly decreases profitability potential.
Wheat Herbicide Tolerant Trait Opportunities
We are pioneering the development of herbicide tolerant traits in wheat without the use of foreign DNA, built using our TALEN gene-editing technology. Herbicides act by inhibiting the activity of certain plant-encoded proteins that promote plant growth. We aim to achieve herbicide tolerance by specifically making a subtle repair to prevent herbicides from being able to recognize and block functions of these proteins, such that the edited plant survives the application of the herbicide. Our product candidate will contain no foreign DNA. We believe this solution, if successfully developed and commercialized, will have the potential to increase the farmer’s yield and revenue. Accordingly, we believe our herbicide tolerant trait would have broad appeal and applicability into the 76 million acres of wheat that are grown in North America each year and potentially into the over 500 million acres grown worldwide. One industry source has suggested that there can be up to $71 per acre of loss for wheat due to weeds. Given the amount of wheat acreage in North America, products with herbicide tolerant traits represent a potential multi-billion dollar industry.
This product candidate is currently in the Discovery phase of our development process. In addition to herbicide tolerance, we are developing traits that are advantageous to the farmer including our variety of wheat that confers resistance to a fungal pathogen, namely powdery mildew. We believe powdery mildew resistance may increase yield and reduce the need for the use of costly fungicides.
Building Up a Licensing Franchise and Supply Chain
We intend to develop our herbicide tolerant wheat technology and plan for initial commercialization in North America in the second half of the coming decade. Under this proposed model, we anticipate that we would establish licensing relationships with seed company customers (and public seed institutions) to enable conversion of elite wheat lines from multiple classes. These seed institutions would then sell seed containing our herbicide tolerant technologies either directly to farmers or through seed distributors. We would seek to receive royalty payments from these seed institutions.
Additional Licensing Growth Opportunities
We believe herbicide tolerant wheat represents the first broad licensing opportunity for a large acre trait for us in a major row crop. Additional licensing opportunities may include:
| • | Herbicide tolerant wheat being developed by us that will enable the application of two different types of chemistries currently available in the market (off patent chemistries). We could pursue a strategy to collaborate with strategic partners, exclusively or on a non-exclusive basis to register the application of chemistries on its herbicide tolerant wheat and seek additional revenue opportunities from such chemistries. |
| • | We could combine our herbicide tolerance technology in wheat with our high fiber wheat products under identity preservation, creating potential synergies between both products being sold on the same acreage. |
| • | Over 500 million acres are cultivated globally, and we could replicate our business model and deploy herbicide tolerant technologies in wheat in other world regions beyond North America. |
86
Table of Contents
| • | Development and out-licensing of additional farmer traits in wheat such as disease resistance, additional herbicide tolerance products, improved yield products and other agronomic characteristics. |
| • | Similar or alternative herbicide tolerant technologies could be developed by Calyxt in other crops such as soybeans and canola, expanding the herbicide tolerance franchise across multiple crops, first in North America and then globally. |
Other Products in Our Development Pipeline
Our extensive product pipeline includes a variety of consumer- and farmer-centric traits for soybean, wheat, canola, alfalfa and potato. We will conduct further development programs to build upon our current pipeline, which currently includes improved oil composition canola, herbicide tolerant canola, improved quality alfalfa and herbicide tolerant alfalfa, late blight resistant potato, cold storable / reduced browning potato, improved protein composition soybean, drought tolerant soybean, herbicide tolerant soybean and improved yield soybean. In the future, we anticipate expanding our product pipeline to include other food crops.
We plan to develop gene-editing automation processes that will enable us to implement a high throughput discovery platform to identify new growth opportunities. This high-throughput platform is intended to allow us to discover more gene traits and make more complex edits, enabling us to drive innovation at a significantly faster rate. We believe all of these steps will enable us to remain at the forefront of food and agriculture innovation.
Technology and Process Overview
Calyxt is a Pioneer in Plant Gene Editing
Gene editing is a technological leapfrog that makes it possible to overcome many of the limitations of traditional trait-development techniques. Gene editing creates novel traits by making minor DNA sequence edits to plant genomes. These edits occur in a precise manner and at high efficiency, providing accelerated development and commercialization timelines and potentially lower regulatory hurdles.
The following chart depicts our development process:
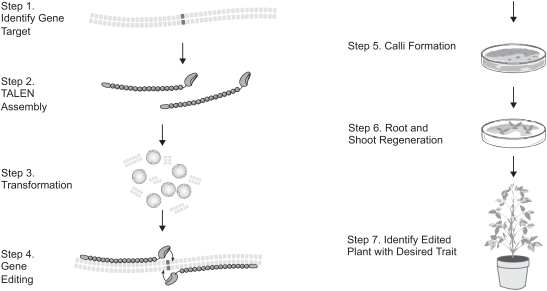
Step 1: We begin by identifying the gene target and the edit we would like to introduce into the plant genome. We do not have an in-house gene discovery platform. Rather, we supplement our own scientific
87
Table of Contents
knowledge and expertise with the efforts of universities, non-profit organizations, government agencies and gene discovery biotech companies to identify genes, which when altered, express desired crop traits. Some of the information on gene targets is in the public domain, and in other instances, we may engage in intellectual property licensing agreements. We believe there are scientific teams around the world continually identifying new gene targets, providing new opportunities for us to expand our product pipeline. We are typically able to assess gene targets and the types of edits to be introduced in several weeks.
Step 2: We create TALEN (Transcription Activator-Like Effector Nucleases) to specifically recognize the DNA target we seek to edit in the plant genome. TALEN were invented by our scientists and are one of the most effective reagents for gene editing. Two TALEN are used to recognize a given target, each of which recognizes 15-20 bases of the genetic code. A 30 base DNA sequence target occurs, on average, once every quintillion bases. Thus it is relatively easy to design TALEN to recognize unique sites in complex genomes. We have unparalleled expertise in TALEN design, and we have an automated TALEN assembly pipeline that allows us to make thousands of TALEN per week—far more than what is needed for product development.
Step 3: We deliver our TALEN and other reagents as necessary to achieve the desired gene editing outcome to plant cells in a process known as transformation. We rely on three methods of transformation—agrobacterium, particle bombardment and protoplast—to deliver reagents to plant cells to achieve targeted edits at high efficiency.
Step 4: Gene editing typically occurs within a few hours after reagent delivery.
Step 5: We place the edited cells in culture and allow them to grow and divide. The cells form a mass of unorganized tissue called a calli. Calli are produced for most plants cells we edit, and the callus stage can last from several weeks to several months.
Step 6: Once calli are obtained, we induce them to differentiate leaf and root tissues through the application of plant hormones. Shoots regenerate first and then roots, ultimately resulting in a “plantlet.” Depending on the plant species, this step can take from a few to several months.
Step 7: We screen the resulting plantlets to identify those with the desired gene edit. We have an automated pipeline to prepare and analyze DNA from plantlets to identify those that have the desired editing outcome.
Once the steps in the chart above are completed, we continue on to Phase II our development process by moving plants with edited genes to soil and growing them in the greenhouse to test for the intended trait and to produce more seed. This step is often the most time-consuming, and is constrained by biology; that is, it takes time for a plant to mature, flower and produce seed. Once we validate the trait in the greenhouse, we advance the edited plants to field trials. We carry out the field trials at multiple locations over multiple growing seasons. In some instances, we carry out field trials in the Southern hemisphere to capture two growing seasons in a given calendar year.
Gene Editing with TALEN
TALEN enable genome editing through a simple two-step process—first by recognizing a specific DNA sequence, and then by precisely inducing a controlled DNA double-strand break. TALEN protein structure comprises a DNA-recognition domain and DNA-cleaving domain. DNA-recognition is achieved by individual repeat domains (depicted in the figure below as an array of spheres); each domain recognizes a specific nucleotide (depicted in the figure below as grey boxes). By rearranging central repeat domains, TALEN can be designed to target nearly any DNA sequence. DNA-cleavage is achieved by a nuclease that is fused to the DNA-recognition domain (depicted in the figure below as large half spheres). The nucleases must interact to cleave DNA, and cleavage occurs only after two TALEN precisely recognize a target DNA sequence. The requirement for two nucleases to interact minimizes cleavage at unintended sites due to binding of a single TALEN.
88
Table of Contents
The following figure depicts the process of making gene edits using TALEN:
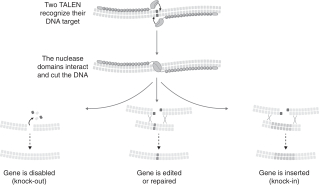
A precisely placed double-strand DNA break is the key to unlocking gene editing. This break can be the substrate for a wide range of outcomes, from single nucleotide deletions, to large DNA insertions. Left alone, a DNA break will result in removal of nucleotides (through a process called non-homologous end-joining). This removal of nucleotides, if placed appropriately, can result in gene inactivation or a gene knock-out. Our high oleic soybean variety, for example, was achieved by gene knock-out. By precisely removing DNA, we inactivated two FAD2 genes, which resulted in the accumulation of oleic acid in the seed.
If a user-supplied DNA fragment with a similar sequence to the TALEN binding site is provided at the time of the DNA break, then novel information in the user-supplied DNA is copied into the plant genome (through a process called homologous recombination). The information that is incorporated can be a single base change that repairs or edits the target gene to improve its function. For example, we believe herbicide tolerance can be achieved in many plant species by specifically making a subtle repair to prevent herbicides from being able to recognize and block functions of certain plant-encoded proteins that promote plant growth. Alternatively, it is possible to incorporate or knock-in one or more transgenes to create a new trait. At present, we are focused on using gene knock-outs and gene repair to create new traits. Other major agricultural biotechnology companies, which commercialize GMOs, are interested in targeted knock-in approaches. Gene editing and gene insertion through homologous recombination are collectively referred to as gene targeting.
Tissue Transformation Systems
Key to achieving a gene-edited plant is the ability to deliver TALEN to plant cells (a process referred to as transformation). Our scientists have proficiency using the three main methods of transforming plant cells—agrobacterium, particle bombardment and protoplast transformation. Agrobacterium is a soil bacterium that naturally delivers DNA to plant cells. This method can be used to deliver our TALEN; it can also be used to deliver templates to copy information to the break site. Particle bombardment uses DNA-coated gold or tungsten particles to deliver DNA to plant cells. The particles are shot at plant tissue at high velocity, and the DNA is either transiently expressed or integrated into the host genome. Protoplasts are plant cells lacking a cell wall. Our TALEN reagents can be directly delivered to protoplasts at high efficiency (between 50% and 90% transformation frequencies). Further, we have shown that purified TALEN proteins can be delivered to plant protoplasts to achieve gene editing, thereby obviating the need to use nucleic acids. Over the past six years our scientists have developed the expertise and know-how to deploy these methods in a variety of plant species.
We believe that our ability to select the appropriate transformation method, our capabilities to use these technologies, as well as our innovative solutions to achieve increased efficiency, creates both a competitive advantage and a barrier to entry. Our technology enables us to precisely and specifically elicit the desired traits with the desired characteristics in a matter of months. For example, in order to develop our high-oleic soybean
89
Table of Contents
oil, we designed TALEN to specifically target two fatty acid desaturase genes (designated FAD2A and FAD2B). These genes convert oleic acid (a mono-unsaturated fatty acid) to linoleic acid (a polyunsaturated fat). By inactivating both the FAD2A and FAD2B genes with TALEN, oleic acid accumulates in the seed—increasing from about 20% to 80%, which improves its characteristics for frying and is healthier for the consumer.
The plant transformation and regeneration steps described above are inherently scalable, and we envision that through the use of robotics, it will be possible to generate thousands of plantlets with a desirable gene edit. By implementing a platform that creates multiple plantlets with the same gene edit, we believe we will be able to greatly accelerate product commercialization. For example, if we are interested in developing wheat flour with a healthier carbohydrate composition, we can harvest enough seed from our population of edited plantlets to produce enough flour to test for functionality and ensure it has the properties desired for our customers. This eliminates the time needed to amplify enough seed over multiple generations from one or a few edited plants.
Key Advantages of TALEN Over Other Gene-Editing Technologies
In addition to TALEN, we are aware of three other classes of nucleases that enable gene editing, including meganucleases, zinc finger nucleases and CRISPR/Cas9. Despite the availability of other gene-editing platforms, we currently rely on TALEN because of the following benefits:
| • | Intellectual property—We have a strong intellectual property position with respect to TALEN technology and its use to make our product candidates. We have actively sought to protect our proprietary technologies and product candidates through the licensing of a portfolio of 81 issued patents and 170 pending patent applications. |
| • | Specificity—TALEN may be designed to limit its DNA cleavage to the desired sequence and to avoid cutting elsewhere in the genome. This parameter is essential as plant genomes are highly complex; for example, the wheat genome comprises 17 billion bases. |
| • | Precision—It is possible to design a TALEN that will cleave at any selected region in any gene. For example, there are four related FAD genes in the soybean genome. Our TALEN edited only the two genes that produce fatty acids in the seed; no edits were introduced into the other related genes. |
| • | Efficiency—A large percentage of cells treated by TALEN bear the desired gene edit. Because of TALEN efficiency, only a handful of plants have to be regenerated to recover those with edits in our target gene. For example, three of 19 transgenic soybean lines expressing the FAD2 TALEN transmitted heritable edits to the next generation. |
| • | Validation—We have a strong track record with respect to our technologies and expertise as we have successfully edited more than 20 unique genes in 6 plant species since our inception in 2010. |
| • | Ease of use—We have extensive expertise in the design and assembly of TALEN and can generate thousands of TALEN per week. |
90
Table of Contents
A Comparison of Crop Development Processes—Traditional vs. Calyxt
Our solution for agriculture biotechnology can provide significant speed and cost advantages as compared to the traditional trait-development methods employed by the current agriculture industry companies. Through our accelerated development process, we believe we are capable of commercializing our product portfolio significantly quicker and at reduced cost.
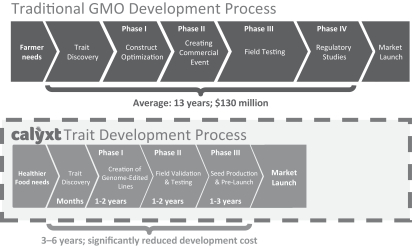
Traditional Agricultural GMO Development Process:
We provide an overview of the development process involved in GMO trait commercialization, which is currently employed by the agricultural industry. This process typically requires an average of 13 years and costs upwards of $130 million to develop a new trait:
| • | Discovery—The screening and identification of potential traits of interest using foreign DNA which many times is from outside the plant kingdom. This process requires the expertise in genetics, molecular biology and bioinformatics/genomics approaches to identify target genes. |
| • | Phase 1 (Proof of Concept)—At this stage of development, tests are performed on a variety of constructs based on lead candidate genes. This is undertaken to achieve the optimal expression of gene in order to obtain the desired trait. |
| • | Phase 2 (Early Development)—Plants with optimized genetic constructs are chosen and evaluated in greenhouse and field trials. This involves upwards of tens of thousands of plants being grown and evaluated. |
| • | Phase 3 (Advanced Development)—Large scale field trials are conducted to measure trait expression and crop yield. |
| • | Phase 4 (Pre-Launch)—Build up seed inventory for commercial launch and regulatory studies to test the safety of the crop. |
Calyxt’s Accelerated Development Process:
We can assess the viability of a trait in less than two years and commercialize it in as short as six years with a significantly reduced development cost.
| • | Discovery (Several Months)—The preliminary screening and identification of genes with the potential to deliver a trait of interest. We do not spend significant resources on the screening and identification of genes in plants that will lead to beneficial traits. Rather, we supplement our own scientific knowledge and expertise with the efforts of universities, non-profit organizations, government agencies and gene discovery biotech companies to identify genes, which when altered, express desired crop traits. |
91
Table of Contents
| • | Phase I: Product Transformation & Event Selection (1-2 Years)—The process to make our product, in which we leverage our gene-editing platform and expertise to induce the expression of a desired trait. We have proficiency in three different tissue transformation systems—agrobacterium, particle bombardment, and direct DNA delivery to protoplasts—which allows us to edit the genome in nearly all major crops. Once a product is created we grow seed in our greenhouse to initiate testing. At the end of Phase I, we submit a petition to the USDA to confirm the product candidate is a non-regulated article. |
| • | Phase II: Trait Validation (1-2 Years)—After obtaining USDA confirmation, trait validation is conducted in small and large scale field trials. Small scale trials take place in a limited number of locations, where we test our products in the field and increase seed production. Larger scale field trials occur across multiple field locations and years and help us ensure our field performance across multiple conditions. At this stage we also test the specialty food ingredients produced from these plants for unique healthier properties. At the end of Phase II, we expect to be able to assess the technical viability of a trait. We can then decide whether to further invest in the product and continue to develop the asset or dispose of the asset with minimal development costs, allowing us to continue to innovate at a rapid pace. |
| • | Phase III: Pre-Commercial (1-3 Years)—This phase is the first commercial scale production and provides us the opportunity to build seed and product inventory prior to commercialization. During this period we also establish our grower supply chains and customer relationships in preparation for commercialization. |
| • | Commercial—When a product is ready to be commercialized, we plan to utilize our innovative commercial strategy to leverage existing supply chains to ensure product quality, manage cyclical demand changes and capture value. Depending on what supply chain relationships may exist at the time when a product candidate is ready to be commercialized, we believe we may be able to leverage our existing supply chain relationships to lower commercialization costs and time to market. We plan to continue to build out our germplasm during commercialization to enhance our seed capability and regional footprint. |
Additional Opportunities
We—through our license agreement with Cellectis—have access to intellectual property that broadly covers the use of engineered nucleases for plant gene editing. This intellectual property covers methods to edit plant genes using “chimeric restriction endonucleases,” which include TALEN, CRISPR/Cas9, zinc finger nucleases, and some types of meganucleases. The granted claims cover methods of introducing chromosomal edits through non-homologous end-joining to create gene knock-outs, as well as methods of introducing chromosomal edits by homologous recombination to produce precise gene edits or gene knock-ins. We believe this umbrella intellectual property applies broadly across gene editing in plants and makes us a key player in the gene editing intellectual property space.
Making precise edits through gene targeting is technically challenging, as frequencies of gene editing or DNA insertion are significantly lower than frequencies of gene knock-outs. Under our license agreement with Cellectis, we have exclusive sublicense rights (subject to existing non-exclusive sublicenses to third parties) to intellectual property exclusively licensed to Cellectis from the University of Minnesota in the field of researching, developing and commercializing agricultural and food products, including traits, seeds, and feed and food ingredients (excluding any application in connection with animals or animal cells). These patent applications cover the use of DNA replicons for gene editing. The replicons carry DNA sequences encoding TALEN as well as a DNA template to copy precise edits into the plant genome. When introduced into plant cells by any of the tissue transformation systems described below, the replicons amplify to a high copy number. This allows us to achieve gene editing at frequencies up to 12-fold higher than the use of traditional methods. The replicon technology, combined with our transformation platforms, expand the versatility of the TALEN technology for generating products.
92
Table of Contents
Intellectual Property
Our success depends in part on our ability to obtain and maintain intellectual property and proprietary protection for our product candidates and technology related to our business, defend and enforce our intellectual property rights, in particular, our patent rights, preserve the confidentiality of our trade secrets, and operate without infringing valid and enforceable intellectual property rights of others. We seek to protect our proprietary position by, among other things, licensing and filing United States and certain foreign patent applications related to our technology, products and product candidates, and improvements that are important to the development of our business, where patent protection is available. We also rely on trade secrets to develop and maintain our proprietary position and protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. We seek to protect our proprietary technologies, in part, by confidentiality agreements with our employees, consultants, scientific advisors, and contractors.
Notwithstanding these efforts, we cannot be sure that patents will be granted with respect to any patent applications we have licensed or filed or may license or file in the future, and we cannot be sure that any patents we have licensed or patents that may be licensed or granted to us in the future will not be challenged, invalidated, or circumvented or that such patents will be commercially useful in protecting our product candidates and technology. Moreover, trade secrets can be difficult to protect. While we have confidence in the measures we take to protect and preserve our trade secrets, such measures can be breached, and we may not have adequate remedies for any such breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. For more information regarding the risks related to our intellectual property, please see “Risk Factors—Risks Related to Intellectual Property.”
As of May 31, 2017, we have licensed approximately 16 U.S. patents, 49 U.S. patent applications, 65 foreign patents, and 121 foreign patent applications. Within this portfolio, approximately 178 patents and patent applications relate to the genetic editing of plants using TALEN technology, 118 patents and patent applications relate to the genetic editing of plants using meganuclease technology, 76 patents and patent applications relate to the genetic editing of plants using CRISPR technology, and 49 patents and patent applications relate to specific plant traits.
The issued patents in our portfolio consist of approximately 6 Cellectis-owned and 10 other in-licensed U.S. patents, 18 Cellectis-owned and 36 other in-licensed European patents, and 2 Cellectis-owned and 9 other in-licensed patents in other jurisdictions, including Japan, China, Australia, Hong Kong, Singapore, Mexico and Canada. The pending patent applications in our portfolio consist of approximately 41 Cellectis-owned and 8 other in-licensed U.S. patent applications, 19 Cellectis-owned and 4 other in-licensed European patent applications, 67 Cellectis-owned and 26 other in-licensed patent applications in other jurisdictions, including Japan, China, Australia, India, Paraguay, Uruguay, Argentina, Brazil, Hong Kong, Singapore, Israel, Mexico, New Zealand and Canada, and 5 Cellectis-owned and 1 other in-licensed Patent Cooperation Treaty (PCT) applications.
Individual patent terms extend for varying periods of time, depending upon the date of filing of the patent application, the date of patent issuance, and the legal term of patents in the countries in which they are obtained. In most countries in which patent applications are filed, including the United States, the patent term is 20 years from the date of filing of the first non-provisional application to which priority is claimed. Under certain circumstances, a patent term can be extended. For example, in the United States, a patent’s term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the U.S. Patent and Trademark Office in reviewing and granting a patent, or may be shortened if a patent is terminally disclaimed over an earlier-filed patent. The issued patents that we have licensed will expire on dates ranging from 2020 to 2033. If patents are issued on the pending patent applications that we have licensed, the resulting patents are projected to expire on dates ranging from 2023 to 2037. However, the actual protection afforded by a patent varies on a product-by-product basis, from country-to-country, and depends upon many factors, including the type of patent, the scope of its coverage, the availability of legal remedies in a particular country, and the validity and enforceability of the patent.
93
Table of Contents
License Agreement with Cellectis
We will be party to a license agreement with Cellectis, pursuant to which we will be granted an exclusive, worldwide license (subject to existing licenses granted by Cellectis to third parties) to use, commercialize and exploit certain intellectual property in the field of researching, developing and commercializing agricultural and food products, including traits, seeds, and feed and food ingredients (excluding any application in connection with animals and animal cells), except that such license will be non-exclusive in such field for any activities relating to researching, developing or commercializing certain modified or mutated I-CreI homing endonucleases. We will also be granted a non-exclusive license to use the TALEN trademark in connection with our exploitation of licensed products under the agreement. Any improvements we make to the licensed intellectual property will be owned by us but licensed back to Cellectis on an exclusive basis for any use outside of our exclusive agricultural field of use. The exclusivity of our license agreement with Cellectis will be subject to existing non-exclusive licenses granted to third parties in the field of research.
In consideration for the license from Cellectis, we will be required to pay to Cellectis, on a product-by-product and country-by-country basis, a royalty of 3% of net sales of any products that are covered by the patents licensed from Cellectis. In addition, we will be required to pay Cellectis 30% of revenue we receive for sublicensing our rights under the agreement to third parties. Our payment obligations to Cellectis will expire upon the expiration of the last-to-expire valid claim of the patents licensed to us by Cellectis.
Under our license agreement with Cellectis, and as between the parties, Cellectis will have the first right to control the prosecution, maintenance, defense and enforcement of the licensed intellectual property and we will have the right to step in and assume such control with respect to the patents owned by Cellectis and exclusively licensed to us under the agreement if Cellectis elects to not prosecute, maintain, defend or enforce such patents. In certain circumstances, if Cellectis elects to abandon any patents owned by Cellectis and exclusively licensed to us under the agreement, we will have the right to assume ownership of such patents. In addition, some of the intellectual property that will be licensed to us by Cellectis consists of a sublicense of intellectual property originally licensed to Cellectis by the University of Minnesota to exploit such intellectual property in our exclusive agricultural field of use. Therefore, as to such sublicensed intellectual property, our license from Cellectis will be subject to the terms and conditions of the license agreement between the University of Minnesota and Cellectis, and to the extent our activities under such sublicense violate any terms and conditions of the license agreement between Cellectis and the University of Minnesota, we will be responsible for any damages that Cellectis may incur. In addition, we will be required to reimburse Cellectis for any and all payments made by Cellectis to the University of Minnesota pursuant to the license agreement between the University of Minnesota and Cellectis to the extent that any such payments are required to be made as a result of our applicable activities. Under the license agreement between Cellectis and the University of Minnesota, the University of Minnesota has the first right to control the prosecution and maintenance of the licensed intellectual property.
Our license agreement with Cellectis will be perpetual. However it may be terminated at any time upon the mutual written agreement of both parties, either party’s uncured material breach of the agreement, or upon certain bankruptcy and insolvency related events.
License Agreement from Regents of the University of Minnesota—TALEN
In January 2011, Cellectis entered into an exclusive license agreement with the University of Minnesota, which was amended in 2012, 2014 and 2015. Pursuant to the agreement, as amended, Cellectis and its affiliates were granted an exclusive, worldwide, royalty-bearing, sublicensable license, under certain patents and patent applications owned by the University of Minnesota, to make, use, sell, import and otherwise dispose of products covered by the licensed patents, in all fields of use. These licensed patents relate to TALEN molecules and their use in gene editing.
Pursuant to the agreement, with respect to the agricultural field, Cellectis is required to pay to the University of Minnesota a low six digit annual fee per year, as well as a low five digit commercialization fee for every seed
94
Table of Contents
variety containing new traits developed using the licensed technology. Cellectis is also required to pay the University of Minnesota, in the aggregate, up to a low seven digit amount of milestone payments based on the net sales of licensed products in the agricultural field. Cellectis must also pay the University of Minnesota certain patent-related expenses for prosecuting and maintaining the licensed patents.
The agreement will expire upon the expiration of the last to expire valid claim of the licensed patents. The University of Minnesota may terminate the agreement upon advance written notice in the event of the insolvency or bankruptcy of Cellectis, and immediately upon written notice in the event that Cellectis challenges the validity or enforceability of any licensed patent in a court or other applicable authority. Cellectis and the University of Minnesota may terminate the agreement by written notice in the event of the other party’s breach that has not been cured within a specified number of days after receiving notice of such breach.
License Agreement from Regents of the University of Minnesota—CRISPR
In December 2014, we entered into an exclusive license with the University of Minnesota, pursuant to which we were granted an exclusive, worldwide, sublicensable license under a specified patent application and any patents that issue therefrom owned by the University of Minnesota relating to the use of the CRISPR-Cas9 technology to make use, and commercialize products covered by the licensed patents in any field of use. Pursuant to the terms of the agreement, we must use commercially reasonable efforts to commercialize the licensed technology and to manufacture, offer to sell, and sell licensed products as soon as practicable and to maximize sales. We must also achieve certain sales- and patent-related milestones.
Pursuant to the terms of the agreement, we paid the University of Minnesota an upfront license fee payment in the amount of $130,000 in connection with entering into the agreement. We are also required to pay the University of Minnesota a tiered annual fee that increases from $20,000 to $225,000 based on the occurrence of certain specified events, including the grant of a sublicense to a third party, as well as patent-related expenses incurred under the agreement in prosecuting and maintaining the licensed patents. We are also required to pay the University of Minnesota a certain percentage of all revenues received by us under sublicenses. If we undergo a change of control and wish to assign all of our rights and duties under the agreement, we must pay the University of Minnesota a specified transfer fee.
Unless earlier terminated, the agreement will continue in effect until no licensed patent is active and until no licensed patent application is pending. The University of Minnesota may terminate the agreement for our uncured breach of the agreement upon 90 days’ prior written notice, or 60 days’ prior written notice if the breach relates to our payment obligations under the agreement. The University of Minnesota may also terminate the agreement, upon 10 days’ prior written notice, if we file for bankruptcy or become insolvent. The University of Minnesota may also immediately terminate the agreement if we or our agents or representatives commences or maintains an action in any court or before any governmental agency asserting or alleging the invalidity or unenforceability of the licensed patent rights. We may terminate the agreement for The University of Minnesota’s uncured breach of the agreement upon 90 days’ prior written notice. We may also terminate the agreement at any time upon 60 days’ prior written notice.
License Agreement from Plant Bioscience Limited
In April 2015, we entered into an exclusive license agreement with Plant Bioscience Limited, or PBL, pursuant to which we were granted an exclusive, worldwide license to use and exploit certain gene-editing technologies related to wheat with endogenous resistance to powdery mildew, and to provide commercial development and technical services to third parties. The technology licensed to us was originally exclusively licensed to PBL by the Institute of Genetics and Developmental Biology, or IGDB, which holds certain rights to which our exclusive license from PBL is subject. Pursuant to the agreement, we are required to use commercially reasonable efforts to achieve certain milestones in a specified development and commercial program, and to exploit the license so as to maximize the sublicensing of the licensed technology and the development and commercial use of the licensed products.
95
Table of Contents
The agreement will expire on the later of the tenth anniversary of the date that sales of the licensed products first occurred or the last expiration of a valid claim of a licensed patent. We may terminate the agreement at any time for any reason by giving PBL written notice. PBL may terminate the agreement upon our material breach of the agreement that has not been cured within a specified number of days after receiving notice of such breach. The agreement will also automatically terminate if we become insolvent or bankrupt.
Commercial License Agreement with Two Blades Foundation
In December 2014, we entered into a commercial license agreement with Two Blades Foundation relating to TAL nuclease technologies, which was amended in 2016. Pursuant to the agreement, we granted Two Blades Foundation a non-exclusive license to TALEN technology for not-for-profit uses within the field of plants genetically engineered by TAL nuclease, including use in Two Blades Foundation’s humanitarian efforts to support subsistence farming, and for certain commercial applications related to Two Blades Foundation’s plant disease resistance programs. The intellectual property licensed to Two Blades Foundation was originally licensed to Cellectis by the University of Minnesota. In addition, pursuant to the agreement and subject to certain restrictions, we received a non-exclusive license under Two Blades Foundation’s TAL Code technology related to nucleases for commercial uses of TAL nucleases in certain specified crop plants. We have an option to expand our license to additional crops under certain conditions.
The agreement will expire upon the expiration of the last to expire valid claim of the licensed patents under the agreement. Either party may terminate the agreement in the event of the insolvency or bankruptcy of the other party, or immediately upon written notice in the event that the other party, or its sublicensees or subcontractors challenges the validity or enforceability of any licensed patent in a court or other applicable authority. Either party may terminate the agreement by written notice in the event of the other party’s breach that has not been cured within a specified number of days after receiving notice of such breach. In the event of termination of license agreement between the University of Minnesota and Cellectis with respect to the TALEN technology, our license from Two Blades Foundation will terminate.
Trademarks
As of May 31, 2017, we had two pending trademark applications in the United States.
Government Regulation and Product Approval
In the United States, the FDA and the USDA Food Safety Inspection Service, or FSIS, are primarily responsible for overseeing food regulation and safety, although as many as fifteen federal agencies also play a role in U.S. food regulation, including several other agencies within USDA.
USDA has regulatory jurisdiction over transgenic crops through the Animal and Plant Health Inspection Service, or APHIS. Under the Plant Protection Act, USDA requires anyone who wishes to import, transport interstate, or plant a “regulated article” to apply for a permit or notify APHIS that the introduction will be made. Regulated articles are defined as “any organism which has been altered or produced through genetic engineering … which USDA determines is a plant pest or has reason to believe is a plant pest.” The petition process can be a multi-year process that varies based on a number of factors, including APHIS’ familiarity with similar products, the type and scope of the environmental review conducted, and the number and types of public comments received. APHIS conducts a comprehensive science-based review of the petition to assess, among other things, plant pest risk, environmental considerations pursuant to the National Environmental Policy Act of 1969, or NEPA, and any potential impact on endangered species. If, upon the completion of the review, APHIS grants the petition, the product is no longer deemed a “regulated article” and the petitioner may commercialize the product, subject to any conditions set forth in the decision. If APHIS does not determine the product to be non-regulated, the product may be subject to extensive regulation, including permitting requirements for import, handling, interstate movement, and release into the environment, and inspections.
96
Table of Contents
We have submitted petitions to APHIS for five of our product candidates to date: high oleic soybeans, high oleic/low lin soybeans, cold storable potatoes, reduced browning potatoes and powdery mildew resistant wheat. We have received confirmation from APHIS for all five product candidates that APHIS does not consider such product candidate to be a “regulated article” under the Plant Protection Act. There can be no guarantee of the timing or success in obtaining nonregulated status from APHIS for our other crops or that the governing regulations will not change. Government regulations, regulatory systems, and the politics that influence them vary widely among jurisdictions and change often.
The FDA has jurisdiction to regulate more than 80 percent of the U.S. food supply. It derives its regulatory power from the FDCA, which has been amended over time by several subsequent laws. The FDA’s oversight of food safety and security is primarily carried out by its Center for Food Safety and Applied Nutrition. To execute its responsibilities, the FDA employs a team of more than 900 investigators and 450 analysts in the foods program who conduct inspections and collect and analyze product samples. The FDA typically does not perform pre-market inspection for foods. The FDA also regulates ingredients, packaging, and labeling of foods, including nutrition and health claims and the nutrition facts panel. Foods are typically not subject to premarket review and approval requirements, with limited exceptions.
For its part, the FDA regulates foods made with GMOs under its 1992 “Statement of Policy: Foods Derived from New Plant Varieties.” Under this policy, the FDA regulates foods derived from genetically modified plant varieties consistent with the framework for non-genetically modified foods. Under Section 409 of the FDCA, any substance that is reasonably expected to become a component of food is considered a “food additive” that is subject to premarket approval by the FDA, unless the substance is generally recognized as safe, or GRAS. Companies are responsible for making an initial determination of whether a food substance falls under an existing food additive regulation, requires a new food additive petition, or is GRAS. A company may market a new food ingredient based on its independent determination that the substance is GRAS; however, the FDA can disagree and take enforcement action. The FDA offers a voluntary consultation process to determine whether foods derived from genetically modified plant varieties will be subject to these more stringent regulatory requirements. In most cases, however, foods derived from genetically modified plant varieties are not subjected to premarket review and approval processes.
The FDA does not currently require manufacturers to label foods made with GMOs as such, but permits voluntary labeling pursuant to a 2001 guidance document. This policy has been the subject of pressure from consumer groups and there can be no guaranty that it will not change in the future.
Competition
The market for agricultural biotechnology products is highly competitive, and we face significant direct and indirect competition in several aspects of our business. Competition for improving plant genetics comes from conventional and advanced plant breeding techniques, as well as from the development of genetically modified traits. Other potentially competitive sources of improvement in crop yields include improvements in crop protection chemicals, fertilizer formulations, farm mechanization, other biotechnology, and information management. Programs to improve genetics and chemistry are generally concentrated within a relatively small number of large companies, while non-genetic approaches are underway with broader set of companies. Additionally, competition for providing more nutritious ingredients for food companies come from chemical-based ingredients, additives and substitutes, which are developed by various companies.
In general, we believe that our direct competitors generally fall into the following categories:
| • | Large Agricultural Biotechnology, Seed, and Chemical Companies—Only a limited number of companies have been actively involved in new trait discovery, development, and commercialization: BASF SE, Bayer AG, DuPont Pioneer, Monsanto Co., Syngenta AG, Takii & Company, LTD and The Dow Chemical Co. Many of these companies have substantially larger budgets for gene discovery, |
97
Table of Contents
| research, development, and product commercialization than we do. Some of these companies also have substantial resources and experience managing the regulatory process for new genetically modified seed traits. Each of Monsanto, DuPont Pioneer, Syngenta, Dow, and Bayer also has significant chemical crop protection background and businesses. The trait pipelines of these companies are heavily weighted toward biotic stress traits, although they also have significant programs aimed at development of abiotic stress traits, and would compete with our farmer-centric agriculturally advantageous trait products. While these companies have internal programs that may compete with our own, they also seek new traits externally and, as such, some of them have been, and may in the future be, our partners. |
| • | Specialty Food Ingredient Companies—Companies focused on providing solutions to the food industry through chemical, synthetic, or other methods. These companies include International Flavors & Fragrances Inc., Givaudan, Kerry Group plc, CSM N.V., FMC Corporation, CP Kelco, Novozymes, Ingredion Incorporated and Royal DSM N.V. These companies develop products that include chemical modification of food ingredients to achieve functional performance, such as chemical additives for food products. Such products currently do, and may in the future, compete with our consumer-centric food ingredient products. While these companies may produce products that directly compete with our own, some may in the future be our partners in developing healthier food ingredients for consumers. |
We also believe that we may face indirect competition from trait research and development companies as well as agricultural research universities and institutions. Given the global importance of agriculture, there are a number of companies, research universities and institutions that specialize in research and development of agricultural yield and product quality traits. Companies such as Evogene Ltd., Ceres, Inc., and Keygene N.V., among others, are competitors in our field but typically focus on a limited number of traits, and do not generally have the product development, gene-editing technologies and regulatory infrastructure necessary to bring traits to market. Therefore, they typically outlicense trait technologies to large industry players with in-house development and regulatory capabilities at a relatively early stage of development. Most publicly funded research is focused on basic research programs that aim to understand basic biological processes and does not necessarily engage in further development and commercialization of discovered traits. While these programs are potentially competitive with us, we view them primarily as sources of innovation that fit with our business model.
Many of our current or potential competitors, either alone or with their R&D or collaboration partners, have significantly greater financial resources and expertise in research and development, manufacturing, testing and marketing approved products than we do. Mergers and acquisitions in the plant science, specialty food ingredient and agricultural biotechnology, seed and chemical industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through R&D and collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel, as well as in acquiring technologies complementary to, or necessary for, our programs.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products faster, with lower research costs or with more desirable traits than we can.
Research and Development
As of May 31, 2017, we had 20 employees dedicated to research and development. Our research and development team has technical expertise in genome engineering, molecular biology, biochemistry, genetics and genetic engineering, plant physiology and plant breeding. Our research and development activities are conducted principally at our Minnesota facilities. We have made, and will continue to make, substantial investments in research and development. Our research and development expenses were $1.3 million, $5.6 million and $2.8 million for the three months ended March 31, 2017 and for the years ended December 31, 2016 and 2015, respectively.
98
Table of Contents
Facilities
We lease a 17,485 square-foot facility in New Brighton, Minnesota, which commenced on October 15, 2012 and expires on October 14, 2017. In March 2016, we acquired a 10-acre parcel in Roseville, Minnesota where we built a 10,900 square-foot greenhouse facility that became operational in July 2016. We plan to build our headquarters facility at the same location and expect the new facility to be composed of a 35,000 square-foot office and lab building, with greenhouses and outdoor research plots. We intend to finance the building for the new facility in whole or in part through a sale leaseback transaction. On June 20, 2017, we signed an agreement with a third party buyer for the sale of the land and warehouse facility. The completion of the sale is conditioned on us and the buyer entering into a new facility construction agreement and a lease in respect of the property in the forms contemplated by the sale agreement. We expect to close on the proposed transaction and construction to begin on the new facilities in the second half of 2017.
Legal Proceedings
We currently are not a party to any material litigation or other material legal proceedings. From time to time, we may be subject to legal proceedings and claims in the ordinary course of business.
Corporate Information
We were incorporated in Delaware on January 8, 2010 and are a wholly owned subsidiary of Cellectis S.A. (société anonyme). Our principal executive offices are located at 600 County Road D West, Suite 8, New Brighton, MN 55112, United States of America, and our telephone number is +1 (651) 683-2807. We also maintain a website at www.calyxt.com. The information contained in, or that can be accessed through, our website is not part of this prospectus.
99
Table of Contents
Directors and Executive Officers
The following table sets forth information regarding our directors and executive officers, as of the date of this prospectus. Upon completion of this offering, our Board of Directors is expected to consist of five members.
| Name |
Age |
Position | ||
| André Choulika, Ph.D. |
52 | Chairman and Director | ||
| Federico A. Tripodi |
40 | Chief Executive Officer | ||
| Bryan W. J. Corkal |
49 | Chief Financial Officer | ||
| Daniel Voytas, Ph.D. |
54 | Chief Science Officer | ||
| Feng Zhang, Ph.D. |
42 | Chief Operations Officer | ||
| Manoj Sahoo |
42 |
Chief Commercial Officer | ||
| Glenn Bowers, Ph.D. |
64 | Vice President of Breeding | ||
| Michel Arbadji |
52 | Director of Business Development | ||
| Joseph B. Saluri, J.D. |
50 | General Counsel, Executive Vice President – Corporate Development | ||
| Philippe Dumont | 66 | Director | ||
| Alain Godard | 71 | Director | ||
| Anna Ewa Kozicz-Stankiewicz | 42 | Director | ||
| Laurent Arthaud | 54 | Director |
Set forth below is information concerning our directors and executive officers as of the date of this prospectus.
André Choulika, Ph.D., has served as Chairman of our Board of Directors since August 2010. Dr. Choulika is one of the founders of Cellectis and has served as Chief Executive Officer of Cellectis since the company’s inception in 1999. He has served as the Chairman of the Board of Directors of Cellectis since 2011. Since December 2014, Dr. Choulika has served as Chief Executive Officer of Cellectis, Inc. From 1997 to 1999, Dr. Choulika worked as a post-doctoral fellow in the Division of Molecular Medicine at Boston Children’s Hospital, where he was one of the inventors of nuclease-based genome editing technologies and a pioneer in the analysis and use of meganucleases to modify complex genomes. After receiving his Ph.D. in molecular virology from the University of Paris VI (Pierre et Marie Curie), he completed a research fellowship in the Harvard Medical School Department of Genetics. His management training is from HEC (Challenge +). Based on Dr. Choulika’s deep knowledge of our company and scientific experience, we believe Dr. Choulika has the appropriate set of skills to serve as a member of our Board of Directors.
Federico A. Tripodi has served as our Chief Executive Officer since May 2016. He holds a Master of Business Administration degree from Washington University’s Olin Business School, as well as an agronomic engineering degree from Buenos Aires University, and has gathered extensive experience in agricultural R&D and product development during his nearly two-decade career in the agriculture biotechnology and seeds industry. Prior to joining Calyxt, Mr. Tripodi worked as General Manager for Monsanto Company’s Sugarcane Division in Brazil for nearly three years. He held other roles for Monsanto in Saint Louis, Missouri, spanning Corporate Strategy (2011– 2013), Omega-3 Program Lead (2009 – 2011), Oilseeds Global Quality Management Lead (2008 – 2009) and multiple other roles that involved managing multidisciplinary research teams in the technology organization between 2001 and 2008. During his tenure at Monsanto, Mr. Tripodi led or participated with early discovery and late commercialization phase product launches across the Americas, which included biotech consumer traits (improved composition soybean oils) and farmer traits (high yield, drought tolerance, insect protection and herbicide tolerance). Mr. Tripodi started his career in Argentina in 1998 in field research of biotechnology traits and chemistry formulations until he moved to Saint Louis in 2001. Mr. Tripodi also has experience as a director of a startup and served on the board of directors for a not-for profit.
Bryan W. J. Corkal has served as our Chief Financial Officer since December 2016. Mr. Corkal received his MBA from York University in Toronto, Canada and a B.Sc. in Civil Engineering from the University of
100
Table of Contents
Manitoba. Mr. Corkal is a CFA charter holder and is a CPA in the state of Missouri. Mr. Corkal brings extensive finance and commercial experience in the seeds and traits agricultural sector having worked over 17 years at Monsanto in a variety of finance and strategy roles including the acquisition and integration of several companies. Prior to joining Calyxt, Mr. Corkal was the North America Supply Chain Finance Lead at Monsanto, a business with a total annual product cost of over $2 billion. Mr. Corkal’s career at Monsanto also included roles as Director of Investor Relations and Director of Finance (regional CFO) for the Latin America North division, a region covering 30 countries throughout the Americas, where he managed the controllership, credit and collections, tax, payroll, treasury, pension plan and financial planning and analysis functions. Early in his career, Mr. Corkal worked for Ernst & Young and Delcan Corporation as a consultant on a number of projects throughout Canada and Latin America.
Daniel Voytas, Ph.D., has served as our Chief Science Officer since May 2010. Dr. Voytas graduated summa cum laude from Harvard College in 1984 and received his Ph.D. in genetics from Harvard Medical School in 1990. He is one of our co-founders and one of the inventors of the TALEN technology. He continues to optimize the use of TALEN for the targeted modification of plant genomes. In addition to his role at Calyxt, Dr. Voytas is a professor in the Department of Genetics, Cell Biology and Development at the University of Minnesota (UMN), which he joined in 2008, and Director of the UMN’s Center for Genome Engineering. In 1992, Dr. Voytas joined the faculty at Iowa State University. Prior to this, he conducted postdoctoral research at Johns Hopkins University School of Medicine. Dr. Voytas is an elected Fellow of the American Association for the Advancement of Science.
Feng Zhang, Ph.D., has served as our Chief Operations Officer since May 2010. Dr. Zeng joined Calyxt in 2010 to develop and lead the trait development programs for crops and vegetables. Dr. Zhang obtained his Ph.D. from Iowa State University working on maize genetics and received post-doctoral training at the University of Georgia. Dr. Zhang has more than 10 years of experience in plant biotechnology development and commercialization and has pioneered development of highly efficient technologies for crop improvement. He has published more than 20 peer-reviewed papers in various journals including Nature, Nature Methods, Proceedings of the National Academy of Sciences and Plant Cell. Dr. Zhang is the co-inventor of more than 10 patents and patent applications. Before joining us, he co-invented TALEN technology with Dr. Voytas at the University of Minnesota and Dr. Adam Bogdanove at Iowa State University.
Manoj Sahoo has served as our Chief Commercial Officer since March 2017. He holds a MBA from the Tuck School of Business at Dartmouth College and a B.S. in Chemical Engineering from the National Institute of Technology in India. Mr. Sahoo has more than two decades of experience working in a variety of roles covering commercial, strategy, business development and mergers and acquisitions for global corporations in agriculture, food, energy and materials fields. Prior to joining us he was Assistant Vice President for Food Ingredients and Bio-industrial Enterprise at Cargill. At Cargill, he was responsible for revenues of over $1 billion, leading the commercial enterprise team to triple its earnings from bio-based products and managing relationships with large institutional customers. His prior roles at Cargill included Business Development Director for Starches and Sweeteners North America as well as serving as an investment team member with the Emerging Business Accelerator, a group structured along corporate venture capital groups, to invest in white space opportunities for Cargill; he also worked in the Corporate Strategy & Development Group. Mr. Sahoo has also served on the boards of both Calysta Inc. and Rivertop Renewables as a Cargill representative. He was responsible for leading Cargill’s equity investments in the industrial biotechnology space including co-investment in real assets with institutional financial investors to build a $600 million commercial-scale aquaculture nutrition plant. He also serves on Industry Advisory Board of Larta Institute which assists the USDA, NIH and NSF with the commercialization assistance program.
Glenn Bowers, Ph.D., has served as our Vice President of Breeding since December 2015. He is responsible for breeding, field trialing and seed production for all crops. Dr. Bowers has M.S. and Ph.D. degrees in Plant Pathology with a focus on breeding and genetics of resistance. He spent 17 years managing a soybean breeding program with Texas A&M University, followed by a year doing the same at Purdue University. He then spent 16
101
Table of Contents
years with Syngenta, first managing a soybean breeding program and then as global head of soybean breeding. He has extensive experience in Argentina and Brazil, in addition to North America. He is also a certified project manager (PMP). Dr. Bowers has extensive experience in delivering products, both global and stateside, through effective collaboration with marketing and supply chain. He is skilled in creating, developing, and managing diverse and globally dispersed teams. He has a deep knowledge of and experience in field trialing, disease phenotyping, and agronomy.
Michel Arbadji has served as our Director of Business Development since July 2015. Mr. Arbadji manages the external supply chain operations. Mr. Arbadji received his degree in Agriculture Engineering and M.A. in Economics and Agriculture Machinery from the Institut National Agronomique Paris Grignon in Paris, France. Prior to joining us, he headed the European and Middle East Operation for Signature Control Systems, build and managed the distribution network, EU marketing and sales. During that period he managed as project manager the new business development of the golf Irrigation division in Europe at John Deere with over 440 accounts. Mr. Arbadji started his career at the Toro Company EMEA, where he held several positions in business development, sales and marketing. Over his 27 years career he successfully built several pioneering businesses from R&D to large scale distribution channels set up. He participated as well in several product launches on the international market. In his career at Cellectis he served as CEO for Sceil the stem cells project (2013 – 2015). He is skilled in negotiation, communication and in delivering complex tasks.
Joseph B. Saluri, J.D., has served as General Counsel and Executive Vice President - Corporate Development since May 2017. He holds a Juris Doctorate from Drake Law School and a B.S.B.A. in investment finance from Drake University. Mr. Saluri has over 20 years of legal and business experience in in the global seeds and biotechnology industry, having served as Chief Legal Officer and Vice President of Business Development for Stine Seed Company, the largest supplier of soybean genetics in the U.S. market, together with its affiliates and related entities. Mr. Saluri was responsible for managing all legal matters related to the Stine Companies. He has a broad depth of experience in litigation, licensing, intellectual property, acquisitions, corporate and regulatory matters. In addition, Mr. Saluri also executed successful business development strategies at Stine that included the acquisitions of seed trait technologies, seed genetics and other ag-biotech technologies. He was involved in the execution and project management of major collaboration projects with various multinational agri-business corporations. Mr. Saluri has served on the public Board of Directors of Newlink Genetics Corporation since 2010, serving on the Compensation and Nominating and Governance Committees and on the Board of Directors of KemPharm, Inc. since 2014, where he chairs the Nominating and Governance Committee and serves on the Audit Committee. Previous to his employment at Stine, Mr. Saluri was an attorney and solicitor at law with Nicholas Critelli & Associates, PC, in Des Moines and London.
Philippe Dumont has served as a member of our Board of Directors since July 2017. Mr. Dumont retired in December 2012 from Bayer CropScience, where he was employed since May 2002. At Bayer he held the position of Head of Technology Management, Seeds, and was responsible for supervising globally the Regulatory Affairs and Regulatory Science functions, Stewardship, Public and Governmental Affairs and Communication impacting GMOs and seeds. Until 2006 Mr. Dumont also supervised the Legal and Intellectual Property functions in the seed business. Mr. Dumont also held the same responsibilities at Aventis Crop Science from December 1998 until April 2002. From 1987 to 1998, Mr. Dumont was General Counsel of Rhône-Poulenc Agrochimie. Prior to moving to France in 1987, Philippe held positions as an associate at Cravath Swaine & Moore (1975 – 1981), international legal counsel at Gulf Oil Corporation (1981-1983) and as solo practitioner in Washington D.C. from 1983 – 1986. Philippe is retired from the New York and District of Columbia Bars and is a graduate of the Georgetown University Law Center (JD 1975) and Columbia University (BA, magna cum laude 1972). Since June 2013 he has been serving as a director of Association Française des Biotechnologies Végétales, responsible for international relations, where he tries to promote public and governmental understanding of new breeding techniques and related regulatory issues. Based on his leadership and regulatory experience in the plant biotechnology industry, we believe Mr. Dumont has the appropriate set of skills to serve as a member of our Board of Directors.
Alain Godard has served as a member of our Board of Directors since July 2017 and is a member of Cellectis’ board of directors since 2007. He is a graduate of the Ecole Nationale Supérieure Agronomique de
102
Table of Contents
Toulouse and began his agronomy career in 1967 in Africa as a researcher at the Institut de Recherche pour les Huiles et Oléagineux. He joined in 1975 the French chemical group Rhône-Poulenc where he held various management positions in France and abroad before becoming CEO of the agrochemical subsidiary in 1991. In 1999 he was directly involved in the merger of Rhône-Poulenc and Hoechst to create Aventis and was appointed CEO of the Aventis CropScience subsidiary with a significant involvement in seeds and agricultural biotechnology. He left Aventis in 2002 to create a consulting company, SARL Godard & Co., specialized in agriculture and biotechnology, where he has served as Chief Executive Officer since 2009. Until 2016, Mr. Godard also served on the board of directors of Fermentalg S.A. We believe Mr. Godard’s leadership and management expertise in the plant biotechnology field qualifies him to serve as a member of our Board of Directors.
Anna Ewa Kozicz-Stankiewicz has served as a member of our Board of Directors since July 2017. Mrs. Kozicz received a BA in Math and Economics from Columbia College and her MBA from Columbia Business School. She started her career in 1996 at Credit Suisse First Boston in its Financial Institutions Group before moving to Goldman Sachs in 2000 where she held multiple positions, including Managing Director, and spent most of her time in the Principal Strategies Group with a focus on the global agricultural sector. She moved to Caxton Associates in 2009 to work as an equity Portfolio Manager before moving to BlackRock in 2012 where she worked as Head of US Strategy and Corporate Development as well as a private assets Portfolio Manager. During her time at BlackRock, she served as a director on the board of a New York-based federal credit union (PSFCU). She left BlackRock in 2017 to start a new investment platform, Anthelion Capital, focused on sustainability in agriculture, energy and transportation services. We believe Mrs. Kozicz’s extensive investment experience in the agriculture industry qualifies her to serve as a member on our Board of Directors.
Laurent Arthaud has served as a member of our Board of Directors since July 2017 and has served as a member of Cellectis’ board of directors since 2011. Mr. Arthaud has been the Managing Director of Life Sciences and Ecotechnologies for Bpifrance Investissement (formerly CDC Enterprises, a subsidiary of Caisse des Dépôts) since 2012. He currently serves on the boards of directors of Kurma Life Sciences Partners, TxCell, Adocia and Sparinvision. From 2006 to 2016, he served on the board of directors of Emertee Gestion. From 2006 to 2012, Mr. Arthaud held the position of Deputy CEO at CDC Entreprises. Since 2009 Mr. Arthaud has also directed InnoBio, an investment fund managed by Bpifrance Investissement as part of the FSI France Investissement program. From 1999 to 2004, he served as Vice President of Aventis Capital, an investment subsidiary of the pharmaceuticals group Aventis, and as President of Pharmavent Partners from 2004 to 2006. Mr. Arthaud is a graduate of the École Polytechnique and the École Nationale de Statistique et d’Administration Économique. We believe Mr. Arthaud’s extensive investment experience in the biotechnology industry qualifies him to serve as a member on our Board of Directors.
Controlled Company Exemption
After completion of this offering, Cellectis will continue to control a majority of the voting power of our outstanding common stock. As a result, we will be a “controlled company” within the meaning of the NASDAQ corporate governance standards. Under the NASDAQ rules, a company of which more than 50% of the voting power is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain NASDAQ corporate governance standards, including the requirements that:
| • | a majority of the board of directors consist of independent directors; |
| • | we have a nominating and corporate governance committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; and |
| • | we have a compensation committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities. |
We intend to utilize certain of these exemptions following the offering, and may utilize any of these exemptions for so long as we are a “controlled company”. Accordingly, you will not have the same protections afforded to stockholders of companies that are subject to all of the NASDAQ corporate governance requirements.
103
Table of Contents
Composition of the Board of Directors After this Offering
Following the completion of this offering, our Board of Directors will consist of five directors: Dr. André Choulika (Chairman), Mr. Philippe Dumont, Mr. Alain Godard, Mrs. Anna Ewa Kozicz-Stankiewicz and Mr. Laurent Arthaud. In connection with this offering, we intend to enter into the a stockholders agreement, which will provide Cellectis with certain rights relating to the composition of our Board of Directors. See “Certain Relationships and Related Party Transactions—Relationship with Cellectis—Stockholders Agreement.”
Board Committees
The standing committees of our Board of Directors are described below.
Audit Committee
The Audit Committee will initially be composed of Mr. Dumont (Chairman), Mrs. Kozicz-Stankiewicz and Dr. Choulika. Our Board of Directors has determined that Mr. Dumont and Mrs. Kozicz-Stankiewicz are independent under the applicable standards of the NASDAQ and the Exchange Act. Mr. Dumont qualifies as an “audit committee financial expert” as such term is defined in the regulations under the Exchange Act. We expect that the Audit Committee will comply with the applicable standards of the NASDAQ and the Exchange Act. The Audit Committee is responsible for, among other things, the oversight of the integrity of our financial statements and system of internal controls, the qualifications and independence of our independent registered accounting firm and the performance of our internal auditor and independent auditor. The Audit Committee also has the sole authority and responsibility to select, determine the compensation of, evaluate and, when appropriate, replace our independent registered public accounting firm. In addition, the Audit Committee will review reports from management, legal counsel and third parties relating to the status of compliance with laws, regulations and internal procedures. The Audit Committee will also be responsible for reviewing and discussing with management our policies with respect to risk assessment and risk management.
A copy of our Audit Committee Charter will be available on our website upon consummation of this offering.
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee will initially be composed of Dr. Choulika (Chairman), Mr. Dumont and Mr. Godard. The Nominating and Corporate Governance Committee is responsible for, among other things, matters of corporate governance and matters relating to the practices, policies and procedures of our Board of Directors, identifying and recommending candidates for election to our Board of Directors and each committee of our Board of Directors, and reviewing, at least annually, our corporate governance principles. The Nominating and Corporate Governance Committee will also advise on and recommend director compensation, which will be approved by the full Board of Directors. As a “controlled company,” we will not be required to have a corporate governance committee comprised entirely of independent directors.
A copy of our Nominating and Corporate Governance Committee Charter will be available on our website upon consummation of this offering.
Compensation Committee
The Compensation Committee will initially be composed of Dr. Choulika (Chairman), Mr. Dumont and Mr. Godard. The Compensation Committee is responsible for, among other things, reviewing and approving our overall compensation philosophy and overseeing the administration of related compensation benefit programs, policies and practices. The Compensation Committee is also responsible for annually reviewing and approving
104
Table of Contents
the corporate goals and objectives relevant to the compensation of our chief executive officer and other executive officers and evaluating their performance in light of these goals, reviewing the compensation of our executive officers and other appropriate officers, and administering our incentive and equity-based compensation plans. As a “controlled company,” we will not be required to have a compensation committee comprised entirely of independent directors.
A copy of our Compensation Committee Charter will be available on our website upon consummation of this offering.
Code of Business Conduct and Ethics
In connection with this offering, we will adopt a Code of Business Conduct and Ethics, or the Code of Conduct that is applicable to all of our employees, executive officers and directors. Following the completion of this offering, the Code of Conduct will be available on our website. The Audit Committee will be responsible for overseeing the Code of Conduct and will be required to approve any waivers of the Code of Conduct for employees, executive officers and directors. We expect that any amendments to the Code of Conduct, or any waivers of its requirements, will be disclosed on our website or in filings under the Exchange Act as required by the applicable rules and exchange requirements.
105
Table of Contents
Summary Compensation Table
The following table sets forth total compensation of our named executive officers for the year ended December 31, 2016. Our NEOs are current executive officers Federico A. Tripodi, Bryan W. J. Corkal and Daniel Voytas and former executive officers Luc Mathis, Gregory Smith and Richard Benish.
| Name and Principal Position |
Fiscal Year |
Salary ($) | Bonus ($) | Option Awards ($)(7) |
All Other Compensation ($)(8) |
Total ($) | ||||||||||||||||||
| Current Executive Officers |
||||||||||||||||||||||||
| Federico A. Tripodi(1) |
2016 | 210,538 | 96,219 | 232,050 | 78,000 | 616,807 | ||||||||||||||||||
| Chief Executive Officer |
||||||||||||||||||||||||
| Bryan W. J. Corkal(2) |
2016 | 16,782 | — | — | 2,538 | 19,320 | ||||||||||||||||||
| Chief Financial Officer |
||||||||||||||||||||||||
| Daniel Voytas(3) |
2016 | 180,000 | 100,000 | 273,000 | 240 | 553,240 | ||||||||||||||||||
| Chief Science Officer |
||||||||||||||||||||||||
| Former Executive Officers |
||||||||||||||||||||||||
| Luc Mathis(4) |
2016 | 65,769 | 9,865 | — | — | 75,634 | ||||||||||||||||||
| Former Chief Executive Officer |
||||||||||||||||||||||||
| Gregory Smith(5) |
2016 | 45,766 | — | — | 43,750 | 89,516 | ||||||||||||||||||
| Former Chief Financial Officer |
||||||||||||||||||||||||
| Richard Benish(6) |
2016 | — | — | — | — | — | ||||||||||||||||||
| Former Interim Chief Financial Officer |
||||||||||||||||||||||||
| (1) | Mr. Tripodi was appointed our CEO, effective on May 23, 2016. |
| (2) | Mr. Corkal was appointed our CFO, effective on December 5, 2016. |
| (3) | Dr. Voytas provided his services to us through a consulting agreement with us. The amount in the “Salary” column represents the consulting fee that he received under this agreement. |
| (4) | During 2016, Dr. Mathis served as our CEO until his resignation on May 22, 2016. Dr. Mathis’s compensation was converted from euros to U.S. dollars using the exchange rate of USD 1.0523 for 1 euro, which was the exchange rate on December 31, 2016. The amount in the “Bonus” column represents a bonus that he received in connection with his secondment to us. |
| (5) | During 2016, Mr. Smith served as our CFO until the termination of his employment on March 22, 2016. |
| (6) | Mr. Benish was appointed our interim CFO, effective on April 11, 2016, and served in that role until the termination of his service on December 4, 2016. Mr. Benish provided his services to us through a consulting agreement with CliftonLarsonAllen LLP (“CLA”), a third party. We paid CLA $262,838 for Mr. Benish’s services as our interim CFO. |
| (7) | This column reflects the fair value of options granted in 2016 based on their grant date fair value. In accordance with FASB ASC Topic 718, dollar amounts are not recognized for financial accounting reporting purposes for the fiscal year until the occurrence of a triggering event, i.e., a change of control, liquidation, dissolution or initial public offering. These amounts reflect our accounting expense for these awards, and do not correspond to the actual value that will be realized by the NEO. The assumptions used in the calculation of the amounts are described in note 9 “Stock-Based Compensation” to our audited financial statements included in this prospectus. The fair value of these awards will be remeasured each reporting period in accordance with liability accounting. |
On April 7, 2016, each of Mr. Tripodi and Dr. Voytas was granted an option to purchase 208,250 and 245,000 common shares, respectively, with an exercise price of $3.59 per share.
106
Table of Contents
| (8) | All other compensation for the year ended December 31, 2016 includes (a) for Mr. Tripodi, relocation assistance of $78,000, (b) for Mr. Corkal, relocation assistance of $2,538, (c) for Dr. Voytas, telephone expenses of $240 and (d) for Mr. Smith, a payment of $43,750 made in connection with his termination of employment. |
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth certain information regarding outstanding equity awards of our NEOs as of December 31, 2016. The market value of the shares in the following table is the fair value of such shares as of December 31, 2016.
| Option Awards(1)(2) | ||||||||||||||
| Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date | ||||||||||
| Federico A. Tripodi |
— | 208,250 | (1) | 3.59 | 4/7/2026 | |||||||||
| Bryan W. J. Corkal |
— | — | — | — | ||||||||||
| Daniel Voytas |
— | 49,000 | (1) | 3.71 | 12/3/2024 | |||||||||
| — | 16,905 | (1) | 21.83 | 9/9/2025 | ||||||||||
| — | 50,000 | (2) | 31.13 | 5/18/2025 | ||||||||||
| — | 50,000 | (2) | 29.52 | 9/8/2025 | ||||||||||
| — | 245,000 | (1) | 3.59 | 4/7/2026 | ||||||||||
| Luc Mathis(3) |
— | |
73,500 4,900 |
(1) (1) |
|
3.71 21.83 |
|
12/3/2024 9/9/2025 | ||||||
| Gregory Smith |
— | — | — | — | ||||||||||
| Richard Benish |
— | — | — | — | ||||||||||
| (1) | The option grants under the Existing Plan vest and become exercisable upon the achievement of two conditions: a time-vesting condition and a liquidity condition. In addition, in order for each such option to become exercisable, a triggering event, i.e., a change of control, liquidation, dissolution or initial public offering, must occur. Options will only become exercisable upon the occurrence of both the time-vesting and liquidity conditions. Options granted on December 3, 2014 vest 20% on January 3, 2015 and 20% on April 10, 2015, with the remainder vesting quarterly in equal installments over the following three years (or with an additional 25% vesting immediately if we undergo a change in control, liquidation, dissolution or initial public offering and the remainder vesting quarterly thereafter). Options granted on September 9, 2015 vest 20% on the grant date and 20% on the first anniversary of the grant date, with the remainder vesting quarterly in equal installments over the following three years (or with an additional 25% vesting immediately if we undergo a change in control, liquidation, dissolution or initial public offering and the remainder vesting quarterly thereafter). Options granted on April 7, 2016 vest 20% on the grant date and 10% on the first anniversary of the grant date, with the remainder vesting quarterly in equal installments over the following 42 months (or with an additional 25% vesting immediately if we undergo a change in control, liquidation, dissolution or initial public offering and the remainder vesting quarterly thereafter). |
On December 3, 2014, each of Dr. Voytas and Dr. Mathis was granted an option to purchase 49,000 and 73,500 common shares, respectively, with an exercise price of $3.71 per share.
On September 9, 2015, each of Dr. Voytas and Dr. Mathis was granted an option to purchase 16,905 and 4,900 common shares, respectively, with an exercise price of $21.83 per share.
On April 7, 2016, each of Mr. Tripodi and Dr. Voytas was granted an option to purchase 208,250 and 245,000 common shares, respectively, with an exercise price of $3.59 per share.
| (2) | Warrants granted by Cellectis on May 8, 2015 and September 8, 2015 vest in equal annual installments over the first, second and third anniversaries of the grant date. |
107
Table of Contents
On May 18, 2015, Dr. Voytas was granted warrants to purchase 50,000 common shares of Cellectis with an exercise price of €29.58 per share, or $31.13 per share using the exchange rate of USD 1.0523 for 1 euro, which was the exchange rate on December 31, 2016.
On September 8, 2015, Dr. Voytas was granted warrants to purchase 50,000 common shares of Cellectis with an exercise price of €28.01 per share, or $29.52 per share using the exchange rate of USD 1.0523 for 1 euro, which was the exchange rate on December 31, 2016.
| (3) | Dr. Mathis received grants of Cellectis free shares and options. However, these grants were made in respect of his services to Cellectis. |
Agreements with Named Executive Officers
We have entered into offer letters with each of Messrs. Tripodi and Corkal. Each of these arrangements provides for at-will employment and generally includes the named executive officer’s initial base salary, an indication of eligibility for an annual cash incentive award opportunity and equity awards. In addition, we have entered into an independent contractor agreement with Dr. Voytas. We and Cellectis previously entered into an employment agreement with Dr. Mathis, and we previously entered into a consulting agreement with CLA, through which we engaged Mr. Benish’s services. We also entered into a separation agreement with Mr. Smith in connection with his departure.
Federico A. Tripodi
We are party to an offer letter with our Chief Executive Officer, Federico A. Tripodi dated May 6, 2016. Pursuant to the offer letter, Mr. Tripodi’s initial base salary was established at $345,000. In addition, Mr. Tripodi is eligible to receive an annual cash bonus with a target value of 50% of his base salary based on his achievement of individual and/or company performance goals as determined by the Board of Directors if he remains employed through the bonus payment date. Mr. Tripodi’s base salary and his target bonus percentage are subject to periodic review, but any modification that results in a reduction of such compensation must be approved in writing by Mr. Tripodi. On April 7, 2016, in accordance with the terms of his offer letter, Mr. Tripodi was granted an option to purchase 208,250 shares of our common stock at an exercise price of $3.59 per share.
The offer letter provides Mr. Tripodi’s principal place of employment is New Brighton, Minnesota and requires that he relocate to within 50 miles by car of our headquarters within 6 months following the date of the agreement. In exchange for his relocation, the offer letter provides for relocation assistance of up to $78,000, which includes reimbursement of home sale assistance, travel relating to house hunting, temporary living and transition expenses, home purchase closing costs, moving expenses and an additional payment of 30% of any amount of reimbursed home sale assistance costs that are not tax deductible, up to $20,000. The offer letter requires Mr. Tripodi to repay all relocation-related amounts paid by us if he has not relocated by November 22, 2016 and he is required to repay a prorated portion of these sums if, prior to May 23, 2019, he resigns or his employment is terminated for “cause.”
While Mr. Tripodi’s offer letter provides that his employment is at-will and may be terminated at any time, with or without cause, his offer letter also provides that he will be entitled to severance benefits in the event his employment is terminated by us without cause (which does not include a termination due to Mr. Tripodi’s death or disability). Upon such a termination, he will be eligible to receive a pro rata bonus and 12 months of base salary severance paid in installments, provided that, upon our request, he executes a release in favor of us.
Under the terms of Mr. Tripodi’s offer letter, he has agreed to maintain the confidentiality of our proprietary or confidential information at all times during his employment and thereafter. He has assigned to us all of the intellectual property rights in any work product created or developed by him during the term of his employment. Mr. Tripodi has also agreed to notify us prior to starting a new position after which we may exercise an election to restrict him from competing for a restricted period of up to 12 months after termination. If Mr. Tripodi is
108
Table of Contents
receiving severance at this time, the severance payments will cease and he will be entitled to receive his base salary during the restricted period. If he breaches the non-competition provision, he is required to reimburse us for all severance and non-competition payments he has received. Furthermore, he has agreed not to solicit any of our customers, employees or prospective customers of any of our subsidiaries for the 24-month period following his termination of employment.
“Cause” means Mr. Tripodi’s (i) conviction, guilty plea or plea of nolo contendere of a felony or misdemeanor involving moral turpitude; (ii) any act of fraud or dishonesty or unauthorized disclosure of confidential information; (iii) failure or refusal to follow lawful directions of the Board of Directors; (iv) gross negligence or willful misconduct; (v) failure to perform his duties (other than resulting from illness) which is not cured within five days after written notice is provided to him; (vi) material failure to comply with written company policy or rule which is not cured within five days after written notice is provided to him; or (vii) material breach of an agreement with us which is not cured within 30 days after written notice is provided to him.
Bryan W. J. Corkal
We are party to an offer letter with our Chief Financial Officer, Bryan W. J. Corkal, dated November 16, 2016. Pursuant to the offer letter, Mr. Corkal’s initial base salary was established at $220,000. In addition, Mr. Corkal is eligible to receive an annual cash bonus with a target value of 30% of his base salary based on his achievement of individual and/or company performance goals as determined by the Board of Directors if he remains employed through the bonus payment date. Mr. Corkal’s base salary and his target bonus percentage are subject to periodic review.
The offer letter provides Mr. Corkal’s principal place of employment is New Brighton, Minnesota and requires that he relocate to within 50 miles by car of our headquarters prior to June 30, 2017. In exchange for his relocation, the offer letter provides for relocation assistance of $30,000, paid after the relocation is complete, and reimbursement of up to $20,000 for temporary living and transition expenses incurred prior to June 30, 2017. The offer letter requires Mr. Corkal to repay all relocation-related amounts paid by us by July 15, 2017 if he has not relocated by June 30, 2017, and he is required to repay a prorated portion of these sums if he resigns prior to the December 5, 2019.
While Mr. Corkal’s offer letter provides that his employment is at-will and may be terminated at any time, with or without cause, his offer letter also provides that he will be entitled to severance benefits in the event his employment is terminated by us without cause (which does not include a termination due to Mr. Corkal’s death or disability). Upon such a termination he will be eligible to receive a pro rata bonus and 12 months of base salary severance paid in installments, provided that, upon our request, he executes a release in favor of us.
Under the terms of Mr. Corkal’s offer letter, he has agreed to maintain the confidentiality of our proprietary or confidential information at all times during his employment and thereafter. He has assigned to us all of the intellectual property rights in any work product created or developed by him during the term of his employment. Mr. Corkal has also agreed to notify us prior to starting a new position after which we may exercise an election to restrict him from competing for a restricted period of up to 12 months after termination. If Mr. Corkal is receiving severance at this time, the severance payments will cease and he will be entitled to receive his base salary during the restricted period. If he breaches the non-competition provision, he is required to reimburse us for all severance and non-competition payments he has received. Furthermore, he has agreed not to solicit any of our customers, employees or prospective customers of any of our subsidiaries for the 24-month period following his termination of employment.
The definition of “cause” is generally consistent with the definition in Mr. Tripodi’s agreement.
109
Table of Contents
Daniel Voytas
Dr. Voytas entered into a consulting agreement with us, dated January 1, 2010, as amended on December 21, 2012, and spends 10 days per month providing his service to us under this agreement. Under the agreement, he renders support and advisory services including providing scientific support during business development missions, running the scientific advisory board and strategic planning services and analyses relating to project review, agricultural research and development and new technologies. Under the agreement, Dr. Voytas receives a monthly fee of $15,000 for 10 days of service per month.
The initial term of the agreement with Dr. Voytas expired on January 1, 2013 and was extended for a period of one year, with automatic renewals for an additional year unless Calyxt delivers written notice of nonrenewal. The agreement also terminates immediately if Dr. Voytas does not comply with the college and university policies at which he is a faculty member and obtain all necessary approvals to provide services and assign work product to us. The agreement provides that we will pay Dr. Voytas a lump sum equal to three months of consultation fees if he executes and does not revoke a release in favor of us and its subsidiaries if we terminate his agreement prior to the end of the term.
Under the terms of Dr. Voytas’ consulting agreement, he has agreed to maintain the confidentiality of proprietary or confidential information of ours and its subsidiaries at all times during his service and thereafter. He has assigned to us all of the intellectual property rights in any work product created or developed by him during the term of his service. Furthermore, he has agreed to not participate in a business that competes with our or solicit any of our customers or employees for the 12-month period following his termination of his consulting term.
Luc Mathis
Cellectis entered into an employment agreement with Dr. Mathis dated as of January 1, 2006, which was subsequently amended several times, with the most recent amendment entered into as of July 1, 2016. Dr. Mathis was our Chief Executive Officer from April 6, 2012 until his resignation on May 22, 2016. On his resignation date, Dr. Mathis was compensated at a monthly rate of $13,154 for his services to Cellectis and at a monthly rate of $1,973 for his secondment to Calyxt using the exchange rate of USD 1.0523 for 1 euro, which was the exchange rate on December 31, 2016.
Under the terms of Dr. Mathis’s employment agreement, he agreed to maintain the confidentiality of the Cellectis group’s proprietary or confidential information at all times during his employment and thereafter. He has assigned to Cellectis all of the intellectual property rights in any work product created or developed by him during the term of his employment. Dr. Mathis agreed with Cellectis to be subject to a non-competition provision for the 24-month period following his termination of employment. If Dr. Mathis breaches the non-competition provision, he is required to reimburse Cellectis a lump sum equal to 6 months’ of his non-competition payment of 60% of base compensation and bonus.
Under his employment agreement, Dr. Mathis became Vice President of New Venture at Cellectis after he ceased to be our Chief Executive Officer. Dr. Mathis’s employment agreement includes an exclusivity waiver, under which Dr. Mathis will not participate in any other professional activity without receiving authorization from the Chief Executive Officer of Cellectis, including any activity that competes with the business of any entities of the Cellectis group worldwide. If Dr. Mathis breaches this exclusivity provision, he may be required to pay a lump sum of 150,000 euros to Cellectis.
Gregory Smith
We entered into an offer letter with our former Chief Financial Officer, Mr. Smith, effective May 19, 2015, pursuant to which he was compensated at a rate of $175,000 per year, and eligible for an annual performance
110
Table of Contents
bonus no greater than 25% of his base salary and a one-time $50,000 bonus subject to certain performance metrics and following our completion of an IPO. Mr. Smith’s offer letter subjected him to various restrictive covenants including perpetual confidentiality obligations, assignment of intellectual property and 12-month noncompetition and 24-month employee and customer non-solicitation covenants. Under the terms of the offer letter, Mr. Smith was also eligible for severance benefits equal to three months of base salary if his employment was terminated without cause.
We and Mr. Smith agreed that he would separate from us and enter into a separation agreement effective March 22, 2016. Under the separation agreement, Mr. Smith is entitled to a sum of $43,750 in respect of specified claims. In addition, the separation agreement contains customary release and non-disparagement provisions and Mr. Smith forfeited all of his options and any rights under such equity documentation.
Richard Benish
We entered into a consulting agreement with a third party, CLA, dated as of April 5, 2016. The agreement provides that a third party, Richard Benish, will serve as interim CFO at a rate of $150 per hour, with travel time billed at half the normal hourly rate. His duties include financial reporting, analysis and planning, merger and acquisition support, management reporting and other accounting duties or special projects as needed. It contains clarifying language that CLA will serve as an independent contractor of ours and that we and CLA have not entered into an employment relationship.
Director Compensation
The following table sets forth the amount of compensation we paid to our sole director during our fiscal year 2016.
| Name |
Fees Earned or Paid in Cash ($) |
Stock Awards ($) |
Option Awards ($)(1) |
All Other Compensation ($) |
Total ($) | |||||||||||||||
| André Choulika |
— | — | 273,000 | — | 273,000 | |||||||||||||||
| (1) | As of December 31, 2016, Dr. Choulika held options to purchase an aggregate of 276,605 common shares. |
This column reflects the fair value of options granted in 2016 based on their grant date fair value. In accordance with FASB ASC Topic 718, dollar amounts are not recognized for financial accounting reporting purposes for the fiscal year until the occurrence of a triggering event, i.e., a change of control, liquidation, dissolution or initial public offering. These amounts reflect our accounting expense for these awards, and do not correspond to the actual value that will be realized by the director. The assumptions used in the calculation of the amounts are described in note 9 “Stock-Based Compensation” to our audited financial statements included in this prospectus.
Other than the equity award grant made to Dr. Choulika, we did not pay any director compensation.
Equity-Based Awards
We believe our ability to grant equity-based awards is a valuable and necessary compensation tool that aligns the long-term financial interests of our personnel with the financial interests of our stockholders. In addition, we believe that our ability to grant stock options and other equity-based awards helps us to attract and retain and top talent, and encourages them to drive company performance to promote the success of our business. The material terms of our equity incentive plans are described below.
Calyxt, Inc. Equity Incentive Plan
General
We adopted the Existing Plan on December 13, 2014.
111
Table of Contents
Awards
Awards granted under the Existing Plan may consist of incentive stock options or non-qualified stock options. Each award is subject to the terms and conditions set forth in the Existing Plan and to those other terms and conditions specified by the committee and memorialized in a written award agreement. The options will only become exercisable in the event that a triggering event or this offering occurs.
Shares Subject to the Existing Plan
Subject to adjustment in certain circumstances as discussed below, the Existing Plan authorizes up to 2,037,175 shares of our common stock for issuance pursuant to the terms of the Existing Plan. The maximum number of shares available for issuance of incentive stock options is 2,037,175. If and to the extent awards granted under the Existing Plan terminate, expire, cancel or are forfeited without being exercised and/or delivered, the shares subject to such awards will again be available for grant under the Existing Plan. Additionally, to the extent any shares subject to an award are tendered and/or withheld in settlement of any exercise price and/or any tax withholding obligation associated with that award, those shares will again be available for grant under the Existing Plan, but shares issued under the Existing Plan and later repurchased by us pursuant to a repurchase right shall not be available for grant under the Existing Plan in the future.
In the event of any stock split, reverse stock split, stock dividend, combination, consolidation, recapitalization or reclassification of the shares, rights offering, reorganization, merger, spin-off, split-up change in corporate structure or similar event or transaction, adjustments will be made by the administrator of the Existing Plan to: (i) to the aggregate number, class and/or issuer of the securities reserved for issuance under the Existing Plan; (ii) to the number, class and/or issuer of securities subject to outstanding awards; (iii) to the exercise price of outstanding options and (iv) any repurchase price per share applicable to shares issued pursuant to an award, in each case in a manner that reflects equitably the effects of such event or transaction.
Administration
The Board of Directors or, to the extent authority is delegated by the Board of Directors, its Compensation Committee or other committee (in either event, the “Administrator”) will administer the Existing Plan and determine the following items:
| • | designate participants; |
| • | determine the fair market value of common stock, provided that the determination is applied consistently to participants under the Existing Plan; |
| • | determine the types of awards to grant, the number of shares to be covered by awards, the terms and conditions of awards, whether awards may be settled or exercised in cash, shares, other awards, other property or net settlement and the circumstances under which awards may be canceled, repurchased or forfeited and vesting will be accelerated or forfeiture restrictions waived; |
| • | interpret and administer the Existing Plan and any instrument or agreement relating to, or award made under, the Existing Plan; |
| • | amend the terms or conditions of outstanding awards, including to accelerate the time or times at which an award becomes vested, unrestricted or may be exercised and to accommodate differences in local law, tax policy or custom which deviate from the terms and conditions of the Existing Plan, provided that the amendment does not materially and adversely affect the rights of any participant without his or her consent; |
| • | implement an option exchange program; |
| • | make any other determination and take any other action that it deems necessary or desirable to administer the Existing Plan. |
112
Table of Contents
Eligibility
Employees, directors, consultants and other of our service providers that provide services to us or our affiliates are eligible to participate in the Existing Plan. Only our employees or employees of our subsidiaries are eligible to receive incentive stock options.
Stock Options
Term, Purchase Price and Vesting. The exercise price of any stock option granted under the Existing Plan will be not less than the fair market value of our common stock, par value $0.0001 per share, on the date the option is granted.
The administrator of the Existing Plan may determine the term for each option, provided that the term of any option may not exceed ten years from the date of grant. The vesting schedule for each option will be determined by the administrator of the Existing Plan.
Effects of Termination of Service
Unless otherwise provided in an award agreement, all unvested awards under the Existing Plan will terminate and be forfeited in the event of any termination of employment. Generally, unless provided otherwise in the award agreement, the right to exercise any vested option terminates three months following termination of the participant’s relationship with us for reasons other than death, disability or termination for cause. If the participant’s relationship with us terminates due to death or disability, unless provided otherwise in the award agreement, the right to exercise a previously vested option will terminate nine months or six months, respectively following such termination. If the participant’s relationship with us is terminated for cause, any option not already exercised will automatically be forfeited as of the date of such termination.
Buyout Provision
The Existing Plan contains a provision that permits the Administrator to buy out using cash or common stock an option that was previously granted under the Existing Plan pursuant to the terms and conditions provided in the option award agreement.
Amendment and Termination of the Existing Plan
The Existing Plan will terminate on the tenth anniversary of the effective date. Our Board of Directors may amend, alter or discontinue the Existing Plan at any time.
Change of Control
In the event of a dissolution, liquidation or change in control of us, our Board of Directors has discretion to cash out all or any portion of outstanding awards, provided that the successor company will assume all or a portion of outstanding awards or substitute for equivalent awards, any combination thereof or, if the successor company does not agree to such treatment, the outstanding awards will terminate upon the transaction and, unless otherwise provided in the award agreement, the administrator may accelerate the vesting and exercisability of outstanding options to be terminated prior to the change in control.
A change of control will be deemed to have taken place upon: (i) the acquisition of all or substantially all of our assets, other than to an entity where the majority of its combined voting power is owned by us, where it is owned by the holders of our capital stock in substantially the same proportions as their ownership in us or where the majority of the voting capital stock retains majority voting power; (ii) a merger, consolidation or other business combination that results in the current holders of a majority of voting stock to cease to hold a majority
113
Table of Contents
of such stock following the transaction; or (iii) the acquisition by any person or group of persons other than the current, directly or indirectly, of more than 50% of the voting power of us or more than 50% of our equity interests, excluding transactions that are determined to be capital raising transactions by the Board of Directors.
2017 Omnibus Incentive Plan
General
Prior to our initial public offering, our Board of Directors adopted a new equity compensation plan in the form of an omnibus incentive plan, or the Omnibus Plan. We have granted awards of restricted stock units to certain employees and awards of restricted stock units and stock options to our employees, directors, officers and consultants and those of our parent or affiliates. The following is a summary of the material terms of the Omnibus Plan.
Purpose
The purpose of the Omnibus Plan is to motivate and reward those employees, directors and consultants who are expected to contribute significantly to our long term success and to further align employee interests to those of our stockholders.
Plan Term
The Omnibus Plan is scheduled to expire after ten years. The term will expire sooner if, prior to the end of the ten-year term or any extension period, the maximum number of shares available for issuance under the Omnibus Plan has been issued or our Board of Directors terminates the Omnibus Plan.
Authorized Shares and Award Limits
Subject to adjustment, 4,900,000 shares of our common stock (representing 19.1% of our issued and outstanding shares as of the effective time of this offering) will be available for awards to be granted under the Omnibus Plan (other than substitute awards; that is, awards that are granted in assumption of, or in substitution for, an outstanding award previously granted by a company or other business acquired by us or with which we combine). The total number of shares available for issuance under the Omnibus Plan will be increased on the first day of each Company fiscal year following the effective date of the Company’s Initial Public Offering in an amount equal to the least of (i) 4,900,000 shares, (ii) 5% of outstanding shares on the last day of the immediately preceding fiscal year of (iii) such number of shares as determined by the Board of Directors in its discretion. An individual who is a non-employee director may not receive an award under the Omnibus Plan that relates to more than $5,000,000 for any calendar year.
Subject to adjustment, the maximum number of shares of our common stock that may be granted to any single individual during any calendar year is as follows: (i) stock options and SARs that relate to no more than 490,000 shares of our common stock; (ii) restricted stock and RSUs that relate to no more than 490,000 shares of our common stock; (iii) performance awards denominated in shares and other share-based awards that relate to no more than 490,000 shares of our common stock; (iv) deferred awards denominated in shares that relate to no more than 490,000 shares of our common stock; (v) deferred awards denominated in cash that relate to no more than $5,000,000; and (vi) performance awards denominated in cash and other cash-based awards that relate to no more than $5,000,000, provided that such no participant may receive awards that relate to more than 490,000 shares of our common stock during the fiscal year of any participant’s initial year of service with the Company.
If an award is forfeited, expires, terminates or otherwise lapses or is settled for cash, the shares covered by such award will again be available for issuance under the Omnibus Plan.
114
Table of Contents
Administration
The Board of Directors or, to the extent authority is delegated by the Board of Directors, its Compensation Committee or other committee will administer the Omnibus Plan.
The Board of Directors or, to the extent authority is delegated by the Board of Directors, its Compensation Committee or other committee (in either event, the “Administrator”) will administer the Omnibus Plan and determine the following items:
| • | determine the fair market value of the common stock; |
| • | select the participants to whom awards may be granted |
| • | approve forms of agreements, amend or modify outstanding awards or award agreements |
| • | correct any defect, supply any omission and reconcile any inconsistency in the Plan or any Award, in the manner and to the extent it will deem desirable to carry the Plan into effect; |
| • | establish, amend, suspend or waive rules and regulations and appoint agents, trustees, brokers, depositories and advisors and determine such terms of their engagement as it will deem appropriate; |
| • | construe and interpret the terms of the plan, any award agreement and any agreement related to any award; and |
| • | make any other determination and take any other action that it deems necessary or desirable to administer the Omnibus Plan |
To the extent not inconsistent with applicable law, the Administrator may delegate to one or more of our officers the authority to grant stock options or SARs or other awards in the form of share rights under the Omnibus Plan.
Types of Awards
The Omnibus Plan provides for grants of incentive and non-qualified stock options, SARs, restricted stock, RSUs, performance awards, deferred awards, other share-based awards and other cash-based awards:
| • | Stock Options. A stock option is a contractual right to purchase shares at a future date at a specified exercise price. The per share exercise price of a stock option (except in the case of substitute awards) will be determined by the Administrator at the time of grant but may not be less than the closing price of a share of our common stock on the day prior to the grant date. The Administrator will determine the date on which each stock option becomes vested and exercisable and the expiration date of each option. No stock option will be exercisable more than ten years from the grant date, except that the Administrator may generally provide for an extension of such ten-year term in the event the exercise of the option would be prohibited by law on the expiration date. Stock options that are intended to qualify as incentive stock options must meet the requirements of Section 422 of the Internal Revenue Code. |
| • | SARs. A SAR represents a contractual right to receive, in cash or shares, an amount equal to the appreciation of one share of our common stock from the grant date over the exercise or hurdle price of such SAR. The per share exercise price of a SAR (except in the case of substitute awards) will be determined by the Administrator but may not be less than the closing price of a share of our common stock on the day prior to the grant date. The Administrator will determine the date on which each SAR may be exercised or settled and the expiration date of each SAR. However, no SAR will be exercisable more than ten years from the grant date. |
| • | Restricted Stock. Restricted stock is an award of shares of our common stock that are subject to restrictions on transfer and a substantial risk of forfeiture. |
| • | RSUs. An RSU represents a contractual right to receive the value of a share of our common stock at a future date, subject to specified vesting and other restrictions. |
115
Table of Contents
| • | Performance Awards. Performance awards, which may be denominated in cash or shares, will be earned upon the satisfaction of performance conditions specified by the Administrator. The performance conditions for awards that are intended to qualify as “performance-based compensation” for purposes of Section 162(m) of the Internal Revenue Code may include, but not be limited to, the following: return measures (including total shareholder return; return on equity; return on assets or net assets; return on risk-weighted assets; and return on capital (including return on total capital or return on invested capital)); revenues (including total revenue; gross revenue; net revenue; and net sales); income/earnings measures (including earnings per share; earnings or loss (including earnings before or after interest, taxes, depreciation and amortization); gross income; net income; operating income (before or after taxes); pre-or after-tax income or loss (before or after allocation of corporate overhead and bonus); pre- or after-tax operating income; net earnings; net income or loss (before or after taxes); operating margin; gross margin; and adjusted net income); expense measures (including expenses; operating efficiencies; and improvement in or attainment of expense levels or working capital levels (including cash and accounts receivable)); cash flow measures (including cash flow or cash flow per share (before or after dividends); and cash flow return on investment); share price measures (including share price; appreciation in and/or maintenance of share price; and market capitalization); strategic objectives (including market share; debt reduction; customer growth; employee satisfaction; research and development achievements; mergers and acquisitions; management retention; dynamic market response; expense reduction initiatives; reductions in costs; risk management; regulatory compliance and achievements; recruiting and maintaining personnel; and business quality); and other measures (including economic value-added models or equivalent metrics; economic profit added; gross profits; economic profit; comparisons with various stock market indices; financial ratios (including those measuring liquidity, activity, profitability or leverage); cost of capital or assets under management; and financing and other capital raising transactions (including sales of the Company’s equity or debt securities; factoring transactions; sales or licenses of the Company’s assets, including its intellectual property, whether in a particular jurisdiction or territory or globally; or through partnering transactions)). These performance criteria may be measured on an absolute (e.g., plan or budget) or relative basis, may be established on a corporate-wide basis or with respect to one or more business units, divisions, subsidiaries or business segments, may be based on a ratio or separate calculation of any performance criteria and may be made relative to an index or one or more of the performance goals themselves. |
| • | Deferred Awards. The Administrator is authorized to grant awards denominated in a right to receive shares of our common stock or cash on a deferred basis. |
| • | Other Share-Based Awards. The Administrator is authorized to grant other share-based awards, which may be denominated in shares of our common stock or factors that may influence the value of our shares. |
| • | Other Cash-Based Awards. The Administrator is authorized to grant other cash-based awards either independently or as an element of or supplement to any other award under the Omnibus Plan. |
116
Table of Contents
Adjustments
In the event that the Administrator determines that, as result of any stock split, reverse stock split, stock dividend, combination, consolidation, recapitalization (including a recapitalization through a large nonrecurring cash dividend) or reclassification of the shares, repurchase, exchange or subdivision of the Shares or other securities of the Company, a rights offering, a reorganization, merger, spin-off, split-up, change in corporate structure or other similar occurrence, in each case excluding any triggering event, i.e., a change in control to an entity of which the Company is not a majority owner, or is not a majority owner of the Company, an adjustment is appropriate to prevent dilution or enlargement of the benefits or potential benefits intended to be made available under the Omnibus Plan, the Administrator will, subject to compliance with Section 409A of the Internal Revenue Code, adjust equitably any or all of:
| • | the number, type and class of shares or other stock or securities: available for future awards and covered by each outstanding award. |
| • | the grant, purchase, exercise or hurdle price covered by each such outstanding award; and |
| • | any repurchase price per share applicable to shares issued pursuant to any award, or, if deemed appropriate, will make a provision for a cash payment to the holder of an outstanding award. |
In addition, the Administrator may adjust the terms and conditions of, and the criteria included in, outstanding awards in recognition of events affecting the Company or the financial statements of the Company, or changes in applicable laws, regulations or accounting principles, whenever the Administrator determines that such adjustments are appropriate in order to prevent dilution or enlargement of the benefits or potential benefits intended to be made available under the Omnibus Plan, subject, in all such instances, to compliance with Section 162(m) of the Internal Revenue Code.
No Repricing
Except as provided the Omnibus Plan’s adjustment provisions, no action will directly or indirectly, through cancellation and regrant or any other method, reduce, or have the effect of reducing, the exercise or hurdle price of any award established at the time of grant thereof without approval of our stockholders.
Termination of Service and Change of Control
The Administrator will determine the effect of a termination of employment or service on outstanding awards, including whether the awards will vest, become exercisable, settle or be forfeited. In the event of a change of control, except as otherwise provided in the applicable award agreement, the Administrator may provide for:
| • | assumption or substitution with equivalent awards of outstanding awards under the Omnibus Plan by us (if we are the surviving corporation) or by the surviving corporation or its parent or subsidiary; |
| • | Termination of outstanding awards under the Omnibus Plan in exchange for a payment of cash, securities and/or other property equal to the excess of the fair market value of the portion of the awards stock that is vested and exercisable immediately prior to the consummation of the corporate transaction over the per share exercise price; |
| • | any combination of assumption, substitution or termination of outstanding awards under the Omnibus Plan as above; provided that outstanding awards of stock options and SARs may be cancelled without consideration if the fair market value on the date of the change in control is greater than the exercise or hurdle price of such award; or |
| • | acceleration of the vesting (including the lapse of any restrictions, with any performance criteria or conditions deemed met at target) and exercisability of outstanding award in full prior to the date of the change of control and the expiration of awards not timely exercised by the date determined by the Administrator. |
117
Table of Contents
Amendment and Termination
Our Board of Directors may amend, alter, suspend, discontinue or terminate the Omnibus Plan. The Administrator may also amend, alter, suspend, discontinue or terminate, or waive any conditions or rights under, any outstanding award. However, subject to the adjustment provision and change of control provision, any such action by the Administrator that would materially adversely affect the rights of a holder of an outstanding award may not be taken without the holder’s consent, except to the extent that such action is taken to cause the Omnibus Plan to comply with applicable law, stock market or exchange rules and regulations, or accounting or tax rules and regulations, or to impose any “clawback” or recoupment provisions on any awards in accordance with the Omnibus Plan. The Company will also seek, to the extent necessary and desirable to comply with applicable laws, the approval of holders of capital stock with respect to any amendment of the Omnibus Plan.
118
Table of Contents
The following table sets forth certain information regarding beneficial ownership of our common stock as of June 30, 2017, and as adjusted to reflect the sale of the shares of common stock in this offering, for:
| • | each person whom we know to own beneficially more than 5% of our common stock; |
| • | each director and named executive officer individually; and |
| • | all directors and executive officers as a group. |
In accordance with the rules of the SEC, beneficial ownership includes voting or investment power with respect to securities and includes the shares issuable pursuant to stock options that are exercisable within 60 days of June 30, 2017. Shares issuable pursuant to stock options are deemed outstanding for computing the percentage of the person holding such options but are not outstanding for purposes of computing the percentage of any other person. The number of shares of common stock outstanding after this offering includes 6,060,606 shares of common stock being offered for sale by us in this offering.
The percentage of beneficial ownership for the following table is based on 19,600,000 shares of common stock outstanding as of June 30, 2017, and 25,660,606 shares of common stock outstanding after the completion of this offering assuming no exercise of the underwriters’ over-allotment option.
The table below does not reflect any shares of our common stock that our directors and executive officers may purchase through the directed share program, described under “Underwriting—Directed Share Program” or any shares of our common stock that may be purchased by Cellectis in this offering.
Unless otherwise indicated, the address for each listed director and named executive officer is c/o Calyxt, 600 County Road D West, Suite 8, New Brighton, MN 55112. The address of Cellectis S.A. is 8, rue de la Croix Jarry, 75013, Paris, France.
| Common Stock Beneficially Owned Prior to the Completion of This Offering |
Common Stock Beneficially Owned Following the Completion of This Offering |
|||||||||||||||
| Name of Beneficial Owner |
Number of Shares |
Percentage of Class |
Number of Shares** |
Percentage of Class |
||||||||||||
| 5% Beneficial Owner: |
||||||||||||||||
| Cellectis S.A. |
19,600,000 | 100 | % | 19,600,000 | 76.4 | % | ||||||||||
| Directors and Executive Officers: |
||||||||||||||||
| André Choulika |
— | — | 161,259 | (1) | * | |||||||||||
| Philippe Dumont |
— | — | — | — | ||||||||||||
| Alain Godard |
— | — | 61,985 | (2) | * | |||||||||||
| Anna Ewa Kozicz-Stankiewicz |
— | — | — | — | ||||||||||||
| Laurent Arthaud |
— | — | — | — | ||||||||||||
| Federico A. Tripodi |
— | — | 114,538 | (3) | * | |||||||||||
| Bryan W. J. Corkal |
— | — | 29,400 | (4) | * | |||||||||||
| Daniel Voytas |
— | — | 197,274 | (5) | * | |||||||||||
| Luc Mathis |
— | — | 57,575 | (6) | * | |||||||||||
| Gregory Smith |
— | — | — | — | ||||||||||||
| Richard Benish |
— | — | — | — | ||||||||||||
| Directors and executive officers as a group (13 persons) |
— | — | 827,684 | 1.9 | % | |||||||||||
| * | Represents beneficial ownerships of less than one percent of our outstanding shares of common stock. |
| ** | Represents shares underlying stock options that will become exercisable as a result of this offering. Outstanding stock options are not exercisable until a triggering event or an initial public offering occurs. |
| (1) | Represents 161,259 shares of common stock issuable upon the exercise of options exercisable within 60 days of June 30, 2017. |
| (2) | Represents 61,985 shares of common stock issuable upon the exercise of options exercisable within 60 days of June 30, 2017. |
119
Table of Contents
| (3) | Represents 114,538 shares of common stock issuable upon the exercise of options exercisable within 60 days of June 30, 2017. |
| (4) | Represents 29,400 shares of common stock issuable upon the exercise of options exercisable within 60 days of June 30, 2017. |
| (5) | Represents 197,274 shares of common stock issuable upon the exercise of options exercisable within 60 days of June 30, 2017. |
| (6) | Represents 57,575 shares of common stock issuable upon the exercise of options exercisable within 60 days of June 30, 2017. |
120
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
We describe below transactions and series of similar transactions, during our last three fiscal years or currently proposed, to which we were a party or will be a party, in which:
| • | the amounts involved exceeded or will exceed $120,000; and |
| • | any of our directors, executive officers or beneficial holders of more than 5% of any class of our capital stock had or will have a direct or indirect material interest. |
Other than as described below, there have not been, nor are there any currently proposed, transactions or series of similar transactions meeting this criteria to which we have been or will be a party other than compensation arrangements, which are described where required under “Executive Compensation.”
Relationship with Cellectis
Prior to the completion of this offering, we were a wholly owned subsidiary of Cellectis. Following this offering, Cellectis will continue to beneficially own a majority of our outstanding common stock, and as a result Cellectis will continue to have significant control of our business, including pursuant to the agreements described below. In addition, we expect that, following this offering, Cellectis will continue to consolidate our financial results in its financial statements.
In connection with this offering, we and Cellectis intend to enter into, or have entered into, certain agreements that relate to our relationship with Cellectis prior to this offering or will provide a framework for our ongoing relationship with Cellectis. Of the agreements summarized below, the material agreements are filed as exhibits to the registration statement of which this prospectus is a part, and the summaries of these agreements set forth the terms of the agreements that we believe are material. These summaries are qualified in their entirety by reference to the full text of such agreements.
Contribution Agreement and Guarantee
On October 1, 2015, we entered into an amended and restated contribution with Cellectis pursuant to which Cellectis contributed $40 million to us in exchange for 17,150,000 shares of our common stock. The $40 million contribution consisted of $30 million of cash and the conversion to equity of $10 million of loans and outstanding obligations owed by us to Cellectis.
Cellectis has guaranteed funding for our operations, which guarantee will not be released, modified or otherwise affected by the timing of, or amount raised in, this offering, as described in Note 1 to the audited financial statements included elsewhere in this prospectus.
Management Services Agreement
We are party to a management services agreement dated January 1, 2016 that we entered into with Cellectis and Cellectis, Inc., a Delaware corporation and wholly owned subsidiary of Cellectis that we refer to as Cellectis, Inc., pursuant to which Cellectis and Cellectis, Inc. provide certain services to us, including certain general management, finance, investor relations, communication, legal, intellectual property, human resources and information technology services. In consideration for such services, we pay to Cellectis and Cellectis, Inc. certain fees, consisting of reimbursement of all costs and expenses reasonably incurred by Cellectis in connection with the provision of such services, payment of a mark-up corresponding to a percentage of certain of the costs and expenses, which range from zero to 10%, and reimbursement of costs and expenses of services that are subcontracted by Cellectis on our behalf.
The management services agreement is automatically renewed for one year periods starting on January 1st of each year. Either party will have the right to terminate the agreement at the anniversary date of the agreement
121
Table of Contents
by giving three months prior notice. We also intend to enter into an amendment to the agreement in connection with this offering to provide that the agreement may otherwise be terminated by Cellectis or by us in connection with certain material breaches by the other party upon prior written notice subject to limited cure periods, the sale of all or substantially all of the assets of either party, certain bankruptcy events or certain judgments.
During fiscal year 2016, we made payments to Cellectis for services provided under our management services agreement of $1.8 million, which excludes direct re-invoicing and royalties paid to Cellectis.
Stockholders Agreement
We intend to enter into a stockholders agreement with Cellectis, which we refer to as the stockholders agreement and which will become effective upon completion of this offering. Pursuant to our stockholders agreement with Cellectis, Cellectis will have certain contractual rights for so long as it beneficially owns at least 50% of the then outstanding shares of our common stock, including:
| • | to approve any modification to our or any future subsidiary’s share capital (e.g., share capital increase or decrease) the creation of any subsidiary, any grant of stock-based compensation, any distributions or initial public offering, merger, spin-off, liquidation, winding up or carve-out transactions; |
| • | to approve the annual business plan and annual budget and any modification thereto; |
| • | to approve any external growth transactions exceeding $500,000 and not included in the approved annual business plan and annual budget; |
| • | to approve any investment and disposition decisions exceeding $500,000 and not included in the approved annual business plan and annual budget (it being understood that this clause excludes the purchase and sale of inventory as a part of the normal course of business); |
| • | to approve any related-party agreement and any agreement or transaction between the executives or shareholders of Calyxt, on the one hand, and Calyxt or any of its subsidiaries, on the other hand; |
| • | to approve any decision pertaining to the recruitment, dismissal/removal, or increase of the compensation of executives and corporate officers; |
| • | to approve any material decision relating to a material litigation; |
| • | to approve any decision relating to the opening of a social or restructuring plan or pre-insolvency proceedings; |
| • | to approve any buyback by us of our own shares; |
| • | to approve any new borrowings or debts exceeding $500,000 and early repayment of loans, if any (it being understood that Cellectis will approve the entering into of contracts for revolving loans and other short-term loans and the repayment of such for financing general operating activities, such as revolving loans for inventory or factoring of receivables); |
| • | to approve grants of any pledges on securities; |
| • | to develop new activities and businesses not described in the annual business plan and annual budget; and |
| • | to approve entry into any material agreement or partnership; and |
| • | to approve any offshore and relocation activities. |
In addition, Cellectis will have the following rights for so long as it beneficially owns at least 15% of the then outstanding shares of our common stock, including:
| • | to nominate the greater of three members of our Board of Directors or a majority of the directors; |
122
Table of Contents
| • | to designate the Chairman of our Board of Directors and one member to each of the audit committee of the Board of Directors, the compensation committee of the Board of Directors and the nominating and corporation governance committee of the Board of Directors; |
| • | to approve any amendments to our amended and restated certificate of incorporation or our amended and restated by-laws that would change the name of our company, its jurisdiction of incorporation, the location of its principal executive offices, the purpose or purposes for which our company is incorporated or the Cellectis approval items set forth in the stockholders agreement; |
| • | to approve the payment of any regular or special dividends; |
| • | to approve the commencement of any proceeding for the voluntary dissolution, winding up or bankruptcy of Calyxt or a material subsidiary; |
| • | to approve any public or private offering, merger, amalgamation or consolidation of us or the spinoff of a business of ours or any sale, conveyance, transfer or other disposition of our assets; and |
| • | to approve any appointment to our Board of Directors contrary to the stockholders agreement or our certificate of incorporation or our by-laws. |
In addition, for so long as Cellectis beneficially owns at least 15% of the then outstanding shares of our common stock, (i) Cellectis will be entitled to certain information rights, including the right to consult with and advise senior management, to receive quarterly and annual financial statements and to review our books and records and (ii) we will also be required to cooperate with Cellectis in connection with certain sales and pledges of our shares or grants of security interests in respect thereof, including in connection with margin loans.
The stockholders agreement will also provide Cellectis with certain registration rights, as follows:
| • | Demand Registration—At any time beginning 180 days after the effective date of the registration statement of which this prospectus forms a part (or such earlier time as agreed by us), Cellectis may request that we register for resale all or a portion of their shares. Any such request must cover a quantity of shares with an anticipated aggregate offering price of at least $25.0 million. To the extent we are a well-known seasoned issuer, Cellectis may also request that we file an automatic shelf registration statement on Form S-3 that covers the registrable securities requested to be registered. Depending on certain conditions, we may defer a demand registration for up to 90 days in any twelve-month period. Cellectis will agree pursuant to a contractual lock-up not to exercise any of its rights under the registration rights agreement during the 180-day restricted period described under “Underwriting.” |
| • | Piggyback Registration Rights—In the event that we propose to register any of our securities under the Securities Act, either for our account or for the account of our other security holders, Cellectis will be entitled to certain piggyback registration rights allowing each to include its shares in the registration, subject to certain marketing and other limitations. As a result, whenever we propose to file a registration statement under the Securities Act, Cellectis is entitled to notice of the registration. |
| • | Expenses; Indemnification—The registration rights agreement will provide that we must pay all registration expenses (other than the underwriting discounts and commissions) in connection with effecting any demand registration or shelf registration. The registration rights agreement will contain customary indemnification and contribution provisions. |
| • | Term—The registration rights will remain in effect with respect to any shares covered by the Stockholders Agreement until (i) all of Cellectis’ shares have been sold pursuant to an effective registration statement under the Securities Act; (ii) all of Cellectis’ shares have been sold to the public pursuant to Rule 144 under the Securities Act; or (iii) Cellectis owns less than 10% of our then outstanding shares of our common stock. |
123
Table of Contents
Separation Agreement
We intend to enter into a separation agreement with Cellectis immediately prior to the completion of this offering. The separation agreement will set forth certain agreements between Cellectis and us that will govern the relationship between Cellectis and us following this offering, including with respect to the following matters:
| • | guarantees; |
| • | insurance policies; |
| • | mutual releases and indemnification matters; |
| • | accounting, financial reporting and internal control issues; |
| • | confidentiality; |
| • | ability of the parties to compete with each other; and |
| • | settlement of intercompany accounts. |
The separation agreement will terminate upon the earlier of (i) mutual written consent of Cellectis and us and (ii) the date on which Cellectis and its affiliates cease to hold at least 15% of the then outstanding shares of our common stock.
License Agreement with Cellectis
We will be party to a license agreement with Cellectis, pursuant to which we will be granted an exclusive, worldwide license (subject to existing licenses granted by Cellectis to third parties) to use, commercialize and exploit certain intellectual property in the field of researching, developing and commercializing agricultural and food products, including traits, seeds, and feed and food ingredients (excluding any application in connection with animals and animal cells), except that such license will be non-exclusive in such field for any activities relating to researching, developing or commercializing certain modified or mutated I-CreI homing endonucleases. We will also be granted a non-exclusive license to use the TALEN trademark in connection with our exploitation of licensed products under the agreement. Any improvements we make to the licensed intellectual property will be owned by us but licensed back to Cellectis on an exclusive basis for any use outside of our exclusive agricultural field of use.
In consideration for the license from Cellectis, we will be required to pay to Cellectis, on a product-by-product and country-by-country basis, a royalty of 3% of net sales of any products that are covered by the patents licensed from Cellectis. In addition, we will be required to pay Cellectis 30% of revenue we receive for sublicensing our rights under the agreement to third parties. Our payment obligations to Cellectis will expire upon the expiration of the last-to-expire valid claim of the patents licensed to us by Cellectis.
Under our license agreement with Cellectis, and as between the parties, Cellectis will have the first right to control the prosecution, maintenance, defense and enforcement of the licensed intellectual property and we will have the right to step in and assume such control with respect to the patents owned by Cellectis and exclusively licensed to us under the agreement if Cellectis elects to not prosecute, maintain, defend or enforce such patents. In certain circumstances, if Cellectis elects to abandon any patents owned by Cellectis and exclusively licensed to us under the agreement, we will have the right to assume ownership of such patents. In addition, some of the intellectual property that will be licensed to us by Cellectis consists of an exclusive sublicense, subject to existing sublicenses granted by Cellectis to third parties, of intellectual property originally licensed to Cellectis by the University of Minnesota to exploit such intellectual property in our exclusive agricultural field of use. Therefore, as to such sublicensed intellectual property, our license from Cellectis will be subject to the terms and conditions of the license agreement between the University of Minnesota and Cellectis, and to the extent our activities under such sublicense violate any terms and conditions of the license agreement between Cellectis and the University of Minnesota, we will be responsible for any damages that Cellectis may incur. In addition, we are required to
124
Table of Contents
reimburse Cellectis for any and all payments made by Cellectis to the University of Minnesota pursuant to the license agreement between the University of Minnesota and Cellectis to the extent that any such payments are required to be made as a result of our applicable activities. Under the license agreement between Cellectis and the University of Minnesota, the University of Minnesota has the first right to control the prosecution and maintenance of the licensed intellectual property.
Our license agreement with Cellectis will be perpetual. However it may be terminated upon the mutual written agreement of both parties, either party’s uncured material breach of the agreement, or upon certain bankruptcy and insolvency related events.
Indemnification Agreements
We expect to enter into indemnification agreements with each of our directors at or prior to the completion of this offering. The indemnification agreements and our Certificate of Incorporation and By-laws will require us to indemnify our directors to the fullest extent permitted by Delaware law.
Policy Concerning Related Person Transactions
Prior to the consummation of this offering, our Board of Directors will adopt a written policy, which we refer to as the related person transaction approval policy, for the review of any transaction, arrangement or relationship in which we are a participant, if the amount involved exceeds $120,000 and one of our executive officers, directors, director nominees or beneficial holders of more than 5% of our total equity (or their immediate family members), each of whom we refer to as a related person, has a direct or indirect material interest. This policy was not in effect when we entered into the transactions described above.
Each of the agreements between us and Cellectis that have been entered into prior to the completion of this offering, and any transactions contemplated thereby, will be deemed to be approved and not subject to the terms of such policy. If a related person, other than Cellectis and its affiliates, proposes to enter into such a transaction, arrangement or relationship, which we refer to as a related person transaction, the related person must report the proposed related person transaction to the chairman of our Audit Committee for so long as the controlled company exception applies and the Nominating and Corporate Governance Committee thereafter (for purposes of this section only, we refer to each of these committees as the Committee). The policy calls for the proposed related person transaction to be reviewed and, if deemed appropriate, approved by the Committee. In approving or rejecting such proposed transactions, the Committee will be required to consider relevant facts and circumstances. The Committee will approve only those transactions that, in light of known circumstances, are deemed to be in our best interests. In the event that any member of the Committee is not a disinterested person with respect to the related person transaction under review, that member will be excluded from the review and approval or rejection of such related person transaction; provided, however, that such Committee member may be counted in determining the presence of a quorum at the meeting of the Committee at which such transaction is considered. If we become aware of an existing related person transaction which has not been approved under the policy, the matter will be referred to the Committee. The Committee will evaluate all options available, including ratification, revision or termination of such transaction. In the event that management determines that it is impractical or undesirable to wait until a meeting of the Committee to consummate a related person transaction, the chairman of the Committee may approve such transaction in accordance with the related person transaction approval policy. Any such approval must be reported to the Committee at its next regularly scheduled meeting.
125
Table of Contents
Below is a description of the material terms and provisions of our amended and restated certificate of incorporation, which we refer to as our Certificate of Incorporation, and our amended and restated bylaws, which we refer to as our By-laws, in each case as in effect and affecting the rights of our stockholders upon the completion of this offering, as well as relevant terms and provisions of our indemnification agreements with directors and Delaware law affecting the rights of our stockholders. This summary does not purport to be complete and is qualified in its entirety by the provisions of our Certificate of Incorporation, By-laws and such indemnification agreements. Copies of our Certificate of Incorporation and By-laws have been or will be filed with the SEC as exhibits to the registration statement of which this prospectus forms a part.
General
Upon the completion of our initial public offering, our authorized capital stock will consist of:
| • | 275,000,000 shares of common stock, par value $0.0001 per share; and |
| • | 50,000,000 shares of preferred stock, par value $0.0001 per share. |
As of June 30, 2017, there were 19,600,000 shares of common stock outstanding, all of which were held by Cellectis. At that date, there were no shares of preferred stock outstanding. Immediately after the completion of this offering, 25,660,606 shares of common stock will be outstanding, assuming the underwriters’ option to purchase additional shares is not exercised, and no shares of preferred stock will be outstanding.
Common Stock
Voting Rights. The holders of our common stock will be entitled to one vote per share on all matters to be voted upon by the stockholders. Holders of our common stock will not have cumulative voting rights in the election of directors. Accordingly, the holders of a majority of the voting power of our common stock could, if they so choose, elect all the directors.
Dividend Rights. Holders of common stock will be entitled to receive dividends if, as and when declared by our Board of Directors, out of our legally available assets, in cash, property, shares of our common stock or other securities, after payments of dividends required to be paid on outstanding preferred stock, if any.
Distributions in Connection with Mergers or Other Business Combinations. Upon a merger, consolidation or substantially similar transaction, holders of common stock will be entitled to receive equal per share payments or distributions.
Liquidation Rights. Upon our liquidation, dissolution or winding up, any business combination or a sale or disposition of all or substantially all of our assets, the assets legally available for distribution to our stockholders will be distributable ratably among the holders of the common stock, subject to prior satisfaction of all outstanding debts and other liabilities and the preferential rights and payment of liquidation preferences, if any, on any outstanding preferred stock.
Stockholders Agreement. In connection with this offering, we will enter into a stockholders agreement with Cellectis, pursuant to which Cellectis will have certain rights so long as it beneficially owns at least 15% of the then outstanding shares of our common stock, as described in “Certain Relationships and Related Party Transactions—Relationship with Cellectis—Stockholders Agreement.”
Other Matters. Our Certificate of Incorporation will not entitle holders of our common stock to preemptive rights. No redemption or sinking fund provisions will be applicable to our common stock. All outstanding shares of our common stock are, and the shares of common stock offered in this offering will be upon payment and delivery in accordance with the underwriting agreement, fully paid and non-assessable.
126
Table of Contents
Preferred Stock
Our Certificate of Incorporation authorizes our Board of Directors to issue preferred stock in one or more series and to determine the preferences, limitations and relative rights of any shares of preferred stock that we choose to issue, without vote or action by the stockholders.
Anti-Takeover Effects of Delaware Law, Our Certificate of Incorporation and Our By-laws
The following provisions may make a change in control of our business more difficult and could delay, defer or prevent a tender offer or other takeover attempt that a stockholder might consider to be in its best interest, including takeover attempts that might result in the payment of a premium to stockholders over the market price for their shares. These provisions also may promote the continuity of our management by making it more difficult for a person to remove or change the incumbent members of our Board of Directors.
Authorized but Unissued Shares; Undesignated Preferred Stock. The authorized but unissued shares of our common stock will be available for future issuance without stockholder approval. These additional shares may be used for a variety of corporate purposes, including future public offerings to raise additional capital, acquisitions and employee benefit plans. In addition, our Board of Directors may authorize, without stockholder approval, the issuance of undesignated preferred stock with voting rights or other rights or preferences designated from time to time by our Board of Directors. The existence of authorized but unissued shares of common stock or preferred stock may enable our Board of Directors to render more difficult or to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise.
Election and Removal of Directors. Our Board of Directors will consist of not less than five nor more than eleven directors, excluding any directors elected by holders of preferred stock pursuant to provisions applicable in the case of defaults, if any. The exact number of directors will be fixed from time to time by resolution of our Board of Directors. Our Board of Directors will initially have five members.
Pursuant to the Stockholders Agreement, Cellectis has the right to nominate the greater of three directors or a majority of directors to our Board of Directors so long as it continues to own at least 15% of our then outstanding shares of our common stock.
At any time after Cellectis beneficially owns less than 50% of our then outstanding common stock, our Certificate of Incorporation provides that directors may be removed only for cause and only by the affirmative vote of holders of a majority of our then outstanding stock. Prior to such time, directors may be removed with or without cause.
Classified Board of Directors. Initially, our Board of Directors will not be classified. However, our Certificate of Incorporation and our By-laws will provide that our Board of Directors will be classified with approximately one-third of the directors elected each year at such time as Cellectis no longer holds at least 50% of our then outstanding common stock. The number of directors will be fixed from time to time by a majority of the total number of directors that we would have at the time such number is fixed if there were no vacancies. The directors will be divided into three classes, designated class I, class II and class III. Each class will consist, as nearly as may be possible, of one-third of the total number of directors constituting the entire Board of Directors. At each annual meeting of stockholders, successors to the class of directors whose term expires at that annual meeting will be elected for a three-year term and until their successors are duly elected and qualified. In addition, if the number of directors is changed, any increase or decrease will be apportioned by our Board of Directors among the classes so as to maintain the number of directors in each class as nearly equal as possible, and any additional director of any class elected to fill a vacancy resulting from an increase in such class or from the removal from office, death, disability, resignation or disqualification of a director or other cause will hold office for a term that will coincide with the remaining term of that class, but in no case will a decrease in the number of directors have the effect of removing or shortening the term of any incumbent director.
127
Table of Contents
Director Vacancies. Our Certificate of Incorporation authorizes only our Board of Directors to fill vacant directorships.
No Cumulative Voting. Our Certificate of Incorporation provides that stockholders do not have the right to cumulate votes in the election of directors.
Special Meetings of Stockholders. At any time after Cellectis beneficially owns less than 50% of our then outstanding common stock, our By-laws and our Certificate of Incorporation provide that special meetings of our stockholders may only be called by the Board of Directors. Prior to such time, a special meeting may also be called by the secretary of the Company at the request of stockholders holding a majority of the outstanding shares entitled to vote.
Advance Notice Procedures for Director Nominations. Our By-laws establish advance notice procedures for stockholders seeking to nominate candidates for election as directors at an annual or special meeting of stockholders. Although our By-laws do not give the Board of Directors the power to approve or disapprove stockholder nominations of candidates to be elected at an annual meeting, our By-laws may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed or may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors or otherwise attempting to obtain control of the Company.
Action by Written Consent. At any time after Cellectis beneficially owns less than 50% of our then outstanding common stock, our By-laws and our Certificate of Incorporation provide that any action required or permitted to be taken by the stockholders must be effected at a duly called annual or special meeting of stockholders and may not be effected by any consent in writing in lieu of a meeting of such stockholders, subject to the rights of the holders of any series of preferred stock. Prior to such time, such actions may be taken without a meeting by written consent.
Amending Our Certificate of Incorporation and Bylaws. At any time after Cellectis beneficially owns less than 50% of our then outstanding common stock, our Certificate of Incorporation and By-laws may be amended by the affirmative vote of the holders of at least two-thirds of our common stock. Prior to such time, our Certificate of Incorporation and By-laws may be amended by the affirmative vote of the holders of a majority of the voting power of our common stock.
Exclusive Jurisdiction. Our Certificate of Incorporation provides that, unless we consent to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a claim of breach of fiduciary duty owed by any of our directors, officers, or other employees to us or to our stockholders, any action asserting a claim arising pursuant to the DGCL, or any action asserting a claim governed by the internal affairs doctrine.
Business Combinations with Interested Stockholders. Subject to certain exceptions, Section 203 of the DGCL prohibits a public Delaware corporation from engaging in a business combination (as defined in such section) with an “interested stockholder” (defined generally as any person who beneficially owns 15% or more of the outstanding voting stock of such corporation or any person affiliated with such person) for a period of three years following the time that such stockholder became an interested stockholder, unless (i) prior to such time the Board of Directors of such corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; (ii) upon consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of such corporation at the time the transaction commenced (excluding for purposes of determining the voting stock of such corporation outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (A) by persons who are directors and also officers of such corporation and (B) by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer); or (iii) at or subsequent to
128
Table of Contents
such time the business combination is approved by the Board of Directors of such corporation and authorized at a meeting of stockholders (and not by written consent) by the affirmative vote of at least 66 2/3% of the outstanding voting stock of such corporation not owned by the interested stockholder.
A Delaware corporation may “opt out” of these provisions with an express provision in its original certificate of incorporation or an express provision in its certificate of incorporation or bylaws resulting from a stockholders’ amendment approved by at least a majority of the outstanding voting shares. We have expressly elected not to be governed by the “business combination” provisions of Section 203 of the DGCL, until after such time as Cellectis no longer beneficially owns at least 50% of our common stock. At that time, such election shall be automatically withdrawn and we will thereafter be governed by the “business combination” provisions of Section 203 of the DGCL.
Conflicts of Interest
Delaware law permits corporations to adopt provisions renouncing any interest or expectancy in certain opportunities that are presented to the corporation or its officers, directors or stockholders. Our Certificate of Incorporation will, to the maximum extent permitted from time to time by Delaware law, renounce any interest or expectancy that we have in, or right to be offered an opportunity to participate in, specified business opportunities that are from time to time presented to our officers, directors or stockholders or their respective affiliates, other than those officers, directors, stockholders or affiliates who are our or our subsidiaries’ employees. Our Certificate of Incorporation will provide that, to the fullest extent permitted by law, none of Cellectis or any of its affiliates or any director who is not employed by us or his or her affiliates will have any duty to refrain from (i) engaging in a corporate opportunity in the same or similar lines of business in which we or our affiliates now engage or propose to engage or (ii) otherwise competing with us or our affiliates. In addition, to the fullest extent permitted by law, in the event that Cellectis or any non-employee director acquires knowledge of a potential transaction or other business opportunity which may be a corporate opportunity for itself or himself or its or his affiliates or for us or our affiliates, such person will have no duty to communicate or offer such transaction or business opportunity to us or any of our affiliates and they may take any such opportunity for themselves or offer it to another person or entity. Our Certificate of Incorporation will not renounce our interest in any business opportunity that is expressly offered to a non-employee director solely in his or her capacity as a director of Calyxt. To the fullest extent permitted by law, no business opportunity will be deemed to be a potential corporate opportunity for us unless we would be permitted to undertake the opportunity under our Certificate of Incorporation, we have sufficient financial resources to undertake the opportunity and the opportunity would be in line with our business.
Registration Rights
We will enter into the Stockholders Agreement prior to the completion of this offering that will provide Cellectis with registration rights relating to shares of our common stock held by Cellectis after this offering. See “Certain Relationships and Related Party Transactions—Relationship with Cellectis—Stockholders Agreement.”
Indemnification and Limitations on Directors’ Liability
Section 145 of the DGCL grants each Delaware corporation the power to indemnify any person who is or was a director, officer, employee or agent of a corporation, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, other than an action by or in the right of the corporation, by reason of serving or having served in any such capacity, if he or she acted in good faith in a manner reasonably believed to be in, or not opposed to, the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful. A Delaware corporation may similarly indemnify any such person in actions by or in the right of the corporation if he or she acted in good faith in a manner reasonably believed to be
129
Table of Contents
in, or not opposed to, the best interests of the corporation, except that no indemnification may be made in respect of any claim, issue or matter as to which the person shall have been adjudged to be liable to the corporation unless and only to the extent that the Delaware Court of Chancery or the court in which the action was brought determines that, despite adjudication of liability, but in view of all of the circumstances of the case, the person is fairly and reasonably entitled to indemnity for expenses which the Delaware Court of Chancery or other court shall deem proper.
Section 102(b)(7) of the DGCL enables a corporation in its certificate of incorporation, or an amendment thereto, to eliminate or limit the personal liability of a director to the corporation or its stockholders for monetary damages for violations of the director’s fiduciary duty as a director, except (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL (providing for director liability with respect to unlawful payment of dividends or unlawful stock purchases or redemptions) or (iv) for any transaction from which a director derived an improper personal benefit.
Our Certificate of Incorporation and By-laws indemnify our directors and officers to the full extent permitted by the DGCL and our Certificate of Incorporation also allows our Board of Directors to indemnify other employees. This indemnification extends to the payment of judgments in actions against officers and directors and to reimbursement of amounts paid in settlement of such claims or actions and may apply to judgments in favor of the corporation or amounts paid in settlement to the corporation. This indemnification also extends to the payment of attorneys’ fees and expenses of officers and directors in suits against them where the officer or director acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, the best interests of Calyxt, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful. This right of indemnification is not exclusive of any right to which the officer or director may be entitled as a matter of law and shall extend and apply to the estates of deceased officers and directors.
We maintain a directors’ and officers’ insurance policy. The policy insures directors and officers against unindemnified losses arising from certain wrongful acts in their capacities as directors and officers and reimburses us for those losses for which we have lawfully indemnified the directors and officers. The policy contains various exclusions that are normal and customary for policies of this type.
We intend to enter into indemnification agreements with our directors providing for certain advancement and indemnification rights. In each indemnification agreement, we agreed, subject to certain exceptions, to indemnify and hold harmless the director or officer to the maximum extent then authorized or permitted by the DGCL or by any amendment(s) thereto.
We believe that the limitation of liability and indemnification provisions in our Certificate of Incorporation, By-laws, indemnification agreements and insurance policies are necessary to attract and retain qualified directors and officers. However, these provisions may discourage derivative litigation against directors and officers, even though an action, if successful, might benefit us and other stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers as required or allowed by these limitation of liability and indemnification provisions.
At present, there is no pending litigation or proceeding involving any of our directors, officers, employees or agents as to which indemnification is sought from us, nor are we aware of any threatened litigation or proceeding that may result in an indemnification claim.
Listing
We have applied to list the common stock on the NASDAQ under the symbol “CLXT.”
130
Table of Contents
Transfer Agent and Registrar
The transfer agent and registrar for the common stock is Computershare Trust Company, N.A.
131
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for shares of our common stock. Future sales of substantial amounts of shares of our common stock, including shares issued upon the exercise of outstanding options, in the public market after this offering, or the possibility of these sales occurring, could adversely affect the prevailing market price for our common stock from time to time or impair our ability to raise equity capital in the future.
Based on the number of shares outstanding as of June 30, 2017, upon the completion of this offering, 25,660,606 shares of our common stock will be outstanding, assuming no exercise of the underwriters’ option to purchase additional shares, no exercise of outstanding options. Of the outstanding shares, 6,060,606 shares sold in this offering will be freely tradable, except that (i) any shares purchased by Cellectis and certain of our or Cellectis’ directors, consultants and employees and their families under the directed share program will be subject to a 180-day lock-up and (ii) any shares acquired by our affiliates, as that term is defined in Rule 144 under the Securities Act, in this offering may only be sold in compliance with the limitations described below.
The remaining 19,600,000 shares of common stock outstanding after this offering are beneficially owned by Cellectis and will be restricted as a result of securities laws and a lock-up agreement, as described below. Following the expiration of the lock-up period, all shares will be eligible for resale in compliance with Rule 144 or Rule 701. “Restricted securities” as defined under Rule 144 were issued and sold by us in reliance on exemptions from the registration requirements of the Securities Act. These shares may be sold in the public market only if registered or pursuant to an exemption from registration, such as Rule 144 or Rule 701 under the Securities Act.
Rule 144
In general, a person who has beneficially owned restricted shares of our common stock for at least six months would be entitled to sell such securities, provided that (i) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days preceding, a sale and (ii) we are subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Persons who have beneficially owned restricted shares of our common stock for at least six months but who are our affiliates at the time of, or any time during the 90 days preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three month period only a number of securities that does not exceed the greater of either of the following:
| • | 1% of the number of shares of our common stock then outstanding, which will equal approximately 256,606 shares immediately after this offering, assuming no exercise of the underwriters’ option to purchase additional shares; or |
| • | the average weekly trading volume of shares of our common stock on the NASDAQ Global Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale; |
provided, in each case, that we are subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Such sales both by affiliates and by non-affiliates must also comply with the manner of sale, current public information and notice provisions of Rule 144 to the extent applicable.
Rule 701
In general, under Rule 701, any of our employees, directors, officers, consultants or advisors who purchases shares from us in connection with a compensatory stock or option plan or other written agreement before the effective date of this offering is entitled to resell such shares 90 days after the effective date of this offering in reliance on Rule 144, without having to comply with the holding period requirements or other restrictions contained in Rule 701.
132
Table of Contents
The SEC has indicated that Rule 701 will apply to typical stock options granted by an issuer before it becomes subject to the reporting requirements of the Exchange Act, along with the shares acquired upon exercise of such options, including exercises after the date of this prospectus. Securities issued in reliance on Rule 701 are restricted securities and, subject to the contractual restrictions described above, beginning 90 days after the date of this prospectus, may be sold by persons other than “affiliates,” as defined in Rule 144, subject only to the manner of sale provisions of Rule 144 and by “affiliates” under Rule 144 without compliance with its one-year minimum holding period requirement.
Registration Rights
Upon completion of this offering, Cellectis will be entitled to various rights with respect to the registration of its shares under the Securities Act. Registration of these shares under the Securities Act would result in these shares becoming freely tradable without restriction under the Securities Act immediately upon the effectiveness of the registration, except for shares purchased by affiliates. See “Certain Relationships and Related Party Transactions—Relationship with Cellectis—Stockholders Agreement.”
Stock Options and RSUs
As of June 30, 2017, options to purchase a total of 1,909,775 shares of common stock were outstanding under the Existing Plan. An additional 127,400 shares of common stock were available for future grants under the Existing Plan. Prior to this offering, we adopted a new equity compensation plan under which 4,900,000 shares of our common stock will be available for awards granted thereunder, as described under “Executive Compensation—2017 Omnibus Incentive Plan.” As of June 30, 2017, options to purchase a total of 2,119,698 shares of common stock and RSUs with respect to 1,452,333 shares of common stock were outstanding under the Omnibus Plan.
Registration Statement
Upon completion of this offering, we intend to file a registration statement on Form S-8 under the Securities Act covering all shares of common stock subject to outstanding options and RSUs or otherwise issuable in the future pursuant to our equity incentive plans. Subject to Rule 144 volume limitations applicable to affiliates, shares registered under any registration statements will be available for sale in the open market, beginning 90 days after the date of the prospectus, except to the extent that the shares are subject to vesting restrictions with us or the contractual restrictions described below.
Lock-up Agreements
Our directors, executive officers and Cellectis have agreed, subject to certain exceptions, not to offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, or enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock for a period of 180 days after the date of this prospectus, without the prior written consent of the representatives of the underwriters. See “Underwriting.”
133
Table of Contents
MATERIAL U.S. FEDERAL TAX CONSIDERATIONS FOR NON-U.S. HOLDERS
The following is a discussion of the material U.S. federal income and estate tax consequences of the ownership and disposition of our common stock acquired in this offering by a “Non-U.S. Holder” that does not own, and has not owned, actually or constructively, more than 5% of our common stock. You are a Non-U.S. Holder if for U.S. federal income tax purposes you are a beneficial owner of our common stock that is:
| • | a nonresident alien individual; |
| • | a foreign corporation; or |
| • | a foreign estate or trust. |
You are not a Non-U.S. Holder if you are a nonresident alien individual present in the United States for 183 days or more in the taxable year of disposition, or if you are a former citizen or former resident of the United States for U.S. federal income tax purposes. If you are such a person, you should consult your tax adviser regarding the U.S. federal income tax consequences of the ownership and disposition of our common stock.
If you are a partnership or other pass-through entity (including an entity or arrangement treated as a partnership or other type of pass-through entity for U.S. federal income tax purposes) that owns our common stock, the U.S. federal income tax treatment of a partner or beneficial owner will generally depend on the status of the partner or beneficial owner and your activities. Partnerships, partners and beneficial owners in partnerships or other pass-through entities that own our common stock should consult their tax advisers as to the particular U.S. federal income tax consequences of the ownership and disposition of our common stock.
This discussion is based on the Internal Revenue Code of 1986, as amended to the date hereof (the “Code”), administrative pronouncements, judicial decisions and final, temporary and proposed Treasury regulations, changes to any of which subsequent to the date of this prospectus may affect the tax consequences described herein, possibly with retroactive effect. This discussion does not describe all aspects of U.S. federal income and estate taxation that may be relevant to you in light of your particular circumstances, does not discuss alternative minimum tax and Medicare contribution tax consequences and does not address any aspect of state, local or non-U.S. taxation. You should consult your tax adviser with regard to the application of the U.S. federal tax laws to your particular situation, as well as any tax consequences arising under the laws of any state, local or non-U.S. taxing jurisdiction.
Dividends
Distributions of cash or other property (other than certain distributions of stock) will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed our current and accumulated earnings and profits, they will constitute a return of capital, which will first reduce your basis in our common stock, but not below zero, and then will be treated as gain from the sale of our common stock, as described below under “—Gain on Disposition of Our Common Stock.”
Dividends paid to you generally will be subject to withholding tax at a 30% rate or a reduced rate specified by an applicable income tax treaty. In order to obtain a reduced rate of withholding, you will be required to provide us or our paying agent with a properly executed applicable Internal Revenue Service, or IRS, Form W-8BEN or IRS Form W-8BEN-E, as applicable, certifying your entitlement to benefits under a treaty. A Non-U.S. Holder eligible for a reduced rate of U.S. withholding tax pursuant to an income tax treaty who fails to timely provide a W-8BEN or W-8BEN-E may obtain a refund of any excess amounts withheld by timely filing an appropriate claim with the IRS.
If dividends paid to you are effectively connected with your conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed
134
Table of Contents
base maintained by you in the United States), you will generally be taxed on the dividends in the same manner as a U.S. person. In this case, you will be exempt from the withholding tax discussed in the preceding paragraph, although you will be required to provide a properly executed IRS Form W-8ECI in order to claim an exemption from withholding. You should consult your tax adviser with respect to other U.S. tax consequences of the ownership and disposition of our common stock, including the possible imposition of a branch profits tax at a rate of 30% (or a lower treaty rate) if you are a corporation.
Gain on Disposition of Our Common Stock
Subject to the discussions below under “—Information Reporting and Backup Withholding” and “—FATCA,” you generally will not be subject to U.S. federal income or withholding tax on gain realized on a sale or other taxable disposition of our common stock unless:
| • | the gain is effectively connected with your conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment or fixed base maintained by you in the United States), or |
| • | we are or have been a “United States real property holding corporation,” as defined in the Code, at any time within the five-year period preceding the disposition or your holding period, whichever period is shorter, and our common stock has ceased to be regularly traded on an established securities market prior to the beginning of the calendar year in which the sale or disposition occurs. |
We believe that we are not, and do not anticipate becoming, a United States real property holding corporation.
If you recognize gain on a sale or other disposition of our common stock that is effectively connected with your conduct of a trade or business in the United States (and if required by an applicable income tax treaty, is attributable to a permanent establishment or fixed base maintained by you in the United States), you will generally be taxed on such gain in the same manner as a U.S. person. You should consult your tax adviser with respect to other U.S. tax consequences of the ownership and disposition of our common stock, including the possible imposition of a branch profits tax at a rate of 30% (or a lower treaty rate) if you are a corporation.
Information Reporting and Backup Withholding
Information returns will be filed with the IRS in connection with payments of dividends on our common stock. Unless you comply with certification procedures to establish that you are not a U.S. person, information returns may also be filed with the IRS in connection with the proceeds from a sale or other disposition of our common stock. You may be subject to backup withholding on payments on our common stock or on the proceeds from a sale or other disposition of our common stock unless you comply with certification procedures to establish that you are not a U.S. person or otherwise establish an exemption. Compliance with the certification procedures required to claim a reduced rate of withholding under a treaty will satisfy the certification requirements necessary to avoid backup withholding as well. Amounts withheld under the backup withholding rules are not additional taxes and may be refunded or credited against your U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
FATCA
Provisions of the Code commonly referred to as “FATCA” require withholding of 30% on payments of dividends on our common stock, as well as payments of gross proceeds of dispositions occurring after December 31, 2018 of our common stock, to “foreign financial institutions” (which is broadly defined for this purpose and in general includes investment vehicles) and certain other non-U.S. entities unless various U.S. information reporting and due diligence requirements (generally relating to ownership by U.S. persons of interests in or accounts with those entities) have been satisfied, or an exemption applies. An intergovernmental
135
Table of Contents
agreement between the United States and an applicable foreign country may modify these requirements. If FATCA withholding is imposed, a beneficial owner that is not a foreign financial institution generally may obtain a refund of any amounts withheld by filing a U.S. federal income tax return (which may entail significant administrative burden). You should consult your tax adviser regarding the effects of FATCA on your investment in our common stock.
Federal Estate Tax
Individual Non-U.S. Holders and entities the property of which is potentially includible in such an individual’s gross estate for U.S. federal estate tax purposes (for example, a trust funded by such an individual and with respect to which the individual has retained certain interests or powers), should note that, absent an applicable treaty exemption, our common stock will be treated as U.S.-situs property subject to U.S. federal estate tax.
136
Table of Contents
Citigroup Global Markets Inc. and Jefferies LLC are acting as representatives of each of the underwriters named below. Subject to the terms and conditions set forth in an underwriting agreement among us and the underwriters, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us, the number of shares of common stock set forth opposite its name below.
| Underwriter |
Number of Shares |
|||
| Citigroup Global Markets Inc. |
||||
| Jefferies LLC |
||||
| Wells Fargo Securities, LLC |
||||
| BMO Capital Markets Corp. |
||||
| Ladenburg Thalmann & Co. Inc. |
||||
|
|
|
|||
| Total |
6,060,606 | |||
|
|
|
|||
Subject to the terms and conditions set forth in the underwriting agreement, the underwriters have agreed, severally and not jointly, to purchase all of the shares sold under the underwriting agreement if any of these shares are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the nondefaulting underwriters may be increased or the underwriting agreement may be terminated.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make in respect of those liabilities.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the shares, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Commissions and Discounts
The representatives have advised us that the underwriters propose initially to offer the shares to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per share. After the initial offering, the public offering price, concession or any other term of the offering may be changed.
The following table shows the public offering price, underwriting discount and proceeds before expenses to us. The information assumes either no exercise or full exercise by the underwriters of their option to purchase additional shares.
| Per Share | Without Option | With Option | ||||||||||
| Public offering price |
$ | $ | $ | |||||||||
| Underwriting discount |
$ | $ | $ | |||||||||
| Proceeds, before expenses, to us |
$ | $ | $ | |||||||||
The expenses of the offering, not including the underwriting discount, are estimated at $2.1 million and are payable by us. We have also agreed to reimburse the underwriters for certain of their expenses, including up to an aggregate of $30,000 in connection with the clearance of this offering with the Financial Industry Regulatory Authority, as set forth in the underwriting agreement.
137
Table of Contents
Option to Purchase Additional Shares
We have granted an option to the underwriters, exercisable for 30 days after the date of this prospectus, to purchase up to 909,091 additional shares at the public offering price, less the underwriting discount. If the underwriters exercise this option, each will be obligated, subject to conditions contained in the underwriting agreement, to purchase a number of additional shares proportionate to that underwriter’s initial amount reflected in the above table.
Reserved Share Program
At our request, the underwriters have reserved for sale, at the initial public offering price, up to 3.0% of the shares offered by this prospectus for sale to some of our and Cellectis’ directors, consultants and employees and their families. Any purchases of reserved shares by these persons would reduce the number of shares available for sale to the general public. Any reserved shares that are not so purchased will be offered by the underwriters to the general public on the same terms as the other shares offered by this prospectus.
No Sales of Similar Securities
We have agreed that, during a period of 180 days from the date of this prospectus, we will not, without the prior written consent of the representatives of the underwriters, (i) directly or indirectly, offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock or file any registration statement under the Securities Act with respect to any of the foregoing or (ii) enter into any swap or any other agreement or any transaction that transfers, in whole or in part, directly or indirectly, the economic consequence of ownership of our common stock, whether any such swap or transaction described in clause (i) or (ii) above is to be settled by delivery of our common stock or other securities, in cash or otherwise. The foregoing sentence will not apply to (A) the shares of common stock to be sold in this offering, (B) any shares of our common stock issued by us upon the exercise of an option or warrant or the conversion of a security outstanding on the date of this prospectus and referred to in this prospectus, (C) any shares of our common stock issued or options to purchase our common stock granted pursuant to our existing employee benefit plans of referred to in this prospectus, (D) any shares of our common stock issued pursuant to any non-employee director stock plan or dividend reinvestment plan referred to in this prospectus, (E) shares of our common stock or other securities issued by us in connection with joint ventures, commercial relationships or other strategic transactions, provided that (x) the aggregate number of securities issued pursuant to this clause (E) shall not exceed 7.5% of the total number of outstanding shares of our common stock immediately following the issuance and sale of the shares of our common stock in this offering and (y) such securities issued pursuant to this clause (E) during the 180-day restricted period described above shall be subject to the restrictions described in the form of lock-up agreement attached to the underwriting agreement for the remainder of such restricted period and the recipient of any such securities shall enter into an agreement substantially in such form or (F) the filing by us of a registration statement on Form S-8 covering the registration of securities issued under our existing employee benefit plans referred to in this prospectus.
In addition, Cellectis and all of our directors and executive officers, whose shares, options and RSUs represent substantially all of our outstanding equity on a fully diluted basis, have agreed that, during the 180-day restricted period described above, such holder will not, without the prior written consent of the representatives of the underwriters, (i) directly or indirectly, offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock, whether now owned or hereafter acquired by such holder or with respect to which such holder has or hereafter acquires the power of disposition (collectively, the “lock-up securities”), or exercise any right with respect to the registration of any of the lock-up securities, or file or cause to be filed any
138
Table of Contents
registration statement in connection therewith, under the Securities Act, or (ii) enter into any swap or any other agreement or any transaction that transfers, in whole or in part, directly or indirectly, the economic consequence of ownership of the lock-up securities, whether any such swap or transaction is to be settled by delivery of our common stock or other securities, in cash or otherwise.
Notwithstanding the immediately preceding paragraph, and subject to the conditions below, such holder may transfer the lock-up securities without the prior written consent of the representatives of the underwriters, provided that (1) the representatives receive a signed lock-up agreement for the balance of the lockup period from each donee, trustee, distributee, or transferee, as the case may be, (2) any such transfer shall not involve a disposition for value, (3) such transfers are not required to be reported with the SEC on Form 4 in accordance with Section 16 of the Securities Exchange Act of 1934, as amended, and (4) such holder does not otherwise voluntarily effect any public filing or report regarding such transfers:
(i) as a bona fide gift or gifts; or
(ii) to any trust for the direct or indirect benefit of such holder or the immediate family of such holder (for this purpose, “immediate family” shall mean any relationship by blood, marriage or adoption, not more remote than first cousin); or
(iii) to a member of the immediate family of such holder; or
(iv) to a corporation, partnership, limited liability company or other entity of which such holder and the immediate family of such holder are the direct or indirect legal and beneficial owners of all the outstanding equity securities or similar interests of such corporation, partnership, limited liability company or other entity; or
(v) by will or intestate succession upon the death of such holder; or
(vi) by operation of law, such as pursuant to a qualified domestic order or in connection with a divorce settlement; or
(vii) as a distribution to limited partners or stockholders of such holder; or
(viii) to such holder’s affiliates or to any investment fund or other entity controlled or managed by such holder; or
(ix) to a nominee or custodian of a person or entity to whom disposition or transfer would be permissible under (i) - (viii) above.
Notwithstanding the foregoing, nothing in the lock-up agreements for such holders will prohibit them from:
(i) entering into a written plan meeting the requirements of Rule 10b5-1 under the Exchange Act of 1934, as amended, relating to the sale of our securities, provided that the securities subject to such plan may not be sold and no public disclosure of any such action shall be required or shall be voluntarily made by any person until after the expiration of the 180-day lock-up period; or
(ii) exercising any stock option to purchase shares of our common stock, or any securities convertible into or exercisable or exchangeable for our common stock, or other similar awards granted on or prior to the date of this prospectus or granted pursuant to our equity incentive plans described in this prospectus; provided that the lock-up agreement shall apply to any securities issued upon such exercise; or
(iii) transferring shares of our common stock or any securities convertible into or exercisable or exchangeable for shares of our common stock from such holder to us (or the purchase and cancellation of the same by us) upon a vesting event of our securities or upon the exercise of options to purchase shares of our common stock that expire during the 180-day lock-up period by such holder, in each case on a “cashless” or “net exercise” basis, or to cover tax withholding obligations of such holder in connection with such vesting or exercise, whether by means of a “net settlement” or otherwise, in each case pursuant to employee benefit plans disclosed in this prospectus; provided that in the case of any transfer or distribution
139
Table of Contents
pursuant to this clause, no filing under Section 16(a) of the Exchange Act (other than a filing on Form 5 after the expiration of the 180-day lock-up period) reporting a reduction in beneficial ownership of shares of our common stock shall be required or shall be voluntarily made during the 180-day lock-up period; or
(iv) transferring shares of our common stock or any security convertible into or exercisable or exchangeable for shares of our common stock that occurs by any order or settlement resulting from any legal proceeding pursuant to a qualified domestic order or in connection with a divorce settlement; provided that in the case of any transfer or distribution pursuant to this clause, any filing under Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of shares of our common stock shall state that such transfer is pursuant to an order of a court or a settlement resulting from a legal proceeding unless such a statement would be prohibited by any applicable law or order of a court; or
(v) if such holder is a corporation, partnership, limited liability company or other entity, transferring shares of our common stock in connection with the sale or other bona fide transfer in a single transaction or all or substantially all of such holder’s capital stock, partnership interests, membership interests or other similar equity interests, as the case may be, or all or substantially all of such holder’s assets, in any such case not undertaken for the purpose of avoiding the restrictions imposed by the lock-up agreement; provided that each transferee shall sign and deliver a lock-up letter substantially in the form contemplated by the underwriting agreement; or
(vi) transferring shares of our common stock or any security convertible into or exercisable or exchangeable for shares of our common stock pursuant to an order of a court or regulatory agency; provided that each transferee shall sign and deliver a lock-up letter substantially in the form contemplated by the underwriting agreement, unless prohibited by any applicable law or order of a court; and provided further that any filing under Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of shares of our common stock shall state that such transfer is pursuant to an order of a court or a settlement resulting from a legal proceeding unless such a statement would be prohibited by any applicable law or order of a court; or
(vii) transferring shares of our common stock or any security convertible into or exercisable or exchangeable for shares of our common stock after the date of this prospectus pursuant to a bona fide third party tender offer, merger, consolidation or other similar transaction made to all holders of the shares of our common stock involving a change of control of us; provided that all of such holder’s common stock subject to the lock-up agreement that are not so transferred, sold, tendered or otherwise disposed of remain subject to the lock-up agreement; provided further that in the event that the tender offer, merger, consolidation or other such transaction is not completed, the shares of our common stock owned by such holder shall remain subject to the restrictions contained in the lock-up agreement (for this purpose, “change of control” shall mean the transfer (whether by tender offer, merger, consolidation, or other similar transaction), in one transaction or a series of related transactions, to a person or a group of affiliated persons, of shares of capital stock if, after such transfer, such person or group of affiliated persons would hold more than 50% of our outstanding voting securities (or the surviving entity)).
Market Listing
We have applied to list the shares offered hereby on the NASDAQ under the symbol “CLXT.”
Before this offering, there has been no public market for our common stock. The initial public offering price will be determined through negotiations between us and the representatives. In addition to prevailing market conditions, the factors to be considered in determining the initial public offering price are:
| • | the valuation multiples of publicly traded companies that the representatives believe to be comparable to us, |
| • | our financial information, |
| • | the history of, and the prospects for, our company and the industry in which we compete, |
140
Table of Contents
| • | an assessment of our management, its past and present operations, and the prospects for, and timing of, our future revenues, |
| • | the present state of our development, and |
| • | the above factors in relation to market values and various valuation measures of other companies engaged in activities similar to ours. |
An active trading market for the shares may not develop. It is also possible that after the offering the shares will not trade in the public market at or above the initial public offering price.
The underwriters do not expect to sell more than 5% of the shares in the aggregate to accounts over which they exercise discretionary authority.
Price Stabilization, Short Positions and Penalty Bids
Until the distribution of the shares is completed, SEC rules may limit underwriters and selling group members from bidding for and purchasing our common stock. However, the representatives may engage in transactions that stabilize the price of the common stock, such as bids or purchases to peg, fix or maintain that price.
In connection with the offering, the underwriters may purchase and sell our common stock in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of shares than they are required to purchase in the offering. “Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares described above. The underwriters may close out any covered short position by either exercising their option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the option granted to them. “Naked” short sales are sales in excess of such option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of shares of common stock made by the underwriters in the open market prior to the completion of the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of such underwriter in stabilizing or short covering transactions.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. The underwriters may conduct these transactions on the NASDAQ, in the over-the-counter market or otherwise.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor any of the underwriters make any representation that the representatives will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Distribution
In connection with the offering, certain of the underwriters or securities dealers may distribute prospectuses by electronic means, such as e-mail.
141
Table of Contents
Other Relationships
Some of the underwriters and their affiliates have engaged in, and may in the future engage in, investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. They have received, or may in the future receive, customary fees and commissions for these transactions.
In addition, in the ordinary course of their business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
European Economic Area
In relation to each member state of the European Economic Area, no offer of ordinary shares which are the subject of the offering has been, or will be made to the public in that Member State, other than under the following exemptions under the Prospectus Directive:
| (a) | to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| (b) | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), subject to obtaining the prior consent of the representatives for any such offer; or |
| (c) | in any other circumstances falling within Article 3(2) of the Prospectus Directive, |
provided that no such offer of ordinary shares referred to in (a) to (c) above shall result in a requirement for the Company or any representative to publish a prospectus pursuant to Article 3 of the Prospectus Directive, or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
Each person located in a Member State to whom any offer of ordinary shares is made or who receives any communication in respect of an offer of ordinary shares, or who initially acquires any ordinary shares will be deemed to have represented, warranted, acknowledged and agreed to and with each representative and the Company that (1) it is a “qualified investor” within the meaning of the law in that Member State implementing Article 2(1)(e) of the Prospectus Directive; and (2) in the case of any ordinary shares acquired by it as a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, the ordinary shares acquired by it in the offer have not been acquired on behalf of, nor have they been acquired with a view to their offer or resale to, persons in any Member State other than qualified investors, as that term is defined in the Prospectus Directive, or in circumstances in which the prior consent of the representatives has been given to the offer or resale; or where ordinary shares have been acquired by it on behalf of persons in any Member State other than qualified investors, the offer of those ordinary shares to it is not treated under the Prospectus Directive as having been made to such persons.
The Company, the representatives and their respective affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgments and agreements.
This prospectus has been prepared on the basis that any offer of shares in any Member State will be made pursuant to an exemption under the Prospectus Directive from the requirement to publish a prospectus for offers of shares. Accordingly any person making or intending to make an offer in that Member State of shares which are the subject of the offering contemplated in this prospectus may only do so in circumstances in which no obligation arises for the Company or any of the representatives to publish a prospectus pursuant to Article 3 of the Prospectus Directive in relation to such offer. Neither the Company nor the representatives have authorized, nor do they authorize, the making of any offer of shares in circumstances in which an obligation arises for the Company or the representatives to publish a prospectus for such offer.
142
Table of Contents
For the purposes of this provision, the expression an “offer of ordinary shares to the public” in relation to any ordinary shares in any Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the ordinary shares to be offered so as to enable an investor to decide to purchase or subscribe the ordinary shares, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive” means Directive 2003/71/EC (as amended) and includes any relevant implementing measure in each Member State.
The above selling restriction is in addition to any other selling restrictions set out below.
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19 (5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the “Order”) and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). This document must not be acted on or relied on in the United Kingdom by persons who are not relevant persons. In the United Kingdom, any investment or investment activity to which this document relates is only available to, and will be engaged in with, relevant persons.
Notice to Prospective Investors in Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company, the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (FINMA), and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (“CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
Notice to Prospective Investors in the Dubai International Financial Centre
This prospectus relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority (“DFSA”). This prospectus is intended for distribution only to persons of a type specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus nor taken steps to verify the information set forth herein and has no responsibility for the prospectus. The shares to which this prospectus relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the shares offered should conduct their own due diligence on the shares. If you do not understand the contents of this prospectus you should consult an authorized financial advisor.
Notice to Prospective Investors in Australia
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission (“ASIC”), in relation to the offering. This
143
Table of Contents
prospectus does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001 (the “Corporations Act”), and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any offer in Australia of the shares may only be made to persons (the “Exempt Investors”) who are “sophisticated investors” (within the meaning of section 708(8) of the Corporations Act), “professional investors” (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the shares without disclosure to investors under Chapter 6D of the Corporations Act.
The shares applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring shares must observe such Australian on-sale restrictions.
This prospectus contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Notice to Prospective Investors in Hong Kong
The securities have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the securities has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to the securities which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
Notice to Prospective Investors in Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948, as amended) and, accordingly, will not be offered or sold, directly or indirectly, in Japan, or for the benefit of any Japanese Person or to others for re-offering or resale, directly or indirectly, in Japan or to any Japanese Person, except in compliance with all applicable laws, regulations and ministerial guidelines promulgated by relevant Japanese governmental or regulatory authorities in effect at the relevant time. For the purposes of this paragraph, “Japanese Person” shall mean any person resident in Japan, including any corporation or other entity organized under the laws of Japan.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of Non-CIS Securities may not be circulated or distributed, nor may the
144
Table of Contents
Non-CIS Securities be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”), (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275, of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the Non-CIS Securities are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| (a) | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| (b) | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, |
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the Non-CIS Securities pursuant to an offer made under Section 275 of the SFA except:
| (a) | to an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA; |
| (b) | where no consideration is or will be given for the transfer; |
| (c) | where the transfer is by operation of law; |
| (d) | as specified in Section 276(7) of the SFA; or |
| (e) | as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore. |
Notice to Prospective Investors in Canada
The securities may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section 3A.4) of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the representatives are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
145
Table of Contents
The validity of the issuance of the shares of common stock offered hereby and certain matters of U.S. federal and New York State law will be passed upon for Calyxt, Inc. by Davis Polk & Wardwell LLP, New York, New York, and for the underwriters by Latham & Watkins LLP, Costa Mesa, California.
The financial statements of Calyxt, Inc. as of December 31, 2016 and 2015, and for the years then ended, appearing in this prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the U.S. Securities and Exchange Commission a registration statement (including amendments and exhibits to the registration statement) on Form S-1 under the Securities Act. This prospectus, which is part of the registration statement, does not contain all of the information set forth in the registration statement and the exhibits and schedules to the registration statement. For further information, we refer you to the registration statement and the exhibits and schedules filed as part of the registration statement. If a document has been filed as an exhibit to the registration statement, we refer you to the copy of the document that has been filed. Each statement in this prospectus relating to a document filed as an exhibit is qualified in all respects by the filed exhibit.
Upon completion of this offering, we will become subject to the informational requirements of the Exchange Act. Accordingly, we will be required to file reports and other information with the SEC, including annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K. You may inspect and copy reports and other information filed with the SEC at the Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet website that contains reports and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
146
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Unaudited Condensed Financial Statements for the Three Months Ended March 31, 2017 and 2016
| Financial Statements |
||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
Financial Statements for the Years Ended December 31, 2016 and 2015
| F-18 | ||||
| Financial Statements |
||||
| F-19 | ||||
| F-20 | ||||
| F-21 | ||||
| F-22 | ||||
| F-23 | ||||
F-1
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
(Amounts in Thousands, Except Share Data and Per Share Data)
| March 31, 2017 |
December 31, 2016 |
|||||||
| (unaudited) | ||||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 3,007 | $ | 5,026 | ||||
| Trade accounts receivable |
— | 110 | ||||||
| Due from related parties |
40 | 47 | ||||||
| Prepaid expenses and other current assets |
650 | 282 | ||||||
|
|
|
|
|
|||||
| Total current assets |
3,697 | 5,465 | ||||||
| Property and equipment, net |
11,173 | 10,994 | ||||||
| Other long-term assets |
222 | 164 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 15,092 | $ | 16,623 | ||||
|
|
|
|
|
|||||
| Liabilities and stockholder’s equity (deficit) |
||||||||
| Current liabilities: |
||||||||
| Due to related parties |
$ | 2,168 | $ | 1,712 | ||||
| Accounts payable |
694 | 357 | ||||||
| Accrued salaries, wages, and other compensation |
243 | 332 | ||||||
| Accrued liabilities |
781 | 363 | ||||||
| Current deferred revenue |
162 | 101 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
4,048 | 2,865 | ||||||
| Other liabilities: |
||||||||
| Non-current deferred revenue |
623 | 639 | ||||||
|
|
|
|
|
|||||
| Total other liabilities |
623 | 639 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
4,671 | 3,504 | ||||||
| Stockholder’s equity: |
||||||||
| Common stock, $0.0001 par value; 30,000,000 shares authorized, 8,000,000 shares issued and outstanding as of March 31, 2017 and December 31, 2016. |
1 | 1 | ||||||
| Additional paid-in capital |
41,820 | 41,686 | ||||||
| Accumulated deficit |
(31,400 | ) | (28,568 | ) | ||||
|
|
|
|
|
|||||
| Total stockholder’s equity |
10,421 | 13,119 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholder’s equity |
$ | 15,092 | $ | 16,623 | ||||
|
|
|
|
|
|||||
See accompanying notes to the Unaudited Condensed Financial Statements.
F-2
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Condensed Statements of Operations
(Amounts in Thousands except Shares Outstanding and per share amounts)
| Three Months Ended March 31, | ||||||||
| 2017 | 2016 | |||||||
| (unaudited) | ||||||||
| Revenue |
$ | 55 | $ | 106 | ||||
| Operating expenses: |
||||||||
| Cost of revenue |
— | 100 | ||||||
| Research and development |
1,266 | 1,370 | ||||||
| Sales, general, and administrative |
1,578 | 1,197 | ||||||
|
|
|
|
|
|||||
| 2,844 | 2,667 | |||||||
|
|
|
|
|
|||||
| Loss from operations |
(2,789 | ) | (2,561 | ) | ||||
| Interest expense |
(14 | ) | (4 | ) | ||||
| Foreign currency transaction (losses) |
(29 | ) | (4 | ) | ||||
|
|
|
|
|
|||||
| Loss before income taxes |
(2,832 | ) | (2,569 | ) | ||||
| Income tax expense |
— | — | ||||||
|
|
|
|
|
|||||
| Net loss |
$ | (2,832 | ) | $ | (2,569 | ) | ||
|
|
|
|
|
|||||
| Basic and diluted loss per share |
$ | (0.35 | ) | $ | (0.32 | ) | ||
|
|
|
|
|
|||||
| Weighted average shares outstanding—basic and diluted |
8,000,000 | 8,000,000 | ||||||
|
|
|
|
|
|||||
See accompanying notes to the Unaudited Condensed Financial Statements.
F-3
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Condensed Statements of Stockholder’s Equity
(Amounts in Thousands except Shares Outstanding)
| Shares Outstanding |
Common Stock |
Additional Paid-In Capital |
Accumulated Deficit |
Total Stockholder’s Equity (Deficit) |
||||||||||||||||
| Balances at December 31, 2015 |
8,000,000 | $ | 1 | $ | 40,738 | $ | (16,482 | ) | $ | 24,257 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Stock-based compensation |
— | 948 | — | 948 | ||||||||||||||||
| Net loss |
— | — | — | (12,086 | ) | (12,086 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2016 |
8,000,000 | $ | 1 | $ | 41,686 | $ | (28,568 | ) | $ | 13,119 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Stock-based compensation |
— | 134 | — | 134 | ||||||||||||||||
| Net loss |
— | — | — | (2,832 | ) | (2,832 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at March 31, 2017 (unaudited) |
8,000,000 | $ | 1 | $ | 41,820 | $ | (31,400 | ) | $ | 10,421 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
See accompanying notes to the Unaudited Condensed Financial Statements.
F-4
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Condensed Statements of Cash Flows
(Amounts in Thousands)
| Three Months Ended March 31, | ||||||||
| 2017 | 2016 | |||||||
| (unaudited) | ||||||||
| Operating activities |
||||||||
| Net loss |
$ | (2,832 | ) | $ | (2,569 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation |
133 | 55 | ||||||
| Stock-based compensation |
134 | 318 | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Trade accounts receivable |
110 | (9 | ) | |||||
| Due to/from related parties |
463 | 363 | ||||||
| Prepaid expenses and other assets |
(426 | ) | (196 | ) | ||||
| Accounts payable |
337 | 379 | ||||||
| Accrued salaries, wages, and other compensation |
(89 | ) | (111 | ) | ||||
| Accrued liabilities |
418 | 111 | ||||||
| Deferred revenue |
45 | 32 | ||||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(1,707 | ) | (1,627 | ) | ||||
| Investing activities |
||||||||
| Purchases of property and equipment |
(312 | ) | (6,780 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
(312 | ) | (6,780 | ) | ||||
| Financing activities |
||||||||
| Capital contribution from Parent |
— | — | ||||||
| Advances from Parent |
— | — | ||||||
| Repayments of advances from Parent |
— | — | ||||||
| Dividend to Parent |
— | — | ||||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
— | — | ||||||
|
|
|
|
|
|||||
| Net (decrease) increase in cash and cash equivalents |
(2,019 | ) | (8,407 | ) | ||||
| Cash and cash equivalents—beginning of year |
5,026 | 24,687 | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents—end of year |
$ | 3,007 | $ | 16,280 | ||||
|
|
|
|
|
|||||
| Supplemental cash flow information |
||||||||
| Interest paid |
$ | 14 | $ | 4 | ||||
|
|
|
|
|
|||||
| Income taxes paid |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
See accompanying notes to the Unaudited Condensed Financial Statements.
F-5
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Condensed Financial Statements
(unaudited)
1. Nature of Business
Calyxt, Inc., formerly known as Cellectis Plant Sciences, Inc. (the Company or Calyxt), was founded in 2010 and incorporated in Delaware. The Company is headquartered in New Brighton, Minnesota. The Company is an agriculture biotechnology company focused on creating healthier specialty food ingredients and agriculturally advantageous food crops through the use of gene editing technology for plants. The Company changed its name from Cellectis Plant Sciences, Inc. to Calyxt, Inc. on May 4, 2015. The Company is a wholly owned subsidiary of Cellectis (Parent).
Going Concern
During the three months ended March 31, 2017 and year ended December 31, 2016, the Company incurred losses from operations and net cash outflows from operating activities as disclosed in the statements of operation and cash flows, respectively. At March 31, 2017, the Company had an accumulated deficit of $31.4 million and expects to incur losses for the foreseeable future. To date, the Company has been funded primarily by various cash and equity infusions by the Parent. Although the Company believes that it will be able to successfully fund its operations through funding from its Parent and cash on hand of $3.0 million at March 31, 2017, there can be no assurance the Company will be able to do so or that the Company will ever operate profitably. The Parent has guaranteed funding for the Company’s operations through August 2018. The ability of the Company to continue as a going concern is subject to this guaranteed funding from its Parent and the ability of the Company to develop and successfully commercialize the Company’s product candidates.
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts and disclosures in the financial statements and accompanying notes. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash and cash equivalents consist of cash and term deposits with original maturities of three months or less. The carrying value of these instruments approximate fair value. The balances, at times, may exceed federally insured limits. The Company has not experienced any losses on its cash and cash equivalents.
Trade Accounts Receivable
Accounts receivable are unsecured, are recorded at net realizable value, and do not bear interest. The Company makes judgments as to its ability to collect outstanding receivables based upon patterns of uncollectability, historical experience, and management’s evaluation of specific accounts and will provide an allowance for credit losses when collection becomes doubtful. The Company performs credit evaluations of its customers’ financial condition on an as-needed basis. Payment is generally due 30 days from the invoice date, and accounts past 30 days are individually analyzed for collectability. When all collection efforts have been exhausted, the account is written off against the related allowance.
As of March 31, 2017, the Company had no trade accounts receivables. The Company considered its trade receivables to be fully collectible at December 31, 2016; accordingly, no allowance for doubtful accounts was considered necessary.
F-6
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Condensed Financial Statements (continued)
(unaudited)
Other Current Assets
Other current assets represent prepayments and deposits made by the Company.
Property and Equipment
Property and equipment is stated at cost less accumulated depreciation. Depreciation is computed based upon the estimated useful lives of the respective assets. Leasehold improvements are amortized using the straight-line method over the shorter of the lease term or the estimated useful life of the assets. Repairs and maintenance costs are expensed as incurred. The cost and accumulated depreciation of property and equipment retired, or otherwise disposed of, are removed from the related accounts, and any residual values are charged to expense. Depreciation expense has been calculated using the following estimated useful lives:
| Buildings and other improvements |
10–20 years | |||
| Leasehold improvements |
Remaining lease period | |||
| Office furniture and equipment |
5–7 years | |||
| Computer equipment and software |
3–5 years |
Long-Lived Assets
Management reviews for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. If the impairment tests indicate that the carrying value of the asset, or asset group is greater than the expected undiscounted cash flows to be generated by such asset or asset group, further analysis is performed to determine the fair value of the asset or asset group. To the extent the fair value of the asset or asset group is less than its carrying value, an impairment loss is recognized equal to the amount the carrying value exceeds the fair value of the asset or asset group. The Company generally measures fair value by considering sale prices for similar assets or asset groups, or by discounting estimated future cash flows from such assets or asset groups using an appropriate discount rate. Assets to be disposed of are carried at the lower of their carrying value or fair value less costs to sell.
There have been no impairment losses recognized for the three months ended March 31, 2017 or March 31, 2016.
Fair Value of Financial Instruments
Pursuant to the requirements of Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) 820, Fair Value Measurement the Company’s financial assets and liabilities measured at fair value on a recurring basis are classified and disclosed in one of the following three categories:
Level 1—Financial instruments with unadjusted quoted prices listed on active market exchanges.
Level 2—Financial instruments lacking unadjusted, quoted prices from active market exchanges, including over-the-counter traded financial instruments. The prices for the financial instruments are determined using prices for recently traded financial instruments with similar underlying terms as well as directly or indirectly observable inputs, such as interest rates and yield curves that are observable at commonly quoted intervals.
Level 3—Financial instruments that are not actively traded on a market exchange. This category includes situations where there is little, if any, market activity for the financial instrument. The prices are determined using significant unobservable inputs or valuation techniques.
F-7
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Condensed Financial Statements (continued)
(unaudited)
The Company has derivative instruments which are classified as Level 2. The Company does not have any financial instruments classified as Level 3, and there were no movements between these categories during the three months ended March 31, 2017 or March 31, 2016.
Forward Purchase Contracts and Derivatives
The Company enters into supply agreements for grain and seed production with settlement values based on commodity market futures pricing. We account for these derivative financial instruments utilizing the authoritative guidance in ASC 815, Derivatives and Hedging. Realized gains and losses from derivative contracts are recorded as research and development (R&D) expenses as a result of breeding contract activity. The fair value for forward purchase contracts is estimated based on exchange-quoted prices.
Unrealized gains and losses on all derivative contracts are recorded in other current assets or other current liabilities on the balance sheet at fair value. The gains and losses recorded by the Company are not significant for the three months ended March 31, 2017 or March 31, 2016.
The table below summarizes the carrying value of derivative instruments as of March 31, 2017 and December 31, 2016.
| Derivatives not ASC 815 |
Asset Derivatives |
Liability Derivatives |
||||||||||||||||||
| Fair Value | Fair Value | |||||||||||||||||||
| Balance Sheet Location |
March 31, 2017 |
December 31, 2016 |
Balance Sheet Location |
March 31, 2017 |
December 31, 2016 |
|||||||||||||||
| (in thousands) | (in thousands) | (in thousands) | (in thousands) | |||||||||||||||||
| Forward Purchase |
Prepaid expenses and other current assets |
$ |
3 |
|
$ |
9 |
|
Accrued liabilities—Current |
$ |
31 |
|
$ |
19 |
| ||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total Derivatives |
$ | 3 | $ | 9 | $ | 31 | $ | 19 | ||||||||||||
Revenue Recognition
The Company enters into R&D agreements that may consist of nonrefundable up-front payments, milestone payments, royalties, and R&D Services. In addition, the Company may license its technology to third parties, which may be part of the R&D agreements.
For agreements that contain multiple elements, each element within a multiple-element arrangement is accounted for as a separate unit of accounting provided the following criteria are met: the delivered products or services have value to the customer on a stand-alone basis and, for an arrangement that includes a general right of return relative to the delivered products or services, delivery, or performance of the undelivered product or service is considered probable and is substantially controlled by the Company. The Company considers a deliverable to have stand-alone value if the product or service is sold separately by the Company or another vendor or could be resold by the customer. Further, the Company’s revenue arrangements do not include a general right of return relative to the delivered products.
Nonrefundable up-front payments are deferred and recognized as revenue over the period of the R&D agreement.
F-8
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Condensed Financial Statements (continued)
(unaudited)
Milestone payments represent amounts received from the Company’s R&D partners, the receipt of which is dependent upon the achievement of certain scientific, regulatory, or commercial milestones. The Company recognizes milestone payments when the triggering event has occurred, there are no further contingencies or services to be provided with respect to that event, and the counterparty has no right to refund of the payment. The triggering event may be scientific results achieved by the Company or another party to the arrangement, regulatory approvals, or the marketing of products developed under the arrangement.
Royalty revenue arises from the Company’s contractual entitlement to receive a percentage of product sales revenues achieved by counterparties. Royalty revenue is recognized on an accrual basis in accordance with the terms of the agreement when sales can be determined reliably and there is reasonable assurance that the receivables from outstanding royalties will be collected.
Licenses revenue from licenses which were granted to third parties is recognized ratably over the period of the license agreements.
Revenue from R&D services is recognized over the duration of the service period.
Cost of Revenue
Cost of revenue relates to the performance of services or contract research and consists of direct external expenses relating to projects and internal costs, including overhead allocated on a full-time equivalent basis.
Research and Development
R&D expenses represent costs incurred for the development of various products using licensed gene editing technology. R&D expenses consist primarily of salaries and related costs of the Company’s scientists, in-licensing of technology, consumables, property and equipment depreciation, and fees paid to third-party consultants. All research and development costs are expensed as incurred.
In the normal course of business, Calyxt enters into R&D arrangements with third parties where the arrangements are contractual agreements, whereby Calyxt performs R&D of certain gene traits for the outside party. The Company has entered into various multiyear arrangements where Calyxt performs the R&D of the gene technology and the third parties generally have primary responsibility for any commercialization of the technology. These arrangements are performed with no guarantee of either technological or commercial success.
The Company in-licenses certain technology from third-parties that is a component of ongoing research and product development. The Company expenses up-front license fees upon contracting due to the uncertainty of future commercial value as well as expensing any ongoing annual fees when incurred. Related-party in-licensing expenses were $6 thousand and $14 thousand for the three months ended March 31, 2017 and 2016, respectively. Third-party in-licensing expenses were $65 thousand and $309 thousand for the three months ended March 31, 2017 and 2016, respectively.
Foreign Currency Transactions
Transactions in foreign currencies are remeasured into the Company’s functional currency, U.S. dollars, at the exchange rates effective at the transaction dates. Assets and liabilities denominated in foreign currencies at
F-9
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Condensed Financial Statements (continued)
(unaudited)
the reporting date are remeasured into the functional currency using the exchange rate effective at that date. The resulting exchange gains or losses are recorded in the statements of operations under sales, general, and administrative expenses. Transaction gains were $29 thousand and $4 thousand for the three months ended March 31, 2017 and 2016, respectively.
Income Taxes
Current income taxes are recorded based on statutory obligations for the current operating period for the jurisdictions in which the Company has operations.
Deferred taxes are provided on an asset and liability method, whereby deferred tax assets are recognized for deductible temporary differences and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax basis. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Deferred tax assets and liabilities are adjusted for the effects of changes in tax laws and rates on the date of enactment.
The tax effects from an uncertain tax position can be recognized in the financial statements only if the position is more likely than not to be sustained on audit, based on the technical merits of the position. Calyxt recognizes the financial statement benefit of a tax position only after determining that the relevant tax authority would more likely than not sustain the position following an audit. For tax positions meeting the more-likely-than-not threshold, the amount recognized in the financial statements is the largest benefit that has a greater than 50% likelihood of being realized upon settlement with the relevant tax authority. The Company is subject to income taxes in U.S. federal and state jurisdictions. With few exceptions, the Company is no longer subject to U.S. federal and state income tax examinations by tax authorities for years ending prior to 2013. In the event of any future tax assessments, the Company’s accounting policy is to record the income taxes and any related interest or penalties as current income tax expense on the statements of operations.
Recent Accounting Pronouncements
In May 2014, the FASB issued Accounting Standards Update (ASU) 2014-09, Revenue from Contracts with Customers, which creates ASC 606, Revenue from Contracts with Customers, and supersedes the revenue recognition requirements in ASC 605, Revenue Recognition. The guidance in ASU 2014-09 and subsequently issued amendments ASU 2016-08, Revenue from Contracts with Customers: Principal versus Agent Considerations (Reporting Revenue Gross versus Net), ASU 2016-10, Revenue from Contracts with Customers: Identifying Performance Obligations and Licensing, and ASU 2016-12, Revenue from Contracts with Customers: Narrow-Scope Improvements and Practical Expedients, outlines a comprehensive model for all entities to use in accounting for revenue arising from contracts with customers as well as required disclosures. Entities have the option of using either a full retrospective or modified approach to adopt the new guidance. For public entities, certain not-for-profit entities, and certain employee benefit plans, the new revenue standard is effective for annual periods beginning after December 15, 2017, including interim periods within that reporting period. For all other entities, the new revenue standard is effective for annual periods beginning after December 15, 2018, and interim periods within annual periods beginning after December 15, 2019. Early adoption is permitted. The Company is evaluating the impact of adopting this pronouncement.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. The
F-10
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Condensed Financial Statements (continued)
(unaudited)
guidance in ASU 2014-15 sets forth management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern as well as required disclosures. ASU 2014-15 indicates that, when preparing financial statements for interim and annual periods, management should evaluate whether conditions or events, in the aggregate, raise substantial doubt about the entity’s ability to continue as a going concern one year from the date the financial statements are issued or are available to be issued. This evaluation should include consideration of conditions and events that are either known or are reasonably knowable at the date the financial statements are issued or are available to be issued, as well as whether it is probable that management’s plans to address the substantial doubt will be implemented and, if so, whether it is probable that the plans will alleviate the substantial doubt. ASU 2014-15 is effective for annual periods ending after December 15, 2016, for both public and non-public entities, and interim periods and annual periods thereafter. The Company adopted this guidance in the current fiscal year, and, accordingly, this is disclosed in Note 1 to these financial statements.
In November 2015, the FASB issued ASU 2015-17, Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes. The amendment simplifies the presentation of deferred income taxes. Instead of separating deferred income tax liabilities and assets into current and non-current amounts in a classified statement of financial position, the amendments in this update require that deferred tax liabilities and assets be classified as non-current in a classified statement of financial position. For public entities, ASU 2015-17 is effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods. For non-public entities, ASU 2015-17 is effective for annual periods beginning after December 15, 2017, and interim periods within annual periods beginning after December 15, 2018. Early adoption is permitted. The Company does not expect the adoption of this standard to have a material impact on its financial statements, as all net deferred tax assets are fully reserved.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). The guidance requires that lessees will be required to recognize assets and liabilities on the balance sheet for the rights and obligations created by all leases with terms of more than 12 months. The amendment also will require disclosures designed to give financial statement users information on the amount, timing, and uncertainty of cash flows arising from leases. These disclosures include qualitative and quantitative information. For public entities, not-for-profit entities, or employee benefit plans, ASU 2016-02 is effective for annual periods beginning after December 15, 2018, and interim periods within those annual periods. For all other entities, ASU 2016-02 is effective for annual periods beginning after December 15, 2019, and interim periods within annual periods beginning after December 15, 2020. Entities are required to use a modified retrospective approach for leases that exist or are entered into after the beginning of the earliest comparative period in the financial statements. Early adoption is permitted. The Company is evaluating the impact of adopting this pronouncement.
In March 2016, the FASB issued ASU 2016-09, Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting. This ASU eliminates the APIC (additional paid-in capital) pool concept and requires that excess tax benefits and tax deficiencies be recorded in the statement of operations when awards are settled. The ASU also addresses simplifications related to statement of cash flows classification, accounting for forfeitures, and minimum statutory tax withholding requirements. For public entities, ASU 2016-09 is effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods. For all other entities, ASU 2016-09 is effective for annual periods beginning after December 15, 2017, and interim periods within annual periods beginning after December 15, 2018. Early adoption is permitted. The Company is evaluating the impact of adopting this pronouncement.
F-11
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Condensed Financial Statements (continued)
(unaudited)
3. Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents, and trade accounts receivable. The Company also has concentrations of revenue with certain customers.
Cash and cash equivalents concentration—The Company places its cash at financial institutions with balances that, at times, may exceed federally insured limits. The Company evaluates the creditworthiness of these financial institutions in determining the risk associated with these deposits. Calyxt has not experienced any losses on such accounts.
Trade accounts receivable concentration—At March 31, 2017, the Company had no trade accounts receivable. At December 31, 2016, one customer accounted for 100% of trade accounts receivable.
Revenue concentration— For the three months ended March 31, 2017, three customers accounted individually for 50%, 30% and 16% of revenue, respectively. For the three months ended March 31, 2016, four customers accounted individually for 32%, 25%, 24%, and 14% of revenue, respectively.
4. Property and Equipment
Property and equipment consists of the following:
| (Amounts in Thousands) | Three months ending March 31, 2017 |
Year ending December 31, 2016 |
||||||
| Land |
$ | 5,690 | $ | 5,690 | ||||
| Buildings and other improvements |
4,304 | 4,304 | ||||||
| Leasehold improvements |
169 | 169 | ||||||
| Office furniture and equipment |
1,560 | 1,506 | ||||||
| Computer equipment and software |
20 | 20 | ||||||
| Assets under construction |
295 | 37 | ||||||
|
|
|
|
|
|||||
| 12,038 | 11,726 | |||||||
| Less accumulated depreciation |
(865 | ) | (732 | ) | ||||
|
|
|
|
|
|||||
| Net property and equipment |
$ | 11,173 | $ | 10,994 | ||||
|
|
|
|
|
|||||
On March 1, 2016, the Company purchased approximately 11 acres of land in Roseville, MN, for approximately $5.7 million to build its new headquarters facility. The Company completed construction of approximately $4.3 million for phase one of the corporate headquarter plan on this parcel of land, which consists of the Company’s R&D green houses and storage facility. The Company began operations in the greenhouse facility on September 1, 2016.
For the three months ended March 31, 2017, the Company recorded $258 thousand in assets under construction for site improvements and architect fees related to phase two of the corporate headquarter plan.
Depreciation expense was $133 thousand and $55 thousand for the three months ended March 31, 2017 and 2016, respectively.
F-12
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Condensed Financial Statements (continued)
(unaudited)
5. Related-Party Transactions
Due to related parties consists of cash advances, license fees, amounts owing under the intercompany management agreement, and interest charged on outstanding amounts due. Amounts due to the Parent bear interest at a rate of the London Interbank Offered Rate plus 5% per annum. All interest expense in the statements of operations is related to interest accruing on amounts due to the Parent.
The Company has a management agreement with its Parent, in which the Company pays the Parent a monthly fee for certain services provided by the Parent, which include general sales and administration functions, accounting functions, legal advice, human resources, and information technology. The Company recorded expenses associated with the management agreement of $412 thousand and $371 thousand for the three months ended March 31, 2017 and 2016, respectively. The Company classified $380 and $325 thousand, for the three months ended March 31, 2017 and 2016, respectively, as a component of sales, general, and administrative expenses, while $32 thousand and $46 thousand was classified as a component of R&D expenses for the three months ended March 31, 2017 and 2016, respectively.
Due from related parties consists of receivables due from another subsidiary of the Parent Company related to employee benefit services provided by Calyxt to the other subsidiary.
TALEN technology was invented by researchers at the University of Minnesota and Iowa State University and exclusively licensed to Cellectis. Calyxt, as a wholly owned subsidiary of Cellectis, obtained an exclusive license to the technology in 2011 for the use in plants and this is the primary technology used by Calyxt. Calyxt seeks to leverage TALEN technology by creating plants and food products with consumer health benefits. TALEN technology works by enabling the Company to edit genes and overcoming many of the limitations of traditional trait-development techniques. The Company will be required to pay a royalty to Cellectis on future sales for the licensing of the technology.
6. Accrued Liabilities
As of March 31, 2017, and December 31, 2016, respectively, the Company had accrued liabilities of $781 thousand and $363 thousand, which consist of capital expenditures and miscellaneous operating expenses.
7. Net Loss per Share
Basic earnings per share is computed based on the net loss allocable to common stockholders for each period, divided by the weighted average number of common shares outstanding. All outstanding stock options are excluded from the calculation since they are anti-dilutive.
8. Stock-Based Compensation
Calyxt, Inc. Equity Incentive Plan
The Company has adopted the Calyxt, Inc. Equity Incentive Plan, which allows for the grant of stock options to attract and retain highly qualified employees. The Company has reserved 831,500 shares of common stock to be issued upon the exercise of stock options under the plan. Option awards are granted with an exercise price equal to the estimated fair value of the Company’s stock at the grant date.
F-13
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Condensed Financial Statements (continued)
(unaudited)
The awards granted under the Calyxt, Inc. Equity Incentive Plan are only exercisable upon a triggering event or Initial Public Offering of the Company, as defined by the plan. These stock awards are accounted for as liability awards and are remeasured each reporting period. Because there were no triggering events that occurred during the three months ended March 31, 2017 or 2016, no compensation expense was recognized in these respective periods. At March 31, 2017 and December 31, 2016, the Company had 825,500 stock options outstanding. Of that total, 201,800 stock options were fully vested at March 31, 2017, and have total unrecognized stock-based compensation expense of $4.54 million. The stock compensation expense related to the awards will be recorded upon either the triggering event or Initial Public Offering of the Company, and the amount recorded will be based upon the stock price at that time.
Valuation Assumptions: The fair value of each stock option is estimated using the Black-Scholes option pricing model at each measurement date. The fair value of stock options under the Black-Scholes model requires management to make assumptions regarding projected employee stock option exercise behaviors, risk-free interest rates, volatility of the Company’s stock price, and expected dividends. The awards were granted with vesting terms between two and four years. Certain awards contain a 25% acceleration vesting clause upon a triggering event or Initial Public Offering of the Company.
The Company has not historically paid cash dividends to its stockholders and currently does not anticipate paying any cash dividends in the foreseeable future. As a result, the Company has assumed a dividend yield of 0%. The risk-free interest rate is based upon the rates of U.S. Treasury bills with a term that approximates the expected life of the option. The Company uses the simplified method to reasonably estimate the expected life of its option awards. Expected volatility is based upon the volatility of comparable public companies.
The following table provides the assumptions used in the Black-Scholes model to measure the unrecognized compensation expense for the Calyxt option awards as of March 31, 2017:
| Expected dividend yield |
0 | % | ||
| Risk-free interest rate |
1.28 | % | ||
| Expected volatility |
25 | % | ||
| Expected life (in years) |
5.75-6.25 |
Parent Awards
The Company’s Parent grants stock options to employees of Calyxt. Compensation costs related to the grant of the Parent company awards to Calyxt’s employees has been recognized in the statements of operations with a corresponding credit to stockholder’s equity, representing the Parent Company’s Capital contribution. The fair value of each stock option is estimated at the grant date using the Black-Scholes option pricing model. The fair value of stock options under the Black-Scholes model requires management to make assumptions regarding projected employee stock option exercise behaviors, risk-free interest rates, volatility of the Company’s stock price, and expected dividends.
F-14
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Condensed Financial Statements (continued)
(unaudited)
The following table provides the range of assumptions used in the Black-Scholes model for the Parent awards:
| Expected dividend yield |
0 | % | ||
| Risk-free interest rate |
0.00-0.42 | % | ||
| Expected volatility |
59.8%-63.2 | % | ||
| Expected life (in years) |
6.11-6.12 |
The Company’s Parent granted certain consultants of Calyxt non-employee warrants to purchase Cellectis stock in exchange for services provided to the Company. The Company recorded the fair value of the warrants as a dividend paid to the Parent in exchange for the warrants issued to them.
The Company recognized stock-based compensation expense related to its Parent’s grants of stock options and warrants to Calyxt employees and consultants of $134 thousand and $318 thousand for the three month period ended March 31, 2017 and 2016, respectively. The following table summarizes the stock-based compensation expense, which was recognized in the statements of operations for the three month period ended March 31:
| (Amounts in Thousands) | Three months ending March 31, 2017 |
Three months ending March 31, 2016 |
||||||
| Selling, general, and administrative |
$ | 3 | $ | 7 | ||||
| Research and development |
131 | 311 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 134 | $ | 318 | ||||
|
|
|
|
|
|||||
9. Income Taxes
The Company provides for a valuation allowance when it is more likely than not that it will not realize a portion of the deferred tax assets. The Company has established a full valuation allowance for deferred tax assets due to the uncertainty that enough taxable income will be generated in the taxing jurisdiction to utilize the assets. Therefore, the Company has not reflected any benefit of such deferred tax assets in the accompanying financial statements.
As of March 31, 2017, there were no material changes to what the Company disclosed regarding tax uncertainties or penalties as of December 31, 2016.
10. Commitments and Contingencies
Litigation and Claims
Various legal actions, proceedings, and claims (generally, “matters”) are pending or may be instituted or asserted against the Company. The Company accrues for matters when losses are deemed probable and reasonably estimable. Any resulting adjustments, which could be material, are recorded in the period the adjustments are identified. The Company has not identified any legal matters needing to be recorded or disclosed as of March 31, 2017.
F-15
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Condensed Financial Statements (continued)
(unaudited)
Leases
The Company leases office space under a non-cancelable operating lease that expires in October, 2017. Rent expense is recognized using the straight-line method over the term of the lease. In addition to minimum lease payments, the office lease requires payment of a proportionate share of real estate taxes and building operating expenses. Total rent expense was $65 thousand and $74 thousand for three months ended March 31, 2017 and 2016, respectively.
Future minimum lease commitments are as follows as of March 31, 2017:
| 2017 |
$ | 83 | ||
| 2018 and beyond |
— | |||
|
|
|
|||
| Total |
$ | 83 | ||
|
|
|
Parent Obligations
As of March 31, 2017 the Company had short-term Parent obligations of $2.1 million consisting of amounts owed under the intercompany management agreement, for certain services provided by Cellectis.
Forward Purchase Commitments
The Company has forward purchase commitments with growers to purchase seed and grain at a future date in the amount of approximately $677 thousand, which are not recorded in the financial statements because the company has not taken delivery of the seed and grain.
11. Employee Benefit Plan
The Company provides a 401(k) defined contribution plan (the Plan) for participation by all regular full-time employees who have completed three months of service. The Plan provides for a matching contribution equal to 100% of the amount of the employee’s salary deduction up to 3% of the salary per employee and an additional 50% match from 3% to 5% of salary. Employees’ rights to the Company’s matching contributions vest immediately. Company contributions to the plan totaled $27 thousand and $18 thousand for the three months ended March 31, 2017 and 2016, respectively.
12. Segment and Geographic Information
The Company has one operating and reportable segment, R&D of plant gene editing. The Company derives substantially all of its revenue from R&D contracts related to plant gene editing.
13. Subsequent Events
On January 4, 2017, the Company signed a Letter of Intent with an unrelated third party (Lessor) for the sale and leaseback of the new Roseville, MN, Greenhouse and Warehouse Facility, and for the construction of the phase 2 expansion of the Roseville Facility, which includes R&D and administrative facilities and a demonstration kitchen. In the second quarter of 2017, the Company plans to enter into a purchase agreement with the Lessor to sell the company’s land and constructed greenhouse facilities and lease said assets back over a 20-year period starting at the completion of phase 2. In addition, the Company plans to enter into a construction
F-16
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Condensed Financial Statements (continued)
(unaudited)
agreement with the Lessor to build the remaining facility, consisting of R&D and administrative facilities, and a demonstration kitchen. At the end of the construction period, the Company plans to lease these R&D, administrative, and demonstration kitchen facilities from the Lessor over a 20-year life. The Company plans to occupy the entire facility in early 2018. There is no guarantee that the Company and the Lessor will consummate these agreements.
On June 15, 2017, the Company received a cash advance from the Parent of $3.0 million to fund ongoing operations.
Pursuant to the authorization provided in a written consent in lieu of a special meeting of the Company by the stockholder dated as of June 14, 2017, the Company effected a stock split of the Company’s common stock at a ratio of 100-for-1 and increased the number of shares of common stock authorized for issuance to 30,000,000 by filing a Certificate of Amendment with the Secretary of State of the State of Delaware. As a result of the stock split, every share of issued and outstanding common stock was converted to 100 issued and outstanding shares of Common Stock, without any change in the par value per share. Since the par value of the common stock remained at $0.0001 per share, the value of “Common stock” recorded to the Company’s balance sheets has been retroactively increased to reflect the par value of restated outstanding shares, with a corresponding decrease to “Additional paid-in capital.” All share and per share data included in this report has been retroactively restated to reflect the stock split.
865,183 stock options were granted under the 2017 Incentive Omnibus Plan on June 14, 2017 with an exercise price of $32.57 per ordinary share, 546,343 of which were granted to our directors, director nominees and executive officers. The remainder of the options were granted to employees and non-employee consultants. In addition, 592,789 restricted stock units were granted under the 2017 Incentive Omnibus Plan on June 14, 2017, 364,229 of which were granted to our directors and executive officers. The remainder of the restricted stock units were granted to employees and non-employee consultants.
The Company has evaluated subsequent events through June 15, 2017, the date the financial statements were available to be issued.
F-17
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Calyxt, Inc.
We have audited the accompanying balance sheets of Calyxt, Inc. (the Company) as of December 31, 2016 and 2015, and the related statements of operations, stockholder’s equity, and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Calyxt, Inc. at December 31, 2016 and 2015, and the results of its operations and its cash flows for the years then ended, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
Minneapolis, MN
May 15, 2017, except for the second paragraph of Note 1, as to which the date is June 15, 2017 and the third, fourth and fifth paragraphs of Note 14, as to which the date is July 10, 2017
F-18
Table of Contents
(A Wholly Owned Subsidiary of Cellectis S.A.)
Balance Sheets
(Amounts in Thousands, Except Share Data and Per Share Data)
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 5,026 | $ | 24,687 | ||||
| Trade accounts receivable |
110 | 217 | ||||||
| Due from related parties |
47 | 117 | ||||||
| Prepaid expenses and other current assets |
282 | 11 | ||||||
|
|
|
|
|
|||||
| Total current assets |
5,465 | 25,032 | ||||||
| Property and equipment, net |
10,994 | 915 | ||||||
| Other long-term assets |
164 | 48 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 16,623 | $ | 25,995 | ||||
|
|
|
|
|
|||||
| Liabilities and stockholder’s equity (deficit) |
||||||||
| Current liabilities: |
||||||||
| Due to related parties |
$ | 1,712 | $ | 80 | ||||
| Accounts payable |
357 | 304 | ||||||
| Accrued salaries, wages, and other compensation |
332 | 244 | ||||||
| Accrued liabilities |
363 | 226 | ||||||
| Current deferred revenue |
101 | 182 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
2,865 | 1,036 | ||||||
| Other liabilities: |
||||||||
| Non-current deferred revenue |
639 | 702 | ||||||
|
|
|
|
|
|||||
| Total other liabilities |
639 | 702 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
3,504 | 1,738 | ||||||
| Stockholder’s equity: |
||||||||
| Common stock, $0.0001 par value; 30,000,000 shares authorized, 8,000,000 shares issued and outstanding as of December 31, 2016; 30,000,000 shares authorized, 8,000,000 shares issued and outstanding as of December 31, 2015. |
1 | 1 | ||||||
| Additional paid-in capital |
41,686 | 40,738 | ||||||
| Accumulated deficit |
(28,568 | ) | (16,482 | ) | ||||
|
|
|
|
|
|||||
| Total stockholder’s equity |
13,119 | 24,257 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholder’s equity |
$ | 16,623 | $ | 25,995 | ||||
|
|
|
|
|
|||||
See accompanying notes.
F-19
Table of Contents
(A Wholly Owned Subsidiary of Cellectis S.A.)
Statements of Operations
(Amounts in Thousands except Shares Outstanding and per share amounts)
| Year Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Revenue |
$ | 399 | $ | 1,272 | ||||
| Operating expenses: |
||||||||
| Cost of revenue |
200 | 751 | ||||||
| Research and development |
5,638 | 2,766 | ||||||
| Sales, general, and administrative |
6,670 | 3,569 | ||||||
|
|
|
|
|
|||||
| 12,508 | 7,086 | |||||||
|
|
|
|
|
|||||
| Loss from operations |
(12,109 | ) | (5,814 | ) | ||||
| Interest expense |
(5 | ) | (261 | ) | ||||
| Foreign currency transaction gains |
28 | 186 | ||||||
|
|
|
|
|
|||||
| Loss before income taxes |
(12,086 | ) | (5,889 | ) | ||||
| Income tax expense |
— | — | ||||||
|
|
|
|
|
|||||
| Net loss |
$ | (12,086 | ) | $ | (5,889 | ) | ||
|
|
|
|
|
|||||
| Basic and diluted loss per share |
$ | (1.51 | ) | $ | (2.15 | ) | ||
|
|
|
|
|
|||||
| Weighted average shares outstanding—basic and diluted |
8,000,000 | 2,745,200 | ||||||
|
|
|
|
|
|||||
See accompanying notes.
F-20
Table of Contents
(A Wholly Owned Subsidiary of Cellectis S.A.)
Statements of Stockholder’s Equity
(Amounts in Thousands except Shares Outstanding)
| Shares Outstanding |
Common Stock |
Additional Paid-In Capital |
Accumulated Deficit |
Total Stockholder’s Equity (Deficit) |
||||||||||||||||
| Balances at December 31, 2014 |
1,000,000 | $ | — | $ | 47 | $ | (10,483 | ) | $ | (10,436 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Stock-based compensation |
— | 692 | — | 692 | ||||||||||||||||
| Net loss |
— | — | (5,889 | ) | (5,889 | ) | ||||||||||||||
| Dividend to Parent |
— | — | (110 | ) | (110 | ) | ||||||||||||||
| Capital contribution from Parent |
7,000,000 | 1 | 39,999 | — | 40,000 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2015 |
8,000,000 | $ | 1 | $ | 40,738 | $ | (16,482 | ) | $ | 24,257 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
— | — | — | (12,086 | ) | (12,086 | ) | |||||||||||||
| Stock-based compensation |
— | — | 948 | — | 948 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2016 |
8,000,000 | $ | 1 | $ | 41,686 | $ | (28,568 | ) | $ | 13,119 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
See accompanying notes.
F-21
Table of Contents
(A Wholly Owned Subsidiary of Cellectis S.A.)
Statements of Cash Flows
(Amounts in Thousands)
| Year Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Operating activities |
||||||||
| Net loss |
$ | (12,086 | ) | $ | (5,889 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation |
345 | 147 | ||||||
| Stock-based compensation |
948 | 692 | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Trade accounts receivable |
107 | 25 | ||||||
| Due to/from related parties |
1,702 | (1,265 | ) | |||||
| Prepaid expenses and other assets |
(387 | ) | 59 | |||||
| Accounts payable |
53 | (28 | ) | |||||
| Accrued salaries, wages, and other compensation |
88 | 93 | ||||||
| Accrued liabilities |
137 | 40 | ||||||
| Deferred revenue |
(144 | ) | (565 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(9,237 | ) | (6,691 | ) | ||||
| Investing activities |
||||||||
| Purchases of property and equipment |
(10,424 | ) | (665 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
(10,424 | ) | (665 | ) | ||||
| Financing activities |
||||||||
| Capital contribution from Parent |
— | 30,000 | ||||||
| Advances from Parent |
— | 2,050 | ||||||
| Repayments of advances from Parent |
— | (200 | ) | |||||
| Dividend to Parent |
— | (110 | ) | |||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
— | 31,740 | ||||||
|
|
|
|
|
|||||
| Net (decrease) increase in cash and cash equivalents |
(19,661 | ) | 24,384 | |||||
| Cash and cash equivalents—beginning of year |
24,687 | 303 | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents—end of year |
$ | 5,026 | $ | 24,687 | ||||
|
|
|
|
|
|||||
| Supplemental cash flow information |
||||||||
| Interest paid |
$ | 5 | $ | 261 | ||||
|
|
|
|
|
|||||
| Income taxes paid |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
| Supplemental schedule of non-cash activities |
||||||||
| Conversion of net payable due to Parent to equity |
$ | — | $ | 10,000 | ||||
|
|
|
|
|
|||||
See accompanying notes.
F-22
Table of Contents
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements
December 31, 2016
1. Nature of Business
Calyxt, Inc., formerly known as Cellectis Plant Sciences, Inc. (the Company or Calyxt), was founded in 2010 and incorporated in Delaware. The Company is headquartered in New Brighton, Minnesota. The Company is an agriculture biotechnology company focused on creating healthier specialty food ingredients and agriculturally advantageous food crops through the use of gene editing technology for plants. The Company changed its name from Cellectis Plant Sciences, Inc. to Calyxt, Inc. on May 4, 2015. The Company is a wholly owned subsidiary of Cellectis (Parent).
Going Concern
During the years ended December 31, 2016 and 2015, the Company incurred losses from operations and net cash outflows from operating activities as disclosed in the statements of operation and cash flows, respectively. At December 31, 2016, the Company had an accumulated deficit of $28.6 million and expects to incur losses for the foreseeable future. To date, the Company has been funded primarily by various cash and equity infusions by the Parent. Although the Company believes that it will be able to successfully fund its operations through funding from its Parent, and cash on hand of $5.0 million at December 31, 2016, there can be no assurance the Company will be able to do so or that the Company will ever operate profitably. The Parent has guaranteed funding for the Company’s operations through May 2018. At June 14, 2017, Cellectis extended the guaranteed funding through August 2018. The ability of the Company to continue as a going concern is subject to this guaranteed funding from its Parent and the ability of the Company to develop and successfully commercialize the Company’s product candidates.
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts and disclosures in the financial statements and accompanying notes. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash and cash equivalents consist of cash and term deposits with original maturities of three months or less. The carrying value of these instruments approximate fair value. The balances, at times, may exceed federally insured limits. The Company has not experienced any losses on its cash and cash equivalents.
Trade Accounts Receivable
Accounts receivable are unsecured, are recorded at net realizable value, and do not bear interest. The Company makes judgments as to its ability to collect outstanding receivables based upon patterns of uncollectability, historical experience, and management’s evaluation of specific accounts and will provide an allowance for credit losses when collection becomes doubtful. The Company performs credit evaluations of its customers’ financial condition on an as-needed basis. Payment is generally due 30 days from the invoice date, and accounts past 30 days are individually analyzed for collectability. When all collection efforts have been exhausted, the account is written off against the related allowance.
The Company considers its receivables to be fully collectible; accordingly, no allowance for doubtful accounts was considered necessary as of December 31, 2016 and 2015.
F-23
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements (continued)
Other Current Assets
Other current assets represent prepayments and deposits made by the Company.
Property and Equipment
Property and equipment is stated at cost less accumulated depreciation. Depreciation is computed based upon the estimated useful lives of the respective assets. Leasehold improvements are amortized using the straight-line method over the shorter of the lease term or the estimated useful life of the assets. Repairs and maintenance costs are expensed as incurred. The cost and accumulated depreciation of property and equipment retired, or otherwise disposed of, are removed from the related accounts, and any residual values are charged to expense. Depreciation expense has been calculated using the following estimated useful lives:
| Buildings and other improvements |
10–20 years | |||
| Leasehold improvements |
Remaining lease period | |||
| Office furniture and equipment |
5–7 years | |||
| Computer equipment and software |
3–5 years |
Long-Lived Assets
Management reviews for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. If the impairment tests indicate that the carrying value of the asset, or asset group is greater than the expected undiscounted cash flows to be generated by such asset or asset group, further analysis is performed to determine the fair value of the asset or asset group. To the extent the fair value of the asset or asset group is less than its carrying value, an impairment loss is recognized equal to the amount the carrying value exceeds the fair value of the asset or asset group. The Company generally measures fair value by considering sale prices for similar assets or asset groups, or by discounting estimated future cash flows from such assets or asset groups using an appropriate discount rate. Assets to be disposed of are carried at the lower of their carrying value or fair value less costs to sell.
There have been no impairment losses recognized for the years ended December 31, 2016 and 2015.
Fair Value of Financial Instruments
Pursuant to the requirements of Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) 820, Fair Value Measurement the Company’s financial assets and liabilities measured at fair value on a recurring basis are classified and disclosed in one of the following three categories:
Level 1—Financial instruments with unadjusted quoted prices listed on active market exchanges.
Level 2—Financial instruments lacking unadjusted, quoted prices from active market exchanges, including over-the-counter traded financial instruments. The prices for the financial instruments are determined using prices for recently traded financial instruments with similar underlying terms as well as directly or indirectly observable inputs, such as interest rates and yield curves that are observable at commonly quoted intervals.
Level 3—Financial instruments that are not actively traded on a market exchange. This category includes situations where there is little, if any, market activity for the financial instrument. The prices are determined using significant unobservable inputs or valuation techniques.
F-24
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements (continued)
The Company has derivative instruments which are classified as Level 2. The Company does not have any financial instruments classified as Level 3, and there were no movements between these categories during the years ended December 31, 2016 and 2015.
Forward Purchase Contracts and Derivatives
The company enters into supply agreements for grain and seed production with settlement values based on commodity market futures pricing. We account for these derivative financial instruments utilizing the authoritative guidance in ASC 815, Derivatives and Hedging. Realized gains and losses from derivative contracts are recorded as research and development (R&D) expenses as a result of breeding contract activity. The fair value for forward purchase contracts is estimated based on exchange-quoted prices.
Unrealized gains and losses on all derivative contracts are recorded in other current assets or other current liabilities on the balance sheet at fair value. The gains and losses recorded by the Company are not significant for the year ended December 31, 2016.
The table below summarizes the carrying value of derivative instruments as of December 31, 2016. The company had no derivative instruments in 2015.
| Derivatives not ASC 815 |
Asset Derivatives |
Liability Derivatives |
||||||||||||||||||
| Fair Value | Fair Value | |||||||||||||||||||
| Balance Sheet Location |
December 31, 2016 |
December 31, 2015 |
Balance Sheet Location |
December 31, 2016 |
December 31, 2015 |
|||||||||||||||
| (in thousands) | (in thousands) | (in thousands) | (in thousands) | |||||||||||||||||
| Forward Purchase Contracts |
Prepaid expenses and other current assets |
|
9 |
|
|
— |
|
Accrued liabilities—Current |
|
19 |
|
|
— |
| ||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total Derivatives |
9 | — | 19 | — | ||||||||||||||||
Revenue Recognition
The Company enters into R&D agreements that may consist of nonrefundable up-front payments, milestone payments, royalties, and R&D Services. In addition, the Company may license its technology to third parties, which may be part of the R&D agreements.
For agreements that contain multiple elements, each element within a multiple-element arrangement is accounted for as a separate unit of accounting provided the following criteria are met: the delivered products or services have value to the customer on a stand-alone basis and, for an arrangement that includes a general right of return relative to the delivered products or services, delivery, or performance of the undelivered product or service is considered probable and is substantially controlled by the Company. The Company considers a deliverable to have stand-alone value if the product or service is sold separately by the Company or another vendor or could be resold by the customer. Further, the Company’s revenue arrangements do not include a general right of return relative to the delivered products.
Nonrefundable up-front payments are deferred and recognized as revenue over the period of the R&D agreement.
Milestone payments represent amounts received from the Company’s R&D partners, the receipt of which is dependent upon the achievement of certain scientific, regulatory, or commercial milestones. The Company
F-25
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements (continued)
recognizes milestone payments when the triggering event has occurred, there are no further contingencies or services to be provided with respect to that event, and the counterparty has no right to refund of the payment. The triggering event may be scientific results achieved by the Company or another party to the arrangement, regulatory approvals, or the marketing of products developed under the arrangement.
Royalty revenues arise from the Company’s contractual entitlement to receive a percentage of product sales revenues achieved by counterparties. Royalty revenues are recognized on an accrual basis in accordance with the terms of the agreement when sales can be determined reliably and there is reasonable assurance that the receivables from outstanding royalties will be collected.
Licenses revenues from licenses which were granted to third parties are recognized ratably over the period of the license agreements.
Revenues from R&D services are recognized over the duration of the service period.
Cost of Revenue
Cost of revenue relates to the performance of services or contract research and consists of direct external expenses relating to projects and internal costs, including overhead allocated on a full-time equivalent basis.
Research and Development
R&D expenses represent costs incurred for the development of various products using licensed gene editing technology. R&D expenses consist primarily of salaries and related costs of the Company’s scientists, in-licensing of technology, consumables, property and equipment depreciation, and fees paid to third-party consultants. All research and development costs are expensed as incurred.
In the normal course of business, Calyxt enters into R&D arrangements with third parties where the arrangements are contractual agreements, whereby Calyxt performs R&D of certain gene traits for the outside party. The Company has entered into various multiyear arrangements where Calyxt performs the R&D of the gene technology and the third parties generally have primary responsibility for any commercialization of the technology. These arrangements are performed with no guarantee of either technological or commercial success.
The Company in-licenses certain technology from third-parties that is a component of ongoing research and product development. The Company expenses up-front license fees upon contracting due to the uncertainty of future commercial value as well as expensing any ongoing annual fees when incurred. Related-party in-licensing expenses were $44 thousand and $86 thousand for the years ended December 31, 2016 and 2015, respectively. Third-party in-licensing expenses were $539 thousand and $165 thousand for the years ended December 31, 2016 and 2015, respectively.
Foreign Currency Transactions
Transactions in foreign currencies are remeasured into the Company’s functional currency, U.S. dollars, at the exchange rates effective at the transaction dates. Assets and liabilities denominated in foreign currencies at the reporting date are remeasured into the functional currency using the exchange rate effective at that date. The resulting exchange gains or losses are recorded in the statements of operations under sales, general, and administrative expenses. Transaction gains were $28 thousand and $186 thousand for the years ended December 31, 2016 and 2015, respectively.
F-26
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements (continued)
Income Taxes
Current income taxes are recorded based on statutory obligations for the current operating period for the jurisdictions in which the Company has operations.
Deferred taxes are provided on an asset and liability method, whereby deferred tax assets are recognized for deductible temporary differences and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax basis. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Deferred tax assets and liabilities are adjusted for the effects of changes in tax laws and rates on the date of enactment.
The tax effects from an uncertain tax position can be recognized in the financial statements only if the position is more likely than not to be sustained on audit, based on the technical merits of the position. Calyxt recognizes the financial statement benefit of a tax position only after determining that the relevant tax authority would more likely than not sustain the position following an audit. For tax positions meeting the more-likely-than-not threshold, the amount recognized in the financial statements is the largest benefit that has a greater than 50% likelihood of being realized upon settlement with the relevant tax authority. The Company is subject to income taxes in U.S. federal and state jurisdictions. With few exceptions, the Company is no longer subject to U.S. federal and state income tax examinations by tax authorities for years ending prior to 2013. In the event of any future tax assessments, the Company’s accounting policy is to record the income taxes and any related interest or penalties as current income tax expense on the statements of operations.
Recent Accounting Pronouncements
In May 2014, the FASB issued Accounting Standards Update (ASU) 2014-09, Revenue from Contracts with Customers, which creates ASC 606, Revenue from Contracts with Customers, and supersedes the revenue recognition requirements in ASC 605, Revenue Recognition. The guidance in ASU 2014-09 and subsequently issued amendments ASU 2016-08, Revenue from Contracts with Customers: Principal versus Agent Considerations (Reporting Revenue Gross versus Net), ASU 2016-10, Revenue from Contracts with Customers: Identifying Performance Obligations and Licensing, and ASU 2016-12, Revenue from Contracts with Customers: Narrow-Scope Improvements and Practical Expedients, outlines a comprehensive model for all entities to use in accounting for revenue arising from contracts with customers as well as required disclosures. Entities have the option of using either a full retrospective or modified approach to adopt the new guidance. For public entities, certain not-for-profit entities, and certain employee benefit plans, the new revenue standard is effective for annual periods beginning after December 15, 2017, including interim periods within that reporting period. For all other entities, the new revenue standard is effective for annual periods beginning after December 15, 2018, and interim periods within annual periods beginning after December 15, 2019. Early adoption is permitted. The Company is evaluating the impact of adopting this pronouncement.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. The guidance in ASU 2014-15 sets forth management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern as well as required disclosures. ASU 2014-15 indicates that, when preparing financial statements for interim and annual periods, management should evaluate whether conditions or events, in the aggregate, raise substantial doubt about the entity’s ability to continue as a going concern one year from the date the financial statements are issued or are available to be issued. This evaluation should include consideration of conditions and events that are either known or are reasonably knowable at the
F-27
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements (continued)
date the financial statements are issued or are available to be issued, as well as whether it is probable that management’s plans to address the substantial doubt will be implemented and, if so, whether it is probable that the plans will alleviate the substantial doubt. ASU 2014-15 is effective for annual periods ending after December 15, 2016, for both public and non-public entities, and interim periods and annual periods thereafter. The Company adopted this guidance in the current fiscal year, and, accordingly, this is disclosed in Note 1 to these financial statements.
In November 2015, the FASB issued ASU 2015-17, Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes. The amendment simplifies the presentation of deferred income taxes. Instead of separating deferred income tax liabilities and assets into current and non-current amounts in a classified statement of financial position, the amendments in this update require that deferred tax liabilities and assets be classified as non-current in a classified statement of financial position. For public entities, ASU 2015-17 is effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods. For non-public entities, ASU 2015-17 is effective for annual periods beginning after December 15, 2017, and interim periods within annual periods beginning after December 15, 2018. Early adoption is permitted. The Company does not expect the adoption of this standard to have a material impact on its financial statements, as all net deferred tax assets are fully reserved.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). The guidance requires that lessees will be required to recognize assets and liabilities on the balance sheet for the rights and obligations created by all leases with terms of more than 12 months. The amendment also will require disclosures designed to give financial statement users information on the amount, timing, and uncertainty of cash flows arising from leases. These disclosures include qualitative and quantitative information. For public entities, not-for-profit entities, or employee benefit plans, ASU 2016-02 is effective for annual periods beginning after December 15, 2018, and interim periods within those annual periods. For all other entities, ASU 2016-02 is effective for annual periods beginning after December 15, 2019, and interim periods within annual periods beginning after December 15, 2020. Entities are required to use a modified retrospective approach for leases that exist or are entered into after the beginning of the earliest comparative period in the financial statements. Early adoption is permitted. The Company is evaluating the impact of adopting this pronouncement.
In March 2016, the FASB issued ASU 2016-09, Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting. This ASU eliminates the APIC (additional paid-in capital) pool concept and requires that excess tax benefits and tax deficiencies be recorded in the statement of operations when awards are settled. The ASU also addresses simplifications related to statement of cash flows classification, accounting for forfeitures, and minimum statutory tax withholding requirements. For public entities, ASU 2016-09 is effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods. For all other entities, ASU 2016-09 is effective for annual periods beginning after December 15, 2017, and interim periods within annual periods beginning after December 15, 2018. Early adoption is permitted. The Company is evaluating the impact of adopting this pronouncement.
3. Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents, and trade accounts receivable, The Company also has concentrations of revenue with certain customers.
F-28
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements (continued)
Cash and cash equivalents concentration—The Company places its cash at financial institutions with balances that, at times, may exceed federally insured limits. The Company evaluates the creditworthiness of these financial institutions in determining the risk associated with these deposits. Calyxt has not experienced any losses on such accounts.
Trade accounts receivable concentration—At December 31, 2016, one customer accounted for 100% of trade accounts receivable. At December 31, 2015, two customers accounted for 50% each of trade accounts receivable.
Revenue concentration—In 2016, four customers accounted individually for 33%, 28%, 19%, and 16% of revenue, respectively. In 2015, three different customers accounted individually for 38%, 26%, and 20% of revenue, respectively.
4. Property and Equipment
Property and equipment consists of the following as of December 31:
| (Amounts in Thousands) | 2016 | 2015 | ||||||
| Land |
$ | 5,690 | $ | — | ||||
| Buildings and other improvements |
4,304 | — | ||||||
| Leasehold improvements |
169 | 161 | ||||||
| Office furniture and equipment |
1,506 | 1,109 | ||||||
| Computer equipment and software |
20 | 20 | ||||||
| Assets under construction |
37 | 12 | ||||||
|
|
|
|
|
|||||
| 11,726 | 1,302 | |||||||
| Less accumulated depreciation |
(732 | ) | (387 | ) | ||||
|
|
|
|
|
|||||
| Net property and equipment |
$ | 10,994 | $ | 915 | ||||
|
|
|
|
|
|||||
On March 1, 2016, the Company purchased approximately 11 acres of land in Roseville, MN, for approximately $5.7 million to build its new headquarters facility. The Company completed construction of approximately $4.3 million for phase one of the corporate headquarter plan on this parcel of land, which consists of the Company’s R&D green houses and storage facility. The Company began operations in the greenhouse facility on September 1, 2016.
Depreciation expense was $345 thousand and $147 thousand for the years ended December 31, 2016 and 2015, respectively.
5. Related-Party Transactions
Due to related parties consists of cash advances, license fees, amounts owing under the intercompany management agreement, and interest charged on outstanding amounts due. Amounts due to the Parent bear interest at a rate of the London Interbank Offered Rate plus 5% per annum. All interest expense in the statements of operations is related to interest accruing on amounts due to the Parent.
The Company has a management agreement with its Parent, in which the Company pays the Parent a monthly fee for certain services provided by the Parent, which include general sales and administration functions, accounting functions, legal advice, human resources, and information technology. The Company recorded expenses associated with the management agreement of $3.15 million and $1.88 million for the years ended
F-29
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements (continued)
December 31, 2016 and 2015, respectively. The Company classified $2.97 million and $1.70 million, in 2016 and 2015, respectively, as a component of sales, general, and administrative expenses, while $0.18 million was classified as a component of R&D expenses in 2016 and 2015.
Due from related parties consists of receivables due from another subsidiary of the Parent Company related to employee benefit services provided by Calyxt to the other subsidiary.
TALEN technology was invented by researchers at the University of Minnesota and Iowa State University and exclusively licensed to Cellectis. Calyxt, as a wholly owned subsidiary of Cellectis, obtained an exclusive license to the technology in 2011 for the use in plants and this is the primary technology used by Calyxt. Calyxt seeks to leverage TALEN technology by creating plants and food products with consumer health benefits. TALEN technology works by enabling the Company to edit genes and overcoming many of the limitations of traditional trait-development techniques. The Company will be required to pay a royalty to Cellectis on future sales for the licensing of the technology.
6. Accrued Liabilities
Accrued liabilities of $363 thousand and $226 thousand for the years ended December 31, 2016 and 2015, respectively, consist of consultant bonuses and other miscellaneous operating expenses.
7. Equity Transactions
On August 1, 2015, the Parent made a $40 million capital contribution to the Company in the form of $30 million cash, and conversion of a $10 million net payable due to the Parent to equity. On October 1, 2015, the Company entered into an amended and restated contribution agreement with Cellectis and, in exchange for the capital contribution, Calyxt issued its Parent 7,000,000 shares of Common Stock.
8. Net Loss per Share
Basic earnings per share is computed based on the net loss allocable to common stockholders for each period, divided by the weighted average number of common shares outstanding. All outstanding stock options are excluded from the calculation since they are anti-dilutive.
9. Stock-Based Compensation
Calyxt, Inc. Equity Incentive Plan
The Company has adopted the Calyxt, Inc. Equity Incentive Plan, which allows for the grant of stock options to attract and retain highly qualified employees. The Company has reserved 831,500 shares of common stock to be issued upon the exercise of stock options under the plan. Option awards are granted with an exercise price equal to the estimated fair value of the Company’s stock at the grant date.
The awards granted under the Calyxt, Inc. Equity Incentive Plan are only exercisable upon a triggering event or Initial Public Offering of the Company, as defined by the plan. These stock awards are accounted for as liability awards and are remeasured each reporting period. Because there were no triggering events that occurred during the years ended December 31, 2016 and 2015, no compensation expense was recognized in these respective periods. At December 31, 2016, the Company had 825,500 stock options outstanding. Of that total, 196,100 stock options were fully vested at December 31, 2016, and have total unrecognized stock-based
F-30
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements (continued)
compensation expense of $1.12 million. The stock compensation expense related to the awards will be recorded upon either the triggering event or Initial Public Offering of the Company, and the amount recorded will be based upon the stock price at that time.
Valuation Assumptions: The fair value of each stock option is estimated using the Black-Scholes option pricing model at each measurement date. The fair value of stock options under the Black-Scholes model requires management to make assumptions regarding projected employee stock option exercise behaviors, risk-free interest rates, volatility of the Company’s stock price, and expected dividends. The awards were granted with vesting terms between two and four years. Certain awards contain a 25% acceleration vesting clause upon a triggering event or Initial Public Offering of the Company.
The Company has not historically paid cash dividends to its stockholders and currently does not anticipate paying any cash dividends in the foreseeable future. As a result, the Company has assumed a dividend yield of 0%. The risk-free interest rate is based upon the rates of U.S. Treasury bills with a term that approximates the expected life of the option. The Company uses the simplified method to reasonably estimate the expected life of its option awards. Expected volatility is based upon the volatility of comparable public companies.
The following table provides the assumptions used in the Black-Scholes model to measure the unrecognized compensation expense for the Calyxt option awards as of December 31, 2016:
| Expected dividend yield |
0 | % | ||
| Risk-free interest rate |
0.64 | % | ||
| Expected volatility |
30 | % | ||
| Expected life (in years) |
5.75-6.25 |
Parent Awards
The Company’s Parent grants stock options to employees of Calyxt. Compensation costs related to the grant of the Parent company awards to Calyxt’s employees has been recognized in the statements of operations with a corresponding credit to stockholder’s equity, representing the Parent Company’s Capital contribution. The fair value of each stock option is estimated at the grant date using the BlackScholes option pricing model. The fair value of stock options under the Black-Scholes model requires management to make assumptions regarding projected employee stock option exercise behaviors, riskfree interest rates, volatility of the Company’s stock price, and expected dividends.
The following table provides the range of assumptions used in the Black-Scholes model for the Parent awards:
| Expected dividend yield |
0 | % | ||
| Risk-free interest rate |
0.00-0.42 | % | ||
| Expected volatility |
59.8%-63.2 | % | ||
| Expected life (in years) |
6.11-6.12 |
The Company’s Parent granted certain consultants of Calyxt non-employee warrants to purchase Cellectis stock in exchange for services provided to the Company. The Company recorded the fair value of the warrants as a dividend paid to the Parent in exchange for the warrants issued to them.
The Company recognized stock-based compensation expense related to its Parent’s grants of stock options and warrants to Calyxt employees and consultants of $948 thousand and $692 thousand for the years ended
F-31
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements (continued)
December 31, 2016 and 2015, respectively. The following table summarizes the stock-based compensation expense, which was recognized in the statements of operations for the years ended December 31:
| (Amounts in Thousands) | 2016 | 2015 | ||||||
| Selling, general, and administrative |
$ | 20 | $ | 16 | ||||
| Research and development |
928 | 676 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 948 | $ | 692 | ||||
|
|
|
|
|
|||||
10. Income Taxes
Deferred income tax assets and liabilities are recognized for the differences between the financial statement and income tax reporting basis of assets and liabilities based on currently enacted rates and laws.
A reconciliation of statutory tax expense to actual tax expense is as follows:
| Year Ended December 31, | ||||||||
| (Amounts in Thousands) | 2016 | 2015 | ||||||
| Federal benefit at statutory rate of 34% |
$ | (4,109 | ) | $ | (2,002 | ) | ||
| Nondeductible expenses |
325 | 237 | ||||||
| Recognized R&D tax credits |
(418 | ) | — | |||||
| Other credits generated |
(58 | ) | — | |||||
| Deferred rate change |
— | 66 | ||||||
|
|
|
|
|
|||||
| Change in valuation allowance |
4,260 | 1,699 | ||||||
|
|
|
|
|
|||||
| Total income tax |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
The total valuation allowance increased by $4,260 thousand and $1,699 thousand for 2016 and 2015, respectively.
F-32
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements (continued)
Deferred assets consist of the following:
| (Amounts in Thousands) | 2016 | 2015 | ||||||
| Deferred tax assets: |
||||||||
| Current: |
||||||||
| Accrued expenses |
$ | 574 | $ | 83 | ||||
|
|
|
|
|
|||||
| Total current deferred tax asset |
574 | 83 | ||||||
|
|
|
|
|
|||||
| Non-current: |
||||||||
| Credits |
592 | — | ||||||
| Tax amortization |
78 | 267 | ||||||
| Other |
11 | — | ||||||
| Net operating loss and credit carryforwards |
8,974 | 5,666 | ||||||
|
|
|
|
|
|||||
| Total non-current deferred tax assets |
9,655 | 5,933 | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
$ | 10,229 | $ | 6,016 | ||||
| Deferred tax liabilities: |
||||||||
| Current: |
||||||||
| Other |
$ | — | $ | (72 | ) | |||
|
|
|
|
|
|||||
| Total current deferred tax liabilities |
— | (72 | ) | |||||
|
|
|
|
|
|||||
| Non-current: |
||||||||
| Property and equipment |
(52 | ) | (27 | ) | ||||
|
|
|
|
|
|||||
| Total deferred tax liabilities |
$ | (52 | ) | $ | (99 | ) | ||
|
|
|
|
|
|||||
| Net deferred tax asset |
10,177 | 5,917 | ||||||
| Less: valuation allowance |
(10,177 | ) | (5,917 | ) | ||||
|
|
|
|
|
|||||
| Total |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
The cumulative net operating loss (NOLs) available to offset future income for federal and state reporting purposes was $24.7 million and $14.9 million at December 31, 2016 and 2015, respectively. Federal and state net operating loss and credit carryforwards will begin to expire in 2032. Due to potential ownership changes that may have occurred or would occur in the future, IRC Section 382 may place additional limitations on the Company’s ability to utilize the net operating loss carryforward.
The net deferred tax assets have a valuation allowance to reserve against those deferred tax assets that Calyxt believes are more likely than not to be realized. In the event that the Company determines that a valuation allowance is no longer required, any benefits realized from the use of the NOLs and credits acquired will reduce its deferred income tax expense. In assessing the recoverability of the deferred tax assets, management considers whether it is more likely than not that a portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income in the periods in which those temporary differences become deductible. Management considers the scheduled reversals of future deferred tax liabilities, projected future taxable income, and tax planning strategies in making this assessment. As such, the Company has recorded a valuation allowance to offset all of its deferred tax assets.
F-33
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements (continued)
11. Commitments and Contingencies
Litigation and Claims
Various legal actions, proceedings, and claims (generally, “matters”) are pending or may be instituted or asserted against the Company. The Company accrues for matters when losses are deemed probable and reasonably estimable. Any resulting adjustments, which could be material, are recorded in the period the adjustments are identified. The Company has not identified any legal matters needing to be recorded, or disclosed as of December 31, 2016.
Leases
The Company leases office space under a non-cancelable operating lease that expires in October, 2017. Rent expense is recognized using the straight-line method over the term of the lease. In addition to minimum lease payments, the office lease requires payment of a proportionate share of real estate taxes and building operating expenses. Total rent expense was $271 thousand and $272 thousand for years ended December 31, 2016 and 2015, respectively.
Future minimum lease commitments are as follows for the years ended December 31:
| 2017 |
$ | 121 | ||
| 2018 and beyond |
— | |||
|
|
|
|||
| Total |
$ | 121 | ||
|
|
|
Parent Obligations
The Company has short-term Parent obligations of $1.7 million consisting of amounts owed under the intercompany management agreement, for certain services provided by Cellectis.
Forward Purchase Commitments
The company has forward purchase commitments with growers to purchase seed and grain at a future date in the amount of approximately $383 thousand, which are not recorded in the financial statements because the company has not taken delivery of the seed and grain.
12. Employee Benefit Plan
The Company provides a 401(k) defined contribution plan (the Plan) for participation by all regular full-time employees who have completed three months of service. The Plan provides for a matching contribution equal to 100% of the amount of the employee’s salary deduction up to 3% of the salary per employee and an additional 50% match from 3% to 5% of salary. Employees’ rights to the Company’s matching contributions vest immediately. Company contributions to the plan totaled $66 thousand and $47 thousand for the years ended December 31, 2016 and 2015, respectively.
13. Segment and Geographic Information
We have one operating and reportable segment, R&D of plant gene editing. The Company derives substantially all of its revenue from R&D contracts related to plant gene editing.
F-34
Table of Contents
Calyxt, Inc.
(A Wholly Owned Subsidiary of Cellectis S.A.)
Notes to Financial Statements (continued)
14. Subsequent Events
The Company has evaluated subsequent events through May 15, 2017, the date the financial statements were available to be issued.
On January 4, 2017, the Company signed a Letter of Intent with an unrelated third party (Lessor) for the sale and leaseback of the new Roseville, MN, Greenhouse and Warehouse Facility, and for the construction of the phase 2 expansion of the Roseville Facility, which includes R&D and administrative facilities and a demonstration kitchen. In the second quarter of 2017, the company plans to enter into a purchase agreement with the Lessor to sell the company’s land and constructed greenhouse facilities and lease said assets back over a 20-year period starting at the completion of phase 2. In addition, the Company plans to enter into a construction agreement with the Lessor to build the remaining facility, consisting of R&D and administrative facilities, and a demonstration kitchen. At the end of the construction period, the Company plans to lease these R&D, administrative, and demonstration kitchen facilities from the Lessor over a 20-year life. The company plans to occupy the entire facility in early 2018. There is no guarantee that the Company and the Lessor will consummate these agreements.
The Company updated its evaluation of subsequent events through July 10, 2017, the date on which the December 31, 2016 financial statements were reissued. There are no additional significant events that require disclosure in these financial statements except as follows:
Pursuant to the authorization provided in a written consent in lieu of a special meeting of the Company by the stockholder dated as of June 14, 2017, the Company effected a stock split of the Company’s common stock at a ratio of 100-for-1 and increased the number of shares of common stock authorized for issuance to 30,000,000 by filing a Certificate of Amendment with the Secretary of State of the State of Delaware. As a result of the stock split, every share of issued and outstanding common stock was converted to 100 issued and outstanding shares of Common Stock, without any change in the par value per share. Since the par value of the common stock remained at $0.0001 per share, the value of “Common stock” recorded to the Company’s balance sheets has been retroactively increased to reflect the par value of restated outstanding shares, with a corresponding decrease to “Additional paid-in capital.” All share and per share data included in this report has been retroactively restated to reflect the stock split.
Pursuant to the authorization provided in a written consent in lieu of a special meeting of the Company by the stockholder dated as of July 7, 2017, the Company will effect a stock split of the Company’s common stock contingent on closing of an initial public offering. The stock split has not occurred and, thus, is not reflected in these financial statements.
F-35
Table of Contents
Through and including , 2017 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
6,060,606 Shares

Calyxt, Inc.
Common Stock
PRELIMINARY PROSPECTUS
, 2017
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
| Amount to Be Paid |
||||
| SEC registration fee |
$ | 14,541 | ||
| FINRA filing fee |
18,819 | |||
| NASDAQ listing fee |
25,000 | |||
| Transfer agent’s and registrar’s fees |
3,500 | |||
| Printing and engraving expenses |
200,000 | |||
| Legal fees and expenses |
1,007,500 | |||
| Accounting fees and expenses |
800,000 | |||
| Blue Sky fees and expenses |
15,000 | |||
| Miscellaneous |
50,000 | |||
|
|
|
|||
| Total |
$ | 2,134,360 | ||
|
|
|
|||
Each of the amounts set forth above, other than the registration fee, the NASDAQ listing fee and the FINRA filing fee, is an estimate.
Item 14. Indemnification of Directors and Officers
Section 145 of the Delaware General Corporation Law provides that a corporation may indemnify directors and officers as well as other employees and individuals against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with any threatened, pending or completed actions, suits or proceedings in which such person is made a party by reason of such person being or having been a director, officer, employee or agent to the registrant. The Delaware General Corporation Law provides that Section 145 is not exclusive of other rights to which those seeking indemnification may be entitled under any bylaw, agreement, vote of stockholders or disinterested directors or otherwise. The registrant’s By-laws provide for indemnification by the registrant of its directors, officers and employees to the fullest extent permitted by the Delaware General Corporation Law. The registrant also expects to enter into indemnification agreements with each of its directors at or prior to completion of the offering contemplated by this registration statement to provide these directors additional contractual assurances regarding the scope of the indemnification set forth in the registrant’s Certificate of Incorporation and amended and restated By-laws and to provide additional procedural protections. There is no pending litigation or proceeding involving a director or executive officer of the registrant for which indemnification is sought.
Section 102(b)(7) of the Delaware General Corporation Law permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for liability (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) for unlawful payments of dividends or unlawful stock repurchases, redemptions or other distributions, or (iv) for any transaction from which the director derived an improper personal benefit. The registrant’s Certificate of Incorporation provides for such limitation of liability.
The registrant maintains standard policies of insurance under which coverage is provided (a) to its directors and officers against loss rising from claims made by reason of breach of duty or other wrongful act, and (b) to the registrant with respect to payments which may be made by the registrant to such officers and directors pursuant to the above indemnification provision or otherwise as a matter of law.
II-1
Table of Contents
The proposed form of underwriting agreement filed as Exhibit 1.1 to this registration statement will provide for indemnification of directors and officers of the registrant by the underwriters against certain liabilities.
Item 15. Recent Sales of Unregistered Securities
We have not sold any securities, registered or otherwise, within the past three years, except for the following:
On October 1, 2015, we entered into an amended and restated contribution with Cellectis pursuant to which Cellectis contributed $40 million to us in exchange for 17,150,000 shares of our common stock. The $40 million contribution consisted of $30 million of cash and the conversion to equity of $10 million of loans and outstanding obligations owed by us to Cellectis. The issuance of the shares was made pursuant to the exemption provided by Section 4(a)(2) of the Securities Act of 1933.
In fiscal year 2017, the Registrant granted to its directors, employees and consultants options to purchase an aggregate of 2,119,698 shares under its equity compensation plans at an exercise price of $13.29 per share and the Registrant granted 1,452,333 restricted stock units to its directors, employees and consultants, 1,436,333 of which remain unvested and all of which had a grant price of $13.29.
In fiscal year 2016, the Registrant granted to its director, employees and consultants options to purchase an aggregate of 1,678,250 shares under its equity compensation plans at an exercise price of $3.59 per share.
In fiscal year 2015, the Registrant granted to its director, employees and consultants options to purchase an aggregate of 113,925 shares under its equity compensation plans at an exercise price of $21.83 per share.
In fiscal year 2014, the Registrant granted to its director, employees and consultants options to purchase an aggregate of 230,300 shares under its equity compensation plans at an exercise price of $3.71 per share.
Option grants, restricted stock unit grants and the issuances of common stock upon exercise of such options were exempt pursuant to Rule 701, Regulation D and Section 4(a)(2) of the Securities Act.
Item 16. Exhibits and Financial Statement Schedules
Reference is herein made to the attached Exhibit Index, which is incorporated herein by reference.
Item 17. Undertakings
The undersigned registrant hereby undertakes:
(a) The undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
(b) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions referenced in Item 14 of this registration statement, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the
II-2
Table of Contents
successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered hereunder, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(c) The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933 shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-3
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in New Brighton, State of Minnesota, on the 10th day of July, 2017.
| CALYXT, INC. | ||
| By: | /s/ Federico A. Tripodi | |
| Name: Federico A. Tripodi | ||
| Title: Chief Executive Officer | ||
KNOW ALL PERSONS BY THESE PRESENTS, that each of Philippe Dumont, Alain Godard, Anna Ewa Kozicz-Stankiewicz and Laurent Arthaud, each of whose signature appears below hereby constitutes and appoints Bryan W.J. Corkal and Joseph B. Saluri each of them, his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement and any and all additional registration statements pursuant to Rule 462(b) of the Securities Act of 1933, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, granting unto each said attorney-in-fact and agents full power and authority to do and perform each and every act in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or either of them or their or his or her substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Federico A. Tripodi |
Chief Executive Officer (principal executive officer) |
July 10, 2017 | ||
| Federico A. Tripodi | ||||
| /s/ Bryan W. J. Corkal |
Chief Financial Officer (principal financial and accounting officer) |
July 10, 2017 | ||
| Bryan W. J. Corkal | ||||
| * André Choulika |
Chairman | July 10, 2017 | ||
| /s/ Philippe Dumont Philippe Dumont |
Director | July 10, 2017 | ||
| /s/ Alain Godard Alain Godard |
Director | July 10, 2017 | ||
| /s/ Anna Ewa Kozicz-Stankiewicz Anna Ewa Kozicz-Stankiewicz |
Director | July 10, 2017 | ||
| /s/ Laurent Arthaud Laurent Arthaud |
Director | July 10, 2017 | ||
| *By: | /s/ Bryan W. J. Corkal | |
| Bryan W. J. Corkal, as Attorney-in-Fact | ||
II-4
Table of Contents
EXHIBIT INDEX
| Exhibit |
Description | |
| 1.1** | Form of Underwriting Agreement | |
| 3.1** | Form of Certificate of Incorporation | |
| 3.2** | Form of By-laws | |
| 5.1 | Opinion of Davis Polk & Wardwell LLP | |
| 10.1** | Management Services Agreement between Cellectis S.A., Cellectis, Inc. and Calyxt, Inc., dated as of January 1, 2016 | |
| 10.2** | Form of Management Services Agreement Amendment | |
| 10.3** | Form of Separation Agreement between Cellectis S.A. and Calyxt, Inc. | |
| 10.4** | Form of Stockholders Agreement | |
| 10.5** | Form of License Agreement between Cellectis S.A. and Calyxt, Inc. | |
| 10.6**# | Exclusive Patent License Agreement between Regents of the University of Minnesota and Calyxt Inc. (f.k.a. Cellectis Plant Sciences, Inc.), dated December 15, 2014 | |
| 10.7**# | Commercial License Agreement between Two Blades Foundation and Calyxt, Inc. (f.k.a. Cellectis Plant Sciences, Inc.), dated December 9, 2014 | |
| 10.8**# | First Amendment to the Commercial License Agreement between Two Blades Foundation and Calyxt, Inc. (f.k.a. Cellectis Plant Sciences, Inc.), dated December 1, 2016 | |
| 10.9**# |
Letter Agreement between Two Blades Foundation and Calyxt, Inc. (f.k.a. Cellectis Plant Sciences, Inc.), dated December 31, 2015 | |
| 10.10**# | Exclusive License Agreement between Plant Bioscience Limited and Calyxt, Inc. (f.k.a. Cellectis Plant Sciences, Inc.), dated April 25, 2015 | |
| 10.11** | Calyxt, Inc. Equity Incentive Plan | |
| 10.12** | Form of Stock Option Agreement pursuant to the Calyxt, Inc. Equity Incentive Plan | |
| 10.13** | Offer Letter between Calyxt, Inc. and Federico A. Tripodi, dated May 6, 2016 | |
| 10.14** | Offer Letter between Calyxt, Inc. and Bryan W. J. Corkal, dated November 16, 2016 | |
| 10.15** | Consulting Agreement between Calyxt, Inc. (f.k.a. Cellectis Plant Sciences, Inc.) and Daniel Voytas, dated January 1, 2010 | |
| 10.16** |
Amendment 1 to Consulting Agreement between Calyxt, Inc. (f.k.a. Cellectis Plant Sciences, Inc.) and Daniel Voytas, dated December 21, 2012 | |
| 10.17** | Composite Employment Agreement among Cellectis, S.A., Calyxt, Inc. (f.k.a. Cellectis Plant Sciences, Inc.) and Luc Mathis, dated January 1, 2006, as last amended July 1, 2016 | |
| 10.18** | Offer Letter between Calyxt, Inc. and Gregory Smith, dated April 24, 2015 | |
| 10.19** | Severance Agreement between Calyxt, Inc. and Gregory Smith, effective March 22, 2016 | |
| 10.20** | Calyxt, Inc. 2017 Omnibus Incentive Plan | |
| 10.21** | Calyxt, Inc. 2017 Stock Option Sub-Plan for French Employees and Directors | |
| 10.22** |
Form of Stock Option Agreement pursuant to the Calyxt, Inc. 2017 Omnibus Incentive Plan | |
II-5
Table of Contents
| Exhibit |
Description | |
| 10.23** | Form of Restrictive Stock Unit Agreement pursuant to the Calyxt, Inc. 2017 Omnibus Incentive Plan | |
| 10.24** | Form of Resolution with regard to the Grant of Warrants to purchase shares of Cellectis S.A. | |
| 10.25** | Calyxt, Inc. 2017 Restricted Stock Unit Sub-Plan for French Employees and Directors | |
| 23.1 | Consent of Independent Registered Certified Public Accounting Firm | |
| 23.2 | Consent of Davis Polk & Wardwell LLP (included in Exhibit 5.1) | |
| 24.1 | Power of Attorney (included on signature page) | |
| ** | Previously filed |
| # | Confidential treatment has been requested for portions of this exhibit. These portions have been omitted from the registration statement and submitted separately to the United States Securities and Exchange Commission. |
II-6
