Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - MEDIZONE INTERNATIONAL INC | ex99-3.htm |
| EX-99.2 - EX-99.2 - MEDIZONE INTERNATIONAL INC | ex99-2.htm |
| EX-99.1 - EX-99.1 - MEDIZONE INTERNATIONAL INC | ex99-1.htm |
| 8-K - 8-K - MEDIZONE INTERNATIONAL INC | medizone8k062217.htm |
Exhibit 99.4

AsepticSure® Medizone International, Inc.Investor Update – June 21, 2017 1 June 21, 2017 – 4:00 – 5:00 PM ESTToll free for North America: 1-800-681-1924Toll call outside North America: 1-303-223-4393A recording of the call will be available from 6/21/17-6/28/17:1-800-633-8284 Conference ID: 21854308 Investor Update 6/21/17

Safe Harbor Statement This investor update contains forward looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act. Our actual results could differ materially from those projected in these forward-looking statements, which involve a number of risks and uncertainties, including global economic conditions generally, government regulation, manufacturing and marketing risks, adverse publicity risks, risks associated with our entry into the US and other markets, expansion and operations. The contents of this update should be considered in conjunction with the risk factors, warnings, and cautionary statements that are contained in our most recent filings with the Securities and Exchange Commission. 2 Investor Update 6/21/17

Agenda Company overviewThe Problem – The SolutionCommercial Strategy UpdateFinancialsQuestion & AnswerClosing comments 3 Investor Update 6/21/17

Executive Summary Medizone International (OTCQB:MZEI) Company formed in 1986 to develop treatments for lipid enveloped virus like Hepatitis B & C and HIV/AIDSStrategic shift in 2007 to develop a disinfectant system capable of addressing a wide range of applications to minimize the transmission of diseaseCommercial systems are operational in several countries AsepticSure® is well positioned to be a superior solution worldwide within healthcare, biodefense, and public health preparednessEPA registered disinfectant system. US regulatory confirmation of disinfectant claimsPeer reviewed/published efficacy against C Difficile, MRSA, VRE and other pathogensDemonstrated efficacy to surrogates of Anthrax, Ebola, SARS and MERS-CoVIntellectual PropertyBroad patent portfolio in the US, Canada, Europe, Mexico and ChinaStrategic Intent Demonstrate market acceptance and superior performance in key addressable marketsSeek global strategic partner for co-commercialization opportunities 4 Investor Update 6/21/17

The Problem Reducing the transmission of disease remains a global health priorityHealthcare facilities110% of inpatients in the U.S. contract infections from the hospital room that is manually cleaned.HAIs are the 4th leading cause of death in the U.S. and cost over $40B a year.Recent survey of hospitals with below average infection rates showed that the cost of HAIs wiped out in-patient hospital profits.Public health preparedness continues to be challenged by pandemic pathogens such as Ebola, MERS, Bird Flu, SARS & H7N9 continue Bioterrorism - The deliberate release of viruses, bacteria, toxins or other harmful agents to cause illness or death is of increasing concern across the worldExposure to healthcare waste in industries such as death care (funeral homes) and sports/fitness equipment presents another growing area for disease transmission 5 1. 2005 - California Air Resources Board’s “Indoor Air Pollution Report” and 2008 Independent studies by AMA, EPA, and CDC Investor Update 6/21/17
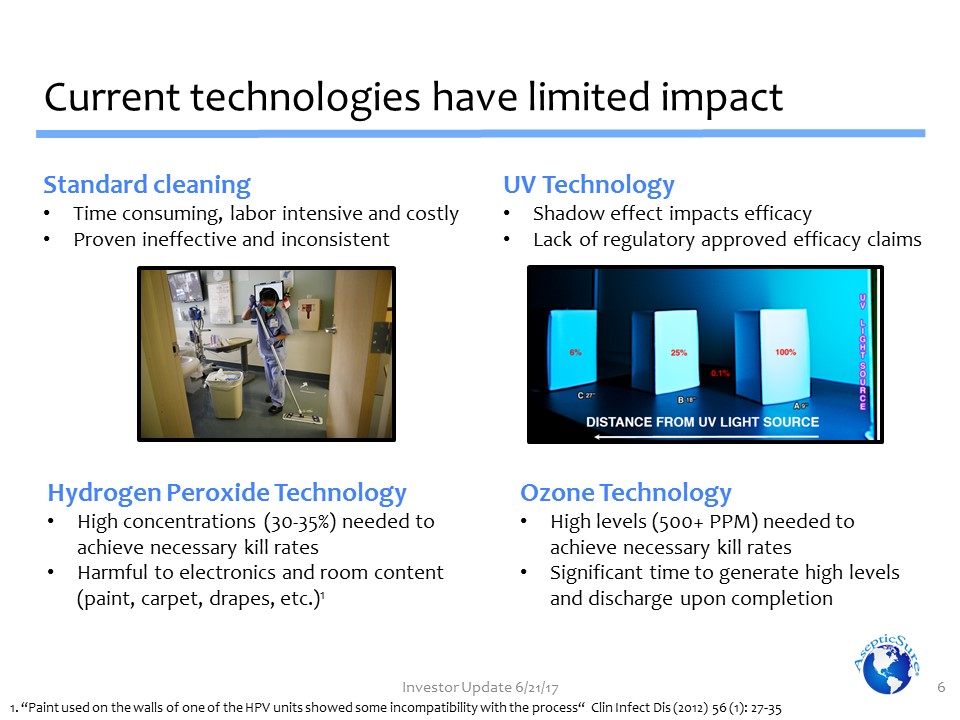
Standard cleaningTime consuming, labor intensive and costlyProven ineffective and inconsistent Current technologies have limited impact 6 UV TechnologyShadow effect impacts efficacyLack of regulatory approved efficacy claims Ozone TechnologyHigh levels (500+ PPM) needed to achieve necessary kill ratesSignificant time to generate high levels and discharge upon completion Hydrogen Peroxide TechnologyHigh concentrations (30-35%) needed to achieve necessary kill rates Harmful to electronics and room content (paint, carpet, drapes, etc.)1 1. “Paint used on the walls of one of the HPV units showed some incompatibility with the process“ Clin Infect Dis (2012) 56 (1): 27-35 Investor Update 6/21/17

7 The Solution - The AsepticSure System Ease of use in a wide range of applications The Science of Synergy EPA validated disinfectant process: AsepticSure produced consistent kill rates across 160 runs using a nominal 1.4% Hydrogen peroxide mixture combined with 80 ppm of OzonePeer reviewed/published efficacy against C Difficile, MRSA, VRE and other pathogensDemonstrated efficacy to surrogates of Anthrax, Ebola, SARS and MERS-CoV Investor Update 6/21/17

Strategic Intent: Establish AsepticSure as a superior solution worldwide to achieve unprecedented kill rates of bacteria and viral pathogens in a wide range of commercial applications including healthcare, biodefense, and public health preparednessCommercial Priorities:Regulatory ApprovalManufacturing Commercial ExecutionIntellectual Property Commercial Strategy Update 8 Investor Update 6/21/17

Regulatory StatusApprovals: United States (EPA), Canada, New Zealand and ChileEPA approval (Nov 2016): AsepticSure Ozone Generator™ fogging system is for use in hospitals, clinics, food industry, sporting venues, and hotels to disinfect hard non-porous surfaces.AsepticSure Oxidative Catalyst is for use in hospitals, clinics, food industry, sporting venues, and hotels to disinfect hard non-porous surfaces.Pending FDA guidance on medical device classification Seeking approvals in priority ex-North American markets: EuropeSouth/Central America (Argentina, Brazil)AustraliaAsia (China, Malaysia) 1) Regulatory Approval 9 Investor Update 6/21/17

Thursday April 6, 2017 teleconference513(g) submission made on May 17, 2017 Expected response within 60 daysHighlights from the submission:Request for “confirmation that the company’s AsepticSure® (“AsepticSure® System” or the “system”) is not considered a medical device when used for general hospital disinfection purposes, and in addition, is exempt from premarket notification requirements.”Rationale for exemption: “Several devices which have been listed with FDA (class I device with pre-market exemptions) make virtually the same claims as the AsepticSure® system.”Hogan Lovells US LLP is leading the communications with the FDA FDA Communications 10 Investor Update 6/21/17

Contract manufacturing support established with CogmedixCurrent FDA and EPA registered manufacturer Establishment registration number has been received by EPAPrepared to manufacture AsepticSure Generation III systemsRecent international electrical safety standards approval is being incorporated into manufacturing plansHydrogen Peroxide supplier: InnovasourceCurrent inventory of AsepticSure systemsDistributors:Canada: 2 systems South America: 3 systems Saudi Arabia: 2 systemsNew Zealand: 1 systemMedizone: 6 systems (prepped for global markets), 1 system (R&D) Ongoing R&D to enhance performance and user experience 2) Manufacturing 11 Investor Update 6/21/17

Addressable MarketsMarketing and sales process Technical support and customer service 3) Commercial Execution 12 Investor Update 6/21/17

Healthcare industryHealthcare waste exposureBio-Terrorism countermeasuresPublic health preparednessBuilding remediation Addressable Markets 13 Investor Update 6/21/17

EPA registered disinfectant systemUS regulatory confirmation of disinfectant claimsCombination of oxidative compounds (O3 and H202) produces a unique mixture of free radicals with oxidation potential much higher than Ozone or Hydrogen Peroxide aloneHospital spaces and other public facilities can effectively and reliably be disinfected in less than 90 minutes TATThe reduced concentration of gases used renders AsepticSure harmless to the environment, electronic equipment, paint and treatment surfacesLethal to all pathogens including spores on all surfaces, fabrics and carpets1No lingering environmental issue affecting patient or staff health after room is disinfected The AsepticSure Difference in Healthcare 14 1. Am J Infect Control 2011;39:873-879: Effectiveness of a novel ozone-based system for the rapid high-level disinfection of health care spaces and surfaces Healthcare Industry Investor Update 6/21/17

Efficacy testing across a wide range of pathogens including spores (C. difficile and B. subtilis)Testing occurred in test chamber and full-size laboratory room/hospital room AsepticSure Efficacy: Am J Infection Control 2011 15 1. Am J Infect Control 2011;39:873-879: Effectiveness of a novel ozone-based system for the rapid high-level disinfection of health care spaces and surfaces Healthcare Industry Investor Update 6/21/17

16 Response to Emerging Pathogens Klebsiella pneumonia is becoming more prevalent in many hospitals and because of its increasing antibiotic resistance it is very dangerousEfficacy tests against Klebsiella pneumoniaeCarbapenemase-resistant strain and β-lactamase resistant strain.Classified an ESKAPE pathogen, one of the leading causes of nosocomial (hospital acquired) infection in the world.Tests were performed as per client request from Chile.Results: Following a standard 40m Asepticsure treatment results in complete kill of Klebsiella pneumoniae.Log reduction: 6.31log10-6.64log10 Multiple tests with multiple samples per test.Samples are placed in multiple locations surrounding AsepticSure machine Klebsiella pneumoniae Healthcare Industry Investor Update 6/21/17

Objectives: (A) Access potential microbiological contamination in a local funeral services mortuary (B) Conduct a pre-treatment and post-treatment assessment of AsepticSure.Process:All pretreatment surface sampling (May 9 and May 12, 2017) and treatment (May 19) were performed in the preparation room of the funeral home.All microbiology work (swab plating, incubation and identification) were performed in the Medizone Int. Microbiology Laboratory in Kingston, ON.Treatment: AsepticSure Efficacy: The Funeral Home Study 17 Two AsepticSure machines inside the prep room running in parallel. Parameters set at 90% RH, 80ppm O3, and treat time of 60 min. Peroxide concentration was 1.4% diluted from 30% stock H2O2 with distilled H2O. Treatment process was successfully completed and post-treatment swabs were taken immediately after. Healthcare Waste Exposure Investor Update 6/21/17

Pre-treatment assessments showed significant bacterial growth in multiple locations. Plates that showed growth exhibited a variety of organisms with no one organism being predominant.Post treatment assessments showed zero growth in all locations assessed after 24h, while one bacterial colony was present at one of the 10 sample locations (top of half-wall) after 48h. Conclusion:A high level of microbial load assessed before AsepticSure treatment was no longer present after a single treatment using the AsepticSure system.Routine cleaning of preparation rooms with AsepticSure can reduce bacterial load The Funeral Home Study: Results 18 Healthcare Waste Exposure Investor Update 6/21/17

Objective: (A) Access potential microbiological contamination in routinely used sports equipment (ice hockey equipment) (B) Conduct a pre-treatment and post-treatment assessment of AsepticSure.Process:All surface sampling, treatment and microbiology work (swab plating, incubation and identification) were performed in the Medizone Int. Microbiology Laboratory in Kingston, ON.Treatment: AsepticSure Efficacy: Athletic Equipment 19 Hockey equipment was separated in treatment room. One AsepticSure machine was used in treatment room.Parameters set at 90% RH, 80ppm O3, and treat time of 60 min. Peroxide concentration was 1.4% diluted from 30% stock H2O2 with distilled H2O. Treatment process was successfully completed and post-treatment swabs were taken immediately after. Healthcare Waste Exposure Investor Update 6/21/17
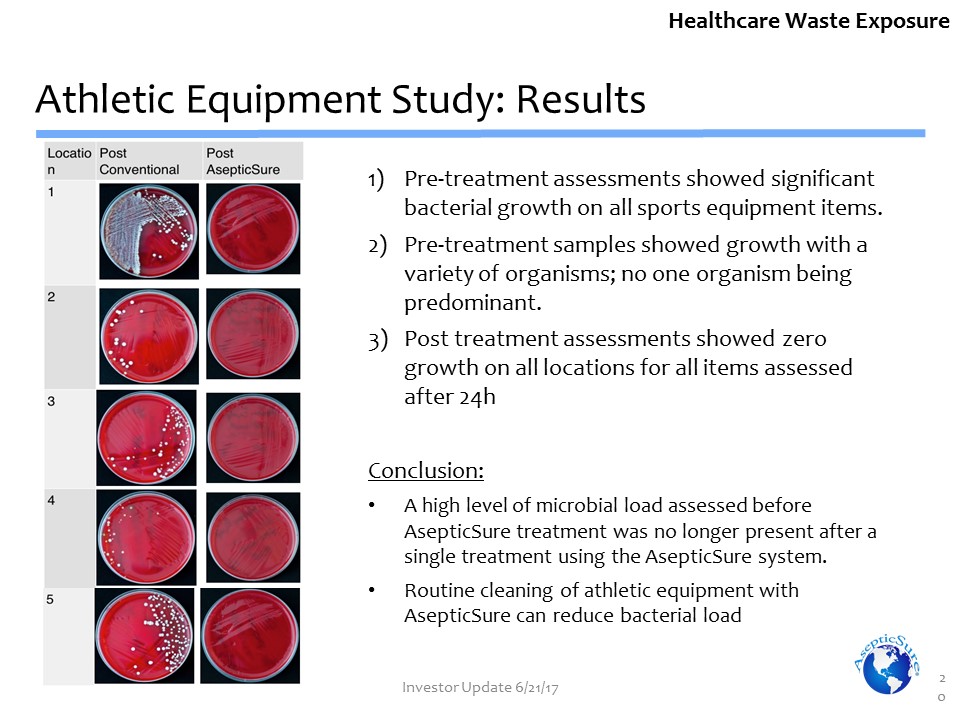
Pre-treatment assessments showed significant bacterial growth on all sports equipment items. Pre-treatment samples showed growth with a variety of organisms; no one organism being predominant.Post treatment assessments showed zero growth on all locations for all items assessed after 24hConclusion:A high level of microbial load assessed before AsepticSure treatment was no longer present after a single treatment using the AsepticSure system.Routine cleaning of athletic equipment with AsepticSure can reduce bacterial load Athletic Equipment Study: Results 20 Healthcare Waste Exposure Investor Update 6/21/17

United States Patent No. 8,636,951 – Bio-terrorism Counteraction Using Ozone and Hydrogen Peroxide (Jan 2014)Europe: Patent No. 252583B - Bio-Terrorism Counter Measures Using Ozone and Hydrogen Peroxide (June 2016)AsepticSure demonstrated bacterial spore efficacy for Anthrax surrogateB. subtilis is routinely used as an acceptable surrogate for accepted surrogate for B. anthracis in scientific literature Bio-Terrorism Countermeasures: Anthrax 21 Bio-Terrorism Countermeasures Investor Update 6/21/17
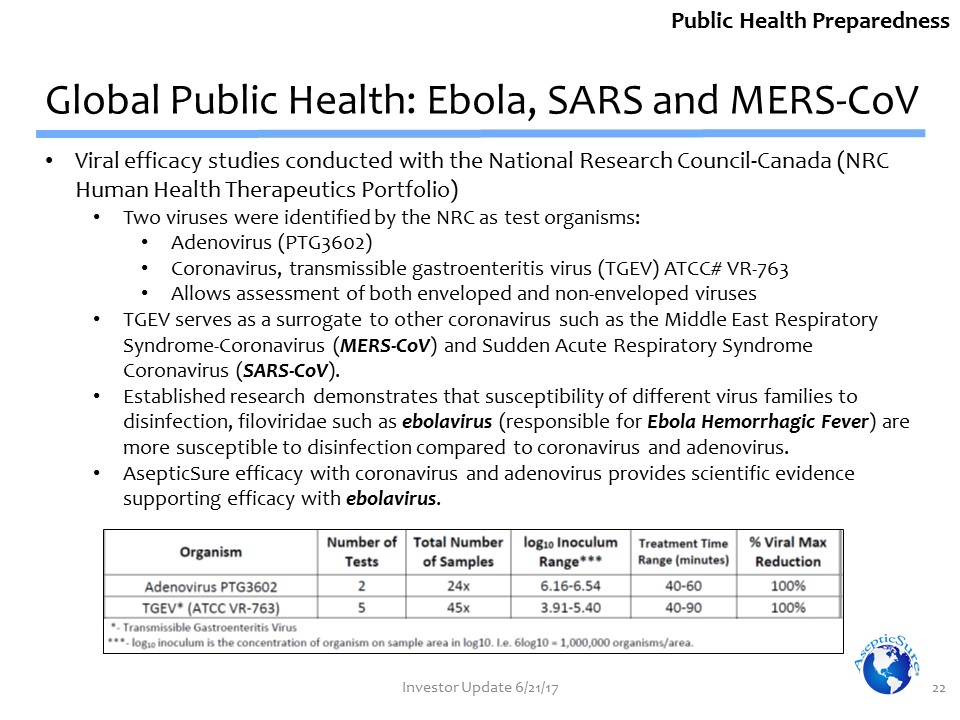
Viral efficacy studies conducted with the National Research Council-Canada (NRC Human Health Therapeutics Portfolio)Two viruses were identified by the NRC as test organisms: Adenovirus (PTG3602)Coronavirus, transmissible gastroenteritis virus (TGEV) ATCC# VR-763Allows assessment of both enveloped and non-enveloped virusesTGEV serves as a surrogate to other coronavirus such as the Middle East Respiratory Syndrome-Coronavirus (MERS-CoV) and Sudden Acute Respiratory Syndrome Coronavirus (SARS-CoV).Established research demonstrates that susceptibility of different virus families to disinfection, filoviridae such as ebolavirus (responsible for Ebola Hemorrhagic Fever) are more susceptible to disinfection compared to coronavirus and adenovirus. AsepticSure efficacy with coronavirus and adenovirus provides scientific evidence supporting efficacy with ebolavirus. Global Public Health: Ebola, SARS and MERS-CoV 22 Public Health Preparedness Investor Update 6/21/17

Active research and development program based on initial successful trialsOverall reduction in bacterial load Mold Smoke Methamphetamine residueResidual effects from water damage Building Remediation 23 Investor Update 6/21/17 Building Remediation
Lead generation:Leverage existing relationships developed by the Medizone teamUtilization of key suppliers as “commissioned” lead generatorsInvestor/shareholder relationships to support leadsTrade show attendance Social media and traditional media outreachResources:Utilize existing media content, AJIC study, and promotional materialScientific overview presentations “White paper” publications on new dataAdditional materials and website updates are in development Marketing and Sales Process 24 Investor Update 6/21/17

Hospital market:Targeted implementation of Product Evaluation Program (PEP)Place instrument, consumables and service/supportPost 3-6 month demonstration, transition to purchase contractPublish resultsExpand key opinion leader network through PEP successesDefense and Public Health markets:Demonstration projects in key areas of needPartner with existing government service providersHealthcare waste exposure and building remediationPartner with leading service providers / cleaning companies Marketing and Sales Process con’t 25 Investor Update 6/21/17

Medizone team in Canada can handle initial product salesOn-site training and support program to address most customersMost technical support issues can be handled remotelyDispatch support to customer as neededOptions to scale technical support and customer service:Success based expansion with placement of local technical support near major customersContract for 3rd party service and support Technical Support and Customer Service 26 Investor Update 6/21/17

4) Intellectual Property Canada: Patent No. 2735739 – Healthcare Facility Disinfection Process and System with Oxygen/Ozone (Nov. 2011)United States: Patent No. 8,551,399 – Healthcare Facility Disinfecting System (Oct 2013). Patent No. 8,636,951 – Bio-Terrorism Counteraction Using Ozone and Hydrogen Peroxide (Jan 2014). Patent No. 9,616,144 - Food-Handling Facility Disinfectant. Patent No. 8,992,829 - Sports Equipment and Facility Disinfection PatentEurope: Patent No. 252583B - Bio-Terrorism Counter Measures Using Ozone and Hydrogen Peroxide (June 2016). Healthcare Facility Disinfection System (Aug 2016)China: Patent No. ZL 201080030657.2 - Healthcare Facility Disinfection System (Nov 2015)Singapore: Patent No.176977 – Healthcare Facility Disinfecting Process and System With Oxygen/Ozone Mixture (Feb 2013)Mexico: Patent – Healthcare Facility Disinfecting Process and System With Oxygen/Ozone Mixture (Nov 2016) 27 Investor Update 6/21/17

Prior budgets supported through exempt private placements of equitySecure Near Term FinancingContinue with private equity offerings in the near termPreferred stock offering with strategic partnersEMA Partners outreach: Focused on securing a development, commercialization, and/or licensing partner Financials 28 Investor Update 6/21/17

Agenda Company overviewThe Problem – The SolutionCommercial Strategy UpdateFinancialsQuestion & AnswerClosing comments 29 Investor Update 6/21/17

Investor Update 6/21/17 30 Summary and Closing Comments Reducing the transmission of disease remains a global health priorityAsepticSure is well positioned to be a superior solution worldwide within healthcare, biodefense, and public health functionsMedizone is continuing to expand the commercial capabilities necessary to deliver on the opportunities in the marketplace The AsepticSure System
