Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AETHLON MEDICAL INC | aethlon_8k.htm |
Exhibit 99.1

Image of Ebola viruses exiting host cells - Courtesy of NIAID BIO I NTERNATIONAL C ONVENTION J IM J OYCE - C HAIRMAN , CEO N ASDAQ - AEMD / J UNE 21, 2017 1

FORWARD LOOKING STATEMENTS The following presentation may contain predictions, estimates, and other forward looking statements that involve risks and uncertainties, including whether and when our products are successfully developed and introduced ; market acceptance of the Aethlon Hemopurifier® and other product offerings ; regulatory delays, manufacturing delays, and other risks detailed in our SEC filings, which are accessible at www . sec . gov or on our website : www . AethlonMedical . com 2

T O A DDRESS U NMET N EEDS IN G LOBAL H EALTH & B IODEFENSE F OCUS 3

T O S AVE L IVES M ISSION 4

T HE T REATMENT OF L IFE - T HREATENING V IRUSES T HAT ARE N OT A DDRESSED WITH A NTIVIRAL D RUGS O UR G LOBAL O PPORTUNITY 5

H UMAN V IRUSES A S IGNIFICANT U NMET N EED IN G LOBAL H EALTH & B IODEFENSE ~300 VIRUSES ARE INFECTIOUS TO HUMANS NINE (9) ARE ADDRESSED WITH AN APPROVED ANTIVIRAL DRUG AGENT 3 - 4 NEW HUMAN VIRUSES ARE IDENTIFIED EACH YEAR * G LOBAL WARMING , URBAN CROWDING AND TRANSCONTINENTAL TRAVEL ARE FUELING AN INCREASED EMERGENCE OF PANDEMIC VIRUS OUTBREAKS I . E .: E BOLA , P ANDEMIC I NFLUENZA , MERS, SARS, Y ELLOW F EVER , Z IKA , ETC N O A NTIVIRAL STRATEGY TO ADDRESS UNKNOWN THREATS , INCLUDING VIRUSES ENGINEERED BY MAN AS AGENTS OF BIOTERRORISM * Center for Immunity and Evolution, University of Edinburgh THE PROBLEM 6

O UR S OLUTION 7

T HE FIRST - IN - CLASS THERAPY TO BE ADVANCED IN FDA APPROVED STUDIES T HE H EMOPURIFIER ® A B ROAD - SPECTRUM T REATMENT C OUNTERMEASURE D ESIGNED FOR THE SINGLE - USE REMOVAL OF VIRAL PATHOGENS FROM BLOOD 8

H EMOPURIFIER ® ATTRIBUTES E LIMINATES VIRUSES FROM THE ENTIRE CIRCULATORY SYSTEM D EPLOYED ON ESTABLISHED GLOBAL INSTRUMENT NETWORK G LYCAN SHIELD (T HE IMMUNE CLOAK ) CAPTURE MECHANISM Image of HIV glycan shield in green Courtesy of Oxford University 9
T HE A ETHLON H EMOPURIFIER ® F ROM THEORETICAL CONCEPT TO CLINICAL REALITY ᣌ >16 IN VITRO VIRUS CAPTURE STUDIES ᣌ F OUR INVESTIGATIONAL HUMAN STUDIES ( OUTSIDE U.S.) ᣌ ~150 HUMAN TREATMENT EXPERIENCES ᣌ ACHIEVED PRIMARY ENDPOINT IN FIRST FDA APPROVED STUDY 10

The Aethlon Hemopurifier® Concluded FDA Approved Feasibility Study on March 13th, 2017 P RIMA R Y O BJECTIVE : T O D EMONSTR A TE S AFETY OF THE H EMOPURIFIER ® IN HEALTH COMPROMISED INDIVIDUALS INFECTED WITH A VIRAL PATHOGEN E ND - S TAGE R ENAL DISEASE SUBJECTS INFECTED WITH HEPATITIS C VIRUS (HCV) A M ODEL TO ADVANCE THE H EMOPURIFIER ® AS A BROAD - SPECTRUM V IRAL COUNTERMEASURE C ONDUCTED AT D A V ITA M ED C ENTER D IALYSIS IN H OUSTON S AFETY IS THE SOLE HUMAN CHALLENGE AGAINST VIRULENT VIRAL PATHOGENS THAT DO NOT PERMIT FOR CONTROLLED CLINICAL STUDIES TO BE CONDUCTED S TUDY PROVIDES A PATHWAY TO CONDUCT P IVOTAL STUDIES AGAINST VIRUSES THAT ALLOW FOR CONTROLLED HUMAN STUDIES W AS WELL W E ACHIEVED OUR PRIMA R Y OBJECTIVE - T HE H EMOPURIFIER ® TOLERATED WITH NO DEVICE - RELATED ADVERSE EVENTS REPORTED 11

FDA - Approved Feasibility Study Feasibility Study Challenges To Overcome H EMOPURIFIER ® MANUFACTURING DELAY R EPLACEMENT OF P RINCIPAL INVESTIGATOR L ACK OF SUBJECTS WHO MET STUDY INCLUSION - EXCLUSION CRITERIA 12

The Aethlon Hemopurifier® Courtesy of Aethlon Medical V IRUS C APTURE V ALIDATIONS A LEADING B ROAD - S PECTRUM C OUNTERMEASURE 13
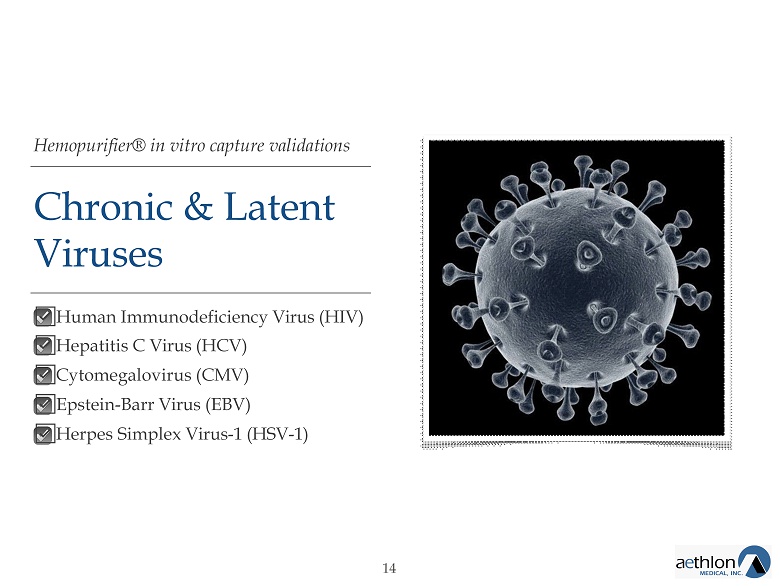
Hemopurifier® in vitro capture validations Chronic & Latent Viruses ᣌ Human Immunodeficiency Virus (HIV) ᣌ Hepatitis C Virus (HCV) ᣌ Cytomegalovirus (CMV) ᣌ Epstein - Barr Virus (EBV) ᣌ Herpes Simplex Virus - 1 (HSV - 1) 14

Hemopurifier® in vitro capture validations Pandemic Influenza Viruses ᣌ H1N1 Swine Flu ᣌ H5N1 Bird Flu ᣌ Spanish Flu of 1918 (reconstructed) Actual Spanish Flu of 1918 pandemic resulted in approximately 50 million deaths worldwide. 15

Hemopurifier® in vitro capture validations Mo s q ui to - B o rne Viruses ᣌ Chikungunya ᣌ Dengue ᣌ West Nile ᣌ Zika 16

Hemopurifier® in vitro capture validations Bioterror & Pandemic Threat Viruses ᣌ Lassa ᣌ MERS - CoV ᣌ Smallpox (based on Monkeypox & Vaccinia models) ᣌ Ebola 17

Ebola Image Courtesy of NIAID T HE TREATMENT OF E BOLA VIRUS A H EMOPURIFIER ® CASE STUDY 18

Frankfurt University Hospital APPRO V AL E MERGENCY - USE FROM G ERMANY ' S F EDERAL I NSTITUTE FOR D RUGS AND M EDICAL D EVICES (B F A R M) TO ADMINISTER H EMOPURIFIER ® THERAPY TO AN E BOLA - INFECTED PHYSICIAN AT F RANKFURT U NIVERSITY H OSPITAL . 19

T HE TREATMENT OF E BOLA VIRUS A SINGLE 6.5 - HOUR ADMINISTRATION THERAPY W AS OF H EMOPURIFIER ® DELIVERED TO THE P A TIEN T , WHO WITH MU L TIPLE W AS COM A T OSE ORGAN FAILURE . 20

T HE TREATMENT OF E BOLA VIRUS D R . S TEFAN B ÜTTNER HOLDING THE H EMOPURIFIER ® AFTER TREATMENT 21

E BOLA T REATMENT R ESULTS P RESENTED AT THE A MERICAN S OCIETY OF N EPHROLOGY A NNUAL M EETING BY H ELMUT G EIGER , M.D., C HIEF OF N EPHROLOGY AT F RANKFURT U NIVERSITY H OSPITAL • H EMOPURIFIER ® THERAPY WAS WELL TOLERATED WITH NO ADVERSE EVENTS • P RE - TREATMENT VIRAL LOAD PRIOR WAS MEASURED TO BE 400,000 COPIES / ML • P OST - TREATMENT VIRAL LOAD WAS MEASURED AT 1,000 COPIES / ML • V IRAL LOAD NEVER AGAIN ROSE ABOVE 1,000 COPIES / ML • P ATIENT MADE A FULL RECOVERY 22

H EMOPURIFIER ® V IRUS C APTURE A SSAY Q UANTIFIES VIRUSES CAPTURED WITHIN THE H EMOPURIFIER ® AND NO LONGER CIRCULATING IN THE PATIENT A SSAY R EINFORCES SINGLE - USE REMOVAL OF VIRAL PATHOGENS LABEL INDICATION P ROVIDES BETTER DEPICTION OF INFECTIOUS VS . NON - INFECTIOUS VIRUS CAPTURE E STABLISHES AN ABSOLUTE VS . OBSERVATION DATAPOINT A SSAY DEVELOPMENT BEGAN DURING PREVIOUS HCV S TUDIES C ONSISTENT CAPTURE OF 100 MILLION + V IRUSES 23

V IRUS C APTURE A SSAY R ESULT 242 MILLION COPIES OF E BOLA VIRUS CAPTURED WITHIN THE HEMOPURIFIER ® DURING 6.5 HOUR TREATMENT Analysis: BSL4 Lab Philipps University Marburg (O. Dolnik/M. Eickmann/S. Becker) 24

The Aethlon Hemopurifier® Courtesy of Aethlon Medical V IRUS C APTURE A SSAY R ESULTS FDA F EASIBILITY S TUDY Values measured post 500 ml/min high pressure 1 - 2 liter saline wash A major deviation from our previous virus capture assay 25
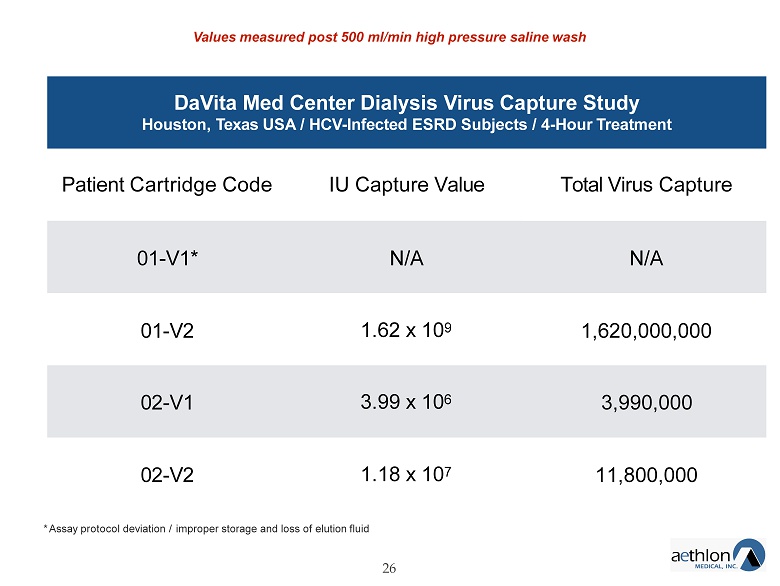
DaVita Med Center Dialysis Virus Capture Study Houston, Texas USA / HCV - Infected ESRD Subjects / 4 - Hour Treatment 26 Patient Cartridge Code IU Capture Value Total Virus Capture 01 - V1* N/A N/A 01 - V2 1.62 x 10 9 1,620,000,000 02 - V1 3.99 x 10 6 3,990,000 02 - V2 1.18 x 10 7 11,800,000 * Assay protocol deviation / improper storage and loss of elution fluid Values measured post 500 ml/min high pressure saline wash
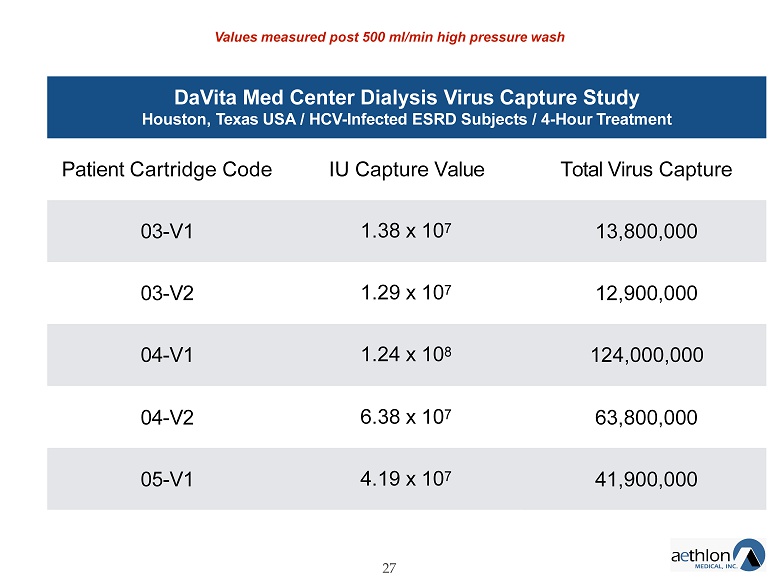
DaVita Med Center Dialysis Virus Capture Study Houston, Texas USA / HCV - Infected ESRD Subjects / 4 - Hour Treatment 27 Patient Cartridge Code IU Capture Value Total Virus Capture 03 - V1 1.38 x 10 7 13,800,000 03 - V2 1.29 x 10 7 12,900,000 04 - V1 1.24 x 10 8 124,000,000 04 - V2 6.38 x 10 7 63,800,000 05 - V1 4.19 x 10 7 41,900,000 Values measured post 500 ml/min high pressure wash
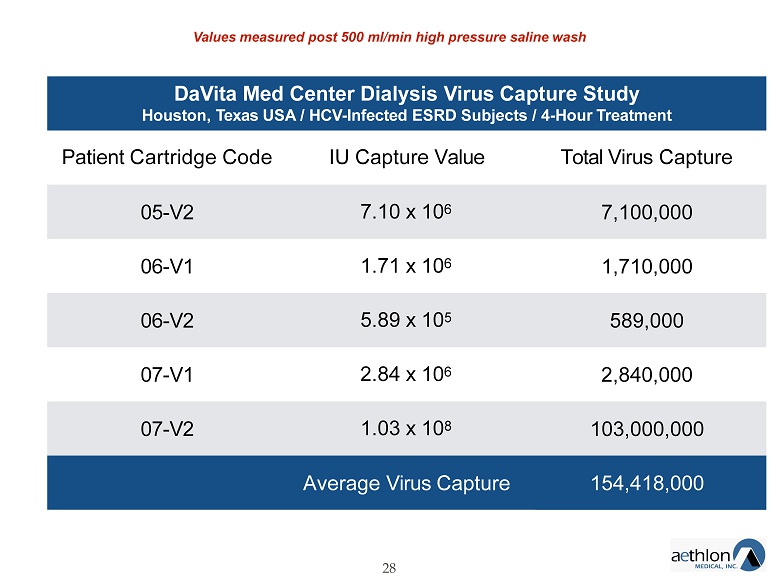
DaVita Med Center Dialysis Virus Capture Study Houston, Texas USA / HCV - Infected ESRD Subjects / 4 - Hour Treatment 28 Patient Cartridge Code IU Capture Value Total Virus Capture 05 - V2 7.10 x 10 6 7,100,000 06 - V1 1.71 x 10 6 1,710,000 06 - V2 5.89 x 10 5 589,000 07 - V1 2.84 x 10 6 2,840,000 07 - V2 1.03 x 10 8 103,000,000 Average Virus Capture 154,418,000 Values measured post 500 ml/min high pressure saline wash

29

The Aethlon Hemopurifier® NEXT STEPS - JULY 2017 S UBMITTING E XPEDITED A CCESS P ATHWAY (EAP) APPLICATION TO FDA. S EEKING "B REAKTHROUGH T ECHNOLOGY " DESIGNATION UNDER 21 ST C ENTURY C URES A CT T O SPEED THE AD V ANCEMENT OF DEVICES FOR UNMET NEEDS AGAINST LIFE - THRE A TENING DISEASE CONDITIONS FOR WHICH NO APPROVED TREATMENTS EXIST L AUNCH B IO D EFENSE & P ANDEMIC T HREAT T REATMENT I NITIATIVE WITH U.S. & F OREIGN G OVERNMENTS S PECIFIC TO VIRULENT VIRUSES THAT DO NOT PERMIT FOR CONTROLLED HUMAN STUDIES AND WHOSE CAPTURE BY THE H EMOPURIFIER ® HAS BEEN VALIDATED T HE H EMOPURIFIER ® F ULFILLS C URRENT U.S. G OVERNMENT OBJECTIVES TO PROTECT CITIZENS FROM B IO T ERROR AND P ANDEMIC THREATS G OAL IS TO BE IN THE S TRATEGIC N ATIONAL S TOCKPILE 30

2016 Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) Strategy and Implementation Plan 31

M EETING THE PHEMCE O BJECTIVES PHEMCE goal is to procure medical countermeasures (MCMs) for the strategic national stockpile ᣌ PHEMCE seeks broad - spectrum MCMs that address high - priority threats and also have commercial viability in other medical applications ᣌ Broad - spectrum approach to address both known and unknown threats ᣌ Broad - spectrum MCM against emerging threats, including Ebola, Zika and MERS - CoV ᣌ MCM against pandemic influenza, including non - pharmaceutical MCMs ᣌ MCMs for at - risk children, pregnant women and older adults for whom first line treatment countermeasures are not recommended Protecting U.S. Citizens from Bioterror & Pandemic Threats 32

T HE A ETHLON H EMOPURIFIER ® A LIGNS TO MEET U.S. PHEMCE G OALS & O BJECTIVES 33

T O A DDRESS U NMET N EEDS IN G LOBAL H EALTH & B IODEFENSE F OCUS 34

T O S AVE L IVES M ISSION 35

T HE T REATMENT OF L IFE - T HREATENING V IRUSES T HAT ARE N OT A DDRESSED WITH A NTIVIRAL D RUGS O UR G LOBAL O PPORTUNITY 36

This presentation may contain predictions, estimates, and other forward looking statements that involve risks and uncertainties, including whether and when our products are successfully developed and introduced ; market acceptance of the Aethlon Hemopurifier® and other product offerings ; regulatory delays, manufacturing delays, and other risks detailed in our SEC filings, which are accessible at www . sec . gov or on our website : www . AethlonMedical . com 9635 Granite Ridge Drive, Suite 100 San Diego, California 92123 858.459.7800 Nasdaq: AEMD ww w .AethlonMedical. c om 37
