Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SELECTA BIOSCIENCES INC | selectabiosciences8k_sel-2.htm |
| EX-99.1 - EXHIBIT 99.1 - SELECTA BIOSCIENCES INC | exhibit991_pressreleasexse.htm |

June 15, 2017
June 2017 Phase 2 Trial Presentation

Safe Harbor / Disclaimer
2
Any statements in this presentation about the future expectations, plans and prospects of Selecta Biosciences, Inc. (“the
company”), including without limitation, the progress of the Phase 1/2 clinical program of SEL-212, the potential of SEL-212 to
treat severe gout patients and resolve their debilitating symptoms, the ability of SEL-212 to avoid unwanted immune
responses, the ability of SVP-Rapamycin to induce immune tolerance against pegsiticase, the ability of SEL-212 to improve
acute symptoms during a short induction cycle, the ability of SEL-212 to be re-administered if severe gout symptoms recur,
whether the company will determine an appropriate dose of SEL-212 for a Phase 3, whether the company will advance to a
Phase 3 for SEL-212 at all, whether the Phase 2 clinical data of SEL-212 demonstrate the potential of SEL-212 to address a
substantial unmet need for gout patients, the ability of the company’s SVP platform, including SVP-Rapamycin, to mitigate
immune response and create better therapeutic outcomes, the potential treatment applications for products utilizing the SVP
platform in areas such as enzyme therapy, gene therapy, oncology therapy, vaccines and treatments for allergies and
autoimmune diseases and other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,”
“expect,” “hypothesize,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” and similar
expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995.
Actual results may differ materially from those indicated by such forward-looking statements as a result of various important
factors, including, but not limited to, the following: the uncertainties inherent in the initiation, completion and cost of clinical
trials including their uncertain outcomes, the availability and timing of data from ongoing and future clinical trials and the
results of such trials, whether preliminary results from a particular clinical trial will be predictive of the final results of that trial or
whether results of early clinical trials will be indicative of the results of later clinical trials, the unproven approach of the
company’s SVP technology, potential delays in enrollment of patients, undesirable side effects of the company’s product
candidates, its reliance on third parties to manufacture its product candidates and to conduct its clinical trials, the company’s
inability to maintain its existing or future collaborations or licenses, and other important factors discussed in the “Risk Factors”
section of the company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission, or SEC, on May
11, 2017, and in other filings that the company makes with the SEC. In addition, any forward-looking statements included in
this presentation represent the company’s views only as of the date of its publication and should not be relied upon as
representing its views as of any subsequent date. The company specifically disclaims any obligation to update any forward-
looking statements included in this presentation.

SEL-212 for Chronic
Severe Gout

Clinical Objectives of SEL-212 Program
Phase 1b
Phase 1a
Phase 2
Demonstrate that SEL 212:
Mitigates ADAs
Enables prolonged control of
uric acid for >30 days
Define effective monthly dose
of pegsiticase
Demonstrate rapid formation
and kinetics of ADAs
Demonstrate SEL-212’s safety,
tolerability and ability to reduce
serum uric acid after multiple
doses
• n = 63
• Single ascending dose of SEL-212
• Hyperuricemic patients
• n = 22
• Single ascending dose of pegsiticase
• Hyperuricemic patients
• n = 60
• 3 monthly doses of SEL-212 +
2 monthly doses of pegsiticase alone
• Symptomatic & hyperuricemic patients
4
+
Nearly 100 patients now dosed with SEL-212
+
Clinicaltrials.gov NCT02464605
Clinicaltrials.gov NCT02648269
Clinicaltrials.gov NCT02959918
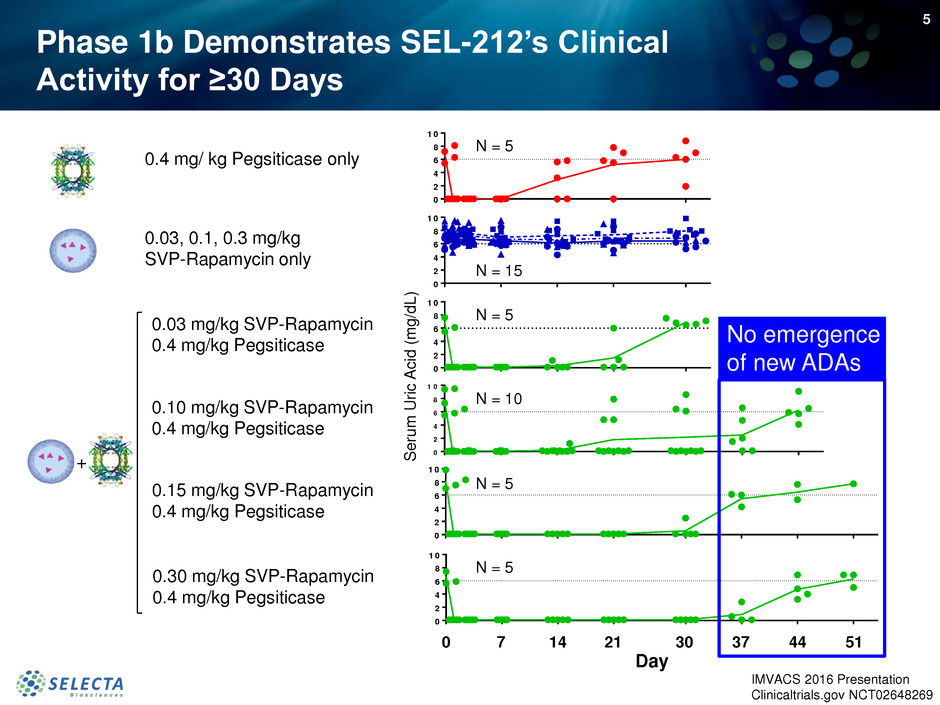
5
0
2
4
6
8
1 0
0
2
4
6
8
1 0
S
e
ru
m
U
ric
A
cid
(
m
g
/d
L
)
0
2
4
6
8
1 0
0
2
4
6
8
1 0
0.03 mg/kg SVP-Rapamycin
0.4 mg/kg Pegsiticase
0.10 mg/kg SVP-Rapamycin
0.4 mg/kg Pegsiticase
0.4 mg/ kg Pegsiticase only
0.03, 0.1, 0.3 mg/kg
SVP-Rapamycin only
0.30 mg/kg SVP-Rapamycin
0.4 mg/kg Pegsiticase
0
2
4
6
8
1 0
0.15 mg/kg SVP-Rapamycin
0.4 mg/kg Pegsiticase
0
2
4
6
8
1 0
0 7 14 21 30 37 44 51
No emergence
of new ADAs
N = 5
N = 15
N = 5
N = 10
N = 5
N = 5
Phase 1b Demonstrates SEL-212’s Clinical
Activity for ≥30 Days
Day
+
IMVACS 2016 Presentation
Clinicaltrials.gov NCT02648269
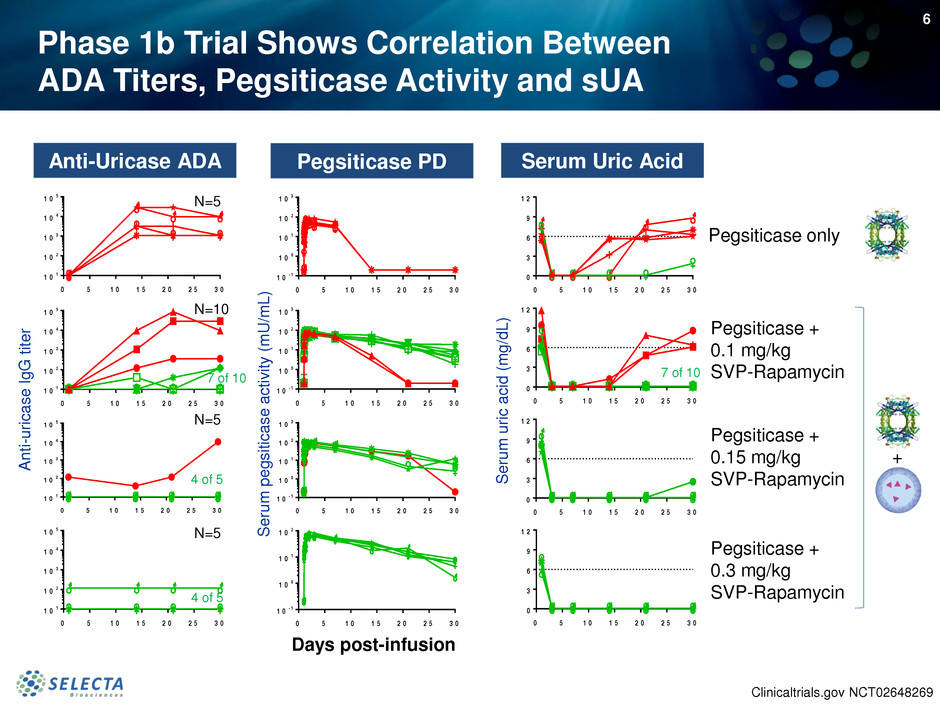
Phase 1b Trial Shows Correlation Between
ADA Titers, Pegsiticase Activity and sUA
6
0 5 1 0 1 5 2 0 2 5 3 0
0
3
6
9
1 2
0 5 1 0 1 5 2 0 2 5 3 0
0
3
6
9
1 2
0 5 1 0 1 5 2 0 2 5 3 0
0
3
6
9
1 2
0 5 1 0 1 5 2 0 2 5 3 0
0
3
6
9
1 2
S
e
ru
m
u
ri
c
a
cid
(
m
g
/d
L
)
0 5 1 0 1 5 2 0 2 5 3 0
1 0
- 1
1 0
0
1 0
1
1 0
2
1 0
3
0 5 1 0 1 5 2 0 2 5 3 0
1 0
- 1
1 0
0
1 0
1
1 0
2
1 0
3
0 5 1 0 1 5 2 0 2 5 3 0
1 0
- 1
1 0
0
1 0
1
1 0
2
3
0 5 1 0 1 5 2 0 2 5 3 0
1 0
- 1
1 0
0
1 0
1
1 0
2
S
e
ru
m
p
e
g
s
iticase
ac
ti
vit
y
(m
U
/m
L)
0 5 1 0 1 5 2 0 2 5 3 0
1 0
1
1 0
2
1 0
3
1 0
4
1 0
5
0 5 1 0 1 5 2 0 2 5 3 0
1 0
1
1 0
2
1 0
3
4
5
0 5 1 0 1 5 2 0 2 5 3 0
1 0
1
1 0
2
1 0
3
4
5
0 5 1 0 1 5 2 0 2 5 3 0
1 0
1
1 0
2
1 0
3
1 0
4
1 0
5
A
n
ti
-u
ri
c
a
s
e
I
g
G
tit
e
r
Days post-infusion
Pegsiticase only
Pegsiticase +
0.1 mg/kg
SVP-Rapamycin
Pegsiticase +
0.15 mg/kg
SVP-Rapamycin
Pegsiticase +
0.3 mg/kg
SVP-Rapamycin
7 of 10
4 of 5
N=5
N=10
N=5
N=5
4 of 5
7 of 10
+
Serum Uric AcidPegsiticase PDAnti-Uricase ADA
Clinicaltrials.gov NCT02648269

Trial Completion
Phase 2 Trial Overview
7
• Patients with symptomatic gout and serum uric acid levels >6 mg/dL
• Safety, tolerability and pharmacokinetics of multiple doses of
SEL-212 and pegsiticase alone
• Reduction of serum uric acid levels
• Reduction of ADA levels
• Multiple ascending dose cohorts
• Control cohorts: pegsiticase alone every 28 days for up to five doses
• All other cohorts: SEL-212 every 28 days for three doses followed
by two doses of pegsiticase alone
• Dosing stopped upon loss of sUA control at Days 21 after a dose
• Expected by the end of 2017
• 60 patients dosed at 11 active U.S. clinical sites
Enrollment Criteria
Primary/Secondary
Endpoints
Design
Dosing
Stopping Rules
As of June 12
Clinicaltrials.gov NCT02959918

8
Status of Phase 2 Trial Cohorts
Cohort
Treatment Weeks 0, 4, 8 Treatment Weeks 12 + 16
Status
Pegsiticase SVP-Rapamycin Pegsiticase
1 0.2 mg/kg None 0.2 mg/kg Enrollment terminated
2 0.4 mg/kg None 0.4 mg/kg Enrollment terminated
3 0.2 mg/kg 0.05 mg/kg 0.2 mg/kg Dosing completed
4 0.4 mg/kg 0.05 mg/kg 0.4 mg/kg Dosing completed
5 0.2 mg/kg 0.08 mg/kg 0.2 mg/kg Dosing completed
6 0.4 mg/kg 0.08 mg/kg 0.4 mg/kg Ongoing
7 0.2 mg/kg 0.1 mg/kg 0.2 mg/kg Ongoing
8 0.4 mg/kg 0.1 mg/kg 0.4 mg/kg Ongoing
9+ Under design Planned
Clinicaltrials.gov NCT02959918

9
Minimal Effective Dose of SEL-212 Now Defined
Unaudited data as of June 12, 2017
Clinicaltrials.gov NCT02959918
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
SU
A
(m
g/d
L)
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
SU
A
(m
g/d
L)
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
SU
A
(m
g/d
L)
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
W e e k s
SU
A
(m
g/
dL
) 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
W e e k s
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
3
6
9
1 2
0.2 mg/kg
None
Single patient
5 patients
Dosing ongoing Dosing ongoing
Cohort terminated for loss of
efficacy and patient safety
Cohort terminated for loss of
efficacy and patient safety
Single
patient
Single
patient
Pegsiticase Dose
0.4 mg/kg
S
V
P
-Rapa
m
y
cin
Dos
e
0.05
0.08
0.1
8 patients8 patients

0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1 0
1 0
2
1 0
3
1 0
4
1 0
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1 0
1 0
2
1 0
3
1 0
4
1 0
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1 0
1 0
2
3
4
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1 0
1 0
2
3
4
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1 0
1 0
2
3
4
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1 0
1 0
2
3
4
5
10
Minimal Effective Dose of SEL-212
Unaudited data as of June 12, 2017
Clinicaltrials.gov NCT02959918
0.4 mg/kg Pegsiticase +
0.08 mg/kg SVP-Rapamycin
0.4 mg/kg
Pegsiticase
114-0001
107-0004
111-0002
110-0008
106-0035
104-0010
Days
S
e
ru
m
U
ric
A
cid
(
m
g
/d
L
)
A
n
ti-U
ricas
e
A
n
ti
b
o
d
y
T
it
e
r
• Sustained reduction
of sUA after two
injections of
pegsiticase alone
suggests induction
of immune tolerance
• Cohort being
expanded to 10
evaluable patients
#
Stopping rules met (sUA levels >1 mg/dL at 21 days after dosing)#
Patient #

11
Higher Dose Cohort: 0.4 mg/kg of Pegsiticase +
0.1 mg/kg of SVP-Rapamycin
Unaudited data as of June 12, 2017
Clinicaltrials.gov NCT02959918
• sUA remains
controlled
in a majority of
patients following
repeat doses
• Two patients met
stopping rules
• One of these
patients was
inadvertently re-
dosed; experienced
an infusion
reaction and fully
recovered
Stopping rules met SAE (infusion reaction) due to protocol deviation# *
0.4 mg/kg Pegsiticase +
0.1 mg/kg SVP-Rapamycin
0.4 mg/kg
Pegsiticase
Patient #
104-0018
110-0015
103-0020
104-0017
106-0054
106-0045
107-0008
103-0019
111-0008
104-0023
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1 0
1 0
2
1 0
3
1 0
4
1 0
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1 0
1 0
2
1 0
3
1 0
4
1 0
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1 0
1 0
2
3
4
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1 0
1 0
2
3
4
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1 0
1 0
2
3
4
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1 0
1 0
2
3
4
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1
1 0
2
3
4
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1
1 0
2
3
4
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1
1 0
2
3
4
5
0 7 1 4 2 1 2 8 3 5 4 2 4 9 5 6 6 3 7 0 7 7 8 4 9 1 9 8 1 0 5 1 1 2 1 1 9 1 2 6 1 3 3 1 4 0
0
2
4
6
8
1
1 0
2
3
4
5
S
e
ru
m
U
ric
A
cid
(
m
g
/d
L
)
A
n
ti-U
ricas
e
A
n
ti
b
o
d
y
T
it
e
r
# *
#

12
• Urate lowering therapies typically increase the incidence of flares at the
beginning of therapy
• SEL-212 lowers flares compared to pegsiticase alone
0 . 0 5 0 . 0 8 0 . 1
0
2 0
4 0
6 0
% P a t i e n t s w i t h f l a r e i n m o n t h s 1 - 3
%
P
a
t
ie
n
t
s
w
it
h
f
la
r
e
s
S V P - R a p a m y c i n ( m g / k g )
+ P e g i s t i c a s e
P
e
g
i s
t i
c
a
s
e
a
l o
n
e
1 1 2 3
0
2 0
4 0
6 0
% P a t i e n t s w i t h f l a r e b y m o n t h
%
P
a
t
ie
n
t
s
w
it
h
f
la
r
e
s
S V P - R a p a m y c i n
+ P e g i s t i c a s e
P
e
g
i s
t i
c
a
s
e
a
l o
n
e
Results to Date Suggest Reduction in Flare
Frequency During SEL-212 Therapy
Unaudited data as of June 12, 2017
Clinicaltrials.gov NCT02959918

Phase 2 Safety Overview
13
• SEL-212 has been generally well tolerated at clinically active doses
following repeated administrations
• SAEs reported to date in the trial:
- Seven infusion reactions, four of which were in cohorts receiving pegsiticase
alone or pegsiticase in combination with the lowest dose of SVP-Rapamycin
and two of which were due to protocol deviations related to dosing errors
- One was for a patient who experienced cholecystitis (inflammation of gall
bladder caused by impacted gall stones), which was determined not to be
related to study drug
• All SAEs were successfully treated and resolved without further issues
Unaudited data as of June 12, 2017
Clinicaltrials.gov NCT02959918

14
Phase 2 Adverse Events
Unaudited data as of June 12, 2017
Clinicaltrials.gov NCT02959918
Cohort Entire
Study
1 2 3 4 5 6 7 8
N(%) 60 3 3 9 10 6 7 10 10
≥ 1TEAE 49(81.7) 2 2 9 8 5 5 3 6
≥ SAE 8 1 1 2 0 0 1* 1#, 1 1*
Death 0 0 0 0 0 0 0 0 0
Discontinuation
due to TEAE
8 1 1 2 0 0 1 2 1
Specific TEAEs
Infusion reaction 8(13.3) 1 1 2 0 0 1*, 1 1 1*
Gout flare 13(21.7) 3 0 2 2 1 2 1 2
Hyperglycemia1 9(15) 0 0 2 0 3 2 1 1
Hypertriglyceridemia1 4(6.7) 0 0 1 0 2 0 1 0
Infection1 9(15) 0 1 4 1 1 1 0 1
Tachycardia1 3(5) 0 0 2 1 0 0 0 0
Headache1 3(5) 0 0 0 3 0 0 0 0
Hypophosphatemia1 4(6.7) 0 0 4 0 0 0 0 0
Stomatitis or oral
lesion1
2(3.3) 0 0 0 0 1 0 0 1
Leukopenia1 10(16.7) 0 0 2 0 2 1 2 3
#Not related to study drug. Patient underwent a cholecystectomy
*Patient incorrectly dosed; protocol deviation
(1) Observed at single data points, transient in nature and mild or moderate

The Unmet Need

Substantial Unmet Need for Chronic Severe
Gout Patients
16
Tophi Significantly Increase Mortality Risk3
No/not
diagnosed tophi
8.3
3.1
5.2 4.7
0.5
Patients
Rx treated
Primary
care, endo,
nephro,
other
Rheumatologists
U.S. Gout Patients (million)1
530,000
370,000
Estimated SEL-212 Target Patient Population1
Gout treated by
rheumatologists
Est. SEL-212
patient pool
Un-diagnosed
or no Rx
treatment
Gout
prevalence
160,000
(1) IMS, Desk Research, Selecta rheumatologist interviews,
Crystal patient registry
(2) Choi HK et al, Trends in Gout and Rheumatoid Arthritis
Hospitalizations in the United States, JAMA, June 2016
(3) Vincent Z et al, Predictors of Mortality in People with Recent Onset of
Gout: A Prospective Observational Study, ACR, Sept. 2016
Gout Admissions Now Exceed RA2

What is Chronic Severe Gout?
(1) IMS, Desk Research
(2) Selecta rheumatologist interviews, Crystal patient registry
(3) Choi HK et al, Tophaceous Gout and the Risk of Mortality: A General
Population-Based Study, ACR, Sept. 2016
(4) Zhu Y, et al, Comorbidities of gout and hyperuricemia in the US general
population: NHANES 2007-2008, Am J Med, July 2012
17
• ~50,000 U.S. gout patients are refractory to standard
therapies and most have existing “tophi”1
• Over 100,000 additional patients have tophaceous
gout and remain symptomatic2
• Tophi are hidden or disfiguring inflammatory nodules
of crystallized uric acid that form in severe gout
patients
- Tend to form primarily in joints and tissues
- Source of recurrent flares and debilitating pain
that cannot be treated effectively by simply
lowering sUA to <6 mg/dL
- Shown to significantly increase morbidity and
mortality if left untreated3,4
Visible tophi
Hidden uric acid deposits
Uric acid
deposits
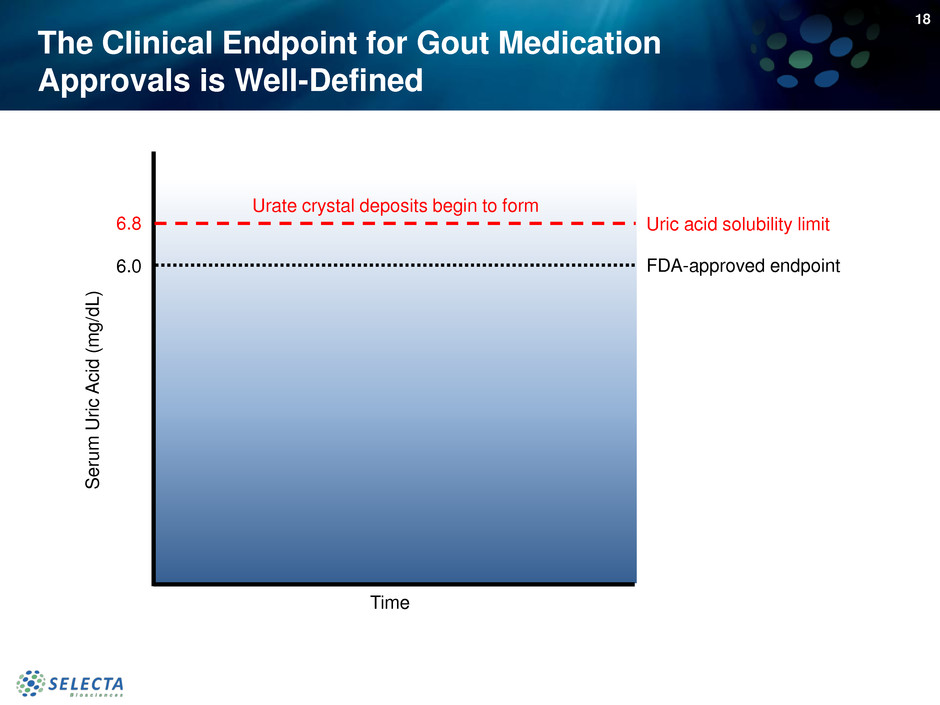
6.8
6.0
Seru
m
U
ri
c
A
c
id
(
m
g
/d
L
)
Time
Uric acid solubility limit
FDA-approved endpoint
Urate crystal deposits begin to form
The Clinical Endpoint for Gout Medication
Approvals is Well-Defined
18

While Oral Therapies May Control sUA,
They do not Effectively Resolve Tophi
19
6.8
6.0
Seru
m
U
ri
c
A
c
id
(
m
g
/d
L
)
Time
Oral therapies
For illustrative purposes only

Resolving Tophi and Uric Acid Deposits
with Monthly Uricase Treatments
20
6.8
6.0
Seru
m
U
ri
c
A
c
id
(
m
g
/d
L
)
Time
Transition from uricase
to oral therapies
Before1 After1
Uric acid deposits
Calcium deposits
Very low sUA levels enable
rapid tophi resolution
12-24 week treatment cycle
(1) Arujo EG et al, Tophus resolution with pegloticase:
a prospective dual-energy CT study, RMD Open, 2015 For illustrative purposes only
Phase 2 Trial Accomplishments to Date
21
Controlled sUA and avoided ADAs after multiple doses
Induced immune tolerance
Demonstrated low incidence of flares
SEL-212 generally well tolerated at active dose levels
Identified minimum effective dose
Data allows initial preparation of Phase 3 program design
Clinicaltrials.gov NCT02959918
Additional cohorts to be enrolled in weeks ahead

Platform Implications

Immunogenicity’s Impact on Biologic Drugs
and Product Candidates is Far-Reaching
COMPROMISED EFFICACY
Anti-drug antibodies (ADAs)
neutralize therapeutic benefit
23
UNPREDICTABLE RESPONSE
Changed PK/PD through
drug-ADA interaction
SAFETY RISK
Hypersensitivity reactions
can impact patients
“Prophylactic immune tolerance induction should be strongly considered in patients who are at risk of
developing immune responses to ERT.”
– Amy Rosenberg, MD, Director, Division of Biotechnology Products Review and Research, FDA

Proprietary program(s) Potential program(s)
24
SEL-212 for Chronic Severe Gout
Commercially attractive
Rare disease
Rapid ADA onset
Clear endpoint
Adult patients
Single
Treatment
Chronic
Treatment
Adult
Patients
Cancer
Patients
Pediatric
Patients
Oncology
(LMB-100)
Enzyme Replacement Therapy
(Pompe Disease)
Gene Therapy
(MMA and OTC)
+
Treatment
Cycle
+
+
+
SEL-212 Trial Data Inform
Platform Expansion and Progression
Ongoing Ph2 trial also
will inform clinical
programs for other
product candidates

Indication Description Preclinical Phase 1 Phase 2
Proprietary ADA Mitigation Programs
Refractory Gout
SVP-Rapamycin
co-administered with
pegsiticase (SEL-212)
Mesothelioma &
Pancreatic Cancer*
SVP-Rapamycin
co-administered with
LMB-100
Methylmalonic Acidemia
(MMA)
SVP-Rapamycin
co-administered with
Anc80 vector
Ornithine
Transcarbamylase
Deficiency (OTC)
SVP-Rapamycin
co-administered with
AAV vector
ADA Mitigation Program License
Hemophilia A
SVP-Rapamycin licensed
for FVIII gene therapy
Immune Tolerance Pipeline
25
* LMB-100 is currently being investigated in two Phase 1 clinical trials at the National Cancer Institute (NCI): one of LMB-100 alone in Mesothelioma and one
of LMB-100 in combination with nab-paclitaxel in Pancreatic Cancer. Selecta and NCI are currently in discussions regarding a planned Phase 1b clinical trial
to evaluate multiple cycles of LMB-100 in combination with SVP-Rapamycin.

