Attached files
| file | filename |
|---|---|
| 8-K - 8-K - YUMANITY THERAPEUTICS, INC. | d411564d8k.htm |
Exhibit 99.1
Exhibit 99.1 Phase 1 Initial Results From The Single Ascending Dose Cohorts Evaluating The Novel CFTR
Amplifier, PTI-428, In Subjects With CF
Proteostasis Therapeutics
ECFS conference
June 9, 2017 1
Outline
Background for PTI-428, a novel CFTR amplifier Review of PTI-428-01 study design Review of initial results from PTI-428-01 study
Summary and Conclusions 2
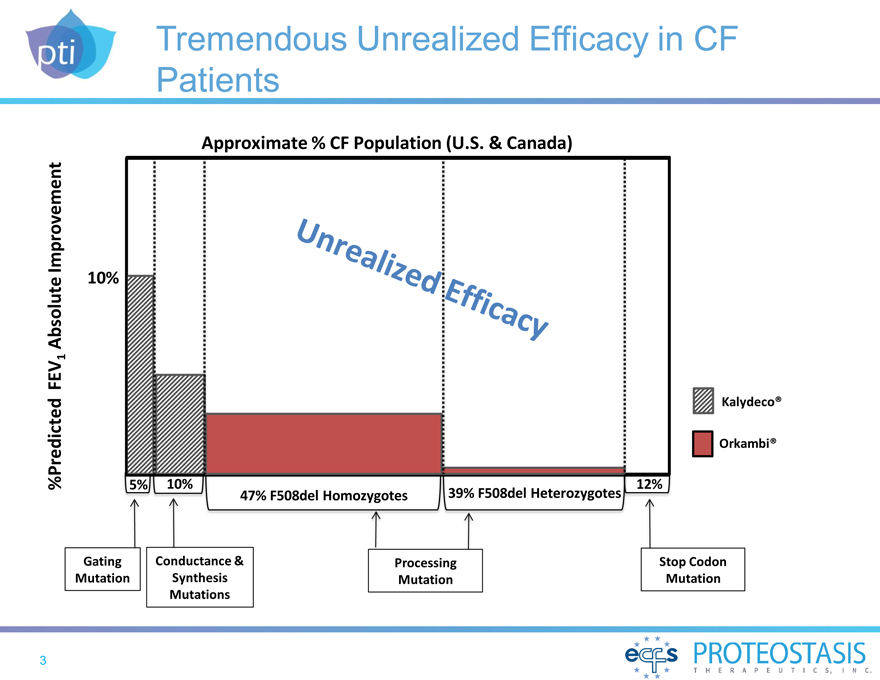
Tremendous Unrealized Efficacy in CF
Patients
Approximate % CF Population (U.S. & Canada)
%Predicted FEV1 Absolute Improvement
10%
Gating Conductance & Processing Stop Codon Mutation Synthesis Mutation Mutation Mutations
5% 10% 47% F508del Homozygotes 39% F508del Heterozygotes 12% Kalydeco®
Orkambi®
Unrealized Efficacy 3
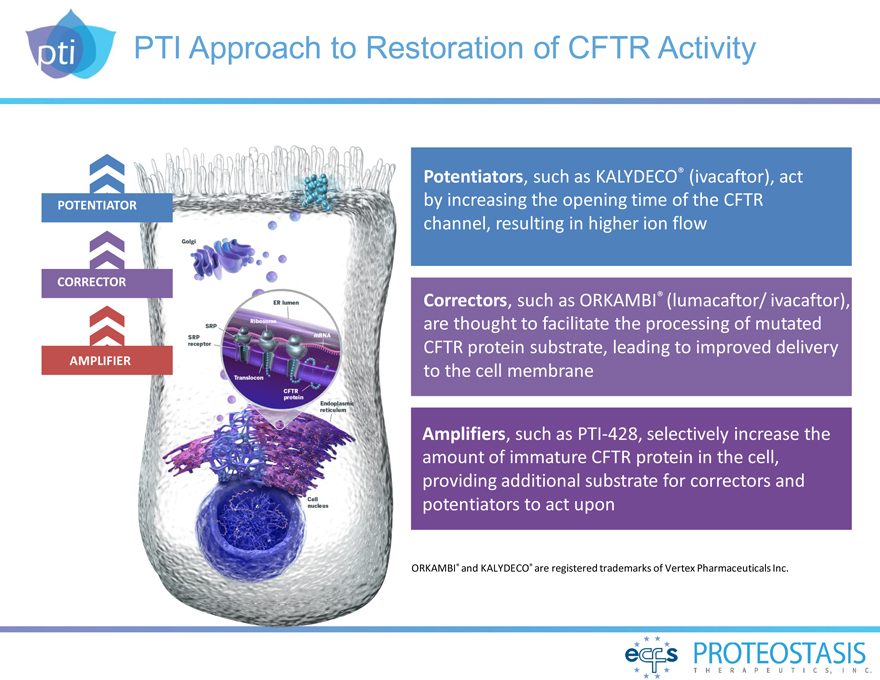
PTI Approach to Restoration of CFTR Activity
POTENTIATOR
CORRECTOR
AMPLIFIER
Potentiators, such as KALYDECO® (ivacaftor), act by increasing the opening time
of the CFTR channel, resulting in higher ion flow
Correctors, such as ORKAMBI® (lumacaftor/ ivacaftor), are thought to facilitate the processing of mutated
CFTR protein substrate, leading to improved delivery to the cell membrane
Amplifiers, such as PTI-428, selectively increase
the amount of immature CFTR protein in the cell, providing additional substrate for correctors and potentiators to act upon
ORKAMBI® and KALYDECO® are
registered trademarks of Vertex Pharmaceuticals Inc.
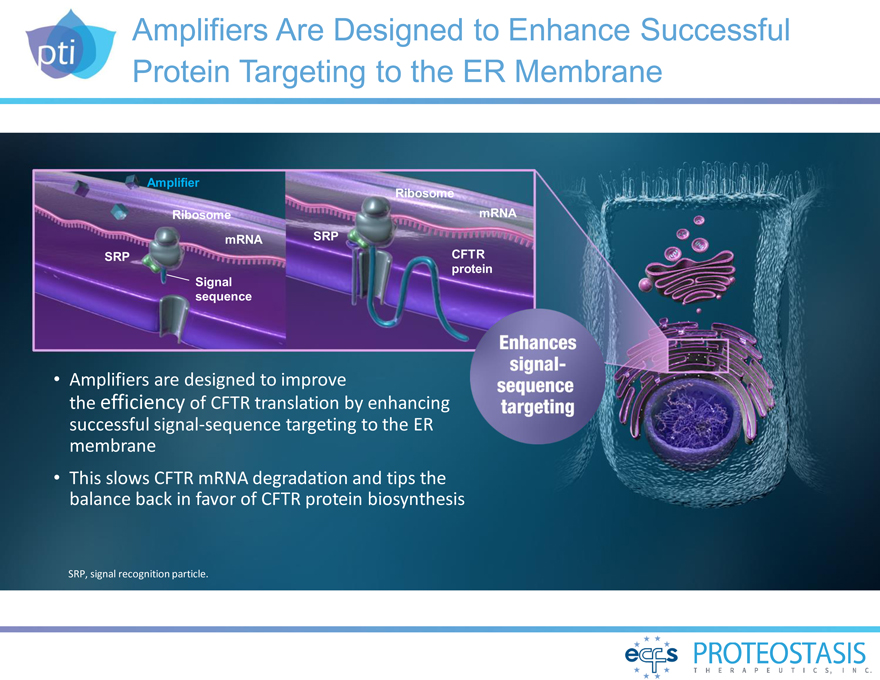
Amplifiers Are Designed to Enhance Successful Protein Targeting to the ER Membrane
Amplifier Ribosome
Ribosome mRNA mRNA SRP
SRP CFTR protein Signal sequence
Amplifiers are designed to improve the efficiency of CFTR
translation by enhancing successful signal-sequence targeting to the ER membrane This slows CFTR mRNA degradation and tips the balance back in favor of CFTR protein biosynthesis
Enhances singal-sequence targeting SRP, signal recognition particle.
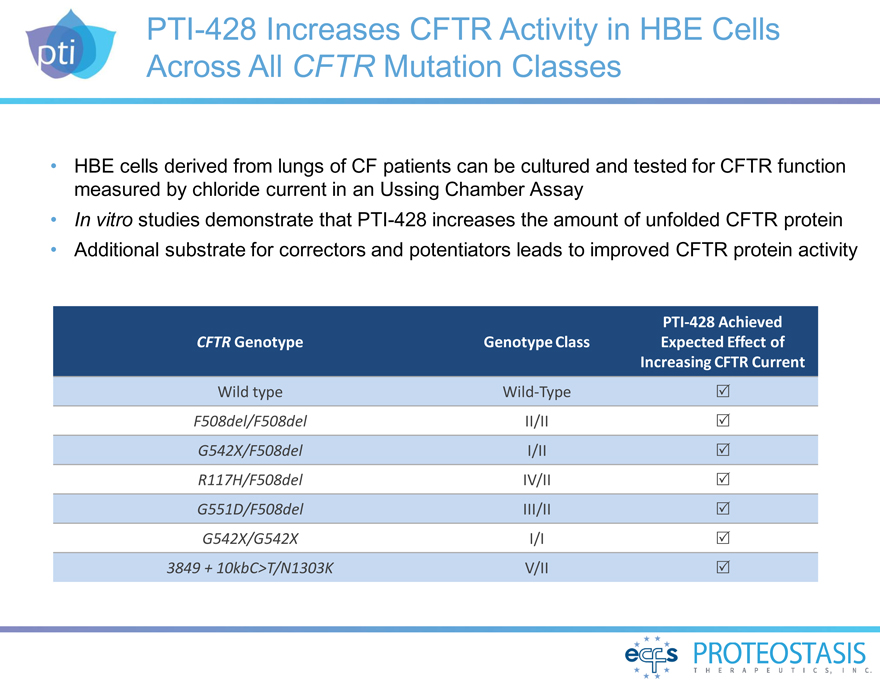
PTI-428 Increases CFTR Activity in HBE Cells Across All CFTR Mutation
Classes
HBE cells derived from lungs of CF patients can be cultured and tested for CFTR function measured by chloride current in an Ussing Chamber Assay In vitro
studies demonstrate that PTI-428 increases the amount of unfolded CFTR protein Additional substrate for correctors and potentiators leads to improved CFTR protein activity
PTI-428 Achieved
CFTR Genotype Genotype
Class Expected Effect of
Increasing CFTR Current
Wild type Wild-Type
F508del/F508del II/II
G542X/F508del I/II
R117H/F508del IV/II
G551D/F508del III/II
G542X/G542X I/I
3849 + 10kbC>T/N1303K V/II
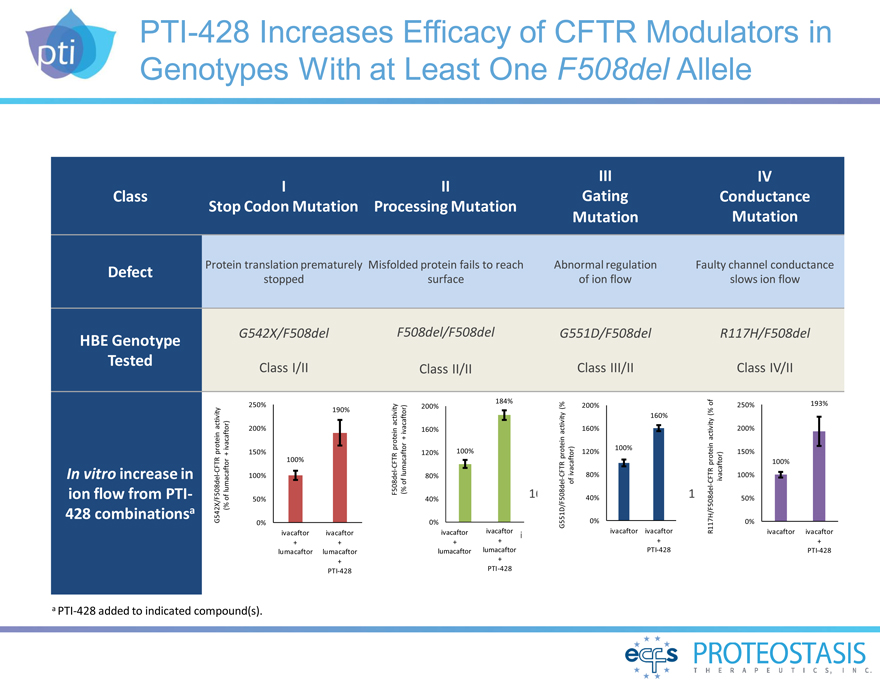
PTI-428 Increases Efficacy of CFTR Modulators in Genotypes With at
Least One F508del Allele
III IV I II
Class Gating Conductance Stop Codon
Mutation Processing Mutation Mutation Mutation
Defect
HBE Genotype Tested
In vitro increase in ion flow from PTI-428 combinationsa
Protein translation prematurely Misfolded protein fails to reach Abnormal regulation Faulty channel conductance
stopped surface of ion flow slows ion flow
G542X/F508del F508del/F508del G551D/F508del
R117H/F508del
Class I/II Class II/II Class III/II Class IV/II
a PTI-428 added to indicated compound(s).
G542X/F508del-CFTR protein activity (% of lumacaftor + ivacaftor)
0% 50% 100%
150% 200% 250%
ivacaftor ivacaftor
+ + lumacaftor lumacaftor + PTI-428
190%
100%
F508del-CFTR protein activity (% of lumacaftor + ivacaftor)
184% 200%
160%
120% 100% 80% 40%
0% ivacaftor ivacaftor
+ + lumacaftor lumacaftor +
PTI-428
G551D/F508del-CFTR protein activity (% of ivacaftor)
200%
160%
160%
100% 120%
80%
40%
0% ivacaftor ivacaftor + PTI-428
R117H/F508del-CFTR protein activity (% of ivacaftor)
250% 193%
200%
150%
100%
100%
50%
0% ivacaftor ivacaftor + PTI-428
1
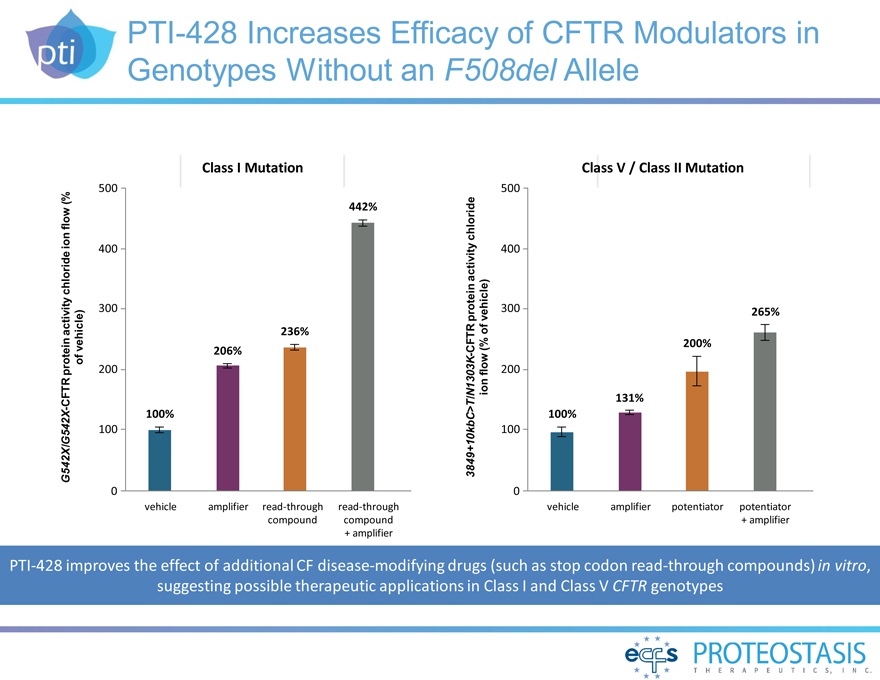
PTI-428 Increases Efficacy of CFTR Modulators in
Genotypes Without an F508del Allele
G542X/G542X-CFTR protein activity chloride ion flow (% of
vehicle)
Class I Mutation
500
442%
400
300
236% 206%
200
100%
100
0 vehicle amplifier read-through read-through compound compound +
amplifier
3849+10kbC>T/N1303K-CFTR protein activity chloride ion flow (% of vehicle)
Class V / Class II Mutation
500
400
300 265%
200%
200
131% 100%
100
0 vehicle amplifier potentiator potentiator + amplifier
PTI-428 improves the effect of additional CF disease-modifying drugs (such as stop codon read-through compounds) in vitro,
suggesting possible therapeutic applications in Class I and Class V CFTR genotypes
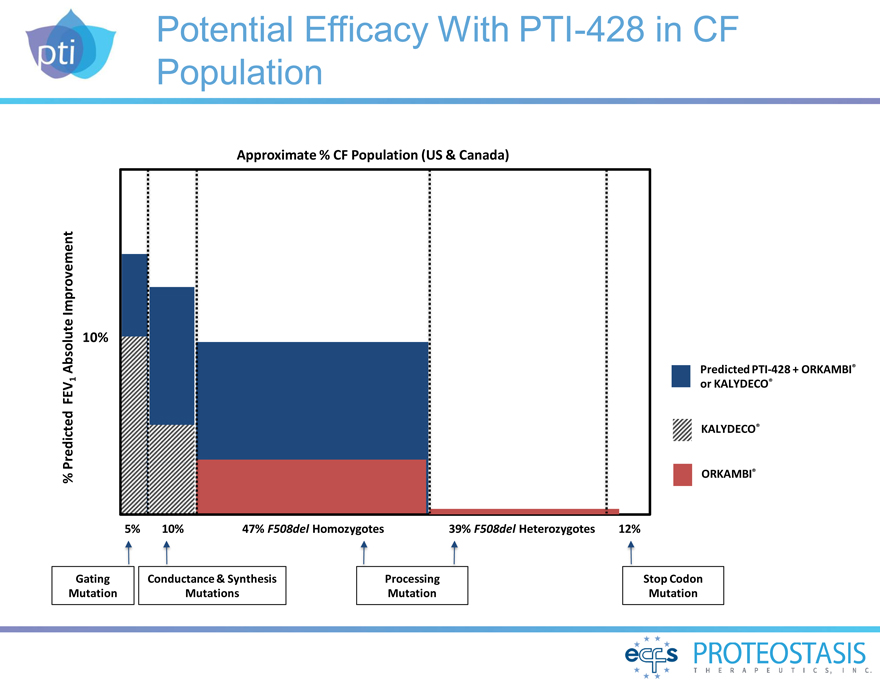
Potential Efficacy With PTI-428 in CF Population
% Predicted FEV1 Absolute Improvement
10%
Approximate % CF Population (US & Canada)
Predicted
PTI-428 + ORKAMBI® or KALYDECO®
KALYDECO®
ORKAMBI®
5% 10% 47% F508del Homozygotes 39% F508del Heterozygotes 12%
Gating Conductance & Synthesis Processing Stop Codon
Mutation Mutations Mutation
Mutation
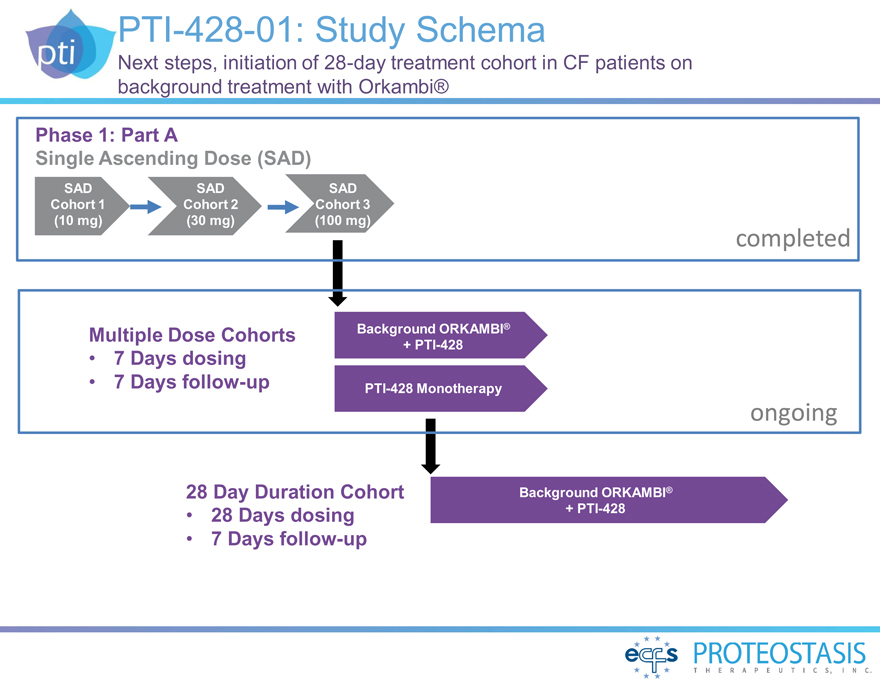
PTI-428-01: Study Schema
Next steps, initiation of 28-day treatment cohort in CF patients on background treatment with Orkambi®
Phase 1: Part A
Single Ascending Dose (SAD)
SAD SAD SAD Cohort 1 Cohort 2 Cohort 3 (10 mg) (30 mg) (100 mg)
completed
Multiple Dose Cohorts Background ORKAMBI®
+
PTI-428
• 7 Days dosing
• 7
Days follow-up PTI-428 Monotherapy
ongoing
28 Day Duration Cohort Background ORKAMBI®
• 28 Days dosing + PTI-428
• 7 Days follow-up
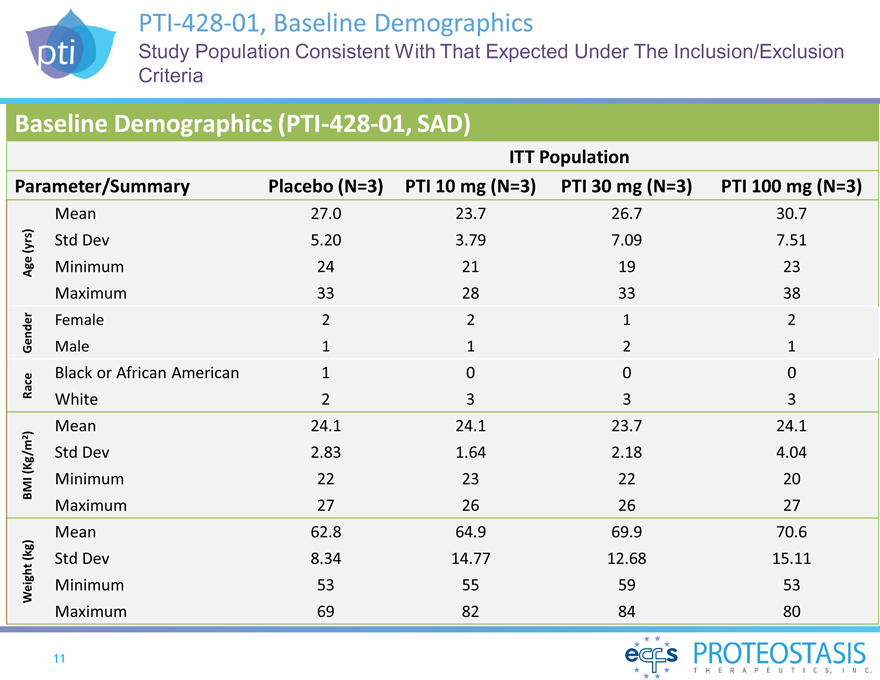
PTI-428-01, Baseline
Demographics
Study Population Consistent With That Expected Under The Inclusion/Exclusion Criteria
Baseline Demographics (PTI-428-01, SAD)
ITT Population
Parameter/Summary Placebo (N=3) PTI 10 mg (N=3)PTI 30 mg (N=3)PTI 100 mg (N=3)
Mean 27.023.726.730.7
(yrs) Std Dev 5.203.797.097.51
Age Minimum 24211923
Maximum 33283338
Female 2212
Gender Male 1121
Black or African American 1000
Race White 2333
Mean 24.124.123.724.1
)
2
(Kg/m Std Dev 2.831.642.184.04
BMI Minimum 22232220
Maximum 27262627
Mean 62.864.969.970.6
(kg) Std Dev 8.3414.7712.6815.11
Weight Minimum 53555953
Maximum 69828480
11
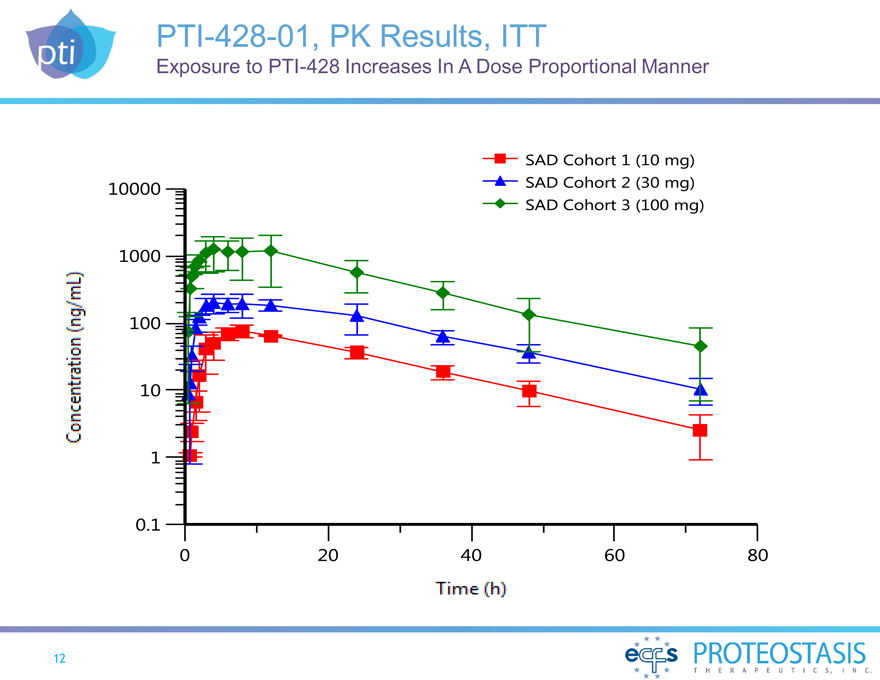
PTI-428-01, PK Results, ITT
Exposure to PTI-428 Increases In A Dose Proportional Manner
Concentration (ng/mL)
10000 1000 100 10 1 0.1 0 20 40 60 80 Time (h)
SAD Cohort 1 (10 mg)
SAD Cohort 2 (30 mg)
SAD Cohort 3 (100 mg)
12
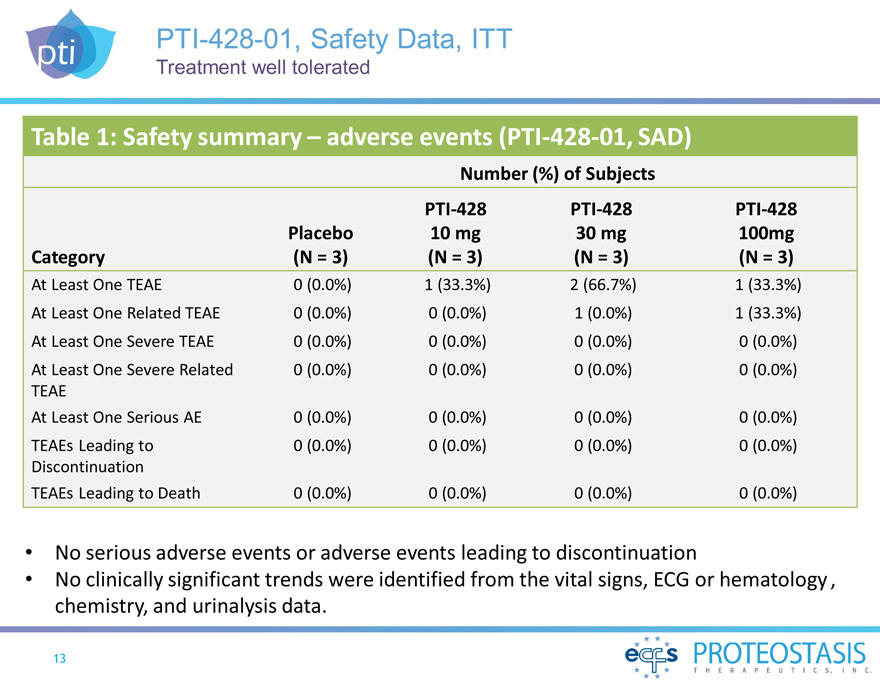
PTI-428-01, Safety Data, ITT
Treatment well tolerated
Table 1: Safety summary – adverse events (PTI-428-01, SAD)
Number (%) of Subjects
PTI-428PTI-428PTI-428
Placebo10 mg30 mg100mg
Category (N = 3)(N = 3)(N = 3)(N = 3)
At Least One TEAE 0(0.0%)1 (33.3%)2 (66.7%)1 (33.3%)
At Least One Related TEAE 0
(0.0%)0(0.0%)1(0.0%)1 (33.3%)
At Least One Severe TEAE 0(0.0%)0(0.0%)0(0.0%)0(0.0%)
At Least One Severe Related 0(0.0%)0(0.0%)0(0.0%)0(0.0%)
TEAE
At Least One Serious AE 0 (0.0%)0(0.0%)0(0.0%)0(0.0%)
TEAEs Leading to
0(0.0%)0(0.0%)0(0.0%)0(0.0%)
Discontinuation
TEAEs Leading to Death 0
(0.0%)0(0.0%)0(0.0%)0(0.0%)
No serious adverse events or adverse events leading to discontinuation
No clinically significant trends were identified from the vital signs, ECG or hematology , chemistry, and urinalysis data.
13
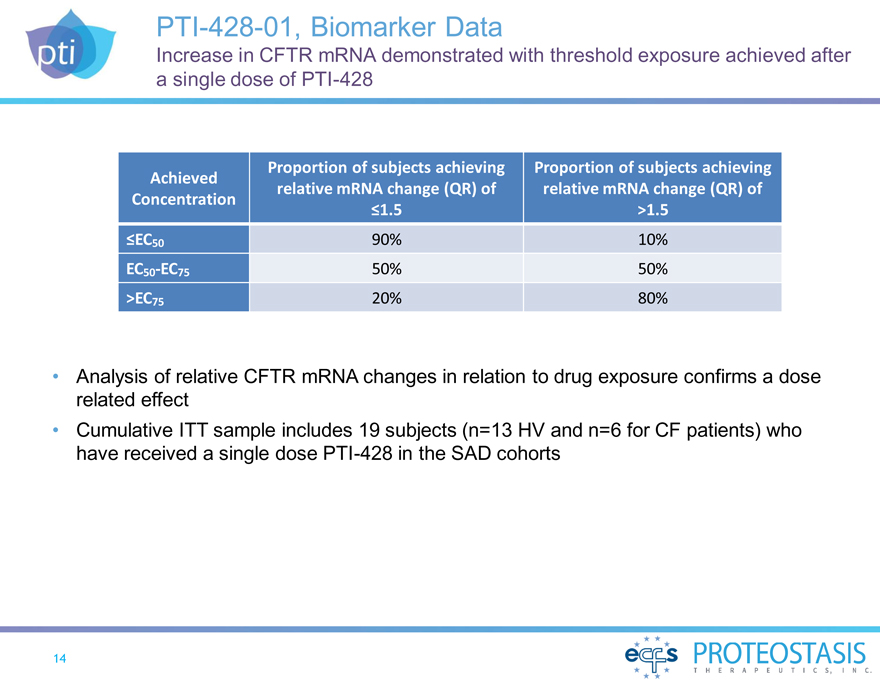
PTI-428-01, Biomarker Data
Increase in CFTR mRNA demonstrated with threshold exposure achieved after a single dose of PTI-428
Proportion of subjects achieving Proportion of subjects achieving Achieved relative mRNA change (QR) of relative mRNA change (QR) of Concentration
£1.5 >1.5
£EC50 90% 10% EC50-EC75 50% 50% >EC75 20% 80%
Analysis of relative CFTR mRNA changes in relation to drug exposure confirms a dose related effect Cumulative ITT sample includes 19 subjects (n=13 HV and n=6 for CF patients) who
have received a single dose PTI-428 in the SAD cohorts
14
Phase 1 Initial Results From The Single Ascending Dose Cohorts Evaluating The Novel CFTR Amplifier, PTI-428, In Subjects With CF
Summary and Conclusions
Amplifiers are a novel CFTR modulator
- Designed to improve the efficiency of CFTR translation
by enhancing successful signal-sequence targeting to the ER membrane
PK
-
Dose levels from 10-100 mg evaluated in PTI-428-01 SAD cohorts
- PK data show dose-proportionality up to 100 mg and support once daily dosing
Treatment well
tolerated
- No safety concerns identified based on SRC review of ECG, Vital Signs, hematology , chemistry, urinalysis and AE data to date
Biomarker data
- Relative CFTR mRNA changes in relation to drug exposure demonstrate a dose
related effect
Next steps
- Multiple dose cohorts are ongoing
- 28 day duration cohort in patients on background treatment with Orkambi® to initiate following completion of the multiple dose cohort
15
