Attached files
| file | filename |
|---|---|
| 8-K - 8-K - EAGLE PHARMACEUTICALS, INC. | a17-14908_18k.htm |
Exhibit 99.1
Eagle Pharmaceuticals June 2017

Forward Looking Statements This presentation contains forward-looking information within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, and other securities laws. Forward-looking statements are statements that are not historical facts. Words such as “will,” “underway,” “allow,” “expect(ed),” “pursuing,” “may,” “would,” “addressing,” “creating,” “intends,” “anticipate(s),” “plan,” “partner,” “could,” “enables,” “potential(ly),” and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to, statements regarding future events such as: the continued commercial performance of our marketed products, including but not limited to BENDEKA, which is marketed by our partner Teva, Argatroban, which is marketed by Chiesi USA and Sandoz pursuant to separate agreements and Ryanodex, which we market ourselves, as well as our ability to replicate our marketing successes for our other product candidates such as Ryanodex for EHS or other additional indications, our pemetrexed product candidate, or our fulvestrant product candidate, either through joint or direct marketing efforts; the potential lack of a need for human safety and efficacy data for the submission of an NDA for Ryanodex and the adequacy of the regulatory pathway to complete an NDA submission; the Company’s share repurchase authorization and timing and ability to continue to repurchase shares of the Company’s common stock under a share repurchase program; the business path forward for the Company between now and beyond 2026; the label expansions of Ryanodex for EHS patients and for the treatment of ecstasy and methamphetamine intoxication; the strength of the Company’s cash position and the ability to optimize the deployment of capital and take advantage of market opportunities; the potential of the Company’s pipeline to drive value between now and beyond 2026; the contribution of the Ryanodex portfolio to the Company’s growth; the timing of Ryanodex for EHS entering the market; and the advancement of any of the Company’s product candidates including but not limited to fulvestrant and pemetrexed, through the development process including FDA approval and the ability of any such products to have commercial success and to access significant new markets; and our ability to use the acquisition of Arsia Therapeutics (now Eagle Biologics) to enter into the biologics market and to effectively carry out our strategy in this new market. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond Eagle’s control, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. Such risks include, but are not limited to: whether our animal studies will support the safety and efficacy of Ryanodex for the treatment of EHS and ecstasy and methamphetamine intoxication; whether the FDA will ultimately approve Ryanodex for these indications; whether the FDA will approve our application for pemetrexed, and, if filed, fulvestrant; fluctuations in the trading volume and market price of shares of the Company's common stock, general business and market conditions and management's determination of alternative needs and uses of the Company's cash resources which may affect the Company's share repurchase program; the success of our commercial relationship with Teva and our other marketing partners and the parties’ ability to work effectively together; whether Eagle and Teva and our other marketing partners will successfully perform their respective obligations under their agreements with us; difficulties or delays in manufacturing; the availability and pricing of third party sourced products and materials; the outcome of litigation involving any of our products or product candidates or that may have an impact on any of our products or product candidates, successful compliance with FDA and other governmental regulations applicable to product approvals, manufacturing facilities, products and/or businesses; general economic conditions; the strength and enforceability of our intellectual property rights or the rights of third parties; competition from other pharmaceutical and biotechnology companies; the timing of product launches; the successful marketing of our products; the risks inherent in the early stages of drug development and in conducting clinical trials; and other factors that are discussed in Eagle’s Annual Report on Form 10-K for the year ended December 31, 2016, Quarterly Report on Form 10-Q for the quarter ended March 31, 2017 and its other filings with the U.S. Securities and Exchange Commission. Readers are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events. 2

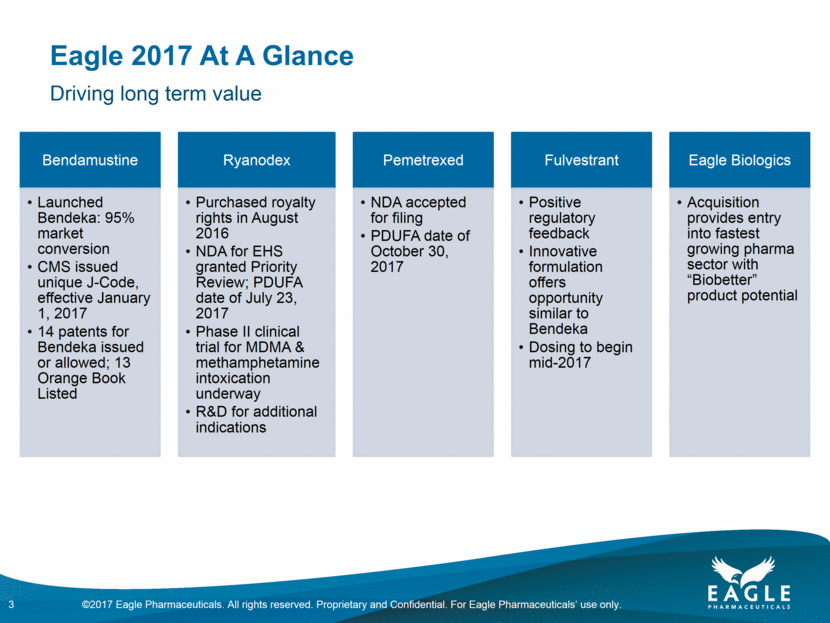
Eagle 2017 At A Glance 3 Driving long term value Bendamustine Launched Bendeka: 95% market conversion CMS issued unique J-Code, effective January 1, 2017 14 patents for Bendeka issued or allowed; 13 Orange Book Listed Ryanodex Purchased royalty rights in August 2016 NDA for EHS granted Priority Review; PDUFA date of July 23, 2017 Phase II clinical trial dosing for MDMA & methamphetamine intoxication expected to begin June 2017 R&D for additional indications Pemetrexed NDA accepted for filing PDUFA date of October 30, 2017 Fulvestrant Positive regulatory feedback Innovative formulation offers opportunity similar to Bendeka to begin mid-2017 Eagle Biologics Acquisition provides entry into fastest growing pharma sector with “Biobetter” product potential
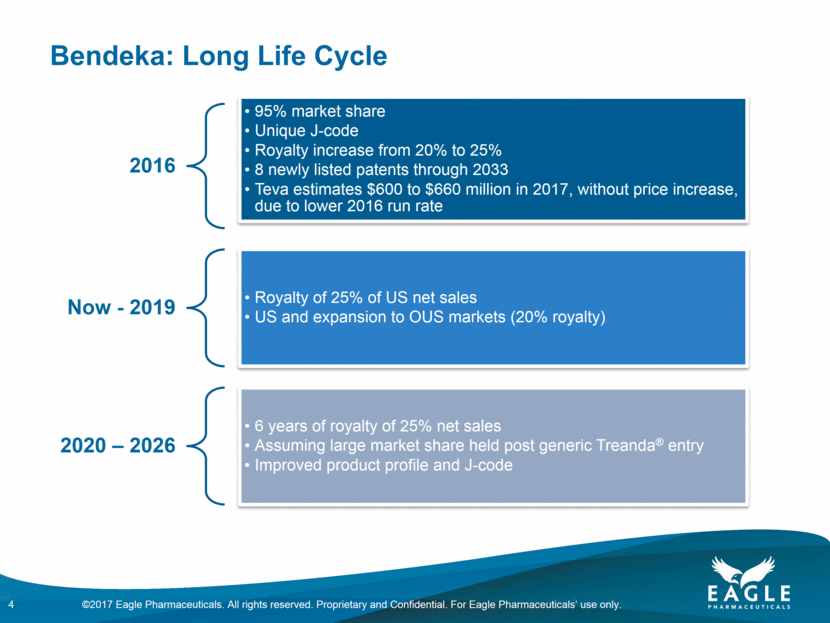
Bendeka: Long Life Cycle 4 2016 95% market share Unique J-code Royalty increase from 20% to 25% 8 newly listed patents through 2033 Teva estimates $600 to $660 million in 2017, without price increase, due to lower 2016 run rate Now - 2019 Royalty of 25% of US net sales US and expansion to OUS markets (20% royalty) 2020 – 2026 6 years of royalty of 25% net sales Assuming large market share held post generic Treanda® entry Improved product profile and J-code
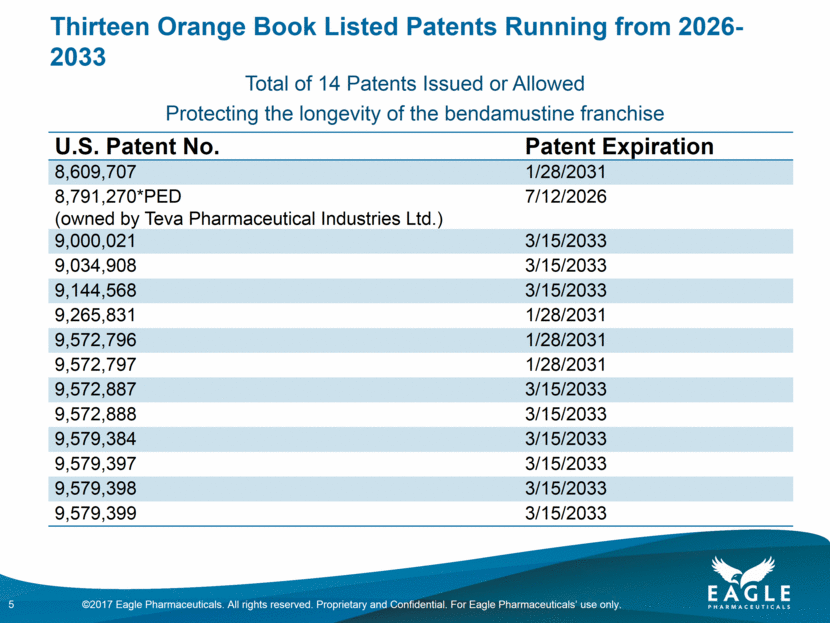
Thirteen Orange Book Listed Patents Running from 2026-2033 5 U.S. Patent No. Patent Expiration 8,609,707 1/28/2031 8,791,270*PED (owned by Teva Pharmaceutical Industries Ltd.) 7/12/2026 9,000,021 3/15/2033 9,034,908 3/15/2033 9,144,568 3/15/2033 9,265,831 1/28/2031 9,572,796 1/28/2031 9,572,797 1/28/2031 9,572,887 3/15/2033 9,572,888 3/15/2033 9,579,384 3/15/2033 9,579,397 3/15/2033 9,579,398 3/15/2033 9,579,399 3/15/2033 Total of 14 Patents Issued or Allowed Protecting the longevity of the bendamustine franchise
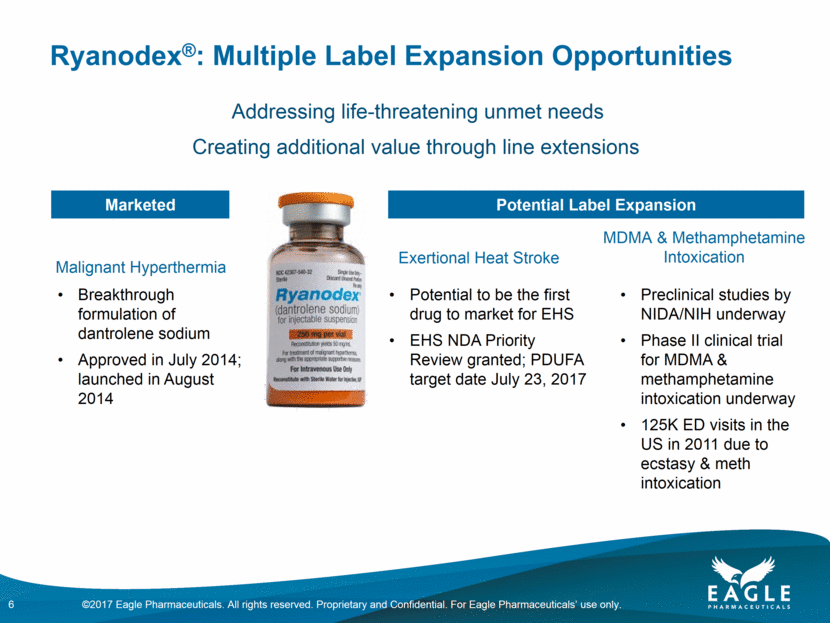
Ryanodex®: Multiple Label Expansion Opportunities Creating additional value through line extensions Breakthrough formulation of dantrolene sodium Approved in July 2014; launched in August 2014 Potential to be the first drug to market for EHS EHS NDA Priority Review granted; PDUFA target date July 23, 2017 Preclinical studies by NIDA/NIH underway Phase II clinical trial for MDMA & methamphetamine intoxication expected underway 125K ED visits in the US in 2011 due to ecstasy & meth intoxication Marketed Potential Label Expansion Malignant Hyperthermia Exertional Heat Stroke MDMA & Methamphetamine Intoxication 6 Addressing life-threatening unmet needs
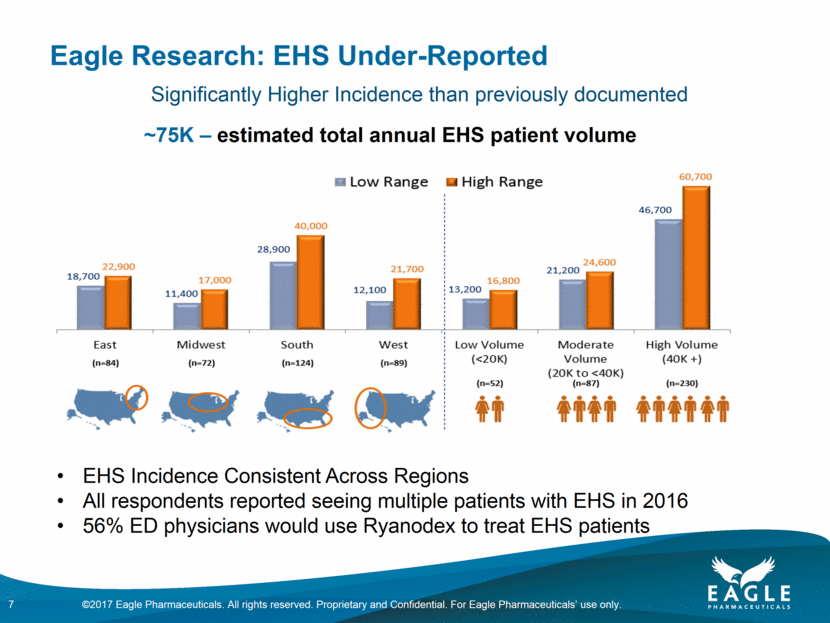
Eagle Research: EHS Under-Reported 7 ~75K – estimated total annual EHS patient volume EHS Incidence Consistent Across Regions All respondents reported seeing multiple patients with EHS in 2016 56% ED physicians would use Ryanodex to treat EHS patients Significantly Higher Incidence than previously documented
References: 1. Center for Behavioral Health Statistics and Quality, SAMHSA, Drug Abuse Warning Network, National Estimates of Drug-Related Emergency Department Visits, 2011. 2. E Musselman M, Saely S. Diagnosis and treatment of drug-induced hyperthermia. Am J Health-Syst Pharm. 2013 Vol 70. Use of illegal stimulants constitutes a growing public health problem in the US and EU1 Over 125,000 ED visits related to MDMA (ecstasy), and methamphetamine use in the US alone (2011)1 ED visits involving MDMA among patients 21 years and younger grew over 128% between 2005-20111 In 2011, 42% of ED visits among people 18-29 years old involved illicit stimulant drugs1 Associated with body and brain hyperthermia, high incidence of severe cardiovascular and neurologic complications, and lifetime neurologic sequelae2 Can be fatal or lead to permanent damage, if not treated promptly2 MDMA (Ecstasy) and Methamphetamine Intoxication 8
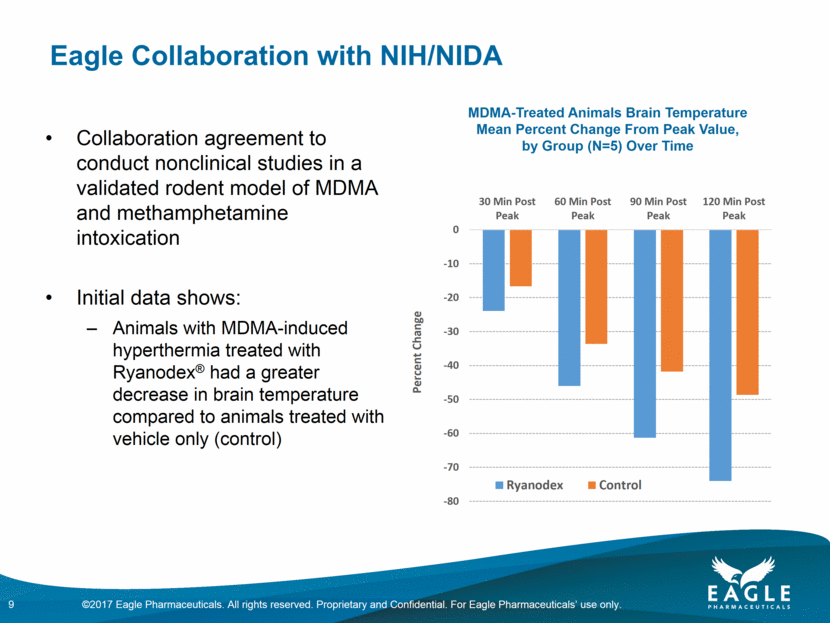
Eagle Collaboration with NIH/NIDA 9 Collaboration agreement to conduct nonclinical studies in a validated rodent model of MDMA and methamphetamine intoxication Initial data shows: Animals with MDMA-induced hyperthermia treated with Ryanodex® had a greater decrease in brain temperature compared to animals treated with vehicle only (control) MDMA-Treated Animals Brain Temperature Mean Percent Change From Peak Value, by Group (N=5) Over Time -80 -70 -60 -50 -40 -30 -20 -10 0 30 Min Post Peak 60 Min Post Peak 90 Min Post Peak 120 Min Post Peak Percent Change Ryanodex Control
MDMA (Ecstasy) & Methamphetamine Intoxication: Positive pre-IND Meeting with FDA FDA suggested broadening the indication to evaluate ‘organ damage and/or severe dysfunction’ in patients with MDMA and Methamphetamine intoxication No additional preclinical work required to support the efficacy of Ryanodex for this indication A single robust, controlled and well powered clinical trial may be sufficient for filing the NDA Planning and execution of a pilot clinical study is underway Phase II clinical trial for MDMA & Methamphetamine intoxication expected to begin June 2017; anticipate pivotal study to begin Q4 2017, if needed 10
Pemetrexed Opportunity At this time, Lilly’s Alimta patent infringement lawsuit prevents current ANDA filers from launching until May 24, 2022 Eagle’s Pemetrexed RTU NDA was filed December 2016 NDA accepted with PDUFA date of October 30, 2017 $1.1B market opportunity1 11 1 Alimta® (pemetrexed) (Eli Lilly & Co.). Source: Eli Lilly & Co. Q2 2016 earnings for MAT 12 mos. ending 6/30/13: (U.S. sales)
Fulvestrant Opportunity INDICATIONS for FASLODEX ® Monotherapy FASLODEX is indicated for the treatment of hormone receptor (HR)-positive metastatic breast cancer in postmenopausal women with disease progression following antiestrogen therapy. Combination Therapy FASLODEX in combination with palbociclib is indicated for the treatment of HR-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer in women with disease progression after endocrine therapy. Currently marketed by AstraZeneca Administered monthly in a doctor’s office as 2 separate intramuscular injections, one in each buttock FDA has recently required revising the Faslodex label 12 Faslodex ® is a registered trademark of AstraZeneca.
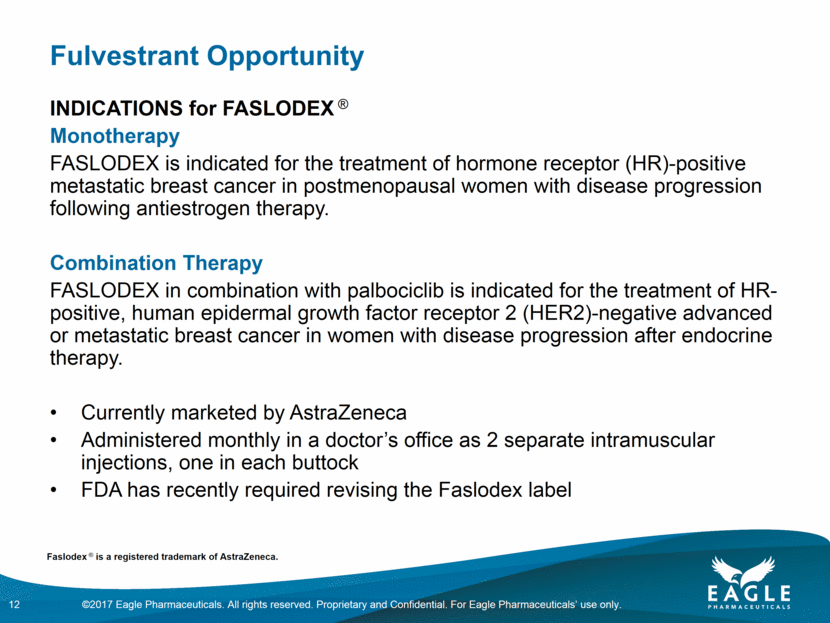
The Problem 2 deep intramuscular injections of high viscosity product per dose of treatment (5 ml each) Administered over 1-2 minutes into each buttock Painful procedure FASLODEX injection reactions have been associated with peripheral nerve adverse reactions, including risk of damaging the sciatic nerve 13
Positive Regulatory Feedback from FDA regarding Fulvestrant Clinical Protocol Pre-IND meeting responses are aligned with Eagle’s development plan A single PK and safety study in healthy female volunteers may be sufficient for filing Expect study to recruit and complete within 12 months FDA has agreed to consider not including Injection Site Reaction (Warning & Precautions) language in Eagle’s label, provided that our clinical data demonstrates a significant safety improvement 14
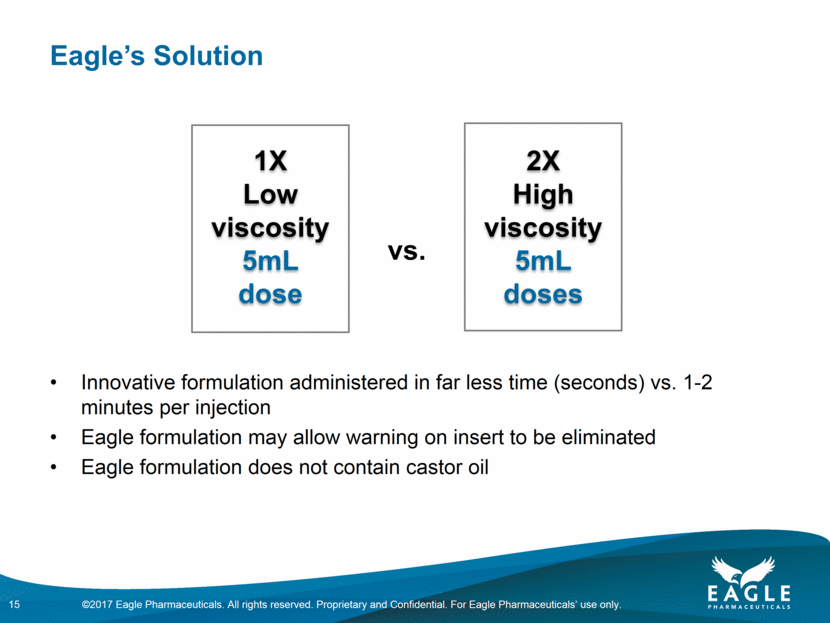
Eagle’s Solution Innovative formulation administered in far less time (seconds) vs. 1-2 minutes per injection Eagle formulation may allow warning on insert to be eliminated Eagle formulation does not contain castor oil 1X Low viscosity 5mL dose 2X High viscosity 5mL doses vs. 15
Faslodex Market Opportunity $400mm+ O.U.S. 2016 total sales of the branded form of fulvestrant, Faslodex, were up 19% to $830 million worldwide 23% growth in US sales to $438m 11% growth in European sales to $228m 25% growth in Emerging Markets sales to $96m; supported by the launch of 500mg Faslodex China sales accelerated in the year to $20m Source: AstraZeneca Financial Summary Full Year and Q4 2016 Results)www.londonstockexchange.com/exchange/news/market-news/market-news 16
Eagle Biologics (Arsia Acquisition) Marks Eagle’s entry into Biologics, fastest growing pharmaceutical sector Enhances Eagle’s formulation capabilities and expands product development opportunities Secures a patent portfolio of viscosity-reducing technology Provides access to leading minds in the field who will work on additional Eagle formulations Extends Eagle’s strategy: plan to partner with key Biosimilar or Bioinnovator companies to alter their existing pipeline into “Biobetters” $78 million investment: largely dependent upon achievement of milestones 17
Biologics Market Opportunity The global biologics market could exceed $390 billion in value over the next five years1 Growing at nearly 2X the rate of pharma1 By the end of 2020, biologics could account for 28% of the global pharmaceuticals market1 The global biosimilar market may reach $20 - $26 billion by 20202 18 References: 1. PRA Health Sciences Whitepaper. The Value of Biobetters. December 2015. 2. IMS Medicines Use and Spending in the U.S. – A Review of 2015 and Outlook to 2020. April 2015.
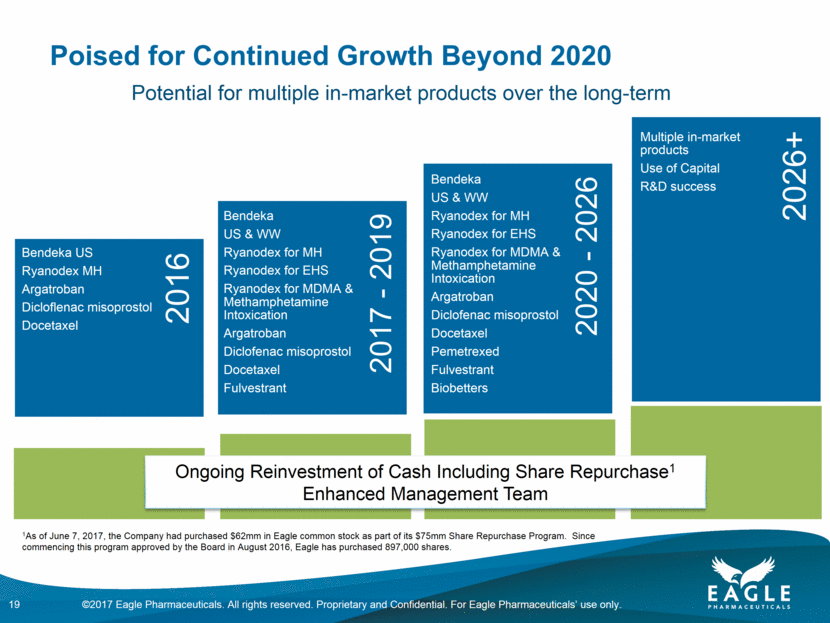
Poised for Continued Growth Beyond 2020 Ongoing Reinvestment of Cash Including Share Repurchase1 Enhanced Management Team 19 Potential for multiple in-market products over the long-term 1As of June 7, 2017, the Company had purchased $62mm in Eagle common stock as part of its $75mm Share Repurchase Program. Since commencing this program approved by the Board in August 2016, Eagle has purchased 897,000 shares. 2017 - 2019 2020 - 2026 2016 2026+ Multiple in-market products Use of Capital R&D success Bendeka US & WW Ryanodex for MH Ryanodex for EHS Ryanodex for MDMA & Methamphetamine Intoxication Argatroban Diclofenac misoprostol Docetaxel Pemetrexed Fulvestrant Biobetters Bendeka US & WW Ryanodex for MH Ryanodex for EHS Ryanodex for MDMA & Methamphetamine Intoxication Argatroban Diclofenac misoprostol Docetaxel Fulvestrant Bendeka US Ryanodex MH Argatroban Dicloflenac misoprostol Docetaxel
Thank You June 2017 20

